Intravenous immunoglobulins for the treatment of mild to moderate Alzheimer’s disease: a phase II, randomised, double-blind, placebo-controlled dose-finding trial (original) (raw)
. Author manuscript; available in PMC: 2016 Aug 16.
Abstract
Background
Three small trials have suggested effects of intravenous immunoglobulins (IVIG) on biomarkers and symptoms of mild-to-moderate Alzheimer’s disease (AD). We explored the safety, the effective dose, and the infusion interval for Octagam_®_10% in this patients’ group.
Methods
The study was a 24-week multicentre, double-blind, placebo-controlled phase II trial with 8 treatment arms at 7 sites in the USA and 5 sites in Germany. Participants aged 50–85 years were randomised (using a computer-generated randomisation sequence) to either 4 weekly infusions (n=22) (0.2 g/0.5 g/0.8 g/kg body weight), 2 weekly infusions (0.1g/0.25 g/0.4 g/kg) (n=21) or to placebo (n=7, 4-weekly, n=8, 2 weekly). The primary endpoint was the mean area under the curve (AUC) of plasma Aβ1–40 after the last infusion for one infusion interval. We considered the AUC of plasma Aβ1–40 being more representative of the potential effect of IVIG than a single time point measurement. Secondary outcomes included changes in (a) the concentrations of Aβ1–40, Aβ1–42, anti-Aβ autoantibodies in CSF/plasma and tau/ptau181 in CSF, (b) cognitive and functional scales, and (c) brain imaging (MRI/FDG-PET). Patients’ safety was assessed by recording of adverse events, clinical examinations, MRI investigations, electrocardiography and laboratory tests. The infusions were performed by site personnel who were otherwise not involved in any other assessments; therefore, the patients, caregivers, and investigators were blinded to the treatment allocations. The study medication was blinded by using intransparent overpouches and infusion lines. The trial is registered at ClinicalTrials.gov (NCT00812565) and controlled-trials.com (ISRCTN64846759).
Findings
Fifty-six patients were randomized. AUC of plasma Aβ1–40, was not significantly different from the placebo for five of the six IVIG arms (median with range: −18.00 [−1347.0; 1068.5] for 0.2 g/kg; 364.25 [−5834.5; 1953.5] for 0.5 g/kg and −351.75 [−1084.0; 936.5] for 0.8 g/kg every 4 weeks compared to −116.25 [−1379.0; 5266.0] for the placebo; −13.75 [−1729.0; 307.0] for 0.1 g/kg, −32.50 [−1102.5; 451.5] for 0.25 g/kg and 47.00 [−341.0; 72.5] for 0.4 g/kg compared to 159.50 [51.5; 303.0] for the placebo; p=0.02 for comparison of the latter two groups). Adverse events were reported in 59.5% and 64.3% of the patients in the IVIG and placebo groups, respectively. No unexpected serious adverse events occurred.
Interpretation
IVIG had a very acceptable safety profile in the patients. The trial did not confirm results from previous studies. Longer trials with greater power are required to assess potential cognitive and functional effects of IVIG in AD.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia in the elderly and available symptomatic treatment options have limited efficacy1.
We and others have reported evidence that intravenous immunoglobulins (IVIG) may have beneficial effects on pathogenic processes in AD (as evaluated by biomarkers) and that IVIG may improve the symptoms in AD patients2–5. IVIG is a fractionated blood product used to treat a variety of medical conditions6. The rationale for using IVIG in AD is based on the existence of naturally occurring antibodies directed against Aβ (nAbs-Aβ); these antibodies may interfere with Aβ metabolism and appear to be reduced in AD patients3, 7. Three small clinical trials using IVIG in mild-to-moderate AD patients have been published. In the initial uncontrolled trial five patients received 1.2 g/kg IVIG every four weeks for six months. The Aβ1–40 level decreased in the CSF and increased in the blood compared to baseline2. There was no cognitive deterioration in these subjects. These results were independently reproduced in an uncontrolled trial with eight patients (0.4 g/kg–2 g/kg for six months)5. Finally, a 6-months, placebo-controlled (saline) multiple dose (0.2g/kg and 0.4g/kg/bi weekly or 0.4g/kg and 0.8g/kg/monthly) study in 24 AD patients was completed. The data have not yet been published in detail8.
The most effective dose and the best treatment interval to maximise IVIG treatment effects while minimising safety risks are not yet known, although preliminary data support a 2-week infusion schedule over a 4-week schedule5. Here, we report the results of a phase II exploratory dose-finding study in mild-to-moderate AD patients.
Methods
Study design
We performed a double-blind, randomised, placebo-controlled, parallel group (with balanced randomisation), multi-centre trial at twelve sites (five and seven sites in Germany and the USA, respectively) to assess efficacy and safety of different IVIG dosages (Octagam®10%) in out-patients with mild-to-moderate AD.
Participants
The inclusion criteria were probable AD according to the NINCDS-ADRDA criteria9, MMSE score between 16–26 (inclusive)10, age between 50–85 years (inclusive) at baseline, a Modified Hachinski-Rosen Score <5 and a MRI-scan consistent with AD. Patients were required to be on stable doses of approved AD medication for at least three months before screening.
Exclusion criteria were any suspected cause of dementia other than AD, history or present diagnosis of another significant disease of the central nervous system, a score of >7 in the geriatric depression scale, present significant psychiatric disorder, insulin-dependent diabetes mellitus, uncontrolled hypertension, severe liver or kidney disease, history of thromboembolic events or history of hypersensitivity to blood or plasma derived products or IVIG use in the previous six months.
The study was approved by each study site’s ethics committee/IRBs and by the regulatory authorities. Each patient provided written informed consent before study participation. An independent data-monitoring committee assessed treatment safety throughout the trial. The trial is registered at ClinicalTrials.gov (NCT00812565) and controlled-trials.com (ISRCTN64846759).
Randomisation and masking procedures
Patients were randomly allocated to receive one of three dosage levels of Octagam®10% (0.2g/kg–0.5g/kg–0.8g/kg bodyweight) or placebo (0.9% isotonic sodium chloride) every four weeks, or half of that dosage (0.1g/kg–0.25g/kg–0.4g/kg) every two weeks.
For allocation of the participants, a computer-generated randomization list was created by the CRO using SAS 9.1.3 (SAS Institute Inc., Cary, USA) allocating patients via an IWRS with a block size of 8. Ethylene vinyl acetate bags containing the study medication were blinded by using intransparent overpouches. It was dispensed by the unblended local pharmacy, which was responsible for study drug preparation. Infusion was performed by a physician who was blinded to the patient’s group assignment and not involved in any assessments.
Procedures
All Octagam® 10% glass bottles and placebo for the calculated individual dose according to the body weight of the patient were pooled aseptically by the hospital pharmacist in three separate EVA bags of 0.5 L volume and were administered intravenously after the ratings at the respective visits. If a patient was randomised to receive placebo (0.9% w/v isotonic sodium chloride solution), the same volume and infusion rate as in the IVIG arms was applied.
Outcomes
Previous studies suggested that peripherally administered antibodies against Aβ induce an Aβ shift from the CSF to the blood, thereby reducing the cerebral Aβ burden (“peripheral sink hypothesis”)11. In agreement, earlier IVIG studies revealed an increase of plasma Aβ and a decrease of CSF Aβ2, 5. Thus, we selected plasma Aβ1–40 as marker of primary interest. We chose the mean area under the curve (AUC)12 of the Aβ1–40 plasma change from the trough level value, prior to the last infusion at week 20 (4-week intervals) or 22 (2-week intervals) to the end of an infusion interval (i.e. 4 weeks, 2 weeks) as the primary outcome measure. Because of the dynamics of the IVIG effect over time, the AUC was considered more appropriate than a single time point measure. The primary endpoint measures of the three different IVIG dosage arms in each of the two treatment interval groups were compared with their corresponding placebo arms.
Blood sampling was performed prior to last infusion and on days 1 (+1), 4 (±1), 7 (±1) and 14 (±2) after the week 22 infusion (2-week interval) or additionally on days 21 (±2) and 28 (±2) after the week 20 infusion (4-week interval).
The secondary outcome measures were the plasma AUC of Aβ1–42, and anti-Aβ autoantibodies, plasma Aβ1–40, Aβ1–42, anti-Aβ autoantibodies single time point measurements at week 24, and change in CSF Aβ1–40, Aβ1–42, anti-Aβ autoantibodies tau and p-tau181 concentration 24±8 hours following the last infusion compared with baseline.
Secondary outcomes included the Alzheimer’s disease Assessment Scale-Cognitive Part (ADAS-cog), the Clinical Dementia Rating Scale – Sum of Boxes (CDR-SOB), the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale (ADCS-ADL), and Mini-Mental-State-Examination (MMSE) at baseline and at week 12 or 24. The raters (physicians, neuropsychologists) were blinded with regard to study medication.
Secondary brain imaging outcomes were changes in whole brain and hippocampal volume from baseline to week 12 and week 24 and changes in glucose metabolism between baseline and week 24. No changes of trial outcomes were implemented after the trial commenced.
Lumbar puncture, CSF and plasma analyses
CSF sampling and analyses are detailed in the supplementary material.
MR-Imaging
For safety and volumetric analyses, MRIs were performed at screening and after 12 and 24 weeks using 1.5T MR scanners. The sequences and procedures are outlined in the supplementary material.
FDG-PET
Fifty-five patients underwent baseline FDG-PET scans. In one centre, a PET-scanner was not available; thus 3 patients were not examined. The details of the FDG-PET are outlined in the supplementary material.
Statistical analysis
As this trial was an exploratory phase II study, no confirmatory hypothesis testing was planned. The sample size was determined empirically. It was estimated that 48 patients with a complete AUC for plasma Aβ1–40 would be appropriate. Accounting for a possible drop-out rate of 14% a total of 56 patients were planned to be randomized.
The AUC was calculated by using the linear trapezoidal rule (i.e., by summing the trapezoid areas formed by the time intervals tn−tn−1 and the change in the baseline plasma concentrations as heights). The AUC was calculated for plasma Aβ1–40, Aβ1–42, and the nAbs-Aβ. The concentrations were determined at five different time points for the patients, who were treated at 2-week intervals (prior to last infusion, days 1/4/7/14) and at seven different time points for those treated at 4-week intervals (prior to last infusion, days 1/4/7/14/21/28). Areas below the abscissa reduced the overall AUC.
The differences in AUC between each IVIG dose and the corresponding placebo was assessed by calculating the Hodges-Lehmann estimate of the difference in two medians and the corresponding non-parametric 95% confidence interval. Further, the _p_-value for a Wilcoxon-rank-sum-test was provided.
For the cognitive and functional scales, plasma biomarkers, and MRI outcomes, the differences between week 24 and baseline were calculated. In case of CSF the difference between the time frame of 24±8 hours after last infusion and baseline were calculated. T-tests were used to assess treatment effects for change in plasma and CSF biomarkers, and MRI measures. The difference in change of cognitive and functional scales was assessed with Wilcoxon-rank-sum-tests, by calculating Hodges-Lehmann estimates, and by the corresponding 95% confidence interval. The results of the individual treatment arms against placebo are reported here. The analyses on the pooled IVIG and placebo groups are detailed in the supplementary material. A correction for multiple comparisons was not applied.
For safety data, continuous variables were analysed using standard summary statistics and qualitatively using frequency tables. The statistical analysis was performed using the software package SAS 9.2 (SAS Institute, Cary, USA).
FDG-PET was analysed with paired _t_-tests of normalised scans in the IVIG and the placebo groups comparing baseline with follow-up data at week 24). The different IVIG doses and placebo arms were combined within dosing intervals to increase power. A significance threshold of p<0.001, uncorrected for multiple comparisons was applied due to small sample sizes. In an additional analysis, all IVIG arms were combined (n=36), and a more conservative significance level of p<0.05, whole brain corrected (false discovery rate, FDR) was applied. Contrasts were calculated for metabolism increases and reductions. Age and gender were used as covariates.
Role of the funding source
The study sponsor and co-investigators (RD, FJ, MF) were jointly responsible for the study design/planning. Data management was outsourced to a CRO (ClinResearch, now Aptiv Solutions, Cologne, Germany). The CRO had responsibility for data monitoring and analysis according to the Statistical Analysis Plan developed by the sponsor as well as for writing the study report. After the database lock and study unblinding, all of the investigators had full access to the study data and shared the final responsibility for data analysis. The manuscript for the publication was written and reviewed by the authors. The decision to submit the manuscript for publication was jointly made by the PI (RD) and the sponsor.
Results
Study group
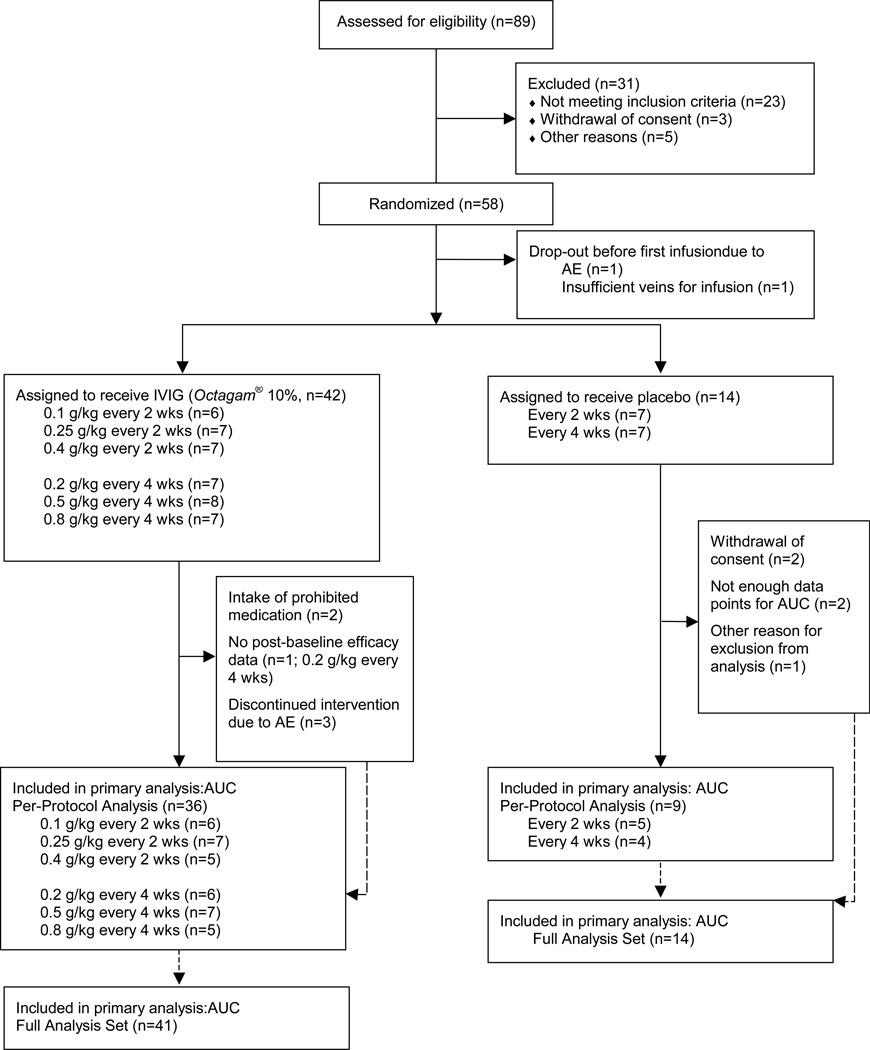
Eighty-nine patients were screened and 58 were randomly assigned to the treatment scheme (Figure 1, period of inclusion: 02 Feb 2009–30 Mar 2010; last patients’ visit on 21 Sep 2010). Forty-nine patients (84%) completed the study. Table 1 and 2 list baseline characteristics of the groups.
Figure 1.
Study flowchart
Table 1.
Baseline characteristics of the patients (Full Analysis Set = Intent-to-treat population; n=55).
| IVIG (n=41) | Placebo (n=14) | |
|---|---|---|
| Demographics | ||
| Age (years; mean ±SD) | 69.4 ± 7.3 | 72.0 ± 10.2 |
| Women n (%) | 15 (36.6) | 9 (64.3) |
| White n (%) | 40 (97.6) | 12 (85.7) |
| Weight (kg ±SD) | 77.6 ± 14.1 | 71.8 ± 17.7 |
| Duration of symptoms of AD (months;mean ±SD) | 29.6 ± 27.3 | 23.6 ± 21.9 |
| APOE ε4 carrier n (%) | 30 (73.2) | 10 (71.4) |
| Use of AChEI or memantine n (%) | 36 (87.8) | 11 (78.6) |
| Clinical measures | ||
| Modified Hachinski score (mean ±SD) | 0.6 ± 0.6 | 1.0 ± 0.4 |
| GDS (mean ±SD) | 1.6 ± 1.5 | 1.7 ± 2.0 |
| MMSE score (mean ±SD) | 21.3 ± 2.8 | 21.9 ± 2.4 |
| ADAS-cog (mean ±SD) | 20.5 ± 8.5 | 19.5 ± 10.5 |
| Clinical dementia rating - sum of boxes(mean ±SD) | 4.7 ± 2.0 | 5.0 ± 3.1 |
| ADCS-ADL (mean ±SD) | 65.1 ± 9.2 | 63.3 ± 11.1 |
| Laboratory measures | ||
| Aβ1–40: CSF (pg/mL) | 8366.8 ± 2428.3 | 9237.0 ± 2799.7 |
| Plasma (pg/mL) | 180.6 ± 43.9 | 174.4 ± 57.1 |
| Aβ1–42: CSF (pg/mL) | 300.6 ± 122.9 | 363.9 ± 138.0 |
| Plasma (pg/mL) | 42.3 ± 11.8 | 38.1 ± 9.2 |
| Tau [pg/ml] | 711.9 ± 432.2 | 600.4 ± 257.7 |
| Tau181 [pg/ml] | 105.7 ± 53.0 | 93.7 ± 28.3 |
| nAbs-Aβ: CSF[RTU] | 3.6 ± 6.7 | 5.8 ± 9.9 |
| Plasma [RTU] | 646.6 ± 1342.2 | 794.1 ± 1062.1 |
| Microbleeds n (%) | 8 (19.5) | 1 (7.1) |
| Normalized whole brain volume | 1288.9 ± 73.3 | 1275.9 ± 120.3 |
| Hippocampal volume left [mm3] | 2650.5 ± 478.3 | 2608.2 ± 491.0 |
| Hippocampal volume right [mm3] | 2829.6 ± 491.8 | 2665.6 ± 434.3 |
Table 2.
Baseline characteristics of the patients stratified by 4-week and 2-week dosing interval (Intent-to-treat population; n=55)
| IVIG(n=21) | Placebo(n=7) | IVIG(n=20) | Placebo(n=7) | |||||
|---|---|---|---|---|---|---|---|---|
| 4-week interval | 2-week interval | |||||||
| 0.2 (n=6) | 0.5 (n=8) | 0.8 (n=7) | 0.1 (n=6) | 0.25 (n=7) | 0.4 (n=7) | |||
| Demographics | ||||||||
| Age (years) | 74.8 ± 5.5 | 65.9 ± 10.2 | 68.4 ± 8.6 | 71.4 ± 11.8 | 66.8 ± 5.5 | 68.3 ± 4.2 | 72.9 ± 5.0 | 72.6 ± 9.2 |
| Women (n) | 2 | 3 | 3 | 5 | 1 | 3 | 3 | 4 |
| White (n) | 5 | 8 | 7 | 6 | 6 | 7 | 7 | 6 |
| Weight (kg) | 72.9 ± 15.9 | 74.8 ± 13.5 | 81.9 ± 13.6 | 65.6 ± 17.9 | 84.8 ± 10.9 | 74.8 ± 14.0 | 77.1 ± 17.6 | 78.1 ± 16.3 |
| Duration of AD (months) | 17.7 ± 16.3 | 17.9 ± 18.3 | 16.0 ± 12.1 | 21.3 ± 16.4 | 38.3 ± 39.6 | 43.8 ± 39.4 | 44.9 ± 15.4 | 25.9 ± 27.6 |
| APOE ε4 carrier | 4 | 3 | 7 | 4 | 6 | 5 | 5 | 6 |
| Use of AChEI or memantine | 5 | 8 | 6 | 5 | 6 | 7 | 5 | 6 |
| Clinical measures | ||||||||
| Modified Hachinski score | 0.3 ± 0.5 | 0.6 ±0.9 | 0.9 ±0.7 | 1.0 ±0.6 | 0.8 ± 0.4 | 0.6±0.5 | 0.1 ±0.4 | 1.0 ±0 |
| GDS | 1.3 ±0.8 | 1.9 ±1.8 | 1.0 ±1.2 | 2.4 ±2.6 | 1.2 ±1.2 | 1.7 ±2.1 | 2.1 ±2.0 | 1.0 ±0.8 |
| MMSE score | 19.3 ± 3.4 | 20.9 ±2.7 | 21.9 ± 2.7 | 22.1 ± 3.1 | 21.8 ±2.6 | 21.4 ±2.4 | 22.4±3.2 | 21.6 ±1.7 |
| ADAS-cog | 27.2 ± 9.7 | 21.0 ± 9.7 | 20.0 ± 9.5 | 21.2 ± 14.7 | 18.5 ± 6.3 | 19.4 ± 8.2 | 17.6 ± 6.5 | 17.7 ± 4.2 |
| CDR - sum of boxes | 6.6 ± 3.3 | 5.1 ± 1.7 | 3.9 ± 2.1 | 5.3 ± 3.7 | 4.4 ± 1.4 | 4.3 ± 1.8 | 4.3 ± 1.1 | 4.8 ± 2.6 |
| ADCS-ADL | 60.5 ± 11.2 | 65.9 ± 8.3 | 67.0 ± 8.3 | 64.4 ± 12.5 | 64.2 ± 6.6 | 65.3 ± 14.0 | 67.1 ± 6.8 | 62.1 ± 10.4 |
| Laboratory measures | ||||||||
| Aβ1–40: CSF (pg/mL) | 8499.2 ± 2030.6 | 8654.6 ± 2357.5 | 7656.0±1882.2 | 10191±2825.4 | 9783.0±2634.0 | 7277.0±2701.1 | 8534.8±3030.3 | 8283.0±2622.3 |
| Plasma (pg/mL) | 169.2 ± 62.7 | 150.9 ± 17.9 | 184.3 ± 43.4 | 194.7 ± 56.5 | 192.5 ± 49.5 | 201.5 ± 37.5 | 189.5 ± 40.6 | 154.1 ± 53.9 |
| Aβ1–42: CSF (pg/mL) | 333.8 ± 156.9 | 279.8 ± 47.5 | 240.0 ± 52.3 | 358.3 ± 161.1 | 355.8 ± 204.5 | 321.4 ± 138.8 | 286.0 ± 101.0 | 369.4 ± 123.5 |
| Plasma (pg/mL) | 42.7 ± 9.4 | 36.6 ± 16.0 | 45.9 ± 8.7 | 42.0 ± 8.5 | 41.8 ± 13.3 | 48.2 ± 12.2 | 39.8 ± 8.7 | 34.3 ± 8.8 |
| Total tau (pg/mL) | 861.3 ± 848.6 | 803.6 ± 239.9 | 629.4 ± 399.3 | 714.9 ± 283.0 | 955.2 ± 327.2 | 468.3 ± 291.5 | 577.2 ± 174.4 | 486.0 ± 182.4 |
| P-tau181 (pg/mL) | 113.8 ± 84.9 | 116.5 ± 27.8 | 91.4 ± 40.9 | 105.9 ± 29.4 | 153.8 ± 68.2 | 77.1 ± 37.1 | 84.8 ± 16.6 | 81.6 ± 22.9 |
| nAbs-Aβ: CSF [RTU] | 7.4 ± 11.9 | 2.2 ± 2.6 | 5.4 ± 6.3 | 4.7 ± 9.2 | 2.1 ± 1.0 | 2.2 ± 2.7 | 2.9 ± 3.1 | 6.9 ± 11.1 |
| Plasma [RTU] | 431.3 ± 161.3 | 493.1 ± 252.7 | 1765.9±3048.1 | 502.1 ± 480.5 | 189.2 ± 72.3 | 562.7 ± 625.1 | 316.0 ± 272.1 | 1086.0±1419.3 |
| Whole brain volume | 1268.2 ± 43.8 | 1319.4 ± 98.1 | 1329.4 ± 90.3 | 1286.3 ± 78.7 | 1276.0 ± 63.0 | 1294.0 ± 47.7 | 1237.1 ± 49.0 | 1265.6 ± 157.9 |
| Hippocampal volume left | 2530.3 ± 387.6 | 2747.6 ± 444.4 | 2568.4 ± 579.9 | 2529.9±353.7 | 2611.0 ± 450.1 | 2818.3 ± 660.9 | 2590.4 ± 392.7 | 2686.6 ± 618.7 |
| Hippocampal volume right | 2693.8 ± 333.9 | 2842.1 ± 557.7 | 2761.9 ± 547.8 | 2679.1±428.4 | 2754.5 ± 609.5 | 2923.6 ± 590.3 | 2969.9 ± 368.2 | 2652.1 ± 474.2 |
Primary endpoint
The effect of treatment on the primary outcome measure is shown in Tables 3 to 4. There was a significant difference in the AUC of plasma Aβ1–40 in the 0.4g/kg every two weeks group compared to placebo (_p_=0.02, decrease). There were no significant differences between the other IVIG groups and the respective placebo groups or in the pooled groups.
Table 3.
AUC of the Aβ plasma change from the trough level in the 4-week dosing interval (Intent-to-treat population; n=55).
| IVIG (n=21) | Placebo (n=7) | Placebo vs. 0.2 IVIG | Placebo vs. 0.5 IVIG | Placebo vs. 0.8 IVIG | |||
|---|---|---|---|---|---|---|---|
| 0.2 (n=6) | 0.5 (n=8) | 0.8 (n=7) | |||||
| Aβ1–40 | −18.00[−1347.0; 1068.5](n=6) | −364.25[−5834.5; 1953.5](n=8) | −351.75[−1084.0; 936.5](n=7) | −116.25[−1379.0; 5266.0](n=6) | −32.50[−1358.0; 4197.5]p=0.8102 | 195.25[−1005.5; 5302.0]p=0.7469 | 235.50[−984.5; 4329.5]p=0.5752 |
| Aβ1–42 | −41.75[−244.40; 336.55](n=6) | −119.25[−1220.60; 375.00](n=8) | −107.50[−173.50; 231.00](n=6) | −20.50[−183.70; 489.00](n=6) | 30.25[−234.55; 346.40]p=0.5752 | 114.75[−64.50; 622.00]p=0.2200 | 87.00[−95.70; 275.50]p=0.2298 |
| nAbs-Aβ | 932.50[−2991.5; 3577.0](n=6) | 5770.00[2892.5; 14426.0](n=8) | 8658.75[5532.5; 11517.5](n=6) | 636.00[−1207.5; 1075.5](n=6) | −915.50[−2844.5; 1784.0]p=0.5752 | −5339.50[−7203.5; −3814.5]p=0.0024 | −8398.25[−10785.0; −5343.5]p=0.0051 |
Table 4.
AUC of the Aβ plasma change from the trough level in the 2-week dosing interval (Intent-to-treat population; n=55).
| IVIG (n=20) | Placebo (n=7) | Placebo vs. 0.1 IVIG | Placebo vs. 0.25 IVIG | Placebo vs. 0.4 IVIG | |||
|---|---|---|---|---|---|---|---|
| 0.1 (n=6) | 0.25 (n=7) | 0.4 (n=7) | |||||
| Aβ1–40 | −13.75[−1729.0; 307.0](n=6) | −32.50[−1102.5; 451.5](n=7) | 47.00[−341.0; 72.5](n=5) | 159.50[51.5; 303.0](n=5) | 159.75[−124.5; 1838.5]p=0.2353 | 200.50[−51.0; 474.5]p=0.0740 | 134.50[4.5; 500.5]p=0.0216 |
| Aβ1–42 | 3.00[−109.50; 74.50](n=6) | −33.50[−190.55; −16.50](n=7) | −9.50[−57.00; 5.00](n=5) | 24.00[2.00; 125.50](n=5) | 26.50[−45.0; 133.5]p=0.1207 | 63.00[40.0; 178.0]p=0.0058 | 39.00[−11.5; −135.0]p=0.0216 |
| nAbs-Aβ | 299.25[−105.0; 987.0](n=6) | 1256.00[1059.0; 5160.0](n=7) | 2172.00[−710.5; 5516.0](n=5) | 593.00[−1090.5; 7747.5](n=5) | 580.75[−1258.0; 7316.5]p=0.4113 | −641.50[−2346.5; 6009.5]p=0.6261 | −380.00[−4923.0; 6600.0]p=1.000 |
Secondary endpoints
Plasma biomarkers
There was a significant change in the plasma AUC of Aβ1–42 in the 2-week interval middle and high-dosage groups compared to the placebo group (_p_=0.01; _p_=0.02, decrease, Table 4). No differences in AUC were found in the 4-week interval groups. In the pooled groups of all six IVIG arms vs. the pooled placebo arms, there was a difference in the AUC of Aβ1–42 (_p_=0.01; decrease; Table 5-SM). There were no significant differences between each dosage group compared to the corresponding placebo group, but there was a significant change in concentrations of plasma Aβ1–42 in the pooled IVIG groups vs. the pooled placebo groups _p_=0.01; decrease, Table 5-SM).
As expected, there was a dose-dependent increase of nAbs-Aβ in plasma from baseline in the 4-week interval groups at week 24 (Tables 5). In the 2-week interval placebo group two patients had tenfold higher nAbs-Aβ values than the others, which prevented significance of the numerical difference compared with the IVIG groups (Table 6).
Table 5.
Mean changes from baseline at week 24 in the 4-week dosing interval (Intent-to-treat population; n=55).
| IVIG (n=21) | Placebo (n=7) | Placebo vs. 0.2 IVIG | Placebo vs. 0.5 IVIG | Placebo vs. 0.8 IVIG | |||
|---|---|---|---|---|---|---|---|
| 0.2 (n=6) | 0.5 (n=8) | 0.8 (n=7) | |||||
| Aβ1–40: | 561.7 ± 1195.5(n=6) | 403.9 ± 588.1(n=8) | 642.2 ± 1171.3(n=6) | 42.0 ± 874.0(n=6) | −519.7 [−1866.7; 827.4]p=0.4102 | −361.9 [−1210.4; 486.6] p=0.3711 | −600.2[−1929.5; 729.2]p=0.3382 |
| CSF* [pg/mL] | |||||||
| Plasma [pg/mL] | −20.3 ± 29.2(n=6) | 15.7 ± 29.7(n=7) | −30.5 ± 42.3(n=6) | −6.0 ± 23.8(n=6) | 14.3 [−19.9; 48.6]p=0.3729 | −21.7 [−55.0; 11.6]p=0.1788 | 24.5 [−19.7; 68.7]p=0.2446 |
| Aβ1–42: | 18.8 ± 40.3(n=6) | 10.5 ± 40.9(n=8) | 24.7 ± 43.2(n=6) | −5.2 ± 36.3(n=6) | −24.0 [−73.3; 25.3]p=0.3037 | −15.7 [−61.6; 30.3]p=0.4718 | −29.8 [−81.1; 21.4]p=0.2239 |
| CSF* [pg/mL] | |||||||
| Plasma [pg/mL] | −3.5 ± 5.8(n=6) | −0.1 ± 4.4(n=8) | −7.0 ± 6.2(n=6) | −2.0 ± 4.8(n=6) | 1.5 [−5.3; 8.3]p=0.6349 | −1.9 [−7.2; 3.5]p=0.4606 | 5.0 [−2.2; 12.2]p=0.1509 |
| nAbs-Aβ: | 0.4 ± 1.4(n=6) | 1.1 ± 1.5(n=8) | 0.7 ± 2.3(n=6) | 0.1 ± 0.3(n=6) | −0.3 [−1.6; 1.1]p=0.6881 | −1.0 [−2.3; 0.4]p=0.1083 | −0.6 [−2.7; 1.5]p=0.5596 |
| CSF* [RTF] | |||||||
| Plasma [RTF] | 96.7 ± 133.7(n=6) | 256.6 ± 214.1(n=8) | 501.3 ± 514.6(n=6) | −56.3 ± 58.3(n=6) | 153.0 [−285.6; −20.4]p=0.0279 | −313.0 [−510.4; −115.6]p=0.0039 | −557.7 [−1028.8; −86.6]p=0.0450 |
| Total tau* [pg/mL] | −141.8 ± 419.5(n=6) | 1.3 ± 86.7(n=8) | 131.3 ± 301.0(n=6) | −33.2 ± 69.7(n=6) | 108.7 [−278.2; 495.5]p=0.5575 | −34.4 [−128.6; 59.8]p=0.4416 | −164.5 [−445.6; 116.6]p=0.2438 |
| P-Tau181* [pg/mL] | 3.3 ± 21.2(n=6) | −2.5 ± 8.6(n=8) | 10.2 ± 19.3(n=6) | −1.3 ± 10.1(n=6) | −4.7 [−26.0; 16.7]p=0.6370 | 1.2 [−9.7; 12.0]p=0.8192 | −11.5 [−31.3; 8.3]p=0.2243 |
| ADAS-cog | 5.33 [−4.7; 8.7](n=6) | 1.84 [−8.0; 24.0](n=8) | −1.50 [−4.3; 18.3](n=6) | 0.33 [−3.3; 5.0](n=5) | −3.84 [−9.34; 4.00]p=0.2353 | −0.34 [7.00; 5.67]p=0.8835 | 0.84 [−13.33; 7.33]p=0.6466 |
| MMSE | −3.0 [−8; 2](n=6) | −1.5 [−4; 1](n=8) | −1.5 [−7; 2](n=6) | −1.5 [−11; 4](n=6) | 2.0 [−6.0; 7.0]p=0.5725 | 0.5 [−5.0; 5.0]p=0.8453 | 0.0 [−6.0; 5.0]p=1.0000 |
| CDR-SOB | 0.50 [−1.0; 3.0](n=6) | 0.00 [−1.0; 5.0](n=8) | 0.25 [−1.5; 3.0](n=6) | −0.50 [−6.0; 0.0](n=5) | −1.50 [−6.50; 0.00]p=0.0641 | −0.50 [−6.00; 0.00]p=0.1879 | −1.25 [−5.50; 0.50]p=0.1961 |
| ADCS-ADL | −3.0 [−31; 11] (n=6) | 0.0 [−15; 11](n=8) | −1.5 [−5; 3](n=6) | −3.0 [−8; 7](n=5) | −1.0 [−13.0; −25.0]p=0.9273 | −4.5 [−14.0; 7.0]p=0.3387 | −1.5 [−8.0; 6.0]p=0.7144 |
| Whole brain volume | −1.4 ± 1.8(n=6) | −1.1 ± 1.0(n=8) | −1.6 ± 1.1(n=6) | −0.9 ± 0.8(n=4) | 0.5 [−1.7; 2.7]p=0.6196 | 0.2 [−1.1; 1.5]p=0.7094 | 0.7 [−0.7; 2.2]p=0.2851 |
| Hippocampal volume left | −191.2 ± 111.7(n=6) | −188.4 ± 228.2(n=8) | −153.5 ± 91.2(n=6) | −137.0 ± 114.8(n=5) | 54.2 [−100.8; 209.1]p=0.4494 | 51.4 [−193.0; 295.7]p=0.6526 | 16.5 [−123.7; 156.7]p=0.7961 |
| Hippocampal volume right | −140.8 ± 60.3(n=6) | −193.1 ± 137.7(n=8) | −230.5 ± 165.8(n=6) | −132.4 ± 122.1(n=5) | 8.4 [−118.9; 135.8]p=0.8842 | 60.7 [−105.2; 226.6]p=0.4375 | 98.1 [−104.6; 300.8]p=0.3021 |
Table 6.
Mean changes from baseline at week 24 in 2-week dosing interval (Intent-to-treat population; n=55).
| IVIG (n=20) | Placebo (n=7) | Placebo vs. 0.1 IVIG | Placebo vs. 0.25 IVIG | Placebo vs. 0.4 IVIG | |||
|---|---|---|---|---|---|---|---|
| 0.1 (n=6) | 0.25 (n=7) | 0.4 (n=7) | |||||
| Aβ1–40: | 266.7 ± 996.0(n=6) | 10.6 ± 1104.0(n=7) | −147.0 ± 1067.9(n=5) | 939.4 ± 1670.8(n=5) | 672.7 [−1160.8; 2506.3]p=0.4280 | 928.8 [844.7; 2702.3]p=0.2703 | 1086.4 [−958.5; 3131.3]p=0.2554 |
| CSF* [pg/mL] | |||||||
| Plasma [pg/mL] | 5.5 ± 64.5(n=6) | −0.3 ± 22.1(n=6) | −6.5 ± 61.4(n=6) | 27.1 ± 40.2(n=7) | 21.6 [−42.8; 86.1]p=0.4755 | 27.5 [−13.2; 68.1]p=0.1650 | 33.6 [−28.7; 96.0]p=0.2602 |
| Aβ1–42: | −8.7 ± 38.2(n=6) | 9.7 ± 54.7(n=7) | 4.0 ± 40.3(n=5) | 32.42 ± 67.0(n=5) | 41.1 [−31.5; 113.7]p=0.2327 | 22.7 [−55.9; 100.9]p=0.5325 | 28.4 [−52.2; 109.0]p=0.4403 |
| CSF* [pg/mL] | |||||||
| Plasma [pg/mL] | −2.3 ± 7.8(n=6) | −4.5 ± 6.3(n=6) | −2.7 ± 7.6(n=6) | 4.1 ± 3.8(n=7) | 6.5 [−0.8; 13.8]p=0.0759 | 8.6 [2.4; 14.8]p=0.0107 | 6.8 [−0.3; 13.9]p=0.0592 |
| nAbs-Aβ: | 0.2 ± 1.8(n=6) | 1.9 ± 1.9(n=7) | −0.8 ± 1.6(n=5) | 0.4 ± 3.7(n=5) | 0.2 [−3.7; 4.0]p=0.9271 | −1.5 [−5.1; 2.2]p=0.3861 | 1.2 [−3.0; 5.4]p=0.5378 |
| CSF* [RTF] | |||||||
| Plasma [RTF] | 74.3 ± 35.0(n=6) | 163.9 ± 136.6(n=7) | 310.2 ± 193.0(n=6) | 109.7 ± 284.8(n=7) | 35.4 [−223.8; 294.6]p=0.7553 | −54.1 [−314.3; 206.0]p=0.6583 | −200.5 [−503.3; 102.4]p=0.1732 |
| Total tau* [pg/mL] | 209.3 ± 416.0(n=6) | −3.4 ± 35.6(n=7) | −7.8 ± 51.3(n=5) | 17.4 ± 38.6(n=5) | −191.9 [−618.1; 234.3]p=0.3110 | 20.8 [−27.2; 68.9]p=0.3571 | 25.2 [−41.0; 91.4]p=0.4056 |
| P-Tau181* [pg/mL] | −3.2 ± 14.7(n=6) | 0.4 ± 3.6(n=7) | 2.6 ± 5.0(n=5) | 0.6 ± 7.5(n=5) | 3.8 [−12.7; 20.2]p=0.6172 | 0.2 [−7.0; 7.4]p=0.9586 | −2.0 [−11.3; 7.3]p=0.6328 |
| ADAS-cog | 2.50 [−3.7; 6.0](n=6) | −1.33 [−7.3; 4.0](n=7) | 4.50 [−4.0; 8.3](n=6) | −0.33 [−5.3; 5.0](n=7) | −3.00 [−7.00; 1.67]p=0.1979 | 2.00 [−4.00; 7.00]p=0.6540 | −4.34 [−10.66; 2.33]p=0.1004 |
| MMSE | −2.0 [−10; 2](n=6) | 0.0 [−3; 4](n=7) | −1.5 [−4; 1](n=6) | −1.0 [−5; 1](n=7) | 1.0 [−4.0; 7.0]p=0.6643 | −1.0 [−5.0; 2.0]p=0.4392 | 1.0 [−2.0; 4.0]p=0.6656 |
| CDR-SOB | 0.00 [−1.0; 5.0](n=6) | 0.50 [−2.0; 2.0](n=7) | 0.75 [−1.5; 4.0](n=6) | 0.00 [−2.5; 1.5](n=7) | −1.25 [−3.50; 0.50]p=0.4236 | −0.50 [−2.50; 1.00]p=0.4356 | −2.50 [−3.50; 0.00]p=0.0953 |
| ADCS-ADL | −0.5 [−11; 4](n=6) | −3.0 [−17; 3](n=7) | −4.0 [−25; 2](n=6) | 2.0 [−6; 10](n=7) | 3.0 [−3.0; 10.0]p=0.3153 | 5.0 [−1.0; 13.0]p=0.0839 | 6.5 [0.0; 18.0]p=0.0734 |
| Whole brain volume | −2.0 ± 0.8(n=5) | −1.5 ± 0.7(n=6) | −1.4 ± 1.7(n=5) | −1.4 ± 1.0(n=4) | 0.6 [−0.8; 2.0]p=0.3620 | 0.1 [−1.1; 1.3]p=0.8766 | −0.0 [−2.3; 2.2]p=0.9899 |
| Hippocampal volume left | −166.2 ± 64.0(n=6) | −183.5 ± 117.4(n=6) | −131.4 ± 98.0(n=5) | −216.5 ± 133.2(n=4) | −50.3 [−193.2; 92.6]p=0.4401 | −33.0 [−216.9; 150.9]p=0.6899 | −85.1 [−266.6; 96.4]p=0.3042 |
| Hippocampal volume right | −106.2 ± 112.7(n=6) | −182.2 ± 179.0(n=6) | −153.6 ± 226.0(n=5) | −198.3 ± 30.5(n=4) | −92.1 [−227.6; 43.4]p=0.1557 | −16.1 [−228.6; 194.4]p=0.8373 | −44.7 [−317.4; 228.1]p=0.6838 |
CSF biomarkers
At 24±8 hours after last infusion at either week 20 or week 22, changes from baseline in Aβ1–40, Aβ1–42, tau and p-tau181 concentrations were neither significant for any individual treatment arm (Table 5,6), nor for the IVIG-pooled group compared to the pooled placebo group (Table 6-SM).
Cognitive and functional outcomes
There were no changes in cognitive and functional scales in any of the individual treatment arms. In the combined analysis of all the IVIG arms, there was a significant difference in CDR-SOB in favour of the placebo (Table 6-SM; improvement of the placebo group). There were no significant differences between the individual treatment arms or the combined IVIG treatment group and placebo with respect to changes in ADAS-cog, MMSE, or ACDS-ADL at 24 weeks (Tables 5, 6, 6-SM). There was also no effect on the respective scales at week 12 (data not shown).
MRI
Neither the mean left and right hippocampal atrophy rates nor whole brain volume atrophy rates differed between either individual treatment arm or between the combined IVIG group and placebo over the course of the study (Tables 5, 6, 6-SM) There was also no effect on the atrophy rates between baseline and week 12 (data not shown).
FDG-PET
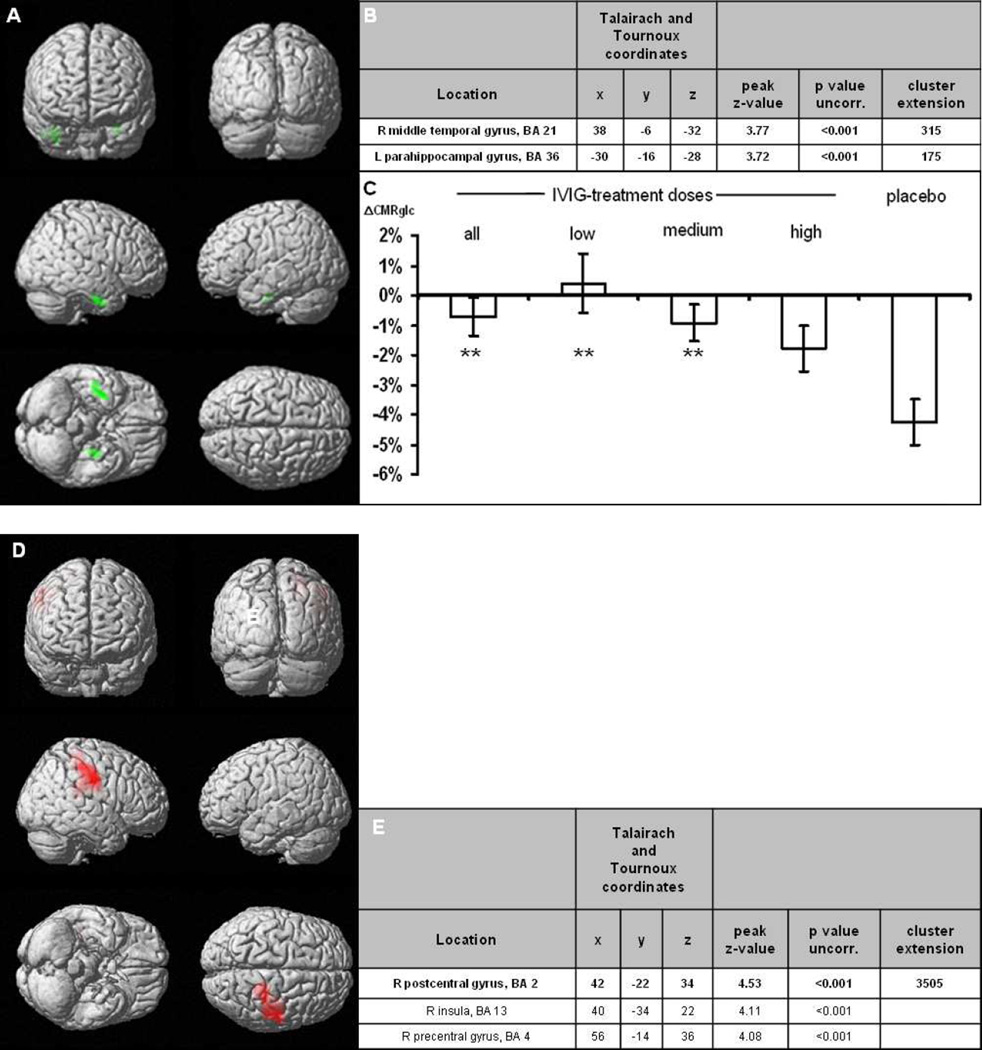
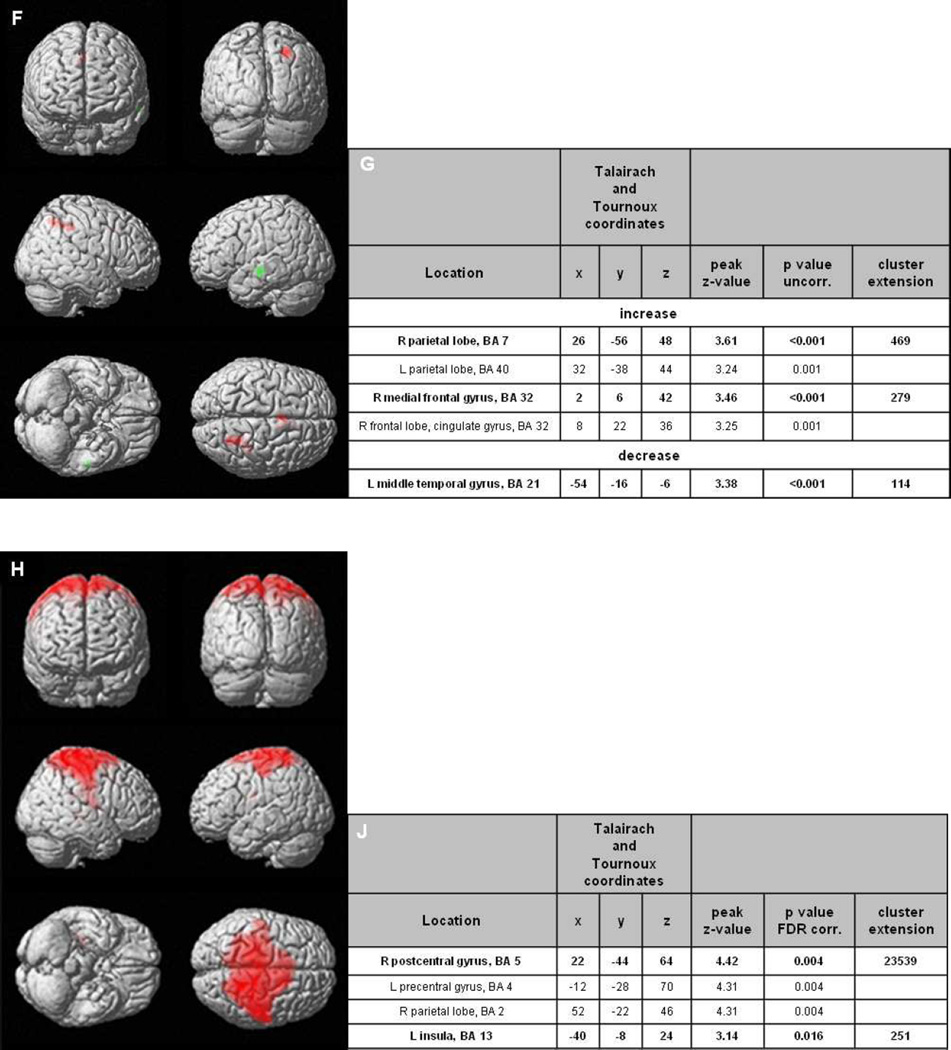
Voxel-wise analyses in the combined placebo arm revealed a decrease in glucose metabolism in the bilateral hippocampal/temporomesial brain regions within the 24 week follow-up period (Figure 2). Stereotactic coordinates including brain area, peak z-scores and cluster sizes are presented in Figure 2b. Within these clusters, a decrease of −4.3% in FDG uptake was calculated. In the same clusters, glucose metabolism was altered in patients receiving low-dose by +0.4%, medium-dose by −0.9%, and high-dose IVIG by −1.8%, respectively (Figure 2c). Across all subjects in the IVIG arms, glucose metabolism was significantly less reduced than in the placebo group (−0.7%, p<0.01). Furthermore, increases in metabolism were observed in the low and high dose group in cortical regions (Figures 2d–g). In the medium dose group, there was no increase, which reached the significance threshold of p<0.001, uncorrected. Across all IVIG groups, there was an increase in metabolism in parietal and frontal regions also after statistical correction for whole brain analysis (p<0.05, FDR-corr, Figures 2h–j). No increases were observed in the placebo group.
Figure 2.
Results of the FDG-PET A) The SPM(T) T-maps are illustrated as surface rendering projection showing significant differences follow-up versus baseline of the placebo-treated group, indicating significant decrease of glucose metabolism in green areas (paired _t_-test, p<0.001, uncorr.; n=8). B) Cluster specifications. C) Changes in CMRglc in these clusters for the different treatment arms as well as placebo (error bars are SEM); ** indicate p<0.01 versus placebo. D) SPM(T) T-maps showing significant differences follow-up versus baseline of low-dose treated patients § (paired _t_-test, p<0.001, uncorr.; n=12). E) Cluster specifications ¥. F) SPM(T) T-maps showing significant differences at follow-up versus baseline of high-dose treated patients, increases in red, decreases in green areas (paired t-test, p<0.001, uncorr.; n=10). G) Cluster specifications ¥. H) SPM(T) T-maps showing significant differences follow-up versus baseline of all IVIG-treated patients § (paired _t_-test, p<0.05, FDR-corr.; n=36) J) Cluster specifications ¥.
§ Significant increase of glucose metabolism in red areas. Cluster sizes of >50 voxels are displayed.
¥ Bold markings delineate a cluster, subsequent non-bold markings identify further peaks within the same cluster.
Adverse events
The safety analysis was based on 56 patients, of whom 42 patients were in the IVIG-group and 14 in the placebo group (Table 7). The number of individuals with one or more adverse events in the placebo group (n=9; 64.3%) and treatment group (n=25; 59.5%) did not differ (_p_=0.75). Similarly, the groups did not differ in the number of serious adverse events with a total of ten SAEs in eight patients (placebo group: n=4, 28.6%; IVIG: n=4, 9.5%). In the IVIG-group, one patient suffered an ischemic stroke that affected ACM territory. Otherwise, most adverse events in the IVIG-group were either mild or moderate in severity. No deaths were observed during the study period.
Table 7.
Individuals in each group experiencing an adverse event
| Symptom | IVIG (n=42) | Placebo (n=14) |
|---|---|---|
| Patients with at least one SAE [%] | 4 [9.5] | 4 [28.6] |
| Knee replacement surgery | 1 [7.1] | |
| Gastric antral vascular ectasia | 1 [7.1] | |
| Post surgery delirium and lumbar laminectomy | 1 [2.4] | |
| Stroke/middle cerebral artery infarct | 1 [2.4] | |
| Nausea and vomiting | 1 [2.4] | |
| Acute aggression | 1 [7.1] | |
| Possible seizure | 1 [7.1] | |
| Progressively severe Alzheimer’sdisease | 1 [2.4] | |
| Patients with at least one AE [%] | 25 [59.5] | 9 [64.3] |
| Microbleeds | 6 [14.3] | |
| Dizziness | 1 [7.1] | |
| Headache | 1 [2.4] | |
| Paraesthesia | 1 [7.1] | |
| Chills | 1 [2.4] | |
| Influenza-likei llness | 1 [2.4] | |
| Hypoaesthesia | 1 [2.4] | |
| Tremor | 1 [2.4] | |
| CSF white blood cell count increased | 1 [7.1] | |
| Muscle spasms | 1 [2.4] | |
| Atrial fibrillation | 1 [7.1] | |
| Procedural hypertension | 1 [2.4] | |
| Agitation | 1 [7.1] | |
| Actinickeratosis | 1 [2.4] | |
| Hyperkeratosis | 1 [2.4] | |
| Pruritus | 1 [2.4] | |
| Headache | 2 [4.8] | 1 [7.1] |
| Hypoaesthesia | 1 [2.4] | |
| Blood pressure fluctuation | 1 [2.4] | |
| Hypotension | 1 [2.4] | 1 [7.1] |
| AST increased | 1 [2.4] | |
| LDH increased | 1 [2.4] | |
| Hearing impaired | 1 [2.4] | |
| Dyspepsia | 1 [2.4] | |
| Post lumbar punctures yndrome | 1 [2.4] | |
| Haematuria | 1 [2.4] | |
| Infusion site extravasation | 1 [2.4] | |
| Fatigue | 1 [2.4] | |
| Pyrexia | 1 [2.4] | |
| Falls | 1 [2.4] |
Microbleeds
At screening, the MRIs of nine patients (1 in the placebo and 8 in the IVIG arms) showed microbleeds. The rate of incident microbleeds in subjects with microbleeds at baseline was 37.5% (in 3 patients of those 8 receiving IVIG). One microbleed was observed at week 12 and two at week 24. The incidence of microbleeds in subjects without microbleeds at baseline was 7.1% (3 patients out of 42 receiving IVIG). One case was observed at week 12. This subject showed 14 new microbleeds and was therefore taken out of the study. The other two patients were observed at week 24. No dosing effects were observed. There were no incident microbleeds in the placebo group. There were no clinical symptoms associated with microbleeds. We did not observe any changes in white matter (amyloid-related imaging abnormalities (ARIA-E) – such as vasogenic oedema or sulcal effusions).
Discussion
This study is currently the largest completed trial evaluating IVIG in AD patients. Our data suggested a very acceptable safety profile for IVIG in patients with mild-to-moderate AD, which is consistent with previous studies2, 5. We recorded no cases of aseptic meningitis, meningoencephalitis, or amyloid-related imaging abnormalities (vasogenic oedema and sulcal effusions, ARIA-E)13. We observed incident microbleeds on MRI in 37.5% and 7.1% of subjects either with or without baseline microbleeds on MRI at baseline (overall incidence 10.7%). Development of microbleeds was slightly higher than expected in AD14. One subject developed 14 microbleeds. In all cases, the microbleeds were asymptomatic. Incident microhaemorrhages and haemosiderin deposits (ARIA-H) have been reported in other immunisation trials in AD. The pooled data on Bapineuzumab revealed an overall incidence of 16%15. The clinically observed side-effects and systemic reactions were comparable to the known adverse events of IVIG-treatment. There was no difference between the IVIG and placebo groups in the rate of serious adverse events. No unexpected serious adverse events occurred. One 68-year old female patient in the high-dose IVIG-group with a medical history of Hashimoto’s thyroiditis and depression experienced a stroke during the trial. The risk for ischemic stroke is known to increase with IVIG-treatment.
We did not observe an effect of IVIG on the primary endpoint, except for the 0.4g/kg dose every two weeks resulting in a decreased AUC of plasma Aβ1–40. We defined AUC of plasma Aβ1–40 as primary endpoint based on the finding of increased plasma Aβ1–40 and reduced CSF Aβ1–40 from previous IVIG studies2, 5, which were interpreted as an indicator of the peripheral sink hypothesis. The secondary analyses of single time point changes of Aβ1–40 and of CSF Aβ1–40 also did not reveal any treatment effects. Thus, our analyses do not confirm the results from previous studies employing 1.2 g/kg or 0.4 and 0.8 g/kg IVIG monthly.
Similarly to the decrease in the AUC of Aβ1–40, we observed a decrease of the secondary endpoint AUC of plasma Aβ1–42 in the 2-week interval middle- and high-dosage arm and in the pooled analysis. The single point measure of plasma Aβ1–42 also revealed a significant decrease at week 24 in the pooled analyses, representing an opposite effect compared to the increase of Aβ1–40 in plasma that has been reported previously. This difference may be related to differences in study designs, such as inter-centre, pre-analytical variation16, different exposure to IVIG or other factors. In addition, the studied sample sizes are still limited. Currently, we cannot explain the differences between our study and the results of previous studies. The available knowledge on the Aβ exchange is still rather limited. Further studies with larger samples and longer exposure to IVIG will be needed before any decisive conclusions can be made regarding effects of IVIG on levels of Aβ1–40, Aβ1–42, total-tau, and p-tau181 in CSF/blood. Based on the data of this study, 0.25g/kg and 0.4g/kg administered at 2-week intervals may have the most pronounced effects on Aβ..
More importantly, our study showed a significant dose-dependent attenuation of decreased glucose metabolism in the temporal/hippocampal brain regions within 24 weeks of treatment. These brain regions are known to be affected in AD patients. Over time, progressive hypometabolism has been well documented in a number of brain regions, including the temporal lobe, the precuneus and cuneus, and the parietal, frontal, and occipital cortices17. Furthermore, IVIG-treated patients displayed significantly increased glucose metabolism in parietal and frontal brain regions at follow-up, even when applying a conservative threshold, whereas placebo-treated patients did not show any increase. However, it needs to be stressed that this increased metabolism is not localized in regions which are typically affected in FDG-PET studies in AD. Thus, the biological meaning of this finding is unclear. Our data suggest that IVIG may reduce the speed of metabolic decline in the MTL in AD, with low doses being more beneficial than high doses. Although these results are promising, they should be interpreted with caution given the limited number of patients in this study.
On the functional and neuropsychological scales, we found effects in favour of the placebo, reaching significance in the case of the CDR-SOB. The decline on the applied functional and cognitive instruments across all the IVIG groups was similar to the decline, which was observed in the natural course of AD and in placebo groups in mild to moderate AD trials18–21. The placebo group in this study showed less decline and even improvement on the CDR-SOB, which may have occurred by chance in this particular group. We conclude that there is most likely no symptomatic effect of IVIG. To detect the effects on slope of decline, longer studies are required.
There are limitations of the study, despite its careful study design and execution. The small size in each treatment group with large variations and heterogeneity in disease trajectories limits the likelihood to achieve clinically significant data favouring one dosage over another. Extrapolation of our findings to other patients’ groups is limited by the small sample size in each group (especially in the placebo groups) and by the inclusion/exclusion criteria applied. In addition, patients were exposed to IVIG only for 6 months, which prevents detection of a disease-modifying effect. Finally, as in other studies with anti-amyloid agents, our study population might have been too advanced in course of AD to detect an effect. Application at an earlier disease stage might be more beneficial, particularly with regard to the clinical outcomes. Although different dosages and intervals have been used in this and previous small trials and are used in an ongoing large trial (ranging from 0.1 to 0.4 g/kg bi-weekly or 0.2 to 1.2 g/kg 4-weekly) it is currently not yet possible to conclude whether higher or more frequent dosages may be needed for achieving substantial effects. Lastly, it cannot be ruled out that IVIG treatment might be not effective in AD patients.”
In conclusion, this phase II trial showed favourable safety and tolerability results and the absence of severe autoimmune reactions. Longer studies with larger study populations are required to assess potential effects on the slope of cognitive and functional decline in patients with Alzheimer’s disease.
Supplementary Material
01
Research in context.
Systematic review
Studies were identified by searches of Medline (2000–2012) and the Cochrane Central Register of controlled trials (trials published up to 2012) with the search terms “Alzheimer’s disease” and “immunotherapy”, “immunoglobulins”. Searches were restricted to human studies. All types of trial design were included. We identified two systematic reviews evaluating immunotherapeutic approaches in clinical development for AD that evaluate trials with active and passive immunisation targeting Aβ. There are currently 6 active and 8 passive immunotherapeutic trials that target Aβ in different stages of development. These trials include ACC-001, CAD-106, ACI-24, UB-311, V950 and Affitopes AD-01/AD-02 investigating active immunotherapy and Bapineuzumab, GSK933776A, Solanezumab, Ponezumab, Gantenerumab, MABT-5102A, BAN-2401 and immunoglobulins as passive immunotherapeutic approaches. Most recently, two large clinical trials, Bapineuzumab and Solanezumab which target the N-terminal and mid-terminal sequence of Aβ, respectively, were found to be negative, with Solanezumab seemingly having a small effect in the mild AD patients’ group. Complete results from these trials are not yet available. With regard to using immunoglobulins to treat AD, two small pilot trials have been published showing an effect on the drain of Aβ from the CSF to the periphery. A large phase III study with immunoglobulins in mild-to moderate AD patients lasting 18 months is currently underway in the USA and a second one planned to start in 2013.
Interpretation
Passive immunisation targeting Aβ is a potentially therapeutic approach: however, several issues must be addressed such as the disease stage at which immunotherapy should be initiated, the role of biomarkers and whether they translate into the clinical course. Further clinical trials of sufficient length and power to assess cognitive and functional outcomes are needed to establish the efficacy of passive immunisation and immunoglobulins for patients with AD.
Acknowledgments
Funding: Octapharma AG, Switzerland.
Richard Dodel received consulting fees or honorarium, support for travel to meetings for the study and fees for participation in review activities from Octapharma. Investigator fees per patient enrolled were paid to his institution by Octapharma. Outside the submitted work he served as a consultant for Eli Lilly, AstraZeneca, Merz, TEVA/Lundbeck, Pfizer and Baxter and served as a board member for Solvay, Affiris, GE Healthcare, Baxter, Lilly. He was a Consultant for Octapharma, TEVA/Lundbeck, Pfizer and Baxter He was paid for lectures from Boehringer Ingelheim, Novartis, Pfizer, Baxter, GSK, Lundbeck, Merz, Solvay, Eisai, Octapharma, Orion Pharma, UCB, CSL Behring, TevaPharma and Canadian Blood Service He received grants from ZLB Behring, Behring-Röntgen-Stiftung, M.J. Fox Foundation, Rentschler, International Parkinson Fond, Faber Stiftung, Movement Disorder Society, Novartis, Hector-Stiftung, Alzheimer Forschung Initiative, DGSM, Lundbeck, Abbott, Baxter. He holds patents on the topic. Axel Rominger received fees for participation in review activities from Octapharma AG paid to his institution. Outside the submitted work he serves as a consultant for Bayer Healthcare and GE. Peter Bartenstein received fees for participation in review activities from Octapharma AG paid to his institution. Outside the submitted work he is a consultant for GE. He is also a consultant for Bayer Healthcare and Siemens with money paid to his institution. He received a grant from Siemens paid to his institution. Frederik Barkhof was paid Octapharma to perform the MRI analysis for this study. Outside the submitted work he is member of the board for Brain, Eur Radiology, Neuroradiology, Multiple Sclerosis, J Neurol with no money paid. He serves as a consultant for Roche, Baxter and Sanofi-Aventis with money paid to himself. He serves as a consultant for Biogen-Idec, Merck-Serono, Novartis, Bayer-Schering, Roche, Synthon BV, Jansen Research and TEVA with money paid to himself and his institution. Kai Blennow received consulting fee or honorarium from Octapharma for attending the Advisory Board in 2011. He received payment from Octapharma for biomarker analyses on CSF and plasma samples paid to his institution Sahlgrenska University Hospital. Outside the submitted work he has served on Advisory Board for Pfizer, Roche, Lilly and Innogenetics and has previously been a Consultant for AstraZeneca. Stefan Förster received fees for participation in review activities from Octapharma paid to his institution. Outside the submitted work he serves as a consultant for Bayer Healthcare, GE and Merck. For his consultancy with Bayer Healthcare and Merck, money was also payed to his institution. Yaroslav Winter had no financial support from Octapharma during the study. Outside the submitted work he is a board member with UCB and received a grant from the Behring-Röntgen Foundation paid to his institution. Jan-Philipp Bach received a grant from Octapharma paid to his institution. Outside the submitted work he received grants from Baxter and Grifols with money paid to the institution. He received payment for a lecture from Teva Pharmaceuticals and travel expenses from Baxter in 2011. Julius Popp had no financial support from Octapharma during the study. He has no relevant financial activities outside the submitted work.
Judith Alferink had no financial support from Octapharma during the study.. She has no relevant financial activities outside the submitted work. Jens Wiltfang reports no disclosures. Katharina Buerger received support for travel and registration to the International Conference of Alzheimer’s Disease in 2009 where a study meeting was held by Octapharma. She received a fee for study performance at the clinical site by Octapharma paid to her institution. Outside the submitted work she received a grant from the DZNE Germany (governmental) paid to her institution. Markus Otto received a grant from Octagam to include patients into the study paid to his institution. Outside the submitted work he is a board member for UCB. He received a grant from Boehringer Ingelheim paid to his institution. He received payment for lectures from Fisher scientific, Beckman Coulter and Teva. He holds a patent regarding CSF markers for Parkinson’s disease dementia with the foundation of the state Baden-Wuerttemberg with money paid to his institution. Piero Antuono had no financial support from Octapharma during the study. He has no relevant financial activities outside the submitted work. Michael Jacoby received a fee for study performance at the clinical site by Octapharma paid to his institution. Outside the submitted work he has no relevant financial activities outside the submitted work. Ralph Richter received a grant for the work under consideration paid to her institution Tulsa Clinical Research LLC. Outside the submitted work he received a grant paid to his institution. James Stevens had no financial support from Octapharma during the study. Outside the submitted work he is a board member with Biogen-Idec, Teva Neuroscience and Genzyme. He received grants from Biogen-Idec, Teva Neuroscience, Sanofi-Aventis and Octapharma paid to his institution. Isaac Melamed had no financial support from Octapharma during the study. He has no relevant financial activities outside the submitted work. Jerome Goldstein received a grant from Octapharma for study subjects paid to his institution San Francisco Clinical Research Center. He received travel support from Octapharma for a poster presentation attending an international Alzheimer meeting in 2010. He has no relevant financial activities outside the submitted work. Martin Farlow received consulting fee or honorarium and support for travel to meetings for the study from Octapharma. He received a grant from Octapharma paid to his institution. Outside the submitted work he serves as a Consultant for Accera, Alltech, Astellas, Avanir, Bayer, Bristol MyersSquibb, Eisai, GE, Helicon, Medavante, Medivation, Merck, Novartis, Pfizer, Prana Biotech, QR Pharma, Roche, Sanofi Aventis, Schering-Plough, Toyama, Eli Lilly, Shire Pharmaceuticals and UCB Pharma. He received grants from Eli Lilly, Novartis, Genentech, Roche and Eisai paid to his institution. He received payment for lectures from Novartis, Pfizer, Forest and Eisai. Frank Jessen received consulting fee or honorarium for a consultation on the study design, support for travel to meetings for the study and support for manuscript writing (non-financial) from Octapharma. He also received case payments for participation in the study from Octapharma paid to his institution. Outside the submitted work he is a board member with AC Immune and serves as a consultant for Lilly, GE Healthcare, Janssen, USB and Schwabe. He received a grant from Schwabe paid to his institution. He was paid for lectures from Pfizer, Novartis, Schwabe, Lilly and GE Healthcare.
We thank the patients and all of the investigators who took part in this study. We also thank members of the data monitoring committee: Prof. Dr. Bengt Winblad, MD (DMC Chairman), Karolinska Institute, KI Alzheimer Disease Research Center. Stockholm, Sweden; Prof. Dr. Joachim Kalden, MD, Dept. of Molecular Immunology, Nikolaus-Fiebiger-Center, Erlangen, Germany; Deborah Kado, MD, MS, MacDonald Research Laboratory, Los Angeles, CA, USA. We thank Dr. Christian Zach (Dept. of Nuclear Medicine LMU Munich) for their contribution to PET studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Prof. Richard Dodel and Prof. Frank Jessen wrote the draft of the manuscript, which was reviewed by Prof. Martin Farlow and Dr. Stefan Wietek.
Prof. Frederik Barkhof wrote the section(s) on MRI.
Prof. Peter Bartenstein, Dr. Axel Rominger, Dr. Stefan Förster wrote the sections on FDG-PET.
Prof. Kai Blennow wrote the section(s) on biomarkers.
The final article was send to all of the co-authors and critically reviewed by all co-authors. The following authors contributed to data collection: Judith Alferink, Piero Antuono, Kaj Blennow (CSF biomarkers), Katharina Bürger, Jan-Philipp Bach, Richard Dodel, Martin Farlow, Jerome Goldstein, Michael Jacoby, Frank Jessen, Isaac Melamed, Markus Otto, Julius Popp, Ralph Richter, James Stevens, Jens Wiltfang, Yaroslav Winter.
Conflicts of interest:
Stefan Haag was an employee of Octapharma AG and has no other conflict of interests to report. Stefan Wietek is employed as Clinical Project Manager at Octapharma PPG (Vienna, Austria) and has no other conflict of interests to report.
References
- 1.Mangialasche F, Solomon A, Winblad B, et al. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 2.Dodel RC, Du Y, Depboylu C, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:1472–1474. doi: 10.1136/jnnp.2003.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Y, Dodel R, Hampel H, et al. Reduced levels of amyloid beta-peptide antibody in Alzheimer disease. Neurology. 2001;57:801–805. doi: 10.1212/wnl.57.5.801. [DOI] [PubMed] [Google Scholar]
- 4.Relkin NR. Current state of immunotherapy for Alzheimer's disease. CNS Spectr. 2008;13:39–41. doi: 10.1017/s1092852900027061. [DOI] [PubMed] [Google Scholar]
- 5.Relkin NR, Szabo P, Adamiak B, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–1736. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Gold R, Stangel M, Dalakas MC. Drug Insight: the use of intravenous immunoglobulin in neurology--therapeutic considerations and practical issues. Nat Clin Pract Neurol. 2007;3:36–44. doi: 10.1038/ncpneuro0376. [DOI] [PubMed] [Google Scholar]
- 7.Weksler ME, Relkin N, Turkenich R, et al. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol. 2002;37:943–948. doi: 10.1016/s0531-5565(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 8.Weksler M, Szabo P, Relkin N. IVIG Therapy of mild to moderate Alzheimer's disease patients showed significant benefits as measured by neuroimaging and neuropsychological testing in a phase II, randomized, double blind placebo controlled clinical study. Gerontologist. 2010;50:449–450. [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.DeMattos RB, Bales KR, Cummins DJ, et al. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 12.Gabrielsson J, Weiner D. Pharmacokinetic & Pharmacodynamic Data Analysis. Concepts and Applications. Stockholm: Swedish Pharmaceutical Press; 2007. [Google Scholar]
- 13.Sperling RA, Jack CR, Jr, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Vlies AE, Goos JD, Barkhof F, et al. Microbleeds do not affect rate of cognitive decline in Alzheimer disease. Neurology. 2012;79:763–769. doi: 10.1212/WNL.0b013e3182661f91. [DOI] [PubMed] [Google Scholar]
- 15.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson N, Blennow K, Zetterberg H. Inter-laboratory variation in cerebrospinal fluid biomarkers for Alzheimer's disease: united we stand, divided we fall. Clin Chem Lab Med. 2010;48:603–607. doi: 10.1515/CCLM.2010.131. [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Langbaum JB, Fleisher AS, et al. Twelve-month metabolic declines in probable Alzheimer's disease and amnestic mild cognitive impairment assessed using an empirically predefined statistical region-of-interest: findings from the Alzheimer's Disease Neuroimaging Initiative. Neuroimage. 2010;51:654–664. doi: 10.1016/j.neuroimage.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tschanz JT, Corcoran CD, Schwartz S, et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams MM, Storandt M, Roe CM, et al. Progression of Alzheimer's disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han L, Cole M, Bellavance F, et al. Tracking cognitive decline in Alzheimer's disease using the mini-mental state examination: a meta-analysis. Int Psychogeriatr. 2000;12:231–247. doi: 10.1017/s1041610200006359. [DOI] [PubMed] [Google Scholar]
- 21.Ito K, Corrigan B, Zhao Q, et al. Disease progression model for cognitive deterioration from Alzheimer's Disease Neuroimaging Initiative database. Alzheimers Dement. 2011;7:151–160. doi: 10.1016/j.jalz.2010.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01