P granules (original) (raw)
. Author manuscript; available in PMC: 2016 Jul 29.
Published in final edited form as: Curr Biol. 2014 Jul 21;24(14):R637–R638. doi: 10.1016/j.cub.2014.06.018
What are they?
P granules are the Caenorhabditis elegans ‘germ granules’, a class of perinuclear RNA granules specific to the germline. The defining components of P granules are two classes of RNA-binding proteins: the RGG-domain proteins, PGL-1 and PGL-3; and the DEAD-box proteins, GLH-1–4 (also related to Drosophila Vasa).
Why are they called P granules?
P granules get their name from the P lineage, the embryonic lineage that gives rise to the germline. P granules segregate asymmetrically with the P lineage during embryogenesis and are maintained in germ cells throughout life (except for mature sperm). P granules should not be confused with P-bodies (also called processing bodies), a different class of RNA granules present in all cells.
What holds P granules together?
Like other RNA granules, P granules are not membrane bound. Granule assembly depends on self-interaction domains in the PGL proteins, and the localization of P granules to nuclei is promoted by FG repeats in the GLH proteins. Despite their structural role, PGL and GLH proteins are highly mobile and readily exchange with the surrounding cytoplasm. When pushed by a needle, P granules deform and ‘drip’ off (i.e. dissociate from) nuclei. In the P lineage, P granules shrink, grow and fuse at each cell division. These properties have suggested that P granules are liquid droplets, held together by low-affinity interactions that cause P-granule proteins to undergo phase separation from the bulk cytoplasm.
What else is in P granules?
Because P granules sit on nuclear pores, most mRNAs transcribed in germ cells likely pass through a P granule on their way to the cytoplasm. Consistent with a role in mRNA surveillance, several members of the Argonaute family of RNA regulators are enriched in P granules, including: CSR-1, which protects germline mRNAs from silencing; and PRG-1 and WAGO-1, which silence transposable elements and foreign genes. The Vasa-like protein RDE-12 associates with WAGO-1 in P granules and is required for siRNA amplification, which has been proposed to occur in ‘mutator loci’ adjacent to the P granules. A link between Vasa, Argonautes and the amplification of small RNAs has also been observed in the perinuclear ‘nuage’ of Drosophila, suggesting a possible conserved role in the synthesis of small RNAs.
What happens when you get rid of P granules?
Mutants that fail to partition P granules to the P lineage are viable and fertile, suggesting that P granules are not essential to distinguish soma from germline in embryos. Mutations in individual P-granule components lead to sterility at high temperature and impaired translational control of at least some mRNAs. What happens when germ cells lack all P granules, however, has been hard to determine due to functional redundancy among P-granule components. A recent study found that simultaneous depletion of PGL-1, PGL3, GLH-1 and GLH-4 gives rise to germ cells that occasionally express somatic markers and form neurite-like extensions. An attractive possibility is that P granules preserve the totipotency of the germline by silencing somatic differentiation programs until fertilization.
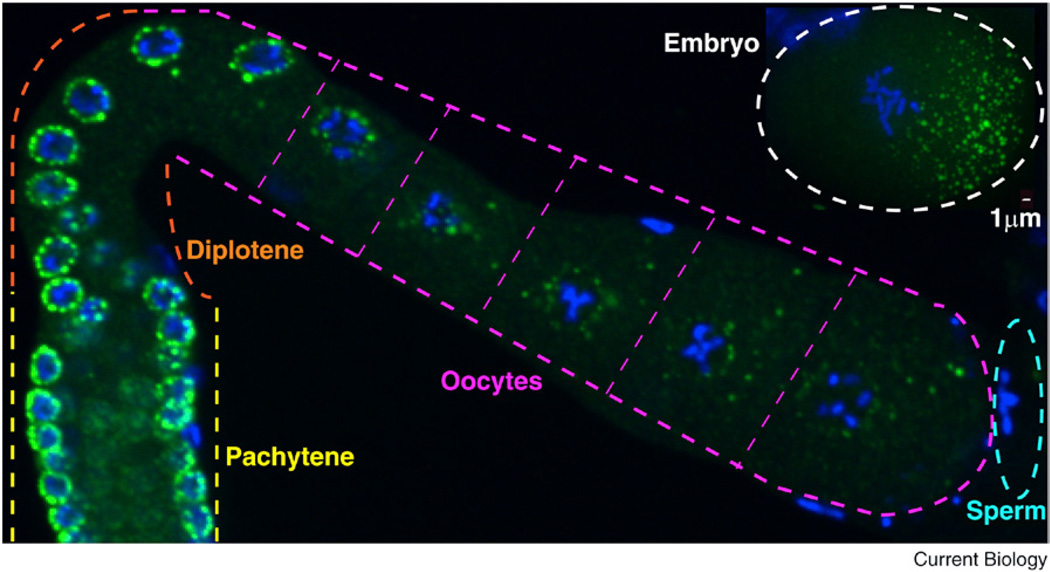
Figure 1. P granules.
P granules (detected using the OIC1D4 antibody) in an adult hermaphrodite gonad. P granules are perinuclear in germ cells in the pachytene and diplotene stages of meiosis and become progressively more cytoplasmic in growing oocytes. The inset at top right shows cytoplasmic P granules (detected with an anti-GLH-2 antibody) in an embryo in the first mitotic prophase: these P granules are enriched on the posterior side. P granules are in green, and DNA is in blue. Scale bar = 1 µm. (Image: Jennifer T. Wang.)
Where can I find out more?
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Claycomb JM, Batista PJ, Pang KM, Gu W, Vasale JJ, van Wolfswinkel JC, Chaves DA, Shirayama M, Mitani S, Ketting RF, et al. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 2009;139:123–134. doi: 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo CM, Wang JT, Motegi F, Seydoux G. Cytoplasmic partitioning of P granule components is not required to specify the germline in C. elegans. Science. 2010;330:1685–1689. doi: 10.1126/science.1193697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa M, Yonetani M, Sugimoto A. PGL proteins self associate and bind RNPs to mediate germ granule assembly in C. elegans. J. Cell Biol. 2011;192:929–937. doi: 10.1083/jcb.201010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U, Pitt J, Dennis S, Priess JR. Perinuclear P granules are the principal sites of mRNA export in adult C. elegans germ cells. Development. 2010;137:1305–1314. doi: 10.1242/dev.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Stanney W, III, Gu W, Seth M, Mello CC. The Vasa homolog RDE-12 engages target mRNA and multiple Argonaute proteins to promote RNAi in C. elegans. Curr. Biol. 2014;24:845–851. doi: 10.1016/j.cub.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D, Strome S. P granule assembly and function in Caenorhabditis elegans germ cells. J. Androl. 2010;31:53–60. doi: 10.2164/jandrol.109.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike DL, Knutson AK, Egelhofer TA, Campbell AC, Strome S. Germ-granule components prevent somatic development in the C. elegans germline. Curr. Biol. 2014;24:970–975. doi: 10.1016/j.cub.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E, Seydoux G, Sassone-Corsi P, Nagamori I. RNA granules in germ cells. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a002774. http://dx.doi.org/10.1101/cshperspect.a002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Vallandingham J, Shiu P, Li H, Hunter CP, Mak HY. The DEAD box helicase RDE-12 promotes amplification of RNAi in cytoplasmic foci in C. elegans. Curr. Biol. 2014;24:832–838. doi: 10.1016/j.cub.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]