Differences in Enzymatic Properties Allow SodCI but Not SodCII To Contribute to Virulence in Salmonella enterica Serovar Typhimurium Strain 14028 (original) (raw)
Abstract
Salmonella enterica serovar Typhimurium produces two Cu/Zn cofactored periplasmic superoxide dismutases, SodCI and SodCII. While mutations in sodCI attenuate virulence eightfold, loss of SodCII does not confer a virulence phenotype, nor does it enhance the defect observed in a sodCI background. Despite this in vivo phenotype, SodCI and SodCII are expressed at similar levels in vitro during the stationary phase of growth. By exchanging the open reading frames of sodCI and sodCII, we found that SodCI contributes to virulence when placed under the control of the sodCII promoter. In contrast, SodCII does not contribute to virulence even when expressed from the sodCI promoter. Thus, the disparity in virulence phenotypes is due primarily to some physical difference between the two enzymes. In an attempt to identify the unique property of SodCI, we have tested factors that might affect enzyme activity inside a phagosome. We found no significant difference between SodCI and SodCII in their resistance to acid, resistance to hydrogen peroxide, or ability to obtain copper in a copper-limiting environment. Both enzymes are synthesized as apoenzymes in the absence of copper and can be fully remetallated when copper is added. The one striking difference that we noted is that, whereas SodCII is released normally by an osmotic shock, SodCI is “tethered” within the periplasm by an apparently noncovalent interaction. We propose that this novel property of SodCI is crucial to its ability to contribute to virulence in serovar Typhimurium.
Superoxide dismutases (SODs) use metal cofactors to dismutate superoxide (O2−) to hydrogen peroxide (H2O2) and molecular oxygen: O2− + O2− + 2 H+ → H2O2 + O2. Superoxide is generated in bacterial cytoplasms as an adventitious by-product of normal metabolism (15, 16, 22). Because this O2− can damage cytoplasmic targets—notably, the [4Fe-4S] clusters of dehydratases (14-16)—virtually all bacteria synthesize manganese- or iron-cofactored cytoplasmic SODs to scavenge it. Mutants that lack these SODs exhibit growth defects due to enzyme inactivation, and they also exhibit high rates of oxidative DNA damage as an indirect consequence of the iron that is released from the degraded clusters (4, 24).
Many gram-negative bacteria also export copper-containing SODs to their periplasm (reference 1 and reference 26 and references therein). The presence of SODs in the periplasm of intracellular pathogens has led to the hypothesis that these enzymes protect bacteria against macrophage-derived superoxide (1). Bacteria internalized in macrophage phagosomes are exposed to a variety of reactive oxygen and nitrogen species: notably O2−, formed by the phagocytic NADPH oxidase (Phox), and nitric oxide, formed by the inducible nitric oxide synthase (32). Periplasmic SODs could plausibly protect periplasmic targets in the captive bacteria from O2−. Further, because O2− could be protonated to HO2• in the acidic interior of the phagolysosome, periplasmic SOD could prevent this neutral species from penetrating the membrane and attacking cytosolic targets (25).
The role of Cu/Zn SODs in virulence has been most closely examined in members of the genus Salmonella, intracellular pathogens that are associated with gastroenteritis, septicemia, and typhoid fever. Salmonellae survive and replicate in macrophages during the course of infection (12, 37), and evidence that phagocyte-produced superoxide is important in Salmonella infection is clear: mice and humans who are genetically defective in superoxide production are significantly more susceptible to infection (29, 42, 44). Many Salmonella strains contain two separate periplasmic SODs, termed SodCI and SodCII (10). SodCII is chromosomally encoded and is the ortholog of the Escherichia coli SodC. SodCI is encoded on the fully functional lambdoid prophage Gifsy-2, which integrates into the Salmonella chromosome at centisome 23.8 (13, 20, 21). The Gifsy-2 phage is preferentially found in the most virulent serovars of Salmonella (10, 21), and Gifsy-2 lysogens are significantly more virulent than nonlysogens (13, 20). We have shown that virulence is independent of Gifsy-2 phage per se, as deletion of regions encoding excision, immunity, and replication functions does not attenuate the bacterium. Thus, the two major virulence factors encoded by Gifsy-2, SodCI and GtgE, are expressed independently of phage induction or DNA replication (20).
All known Cu/Zn SODs are structurally related. However, SodCI and SodCII are clearly divergent. The mature SodCI protein shares only 60% identity with SodCII and 58% identity with E. coli SodC. SodCII and E. coli SodC are 85% identical. The crystal structures of both SodCI (34) and E. coli SodC (35) have been determined. Although the overall structures are quite similar, SodC and its close orthologs are monomeric, whereas most Cu/Zn SODs, including SodCI, are dimers.
Periplasmic SOD contributes to virulence in all Salmonella strains that have been tested, including S. enterica serovars Typhimurium, Dublin, and Choleraesuis. Farrant et al. (11) showed that sodCI mutants in all three backgrounds were recovered in lower numbers than the parental wild-type strains from the spleens and livers of mice 4 days after infection. DeGroote et al. (6) showed that the time to death was significantly longer in mice infected with an S. enterica serovar Typhimurium sodCI strain. This phenotype was not observed when Phox−/− mice were infected, showing that the defect conferred by sodCI is dependent on the oxidative burst of phagocytes (6). Our laboratory has shown that sodCI mutants are 7- to 10-fold reduced in virulence as measured in an intraperitoneal competition assay (20, 21). These virulence defects are seen in both Nramp1+ and Nramp1− mice (references 10, 38, and 41 and data from this study).
Although there is agreement that SodCs have a role in virulence, there is controversy regarding the relative contributions of SodCI and SodCII. Fang et al. (10) concluded that, in serovar Typhimurium strain 14028, both SodCI and SodCII contributed equally to virulence in Nramp+ mice, with the double mutant showing a more severe virulence defect than either single mutant. Sly et al. (39), using Fang's exact strains, came to the same conclusion by examining killing of sodC mutants by a vitamin D3-induced human macrophage cell line. Sansone et al. (38), using Fang's sodCII allele moved into serovar Choleraesuis, also concluded that both SodCI and SodCII contribute to resistance to phagocytic superoxide, as shown by in vitro and in vivo assays. However, they observed no further defect when both genes were mutant. In contrast, Uzzau et al. (41) showed that, while loss of SodCI conferred a clear virulence defect, deletion of sodCII in serovar Typhimurium strain 14028 had no apparent effect.
In this study we confirm that, while sodCI mutations confer a virulence phenotype in serovar Typhimurium 14028, deletion of sodCII does not. Moreover, loss of SodCII does not further decrease virulence in a sodCI mutant background. By exchanging the open reading frames of SodCI and SodCII and studying the virulence phenotypes of these hybrid constructs, we have found that the sodCII promoter is capable of supporting virulence when it drives the expression of SodCI. The SodCI enzyme apparently possesses some unique property that allows only this enzyme and not SodCII to increase survival in the host.
MATERIALS AND METHODS
Strain and plasmid construction.
Bacterial strains and plasmids are described in Table 1. Unless otherwise noted, all serovar Typhimurium strains created for this study are isogenic derivatives of strain 14028 (American Type Culture Collection). Strains were constructed by using P22 HT105/1 _int_-201_-mediated transduction (28). Insertion-deletion mutations in the sod genes were obtained by λ Red-mediated recombination (5, 45) as described elsewhere (8). In all cases, the appropriate insertion of the antibiotic resistance marker was checked by P22 linkage to known markers and/or PCR analysis. The constructs resulting from this procedure were then transduced into a clean wild-type background (strain 14028) by using phage P22. The Δ_sodCI-1::aph mutation was described by Fang et al. (10). This mutant allele was transduced into a clean background for mouse virulence assays. The sodCI gene was cloned into the vector pWKS30 (43) by using a natural BglII site and an engineered BamHI site, giving pMC101. The sodCII gene was cloned into pWM73 (31) at the XhoI and SalI sites, giving pMC102. All plasmids were passaged through a restriction-minus modification-plus Salmonella strain (JS198 [8]) prior to transformation into derivatives of strain 14028.
TABLE 1.
Bacterial strains and plasmids
| Strain | Genotypea | Deletion or cloned endpoints | Source or referenceb |
|---|---|---|---|
| 14028 | Wild type | ATCCc | |
| JS135 | zii-8104::Tn_10_dTc | 40 | |
| JS450 | Δ_sodCI-1_::aph zii-8104::Tn_10_dTc | ||
| JS451 | Δ_sodA101_::Cm | 4266593-4267101 | |
| JS452 | Δ_sodB102_::Kn | 1509923-1509486 | |
| JS453 | Δ_sodA101_::Cm Δ_sodB102_::Kn | ||
| JS454 | Δ_sodCII-103_::Cm | 1516106-1516488 | |
| JS455 | Δ_sodCII-103_::Cm zii-8104::Tn_10_dTc | ||
| JS456 | Δ_sodCI-1_::aph Δ_sodCII-103_::Cm | ||
| JS457 | Δ_sodCI-104_::Cm | 1130586-1129969 | |
| JS458 | Δ_sodCII-105_::Cm | 1516050-1516703 | |
| JS459 | Δ_sodA101_ Δ_sodB102_ Δ_sodCI-104_::Cm | ||
| JS460 | Δ_sodA101_ Δ_sodB102_ Δ_sodCII-105_::Cm | ||
| JS461 | Δ_sodA101_ Δ_sodB102_ | ||
| JS462 | Δ_sodCII-106_::tet | 1515982-1516773 | |
| JS463 | Δ_sodCII-107_::sodCI+-Km | 1516050-1516703 | |
| JS464 | Δ_sodCI-109_::sodCII+-Km | 1130586-1129969 | |
| JS465 | Δ_sodCII-107_::sodCI+-Km Δ_sodCI-104_::Cm | ||
| JS466 | Δ_sodCI-109_::sodCII+-Km Δ_sodCII_::tet | ||
| JS467 | Δ_sodCII-107_::sodCI+-Km Δ_sodA101_ Δ_sodB102_ Δ_sodCI-104_::Cm | ||
| JS468 | Δ_sodCI-109_::sodCII+-Km Δ_sodA101_ Δ_sodB102_ Δ_sodCII-105_::Cm | ||
| JS469 | Δ_sodA101_ Δ_sodB102_ Δ_sodCII-105_::Cm / pMC101 | ||
| JS470 | zjg-8103::pir | ||
| JS471 | sodCI-1::aph Δ_sodA101_::Cm Δ_sodB102_ zjg-8103::pir/pMC102 | ||
| SL1344 strains | |||
| JS472 | Δ_sodCI-1_::aph | ||
| JS473 | Δ_sodCII-103_::Cm | ||
| JS474 | Δ_sodCI-1_::aph Δ_sodCII-103_::Cm | ||
| E. coli strains | |||
| JI132 psodC2.3 | AB1157 (F-thr-1 leuB6 proA2 his-4 thi-1 argE2 lacY1 galK2 rspL supE44 ara-14 xyl-15 mtl-1 tsx-33) plus (sodA::MudPR13)25 (_sodB_-Km)1-Δ2/psodC2.3 | 24 | |
| AS391 | AB1157 plus (sodA::MudPR13)25 (_sodB_-Km)1-Δ2 sodC::Spec | 17 | |
| RK94 | AB1157 plus (sodA::MudPR13)25 (_sodB_-Km)1-Δ2 sodC::Spec/pMC101 | ||
| Plasmids | |||
| pMC101 | pWKS30 sodCI | 1129594-1130988 | |
| pMC102 | pWM73 sodCII | 1515730-1516874 |
Media and growth of strains.
Cultures were maintained in Luria-Bertani (LB) medium (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl per liter) with 15 g of agar per liter for solid medium. LB was supplemented with 0.2% glucose where noted. The concentrations of the antibiotics used were as follows: ampicillin and kanamycin, 50 μg/ml; chloramphenicol, 20 μg/ml; and tetracycline, 25 μg/ml.
Exchanging the open reading frames of sodCI and sodCII.
The open reading frames of sodCI and sodCII were exchanged using the λ Red recombinase method (5, 8). Kanamycin resistance cassettes (from plasmid pKD4 [5]) were inserted immediately downstream of the sodCI (114 bp downstream of the termination codon) or sodCII (131 bp downstream of the termination codon) open reading frames. PCR primers were designed to amplify the region containing the sodCII open reading frame and the downstream kanamycin resistance marker. These primers had 5′ extensions of homology to the sodCI locus, allowing precise replacement of the sodCI open reading frame with sodCII starting at the methionine codon. The recipient strain had an insertion-deletion of sodCII and harbored the pKD46 plasmid (5). The hybrid construct in which the sodCI promoter controls the expression of SodCII is described as PCI:: sodCII+ Δ_sodCI_ in the text. An analogous procedure was used to place SodCI under the control of the sodCII promoter and is described as PCII:: sodCI+ Δ_sodCII_ in the text. In this case the primers used to amplify SodCI had 5′ extensions of homology to the sodCII locus.
Preparation of cellular fractions.
Whole-cell lysates were prepared in ice-cold 50 mM potassium phosphate buffer (pH 7.8) using the French pressure cell and clarified by centrifugation at 13,000 × g for 10 min at 4°C. The supernatants were used to determine SodC activity. When indicated, the whole-cell lysates were centrifuged at 13,000 × g to remove cell debris, and the supernatant was centrifuged at 141,370 × g for 1 h at 4°C in a Beckman ultracentrifuge. The pellet obtained from this centrifugation step was considered the membrane fraction.
Periplasmic extracts were prepared by osmotic shock (23). Briefly, 25-ml overnight cultures were centrifuged, washed in ice-cold 50 mM potassium phosphate buffer (pH 7.4), and resuspended in 5 ml of room temperature plasmolysis buffer (50 mM Tris, 2.5 mM EDTA, 20% [wt/vol] sucrose; pH 7.4). After sitting at room temperature for 10 min, the cells were centrifuged, resuspended in 2.5 ml of ice-cold deionized water, and incubated on ice for 15 min. The cells were recovered by centrifugation, and the supernatant was considered the osmotic shockate.
Periplasmic extracts were also prepared by the lysozyme-EDTA method described by Battistoni et al. (3). Cells were centrifuged, resuspended in a 1/10 volume of an ice-cold solution containing 20% sucrose, 30 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 1 mg of lysozyme/ml, and incubated on ice for 10 min. Cells were recovered by centrifugation, and the supernatant was used as the periplasmic fraction.
Enzyme assays.
SOD activity was assayed by the xanthine oxidase-cytochrome c method (30). Glucose-6-phosphate dehydrogenase was assayed as described elsewhere (24). Protein content of the cell extracts was determined using the Coomassie dye-based assay by Pierce (Rockford, Ill.).
For determining SodCI and SodCII activity, SOD mutant strains were used in which both the cytosolic SODs and the complementary periplasmic SOD were deleted. The strains were grown as indicated, and whole-cell extracts were made using the French press. When osmotic shocking was used to release the periplasmic SODs in a background where cytosolic SODs were present, parallel assays were conducted with and without 2 mM potassium cyanide in order to differentiate the cyanide-sensitive Cu**/**Zn SOD activity from the Mn and Fe SOD activities, which are cyanide resistant. For determining the activity and stability of SodCI and SodCII at various pH, the cytochrome c reduction assay was performed in the buffer solutions maintained at the indicated pH.
For peroxide treatment, the extracts were treated with 10 mM hydrogen peroxide for the stated period of time. Since kat+ strains were used to assay SOD activity, the residual H2O2 in the extracts was determined spectrophotometrically at 240 nm. Approximately 80% of the peroxide remained after 5 min of incubation. After incubation, 100 U of catalase was added per ml of extract to remove the peroxide, and the extracts were assayed for SodC activity. The SOD activity recovered from the peroxide-treated samples was compared to that of untreated samples.
To compare the ability of SodCI and SodCII to obtain copper in a copper-deficient environment, the high-affinity Cu(II) chelator N,_N_′-bis(2-aminoethyl)-1,3-propanediamine (TETA; Aldrich) was used to decrease the concentration of available copper in the growth medium. Strains overexpressing SodCI and SodCII were grown for 16 h in LB medium or LB with 0.01 μM to 8 mM TETA. Whole-cell extracts were assayed for SodC activity. The extracts were then dialyzed against 10 mM Tris-HCl (pH 7.8) containing 15 μM CuCl2 to reactivate any apoenzymes that were synthesized in the absence of copper and assayed again.
Mouse virulence assays.
Strains were grown overnight (16 h) in LB medium, washed, and diluted in sterile 0.15 M NaCl. For competition assays, female BALB/c or C3H/HeN mice (Harlan Sprague Dawley, Inc.) were inoculated intraperitoneally (i.p.) in groups of 4 to 10 with an equal mixture of mutant and wild-type bacteria (approximately 500 total bacteria). Inocula were plated on LB and then replica plated onto the appropriate selective media to determine the total number and percentage of mutant and wild type bacteria used for the infection. Mice were sacrificed after 4 to 5 days of infection, and their spleens were removed. The spleens were homogenized, diluted, plated on LB medium, and then replica plated onto the selective medium to determine the percent mutant bacteria recovered. The competitive index (CI) was calculated as follows: (percent strain A recovered/percent strain B recovered)/(percent strain A inoculated/percent strain B inoculated). The CI of each set of assays was analyzed statistically using Student's t test. In each case, the strains were rebuilt by P22 transduction, and the mouse assay was repeated to ensure that the virulence phenotype was the result of the designated mutation. For time-to-death assays, six C3H/HeN mice per group were injected i.p. with 2,000 bacteria on day zero, and mortality was assessed daily. Mice were humanely euthanized upon becoming moribund.
RESULTS
SodCI, but not SodCII, contributes to virulence in serovar Typhimurium.
Our group's previous studies (21) have suggested that only SodCI has a role in pathogenesis. Yet others have reported that SodCII mutants of Salmonella are attenuated (10, 38, 39). To distinguish between these two possibilities, serovar Typhimurium 14028 strains mutant in sodCI, sodCII, or both genes were tested in i.p. competition assays versus the isogenic wild-type strain. The results (Table 2) showed that the sodCI mutant was eightfold attenuated, as previously observed (20, 21). SodCII, however, did not significantly contribute to bacterial survival in the animal. Indeed, even in the absence of SodCI, there was no further effect of knocking out SodCII. Note that the sodCII mutation used in these studies is a complete deletion, which has been confirmed using genetic, molecular, and biochemical methods. We reconstructed these strains and repeated the assays many times and always obtained the same results. We also performed the same experiments in the serovar Typhimurium SL1344 background and reached the same conclusion (Table 2). Overall, our results are virtually identical to those obtained by Uzzau et al. (41).
TABLE 2.
sodCI and sodCII competition assays in BALB/c mice
| Strain Aa | Strain B | Median CIb | No. of micec | pd |
|---|---|---|---|---|
| Background strain 14028 | ||||
| sodCI | WT | 0.13 | 16 | <0.0005 |
| sodCII | WT | 0.74 | 6 | NS |
| sodCI sodCII | WT | 0.14 | 4 | 0.011 |
| sodCI sodCII | sodCI | 0.97 | 10 | NS |
| Background strain SL1344 | ||||
| sodCI | WT | 0.031 | 4 | 0.0017 |
| sodCII | WT | 0.71 | 10 | NS |
| sodCI sodCII | sodCI | 2.1 | 10 | NS |
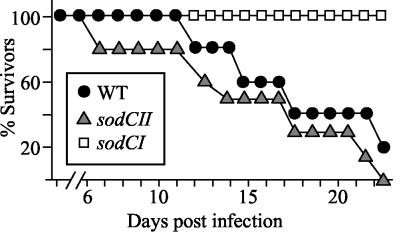
The competition assay allows us to directly compare levels of attenuation in different host backgrounds. In C3H/HeN (Nramp1**+**) mice, the sodCI strain was 12-fold attenuated and there was no significant effect from loss of sodCII, in either the wild-type or sodCI backgrounds (data not shown). To further confirm the relative contribution of the two enzymes, we tested our strains in time-to-death assays in C3H/HeN mice (the assay used in reference 10). These results indicated no difference between the wild type and sodCII mutant, whereas the sodCI mutant was significantly attenuated (Fig. 1). Thus, our results are not dependent on the Nramp status of the mice or the virulence assay.
FIG. 1.
Relative virulence of sodCI and sodCII single mutants. Six C3H/HeN mice per group were injected i.p. with 2,000 bacteria on day zero, and mortality was assessed daily. Mice were euthanized upon becoming moribund. Strains used were JS455 and JS450.
SodCI and SodCII are produced in laboratory culture.
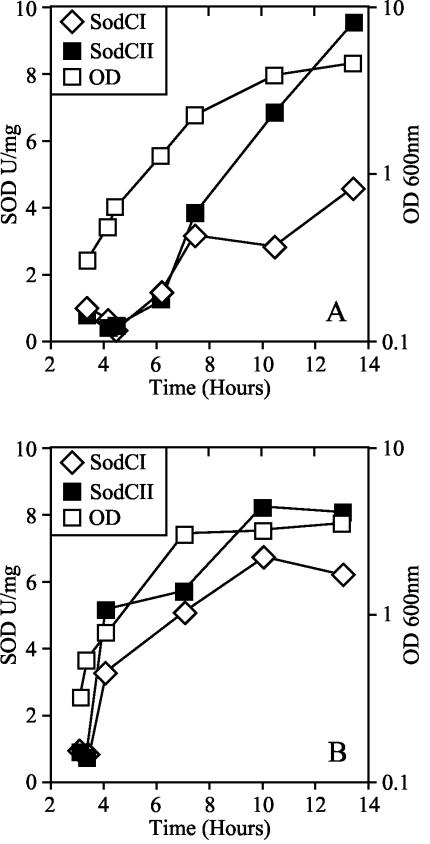
We sought to identify the feature of SodCI that allowed it but not SodCII to contribute to virulence. It seemed possible that SodCI was expressed at a higher level than SodCII and/or that it was produced during a growth phase when SodCII was not. We determined SodCI and SodCII activity in whole-cell extracts. In order to avoid interference in the assay, we used a genetic background where the cytosolic MnSOD and FeSOD, and the complementary periplasmic SOD, were all absent. Neither SodCI nor SodCII was detectable when cells were harvested in exponential phase. In contrast, SodCI and SodCII were induced 5- and 13-fold as cells reached stationary phase in LB (Fig. 2A), and 8- and 16-fold in LB supplemented with 0.2% glucose (Fig. 2B). These data are consistent with published results (10, 40). SodCII was produced at slightly higher levels (twofold over SodCI) in stationary phase, and the specific activities of both enzymes were higher when cultures were harvested from LB supplemented with glucose. Loss of either enzyme does not apparently affect the activity of the other: the specific SodC activity was simply additive when both enzymes were present (data not shown). Thus, neither the magnitude nor pattern of SodCI synthesis in vitro explained its phenotypic dominance over SodCII in vivo.
FIG. 2.
Specific activities of SodCI and SodCII as a function of growth phase. Δ_sodA101_ Δ_sodB102_ Δ_sodCI-104_::Cm and Δ_sodA101_ Δ_sodB102_ Δ_sodCII-105_::Cm strains were grown overnight for 16 h in LB or LB supplemented with 0.2% glucose, diluted to an optical density at 600 nm (OD600) of 0.01, and subcultured until an OD of 0.2 was reached. These log-phase cells were then diluted back to 0.01 in LB (A) or LB plus glucose (B), and aliquots were removed at the specified time to assay SodC activity. The growth curves of both strains were indistinguishable. A representative growth curve is shown in both panels.
SodCI contributes to virulence even when regulated by the sodCII promoter.
Simplistically, there are two models to explain the differential roles of SodCI and SodCII in the infection process. First, the two enzymes could be differentially regulated such that only SodCI is produced at the time that resistance to extracytoplasmic superoxide is critical. Second, the two proteins could have different physical properties such that SodCII is incapable of acting to protect the cell. The SodCII enzyme could be enzymatically or structurally unstable, or specific interaction between SodCI and some other component in the periplasm could be critical for its stability or role in protection. Note that these models are not mutually exclusive.
To test the above hypotheses, we exchanged the open reading frames of sodCI and sodCII and compared the relative contributions of the two proteins in vitro and in vivo. If the in vivo phenotypes were simply dependent on differential transcriptional regulation of the two proteins, then SodCII would be functional when under the control of the sodCI promoter. If SodCII cannot functionally replace SodCI, this would suggest that there is some difference between the two proteins rather than or in addition to differences in expression. In this case, SodCI should be fully functional at the sodCII locus, confirming that expression of SodCII is sufficient but that the enzyme cannot fulfill the function of SodCI.
The appropriate strains were constructed using the λ Red recombinase method (5). We inserted kanamycin resistance cassettes downstream of the sodCI and sodCII open reading frames such that expression of the genes was unaffected. We confirmed that the insertion downstream of sodCI did not affect virulence and that neither insertion affected in vitro enzymatic activity (data not shown). To swap the open reading frames, PCR primers were designed to amplify the sodCII open reading frame with the downstream kanamycin resistance marker. This PCR product was integrated at the SodCI locus, precisely replacing the open reading frame beginning at the methionine start codon. Thus, SodCII was produced under the normal transcriptional and translational control of sodCI. An analogous procedure was used to replace the SodCII open reading frame precisely with SodCI. The normal sodCII or sodCI allele was deleted. Thus, the resulting strains each produced a single SodC enzyme. There was no significant difference in the amount of enzyme produced from the hybrid constructs in comparison to that from the wild-type genes. At 16 h, the specific activity obtained from PCII:: sodCI+ (JS 467) was 12.2 ± 5.3 U/mg and from PCI:: sodCII+ (JS 468) it was 7.1 ± 1.5 U/mg. These results suggest that the two enzymes are not only expressed equally in vitro but also have similar turnover numbers.
The PCII:: sodCI+ Δ_sodCII_ Δ_sodCI_ strain was competed against the wild type and the sodCII mutant in separate competition assays. In both cases, the hybrid strain was essentially wild type in virulence (Table 3). When the PCII:: sodCI+ Δ_sodCII_ Δ_sodCI_ construct was competed against the Δ_sodCI_ Δ_sodCII_ double mutant, the hybrid strain out-competed the double mutant by 2.6-fold. This relative level of attenuation of the sodCI sodCII double mutant was slightly less than it would be when competed against the wild type (eightfold) (Table 2). Nevertheless, this result confirms that the sodCII promoter is capable of supporting virulence, but only when it drives the synthesis of SodCI rather than SodCII. Wild-type regulation of SodCI in the host is not essential for virulence.
TABLE 3.
Competition assay of hybrid constructs with the wild type
| Strain Aa | Strain B | Median CIb | No. of micec | Pd |
|---|---|---|---|---|
| PCII::sodCI+ Δ_sodCII_ Δ_sodCI_ | WT | 0.76 | 6 | NS |
| PCII::sodCI+ Δ_sodCII_ Δ_sodCI_ | Δ_sodCII_ | 0.83 | 4 | NS |
| PCII::sodCI+ Δ_sodCII_ Δ_sodCI_ | Δ_sodCI_ Δ_sodCII_ | 2.6 | 5 | 0.028 |
| PCI::sodCII+ Δ_sodCI_ Δ_sodCII_ | WT | 0.10 | 4 | 0.002 |
| PCI::sodCII+ Δ_sodCI_ Δ_sodCII_ | Δ_sodCI_ Δ_sodCII_ | 0.26 | 5 | NS |
| PCI::sodCII+ Δ_sodCI_ Δ_sodCII_ | Δ_sodCI_ | 0.25 | 5 | 0.017 |
SodCII regulated by the sodCI promoter cannot replace SodCI function.
The data above suggest that, although SodCII is normally produced during infection, it does not contribute to virulence. To confirm this conclusion, the hybrid strain PCI:: sodCII+ Δ_sodCII_ Δ_sodCI_ was competed against the wild-type strain. This construct should be attenuated in comparison to the wild type if the difference lay in the identity of the protein rather than the promoter. Indeed, the PCI:: sodCII+ ΔsodCII ΔsodCI strain was 10-fold attenuated in virulence compared to the wild type (Table 3). This level of attenuation is apparently greater than that observed in a sodCI sodCII double mutant. Indeed, when the hybrid strain was competed against a sodCI mutant and a sodCI sodCII double mutant, it was fourfold attenuated. Thus, expressing SodCII under sodCI control somehow attenuates the bacterium. Note also that these data are independent confirmation that SodCII does not contribute to survival in the animal.
We conclude that differences in enzyme structure or function are primarily responsible for the ability of SodCI, but not SodCII, to contribute to virulence. SodCII produced by the sodCI promoter attenuates virulence, perhaps as a result of overproduction. This suggests that expression from the sodCI promoter in the animal may be higher than expression from sodCII, although these differences are not essential for the contribution of SodCI to virulence. This conclusion warrants further confirmation through studies of expression patterns of the two enzymes in vivo. Thus, differences in expression could also contribute to the differential roles of SodCI and SodCII during infection.
SodCI and SodCII are enzymatically similar in vitro.
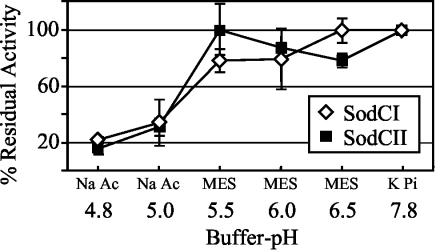
SodCII is apparently made but is nonfunctional during infection. We considered the possibility that SodCI is better suited than SodCII to function in a macrophage. To test this hypothesis, the activities of SodCI and SodCII were assayed under a variety of conditions that could prevail inside a phagosome. For example, the _Salmonella_-containing vacuole ranges between pH 4.0 and 5.0 (36), and so we tested the sensitivity of the two enzymes to acid. We found no significant difference (Fig. 3). Compared to the activity at pH 7.8, which was considered 100%, both enzymes retained only about 20% activity at pH 4.8, the lowest pH at which the xanthine oxidase system could generate O2−. Both SodCI and SodCII retained 100% activity when the extracts containing the enzymes were incubated at pH 4.6 for 2 hours and then assayed at pH 7.8 (data not shown). Thus, SodCII is not detectably more sensitive than SodCI to acid pH in vitro.
FIG. 3.
Activities of SodCI and SodCII at various pH. SodC activity was assayed as described elsewhere (30) except at the indicated pH and in the designated buffer. Activity at pH 7.8 was considered 100% activity. KPi, phosphate buffer; MES, 4-morpholineethanesulfonic acid buffer; Na Ac, sodium acetate buffer. Strains used were JS471 and JS469.
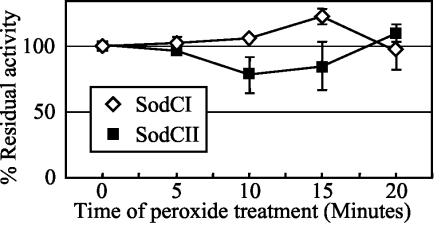
Spontaneous or enzymatic dismutation of superoxide produces H2O2, and the eukaryotic Cu/Zn SODs are inactivated by peroxide (2, 27). Therefore, we tested the influence of peroxide on the activity of both SodCI and SodCII. Both enzymes were equally resistant to peroxide treatment over a period of 20 min (Fig. 4).
FIG. 4.
Sensitivities of SodCI and SodCII to hydrogen peroxide. The activity recovered from the peroxide-treated samples was compared to that of untreated samples to determine the residual activity after treatment. Activity of the untreated sample at pH 7.8 was considered 100%. Strains used were JS471 and JS469.
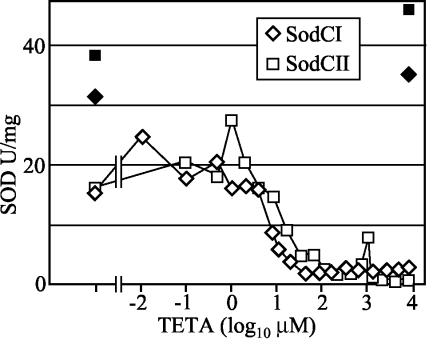
Another potential difference between the enzymes could be their affinity for copper. It is not clear how these periplasmic enzymes acquire copper, since there are no known copper chaperones for prokaryotic Cu/Zn SODs (17). The simplest model is that the apo-SODs abstract copper from adventitious copper chelates that passively diffuse into the periplasm. The ability to obtain and retain copper could be important, particularly in a copper-deficient environment. To determine if SodCI was able to obtain copper more efficiently than SodCII, the amount of SodCI and SodCII activity was measured from cells grown in the presence of the high-affinity Cu(II) chelator TETA. As shown in Fig. 5, the chelator completely inactivated SOD activity when added to cultures at ∼100 μM. (Bacterial growth inhibition was not observed until the TETA concentration reached 6 mM). However, there was no significant difference between the amount of enzymatically active SodCI and SodCII from stationary-phase cells that were grown in various concentrations of the chelator (Fig. 5). Both SodCI and SodCII proteins were synthesized and maintained in the inactive form in TETA-treated cells, and the amount of total enzyme present in cells grown in 8 mM TETA was almost identical to the amount found in the control cells without TETA (Fig. 5). The enzymes also regained full activity when copper was added back by dialysis to TETA-treated whole cells (data not shown). These data suggest that the two enzymes do not differ in copper affinity.
FIG. 5.
Specific activities of SodCI and SodCII from cells grown in various concentrations of TETA. Strains JS471 and JS469 were cultured for 16 h in either LB without chelator (first point) or LB containing the indicated concentration of chelator. The filled symbols specify the activity recovered from the indicated samples after extracts were dialyzed against copper-containing buffer.
SodCI is not released by osmotic shock.
As shown above, SodCI and SodCII behave similarly under a variety of conditions. However, we discovered a fundamental difference in the two enzymes: only SodCII is released by standard osmotic shock. As shown in Table 4, only 5% of SodCI activity was released into osmotic shockates. This was in striking contrast to isogenic strains producing SodCII, where >50% of the enzyme was released. This phenomenon was observed even when the enzymes were 5- to 10-fold overproduced, and it was true in both Salmonella and E. coli. In both backgrounds, <1.5% of the plasmid-encoded SodCI activity was released by osmotic shock compared to 75 to 100% of either SodCII or E. coli SodC (Table 4). Thus, whatever factor keeps SodCI in the periplasm is not specific to Salmonella and is apparently not saturable. SodCI was not inactivated during the process of osmotic shocking; the enzyme was quantitatively recovered when the cell pellet left after osmotic shock was lysed by French press (data not shown). These results suggest that SodCI is somehow “tethered” within the periplasm.
TABLE 4.
Release of SodCI, SodCII, and SodC by various methods
| Enzymea | Strainb | Sp actc (U/ml/OD600) (% of total)d | ||
|---|---|---|---|---|
| French press | Lysozyme treatment | Osmotic shock | ||
| SodCI | Serovar Typhimurium | 0.4 | ND | 0.02 (5) |
| SodCII | Serovar Typhimurium | 0.7 | ND | 0.37 (53) |
| SodCI | Serovar Typhimurium p_sodCI_ | 3.35 ± 0.21 | ND | 0.12 ± 0.04 (1.2) |
| SodCII | Serovar Typhimurium p_sodCII_ | 5.5 ± 0.77 | ND | 4.55 ± 0.77 (75) |
| SodCI | E. coli p_sodCI_ | 4.7 | 1.5 (32) | 0.01 (0.2) |
| SodCec | E. coli p_sodCec_ | 2.6 | 1.1 (42) | 3.7 (142) |
| GPDH | E. coli p_sodCI_ | 1.9 | 0.02 (1) | ND |
This tethering does not appear to involve a covalent interaction. Several results support this interpretation. First, enzyme released by French press remained in the soluble fraction after membranes were pelleted (92% soluble). Thus, SodCI is not membrane bound. Second, we could release a significant fraction (32%) of SodCI by treating cells with lysozyme. This same treatment caused release of less than 1% of the cytoplasmic enzyme glucose-6-phosphate dehydrogenase (Table 4). Third, the crystal structure of SodCI does not reveal any modifications of the protein (34). Thus, tethering of SodCI within the periplasm is apparently via a noncovalent interaction. Clearly, SodCI and SodCII differ in their association within the periplasm. It is possible that this difference confers the unique virulence property on SodCI.
DISCUSSION
SodCI contributes significantly to Salmonella virulence by combating the oxidative burst of phagocytes. Mutants lacking SodCI are attenuated in systemic infection by a variety of assays, and this defect is evident in all Salmonella serovars that have been tested (6, 10, 11, 20, 21, 38, 41) (Table 2; Fig. 1). Attenuation is not observed in Phox−/− mice, which lack an oxidative burst (6). Sensitivity of sodCI mutants to reactive oxygen species can also be mimicked in vitro (6, 11, 38). Importantly, these mutants show a defect in macrophage survival in tissue culture (6, 38). The simplest interpretation of these data is that SodCI is required for full resistance to superoxide generated in the phagosome of the macrophage.
In this study we have confirmed that only SodCI is important in the virulence of serovar Typhimurium 14028. Although our data are in agreement with those of Uzzau et al. (40), they contradict earlier reports that both SodCI and SodCII contribute to the virulence of serovar Typhimurium. Fang et al. (10) reported that the sodCI sodCII double mutants caused significantly less mortality in _Ity_s C57BL/6 mice and that the single mutants were significantly attenuated in the more resistant _Ity_r C3H/HeN mouse strain. We have found no contribution of sodCII towards virulence in competition assays (Table 2) or time-to-death assays (Fig. 1) in _Ity_r or _Ity_s mice. Currently, we are unable to explain this discrepancy. However, several points should be noted. First, the sodCII alleles used here and by Uzzau et al. (41) are both complete deletions. The allele originally constructed by Fang et al. (10) and used in several studies (10, 38, 39) is a replication-defective plasmid inserted by homologous recombination. It is possible that production of the resulting truncated SodCII protein causes a defect unrelated to the lack of enzymatic activity. Indeed, we found that producing SodCII under the control of the sodCI promoter attenuated the bacterium. The simplest interpretation of this result is that overproduction of even wild-type SodCII is detrimental. This effect must be independent of SOD activity, because the hybrid strain produces less activity than the wild type. Second, several published studies have apparently been carried out using a single isolate containing the plasmid-inactivated sodCII allele, and it is not clear that this allele has been transduced into a clean background to confirm that the virulence defect is attributable to the mutation. Third, some studies have been performed using different strain backgrounds and in different Salmonella serovars. There could be differences in the sodCII sequences such that some alleles do contribute during infection.
Uzzau et al. (41), using epitope-tagged constructs, reported that, whereas SodCII accumulated to higher levels than SodCI in laboratory medium, SodCII protein was essentially undetectable and SodCI clearly predominated in vivo. It was suggested that this difference in accumulation of the two Cu/Zn SODs was due to a difference at the transcriptional level and that this explained the selective contribution of SodCI to virulence. Our results do not support this interpretation. Although our data and other published data (9) are consistent with a higher level of expression of SodCI than SodCII during infection, the sodCII promoter is clearly active and capable of supporting virulence. Thus, we believe that the two enzymes have structural differences that dictate their activities in the host such that the SodCI protein is better suited to function as a virulence factor.
We have attempted to determine the feature of SodCI that is important for virulence. We have ruled out three important factors: sensitivity to acid and H2O2 and affinity for copper. Indeed, both SodCI and SodCII are stable in the absence of copper. However, we have noted a fundamental difference between the two periplasmic enzymes: SodCI is not released from the periplasm by osmotic shock. We are calling this phenomenon tethering. To our knowledge, the inability to release a periplasmic protein by osmotic shock is novel. The size of SodCI alone certainly does not account for tethering; proteins substantially larger than the SodCI dimer are released by osmotic shock (33).
The simplest explanation is that SodCI is in a complex with some periplasmic component. We hypothesize that this association affects the stability or function of SodCI in the phagosome, contributing to its preferential role in virulence. Tethering of SodCI might help the bacterium retain periplasmic SOD activity if the outer membrane were damaged. It is known that modification and stabilization of the outer membrane by components of the PhoPQ and PmrAB regulons are important for virulence (18, 19). Indeed, it has been suggested that host proteases gain access to the periplasm and that enterics, including Salmonella, produce a periplasmic serine protease inhibitor that protects against a subset of these proteases and allows the bacteria to recover, even after the outer membrane has been compromised (7). Another possibility is that SodCI adheres to an unidentified target or source of O2− in the periplasm. This idea seems unlikely, however, because tethering is apparently not saturable with a 10-fold overproduction of SodCI in serovar Typhimurium or E. coli.
During the course of these studies we have made other interesting observations regarding the periplasmic SodCs. For example, both SodCI and SodCII were synthesized and stable in the apo-enzyme form in the absence of copper and could be spontaneously remetallated by the addition of copper to the growth medium or to the extracts containing the enzymes. The amount of periplasmic Cu/Zn SODs produced in serovar Typhimurium is also striking. The periplasmic SODs of serovar Typhimurium compose almost 50% of the total cellular SOD specific activity (data not shown). Since the periplasm comprises approximately 30% of the total cell volume, it appears that serovar Typhimurium has more SOD (in units per milliliter) in the periplasm than in the cytosol. The abundance of periplasmic SODs in serovar Typhimurium, along with the phenotype exhibited by sodCI mutants in vivo and by sodC mutants of E. coli in vitro (17), strongly suggest that the presence of periplasmic SODs in these organisms confers a certain advantage.
The physiological need for periplasmic SOD in nonpathogens or outside phagocytes is still unclear. While some O2− is released from the periplasmic face of the cytoplasmic membrane (S. S. Korshunov and J. A. Imlay, unpublished data), no periplasmic biomolecules have yet been shown to be vulnerable to O2−. The periplasm apparently lacks labile dehydratases of the iron-sulfur class, and sodC mutants that lack periplasmic SODs grow at normal rates in laboratory cultures. Still, some target must exist, since Salmonella and E. coli sodC strains exhibit aberrant sensitivities to oxidants in vitro (6, 11, 17, 38).
Acknowledgments
This work was supported by National Institutes of Health grant GM49640 to J.A.I.
We thank the Slauch lab and the Imlay lab for valuable comments.
REFERENCES
- 1.Battistoni, A. 2003. Role of prokaryotic Cu,Zn superoxide dismutase in pathogenesis. Biochem. Soc. Trans. 31**:**1326-1329. [DOI] [PubMed] [Google Scholar]
- 2.Battistoni, A., G. Donnarumma, R. Greco, P. Valenti, and G. Rotilio. 1998. Overexpression of a hydrogen peroxide-resistant periplasmic Cu,Zn superoxide dismutase protects Escherichia coli from macrophage killing. Biochem. Biophys. Res. Commun. 243**:**804-807. [DOI] [PubMed] [Google Scholar]
- 3.Battistoni, A., A. P. Mazzetti, R. Petruzzelli, M. Muramatsu, G. Federici, G. Ricci, and B. M. Lo. 1995. Cytoplasmic and periplasmic production of human placental glutathione transferase in Escherichia coli. Protein Expr. Purif. 6**:**579-587. [DOI] [PubMed] [Google Scholar]
- 4.Carlioz, A., and D. Touati. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 5**:**623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97**:**6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vazquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 94**:**13997-14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggers, C. T., I. A. Murray, V. A. Delmar, A. G. Day, and C. S. Craik. 2004. The periplasmic serine protease inhibitor ecotin protects bacteria against neutrophil elastase. Biochem. J. 379**:**107-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellermeier, C. D., A. Janakiraman, and J. M. Slauch. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290**:**153-161. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47**:**103-118. [DOI] [PubMed] [Google Scholar]
- 10.Fang, F. C., M. A. DeGroote, J. W. Foster, A. J. Baumler, U. Ochsner, T. Testerman, S. Bearson, J. C. Giard, Y. Xu, G. Campbell, and T. Laessig. 1999. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl. Acad. Sci. USA 96**:**7502-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrant, J. L., A. Sansone, J. R. Canvin, M. J. Pallen, P. R. Langford, T. S. Wallis, G. Dougan, and J. S. Kroll. 1997. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis. Mol. Microbiol. 25**:**785-796. [DOI] [PubMed] [Google Scholar]
- 12.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83**:**5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueroa-Bossi, N., and L. Bossi. 1999. Inducible prophages contribute to Salmonella virulence in mice. Mol. Microbiol. 33**:**167-176. [DOI] [PubMed] [Google Scholar]
- 14.Flint, D. H., J. F. Tuminello, and M. H. Emptage. 1993. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 268**:**22369-22376. [PubMed] [Google Scholar]
- 15.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J. Biol. Chem. 266**:**1478-1483. [PubMed] [Google Scholar]
- 16.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli aconitase. J. Biol. Chem. 266**:**19328-19333. [PubMed] [Google Scholar]
- 17.Gort, A. S., D. M. Ferber, and J. A. Imlay. 1999. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol. Microbiol. 32**:**179-191. [DOI] [PubMed] [Google Scholar]
- 18.Gunn, J. S. 2001. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotoxin Res. 7**:**57-62. [PubMed] [Google Scholar]
- 19.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes, phoP-phoQ. Science 276**:**250-253. [DOI] [PubMed] [Google Scholar]
- 20.Ho, T. D., N. Figueroa-Bossi, M. Wang, S. Uzzau, L. Bossi, and J. M. Slauch. 2002. Identification of GtgE, a novel virulence factor encoded on the Gifsy-2 bacteriophage of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184**:**5234-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho, T. D., and J. M. Slauch. 2001. Characterization of grvA, an antivirulence gene on the Gifsy-2 phage in Salmonella enterica serovar typhimurium. J. Bacteriol. 183**:**611-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imlay, J. A., and I. Fridovich. 1991. Assay of metabolic superoxide production in Escherichia coli. J. Biol. Chem. 266**:**6957-6965. [PubMed] [Google Scholar]
- 23.Imlay, K. R., and J. A. Imlay. 1996. Cloning and analysis of sodC, encoding the copper-zinc superoxide dismutase of Escherichia coli. J. Bacteriol. 178**:**2564-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keyer, K., and J. A. Imlay. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 93**:**13635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korshunov, S. S., and J. A. Imlay. 2002. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of gram-negative bacteria. Mol. Microbiol. 43**:**95-106. [DOI] [PubMed] [Google Scholar]
- 26.Kroll, J. S., P. R. Langford, K. E. Wilks, and A. D. Keil. 1995. Bacterial [Cu,Zn]-superoxide dismutase: phylogenetically distinct from the eukaryotic enzyme, and not so rare after all! Microbiology 141**:**2271-2279. [DOI] [PubMed] [Google Scholar]
- 27.Liochev, S. I., and I. Fridovich. 2002. Copper, zinc superoxide dismutase and H2O2. Effects of bicarbonate on inactivation and oxidations of NADPH and urate, and on consumption of H2O2. J. Biol. Chem. 277**:**34674-34678. [DOI] [PubMed] [Google Scholar]
- 28.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogenic bacteria: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 29.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192**:**237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCord, J. M., and I. Fridovich. 1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244**:**6049-6055. [PubMed] [Google Scholar]
- 31.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35**:**1-13. [DOI] [PubMed] [Google Scholar]
- 32.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10**:**1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nossal, N. G., and L. A. Heppel. 1966. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J. Biol. Chem. 241**:**3055-3062. [PubMed] [Google Scholar]
- 34.Pesce, A., A. Battistoni, M. E. Stroppolo, F. Polizio, M. Nardini, J. S. Kroll, P. R. Langford, P. O'Neill, M. Sette, A. Desideri, and M. Bolognesi. 2000. Functional and crystallographic characterization of Salmonella typhimurium Cu,Zn superoxide dismutase coded by the sodCI virulence gene. J. Mol Biol. 302**:**465-478. [DOI] [PubMed] [Google Scholar]
- 35.Pesce, A., C. Capasso, A. Battistoni, S. Folcarelli, G. Rotilio, A. Desideri, and M. Bolognesi. 1997. Unique structural features of the monomeric Cu,Zn superoxide dismutase from Escherichia coli, revealed by X-ray crystallography. J. Mol. Biol. 274**:**408-420. [DOI] [PubMed] [Google Scholar]
- 36.Rathman, M., M. D. Sjaastad, and S. Falkow. 1996. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 64**:**2765-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richter-Dahlfors, A., A. M. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186**:**569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sansone, A., P. R. Watson, T. S. Wallis, P. R. Langford, and J. S. Kroll. 2002. The role of two periplasmic copper- and zinc-cofactored superoxide dismutases in the virulence of Salmonella choleraesuis. Microbiology 148**:**719-726. [DOI] [PubMed] [Google Scholar]
- 39.Sly, L. M., D. G. Guiney, and N. E. Reiner. 2002. Salmonella enterica serovar Typhimurium periplasmic superoxide dismutases SodCI and SodCII are required for protection against the phagocyte oxidative burst. Infect. Immun. 70**:**5312-5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanley, T. L., C. D. Ellermeier, and J. M. Slauch. 2000. Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar Typhimurium survival in Peyer's patches. J. Bacteriol. 182**:**4406-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uzzau, S., L. Bossi, and N. Figueroa-Bossi. 2002. Differential accumulation of Salmonella [Cu, Zn] superoxide dismutases SodCI and SodCII in intracellular bacteria: correlation with their relative contribution to pathogenicity. Mol. Microbiol. 46**:**147-156. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192**:**227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100**:**195-199. [PubMed] [Google Scholar]
- 44.Winkelstein, J. A., M. C. Marino, R. B. Johnston, Jr., J. Boyle, J. Curnutte, J. I. Gallin, H. L. Malech, S. M. Holland, H. Ochs, P. Quie, R. H. Buckley, C. B. Foster, S. J. Chanock, and H. Dickler. 2000. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79**:**155-169. [DOI] [PubMed] [Google Scholar]
- 45.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97**:**5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]