Impact of low-magnitude, high frequency mechanical stimulation on bone mineral density among young childhood cancer survivors: a double-blind randomized controlled trial (original) (raw)
. Author manuscript; available in PMC: 2017 Jul 1.
Abstract
Importance
Bone accrual during youth is critical to establish sufficient strength for lifelong skeletal health. Children with cancer may develop low bone mineral density any time before or after diagnosis.
Objective
To evaluate the ability of low magnitude, high frequency mechanical stimulation to enhance bone mineral density among childhood cancer survivors.
Design
Double-blind randomized controlled trial from June 1, 2010-January 22, 2013. Participants were randomized (stratified by sex and Tanner stage) to either a placebo device or low magnitude, high frequency mechanical stimulation.
Setting
St. Jude Children’s Research Hospital; intervention completed at home.
Participants
Survivors, ages 7-17 years, previously treated at St. Jude Children’s Research Hospital, in remission, at least five years from diagnosis, with whole body or lumbar spine bone mineral density Z-scores ≤−1.0.
Intervention
Placebo or low magnitude, high frequency mechanical stimulation (0.3 g, 32-37Hz) for two 10-minute sessions, seven days a week for one year. All participants were prescribed daily vitamin D and calcium.
Main outcome measures
Changes in areal and volumetric bone mineral density and bone biomarkers were compared by analysis of variance, adjusted for strata.
Results
Forty-eight of 65 randomized participants completed this double-blind study with median adherence of 70.1% for intervention and 63.7% for placebo groups. With intention-to-treat analysis, mean whole body bone mineral density Z-score by dual x-ray absorptiometry improved by 0.25±0.78 in the intervention (N=22), but decreased by −0.19±0.79 in the placebo group (N=26) p=0.05). Circulating osteocalcin at 12 months correlated with change in total body bone mineral density (r=0.35, p=0.02). Participants completing ≥70% of prescribed sessions increased 11.2±11.3% in tibial trabecular bone volume compared to those completing <70% who decreased −1.3±9.9% (p=0·02). Change in circulating receptor activator of nuclear factor kappa-B ligand was higher in the intervention than in the placebo group (0.06±0.16 vs. −0.04±0.17 pmol/L, p=0.04).
Conclusions and relevance
Pediatric cancer survivors with low bone mineral density may benefit from low magnitude, high frequency mechanical stimulation as a novel, safe, and convenient intervention to optimize peak bone mass during youth, alone or in conjunction with other therapies.
Trial Registration
NCT01010230. Vibration Intervention For Bone Enhancement In Childhood Cancer Survivors, www.clincialtrials.gov
INTRODUCTION
Accrual of bone mineral density (BMD) during childhood and adolescence is critical to establish sufficient bone mass to support and maintain skeletal health throughout life. For children diagnosed with cancer, the cumulative effects of disease,1,2 chemotherapy,3 radiation exposure,4 physical inactivity,5 and poor nutrition6 are detrimental to bone mineralization and may result in sub-optimal BMD that persists into adulthood.7,8 The percentage of childhood cancer survivors with low BMD is reported as high as 47%, depending on tumor type and treatment exposure.7-10 Bisphosphonate therapy successfully improved BMD in small studies among childhood cancer survivors,11 but has potential adverse effects.12,13 Randomized trials evaluating weight-bearing exercise14 or nutritional supplementation15 have not benefited BMD in this population.
Designed to harness bone’s sensitivity to mechanical signals,16 low magnitude (<1.0g) mechanical stimulation (LMS) improves quantity and quality of bone in animal models17 and humans, with particular efficacy in younger populations.18-20 In humans, low magnitude acceleration is applied through the feet, by standing on a platform oscillating at relatively high frequency. The mechanical signal transmits to the hip and spine with approximately 80% efficiency21 and is anabolic to bone,22 biasing mesenchymal stem cell populations towards osteoblastogeneis23 and enhancing cytoskeletal adaptation in stem cell progenitors.24 Examined in mouse models of cancer,25,26 LMS protects bone tissue without compromising longevity or promoting tumor growth. Because LMS is non-invasive and non-pharmacologic with minimal risk for adverse events, the aims of this study were to evaluate the effects of LMS on BMD and markers of bone turnover among survivors of childhood cancer with low BMD.
METHODS
Design
This prospective, double-blind, placebo-controlled trial (NCT01010230) was conducted at St. Jude Children’s Research Hospital (SJCRH), and approved by the Institutional Review Board.
Participants
Childhood cancer survivors, seven to 17 years of age, ≥five years from diagnosis and not currently receiving treatment for cancer, with age- and sex-specific lumbar or whole body BMD Z-scores <−1.0 were contacted for recruitment one month prior to annual After Completion of Therapy Clinic appointments. Eligibility criteria included verification of BMD Z-score <−1.0, ability to stand independently for ten minutes, and ability to tolerate calcium and vitamin D supplements. Children requiring chronic oral glucocorticoid therapy, pharmacologic agents for reduced BMD other than calcium or vitamin D, with metal implants or spinal deformity requiring bracing, and pregnant females were not eligible. Potentially eligible participants with baseline 25-hydroxy vitamin D (25(OH)-D) levels <50 nanomoles per liter (nmol/L) were treated with 5,000 international units (IU) of vitamin D to normalize 25(OH)-D status prior to randomization.27 Written informed consent was obtained from parents/legal guardians.
Randomization
Personnel not involved with study participants randomized those enrolled using a computer program written in C++ to intervention or placebo groups in block sizes of four by Tanner Stage (I, II, III versus IV, V) and sex.28
Intervention
Participants were instructed to stand on a platform for 10 minutes twice daily29 for one year. Those assigned to the intervention stood on an active platform. The mechanical signal (0.3g at 32-37Hz) produced a subtle, sinusoidal, vertical translation <100 microns via a linear electromagnetic actuator.30 The placebo group stood on a device identical in appearance to the active platform. The placebo device emitted a 500Hz audible hum, but did not deliver the signal. Both groups received calcium (800-1,200 mg/day) and vitamin D supplements (cholecalciferol 400 IU/day) as recommended by the Children’s Oncology Group.31
Adherence
Participants received log sheets to record daily sessions. An inboard monitor32 also recorded the number and time of each session. Session counts were visible when the device was turned on. To encourage adherence, participants were contacted weekly to inquire how many sessions they completed, and provided with problem solving strategies if they had difficulty. Participants were encouraged to put the device in an accessible location and to use it while engaged in another activity (e.g., watching television, listening to music). In addition, $5 gift cards were mailed after completion of every 50 sessions, report cards were sent monthly with statements to reinforce adherence, and participants received an iPod™ shuffle at the end of the study. Actual adherence (ratio of time the device was used/total prescribed use) was determined from device monitor data at study completion.
Assessments
Areal bone mineral density was measured with dual X-ray absorptiometry (DEXA, 4500 QDR-A/Discovery fan beam, Hologic, Bedford, MA). Scans were performed in fast transverse speed mode; the scanner was calibrated monthly. Data were analyzed using QDR software for Windows (version 13.3:3) (CV <1%). Normative data provided by the manufacturer were used to calculate age- and sex-specific Z-scores.
Average lumbar volumetric BMD was assessed with quantitative computed tomography (QCT, Lightspeed Ultra 8-detector, GE Healthcare, Pittsburgh, PA). Participants were scanned lying on cushions containing phantoms of K2HPO4 mineral equivalents (Mindwaves, San Francisco, CA) (CV <2.8%). Mineral content was determined via axial images of mid bodies of L1 and L2, using sagittal or coronal scout images of the upper abdomen.33 Between 28 and 32 three-millimeter slices were obtained for complete assessment of target vertebrae.
Tibial cortical and trabecular bone content were also assessed with QCT. To assure identical scan locations, left tibial length was measured with a Segmometer (Rosscraft, Blain, WI). A scout view was used to position the scanner 50% from the distal tibial endplate. A single slice of 2.3 mm was taken with a voxel size of 0.4 mm. Image processing and numerical values were generated (Mindwaves QCT ProTM BIT) to calculate cortical cross-sectional area (mm2 between endosteal-periosteal borders). A region 2×2 voxels between endosteal-periosteal bone envelopes defined the cortical compartment.
Fasting blood and urine samples were taken at baseline and 12-months. To detect and resolve potential endocrinopathies, including vitamin D deficiency, prior to randomization, serum was assayed for estradiol (female), testosterone (male), free thyroxine, triiodothyronine, thyroid stimulating hormone, intact parathyroid hormone, 25(OH)D, and 1,25-dihydroxyvitamin D2 [1,25(OH)2-D2].27 To evaluate bone turnover, thawed samples obtained at baseline and 12-months (stored at −80°C) were assayed for osteocalcin (OC), skeletal alkaline phosphatase (BSAP), carboxyterminal telopeptide of type I collagen (CTx), collagen cross-linked N-telopeptide (NTx), N-terminal propeptide of Type I procollagen (PINP), osteoprotegerin (OPG), and receptor activator of nuclear factor kappa-B ligand (RANKL).
Dietary intake was assessed with the Block Kids Frequency Questionnaire 2004 (NutritionQuest, Berkeley, CA).34 Average daily minutes of moderate/vigorous physical activity was measured over seven days with an accelerometer (GT3X, ActiGraph, Pensacola, FL) worn over the right hip during waking, non-bathing hours.35,36
Outcomes
Primary outcome measures were changes from baseline to 12-months in age- and sex-specific Z-scores for total body and lumbar spine areal BMD, in total and lumbar areal BMD, and in lumbar volumetric bone mineral density, tibia bone content and strength. Secondary outcome measures were changes in biomarkers of bone turnover. Adverse events were collected from on-study date until 12 months after start of the intervention and reported in real time.
Statistical Analysis
Descriptive statistics summarized demographic, skeletal, endocrine characteristics, macronutrient intake, and daily physical activity among participants. Two-sample t-tests compared characteristics of intervention and control groups. Mean changes in BMD measures and biomarkers of bone turnover were calculated from baseline to 12-months. Pre-established comparisons (intention-to-treat) between intervention and placebo groups, and post-hoc comparisons (per-protocol analyses) between intervention group participants who completed ≥70% to those who completed <70% of sessions, utilized analyses of variance, adjusting for Tanner stage and sex. Associations between measures of BMD and biomarkers of bone turnover were evaluated with Pearson product-moment correlation. SAS version 9.3 (SAS Institute, Inc., Cary, NC) was used for all analyses.
Power estimates for primary outcomes were based on two-sided two-sample t-tests. A priori, we estimated that 30 subjects in each group, assuming no change in the placebo group, and a common standard deviation (SD) of 6.8%,29 resulted in 87% power to detect a 5.5% change in BMD measures in the intervention group, with type I error ± 0.05.30,31 We did not adjust for multiple comparisons.
RESULTS
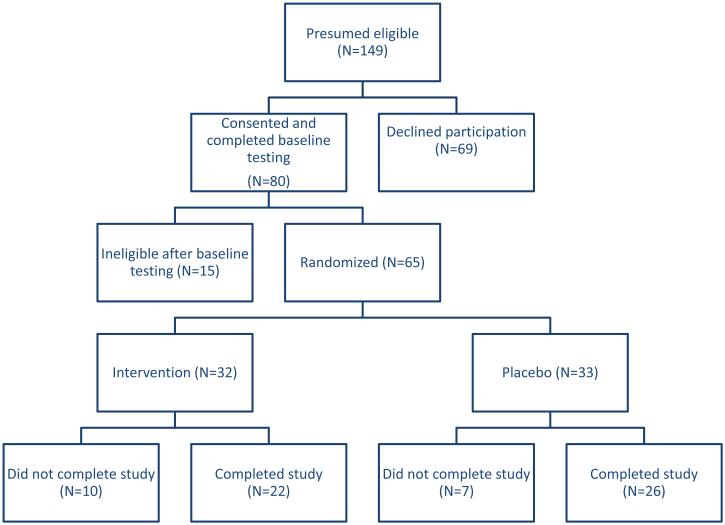
Among 149 children scheduled for follow-up between May 13, 2010 and November 9, 2011, with previous total body or lumbar BMD Z-scores <−1.0, 80 (54%) consented and completed testing. Twelve were not eligible because Z-scores had improved to >−1.0 since their last visit, two had vitamin D levels that did not recover enough to allow participation, and one was on chronic glucocorticoid therapy, leaving 65 for randomization. Thirty-two were randomized to LMS and 33 to placebo. The groups shared similar baseline characteristics (Supplemental Table 1). Ten participants in the intervention and seven in the placebo group did not complete the study (Figure 1). Nine participants reported being too busy, one had attention issues that precluded standing on the device, seven were lost to follow-up. Those completing did not differ from those not completing the study with respect to sex, race, Tanner stage, age, diagnostic group, or treatment exposure. Adherence did not differ between groups with median (interquartile range) values of 70.1% (35.4-91.5%) in the intervention and 63.7% (33.3-86.5%) in the placebo group (p=0.40). There were no adverse events associated with standing on either device.
Figure 1.
Consort
Table 1 displays changes in BMD measures by treatment group. In an intention to treat analysis, age- and sex-specific total body BMD Z-scores improved, on average, by 0.25 in the intervention and decreased by −0.19 in the placebo group (p=0.05). Because there were no differences between groups in changes in lumbar BMD measures, this difference is likely accounted for by accrual of bone in the lower extremity or other sites. Percent changes in tibia cortical and trabecular bone trended greater in the intervention than in the placebo group, but did not appear to keep up with linear growth as percent change in tibial cortical bone content per length declined in both groups.
Table 1.
Mean change in bone density, content, and strength from baseline to 12 months
| Intervention (N=22) | Placebo (N=26) | p - value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean* | SD | 95% CI | Mean* | SD | 95% CI | ||||
| Total body BMD Z-score | 0.25 | 0.78 | −0.09 | 0.59 | −0.19 | 0.79 | −0.51 | 0.12 | 0.05 |
| L1,L2 BMD Z-score | 0.08 | 0.51 | −0.13 | 0.30 | 0.14 | 0.51 | −0.06 | 0.35 | 0.68 |
| Percent bone mineral content/height (total) | 1.71 | 9.01 | −2.15 | 5.60 | 3.99 | 8.97 | 0.44 | 7.55 | 0.38 |
| Percent bone mineral density/height (total) | 6.56 | 7.64 | 3.27 | 9.85 | 3.45 | 7.60 | 0.43 | 6.46 | 0.12 |
| Percent L1, L2 bone mineral content/height | 3.70 | 21.20 | −5.41 | 12.82 | 2.54 | 21.06 | −5.81 | 10.89 | 0.84 |
| Percent L1, L2 bone mineral density/height | 4.91 | 10.34 | 0.46 | 9.36 | 5.01 | 10.29 | 0.94 | 9.06 | 0.97 |
| Percent L1, L2 volumetric bone mineral density | 5.64 | 10.83 | 0.98 | 10.31 | 5.30 | 11.06 | 0.92 | 9.68 | 0.91 |
| Percent tibia cortical bone | 3.00 | 4.69 | 0.87 | 5.02 | 1.77 | 4.90 | −0.17 | 3.71 | 0.40 |
| Percent tibia trabecular bone | 4.89 | 10.27 | 0.47 | 9.31 | 0.64 | 10.45 | −3.51 | 4.79 | 0.08 |
| Percent tibia cortical bone/length | −1.19 | 6.63 | −4.05 | 1.66 | −1.86 | 6.77 | −4.54 | 0.82 | 0.73 |
In a per protocol analysis, changes in percent tibial trabecular bone were associated with adherence in the intervention group. After adjusting for sex and Tanner stage, the 11 intervention group participants who completed ≥70% of sessions had a mean 11.2±11.3% increase whereas the 11 who completed <70% of sessions had a mean 1.3±9.9% decrease in tibia trabecular bone (p=0·02). There was no association between adherence and change in percent tibial trabecular bone in the placebo group (p=0.49).
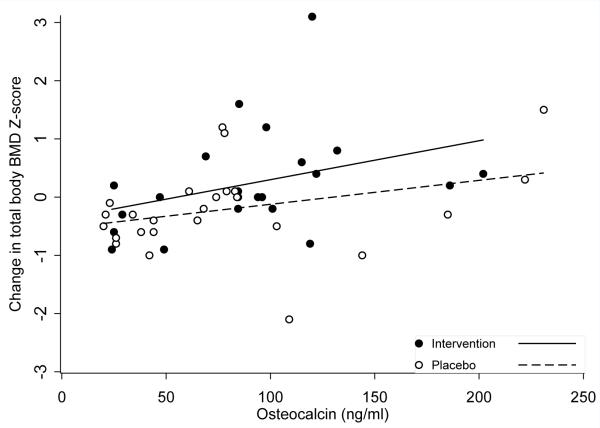
Table 2 shows changes from baseline to 12-months among biomarkers of bone turnover. Although differences between groups were not statistically significant, after accounting for sex and Tanner stage, means for biomarkers of bone formation (PINP, OC, and BSAP) tended to increase or slightly decrease from baseline to 12-months in the intervention group, with a larger magnitude decrease in the placebo group. OC values at 12-months correlated positively with change in total body BMD Z-score (r=0.35, p=0.02, Figure). Changes in biomarkers of bone resorption did not differ between groups. RANKL values increased in the intervention and decreased in the placebo group.
Table 2.
Mean change from baseline to 12 months in biomarkers of bone turnover
| Intervention (N=22) | Placebo (N=26) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean* | SD | 95% CI | Mean* | SD | 95% CI | ||||
| PINP (μg/L) | 22.75 | 241.40 | −81,22 | 126.71 | −62.41 | 235.98 | −159.56 | 34.73 | 0.23 |
| OC (ng/ml) | −5.87 | 40.61 | −23.38 | 11.65 | −14.15 | 39.36 | −29.77 | 1.47 | 0.47 |
| BSAP (g/L) | −3.90 | 18.71 | −11.97 | 4.17 | −13.96 | 19.01 | −21.51 | −6.41 | 0.07 |
| CTx (μg/ml) | −21.36 | 466.50 | −222.24 | 179.52 | −187.57 | 473.90 | −375.27 | 0,13 | 0.23 |
| NTx (mmol BCE/mmol creatinine) | −61.29 | 207.22 | −150.52 | 27.94 | −46.35 | 210.48 | −129.74 | 37.02 | 0.81 |
| OPG (pmol/L) | −0.29 | 1.43 | −0.90 | 0.33 | 0.30 | 1.41 | −0.26 | 0.86 | 0.16 |
| RANKL (pmol/L) | 0.06 | 0.16 | −0.01 | 0.13 | −0.04 | 0.17 | −0.11 | 0.02 | 0.04 |
| RANKL/OPG index | 0.13 | 0.50 | −0.09 | 0.34 | −0.13 | 0.50 | −0.33 | 0.08 | 0.09 |
DISCUSSION
To our knowledge, this is the first prospective, randomized trial that suggests benefit of LMS to prevent or reverse decline in bone density among young survivors of childhood cancer. Participants in the intervention group demonstrated increased total body BMD, in contrast to decreased BMD among those in the placebo group. Overall trends in bone formation biomarkers (OC, PINP, BSAP) among those who received the intervention, in combination with a suggested increase in tibial trabecular and cortical bone, indicate effects favoring bone formation as a result of LMS. Moreover, intervention adherence influenced favorable change in BMD; those in the LMS group who completed ≥70% of prescribed sessions displayed a better outcome than those less adherent.
Accruing bone tissue during childhood and adolescence carries lifelong benefits.37 Although reports fail to demonstrate an association between low BMD and idiopathic fracture,38 and do not provide evidence of increased fracture risk among adult survivors of childhood cancer when compared to siblings,39 such studies included survivors who were in their second and fourth decades of life. The impact of persistently low BMD on fracture risk among childhood cancer survivors as they enter their fifth and sixth decades of life is not known. However, it is clear from the general population literature that the higher the BMD, the lower the risk of complications of osteoporosis. Models that incorporate BMD with clinical risk factors among post-menopausal women, to predict age at onset of osteoporosis, indicate that a 10% increase in peak bone mass is associated with delayed onset of osteoporosis of 13 years.40 Additionally, epidemiologic data indicate that a 10% increase in peak bone mass reduces risk for fracture among women by 50% after menopause.41 Thus, gaining BMD during adolescence, as demonstrated here following one year of LMS, has potential to reduce long-term risk for osteoporosis, fractures, and associated mortality among childhood cancer survivors. Although prospective studies that follow survivors through adulthood will be required to determine if this hypothesis is valid, LMS appears to be a convenient, safe, and non-pharmacologic intervention that could be started during or immediately following therapy to boost bone health among youth whose cancer and/or therapy interferes with achieving optimal skeletal health.
Bone metabolism is a complex process involving resorption and formation, mediated by osteoclasts and osteoblasts, enabled by hematopoietic and mesenchymal stem cells that reside in a bone marrow niche susceptible to disruption by cancer and/or its treatment.42 These processes are tightly coupled during bone cell recruitment and remodeling, and are influenced by hormonal status, especially during childhood and adolescence, the most important period of bone growth.43 Balanced hormonal status across treatment and placebo groups in our study allowed evaluation of changes in biomarkers of bone formation and resorption. Although we found a modest correlation between OC values at the end of the intervention and change in total body BMD Z-score, RANKL was the only biomarker of bone turnover where change over 12-months differed between groups. Mean RANKL increased in the intervention, but decreased in the placebo group, indicating increased osteoclast activity and elevated bone resorption.44 This, associated with a trend toward an increase in BSAP (index of bone formation) among those in the intervention group, suggests that mechanical signals promote bone turnover, with a net balance towards bone accrual. It is possible, although not demonstrated here, that a bone-organ system suppressed by disease and/or treatments is ‘activated’ by the mechanical signals, either mobilizing stem cell progenitors or stimulating resident bone cell populations. We did not observe a correlation between RANKL and any BMD measures. This may be because peak RANKL and other bone turnover markers occurred after baseline but prior to 12-month follow-up.
Our results suggest an increase in appendicular rather than spinal bone mass, results similar to those reported by Ward et al20 in their study of 20 children with cerebral palsy where they reported increased volumetric tibial trabecular bone (6.3% intervention vs. −11.9% control), but not lumbar spine or tibial cortical bone, after six months of five weekly, 10-minute LMS sessions (0.3 g, 90Hz). Wren et al45 reported changes in tibial cortical bone density, but not tibial or spinal trabecular BMD among 31 children with cerebral palsy during LMS, versus a standing period. Studies of LMS among young females with idiopathic scoliosis and low BMD,46 or with fracture history and low BMD,18 also reported improvements in appendicular rather than in spinal BMD. These regional differences in the effects of LMS on BMD may relate to the proximity of the device to body region, with potential loss of vibratory energy as the signal travels from the distal lower extremity to the trunk,47 or to differences in capacity to respond to mechanical signals, with some regions more attuned to functional adaptation than others.48
These data point toward the anabolic potential of LMS for the skeleton. Considering the health history of children previously treated for malignancy, it is important to ensure that any intervention, even one that is non-pharmacologic, is safe. Vibration is recognized as a pathogen, provoking low back pain, white finger diseases, blurred vision and hearing loss.49 Indeed, measures of human exposure to high magnitude vibration emphasize that its use should be approached with caution, particularly among those at greatest risk for fracture.50-52 That being said, ISO-2631 standards for human exposure to vibration indicate that the frequency and intensity of the signal in this study is considered safe for up to four hours each day.53 Additionally, in murine models of ovarian cancer and myeloma, LMS did not compromise survival or exacerbate disease, yet protected bone quality in the axial and appendicular skeleton and reduced pathologies in skeletal tissue,25,26 suggesting that mechanical signals that are salutary to bone tissue do not result in progression of malignant disease. Finally, in our study, we observed no adverse events associated with LMS over one year of twice daily use.
Our results should be considered in the context of some limitations. First, although 74% of enrolled participants completed the study, retaining our ability to detect a change in total body BMD Z-score, our final sample size limited statistical power to detect differences in other outcomes. Second, only half of participants in the intervention group completed 70% or more of prescribed sessions. This may limit the feasibility of this intervention in a non-research setting. Third, our data lack early and repeated measures of the biomarkers of bone turnover, limiting ability to determine and understand initial and longitudinal metabolic responses of bone to LMS in this population. Failure to detect differences between groups, with respect to mean changes in some biomarkers of bone turnover, may be because the biomarker response to LMS was acute (within days or weeks of the intervention start), with values attenuating over the study period.54 Finally, even though we employed a double-blind, placebo-controlled study design, we could not control for potential confounding by differences in other factors that influence bone growth (e.g., genetics, sun exposure).
In summary, this study suggests that pediatric cancer survivors with low BMD may benefit from LMS as a safe, convenient intervention to promote accrual of bone mass during childhood and adolescence. These results are preliminary. Further work needs to be done to define mechanisms of action of LMS in this population, and to determine if modifications in dose/duration of LMS can provide greater improvements in BMD. There is also a need to determine if LMS works synergistically with pharmacologic or exercise interventions, prevents bone loss among children during cancer therapy, or improves BMD among survivors who have achieved skeletal maturity, and to determine if bone accrual is retained following cessation of use.
Supplementary Material
Supplemental Table 1
Figure 2.
Correlation between osteocalcin at 12 months and change in total body BMD Z-score
ACKNOWLEDGEMENTS
KKN and DKS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by Gabrielle’s Angel Foundation, National Institutes of Health (HD059292, CA21765, CA195547) and the American Lebanese Syrian Associated Charities (ALSAC). The funding sources had no role in the design and conduct of the study, collection, management, analysis or interpretation of data, or in preparation, review, approval or decision to submit the manuscript for publication.
The authors would like to thank Clinton Rubin, SUNY Distinguished Professor and Chair, Department of Biomedical Engineering, Stony Brook University, Stony Brook, NY for his assistance editing the manuscript (he received no compensation) and Tracie Gatewood for her assistance formatting the manuscript for submission.
Footnotes
The authors declare no conflicts of interest.
KKN, SCK, RJF, LLR, and DKS designed the study. RJM, SCK, RJF, MMH, CRH, REP, and KKN participated in data collection. KKN, RJM, and DKS analyzed the data. All authors interpreted the data and participated in writing and revising the manuscript.
Reference List
- 1.Halton JM, Atkinson SA, Fraher L, et al. Mineral homeostasis and bone mass at diagnosis in children with acute lymphoblastic leukemia. J Pediatr. 1995;126(4):557–564. doi: 10.1016/s0022-3476(95)70349-7. [DOI] [PubMed] [Google Scholar]
- 2.Ness KK, Kaste SC, Zhu L, et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk Lymphoma. 2015;56(4):1004–1011. doi: 10.3109/10428194.2014.944519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nysom K, Holm K, Michaelsen KF, Hertz H, Muller J, Molgaard C. Bone mass after treatment for acute lymphoblastic leukemia in childhood. J Clin Oncol. 1998;16(12):3752–3760. doi: 10.1200/JCO.1998.16.12.3752. [DOI] [PubMed] [Google Scholar]
- 4.Gilsanz V, Carlson ME, Roe TF, Ortega JA. Osteoporosis after cranial irradiation for acute lymphoblastic leukemia. J Pediatr. 1990;117(2):238–244. doi: 10.1016/s0022-3476(05)80536-0. Pt 1. [DOI] [PubMed] [Google Scholar]
- 5.Heath JA, Ramzy JM, Donath SM. Physical activity in survivors of childhood acute lymphoblastic leukaemia. J Paediatr Child Health. 2010;46(4):149–153. doi: 10.1111/j.1440-1754.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson SA, Halton JM, Bradley C, Wu B, Barr RD. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: influence of disease, drugs and nutrition. Int J Cancer Suppl. 1998;11:35–39. [PubMed] [Google Scholar]
- 7.Gurney JG, Kaste SC, Liu W, et al. Bone mineral density among long-term survivors of childhood acute lymphoblastic leukemia: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2014;61(7):1270–1276. doi: 10.1002/pbc.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaste SC, Rai SN, Fleming K, et al. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46(1):77–87. doi: 10.1002/pbc.20553. [DOI] [PubMed] [Google Scholar]
- 9.Barr RD, Simpson T, Webber CE, et al. Osteopenia in children surviving brain tumours. Eur J Cancer. 1998;34(6):873–877. doi: 10.1016/s0959-8049(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaste SC, Ahn H, Liu T, et al. Bone mineral density deficits in pediatric patients treated for sarcoma. Pediatr Blood Cancer. 2008;50(5):1032–1038. doi: 10.1002/pbc.21281. [DOI] [PubMed] [Google Scholar]
- 11.Bryant ML, Worthington MA, Parsons K. Treatment of osteoporosis/osteopenia in pediatric leukemia and lymphoma. Ann Pharmacother. 2009;43(4):714–720. doi: 10.1345/aph.1L567. [DOI] [PubMed] [Google Scholar]
- 12.Green SB, Pappas AL. Effects of maternal bisphosphonate use on fetal and neonatal outcomes. Am J Health Syst Pharm. 2014;71(23):2029–2036. doi: 10.2146/ajhp140041. [DOI] [PubMed] [Google Scholar]
- 13.Sebestyen JF, Srivastava T, Alon US. Bisphosphonates use in children. Clin Pediatr (Phila) 2012;51(11):1011–1024. doi: 10.1177/0009922812452118. [DOI] [PubMed] [Google Scholar]
- 14.Hartman A, te Winkel ML, van Beek RD, et al. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53(1):64–71. doi: 10.1002/pbc.21942. [DOI] [PubMed] [Google Scholar]
- 15.Kaste SC, Qi A, Smith K, et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2014;61(5):885–893. doi: 10.1002/pbc.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff J. Das Gesetz det Transformation der Knochen (The Law of Bone Remodeling) Verlag von August Hirschwald; Berlin: 1892. [Google Scholar]
- 17.Judex S, Rubin CT. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J Musculoskelet Neuronal Interact. 2010;10(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- 18.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21(9):1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 19.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19(3):343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 20.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19(3):360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 21.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28(23):2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 22.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412(6847):603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 23.Rubin CT, Capilla E, Luu YK, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104(45):17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uzer G, Thompson WR, Sen B, et al. Cell Mechanosensitivity to Extremely Low-Magnitude Signals Is Enabled by a LINCed Nucleus. Stem cells. 2015;33(6):2063–2076. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagnotti G, Adler B, Chan ME, Korman M, Shroyer KR, Rubin C. Osteopenia and osteolysis resulting from multiple myeloma partially suppressed through low intensity mechanical signals. J Bone Miner Res. 2014;29(Suppl 1) [Google Scholar]
- 26.Pagnotti GM, Adler BJ, Green DE, et al. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone. 2012;51(3):570–577. doi: 10.1016/j.bone.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 28.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7-8):365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 29.Sen B, Xie Z, Case N, Styner M, Rubin CT, Rubin J. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech. 2011;44(4):593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritton JC, Rubin CT, Qin YX, McLeod KJ. Whole-body vibration in the skeleton: development of a resonance-based testing device. Ann Biomed Eng. 1997;25(5):831–839. doi: 10.1007/BF02684167. [DOI] [PubMed] [Google Scholar]
- 31.Children’s Oncology Group The Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. 2006;2.0 www.survivorshipguidelines.org. Accessed November 12, 2015. [Google Scholar]
- 32.Jeffrey BA, Hannan MT, Quinn EK, et al. Self-reported adherence with the use of a device in a clinical trial as validated by electronic monitors: the VIBES study. BMC Med Res Methodol. 2012;12:171. doi: 10.1186/1471-2288-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cann CE. Quantitative CT for determination of bone mineral density: a review. Radiology. 1988;166(2):509–522. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 34.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92(6):686–693. [PubMed] [Google Scholar]
- 35.National Cancer Institute. SAS Programs for Analyzing NHANES 2003-2004 Accelerometer Data http://riskfactor.cancer.gov/tools/nhanes_pam/. Accessed November 12, 2015.
- 36.National Center for Health Statistics National Health and Nutrition Examination Survey 2003-2004 Examination Files. http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/exam03_04.htm. Accessed November 12, 2015.
- 37.Melton LJr, Atkinson EJ, Khosla S, Oberg AL, Riggs Evaluation of a prediction model for long-term fracture risk. J Bone Miner Res. 2005;20(4):551–556. doi: 10.1359/JBMR.041206. [DOI] [PubMed] [Google Scholar]
- 38.Kaste SC, Tong X, Hendrick JM, et al. QCT versus DXA in 320 survivors of childhood cancer: association of BMD with fracture history. Pediatr Blood Cancer. 2006;47(7):936–943. doi: 10.1002/pbc.20854. [DOI] [PubMed] [Google Scholar]
- 39.Wilson CL, Dilley K, Ness KK, et al. Fractures among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2012;118(23):5920–5928. doi: 10.1002/cncr.27626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez CJ, Beaupre GS, Carter A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14(10):843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 41.Marshall D, Johnell O, Wedel Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green DE, Adler BJ, Chan ME, Rubin Devastation of adult stem cell pools by irradiation precedes collapse of trabecular bone quality and quantity. J Bone Miner Res. 2012;27(4):749–759. doi: 10.1002/jbmr.1505. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Lopez FR, Chedraui P, Cuadros-Lopez Bone mass gain during puberty and adolescence: deconstructing gender characteristics. Curr Med Chem. 2010;17(5):453–466. doi: 10.2174/092986710790226138. [DOI] [PubMed] [Google Scholar]
- 44.Khosla Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 45.Wren TA, Lee DC, Hara R, et al. Effect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsy. J Pediatr Orthop. 2010;30(7):732–738. doi: 10.1097/BPO.0b013e3181efbabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam TP, Ng BK, Cheung LW, Lee KM, Qin L, Cheng Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int. 2013;24(5):1623–1636. doi: 10.1007/s00198-012-2144-1. [DOI] [PubMed] [Google Scholar]
- 47.Abercromby AF, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Paloski Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc. 2007;39(9):1642–1650. doi: 10.1249/mss.0b013e318093f551. [DOI] [PubMed] [Google Scholar]
- 48.Wallace IJ, Pagnotti GM, Rubin-Sigler J, et al. Focal enhancement of the skeleton to exercise correlates to mesenchymal stem cell responsivity rather than peak external forces. J Exp Biol. 2015 doi: 10.1242/jeb.118729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernard B, Nelson N, Estill CF, Fine The NIOSH review of hand-arm vibration syndrome: vigilance is crucial. National Institute of Occupational Safety and Health. J Occup Environ Med. 1998;40(9):780–785. doi: 10.1097/00043764-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Kiiski J, Heinonen A, Jarvinen TL, Kannus P, Sievanen Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23(8):1318–1325. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 51.Muir J, Kiel DP, Rubin Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. J Sci Med Sport. 2013;16(6):526–531. doi: 10.1016/j.jsams.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pel JJ, Bagheri J, van Dam LM, et al. Platform accelerations of three different whole-body vibration devices and the transmission of vertical vibrations to the lower limbs. Med Eng Phys. 2009;31(8):937–944. doi: 10.1016/j.medengphy.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 53.International Organization for Standardization ISO 2631-1:1997(en) Mechanical vibration and shock — Evaluation of human exposure to whole-body vibration — Part 1: General requirements. 1997 http://www.iso.org/iso/catalogue_detail.htm?csnumber=7612. Accessed October 15, 2015.
- 54.Harrison R, Ward K, Lee E, Razaghi H, Horne C, Bishop Acute bone response to whole body vibration in healthy pre-pubertal boys. J Musculoskelet Neuronal Interact. 2015;15(2):112–122. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1