Risk stratification and prognosis in sepsis: what have we learned from microarrays? (original) (raw)
. Author manuscript; available in PMC: 2017 Jun 1.
Published in final edited form as: Clin Chest Med. 2016 Mar 10;37(2):209–218. doi: 10.1016/j.ccm.2016.01.003
Introduction
Sepsis kills over 750,000 people in the U.S. annually1; mortality rates have fallen in recent years as a result of clinical process improvements such as adherence to resuscitation protocols and timely administration of antibiotics2, but remain unacceptably high. Risk stratification and prognostication in sepsis is of particular importance, as high-risk patients may benefit from earlier clinical interventions, whereas low-risk patients may benefit from not undergoing unnecessary procedures. Prognostication in sepsis is currently done mostly via clinical criteria (such as organ dysfunction and/or presence of shock) and blood lactate levels. While useful, these approaches may not adequately reflect the diversity of clinical presentations seen. In addition, the lack of biomarkers to adequately quantify the heterogeneity of patients with sepsis may have contributed to the numerous failed drug trials in sepsis.3 Better risk stratification could lead to successful clinical trials through predictive enrichment and prognostic enrichment.4 Eventually, such biomarkers could personalize treatment based on where a patient resides on the spectrum of inflammation, or whether specific organs are failing.
There are multiple approaches to discovering and developing biomarkers. One such approach leverages the high throughput capabilities of transcriptomics in which thousands of genes can be simultaneously measured. These data-driven, systematic studies are particularly amenable to highly complex syndromes such as sepsis, since so many changes are occurring at once. Sepsis induces profound changes in the peripheral blood transcriptome, with 70–80% of all genes undergoing significant changes in expression.5,6 To understand and make sense of these changes thus requires a comprehensive view of the transcriptome. As a result, dozens of whole-genome expression studies in clinical human sepsis have now been completed. These studies mostly fall into three broad, often overlapping categories: (1) studies of sepsis at onset; (2) longitudinal studies of sepsis, and (3) studies of organ-specific outcomes in sepsis.
The complexity of changes at the molecular level has made interpreting individual studies difficult for the casual reader; we thus performed a summary of the literature. We did not review: (1) animal studies of sepsis, (2) studies of critical illness (i.e., traumatic injuries) without sepsis, (3) studies that only sampled later time-points in sepsis (more than 48 hours after onset), (4) studies of acute infection only without sepsis, and (5) studies of sepsis that only examined small numbers of genes (i.e., studies not using highly parallel technologies). We focused on synthesizing both validated clinical findings and recurring themes across studies of whole blood or sorted leukocytes evaluating gene expression in sepsis.
Prognostication of mortality at admission: gene expression at the onset of sepsis
The first microarray study in clinical sepsis was published in 2004; the principle findings were that two thirds of all genes assayed were differentially expressed, and that septic inflammation was distinctly different from the inflammation that underlies other critical illness.7 Since that time, both findings have been confirmed my much larger studies with more advanced technologies.5,6,8,9 Having found that septic inflammation can be distinguished from non-septic inflammation, the next question was whether subtypes of sepsis both known (i.e., survivors and non-survivors) and unknown (i.e., new classifications based on gene expression patterns) could be discovered in gene expression data. Pachot et al. established that within a cohort, gene expression patterns in early sepsis could divide survivors from non-survivors10; however, these results were likely overfit (a common failing in high-dimensional data) as the gene expression pattern that distinguished survivors from non-survivors has not been independently validated.
Several other studies have also examined how gene expression in early sepsis differs between eventual survivors and non-survivors. Reproduced findings in non-survivors include an early decrease in adaptive immunity as compared to innate immunity 11–13, disrupted cell cycle control genes12,14,15, increases in protease and metal-ion regulation pathways 12,14, and increased expression of innate inflammatory cytokines such as IL-1, IL-6, and IL-18 (Table 1).11,12,16 On the other hand, two studies reported either no or very few genes significantly differently regulated between eventual survivors and non-survivors at sepsis onset.5,17
Table 1.
Validated findings of changes in non-survivors or higher-mortality subgroups in sepsis. Reference is always survivors; thus the direction of change refers to the changes in non-survivors. SOFA, sequential organ failure assessment score.
| Wong et al. 2007 | Parnell et al. 2011 | Dolinay et al. 2012 | Parnell et al. 2013 | Severino et al. 2014 | Tsalik et al. 2014 | Almansa et al. 2015 | Scicluna et al. 2015 |
|---|---|---|---|---|---|---|---|
| Suppressed adaptive immune genes | Greater “Immune Suppression Integer”, decreased lymphocytes | Decreased MHC I and MHC II genes | T-Cell response genes negatively correlated with SOFA | ||||
| Disrupted cell cycle control | Increased cyclins and CDKs in higher-mortality group | Cell cycle control genes correlate with SOFA | |||||
| Increased neutrophil-specific proteases | Increased metallothionein, granzyme B | Increased metallothioneins and MMP8/MMP9 | Increased ELANE, MPO, MMP8, CTSG with increased SOFA | ||||
| Increased inflammatory cytokines | Increased IL8 gene and protein levels | Increased IL18 gene and protein levels | Increased IL1R2, IL18R1, IL18RAP | Increased IL1R2, IL18R1 with increased SOFA | |||
| Other | No significant difference shown, trend towards mitochondrial dysfunction | Only three genes significantly different in non-survivors (BCL2L15, NNMT, PRTN) |
Another way to stratify patients in a supervised fashion is to examine illness severity instead of the binary outcome of mortality. While such studies might not be immediately clinically actionable (since a gene expression model to predict a clinical score is made redundant by that clinical score), they might provide pathophysiologic insights or potential markers for risk stratification. Results have been mixed; one group reported that patients with worse outcomes show a greater degree of change in their gene expression profiles18, while another reported that among patients with septic shock, more genes were differentially regulated in the lower-severity group than in the higher-severity group (as measured by SAPS score).19 A more targeted approach is to study correlation coefficients between severity scores and gene expression20; Almansa et al. found modest but significant correlation (mostly absolute Spearman r<0.5) between the expression levels of 55 genes and SOFA scores of patients with sepsis; however, no model of severity was constructed.12 Such widely divergent results in the study of sepsis severity and mortality are likely explained both by differences in underlying biology between different cohorts, as well as strong confounding from technical and informatics approaches, sampling time, and study design.
More important than qualitative differences in the transcriptome of survivors is a testable clinical model. Here the hypothesis is that a set of genes with a trained predictive model could give a probability of mortality at the time of admission. The Pediatric Sepsis Biomarker Risk Model (PERSEVERE) is probably the best-validated biomarker set to estimate the probability of mortality at the time of admission.21,22 The PERSEVERE model is a decision tree that incorporates the plasma levels of the protein products of 5 genes (initially identified via microarray but subsequently tested with targeted assays) plus patient age; it can accurately predict mortality (AUC 0.81) in septic children at admission with a high level of sensitivity, and its level is correlated with severity.21 PERSEVERE also added significant classification power to the PRISM score, with a net reclassification index greater than 0.9. A second model has been developed for adults, which includes 4/5 of the same protein products, plus age, lactate level, and presence of chronic disease; this model outperforms APACHE II at predicting mortality, with an AUC of 0.76.23 These sepsis severity models are based on protein assays, and so should be translatable to clinically actionable predictions of disease severity.
The importance of time in studies of acute critical illness
Several studies have shown the importance of time since illness onset in interpreting the underlying genomic response. The host response to sepsis changes significantly even within the first 24–48 hours; in fact, the set of genes differentially expressed in sepsis changes by 20–50% within a 24-hour period.19,27 Ongoing nonlinear changes have been confirmed in the following 5–7 days. Similar non-linear changes in host gene expression have been found as late as a year after traumatic injuries and burn injuries.6 Two main approaches have thus been taken in longitudinal studies of sepsis: one is to compare cases and controls at matched time points in short enough time windows such that the underlying response approaches linearity8,9; the other is to use advanced nonlinear functions to model the underlying changes.28,29 Both have been leveraged to make use of several longitudinal studies of sepsis.
Findings from longitudinal studies: predicting hospital-acquired sepsis
There are several studies that have focused not on prognosis within sepsis but rather prognosis of sepsis in hospitalized patients. Here the hypothesis is that a set of genes can predict infections earlier than clinical diagnosis (there are several other studies that focus on diagnostic gene sets at the time of diagnosis, but pure diagnosis is outside the scope of this manuscript). A summary of these studies can be found in Table 2.
Table 2.
Findings in the studies examining prediction of onset of sepsis in hospitalized patients. TLR, toll-like receptor; MAPK, mitogen-activated protein kinase; GO, gene ontology; IPA, Ingenuity Pathway Analysis.
| Johnson et al., 2007 | McDunn et al., 2008 & Cobb et al., 2009 | Yan et al., 2015 | Sweeney et al., 2015 | |
|---|---|---|---|---|
| Clinical Setting | Prediction of sepsis as compared to ICU patients with non-infectious SIRS | Prediction of VAP in ICU patients | Prediction of multiple infections in burn patients | Prediction of sepsis as compared to non-infectious SIRS in multi-cohort analysis |
| Activated inflammatory pathways | KEGG pathways activated: TLR signalling, MAPK signalling, IL-22 signalling, IL-1 signaling | Enriched defense response GO codes; enriched neutrophil activation genes | Multiple pathways, including interleukin and JAK/STAT signalling | Convergence on IL-6 and JUN signalling in IPA |
| Changes in T-cell signalling | Increased Th1 differentiation | Impaired T-cell signalling | Trend towards Treg enrichment | |
| Other findings | Increased apoptosis-associated genes | Enriched GO codes for metal ion binding, protein binding, and ATP binding | Enriched epigenetic modulation genes | Signature may be present in band neutrophils |
| Prognostic gene set | None | 85-gene set; see manuscript | Up: THBS1, ARHGEF7; Down: MDFIC, CCND2, OSBPL8, DCAF7, TMEM50B, GOLGA8A/B, SMARCA4, WHSC1L1 | Up: CEACAM1, ZDHHC19, C9orf95, GNA15, BATF, C3AR1; Down: KIAA1370, TGFBI, MTCH1, RPGRIP1, HLA-DPB1 |
Cobb et al. discovered and validated a principal components model of an 85-gene set called the ‘riboleukogram’ that they used to predict eventual ventilator-associated pneumonia (VAP) in critically ill patients.28,29 In principal component space, the paths for patients with eventual VAP and those without were significantly different several days before VAP diagnosis.
Johnson et al. examined time-matched ICU patients with SIRS who eventually developed infections and showed that 12, 36, and even 60 hours prior to eventual diagnosis of sepsis, compared to never-infected patients, pre-septic patients showed upregulation of innate inflammatory networks and pattern recognition receptor pathways.8 That group did not build models for prognosis.
Yan et al. studied patients with severe burns to predict which patients would go on to have multiple episodes of infection.30 They showed that a 14-probe (10-gene) signature could improve on a regression model for multiple infections built from only clinical parameters, suggesting the added utility of genomic markers in clinical medicine.
Sweeney et al. performed a meta-analysis of gene expression in nine cohorts of patients with non-infectious SIRS versus patients sepsis and discovered a diagnostic 11-gene set.9 They validated this 11-gene set in multiple independent public sepsis gene expression datasets. They further found that the 11-gene set was prognostic of sepsis in both discovery and validation in longitudinal trauma patient cohorts 2–5 days prior to onset of sepsis, raising the possibility of earlier diagnosis than is currently clinically possible.
Prognosis of sepsis in the hospitalized patient could potentially reduce both morbidity and mortality, but its applications are not yet fully understood. Daily testing for monitoring of prognostic gene sets seems both costly and disruptive; more likely is an integrated model of a clinical score that triggers a gene expression test. Further work is necessary prior to clinical implementation of any of the sepsis-prognostic models.
“Immune paralysis” in sepsis: an updated view from CARS to PICS
The impaired adaptive immune response in sepsis has been previously referred to as a compensatory anti-inflammatory response syndrome (CARS), also known as ‘immune paralysis’. Hypothesis-driven mechanistic studies of immune paralysis in sepsis have been invaluable in establishing some key events. However, computational analysis of transcriptomic studies has allowed significant advances in understanding the multiple nonlinear changes that occur in the different portions of the immune system. Severe immune dysregulation is present not only in sepsis but also in other severe critical illness, such as the response to traumatic injury. The Glue Grant, a multicenter, longitudinal study of trauma and burn patients, performed microarray gene expression profiling on both whole blood and sorted-cells of these patients. Their findings have, in particular, driven and confirmed two major paradigmatic shifts in the understanding of immune paralysis.
Xiao et al. showed that adaptive immune gene modules were rapidly and persistently depressed in trauma patients (and to a greater degree in patients with complicated ICU course).31 This showed that, rather than being an adaptive response to critical inflammation, immune paralysis is induced simultaneously with SIRS.
Desai et al. looked at the same patients over their entire hospital stays, and divided them into five ordered groups depending on multi-organ dysfunction scores and long-term outcomes.20 They showed that gene expression modules for adaptive immunity (among others) became more dysregulated over time in patients with worse outcomes. In particular, the expression of genes associated with antigen-presenting cells fell in correlation with worsening outcomes.
A new characterization of immune dysregulation in chronic critical illness has been proposed in response to this and other studies, the “persistent inflammation-immunosuppression catabolism syndrome” (PICS).32 PICS describes the state of ongoing immune dysregulation in chronically critically ill patients. Notably, the group that described PICS was able to validate it with a mix of clinical and microarray data from the Glue Grant database.33 In particular, they showed that T-cells from complicated patients late in their course showed gene expression patterns consistent with T-reg-induced suppression, while the neutrophils and monocytes from the same patients still showed persistent activation of innate immune inflammation.
Interestingly, a separate group has used totally separate methods to come to similar conclusions. Pena et al. studied the transcriptomes from monocytes with endotoxin tolerance (induced with a two-hit endotoxin exposure) for a conserved set of ‘endotoxin tolerance’ genes similar to M2 polarization.34 They then used RNA-seq in a validation cohort to show that the endotoxin tolerance signature is more enriched in patients with more severe sepsis at admission (more organ failure) than in less-complicated cases.35 They further showed that their endotoxin tolerance module can be found in several independent sepsis datasets. This confirms that the immune paralysis/tolerance seen in late critical illness is also present in early severe sepsis, and may contribute to the worse outcomes of high-risk patients.
Markers of organ-specific outcomes in peripheral blood
Of particular interest in stratifying patients with sepsis is the notion of predicting specific organ dysfunctions, as such predictions might be more clinically actionable than simply stratifying a patient to a higher-mortality group. Predicting lung injury could allow for early low-tidal volume ventilation, for instance, or predicting kidney injury could lead to avoidance of further nephrotoxicity. Here the hypothesis is that leukocytes (or the sorted cell type being profiled) carry gene expression patterns that are specific for a distant site of injury (i.e. the failing organ system being studied). Circulating cells are, in some sense, one step removed from the organ in question (unlike in studies of systemic inflammation, where circulating cells are largely thought to be the mediators of the effect). However, peripheral blood (or sorted cell) gene expression may still reveal patterns of inflammation that are specific to a failing organ or organ system.
Several studies have examined the transcriptomic whole blood response in lung injury or acute resipiratory distress syndrome (ARDS) in sepsis.16,36–38 The gene sets found to be differentially expressed in ARDS are not highly overlapping in any of the studies. Interestingly, the genes found to be increased in ARDS are often genes found to be associated with more-severe sepsis or septic shock in other studies (such as MMP8, RETN, IL1B, IL18, OLFM4). The most likely explanation for this is that the lung injury signal is strongly confounded by worse systemic inflammation in patients with lung injury; it is also possible, though, that the higher severity of sepsis in other studies also included some degree of lung injury.
Similar results were found in relation to septic-shock associated kidney injury (AKI), with MMP8, IL1R2, OLFM4 and other genes previously associated with severity increased in patients with AKI.39 Here, however, all patients were children with septic shock, which removes at least some confounding from clinical severity.
Not all genes associated with specific organ failure have been shown to be associated with sepsis severity. However, they were also not reproduced between studies. In general, the effects of any organ-specific pattern of gene expression may be small compared to the profound changes in systemic inflammation in severe sepsis. Coupled with generally small sample sizes in the studies done so far, the only firm conclusions are that more studies are necessary, and that controlling for severity with advanced informatics approaches will likely be necessary.
Unsupervised learning of sepsis subtypes
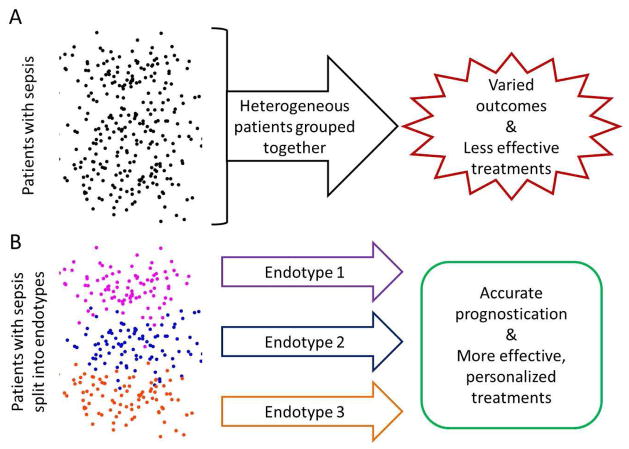
Another approach to risk stratification is to discovery novel subtypes of gene expression (‘endotypes’) in sepsis. The hypothesis is that we may be clinically grouping all patients of one class together (for instance, all those with septic shock), when potentially there are really two or more subtypes (for instance, one group with severe endothelial cell dysfunction, another with mitochondrial dysfunction, etc.) within that class that may help stratify patients (Figure 1). All such unsupervised clustering studies are highly dependent on clustering method, validation metric, and interpretation, and yet they can yield valuable insights in high-dimensional datasets that are not easily classified by human intuition alone.
Figure 1.
Schematic of the discovery and use of endotypes in sepsis. (A) All patients in a heterogeneous cohort of septic patients are treated similarly, reducing the signal-to-noise ratio and rendering prognostic and therapeutic differences less effective. (B) The same cohort is split into multiple (here three, arbitrarily) subgroups based on transcriptomic biomarkers. Each group is treated with a therapy best suited to it, and prognostic predictions are more accurate.
Maslove et al. performed unsupervised clustering on 365 sepsis-related genes from two microarray studies of neutrophils in clinical sepsis; they identified two main clusters of septic patients.24 Interestingly, they found that the two subgroups stratified patients with severe sepsis, but that septic shock patients were evenly distributed, suggesting that shock may be less associated with underlying changes in the transcriptome than other kinds of organ dysfunction.
Wynn et al. used 2955 probes to cluster the gene expression profiles of neonates with sepsis; here they found that ‘early’ day of life (<3 days) was distinct from ‘late’ day of life episodes of sepsis at the molecular level.25 This points to the utility of unsupervised transcriptomic clustering to discover important clinical differences in sepsis.
Wong et al. used 6,934 genes to cluster children with septic shock only; they identified three clusters of patients (A–C).26 They found that group A had more deaths and organ failures, and group B had older children and more children receiving corticosteroids. Interestingly, the group was able to reduce the number of classifying genes to 100, and has tested them in independent patients using visual gene expression mosaics. Here they found that matched patients in group A, but not group B, carried a significant increased risk of mortality when receiving adjunctive cortisosteroids. This type of ‘theranostic’ approach illustrates the potential power of identifying new endotypes in sepsis.
Caveats in gene expression profiling: considerations for future studies
As with all ‘omics’ (massively parallel) technologies, transcriptomic profiling via microarrays often leads to significant rates of both type I and type II errors. The number of samples is often far less than the number of variables measured, and studies often thus report false positives. However, the expense of running these studies often leads to publications underpowered to find true effects. Independent validation and/or meta-analysis (either by simple ‘vote counting’ of study replications, or by true random-effects or Bayesian models) are thus absolutely required prior to clinical application.
Many of the studies of sepsis and critical illness focus (appropriately) on whole blood, since systemic inflammation is likely largely mediated by circulating leukocytes. However, a gene expression profile of whole blood is in fact a profile of the mixtures of individual cell types present. Thus, differential gene expression can be caused both by actual changes in expression within a cell type and by changes in cell type ratios, since each cell type has different background patterns of gene expression. For simple clinical tools, this may not matter, since a biomarker must be primarily reliably reproducible, not grounded in pathophysiology. However, for interpretation of pathophysiology, prediction of therapies, and further research directions, cell-type-specific gene expression (either via direct measurement or via bioinformatic deconvolution analysis40) will become important. Several studies have thus focused solely on gene expression profiling of peripheral blood mononuclear cells or further-sorted subsets. These studies often have significantly different findings from whole-blood studies, whose signals are dominated by neutrophils, since these are the largest blood cell compartment during acute inflammation. Both approaches offer important insight into sepsis pathophysiology.
Finally, the application of gene expression biomarkers in sepsis has a unique challenge, which is clinical time. In critical illness, hours count, and accurate gene expression quantitation takes time. While some technologies are in development to reduce the time required for gene expression quantitation to an hour or less, a standard multiplex PCR still takes 3–4 hours, and technologies that can measure more than a handful of genes at once often take much longer. Some gene expression biomarkers have been converted to protein assays; the rest will require careful optimization prior to clinical translation.
What we’ve learned so far, and where we go from here
Despite the caveats, genome-wide expression profiling has yielded some tools which are rapidly approaching clinical deployment, and has led to several remarkable, reproducible insights into sepsis pathophysiology. Risk stratification via biomarker sets such as PERSEVERE initially discovered in microarray studies have been validated in the research setting to better predict mortality than other clinical scoring systems (such as PRISM and APACHE II). Further, these models have been reduced to protein assays, making their eventual translation to clinical practice easier.
Our understanding of the pathophysiology of severe sepsis has been greatly advanced by transcriptomic studies. Parallel immune dysregulations – overactive innate inflammation and collapse of adaptive immunity – are now known to co-exist in sepsis. SIRS appears to be driven largely by activated and immature neutrophils, as evidenced by repeated findings of both increased typical pro-inflammatory cytokines (IL-1, IL-6, IL-18) and related networks, as well as increased neutrophil proteinase and secretory genes (MMP8, RETN). Immune paralysis, shown to be activated along with SIRS, is evidenced by both acute and chronic down-regulation of adaptive immune genes, possibly by increases in the T-regulatory compartment. However, immune paralysis is not limited to adaptive immunity, as a signature of endotoxin tolerance initially derived in monocyte in vitro has been shown in multiple studies of septic whole blood. Further, as clinical improvements have decreased mortality in the initial acute phases in sepsis, the chronic stage of PICS appears to gaining more relevance. Immune dysregulation in sepsis has been definitively linked to worse outcomes and greater severity (although the causal mechanisms are unknown). Future immunomodulatory therapies for sepsis will likely need to take our new understanding of immune dysregulation into account in order to succeed.
Stratification of risk for specific types of organ failure is an important area of study in which more research is needed. While many types of ‘omics’ technologies (metabolomics, lipidomics, proteomics, etc.) can potentially find ‘leaked’ circulating molecules of interest from the target organ in whole blood41, transcriptomics has a somewhat greater challenge. Most gene expression signal in the whole blood comes from leukocytes, and will thus primarily show patterns of systemic inflammation. Still, decoding this signal (i.e., searching for the organ-specific needle in the transcriptomic haystack) could potentially allow for a single diagnostic to yield multiple dimensions of risk stratification, with obvious clinical benefit.
Summary
Sepsis is a complex, heterogeneous clinical syndrome. At its most basic, better risk stratification can help determine level of care. As our understanding of gene expression in sepsis grows, however, so too does the potential to move sepsis care into the era of precision medicine. Immunomodulatory therapies have failed when applied broadly, but better biomarkers that quantitatively stratify patients will allow for clinical trials based on predictive and prognostic enrichment, both saving costs and increasing positive trial outcomes. Further, this better understanding of the underlying immune dysregulation will allow different classes of septic patients to be treated differently, ultimately reducing morbidity and mortality. We have learned and validated much already, but more research is needed, both in targeted validation of proposed gene sets and further data-driven microarray studies of sepsis severity and organ-specific outcomes.
Key Points.
- Whole-genome expression studies are a useful tool in making sense of the complex and heterogeneous changes that occur in sepsis.
- Prognosis of sepsis at admission is becoming more feasible, with recent validation of some stratification markers such as PERSEVERE.
- Splitting patients into subgroups (‘endotypes’) based on gene expression markers may be a way to identify more homogeneous populations of patients with sepsis.
- Better biomarkers may provide for prognostic and phenotypic enrichment strategies in future therapeutic trials.
- Both time and illness severity must be controlled for in future studies of sepsis.
Acknowledgments
TES is funded by a Stanford Child Health Research Institute Young Investigator Award (through the Institute for Immunity, Transplantation and Infection), the Society for University Surgeons, and the Stanford Department of Surgery. HRW is funded by the National Institutes of Health (R01GM099773, R01GM096994, and R01GM108025).
Footnotes
Disclosures: None
Disclosures
TES is a scientific advisor to Multerra Biosciences, and is a party to a provisional patent filed on the 11-gene sepsis diagnostic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lagu T, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 4.Temple R. Enrichment of clinical study populations. Clin Pharmacol Ther. 2010;88:774–778. doi: 10.1038/clpt.2010.233. [DOI] [PubMed] [Google Scholar]
- 5.Scicluna BP, et al. A Molecular Biomarker to Diagnose Community-acquired Pneumonia on Intensive Care Unit Admission. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201502-0355OC. [DOI] [PubMed] [Google Scholar]
- 6.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prucha M, et al. Expression profiling: toward an application in sepsis diagnostics. Shock. 2004;22:29–33. doi: 10.1097/01.shk.0000129199.30965.02. [DOI] [PubMed] [Google Scholar]
- 8.Johnson SB, et al. Gene expression profiles differentiate between sterile SIRS and early sepsis. Ann Surg. 2007;245:611–621. doi: 10.1097/01.sla.0000251619.10648.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney TE, Shidham A, Wong HR, Khatri P. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci Transl Med. 2015;7:287ra271. doi: 10.1126/scitranslmed.aaa5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pachot A, et al. Systemic transcriptional analysis in survivor and non-survivor septic shock patients: a preliminary study. Immunol Lett. 2006;106:63–71. doi: 10.1016/j.imlet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Tsalik EL, et al. An integrated transcriptome and expressed variant analysis of sepsis survival and death. Genome Med. 2014;6:111. doi: 10.1186/s13073-014-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almansa R, et al. Transcriptomic correlates of organ failure extent in sepsis. J Infect. 2015;70:445–456. doi: 10.1016/j.jinf.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Parnell GP, et al. Identifying key regulatory genes in the whole blood of septic patients to monitor underlying immune dysfunctions. Shock. 2013;40:166–174. doi: 10.1097/SHK.0b013e31829ee604. [DOI] [PubMed] [Google Scholar]
- 14.Wong HR, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007;30:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parnell G, et al. Aberrant cell cycle and apoptotic changes characterise severe influenza A infection--a meta-analysis of genomic signatures in circulating leukocytes. PLoS One. 2011;6:e17186. doi: 10.1371/journal.pone.0017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolinay T, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Severino P, et al. Patterns of gene expression in peripheral blood mononuclear cells and outcomes from patients with sepsis secondary to community acquired pneumonia. PLoS One. 2014;9:e91886. doi: 10.1371/journal.pone.0091886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pankla R, et al. Genomic transcriptional profiling identifies a candidate blood biomarker signature for the diagnosis of septicemic melioidosis. Genome Biol. 2009;10:R127. doi: 10.1186/gb-2009-10-11-r127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MAC, et al. Early and dynamic changes in gene expression in septic shock patients: a genome-wide approach. Intensive Care Medicine Experimental. 2014;2 doi: 10.1186/s40635-014-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai KH, et al. Dissecting inflammatory complications in critically injured patients by within-patient gene expression changes: a longitudinal clinical genomics study. PLoS Med. 2011;8:e1001093. doi: 10.1371/journal.pmed.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong HR, et al. Testing the prognostic accuracy of the updated pediatric sepsis biomarker risk model. PLoS One. 2014;9:e86242. doi: 10.1371/journal.pone.0086242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong HR, et al. The temporal version of the pediatric sepsis biomarker risk model. PLoS One. 2014;9:e92121. doi: 10.1371/journal.pone.0092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong HR, et al. A multibiomarker-based outcome risk stratification model for adult septic shock*. Crit Care Med. 2014;42:781–789. doi: 10.1097/CCM.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maslove DM, Tang BM, McLean AS. Identification of sepsis subtypes in critically ill adults using gene expression profiling. Crit Care. 2012;16:R183. doi: 10.1186/cc11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynn JL, et al. Post-natal age is a critical determinant of the neonatal host response to sepsis. Mol Med. 2015 doi: 10.2119/molmed.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong HR, et al. Validation of a gene expression-based subclassification strategy for pediatric septic shock. Critical care medicine. 2011;39:2511–2517. doi: 10.1097/CCM.0b013e3182257675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwan A, Hubank M, Rashid A, Klein N, Peters MJ. Transcriptional instability during evolving sepsis may limit biomarker based risk stratification. PLoS One. 2013;8:e60501. doi: 10.1371/journal.pone.0060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cobb JP, et al. Validation of the riboleukogram to detect ventilator-associated pneumonia after severe injury. Ann Surg. 2009;250:531–539. doi: 10.1097/SLA.0b013e3181b8fbd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDunn JE, et al. Plasticity of the systemic inflammatory response to acute infection during critical illness: development of the riboleukogram. PLoS One. 2008;3:e1564. doi: 10.1371/journal.pone.0001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan S, et al. Prediction of multiple infections after severe burn trauma: a prospective cohort study. Ann Surg. 2015;261:781–792. doi: 10.1097/SLA.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao W, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentile LF, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanzant EL, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76:21–29. doi: 10.1097/TA.0b013e3182ab1ab5. discussion 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock RE. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol. 2011;186:7243–7254. doi: 10.4049/jimmunol.1001952. [DOI] [PubMed] [Google Scholar]
- 35.Pena OM, et al. An Endotoxin Tolerance Signature Predicts Sepsis and Organ Dysfunction at Initial Clinical Presentation. E Bio Medicine. 2014;1:64–71. doi: 10.1016/j.ebiom.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Shi JX, Pan XF, Feng J, Zhao H. DNA microarray-based screening of differentially expressed genes related to acute lung injury and functional analysis. Eur Rev Med Pharmacol Sci. 2013;17:1044–1050. [PubMed] [Google Scholar]
- 37.Howrylak JA, et al. Discovery of the gene signature for acute lung injury in patients with sepsis. Physiol Genomics. 2009;37:133–139. doi: 10.1152/physiolgenomics.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kangelaris KN, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1102–1113. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu RK, et al. Identification of candidate serum biomarkers for severe septic shock-associated kidney injury via microarray. Crit Care. 2011;15:R273. doi: 10.1186/cc10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen-Orr SS, et al. Cell type-specific gene expression differences in complex tissues. Nat Methods. 2010;7:287–289. doi: 10.1038/nmeth.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.JHB, MM, KRW Acute organ injury is associated with alterations in the cell-free plasma transcriptome. Intensive Care Med Exp. 2014;2:5. doi: 10.1186/2197-425X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]