Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy (original) (raw)
. Author manuscript; available in PMC: 2017 Aug 1.
Abstract
It remains unclear how obesity worsens treatment outcomes in patients with pancreatic ductal adenocarcinoma (PDAC). In normal pancreas, obesity promotes inflammation and fibrosis. We found in mouse models of PDAC that obesity also promotes desmoplasia associated with accelerated tumor growth and impaired delivery/efficacy of chemotherapeutics through reduced perfusion. Genetic and pharmacological inhibition of angiotensin-II type-1 receptor (AT1) reverses obesity-augmented desmoplasia and tumor growth and improves response to chemotherapy. Augmented activation of pancreatic stellate cells (PSCs) in obesity is induced by tumor-associated neutrophils (TANs) recruited by adipocyte-secreted IL-1β. PSCs further secrete IL-1β, and inactivation of PSCs reduces IL-1β expression and TAN recruitment. Furthermore, depletion of TANs, IL-1β inhibition, or inactivation of PSCs prevents obesity-accelerated tumor growth. In pancreatic cancer patients, we confirmed that obesity is associated with increased desmoplasia and reduced response to chemotherapy. We conclude that crosstalk between adipocytes, TANs, and PSCs exacerbates desmoplasia and promotes tumor progression in obesity.
Keywords: Obesity, pancreatic cancer, desmoplasia, IL-1β, tumor-associated neutrophil
Introduction
Pancreatic cancer is the fourth-leading cause of cancer-associated death worldwide, with an overall five-year survival rate of 7 % (1). The risk of pancreatic cancer is about 50% greater for individuals with obesity, particularly those with increased abdominal adiposity (2). Excess body weight also worsens the already dismal outcome of pancreatic ductal adenocarcinoma (PDAC) patients by increasing the relative risk of cancer mortality by more than 2-fold (3–7). As a consequence of the obesity pandemic – with nearly 70% of the United States adult population being either overweight or obese (8) – the majority of PDAC patients present with excess weight at diagnosis (6). Thus, understanding why obesity is associated with a worse prognosis might lead to novel treatments and enhance the outcome of current therapies.
PDAC is a highly desmoplastic cancer characterized by activated pancreatic stellate cells (PSCs) and an excessive accumulation of extracellular matrix (ECM) (9, 10). ECM components and PSCs directly promote the survival and migration of cancer cells (11–13). In addition, the interaction between cancer cells, stromal cells (e.g. PSCs) and the ECM produces physical forces (solid stress - pressure from solid tissue components) which compress tumor blood vessels thus causing poor and heterogeneous tumor perfusion (14, 15). These mechanically-induced changes in vascular perfusion create a formidable barrier to delivery and efficacy of chemotherapeutics leading to poorer treatment outcomes (11, 16–19). Importantly, obesity itself is a pro-desmoplastic condition. The hypoxia that results from abnormal blood vessels and decreased blood flow due to the rapidly expanding white adipose tissue (WAT) in obesity causes adipocyte dysfunction and immune cell recruitment (20–22). The latter leads to cytokine production, inflammation, and ultimately fibrosis (20–22). In particular, adipocytes in WAT abundantly express the angiotensin II type-1 receptor (AT1) (23, 24), a major pro-fibrotic pathway that becomes activated in a pro-inflammatory environment (23, 24). Obesity also leads to fat accumulation in the normal pancreas (steatosis), which generates a similar inflammatory process within the pancreas itself, with increased expression of cytokines, extracellular matrix remodeling, and fibrosis (7, 25, 26). Importantly, cancer lesions in obese mice and patients have an increased adipocyte content (27, 28). Of clinical relevance, the interaction of cancer cells with adipocytes – both in the form of accumulation of fat in the pancreas and at the invasive tumor front into the local adipose tissue - is associated with worse outcomes in PDAC patients (7, 29). However, the role of adipocytes during obesity-induced PDAC progression remains unclear.
We have previously reported that obesity promotes inflammation (increased IL-1β levels) and immune cell infiltration in PDACs, which associated with increased tumor growth and metastasis (30). Here, we hypothesized that the obesity-associated adipocyte accumulation in PDACs generates a pro-inflammatory and pro-fibrotic microenvironment, which promotes tumor progression, and hinders the delivery and efficacy of chemotherapy. In this study, we tested this hypothesis in pancreatic cancer patients as well as in clinically relevant orthotopic and genetically-engineered mouse models (GEMMs) of PDAC. To reduce the obesity-instigated desmoplastic reaction in PDAC, we used AT1 knockout (Agtr1a−/−) mice and the FDA approved AT1 inhibitor (AT1 blocker, ARB) losartan, which as we previously demonstrated reduces PSC activation, desmoplasia and solid stress in PDAC (18). In addition, we examined whether the obesity-exacerbated desmoplasia in PDAC results from increased inflammation and immune cell infiltration, and we uncovered the cellular and molecular mechanisms involved.
Results
Diet- or genetically-induced obesity promotes pancreatic tumor progression
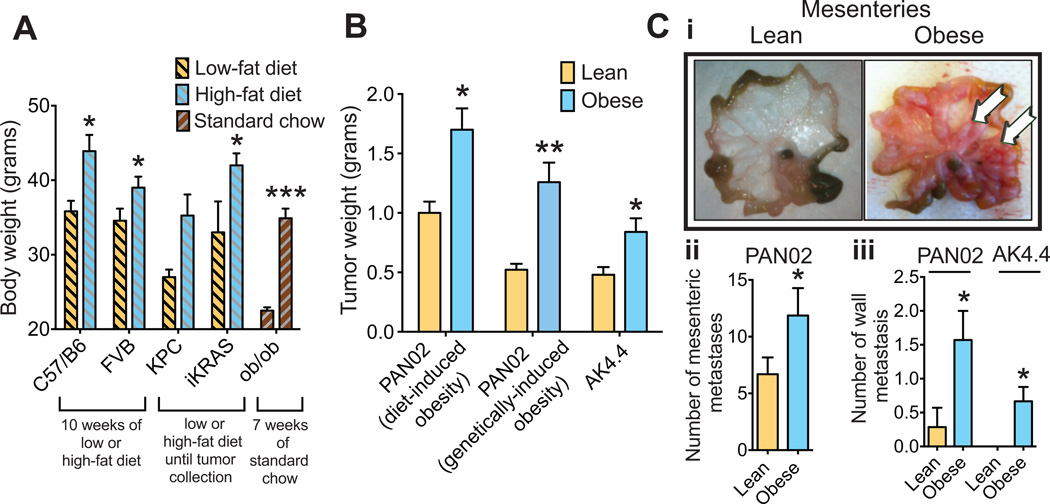
We fed a high-fat diet (HFD) to four different strains of mice to generate diet-induced obesity (DIO) (Fig. 1A). In addition, we used a genetic model of leptin deficiency (ob/ob) (Fig. 1A). Consistent with previous studies including from our laboratory (27, 30, 31), obesity promoted tumor initiation and progression across various tumor models. Using spontaneous PDAC models – KPC (Ptf1-Cre/ KrasLSL-G12D/+ / p53LSL-R172H/+) and iKRAS (Ptf1-Cre / ROSA26-LSL-rtTa-IRES-eGFP / TetO-KrasTetO-LSL-G12D / p53L/+) mice (32–35) – we found that obese animals tended to develop tumors earlier than lean mice (Fig. S1). Furthermore, DIO and genetically-induced obesity accelerated the growth of implanted tumors in two orthotopic syngeneic PDAC models – PAN02 and AK4.4. Similar to our previous observations (30), in comparison to lean mice, obese mice presented with increased tumor weight (Fig. 1B), increased metastatic dissemination to the mesenteric peritoneum in the PAN02 model (Fig. 1Ci–ii), and increased local invasion into the retroperitoneum in both models (Fig. 1Ciii). Taken together, the mice models used confirmed the tumor-promoting effect of obesity in PDAC.
Figure 1. Obesity promotes tumor initiation and progression.
(A) Generation of obese mouse models. High-fat (60%) versus low-fat (10%) diets started at six weeks of age generated a difference in body weight (BW) in multiple models. Data represents the body weight of wild-type C57BL/6 and FVB mice after ten weeks on diet, and of the spontaneous PDAC models (KPC and iKRAS) at the time of tumor collection (between 15–20 weeks of diet for KPC, 12–16 weeks for iKRAS). Body weight of mice genetically deficient for leptin (ob/ob) or age-matched WT mice was recorded after seven weeks on a standard chow (n = 8–10/ group for C57Bl/6, FVB, and ob/ob, 4–10/group for KPC, 7–21/group for iKRAS). (B) Effect of obesity on tumor growth. Data in this panel represent the weight of tumors collected three weeks after implantation. PAN02 and AK4.4 syngeneic tumors were orthotopically implanted in C57BL/6 and FVB mice respectively at ten weeks of diet (diets were continued until tumor collection); ob/ob mice and corresponding age-matched WT mice were implanted with PAN02 tumors at seven weeks of age (mice under standard chow). Obese animals presented with higher tumor weights than lean counterparts in all models (n = 8–10/group). (C) Effect of obesity on metastasis. (C-i): Representative images of mesenteric peritoneal dissemination observed in lean and obese mice three weeks after implantation of PAN02 tumors. (C-ii): Quantification of mesenteric peritoneal metastasis in the PAN02 model (n = 26–30/group). (C-iii): Quantification of retro-peritoneal metastasis in the PAN02 model and AK4.4 model (n = 4–7/group). Data are shown as mean ± standard error of the mean (SEM). P values were determined by the Student t-test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Obesity induces a steatotic and fibrotic microenvironment in PDACs
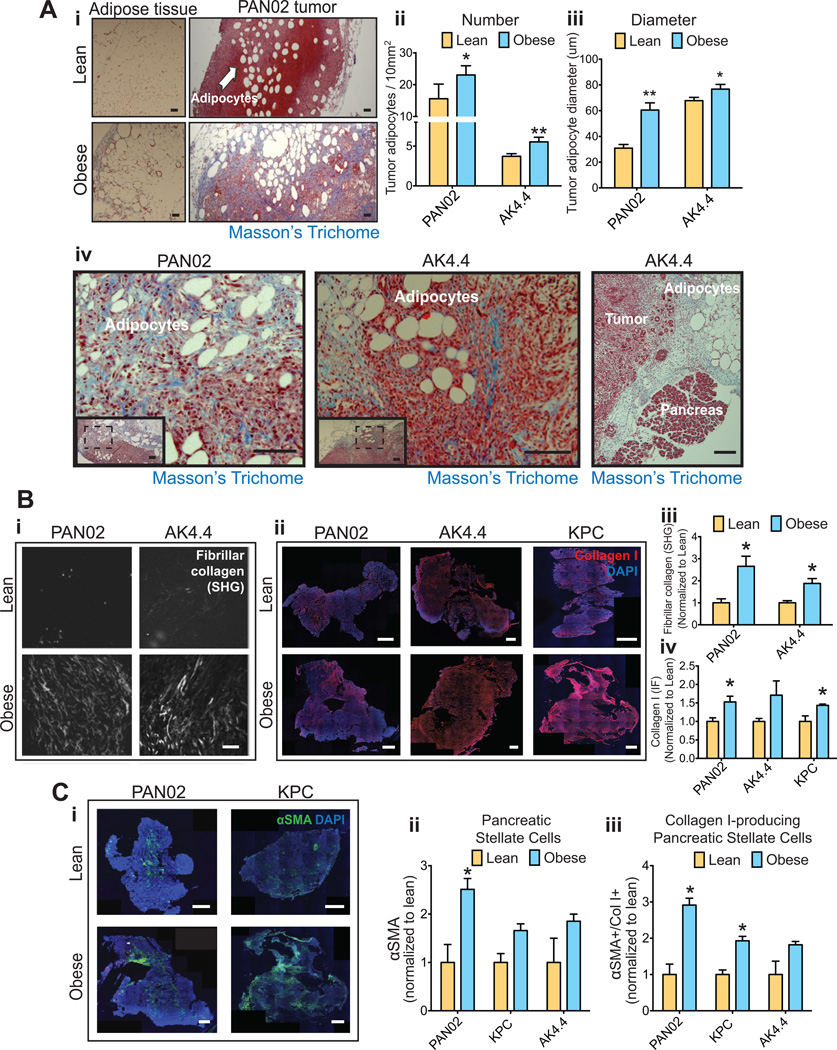
The dysfunctional hypertrophic adipocytes that accumulate in visceral adipose and pancreatic tissues in obesity lead to the development of a local desmoplastic reaction characterized by fibrosis and inflammation (20–22). In tumors, desmoplasia promotes tumor growth and impairs response to chemotherapy via reduced vessel perfusion (18). We hypothesized that obesity augments desmoplasia in the pancreatic tumor microenvironment, thus stimulating tumor progression. As expected (24), in obese mice we observed hypertrophic adipocytes and associated fibrosis in the visceral adipose tissue (Fig. 2Ai). Importantly, we found that the tumor microenvironment also contained more and larger adipocytes (Fig. 2Ai–iii). In part, this was due to tumors invading the neighboring visceral white adipose tissue (WAT) (Fig. S2A–B), as reported in pancreatic cancer patients (7, 29). Furthermore, Masson’s trichrome staining revealed an abundant fibrosis in tumor areas enriched in adipocytes or alongside adjacent visceral adipose tissues (Fig. 2Ai and iv, Fig. S2C, Fig. S3A). These data suggest that, in obesity, PDACs adopt a fibrotic adipose microenvironment as they invade the adjacent fibrotic adipose tissues. We next determined whether the abundance of fibrotic adipocyte-rich areas in tumors from obese mice led to an overall increase in tumor fibrosis. Using second harmonic generation (SHG) imaging we found that obesity significantly increased the SHG signal, which represents fibrillar collagen, in orthotopically grown PAN02 and AK4 tumors (Fig. 2Bi and iii). Obesity also significantly increased collagen I expression (immunofluorescence) in the lesions of orthotopic and spontaneous PDACs (Fig. 2Bii and iv). Of note, within each body weight setting, collagen-I levels did not correlate with tumor area, indicating that tumor size alone (increased in obesity) is not responsible for the observed increase in fibrosis in obese mice (Fig. S3B). The tumor levels of hyaluronan (HA) – a ECM molecule also associated with desmoplasia – also tended to be higher in obese mice (Fig. S3C–D). We then determined whether the presence of activated PSCs was also increased in tumors in obese mice. In obese animals, immunofluorescence staining revealed a significant increase in the density of αSMA-positive PSCs in PAN02 (Fig. 2Ci–ii). The numbers of αSMA-positive PSCs in AK4.4 and KPC models also tended to be higher in obese mice, and immunoblotting confirmed significant increases of αSMA in PAN02 and AK4.4 tumors (Fig. 2Ci–ii, Fig. S3E. See also Fig 4C). We confirmed that αSMA-expressing PSCs associate with collagen I expression in our PDAC models (Fig. S3F–G). Importantly, the percentage of PSCs associated with collagen I expression increased by 2–3 fold in the obese setting (Fig. 2Ciii). Consistent with the increase in the fibrotic reaction in adipocyte-rich regions in tumors, the expression of αSMA also increased in these regions (See Fig. 6Bi–ii). Taken together, we found that tumors in the obese setting are enriched in enlarged adipocytes, activated PSCs, and collagen I.
Figure 2. Obesity aggravates tumor desmoplasia.
(A) Description of the adipose microenvironment in tumors from obese mice. (A-i) Adipocyte enlargement and fibrosis in visceral adipose tissue and tumors from obese mice. Masson’s Trichrome staining denotes fibrosis in blue. Arrows: Adipocytes. Scale bars: 200 µm. Adipocyte count (A-ii) and size (A-iii) in PAN02 and AK4.4 tumors, indicating an enrichment for enlarged adipocytes in the tumor microenvironment in obese mice (n=3 tumors/group, 8 ROIs/tumor). (A-iv) Representative images of the adipose tissue-tumor interaction, revealing increased expression of fibrosis where tumors invade the adjacent adipose tissue. On the far right, tumor epithelium is observed in close proximity to fibrotic adipose tissue and normal pancreas. Tumor sections were stained for Masson’s Trichrome. Scale bars: 100 µm (PAN02), 200 µm (AK4.4), 500 µm (Ak4.4 far right). (B) Collagen levels in PDACs. (B-i) Representative pictures of fibrillar collagen in tumors using second harmonic generation (SHG). Scale bars: 100 µm. (B-ii) Representative pictures of collagen I staining (immunofluorescence) in tumors. Scale bars: 1 mm. (B-iii) Quantification of fibrillar collagen normalized to lean animals. Tumors from obese mice presented with increased expression of fibrillar collagen in PAN02 and AK4.4 orthotopic PDACs. (B-iv) Quantification of collagen expression normalized to lean animals. Tumors from obese mice presented with increased collagen I expression in three different tumor models (n=3–6/group). (C) Effect of obesity on αSMA expression in PDACs. (C-i) Representative pictures of αSMA expression in AK4.4, PAN02 and KPC tumors by immunofluorescence. (C-ii) Quantification of αSMA expression by immunofluorescence was performed as a % of αSMA (C-ii), as well as a % of double positive αSMA/Col-I expression normalized to lean animals (C-iii) (n=3–6/group). For representative pictures of αSMA/Col-I double staining in PAN02 and AK4.4 tumors, please see Figs. S3C–D. Data are shown as mean ± SEM. P values were determined by the Student t-test. *, P < 0.05; **, P < 0.01.
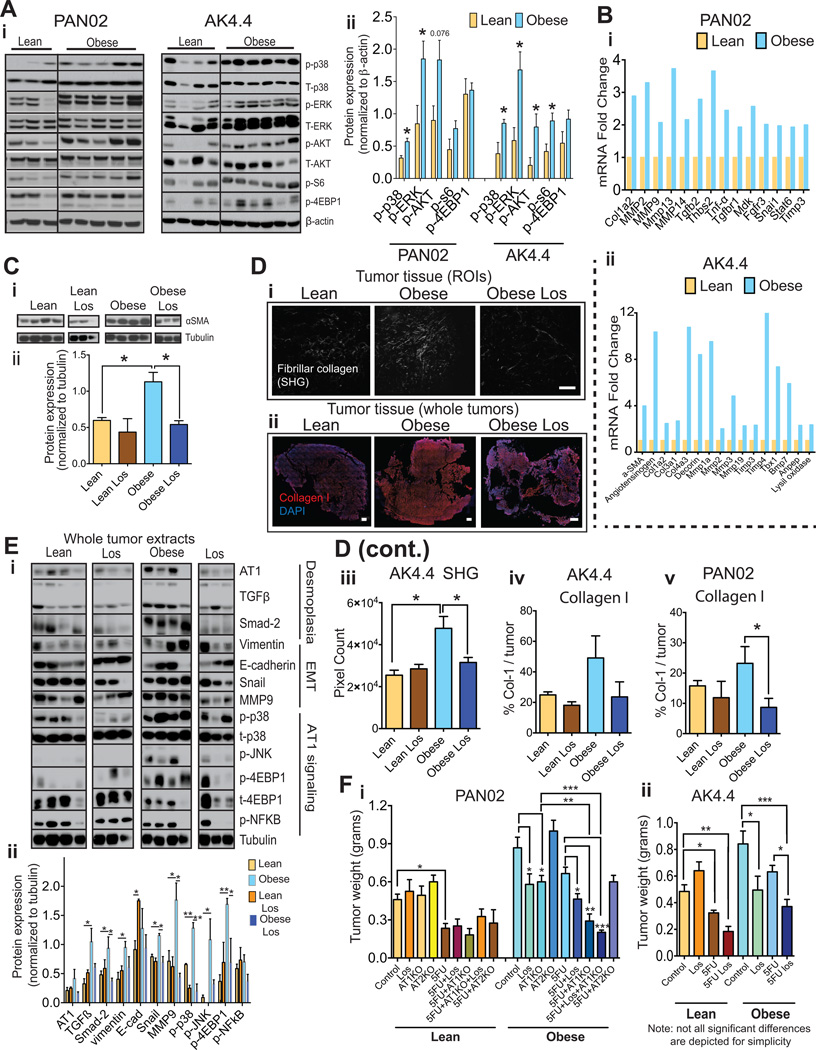
Figure 4. Blockade of AT1 reverses the obesity-aggravated desmoplasia and improves response to chemotherapy.
(A) Effect of obesity on activation of major signaling pathways in tumors. (A-i) Each lane represents the protein expression in individual tumors. (A-ii) Densitometric analysis normalized to β-actin. Obesity associates with increased signaling activity. (B) Effect of obesity on target genes of AT1 signaling. Depicted genes where at least a ~ 2-fold change in mRNA expression was observed in either tumor model. Data normalized to lean group. 3–4 samples per group pooled in one single PCR array fibrosis gene set plate. Expression of genes associated with AT1 pathway activation and fibrosis/desmoplasia is increased in PAN02 and AK4.4 tumors from obese mice. (C) Effect of losartan (Los) on αSMA expression in AK4.4 tumors. (C-i) Each lane represents the protein expression in individual tumors. Losartan reduced tumor αSMA protein expression more dramatically in the obese setting. (C-ii) Densitometric analysis normalized to tubulin. (D) Effect of losartan on collagen levels. Losartan reduced tumor fibrillar collagen as determined by second harmonic generation (SHG) (D-i) as well as collagen-1 (Col-I) expression (D-ii) in AK4.4 tumors from obese mice. Scale bars: 100 µm (SHG) and 1 mm (Col-I). (D-iii-v) Quantification of collagen was performed as a % of a region of interest (ROI) for SHG (n=4 tumors/group, 8 ROIs per tumor) and as a % of viable tumor area in the whole tumor for collagen-1 immunofluorescence (n=4–6 tumors/group). (E) Effect of losartan on tumor desmoplasia and AT1 signaling related markers in AK4.4 tumors from lean and obese mice. (D-i) Protein expression by Western blotting of AK4.4 tumors (each lane represents the protein expression in individual tumors) revealed that losartan normalized the obesity-augmented expression of several AT1 signaling and desmoplasia-related markers, i.e. AT1, TGFB, SMAD2, vimentin, snail, MMP9, and phospho-p38. Of note, similar to αSMA, the changes in AT1, as well as other desmoplasia related markers, were relatively mild in the lean setting. (E-ii) Densitometric analysis normalized to tubulin (Depicted are significant differences between control and losartan treatment). (F) Effect of pharmacological and genetic blockade of AT1 on the efficacy of chemotherapy in the lean and obese setting. (F-i) In the PAN02 model, losartan and AT1 genetic deficiency (Agtr1a−/− mice) improved response to chemotherapy in obese, but not in lean animals. (F-ii) In the AK4.4 model, losartan improved response to chemotherapy in both lean and obese settings but with a higher magnitude in obese setting (i–ii: Two-way ANOVA, n=4–8 tumors/group. Depicted are significant differences between treatment groups compared to control or 5-FU groups). Data are shown as mean ± SEM with the exception of panels Ai–ii. P values were determined by the Student t-test unless otherwise stated. *, P < 0.05; **, P < 0.01, *** p < 0.001.
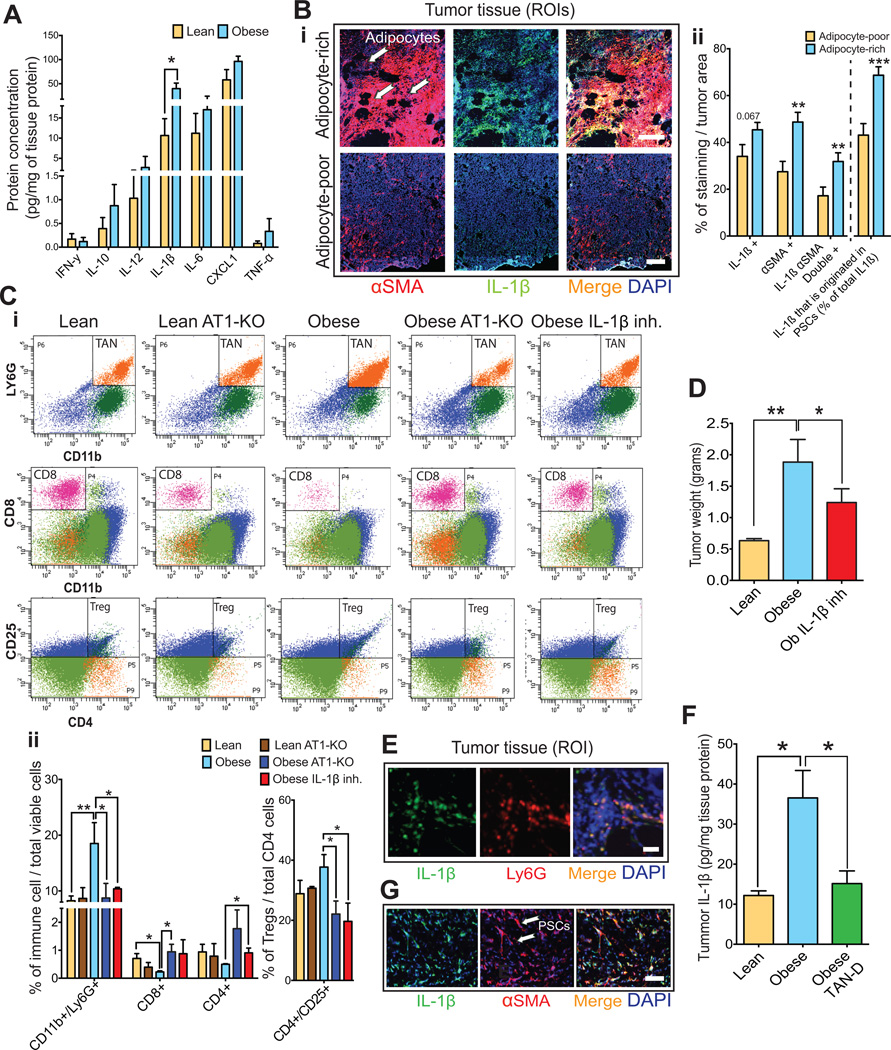
Figure 6. The adipose microenvironment promotes TAN infiltration and fibrosis via IL-1β.
(A) Effect of obesity on cytokine expression in PAN02 tumors. Multiplex protein revealed that PAN02 tumors from obese mice had increased expression of IL-1β (n=4–6 tumors/group). (B) Effect of adipocytes on IL-1β and αSMA expression in PAN02 tumors. (B-i) Representative picture of αSMA and IL-1β expression in adipocyte-rich and poor regions. Scale bars: 200 µm. (B-ii) Quantification of αSMA and IL-1 β expression in adipocyte-rich and poor regions (n = 20 samples/region). IL-1β was abundantly expressed in adipocytes and PSCs in the adipocyte-rich areas where PSCs predominate. (C) Effect of IL-1β and AT1 blockade on immune cell profile. (C-i) Representative flow cytometry scatter plots of CD45(+)CD11b(+)Ly6G(+) tumor-associated neutrophils (TANs), CD8(+) cytotoxic lymphocytes and CD4(+)CD25(+) regulatory T cells in PAN02 tumors in lean and obese setting. Quantification normalized by total viable cells (ii) or total CD4 cells (iii) (n=3–6 tumors/group). Obesity promoted an increase in TANs, a decrease in CD8(+) lymphocytes, and a strong tendency for increased Tregs. An anti-IL1β neutralizing antibody or genetic AT1 blockade decreased CD45(+)CD11b(+)Ly6G(+) TAN infiltration while recovering CD4+ and CD8+Tcells (i-ii) and decreasing Tregs (iii) in obese mice (one-way ANOVA, n=3–6 tumors/group). (D) IL-1β blockade normalized obesity-aggravated tumor growth (one-way ANOVA, n=3–6 tumors/group). (E) IL-1β expression in TANs. Immunofluorescence for PAN02 tumor sections denoting co-localization. Scale bar: 30 µm. (F) TAN depletion using Ly6G specific antibody abolished obesity-induce IL1-β expression in PAN02 tumors. (one-way ANOVA, n=4–6 tumors/group). (G) Representative picture of αSMA and IL-1β expression in PAN02 tumors. Scale bar: 30 µm. Data are shown as mean ± SEM. p values were determined by the Student t-test unless otherwise stated. *, p < 0.05; **, p < 0.01, ***, p < 0.001.
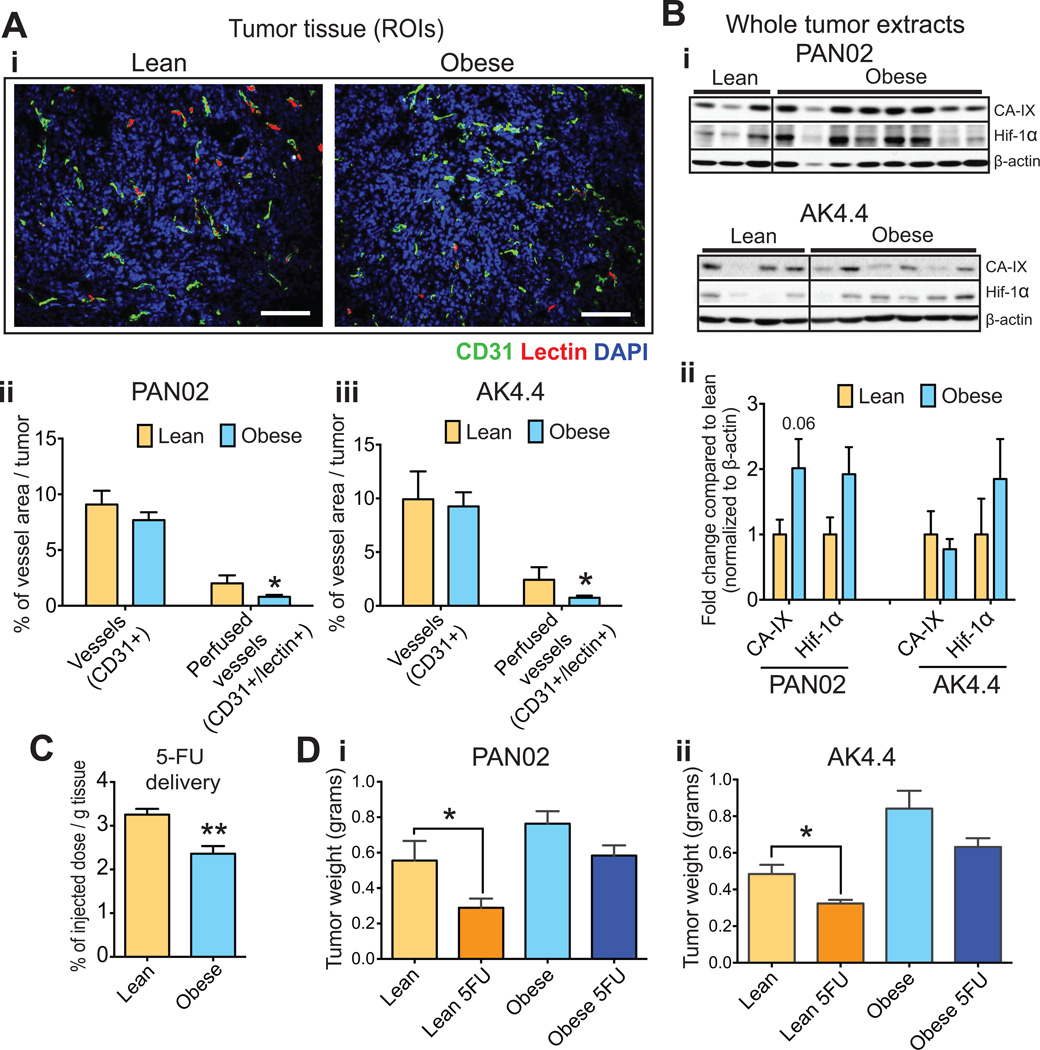
Obesity-aggravated desmoplasia is associated with impaired vascular perfusion, intratumoral drug delivery and treatment efficacy
We have reported that desmoplasia reduces the delivery and efficacy of chemotherapy in PDAC by decreasing vessel perfusion (18). Therefore, we tested if obesity-augmented desmoplasia reduced the efficacy of chemotherapy. Indeed, we found that in obese animals the tumor vascular perfusion was reduced (Fig. 3Ai–iii). In both PAN02 and AK4.4 tumors, the percentage of perfused vessels per total tumor area significantly decreased from 2.5% to 0.75% (from ~25 % to 10 % of total vessel area). Consistent with this finding, we observed an increase in hypoxia markers in tumors from obese mice (Fig. 3B, Fig. S4). To determine if reduced perfusion impairs the delivery of chemotherapeutic agents, we measured the uptake of 5-Fluorouracil (5-FU), an approved cytotoxic agent used in the treatment of PDAC (36). Obesity significantly decreased the uptake of 5-FU in PAN02 tumors (Fig. 3C) and reduced the efficacy of 5-FU chemotherapy in both PAN02 and AK4.4 tumors (Fig. 3D). The 5-FU treatment significantly reduced the tumor weight in lean mice (Fig. 3D). In contrast, in obese mice 5-FU did not significantly affect the growth of PAN02 and AK4.4 tumors (Fig. 3D). Collectively, these data show that, in addition to directly promoting PDAC growth and metastasis, obesity reduces vascular perfusion, drug delivery and the efficacy of 5FU chemotherapy.
Figure 3. Obesity-aggravated desmoplasia is associated with impaired perfusion, oxygenation, drug delivery and resistance to chemotherapy.
(A) Effect of diet-induced obesity (DIO) on tumor perfusion. (A-i) Representative pictures of CD31(+) vessels and lectin in PAN02 tumors. Scale bars: 200 µm (A-ii) Quantification of total (CD31+) and lectin-positive (CD31/lectin+) vessel area in PAN02 and AK4.4 tumors. Obese mice presented with decreased perfusion (n = 3–12 tumors/group). (B) Effect of DIO on tumor hypoxia. (B-i) Each lane represents the protein expression of hypoxia markers in individual PAN02 or AK4.4 tumors. (B-ii) Densitometric analysis (fold-change compared to the lean group) normalized to β-actin (n = 4–15. See additional bands in Supplementary Figure 4). Obese mice presented with increased hypoxia in tumors. (C) Effect of DIO on the delivery of chemotherapy to PAN02 tumors. 5-FU quantified via high-performance liquid chromatography (HPLC). Obesity decreased delivery of the chemotherapeutic agent (n=4 tumors/group). (D) Effect of DIO on response to chemotherapy. PAN02 and AK4.4 tumors were orthotopically implanted at ten weeks of diet, treatments were initiated at day seven post-implantation and tumors resected at day 19 (after three cycles of 5-FU 30mg/kg q4d). 5-FU was less effective in preventing tumor growth in obese animals than in lean (two-way ANOVA, n=6–10/group). Data are shown as mean ± SEM. P values were determined by the Student t-test unless otherwise stated. *, P < 0.05; **, P < 0.01.
The inhibition of AT1 signaling reduces obesity-induced desmoplasia, tumor growth, and increases the response to chemotherapy
We have shown that tumor desmoplasia results in part from the activation of PSCs through AT1 signaling (18). We confirmed the expression of AT1 in PSCs (AT1/αSMA double staining), which ranged from 70.3% (SEM = 5.7) in PSCs in PAN02 tumors to 35.3% (SEM = 12.8) in AK4.4 tumors (Fig. S5A). We next analyzed the effects of obesity on AT1 downstream pathways. In both PAN02 and AK4.4 tumors, obesity increased the activation of p38, ERK, AKT and their targets pS6 and 4EBP1 (Fig. 4A) (18, 37–39). In addition, several target genes of the AT1 pathway, including Col1a2, Tgfβ1 and matrix metalloproteinases genes were upregulated (Fig. 4Bi–ii, Tables S1–2).
To test if the inhibition of AT1 signaling could reverse desmoplasia in obese mice, we used the angiotensin II receptor 1 blocker (ARB) losartan and mice deficient in AT1 (Agtr1a−/−). In AK4.4 tumors in obese, but not lean mice, the 16 days losartan treatment significantly reduced the gene and protein expression of the PSC-activation marker αSMA (Fig. 4Ci–ii, Fig. S5B). Similarly, in obese mice losartan significantly decreased the SHG signal for fibrillar collagen, and reduced collagen I immunofluorescence, though not significantly (Fig. 4Di–iv). Moreover, in AK4.4 losartan normalized the obesity-induced expression of desmoplasia-related markers (αSMA, decorin, Smad-2, p-P38) and ECM remodeling (MMP9) (Fig. 4Ei–ii and Fig. S5B). These effects were absent or only modest in lean animals, consistent with the small reduction in αSMA expression in this weight setting. Similarly, in obese mice with PAN02 tumors, losartan significantly reduced the collagen I content (Fig. 4Dv), and losartan or AT1 deletion reduced the expression of αSMA (Fig. S3Ei–ii, Fig. S5C). Furthermore, intimately associated with AT1 signaling, the epithelial-to-mesenchymal (EMT) markers MMP9, snail and vimentin were increased in AK4.4 tumors from obese mice in both models and were subsequently normalized by losartan (Fig.4E, Fig. S5B). In PAN02 tumors, we confirmed that AT1 deletion normalized the obesity-increased expression of vimentin (Fig. S3Ei and iii).
We then determined whether AT1 blockade could improve the response to chemotherapy, particularly in obese mice with desmoplastic tumors. In a previous study we found that losartan combined with 5FU significantly inhibited the growth of AK4.4 tumors in mice fed a regular diet, but losartan alone had no effect on tumor growth (18). Consistent with this, in the current study in lean mice the genetic deletion of AT1 or the losartan treatment had no effect on tumor size in PAN02 and AK4.4 models. However, in obese mice with a genetic deletion of AT1 or treated with losartan the tumor sizes were significantly smaller (Fig. 4Fi–ii). Similar to the data of figure 3D, 5FU alone significantly reduced the size of AK4.4 and PAN02 tumors in lean but not in obese mice. The combination of genetic AT1 blockade plus 5FU significantly reduced tumor size compared with either treatment alone in obese but not in lean animals (Fig. 4F-i). Similarly, in both AK4.4 and PAN02 tumors, losartan significantly enhanced the response to 5FU in obese but not in lean animals, although it was still somewhat effective in the lean setting in the AK4.4 model (Fig. 4Fi–ii). However, since losartan alone was effective in decreasing tumor growth in obese mice (Fig. 4Fi–ii, Fig. S5D), the tumor size in losartan versus losartan plus 5-FU treated groups in obese mice did not significantly differ (Fig. 4Fi–ii). Importantly, we confirmed via abdominal ultrasound that, when treatment was initiated, tumors had similar size in all groups (lean and obese, control vs. losartan) (Fig. S5D). In addition to AT1, we recently observed that PSCs also express the angiotensin II type-2 receptor (AT2), which has anti-fibrotic effects as opposed to the pro-fibrotic effects of AT1 signaling (18). However, we found here that the PAN02 tumor response to chemotherapy in AT2−/− mice was similar to that in WT, regardless of diet group (Fig. 4F-i).
Similar to our previous study (18) we analyzed whether the increase in 5FU anti-tumor efficacy induced by losartan in obese animals could be at least in part due to improvements in vascular perfusion and drug delivery. In both AK4.4 and PAN02 models we found a trend for increased tumor perfusion and increased delivery of chemotherapeutics by AT1 blockade (Fig. S5E–F). The mild effects on tumor perfusion could be the consequence of a reduced mean arterial blood pressure (MABP) induced by the relatively high dose of losartan (90 mg/kg/day). In fact, losartan reduced the MABP by ~ 27% in both lean and obese mice (Fig. S5G). Of note, AT1 blockade did not induce greater loss in body weight in tumor-bearing mice compared to WT, suggesting that based on this parameter, it does not appear to cause overall toxicity (Fig. S5H). In conclusion, we found that obesity is associated with increased AT1 signaling in tumors; that in obese animals, losartan alone or the genetic blockade of AT1 have direct anti-tumor effects; and that AT1 inhibition can also enhance the efficacy of 5FU chemotherapy.
Tumor-associated neutrophils mediate obesity-induced PSC activation and tumor progression
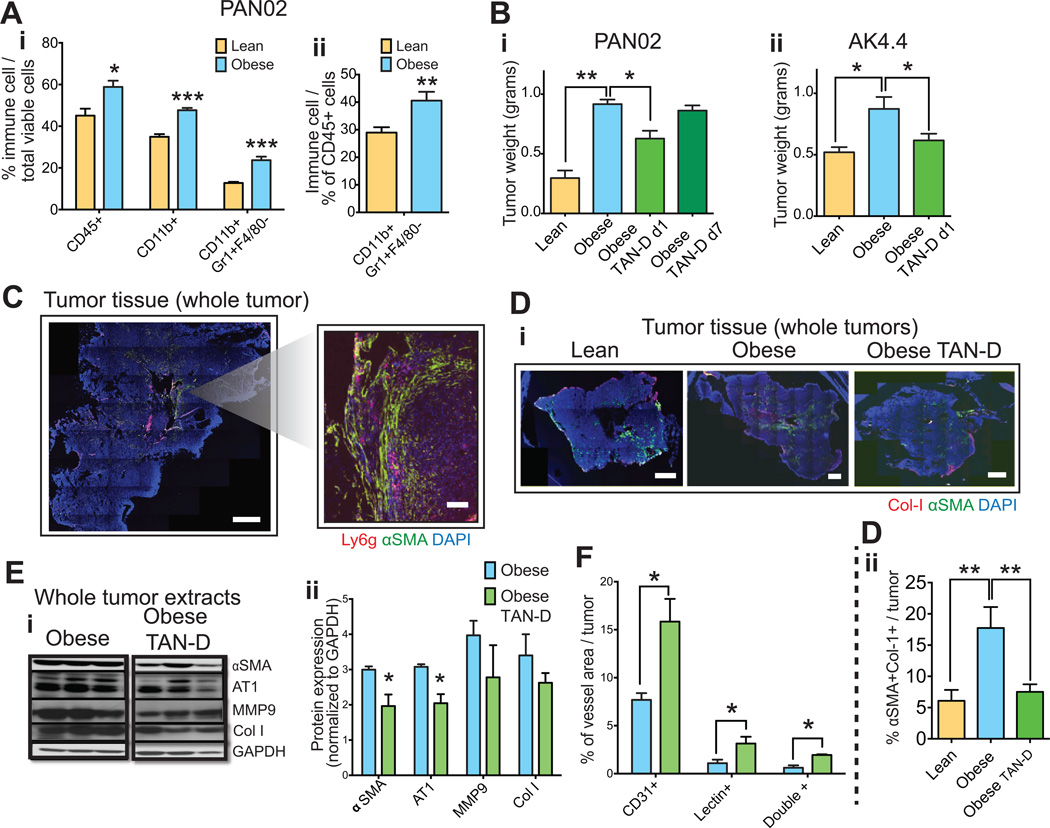
The fibrotic phenotype in adipose tissues and normal pancreas in obesity is largely the consequence of a persistent pro-inflammatory state, which is characterized by the production of cytokines by hypoxic and dysfunctional hypertrophic adipocytes and the recruitment of immune cells (21, 22, 34). In addition, we have previously described increased inflammation and immune cell recruitment in PAN02 tumors implanted in obese mice (30). Hence, we next determined here whether adipocyte-associated inflammation was responsible for the increased tumor desmoplasia and accelerated tumor growth observed in obese animals. Flow cytometric analysis revealed that obesity promotes the infiltration of CD11b(+)Gr-1(+)F4/80(−) myeloid cells in orthotopic AK4.4 and PAN02 PDAC models in obese mice (Fig. 5A, Fig. S6A). We subsequently confirmed that the majority of infiltrated myeloid cells were Ly6G(+) tumor-associated neutrophils (TANs) (Fig. S6B). In both PAN02 and AK4.4 tumors, TAN depletion (TAN-D) – with an anti-Ly6G antibody that decreased TAN infiltration by 90% (Fig. S7A) - reverted the increased tumor growth in obese mice to levels in lean mice (Fig 5Bi–ii). This effect only occurred when TAN depletion was initiated on day 1 but not on day 7 of the experiment, indicating the relative importance of TANs in tumor progression at an early stage (Fig 5Bi). In addition to a direct effect on tumor growth, we determined whether TANs could affect desmoplasia. We observed the preferential accumulation of TANs in areas with activated PSCs (Fig. 5C), suggesting a potential paracrine crosstalk between TANs and PSCs. Indeed, TAN depletion in PAN02 tumors in obese animals decreased PSCs and the number of activated PSCs associated with collagen I to levels in lean animals (Fig. 5Di–ii, E). Consistent with this we observed a significant reduction in AT1 expression and a trend for decreased collagen I and MMP9 expression in PAN02 tumors (Fig. 5E). In addition, TAN depletion significantly increased the vascular surface area and the number of perfused vessels in PAN02, but not significantly in AK4.4 tumors (Fig. 5F, Fig. S7Bi–ii). Our data suggests that obesity increases TAN recruitment in PDAC, which mediates the activation of PSCs and tumor growth.
Figure 5. Tumor-associated neutrophils mediate obesity-induced tumor progression and aggravated desmoplasia.
(A) Effect of obesity on immune cell infiltration in PDACs. Obesity promoted infiltration of myeloid CD11b(+)Gr-1(+)F4/80(−) cell population in PAN02 tumors in obese mice. Quantification normalized by total viable cells (i) or total CD45 leucocytes (ii) (n=4–6 tumors/group). (B) Effect of TAN depletion (TAN-D) on PDAC growth in obese mice. TAN depletion from day 1 using anti-Ly6G specific pharmacological inhibitory antibody in obese mice significantly reverted the obesity-increased tumor weight in PAN02 and AK4.4 models (n=4–6 tumors/group). (C) Preferential accumulation of TANs in areas with activated PSCs. Scale bars: 1 mm (whole tumors) and 100 µm (caption). (D) TAN depletion decreased activated PSCs in obese PDACs to the level of lean tumors. Representative pictures (i) and quantification of αSMA(+)Col-1(+) double positive cells (ii) (n=4–6 tumors/group). (E) TAN depletion reduced AT1 expression, collagen production, and MMP9 expression in PAN02 tumors in obese animals. (F) TAN depletion led to increasing in perfusion in PAN02 tumors in obese animals. (F-i) % of CD31(+), lectin(+) or double positive vessel density in the viable area of whole tumors. (F-ii) % of CD31 positive vessels co-stained with lectin (n=4–6 tumors/group). Data are shown as mean ± SEM. p values were determined by the Student t-test, or one-way ANOVA for panels C and E. *, p < 0.05; **, p < 0.01, *** p < 0.001.
IL-1β mediates obesity-induced tumor-associated neutrophil infiltration and PSC activation in PDACs
The obesity-induced pro-inflammatory/pro-fibrotic response and immune cell recruitment that occur in adipose tissue are mediated by cytokine/chemokine production from dysfunctional adipocytes, such as IL-1β and IL-6 (21, 22, 40). Hence, we determined whether these inflammatory cytokines mediated obesity-induced fibrotic processes and TAN infiltration in the tumor microenvironment. We confirmed our previous observation (30) that in PAN02, obesity significantly increased the expression of IL1-β by 5 fold, and induced a trend towards increased expression of IL-6, TNF-α, IL-12 or CXCL1 (Fig. 6A). Obesity also increased the levels of IL-1β in AK4.4 tumors (Fig. S8). Not surprisingly, IL-1β was abundantly expressed by adipocytes in adipocyte-rich areas where activated PSCs predominate (Fig. 6B-i upper row figure, 6Bii). Antibody-neutralization of IL-1β (MM425B, Endogen/Pierce Biotechnology, 2mg/Kg i.p. q4d) – in obese mice implanted with PAN02 tumors – significantly decreased TAN infiltration. In addition, the decreased TAN recruitment was associated with an increase in CD8(+) cytotoxic T cell population (decreased in obese mice compared to lean) and a decrease in regulatory T cells (Tregs) (Fig. 6Ci and ii). Similar to TAN depletion effects, IL-1β inhibition in obese animals significantly reduced the growth of PAN02 tumors (Fig. 6D) as well as the expression levels of αSMA (Fig. S3E). In addition, the EMT marker vimentin was also reduced by IL-1β inhibition in obese mice (Fig. S3E). In addition to adipocytes, IL-1β was also expressed in about 70% of TANs themselves (Fig. 6E), and TAN depletion reduced tumor IL-1β levels (Fig. 6F). This suggests the presence of an autocrine mechanism that enables further TAN recruitment and potentiates inflammation and fibrosis. Furthermore, TAN depletion significantly reduced the levels of CXCL1, indicating that this cytokine may also play a role in obesity-induced inflammation (Fig. S9). In conclusion, our findings suggest IL-1β is involved in obesity-induced TAN recruitment, immunosuppressive microenvironment and PDAC progression.
AT1 blockade can reciprocally inhibit inflammation and immune cell infiltration in obesity
We observed that αSMA-positive PSCs also abundantly (~70% of PSCs) expressed IL-1β (Fig. 6B-ii and G) and that of the total cells expressing IL-1β, PSCs correspond to 40% of these cells in adipocyte-poor but 70% in adipocyte-rich (~ 70% increase) regions (Fig. 6Bi–ii). Since IL-1β recruits TANs, which localize in close proximity to PSCs as shown above, we next examined whether targeting PSCs could also interfere with IL-1β production and TAN recruitment, particularly in obese mice. Indeed, in obese mice prevention of PSC activation by losartan-inhibition of AT1 signaling decreased IL-1β levels in AK4.4 and PAN02 tumors (Fig. S10A). In addition, the genetic deletion of AT1 decreased TAN recruitment in PAN02 tumors in obese but not lean mice (Fig. 6Ci–ii). Consequently, recapitulating IL-1β inhibition, AT1 blockade increased CD8(+) T cells and reduced Tregs in tumors from obese but not lean mice (Fig. 6Fi–iii). Similar trends were observed for losartan in the AK4.4 tumor model (Fig. S10Bi–ii). The effects of AT1 blockade on the expression of IL-1β and immune cell recruitment are consistent with the prevention of obesity-induced tumor growth (Fig. 4F) and indicate that AT1 signaling mediates obesity-augmented tumor progression via modulation of the immune microenvironment.
PDAC in obese patients presents with increased adipocyte area and fibrosis
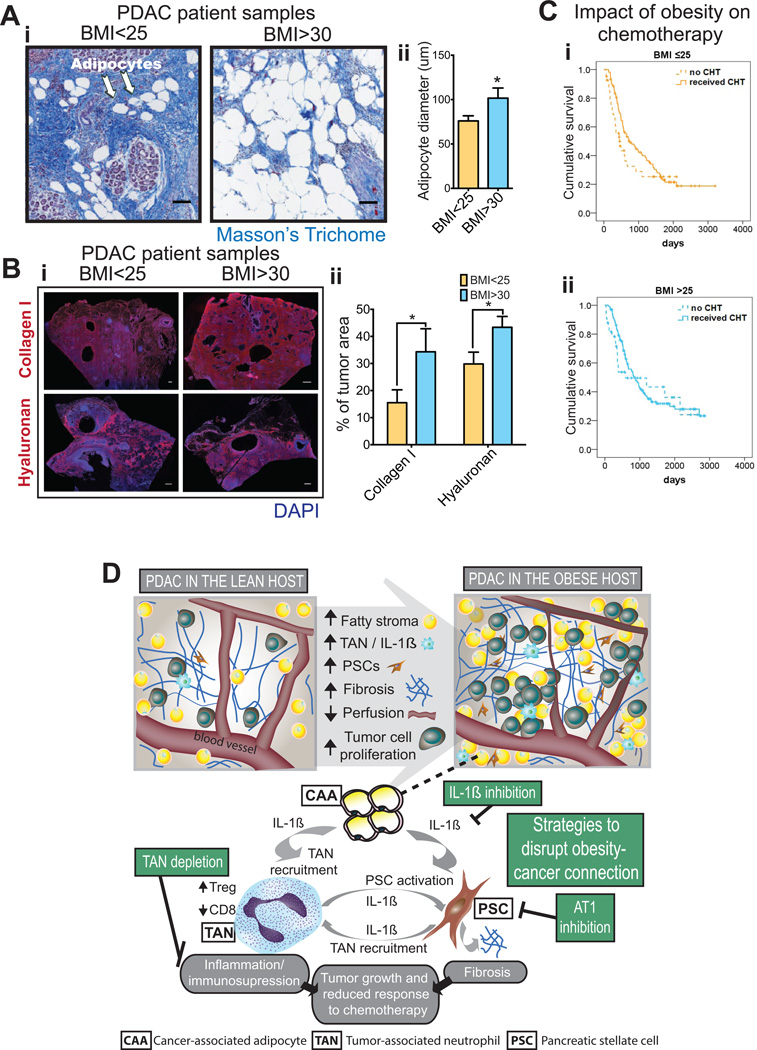
To validate the findings from mouse models of PDAC, we obtained samples of PDAC from treatment-naïve patients that presented with a body mass index (BMI) either below 25 (normal weight) or above 30 (obese). As in the mouse models, we found that tumors from obese patients presented with hypertrophic adipocytes (Fig. 7A) and more pronounced ECM deposition – as shown by increased collagen I and hyaluronan expression (Fig. 7B).
Figure 7. PDACs from obese patients recapitulate the findings in pre-clinical models.
(A) Adipocytes in human PDACs in lean and obese patients. (A-i) Representative pictures of adipocytes in human PDAC from patients with normal weight [Body mass index (BMI) <25] and obesity (BMI>30). Scale bars: 100 µm. (A-ii). Quantification of adipocyte size in human PDACs. Tumors from obese patients presented with hypertrophied adipocytes (n=8 tumors/group). (B) Collagen-I and hyaluronan (HA) expression in human PDACs in lean and obese patients. (B-i) Representative pictures of Collagen-I and HA in human PDAC from patients with normal weight (BMI<25) and obesity (BMI>30). Scale bars: 1mm. (B-ii) Quantification of Collagen-I and HA in human PDACs (n=8 tumors/group). Obesity associated with increased levels of collagen-I and hyaluronan. (C) Cumulative survival curves in PDAC patients stratified by chemotherapy in patients with (i) BMI ≤ 25 or (ii) BMI >25. The survival advantage present in the BMI ≤ 25 subgroup is lost in the >25 BMI subgroup. (D) Graphical summary of the key findings of this study. PDACs in obese hosts present with increased fatty stroma, inflammation, and desmoplasia. The amplified crosstalk between CAAs, TANs, and PSCs that occurs in obesity leads to an aggravation of desmoplasia, increased tumor progression and reduced response to chemotherapy.
Excess weight in PDAC patients is associated with worse response to chemotherapy
To validate our preclinical findings, we analyzed data collected from patients admitted to MGH with a diagnosis of PDAC that underwent surgical resection. A total of 309 patients were included in this study. The average BMI of patients was 26.25 (BMI distribution in Fig. S11). In normal weight patients (BMI ≤25), adjuvant chemotherapy was a significant predictor of overall survival on multivariate analysis (HR, 0.5; 95% CI, 0.3–0.8; p<0.005), independent of other tumor characteristics including tumor size or lymph node positivity (Fig. 7Ci, Tables S3–4). In overweight and obese patients (BMI >25), we did not observe a significant survival advantage of adjuvant chemotherapy on either univariate or multivariate analysis (Fig. 7Cii, Tables S5–6). In conclusion, these results support our preclinical findings and suggest less efficacy of chemotherapy in overweight/obese patients. In conclusion, our pre-clinical and clinical studies indicate that excess weight alters the tumor microenvironment to augment the crosstalk between CAAs, TANs, and PSCs, which untimately leads to increased tumor progression and reduced response to chemotherapy (Fig. 7D).
Discussion
Obesity promotes AT1-dependent PSC activation, PDAC desmoplasia and reduces the efficacy of 5-FU chemotherapy
We and others have shown that obesity promotes pancreatic tumor initiation, growth, and metastasis in preclinical models (27, 30, 41–44). Here we show in established PDAC models that obesity stimulates the activity of IL1β and the crosstalk between adipocytes, TANs, and PSCs, to worsen the fibro-inflammatory microenvironment. The increased fibro-inflammatory activity then promotes tumor progression and reduces vascular perfusion, drug delivery and the efficacy of chemotherapy. We also uncovered that AT1 signaling can mediate obesity-induced PSC activation and increase collagen I accumulation. The inhibition of AT1 signaling is particularly effective in preventing these effects in obese animals, reverting the obesity-accelerated tumor progression and re-sensitizing tumors to the chemotherapeutic agent 5FU in obese mice.
In obese mice the abundance of adipocytes and TANs increases PSC activation via IL-1β particularly in adipocyte-rich regions
In more than half of PDAC patients, adipocytes infiltrate >20% of pancreatic tissue (28), Similar to previous reports (27, 28, 45), we demonstrated that hypertrophic tumor adipocytes - referred to as cancer-associated adipocytes (CAAs) (45, 46) - accumulate in murine and human PDAC in the obese setting. It is well known that obesity promotes the secretion of inflammatory cytokines from hypertrophic adipocytes in adipose tissues and the pancreas, which ultimately leads to local tissue fibrosis (21, 22, 25, 47, 48). However, how hypertrophic CAAs facilitate the interaction between inflammation and desmoplasia to ultimately promote PDAC progression in the obese setting was unknown. We observed here an accumulation of activated PSCs and fibrosis surrounding CAA-rich areas. Given the increased adipocyte burden, the overall PSC activation and fibrotic content in tumors was consequently increased. We also found in obese mice that CAAs express IL-1β, which increases the production of IL-1β in PDAC, especially in CAA-rich areas. In line with IL-1β being an activator of PSCs (49), we found that IL-1β inhibition reduces PSC activation in PDACs in obese animals, indicating that the elevated production of IL-1β in CAA-rich regions leads to activation of PSCs. This is in agreement with previous reports showing that in the pancreas of mice fed a high-fat diet, fibrosis is also accompanied by inflammation in early-stage neoplastic lesions (43, 44); and with our previous observation that metformin reduces IL-1β and desmoplasia in tumors implanted in obese mice (50). It was shown before that neutrophil infiltrates can be observed in the vicinity of tumor cells and in the desmoplastic tumor stroma, which correlates with undifferentiated tumor growth and poor prognosis (51). We found that TAN specific depletion reduces PSC activation and the number of αSMA-positive cells associated with collagen I. In addition, we discovered that IL-1β could recruit/activate TANs. This is consistent with previous reports of increased levels of myeloperoxidase - a marker of intrapancreatic neutrophil sequestration/activation – associating with IL-1β in the steatotic pancreas of obese mice (25, 52). Furthermore, our results show that TANs also secrete IL-1β, which may then activate PSCs and further increase TAN recruitment. Our results explain the findings that, in patients with pancreatic cancer the intra-pancreatic fat correlates with the increased incidence of PDAC, lymphatic metastasis and decreased survival (28, 53). In conclusion, in obese mice the crosstalk between adipocytes, TANs, and PSCs – mediated in part by IL-1β – stimulates the activation of PSCs.
Obesity-induced TAN recruitment and IL-1β promote immunosuppression and the growth of PDAC
We also found here that TAN depletion in obese mice reduced tumor growth, which is consistent with the correlation between TAN infiltration and more aggressive types of pancreatic tumors (54). Importantly, the obesity-induced increase in TANs occurred concomitantly with decreased CD8(+) T cells and increased Tregs, which is typical of an immunosuppressive microenvironment that promotes tumor progression (55, 56). In fact, we previously found that obesity is associated with an increased expression of the immunosuppressive molecules IL-4 and IL-5 in both PAN02 (30), which was confirmed here in the AK4.4 model (Fig. S8B). Furthermore, we found here that IL-1β inhibition recapitulates the effect of TAN depletion on the immune phenotype and tumor growth, consistent with the TAN-recruiting effect of IL-1β. These findings extend our previous observations that obesity is associated with inflammation, immunosuppression, and macrophage recruitment in PAN02 tumors, and that reducing IL-1β production via VEGFR-1 inhibition leads to a reduction in tumor progression in obese mice (30). Considering that the immune environment in obesity appears to be suppressed, and the unprecedented success of therapies that block immune checkpoint pathways (55), it would be interesting to evaluate whether these therapies will be particularly effective in obese hosts.
Obesity promotes the crosstalk between CAAs, TANs and PSCs that leads to PDAC growth
We found that the reduction in PSC activation after AT1 blockade, in addition to decreasing obesity-associated desmoplasia, also reduces the levels of IL-1β, decreases the infiltration of TANs and Tregs and increases the number of CD8(+) T cells, and reduces obesity-associated tumor growth. These findings suggest that the interaction of inflammatory cells and PSCs is bidirectional - targeting TANs reduces PSC activation, and in turn targeting PSCs reduces the recruitment of TANs, with both approaches reducing tumor growth. This is consistent with previous in vitro work demonstrating that neutrophils interact reciprocally with myofibroblasts and PSCs (57, 58). In conclusion, our work reveals a crosstalk between CAAs, TANs, and PSCs that promotes tumor progression in obese hosts, with IL-1β (secreted by all these cells) playing a major role in this cooperation (Fig. 7D).
AT1 inhibition reverses obesity-induced PDAC growth
Similar to our previous results in mice fed a regular diet (18), losartan or AT1 deletion did not affect the growth of PDAC in lean mice. In contrast in obese animals, AT1 inhibition reduced PDAC growth, which was likely due to the inhibition of the pro-tumorigenic activity of TANs and IL-1β, which modulate the recruitment / activity of CD8-positive T cells and Tregs, as discussed above. The reduction of TAN recruitment after AT1 inhibition may result from a direct effect, since neutrophils have been shown to express AT1 (59, 60). Indeed, we found that ~40% of TANs expressed AT1 in the PAN02 model (Fig. S12) and similarly in AK4.4 and KPC models (data not shown). Alternatively, the effects of AT1 blockade on immune cell recruitment could also be indirect. We showed that IL-1β recruits TANs, and AT1 inhibition reduced the production of IL-1β. This could be due to AT1 inhibition of PSCs or tumor adipocytes, as both cells express AT1 and produce IL-1β. In fact, AT1 is highly expressed in normal adipocytes (23), its activity is increased in adipose tissues in obesity (23, 24), and we show here to be expressed in CAA as well (Fig. S13). Moreover, AT1 deletion reduced the levels of the TAN chemoattractant CXCL-1 and TAN activation molecule INF-γ in tumors (Fig. S14A), which can also explain the reduced TAN infiltration.
On the other hand, AT1 inhibition in obese mice reduced the expression of hypoxia and EMT markers in tumors (Fig. S3E and S14B), likely due to its effects on the desmoplastic microenvironment (Fig. S3E). These effects of AT inhibition may have also contributed to the reduction of tumor progression in obesity. In addition, obesity also increased AKT/ERK, p38, JNK and ps6/4EBP1 signaling, which is involved not only in AT1 signaling/desmoplasia in PSCs, but also in proliferation and survival of PDAC cells (61–63). Since losartan reduced the activation of some of these signaling pathways (p38/JNK/4EBP1), its effects on tumor growth may also be explained by a direct effect on cell proliferation/survival.
AT1 ablation reverses obesity-induced chemoresistance
In contrast to the anti-tumor effects of losartan, 5FU reduced PDAC growth in lean but not obese mice, which could be due to the significantly lower intratumoral vascular perfusion and 5FU-uptake in obese than lean mice. In obese mice, AT1 deletion significantly increased the anti-tumor activity of 5FU, whereas the effect of the combination of losartan and 5-FU was not superior to 5-FU alone. In fact, and in contrast to our previous study (18), the increased 5FU-activity induced by losartan was not related to a significant increase in vascular perfusion or drug uptake. Blood flow in tumors is known to be highly dependent on the MABP (64), thus it is possible that the significant decrease in MABP induced by the relatively high dose of losartan (90 mg/kg) may have affected the tumor blood flow thus minimizing the positive-effects of losartan on vascular perfusion. Apart from the effects of losartan on TANs or the adaptive immune response, the losartan-induced decrease in Snail expression and phospho-p38 may also increase the cytotoxic activity of 5FU. P38 is known to be involved in chemoresistance (65), and in mice models of pancreatic cancer, Snail deletion increased the anti-tumor activity of gemcitabine (66).
Of note, consistent with previous work in PAN02 tumors (67), by using both genetic and diet-induced obese mouse models, we determined that obesity promotes tumor growth independently of diet. This was also confirmed by the finding that FVB mice that did not gain weight despite a HFD (~25% of all mice on a HFD) observed tumor growth similar to that in lean animals (not shown).
Validation of preclinical findings in PDAC patients
We found that our key observations in mice were also present in patients. In particular, excess weight was associated with increased desmoplasia in tumors and reduced response to chemotherapy. Moreover, the high incidence (~85% in our cohort – Fig. S15) of hypertension in obese patients makes it an ideal target population of AT1 inhibitors (ACERi/ARBs), considering the blood pressure lowering effect of these drugs.
Conclusions
Obesity is considered to be responsible for 14–20% of all cancer-related deaths in the United States (4). As a result, the obesity epidemic – which also affects the majority of PDAC patients – highlights the importance of understanding the pathophysiology underlying the obesity-cancer connection. We had previously found that obesity associates with increased tumor inflammation, immune cell recruitment and tumor growth. We discovered here that obesity-induced inflammation and TAN infiltration leads to a desmoplastic tumor microenvironment, which directly promotes tumor growth and impairs the response to chemotherapy. Both of these factors may explain the poor outcomes in obese patients. The finding that obesity-induced desmoplasia responds to clinically available anti-fibrotic therapies (e.g., ARBs) is extremely encouraging and strongly supports the implementation of clinical trials to determine the efficacy of these therapies in obese PDAC patients in combination with the current standard-of-care. Since epidemiological and molecular evidence suggests a link between obesity and other desmoplastic cancer types, the strategies established in this study may also apply to a broader patient population. In conclusion, obesity and the obesity-related markers described here (increased desmoplasia, TAN infiltration, IL-1 β expression) may help explain the poorer prognosis in PDAC patients.
Methods
Obese mouse models
C57BL/6 WT, leptin-deficient (ob/ob), AT1-knockout (KO) _(agtr1a_−/−) or AT2-KO (_agtr2_−/−) (all C57BL/6 background) and FVB mice were originally obtained from Jackson Laboratory (The Jackson Laboratory, Bar Harbor, Maine) and bred and maintained in our defined-flora animal colony. KPC (Ptf1-Cre/LSL-KRASG12D/p53-R172H, mixed C57BL/6 and FVB background) and iKRAS (p48-Cre;R26-rtTa-IRES-EGFP;TetO-KrasG12D mice, FVB background) (32–35, 68) mice were obtained form our collaborators. To generate an obese model, mice (6-week old) were given either 10% or a 60% fat diet (D12450J and D12492, Research Diets, New Brunswick, NJ) for 10 weeks (or until tumor collection in KPC spontaneous models), as previously described (68–70). iKRAS mice were given 10 and 60% doxycycline (650 mg/kg) - containing diet for induction of PDAC development (D14071902 and D14071903, Research Diets, New Brunswick, NJ). ob/ob mice remained on standard chow. For implanted tumor experiments, AK4.4 cells (KrasG12D and p53 + / −) were kindly provided by Dr Nabeel Bardeesy, and were isolated from mice generating spontaneous pancreatic tumors (Ptf1-Cre/LSL-KrasG12D/p53Lox/+) (71). PAN02 cells (SMAD4-m174) (72) were obtained from ATCC. Orthotopic pancreatic tumors were generated by implanting a small piece (1 mm3) of viable tumor tissue (from a source tumor in a separate donor animal) into the pancreas of a 6–8-week-old male lean or obese FVB (AK4.4 model) or C57BL/6 background (PAN02 model) mice. PAN02 tumor chunks and AK4.4 cells were authenticated by IDEXX laboratories. (PAN02: IDEXX RADIL Case # 22366, 2013. AK4.4: IDEXX RADIL Case # 27818, 2014. Please see more details in Supplementary Methods). With exception of PAN02, these orthotopically grown pancreatic cancers are characterized by a dense collagenous stroma, a hallmark of pancreatic cancer desmoplasia (73). All experimental use of animals abide by the Public Health Service Policy on Humane Care of Laboratory Animals, and was approved by the institutional animal care and use committee (IACUC) at Massachusetts General Hospital.
Pancreatic tumor growth studies
For all experiments unless specified below, mice bearing orthotropic PAN02 or AK4.4 pancreatic tumors were randomized into treatment groups (or controls) and tumors were collected at day 21 after implantation. For tumor growth study with chemotherapy, mice were divided into treatment groups 7 days after implantation when tumors in lean and obese mice have similar sizes, as determined by ultrasound (Fig. S5J), and treated with either 5-FU (30mg/Kg i.v. every 4 days) or an equal volume of saline by intravenous injection on days 7, 11 and 15 after implantation, with tumors collected at day 19. For tumor growth study with losartan, mice were treated with losartan (90 mg/Kg i.p. every day) or an equal volume of PBS intraperitoneally starting on day 5 after implantation for the duration of the study. In the combined experiment of losartan and 5-FU, the same protocol was used for each drug as described above. TAN depletion by a Ly6G specific inhibitor (BioExcell, 4 mg/Kg i.p. every 2 days) was administered starting at day 1 or day 7. IL1-β inhibition (MM425B, Endogen/Pierce Biotechnology, 2mg/Kg i.p. every 2 days) was administered to PAN02 bearing animals starting at day 7. At the completion of the study, adipose tissue and tumor samples were collected, weighed, and processed for further analysis (for information on rtPCRarray, immunohistochemistry/immunofluorescence, Western blotting, ELISA and Flow cytometry, please see Supplementary Methods). When spontaneous models were used, tumors were collected when palpable. For drug delivery in tumors, drug preparation, and ultrasound and blood pressure measurements, please refer to Supplementary Methods.
Human samples / Clinical Studies
Human samples of pancreatic cancer were obtained from the MGH tissue repository. Tumors selected received no prior chemotherapy or radiation therapy before the surgical specimen was collected at the time of tumor resection. Body mass index (BMI) was obtained for the respective sample. A total of 16 samples were randomly selected from this subset of samples (8 with BMI<25 and 8 with BMI>30). Paraffin sections were stained for collagen-I and HA as described below. Images are acquired using confocal microscopy and quantified using Matlab. Data were analyzed anonymously. For survival studies, 309 consecutive pancreatic cancer patients who received surgical resection that were followed up in MGH between 2006–2010 with information, including clinicopathological details, treatment received and BMI at the time of diagnosis, were included. Written informed consent from the patient or the next of kin was obtained for the use of pancreatic cancer samples or clinical data. All studies were conducted in accordance with the Declaration of Helsinki, and were approved by an institutional review board. All patients have pathology confirmed diagnosis of pancreatic ductal adenocarcinoma..
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 6.0f (GraphPad Software, San Diego, California) and SPSS software (for clinical studies), version 22 (SPSS, Cary, North Carolina). Error bars indicate the standard error of the mean of data from replicate experiments. The significance of difference between samples within figures was confirmed using unpaired t-tests, one-way ANOVA, chi-square test or two-way ANOVA, depending on the experimental setting. In clinical studies, patients were divided into BMI ≤ 25 (n=145), or BMI >25 (n=164), with mean BMI being 26.25. The values of BMI represent the BMI at the time of diagnosis. Overall survival (OS) was defined as the time from date of diagnosis until date of death or last contact. Survival curves were calculated using Kaplan-Meier methods and were compared using log rank test. Covariates with a p-value <0.150 in univariate analysis were included into multivariate analyses. Cox proportional hazards regression analyses were used to conduct comparisons for chemotherapy efficacy. The association of adjuvant chemotherapy and overall survival was adjusted for tumor size (continuous variable), lymph vessel and perineural invasion (absent vs. present), lymph nodes (negative vs. positive), surgical margin (negative vs. positive), pathological grading (1–2 vs. 3–4), adjuvant radiation (yes vs. no), and adjuvant chemotherapy (yes vs. no). Time to event was diagnosis of pancreatic cancer until death or last follow-up with individuals still alive at last follow-up being coded as censored for the event (death) as of that date. For comparison between prevalence of hypertension, a Chi-Square test was performed. In all analysis, a p value of less than 0.05 was considered significant.
Supplementary Material
1
2
3
Statement of Significance.
Considering the current obesity pandemic, unraveling the mechanisms underlying obesity-induced cancer progression is an urgent need. We found that the aggravation of desmoplasia is a key mechanism of obesity-promoted PDAC progression. Importantly, we discovered that clinically available anti-fibrotic/inflammatory agents can improve the treatment response of PDAC in obese hosts.
Acknowledgments
Financial support: This study was supported in part by the National Institutes of Health (CA80124, CA85140, CA96915, CA115767, CA126642 to R.K.J. and D.F.), the Lustgarten Foundation (research grant to R.K.J.), and the Foundation for Science and Technology (Portugal, POPH/FSE funding program, fellowship and research grant to J.I.).
Conflict of interest statement: R.K.J. received consultant fees from Ophthotech, SPARC, SynDevRx and XTuit. RKJ owns equity in Enlight, Ophthotech, SynDevRx, and XTuit and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund. No reagents or funding from these companies was used in these studies.
We would like to thank Sylvie Roberge for assistance with tumor implantation and blood pressure measurements, Sergey Kozin for assistance with blood pressure measurements, Penny Woo for assistance with animal husbandry, Carolyn Smith for assistance with immunohistochemical studies, Lance Munn for his contribution to illustrations, and Ana Batista and Peter Blume-Jensen for suggestions and critiques to the manuscript.
References
- 1.American Cancer Society: Cancer Facts and Figures. 2015 [Google Scholar]
- 2.Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. International journal of cancer Journal international du cancer. 2011;129:1708–1717. doi: 10.1002/ijc.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan C, Bao Y, Wu C, Kraft P, Ogino S, Ng K, et al. Prediagnostic body mass index and pancreatic cancer survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4229–4234. doi: 10.1200/JCO.2013.51.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.McWilliams RR, Matsumoto ME, Burch PA, Kim GP, Halfdanarson TR, de Andrade M, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116:5054–5062. doi: 10.1002/cncr.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA : the journal of the American Medical Association. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nature reviews Gastroenterology & hepatology. 2011;8:169–177. doi: 10.1038/nrgastro.2011.4. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA : the journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 10.Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, et al. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12325–12330. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauhan VP, Boucher Y, Ferrone CR, Roberge S, Martin JD, Stylianopoulos T, et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell. 2014;26:14–15. doi: 10.1016/j.ccr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. British Journal of Cancer. 2013;109:926–933. doi: 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hidalgo M, Von Hoff DD. Translational Therapeutic Opportunities in Ductal Adenocarcinoma of the Pancreas. Clinical Cancer Research. 2012;18:4249–4256. doi: 10.1158/1078-0432.CCR-12-1327. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumor blood vessels. Nature Communications. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endrich B, Reinhold HS, Gross JF, Intaglietta M. Tissue perfusion inhomogeneity during early tumor growth in rats. J Natl Cancer Inst. 1979;62:387–395. [PubMed] [Google Scholar]
- 20.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 21.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Perez RR, Rueda BR. Tumor angiogenesis regulators. Boca Raton: Taylor & Francis; 2013. [Google Scholar]
- 23.AGTR1 (angiotensin II receptor, type 1) gene expression. Biogps.org. 2015 [Google Scholar]
- 24.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell metabolism. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur A, Marine M, Lu D, Swartz-Basile DA, Saxena R, Zyromski NJ, et al. Nonalcoholic fatty pancreas disease. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2007;9:312–318. doi: 10.1080/13651820701504157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda A, Makino N, Tozawa T, Shirahata N, Honda T, Ikeda Y, et al. Pancreatic fat accumulation, fibrosis, and acinar cell injury in the Zucker diabetic fatty rat fed a chronic high-fat diet. Pancreas. 2014;43:735–743. doi: 10.1097/MPA.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Hori M, Takahashi M, Hiraoka N, Yamaji T, Mutoh M, Ishigamori R, et al. Association of pancreatic Fatty infiltration with pancreatic ductal adenocarcinoma. Clinical and translational gastroenterology. 2014;5:e53. doi: 10.1038/ctg.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hefetz-Sela S, Scherer PE. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacology & therapeutics. 2013;138:197–210. doi: 10.1016/j.pharmthera.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Incio J, Tam J, Rahbari NN, Suboj P, McManus DT, Chin SM, et al. PlGF/VEGFR-1 Signaling Promotes Macrophage Polarization and Accelerated Tumor Progression in Obesity. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1839. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Molecular carcinogenesis. 2012;51:53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nature genetics. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 34.Kanasaki K, Koya D. Biology of obesity: lessons from animal models of obesity. Journal of biomedicine & biotechnology. 2011;2011:197636. doi: 10.1155/2011/197636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA : the journal of the American Medical Association. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 37.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 38.Hama K, Ohnishi H, Yasuda H, Ueda N, Mashima H, Satoh Y, et al. Angiotensin II stimulates DNA synthesis of rat pancreatic stellate cells by activating ERK through EGF receptor transactivation. Biochemical and Biophysical Research Communications. 2004;315:905–911. doi: 10.1016/j.bbrc.2004.01.155. [DOI] [PubMed] [Google Scholar]
- 39.Hama K, Ohnishi H, Aoki H, Kita H, Yamamoto H, Osawa H, et al. Angiotensin II promotes the proliferation of activated pancreatic stellate cells by Smad7 induction through a protein kinase C pathway. Biochemical and Biophysical Research Communications. 2006;340:742–750. doi: 10.1016/j.bbrc.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 40.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukumura D, Incio J, Shankaraiah RC, Jain RK. Obesity and Cancer: An Angiogenic and Inflammatory Link. Microcirculation. 2016;23:191–206. doi: 10.1111/micc.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White PB, True EM, Ziegler KM, Wang SS, Swartz-Basile DA, Pitt HA, et al. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010;14:1888–1893. doi: 10.1007/s11605-010-1349-x. discussion 93-4. [DOI] [PubMed] [Google Scholar]
- 43.Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, Gomez SB, et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145:1449–1458. doi: 10.1053/j.gastro.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khasawneh J, Schulz MD, Walch A, Rozman J, Hrabe de Angelis M, Klingenspor M, et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci U S A. 2009;106:3354–3359. doi: 10.1073/pnas.0802864106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer research. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 46.Wang YY, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L, et al. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer letters. 2012;324:142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Hursting SD. Minireview: the year in obesity and cancer. Molecular endocrinology. 2012;26:1961–1966. doi: 10.1210/me.2012-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Annals of the New York Academy of Sciences. 2012;1271:82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masamune A, Satoh M, Kikuta K, Sakai Y, Satoh A, Shimosegawa T. Inhibition of p38 mitogen-activated protein kinase blocks activation of rat pancreatic stellate cells. The Journal of pharmacology and experimental therapeutics. 2003;304:8–14. doi: 10.1124/jpet.102.040287. [DOI] [PubMed] [Google Scholar]
- 50.Incio J, Suboj P, Chin SM, Vardam-Kaur T, Liu H, Hato T, et al. Metformin Reduces Desmoplasia in Pancreatic Cancer by Reprogramming Stellate Cells and Tumor-Associated Macrophages. PloS one. 2015;10:e0141392. doi: 10.1371/journal.pone.0141392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felix K, Gaida MM. Neutrophil-Derived Proteases in the Microenvironment of Pancreatic Cancer -Active Players in Tumor Progression. International journal of biological sciences. 2016;12:302–313. doi: 10.7150/ijbs.14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zyromski NJ, Mathur A, Pitt HA, Lu D, Gripe JT, Walker JJ, et al. A murine model of obesity implicates the adipokine milieu in the pathogenesis of severe acute pancreatitis. American journal of physiology Gastrointestinal and liver physiology. 2008;295:G552–G558. doi: 10.1152/ajpgi.90278.2008. [DOI] [PubMed] [Google Scholar]
- 53.Mathur A, Zyromski NJ, Pitt HA, Al-Azzawi H, Walker JJ, Saxena R, et al. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg. 2009;208:989–994. doi: 10.1016/j.jamcollsurg.2008.12.026. discussion 94-6. [DOI] [PubMed] [Google Scholar]
- 54.Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:1612–1619. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. The Journal of pathology. 2014;233:294–307. doi: 10.1002/path.4359. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan X, et al. The IL-6-STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell death & disease. 2014;5:e1295. doi: 10.1038/cddis.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raiden S, Pereyra Y, Nahmod V, Alvarez C, Castello L, Giordano M, et al. Losartan, a selective inhibitor of subtype AT1 receptors for angiotensin II, inhibits neutrophil recruitment in the lung triggered by fMLP. J Leukoc Biol. 2000;68:700–706. [PubMed] [Google Scholar]
- 60.Raiden S, Giordano M, Andonegui G, Trevani AS, Lopez DH, Nahmod V, et al. Losartan, a selective inhibitor of subtype AT1 receptors for angiotensin II, inhibits the binding of N-formylmethionyl-leucyl-phenylalanine to neutrophil receptors. The Journal of pharmacology and experimental therapeutics. 1997;281:624–628. [PubMed] [Google Scholar]
- 61.Preis M, Korc M. Signaling pathways in pancreatic cancer. Critical reviews in eukaryotic gene expression. 2011;21:115–129. doi: 10.1615/critreveukargeneexpr.v21.i2.20. [DOI] [PubMed] [Google Scholar]
- 62.Freeman JW, DeArmond D, Lake M, Huang W, Venkatasubbarao K, Zhao S. Alterations of cell signaling pathways in pancreatic cancer. Frontiers in bioscience : a journal and virtual library. 2004;9:1889–1898. doi: 10.2741/1388. [DOI] [PubMed] [Google Scholar]
- 63.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 64.Gullino PM, Grantham FH. Studies on the exchange of fluids between host and tumor. III. Regulation of blood flow in hepatomas and other rat tumors. Journal of the National Cancer Institute. 1962;28:211–229. [PubMed] [Google Scholar]
- 65.Guo X, Ma N, Wang J, Song J, Bu X, Cheng Y, et al. Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC cancer. 2008;8:375. doi: 10.1186/1471-2407-8-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White PB, Ziegler KM, Swartz-Basile DA, Wang SS, Lillemoe KD, Pitt HA, et al. Obesity, but not high-fat diet, promotes murine pancreatic cancer growth. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012;16:1680–1685. doi: 10.1007/s11605-012-1931-5. [DOI] [PubMed] [Google Scholar]
- 68.Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PloS one. 2009;4:e4974. doi: 10.1371/journal.pone.0004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circulation research. 2003;93:e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism: clinical and experimental. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 71.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Zhang Y, Yang J, Ni X, Liu S, Li Z, et al. Genomic sequencing of key genes in mouse pancreatic cancer cells. Current molecular medicine. 2012;12:331–341. doi: 10.2174/156652412799218868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3