Secondary solid cancer screening following hematopoietic cell transplantation (original) (raw)
. Author manuscript; available in PMC: 2016 Aug 18.
Published in final edited form as: Bone Marrow Transplant. 2015 Mar 30;50(8):1013–1023. doi: 10.1038/bmt.2015.63
Abstract
Hematopoietic stem cell transplant (HCT) recipients have a substantial risk of developing secondary solid cancers, particularly beyond 5 years after HCT and without reaching a plateau overtime. A working group was established through the Center for International Blood and Marrow Transplant Research and the European Group for Blood and Marrow Transplantation with the goal to facilitate implementation of cancer screening appropriate to HCT recipients. The working group reviewed guidelines and methods for cancer screening applicable to the general population and reviewed the incidence and risk factors for secondary cancers after HCT. A consensus approach was used to establish recommendations for individual secondary cancers. The most common sites include oral cavity, skin, breast and thyroid. Risks of cancers are increased after HCT compared with the general population in skin, thyroid, oral cavity, esophagus, liver, nervous system, bone and connective tissues. Myeloablative TBI, young age at HCT, chronic GVHD and prolonged immunosuppressive treatment beyond 24 months were well-documented risk factors for many types of secondary cancers. All HCT recipients should be advised of the risks of secondary cancers annually and encouraged to undergo recommended screening based on their predisposition. Here we propose guidelines to help clinicians in providing screening and preventive care for secondary cancers among HCT recipients.
INTRODUCTION
Advances in hematopoietic cell transplantation (HCT) and supportive care measures have led to substantial improvements in long-term survival for HCT recipients.1 Transplant survivors are at considerable risk for developing significant late effects, and general recommendations regarding the multi-faceted approach required in their evaluation and treatment have been recently published.2 One particular challenge faced in the post-HCT setting is the risk of development of secondary malignancies. There is an increased risk of secondary malignancies following both autologous and allogeneic HCT, which may be further impacted by pretransplant therapies. Secondary malignancies can be grouped into post-transplant lymphoproliferative diseases, leukemia or myelodysplasia and solid cancers. The incidence of secondary solid malignancies can continue to rise over time and studies with follow-up to 20 years have not shown a plateau in their occurence.3–5
Several organizations (for example, National Comprehensive Cancer Network (NCCN), American Cancer Society (ACS), United States Preventative Services Task Force) have developed standardized cancer screening methods that are currently implemented as part of a routine general health evaluation for certain malignancies in the general population, but application of these guidelines to HCT recipients have not been reviewed. In acknowledging the increased risk of secondary solid cancers among HCT recipients, a working group was established through the Center for International Blood and Marrow Transplant Research Late Effects and Quality of Life Working Committee and the European Group for Blood and Marrow Transplantation Complications and Quality of Life Working Party with the goal to facilitate implementation of cancer screening appropriate to HCT recipients. As a clinical output of this working group’s effort, we offer consensus-based recommendations applicable for screening and prevention of individual secondary solid cancers among HCT recipients.
METHODS
The goals of this document were to provide an expert review of existing, evidence-based, cancer screening guidelines applicable to the general population and adopt them to the post-HCT setting. In order to integrate the incidence and risk factors of individual secondary cancers after HCT, an incidence rate and a standardized incidence ratio (SIR) (the ratio of observed cancer cases in a HCT cohort to expected cancer cases in the general population of similar age and gender) were summarized from existing literatures (Table 1) and reported risk factors for secondary cancer among HCT recipients were reviewed (Table 2). Cancer screening guidelines for the general population were primarily based on those established by ACS or NCCN, unless otherwise specified (Table 3). Details of screening guidelines and methods in the general population are summarized in the Supplementary Appendix. Adult and pediatric HCT recipients were considered in the development of these guidelines.
Table 1.
Incidence rate of secondary cancers according to time after transplantation
| Site | Incidence rate per 100 000 person-years according to time after transplantation | SIR | Reference | ||||
|---|---|---|---|---|---|---|---|
| < 1 years | 1–4 years | 5–9 years | ⩾ 10 years | Total | |||
| Any skin | 16 | 20 | 16 | 28 | 19a | 7.2 | 19 |
| Melanoma | 0–21 | 10–31 | 10–30 | 50–58 | 18–76 | 1.4–8.3 | 3, 5, 16, 17 |
| Thyroid | 8–16 | 9–10 | 20–24 | 83–170 | 6–34 | 5.8–6.6b | 5, 16, 19, 32 |
| Female breast | 0–8 | 10–51 | 5–60 | 0–83 | 10–41 | 0.3–2.0 | 3, 5, 16, 17, 19, 31 |
| Lung | 5–57 | 4–40 | 0–48 | 0–150 | 4–50 | 0.7–2.6c | 3, 5, 16, 17, 19 |
| Mouth/pharynx | 0 | 10–52 | 76–140 | 130–290 | 32–92 | 7.0–16 | 5, 16, 19 |
| Lip | 0 | 4–30 | 10–15 | 0–33 | 4–18 | 19–27 | 3, 5, 16 |
| Tongue | 0 | 3–20 | 25–30 | 0–170 | 12–18 | 9.3–20 | 3, 5, 16 |
| Oral cavity | 0 | 0 | 30 | 50 | 14–100 | 7.3–17 | 3, 17, 31 |
| Salivary gland | 0 | 0 | 20–36 | 0 | 5–7 | 14–23 | 3, 16 |
| Gum or other | 0 | 3–4 | 10–24 | 33–120 | 6–11 | 5.1–13 | 3, 16 |
| Pharynx | 0 | 0 | 10–15 | 0 | 2 | 1.2–5.4d | 3, 5, 16 |
| Esophagus | 0 | 20–43 | 60–90 | 0–150 | 4–59 | 8.5–11e | 3, 5, 19 |
| Stomach | 16 | 23 | 32 | 14 | 23a | 0.6 | 19 |
| Colon | 0 | 0–5 | 0–15 | 0–100 | 2–39 | 0.5–1.2f | 3, 5, 16, 17, 19 |
| Rectum | 0 | 3–7 | 0 | 17–69 | 4–9 | 0.6–2.2 | 5, 16, 19 |
| Liver | 5–8 | 4–5 | 0–5 | 50–58 | 7–34 | 6.3–28b | 5, 16, 19, 31 |
| Gallbladder | 16 | 7 | 11 | 0 | 9a | 2.6 | 19 |
| Pancreas | 0 | 7 | 32 | 14 | 13a | 1.9 | 19 |
| Adrenal gland | 29 | 0 | 0 | 0 | 5 | 23.2 | 3 |
| Kidney | 0–29 | 0–3 | 0–5 | 0 | 3–10 | 0.5–2.5 | 3, 17, 19 |
| Bladder | 8–29 | 0–10 | 0 | 0 | 5–6 | 0.8–1.4 | 3, 19 |
| Cervix | 0–29 | 4–13 | 0–15 | 0–17 | 2–67 | 0.7–2.3g | 3, 5, 16, 19 |
| Endometrium | 0–16 | 0–3 | 10–12 | 0 | 2–7 | 1.1–1.4 | 5, 16, 19 |
| Ovary | 0 | 3 | 5 | 14 | 4a | 0.8 | 19 |
| Prostate | 0–8 | 0–10 | 0–5 | 14–50 | 4–10 | 0.5–0.7 | 3, 17, 19 |
| Testis | 0 | 0–9 | 0–15 | 0–17 | 4–5 | 0.9–1.2 | 3, 5, 16 |
| Brain/nervous system | 0–16 | 3–20 | 5–84 | 0–55 | 10–38 | 3.8–9.5 | 3, 5, 16, 17, 19 |
| Bone | 0 | 7–13 | 10–12 | 17–58 | 7–11 | 8.5–13 | 3,16 |
| Connective tissue | 5–29 | 5–10 | 15–20 | 0 | 8–14 | 6.5–8.0 | 3, 5, 16 |
Table 2.
Reported risk factors for individual secondary cancers among HCT population
| Site | Risk factor |
|---|---|
| Skin | Chronic GVHD19 |
| SCC | Acute GVHD,20 chronic GVHD,4,5,20 male,5 age < 18years at transplantation20 |
| BCC | Age < 18 years at transplantation,20 myeloablativeTBI conditioning,20,22 white,20,22 chronic GVHD,19,20attained age22 |
| Melanoma | Myeloablative TBI conditioning,5,16 T-cell depletion,5female5 |
| Thyroid | Radiation conditioning,5,32 female,32 age ⩽ 20 yearsat transplantation,32 chronic GVHD32 |
| Oral | Persistent chronic GVHD,4,5,16,18–20,36–38 cumulativeduration of immunosuppressive therapy, includingprophylaxis > 24 months,4 history of localized fieldirradiation,5,16 ages < 10 y.o. at the time of transplant,5male gender5,16 |
| Esophagus | Persistent chronic GVHD,4,19 prolongedimmunosuppressive therapy > 24 months4 |
| Stomach | None reported |
| Colorectal | None reported |
| Liver | TBI-based conditioning, younger age (< 34 years) atHCT, liver cirrhosis, chronic hepatitis C infection54 |
| Lung | Tobacco use prior to transplantation3 |
| Breast | History of myeloablative TBI or radiation treatment,5,74longer time since HCT,5,16,39,74 age < 18 years atHCT,17,39,74 use of growth factors,3 antithymocyteglobulin5 |
| Cervix | Chronic GVHD with systemic immunosuppressivetherapy > 3 years,83 age > 34 years31 |
| Endometrial | None reported |
| Ovary | None reported |
| Prostate | None reported |
| Testis | None reported |
| Brain/CNS | None reported, prior history of CNS irradiation mayincrease risk.115 |
| Sarcoma | None reported |
Table 3.
Cancer screening guidelines for the general and HCT populations
| Site | American Cancer Society (ACS) | National Comprehensive Cancer Network(NCCN) | Consensus recommendationsfor post-HCT screening |
|---|---|---|---|
| Skin | General cancer-related checkupfor men and women ⩾ 20 yearsa | No specific guidelines | Routine skin examination in alltransplant survivors, particularly forpatients who had myeloablative TBI,HCT at ages < 18 years or GVHD |
| Thyroid | General cancer-related checkupfor men and women ⩾ 20 yearsa | No specific guidelines | Annual physical examination.Heightened awareness for patients aged⩽ 20 years at HCT, female patients, thosereceiving TBI-containing conditioningregimens and those who developchronic GVHD |
| Oropharyngeal | General cancer-related checkupfor men and women ⩾ 20 yearsa | No specific guidelines | Screening every 12 months. Screeningevery 6 months may be considered forpatients with risk factors summarized inTable 2 |
| Esophagus | No specific guidelines | Surveillance upper endoscopies withbiopsies for patients with certainhereditary predisposition cancersyndromes (for example, Familial Barrett’s,Bloom syndrome and Fanconi anemia) | Upper GI endoscopy for patients whohave persistent GERD symptoms ordysphagia.Endoscopic screening may beconsidered for patients with prolongedimmunosuppressive treatment(> 24 months) for chronic GVHD |
| Stomach | No specific guidelines | No specific guidelines | Follow guidelines for the generalpopulation |
| Colorectal | Starting at ⩾ 50 years for average risk.Sigmoidoscopy: every 5 years.Colonoscopy: every 10 years.Barium enema: every 5 years.CT colonography: every 5 years.More frequent screening guidelinesavailable for those at increased risk | Starting at ⩾ 50 years for those withaverage risk.Sigmoidoscopy: every 5 years with orwithout stool testing at year 3.bColonoscopy: every 10 years.cGuaiac-based or immunochemical-basedstool testing (multitarget stool DNAtesting: Cologuard52): annually.dMore frequent screening guidelinesavailable for those at increased risk | Follow guidelines for the generalpopulation |
| Liver | No screening recommendations for thoseat low risk.For those at risk for hepatocellularcarcinoma, including those with cirrhosisor chronic hepatitis B, consider alfa-fetoprotein (AFP) and ultrasoundevery 6–12 months | No screening recommendations for thoseat low risk, without cirrhosis or chronichepatitis.For those at risk for hepatocellularcarcinoma, including those with cirrhosisor chronic hepatitis B, consider AFP andultrasound every 6–12 months | Follow guidelines for the generalpopulations.Consider liver ultrasound every6 months in HBV seropositive recipientsor in patients with history of cirrhosis |
| Lung | Consider screening in patients aged 55–74years who have at least a 30 pack-yearsmoking history and who currently smokeor have quit within the past 15 years.Discuss risk/benefits prior to any screeningfor lung cancer with low-dose CT scan.Encourage smoking cessation | Screening with low-dose CTrecommended for two high-risk groups: > 55 years and ⩾ 30 pack-year smoking history (those who quit smoking > 15 years ago are excluded) ⩾ 50 and have ⩾ 20 pack-year smoking history with one additional risk factor (for example, radon, asbestos, family history of lung cancer, second-hand smoke exposure) | Follow guidelines for the generalpopulation.Encourage smoking cessation.Consider screening if there areadditional risk factors (for example,smoking history) |
| Breast | Average risk: age 20–40 years: -Clinical breast exam every 3 years age > 40 years: -Annual mammography and clinical breast exam. High risk,e including women treated forHodgkin lymphoma:Annual mammogram and MRI starting at age 30 years | Breast awareness for all risk groups whichcan be attained by periodic, consistentbreast self-exams.Average risk: age 25–40 years: -Clinical breast exam every 1–3 years age > 40 years -Annual clinical breast exam -Annual mammogram _I_ncreased risk,g including prior thoracicradiation therapy between 10 and 30 years(for example, mantle): age < 25 years: -Annual clinical breast exam from 8 to 10 years after radiation therapy age ⩾ 25 years: -Annual mammogram + clinical breast exam every 6–12 months from 8 to 10 years after radiation or age 40 years, whichever comes first + annual breast MRI | Breast awareness for all patientsAverage risk: age 20–40 years: -Clinical breast exam every 1–3 years age > 40 years: -Annual clinical breast exam -Annual mammogram Prior radiation therapy or TBI: age 25 years or 8 years after radiation therapy/TBI, whichever comes first, but no later than age 40 years: -Annual clinical breast exam -Annual mammogram -Annual breast MRI |
| Cervix | Pap test is recommended for women aged21–29 years every 3 years. Both Pap testand HPV DNA test are recommended forwomen aged 30–65 years every 5 years.Annual screening is recommended forimmunocompromised women | Pap test is recommended for women aged21–29 years every 3 years. Both Pap testand HPV DNA test are recommended forwomen aged 30–65 years every 5 years.Annual screening is recommended forimmunocompromised women | Annual Pap test and HPV DNA test |
| Endometrial | Information about the risks (for example,replacement of estrogen alone) andsymptoms (unexpected bleeding orspotting) of endometrial cancers shouldbe provided to women at average risk atthe time of menopause | No specific guidelines | Follow guidelines for the generalpopulation |
| Ovary | No recommended specific screeningguidelines in average-risk women. Generalcancer-related checkup for men andwomen ⩾ 20 years.aIn those with a hereditary bilateral ovariancancer syndrome (BRCA1/2 mutation orhistory of Lynch syndrome), do annualpelvic exam, CA-125 and transvaginalultrasounds until childbearing is completeor until age 35 years, at which prophylacticbilateral oophorectomy is recommendedf | No recommended specific screeningguidelines in average-risk women. Generalcancer-related checkup for men andwomen ⩾ 20 years.aIn those with a hereditary bilateral ovariancancer syndrome (BRCA1/2 mutation orhistory), consider screening every6 months with CA-125 and transvaginalultrasound starting at age 30 years or 5–10years earlier than the earliest diagnosis ofovarian cancer in the familyf | Follow guidelines for the generalpopulation |
| Prostate | PSA ± digital rectal examination afterinformed decision-making | PSA ± digital rectal examination afterinformed decision-making | Follow guidelines for the generalpopulation |
| Testis | General cancer-related checkup for men⩾ 20 yearsa | No specific guidelines | Follow guidelines for the generalpopulation |
| Brain/CNS | No specific guidelines | No specific guidelines | No specific guidelines |
| Sarcoma | No specific guidelines | No specific guidelines | No specific guidelines |
Skin cancer
Overview
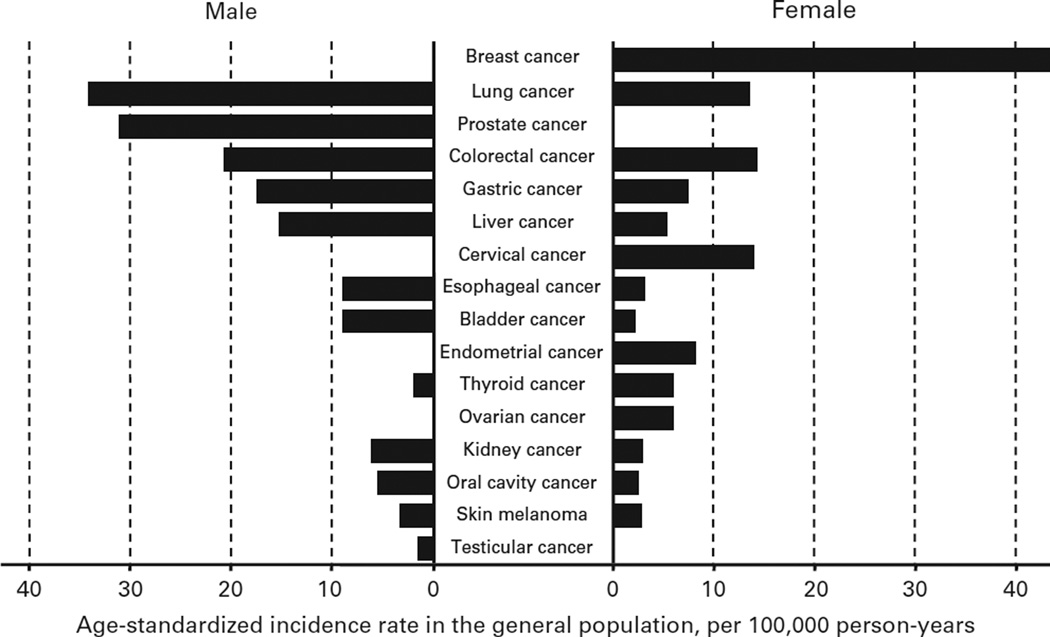
There are three main types of skin cancer, including basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and melanoma. Age-standardized incidence rates of melanoma in men and women were 3.3 and 2.8, respectively, per 100 000 person-years worldwide in 2012 (Figure 1).6 Age-standardized incidence rates of other skin cancers have not been reported. Incidence varies 100-fold between countries and ethnicities and is the highest in Caucasians. BCC and SCC are the most common and have better prognosis than other skin cancers in the general population. Actinic keratoses have been considered as direct precursors of both BCC and SCC, and the annual rate of transformation is estimated as 0.03–20%.7 Exposure to UV radiation is a well-known risk factor for melanoma and non-melanoma skin cancers.8,9 The risk of developing skin cancers is incredibly high after solid organ transplantation owing to the chronic use of potent immunosuppression.10
Figure 1.
Age-standardized cancer incidence rate in the general population. Data were based on Cancer Incidence in Five Continents Time Trends in 2012.85
Incidence and risk factors after transplantation
The reported incidence rates of skin cancers after HCT were 105 per 100 000 person-years for BCC, 76 for SCC and 18–76 for melanoma.3,5,11,12 Reports describing skin cancer following HCT are dominated by case reports, but at least five large studies have reported an increased incidence of melanoma after allogeneic HCT compared with the general population (SIR 1.4–8.3).3,5,11–13 The risk of developing melanoma was also increased compared with the general population after HCT with reduced-intensity conditioning (RIC) regimens (SIR 3.04 for leukemia or myelodysplastic syndrome patients and SIR 3.52 for lymphoma patients).13 An Asian study reported an increased incidence of skin cancers without distinguishing cancer subtypes after allogeneic HCT (SIR 7.2).14 One study reported that the cumulative incidences of BCC and SCC were 6.5% and 3.4% at 20 years after allogeneic HCT in a predominantly Caucasian cohort.15 Although it is rare, merkel cell carcinoma can occur after HCT,16 which is fatal in approximately one-third of patients and requires immediate treatment. Chronic GVHD (cGVHD), younger age at HCT and myeloablative TBI (that is, single doses ⩾ 10 Gy or fractionated doses ⩾ 13 Gy) are consistently reported risk factors for secondary skin cancer (Table 2).4,5,11,14,15,17 Voriconazole might increase the risk of skin cancer in organ transplant recipients, including HCT.18 The risk of skin cancer for patients who had allogeneic HCT for non-malignant disease remains undefined. A report restricted to autologous HCT demonstrated a SIR of 2.55 for melanoma, and multivariate analysis revealed age ⩾ 45 years, male patients and relapse following HCT as risk factors for melanoma development.19
Recommendation for transplant recipients
Given the considerations above, HCT recipients should maintain at least annual follow-up with their provider to incorporate skin cancer awareness and early disease recognition into routine late effects management. Photoprotection should be counseled to all HCT recipients as it can reduce the risk of skin cancer.20–22 Physical examination is the primary method for skin cancer screening. Heightened awareness is warranted for patients who received myeloablative TBI, those who had HCT at ages < 18 years, those with prior or concurrent GVHD of any form and those who had HCT for hereditary disorders, such as Fanconi anemia. Suspicious lesions should be addressed promptly, with management complying with standard practices.23
Thyroid cancer
Overview
Thyroid cancer is the most common endocrine cancer. Age-standardized incidence rates in men and women were 1.9 and 6.1, respectively, per 100 000 person-years worldwide in 2012 (Figure 1).6 The incidence of thyroid cancer has steadily risen over the past decades with no change in mortality rate (0.5 deaths per 100 000 person-years), which has largely been attributed to earlier detection.24 The incidence in women is approximately three times that in men and the median age at diagnosis is 50 years. Radiation and exposure to radioactive iodine isotopes are known risk factors for the development of thyroid cancer, though the majority of well-differentiated thyroid cancers (that is, papillary and follicular thyroid cancer) are unrelated to radiation exposure.
Incidence and risk factors after transplantation
Results have been conflicting in studies investigating the incidence of secondary thyroid cancer after HCT. Although some have found an increased incidence of thyroid cancers (SIR 5.8–6.5),5,11 others have not found any difference.12,25 In an attempt to better characterize the risk of secondary thyroid cancer, the European Group for Blood and Marrow Transplantation Late Effect Working Party reviewed data on 68 936 patients undergoing HCT between 1985 and 2003.26 They found an increased risk of secondary thyroid cancers when compared with the general population, with a SIR of 3.26. Multivariate analysis revealed that age ⩽ 20 years at HCT was the strongest risk factor for secondary thyroid cancer (relative risk (RR) 24.6 for age 0–10 years; RR 4.80 for age 11–20 years). Other risk factors were irradiation history (RR 3.44), female gender (RR 2.79) and history of cGVHD (RR 2.94). The median interval between HCT and secondary thyroid cancer was 8.5 years.
Recommendations for transplant recipients
Physical examination (neck palpation) at least once a year remains the cornerstone of thyroid cancer screening. There is no evidence for routine imaging (for example, thyroid ultrasound) in screening. Heightened awareness is warranted for pediatric long-term survivors, female patients, TBI recipients and patients with cGVHD.
Oropharyngeal cancer
Overview
Age-standardized incidence rates of oropharyngeal cancer in men and women were 5.5 and 2.5, respectively, per 100 000 person-years worldwide in 2012 (Figure 1).6 Oral squamous cell cancer (SCC) is the most common type and the major risk factors in the general population are tobacco and alcohol use.27 Human papilloma virus (HPV) and other viruses have also been implicated in the pathogenesis.28 Other reported risk factors are low socioeconomic status, dietary, tylosis, poor oral hygiene, use of chewing tobacco, betel nut chewing, occupational exposure, radiation and genetic predispositions.
Incidence and risk factors after transplantation
Among secondary cancers after HCT, oropharyngeal cancer is the most frequent (Table 1). The incidence rate of oropharyngeal cancer after HCT ranged from 32 to 92 per 100 000 person-years with a 7–16-fold higher risk than the general population.3,5,11,14 The risk of oropharyngeal cancers was also elevated after HCT with RIC regimens (SIR 46.7).13 The risk of oral SCC is particularly high in patients with cGVHD receiving long-term immunosuppressive therapy ⩾ 24 months.4,5,11,13–15,29–31 There was an association between oral cancer and male gender.5,11 In addition to cGVHD, conditioning regimens with limited field irradiation was associated with an increased risk of SCC of the oral cavity (RR = 4.7).5,11 A pediatric study showed an increased incidence of pharyngeal cancer after autologous HCT,32 whereas an adult study did not report oropharyngeal cancer after autologous HCT.33 Patients with hereditary disorders such as Fanconi anemia are at a significantly increased risk of oropharyngeal cancer. In immunocompromised patients, oncogenic viruses such as HPV may contribute to oropharyngeal SCC.34
Recommendation for transplant recipients
We recommend that all HCT recipients should be informed about the risks and symptoms of oropharyngeal cancer. Evaluation for premalignant lesions or oral cancer by a dentist or an oral surgeon is recommended annualy.2 Alternatively, primary care providers can include careful oral examination as part of an annual physical exam. Screening every 6 months may be considered for patients at high risk for developing oropharyngeal cancer (for example, cGVHD; Table 2). No definitive preventive interventions are recommended beyond general lifestyle counseling (for example, smoking cessation). Children who received radiation-containing treatment at a young age may require particular attention to screening for oropharyngeal cancer.
Esophageal cancer
Overview
Age-standardized incidence rates of esophageal cancer in men and women were 9.0 and 3.1, respectively, per 100 000 person-years worldwide in 2012 (Figure 1).6 Adenocarcinoma is the most common subtype in countries where smoking rate is low, and SCC is the most common subtype in countries where smoking rate is high. Reported risk factors are tobacco use, alcohol use, low socioeconomic status, dietary, other esophageal diseases, previous head and neck cancer, bisphosphonate use, occupational exposure, radiation and genetic predispositions. Barrett’s esophagus is the major risk factor for esophageal adenocarcinoma in the general population. Factors known to increase the risk for Barrett’s esophagus include White ethnicity, older age, obesity and longstanding gastroesophageal reflux disease symptoms.35
Incidence and risk factors after transplantation
The incident rate of esophageal cancer after HCT ranges from 4 to 59 per 100 000 person-years and the SIR compared with the general population is 8.5–11-fold.3,5,14 The risk of secondary esophageal cancer was increased in patients with persistent cGVHD and those receiving long-term immunosuppressive therapy ⩾ 24 months.4,14 In particular, extensive-type cGVHD was a risk factor for esophageal cancer (RR = 5.3). Information is limited in the pediatric population. An adult study did not show an increased incidence of esophageal cancer after autologous HCT.33
Recommendation for transplant recipients
HCT recipients should be informed about the risks and symptoms of secondary esophageal cancer. Patients who have persistent gastroesophageal reflux disease symptoms or dysphagia should be evaluated by upper gastrointestinal (GI) endoscopy. Endoscopic screening can be considered in patients receiving prolonged immunosuppressive treatment ⩾ 24 months for cGVHD, especially if symptoms such as dysphagia and persistent heartburn are present. Barium swallow is inexpensive and could be used for screening, but its sensitivity is unknown.36
Gastric cancer
Overview
Gastric cancer was the fifth most common cancer worldwide in 2012 and age-standardized incidence rates of gastric cancer in men and women were 17.4 and 7.5, respectively, per 100 000 person-years (Figure 1).6 Almost three quarters of the new cases occurred in Asia. Incidence varies 10-fold between countries, with the highest incidence in East Asia and central and Eastern Europe. Incidence rates are low in Africa and in North America. Helicobacter pylori infection is a well-established carcinogen for gastric cancer and is found in approximately 75% of all cases of gastric cancer.37
Incidence and risk factors after transplantation
Although GI tract cancers are among the most common cancers worldwide, current data in HCT survivors have not necessarily indicated an increased risk of secondary gastric cancer relative to the general population.3,11,12,38–41 In the largest study of long-term HCT survivors, no gastric cancers were reported.5 Although cGVHD has been linked to secondary SCC, cGVHD does not appear to be associated with gastric cancer. Given the long latency of GI cancers, studies with longer-term follow-up are needed to clarify the risk of GI cancers after HCT. Of note, most secondary cancer studies have included patients from regions with a low prevalence of gastric cancer.
Recommendation for transplant recipients
There are currently no data indicating an increased risk of secondary gastric cancers in HCT recipients. Routine screening for gastric cancer in HCT recipients is not recommended, but following local screening recommendations based on geographic location and associated predisposition may be considered.
Colorectal cancer
Overview
Colorectal cancer represents almost 10% of the global cancer incidence burden in 2012 and is the third most common cancer in men and the second most common cancer in women. Age-standardized incidence rates in men and women were 20.6 and 14.3, respectively, per 100 000 person-years worldwide in 2012 (Figure 1).6 Incidence varies 10-fold between countries in both genders and rates tend to be low in many African countries.
Incidence and risk factors after transplantation
Limited data in the post-HCT setting do not indicate a greatly increased risk of secondary colorectal cancers relative to the general population.3,11,12,38–41 In the largest study of long-term HCT survivors, there were only two cases of colon cancer (observed/expected (O/E), 0.52; 95% confidence interval (CI), 0.06–1.89) and four cases of rectal cancer (O/E, 1.67; 95% CI, 0.46–4.29) described among 28 874 patients.5 Secondary GI cancers were similarly infrequent in a recent cohort of 4269 long-term survivors of HCT with RIC regimens (1 small intestinal cancer, 3 colon cancers, and 2 rectal cancers).13 A more recent study that included 17 545 HCT recipients, in contrast to prior reports, did find an increased risk (SIR 1.9) of colon cancer.14
Recommendation for transplant recipients
The data indicating an increased risk of secondary colorectal cancers in HCT recipients is limited, but has been reported.14 It is recommended that HCT recipients undergo screening for secondary colorectal cancers according to the current general population guidelines (Table 3). This would include consideration of annual stool guaiac testing, flexible sigmoidoscopy every 5 years or colonoscopy every 10 years, with earlier and more frequent screening in high-risk patients.
Liver cancer
Overview
The annual incidence of hepatocellular carcinoma (HCC) is rising worldwide. Age-standardized incidence rates in men and women were 15.3 and 5.4, respectively, per 100 000 person-years worldwide in 2012 (Figure 1).6 A history of hepatitis B, hepatitis C, chronic active hepatitis and cirrhosis are potential risk factors for the development of HCC.
Incidence and risk factors after transplantation
An increased incidence of liver cancer in long-term survivors of both autologous and myeloablative allogeneic HCT has been reported (6–28-fold increased risk compared with the general population) by some5,11,25 but not all studies (Table 1).12 Similarly, a trend towards increased incidence of liver cancer is also noted in recipients of allogeneic HCT with RIC regimens (SIR, 5.96; 95% CI, 0.72–21.54).13 Risk factors of liver cancer in HCT survivors include younger age (< 34 years) at HCT (SIR, 200; 95% CI, 18.9–573.2), history of chronic hepatitis C infection, liver cirrhosis and TBI-based conditioning (SIR, 20.0; 95% CI, 1.9–57.3)(Table 2).42 No data indicate that GVHD increases the risk of liver cancer in allogeneic HCT survivors. An additional consideration in the post-HCT population is the potential for iron overload from prior transfusions.43,44 Iron overload can contribute to the development of chronic liver disease and/or subsequent HCC in cases of both hereditary45 and acquired iron overload syndromes,46 but further studies are needed to determine whether there would be similar implications of iron overload in the post-HCT setting.
Recommendation for transplant recipients
General population guidelines can be followed for screening for HCC in HCT survivors (screening liver ultrasound every 6 months in patients at increased risk for developing HCC, as defined in AASLD guidelines).54
Lung cancer
Overview
Lung cancer is the most frequent cancer worldwide and is the leading cause of cancer death in men and in women. Age-standardized incidence rates in men and women were 34.2 and 13.6, respectively, per 100 000 person-years worldwide in 2012 (Figure 1).6 Incidence varies 80-fold between countries, with the highest incidence in North America, Europe and East Asia and relatively low incidence in many African countries. The two main classes of lung cancer are small cell lung carcinoma (SCLC) and non-SCLC. Non-SCLC is further subdivided into adenocarcinoma, squamous cell carcinoma and large cell carcinoma. SCLC is strongly associated with tobacco use in the general population.
Incidence and risk factors after transplantation
Reported SIRs for secondary lung cancer in HCT survivors compared with the general population vary among large studies from 0.7 to 2.6.3,5,11–14 A recent study evaluated the incidence and risk factors for solid cancers after HCT using high-dose BU and CY conditioning in 4318 recipients of first allogeneic HCT. In this study, lung cancer was the most common site (n = 11, including 9 with non-SCLC, among 66 patients with all solid cancers).3 Median age at HCT for these 11 patients was 48 (range, 24–58) years, and lung cancer occurred at a median of 4.5 years after HCT. The association of radiation (for example, TBI) and lung cancer risk in HCT survivors is not well established, especially when lung shielding is applied. Limited data in the setting of radiation used for the treatment of Hodgkin lymphoma, however, suggest an increase in the risk of lung cancer following radiation therapy.47,48 Tobacco use prior to HCT was a strong risk factor for developing secondary lung cancer (RR = 11.6, P = 0.02).3 In other large studies, however, overall SIRs of developing secondary lung cancers were not significantly elevated compared with the general population.5,11–14
Recommendation for transplant recipients
Patients preparing to undergo HCT who are current smokers should be counseled to quit immediately to avoid the risks of secondary lung cancers and other adverse post-HCT health effects. Past or current smokers who are HCT survivors should be counseled about the risks of secondary lung cancer. Assessment of tobacco use, counseling against smoking and smoking cessation guidance for current smokers should be routinely included as part of post-transplant assessment.2 Screening with low-dose computed tomography may be offered to high-risk smokers and former smokers according to general population guidelines.49
Breast cancer
Overview
Breast cancer is the most frequently diagnosed and the leading cause of cancer death in women worldwide.50,51 An age-standardized incidence rate of breast cancer was 43.3 per 100 000 person-years worldwide in 2012 (Figure 1).6 Introduction of screening programs has reduced breast cancer-related mortality by 20–48%.52,53
Incidence and risk factors after transplantation
Overall incidence of breast cancer is not increased among HCT survivors as compared with the general population (Table 1). Among HCT survivors, however, patients who had HCT at a younger age have an increased risk of developing breast cancer, with the highest incidence in young females < 18 years at HCT. The risk remains elevated for subsequent age groups.12,32,54 The incidence of breast cancer was increased markedly from 0.8% at 10 years to 4.6% at 20 years, reaching 11% at 25 years after HCT.54 Similar results have been observed in other studies.5,11,32 The use of TBI-based conditioning regimens has been associated with an increased risk of breast cancer after allogeneic HCT.5,54 The effect of TBI seems to be greater in children irradiated before 10 years of age with a 55-fold increase in the risk of developing solid cancers, including breast cancer. The risk remains elevated at 4–6-fold among recipients irradiated between 10 and 19 years and 20 and 29 years. The incidence of breast cancer was increased among patients > 50 years of age who received BU–CY conditioning regimen,3 but the incidence was not increased among patients of similar age in another study.14 Other studies have reported an increased risk of breast cancer associated with antithymocyte globulin5 or growth factors.3 One study, in which many patients received radiation therapy before HCT or as part of conditioning, showed a higher risk of developing breast cancer in pediatric patients after autologous HCT.32 In contrast, none of 1482 adult patients in another large cohort of autologous HCT recipients developed breast cancer.33
Recommendation for transplant recipients
Breast awareness should be emphasized for all long-term survivors after HCT, with prompt reporting of any new breast symptoms (for example, new nodules) to a health-care professional. Mammograms and clinical breast exam beginning at age 40 years and occurring every 1–2 years are recommended for all HCT recipients. For patients who received chest radiation or TBI, special consideration should be given for annual clinical breast exam, mammography and breast magnetic resonance imaging beginning at age 25 years or 8 years after radiation or TBI, whichever occurs later, but no later than 40 years of age.2 Screening at an earlier age may also be considered in women at high risk for breast cancer (for example, known BRCA mutations) and should follow general population screening guidelines. Breast ultrasound may be considered in addition to mammography for high-risk women, but additional benefits over magnetic resonance imaging are undetermined.55
Cervical and endometrial cancer
Overview
Age-standardized incidence rate of cervical cancer was 14.0 per 100 000 person-years worldwide in 2012 (Figure 1).6 The incidence and mortality rate of cervical cancer have declined since the introduction of the Papanicolaou (Pap) test. More than 80% cases are SCC and 10–20% are adenocarcinomas. Cervical cancers present with no symptoms in early stages but progresses to abnormal vaginal bleeding, pelvic pain or pain during intercourse in advanced stages. As HPV of high-risk subtypes such as HPV16 and HPV18 causes > 90% of cases, routine HPV vaccination is recommended for females aged 9–26 years.56,57 The Centers for Disease Control and Prevention recommends HPV vaccination for girls and boys at age 11 or 12 years. Age-standardized incidence rate of endometrial cancer was 8.2 per 100 000 person-years worldwide in 2012 (Figure 1).6 Endometrial cancers typically occur after menopause, and the first symptom is often vaginal bleeding. Obesity and excessive estrogen exposure are known risk factors.58 It often develops from endometrial hyperplasia and 80% of cases are endometrioid carcinoma.
Incidence and risk factors after transplantation
The reported incidence rates of cervical and endometrial cancers occurring after HCT are 2–67 and 2–7 per 100 000 person-years.3,5,11,14 Several studies have shown that the overall incidence rates of these cancers did not differ from the general population,3,5,11,14 but one study reported a 13-fold increased risk of cervical cancer compared with the general population (Table 1).25 Age > 34 years and systemically treated cGVHD for > 3 years were associated with an increased risk of cervical cancer (Table 2).25,59 HPV-related cervical dysplasia occurred in more than one-third of long-term survivors after allogeneic HCT, and most cases had high-risk HPV subtypes 16 and 18.59 Risk factors for endometrial cancer after HCT have not been studied. Information on cervical or endometrial cancer after autologous HCT or in the pediatric population are limited, although some studies have included adult survivors of HCT during childhood.32
Recommendation for transplant recipients
The screening and preventive practice guidelines for HCT recipients recommend annual pelvic exam for all female HCT survivors, ideally to begin in the year following transplant, as age appropriate.2,60 Screening methods recommended for the general population by expert societies apply to HCT recipients as well.61 Although the efficacy of HPV vaccination in HCT recipient remains to be determined, the Centers for Disease Control and Prevention has recommended vaccination in those who are immunocompromised.57,62 Three doses of HPV vaccination starting at 1 year after allogeneic HCT are provided in some transplant centers for males and females aged 9–26 years. Although there are no clear contraindications to vaccination, studies demonstrating its effectiveness in the post-transplant setting are lacking and clinical studies are needed to evaluate the benefit in this subpopulation. Information about the risks and symptoms of endometrial cancers (any unexpected bleeding or spotting) should be provided to female HCT recipients at the time of menopause. Combination of estrogen and progestogens should be used for HCT recipients who need hormone-replacement therapy,63,64 as estrogen alone increases risk of endometrial cancer in the general population.65,66
Ovarian cancer
Overview
Ovarian cancer is the second most common gynecological cancer in developed countries. Age-standardized incidence rate of ovarian cancer was 6.1 per 100 000 person-years worldwide in 2012 (Figure 1).6 The fatality rate of ovarian cancer is higher than other cancers of the female reproductive organs. Ovarian carcinomas are the most common type and nulliparous women are more frequently affected than those with suppressed ovulation typically by pregnancy or oral contraceptives.
Incidence and risk factors after transplantation
Few studies have reported on the incidence of ovarian cancer after HCT. In long-term Asian survivors of allogeneic HCT compared with the general population, the incidence of ovarian cancer does not appear to be increased (SIR 0.8; 95% CI 0.2–2.4%; Table 1).14 No specific risk factors predisposing to secondary ovarian cancer among HCT recipients are known.
Screening recommendations for transplant recipients
Routine ovarian cancer screening in average-risk transplant survivors is not recommended. Evaluation should instead focus on early detection in symptomatic patients. Such evaluation includes physical exam, transvaginal ultrasound and serum CA-125 levels.67
Prostate cancer
Overview
Prostate cancer is most frequent in men > 65 years but rarely reported as a primary cause of death.68 Age-standardized incidence rate was 31.1 per 100 000 person-years worldwide in 2012 (Figure 1).6 In the general population, risk factors for prostate cancer are family history, African American ethnicity, older age and abnormal digital rectal examination.23
Incidence and risk factors after transplantation
The incidence rate of prostate cancer after HCT is 4–10 per 100 000 patient-years, and the risk has not exceeded the risk in the general population.3,5,11,12,14,38,69 No specific risk factors predisposing to secondary prostate cancer among HCT recipients are known.
Recommendation for transplant recipients
HCT survivors should follow guidelines for the general population. For prostate cancer, after an informed decision-making process for asymptomatic men aged > 50 years with life expectancy of at least 10 years, or for those considered to be at higher risk, consideration should be made for routine digital rectal examination with or without prostate-specific antigen.23,70–72
Testicular cancer
Overview
Testicular cancer usually presents between 20 and 30 years of age and represents about 1% of male malignant tumors.73 Age-standardized incidence rate was 1.5 per 100 000 person-years worldwide in 2012 (Figure 1).6 Risk factors for testicular cancer include cryptorchidism/hypospadias, a personal or family history of testicular cancer, infertility, tobacco use, increased height or weight, HIV infection, Caucasian or Hispanic ethnicity and exposure to organochlorine compounds.73–82
Incidence and risk factors after transplantation
The incidence rate of testicular cancer after HCT is 4–5 per 100 000 patient-years, and the risk after HCT does not seem to exceed the risk in the general population.3,5,11,12,14,38,69 No specific risk factors predisposing to secondary testicular cancer among HCT recipients are known.
Recommendation for transplant recipients
Screening recommendations for testicular cancer among HCT recipients should mirror screening recommendations in the general population, including performance of a testicular exam as part of a routine annual physical exam.
Other secondary solid cancers
In addition to screening for cancers that the general population is also at risk for, it is notable that patients with history of cancer or who are post-transplant are at higher risk for cancers where there are no routine screening recommendations. These include central nervous system tumors and sarcomas (Table 1).3 Although there is no evidence to suggest clear recommendations for screening, providers should maintain vigilance and have a high level of suspicion in patients who present with relevant symptoms. Central nervous system tumors may be of particular concern in those who have previously undergone central nervous system irradiation as part of prior therapy or have received TBI.83
Additional considerations
The incidence of secondary solid cancers did not differ according to donor type or HLA matching in many previous studies.3–5,11,12,14,15,54 As previous studies included few cord blood or haploidentical transplantation, further studies are warranted to characterize outcomes after the use of these new graft sources. One recent study found a similar risk of secondary cancers between RIC and myeloablative conditioning for leukemia or myelodysplastic syndrome patients and a lower risk of secondary cancers after RIC than after myeloablative conditioning for lymphoma patients.13 Compared with allogeneic HCT, data of secondary solid cancers after autologous HCT are relatively lacking partly because recurrent malignancy occurs frequently after autologous HCT, precluding long-term follow-up to investigate secondary cancers. Some studies found different risks of secondary cancers according to diseases indicated for HCT or recipient genders. The risk of secondary cancers was increased in patients with acute leukemia or chronic myeloid leukemia but not in those with lymphoma or severe aplastic anemia.25 The risk of skin BCC was higher in patients with hematological malignancies than those with benign diseases.15 The risk of SCC in the skin and mouth was higher in male recipients than in female recipients.5,11 The risks of thyroid cancer and melanoma were higher in female recipients than in male recipients.5,26
Although the consensus recommendations and this manuscript provide a general overview of risks related to development of secondary solid cancers, a few caveats have to be considered. First, incidence of cancers may differ by race and ethnicity and the practice of cancer screening may differ among countries. As available literature is dominated by studies of Western populations with an overrepresentation of Caucasians, our recommendations would require careful application in other races, ethnicities and regions (for example, risks of gastric cancer in Asian populations). Second, reported incidences of secondary malignancies after HCT rely largely on registry data, which may underestimate the true incidence as transplant centers may not have information on long-term HCT survivors. Hence, we would emphasize that transplant centers keep in touch with long-term survivors and report events in order to accurately estimate the incidence of secondary cancers. Finally, establishing the appropriate time after transplant to initiate screening for secondary malignancies is not clear, especially given the long latency of certain solid cancers and the steady rise in incidence, which is especially important when considering secondary cancer screening in those who underwent HCT as children. This is demonstrated by a Nordic study consisting of 47 697 patients with childhood cancers followed up to age 79 years.84 The cumulative risk of a secondary cancer increased substantially at the age of 60–80 years, with a predominance of cancers of the breast, GI, respiratory or genitourinary organs in patients ⩾ 60 years, which was a different distribution compared with secondary cancers seen in younger patients. Certainly, children who undergo HCT will continue to be at increased risk for development of secondary solid cancers over the course of their lifetime, but adopting standard practice guidelines that are applicable in the general adult population may be neither feasible nor reliable in a younger population. Hence, for children it will be even more imperative to rely on clinical signs/symptoms that would prompt early initiation of screening as opposed to only that which would be initiated solely based on age.
CONCLUSION
In conclusion, we provide our consensus recommendations for secondary solid cancer screening in Table 3. As risks of cancers in the skin, thyroid, oral cavity, esophagus, liver, brain/nervous system, bone and connective tissues are increased after HCT compared with the general population, heightened awareness is warranted for these sites. These recommendations will inform future iterations of screening and preventive practice guidelines for HCT survivors.
Supplementary Material
Supplemental Materials
Acknowledgments
The Center for International Blood and Marrow Transplant Research (CIBMTR) is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia and Lymphoma Society; The Medical College of Wisconsin; Merck and Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense or any other agency of the US Government.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest
REFERENCES
- 1.Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19:1498–1501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117:316–322. doi: 10.1182/blood-2010-07-294629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Stewart BW, Wild CP, editors. 1st edition. International Agency for Research on Cancer (IARC) 2014
- 7.Criscione VD, Weinstock MA, Naylor MF, Luque C, Eide MJ, Bingham SF, et al. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 8.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 9.Preston DS, Stern RS. Nonmelanoma cancers of the skin. N Engl J Med. 1992;327:1649–1662. doi: 10.1056/NEJM199212033272307. [DOI] [PubMed] [Google Scholar]
- 10.Le Mire L, Hollowood K, Gray D, Bordea C, Wojnarowska F. Melanomas in renal transplant recipients. Br J Dermatol. 2006;154:472–477. doi: 10.1111/j.1365-2133.2005.07094.x. [DOI] [PubMed] [Google Scholar]
- 11.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 12.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 13.Ringden O, Brazauskas R, Wang Z, Ahmed I, Atsuta Y, Buchbinder D, et al. Second solid cancers after allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20:1777–1784. doi: 10.1016/j.bbmt.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atsuta Y, Suzuki R, Yamashita T, Fukuda T, Miyamura K, Taniguchi S, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014;25:435–441. doi: 10.1093/annonc/mdt558. [DOI] [PubMed] [Google Scholar]
- 15.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 16.Iyer JG, Storer BE, Paulson KG, Lemos B, Phillips JL, Bichakjian CK, et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J Am Acad Dermatol. 2014;70:637–643. doi: 10.1016/j.jaad.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz JL, Kopecky KJ, Mathes RW, Leisenring WM, Friedman DL, Deeg HJ. Basal cell skin cancer after total-body irradiation and hematopoietic cell transplantation. Radiat Res. 2009;171:155–163. doi: 10.1667/RR1469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams K, Mansh M, Chin-Hong P, Singer J, Arron ST. Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis. 2014;58:997–1002. doi: 10.1093/cid/cit940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilmon IA, Ashton LJ, Le Marsney RE, Dodds AJ, O’Brien TA, Wilcox L, et al. Second cancer risk in adults receiving autologous haematopoietic SCT for cancer: a population-based cohort study. Bone Marrow Transplant. 2014;49:691–698. doi: 10.1038/bmt.2014.13. [DOI] [PubMed] [Google Scholar]
- 20.Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354:723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn KG, Boesen E, Ross L, Johansen C. Evaluation and outcome of behavioural changes in the rehabilitation of cancer patients: a review. Eur J Cancer. 2005;41:216–224. doi: 10.1016/j.ejca.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Lau SC, Chen L, Cheung WY. Protective skin care behaviors in cancer survivors. Curr Oncol. 2014;21:e531–e540. doi: 10.3747/co.21.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith RA, Brooks D, Cokkinides V, Saslow D, Brawley OW. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin. 2013;63:88–105. doi: 10.3322/caac.21174. [DOI] [PubMed] [Google Scholar]
- 24.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia S, Louie AD, Bhatia R, O’Donnell MR, Fung H, Kashyap A, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 26.Cohen A, Rovelli A, Merlo DF, van Lint MT, Lanino E, Bresters D, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J Clin Oncol. 2007;25:2449–2454. doi: 10.1200/JCO.2006.08.9276. [DOI] [PubMed] [Google Scholar]
- 27.Moyer VA, Force USPST. Screening for oral cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:55–60. doi: 10.7326/M13-2568. [DOI] [PubMed] [Google Scholar]
- 28.Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 29.Baker KS, Gurney JG, Ness KK, Bhatia R, Forman SJ, Francisco L, et al. Late effects in survivors of chronic myeloid leukemia treated with hematopoietic cell transplantation: results from the Bone Marrow Transplant Survivor Study. Blood. 2004;104:1898–1906. doi: 10.1182/blood-2004-03-1010. [DOI] [PubMed] [Google Scholar]
- 30.Martin PJ, Counts GW, Jr, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolb HJ, Socie G, Duell T, Van Lint MT, Tichelli A, Apperley JF, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 32.Danner-Koptik KE, Majhail NS, Brazauskas R, Wang Z, Buchbinder D, Cahn JY, et al. Second malignancies after autologous hematopoietic cell transplantation in children. Bone Marrow Transplant. 2013;48:363–368. doi: 10.1038/bmt.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jantunen E, Itala M, Siitonen T, Koivunen E, Leppa S, Juvonen E, et al. Late non-relapse mortality among adult autologous stem cell transplant recipients: a nation-wide analysis of 1,482 patients transplanted in 1990–2003. Eur J Haematol. 2006;77:114–119. doi: 10.1111/j.1600-0609.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 34.Alani RM, Munger K. Human papillomaviruses and associated malignancies. J Clin Oncol. 1998;16:330–337. doi: 10.1200/JCO.1998.16.1.330. [DOI] [PubMed] [Google Scholar]
- 35.Shaheen NJ, Weinberg DS, Denberg TD, Chou R, Qaseem A, Shekelle P, et al. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808–816. doi: 10.7326/0003-4819-157-11-201212040-00008. [DOI] [PubMed] [Google Scholar]
- 36.Gerson LB, Triadafilopoulos G. Screening for esophageal adenocarcinoma: an evidence-based approach. Am J Med. 2002;113:499–505. doi: 10.1016/s0002-9343(02)01234-2. [DOI] [PubMed] [Google Scholar]
- 37.Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: Prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842–13862. doi: 10.3748/wjg.v20.i38.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota A, Ozawa S, Masanori T, Akiyama H, Ohshima K, Kanda Y, et al. Secondary solid tumors after allogeneic hematopoietic SCT in Japan. Bone Marrow Transplant. 2012;47:95–100. doi: 10.1038/bmt.2011.23. [DOI] [PubMed] [Google Scholar]
- 39.Shimada K, Yokozawa T, Atsuta Y, Kohno A, Maruyama F, Yano K, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36:115–121. doi: 10.1038/sj.bmt.1705020. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher G, Forrest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007;109:84–92. doi: 10.1002/cncr.22375. [DOI] [PubMed] [Google Scholar]
- 41.Forrest DL, Nevill TJ, Naiman SC, Le A, Brockington DA, Barnett MJ, et al. Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant. 2003;32:915–923. doi: 10.1038/sj.bmt.1704243. [DOI] [PubMed] [Google Scholar]
- 42.Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruccione KS, Wood JC, Sposto R, Malvar J, Chen C, Freyer DR. Characterization of transfusion-derived iron deposition in childhood cancer survivors. Cancer Epidemiol Biomarkers Prev. 2014;23:1913–1919. doi: 10.1158/1055-9965.EPI-14-0292. [DOI] [PubMed] [Google Scholar]
- 44.Rascon J, Rageliene L, Stankeviciene S, Palionis D, Tamosiunas AE, Valeviciene N, et al. An assessment of iron overload in children treated for cancer and non-malignant hematologic disorders. Eur J Pediatr. 2014;173:1137–1146. doi: 10.1007/s00431-014-2295-5. [DOI] [PubMed] [Google Scholar]
- 45.Ko C, Siddaiah N, Berger J, Gish R, Brandhagen D, Sterling RK, et al. Prevalence of hepatic iron overload and association with hepatocellular cancer in end-stage liver disease: results from the National Hemochromatosis Transplant Registry. Liver Int. 2007;27:1394–1401. doi: 10.1111/j.1478-3231.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 46.Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: new insights into the relationship between iron overload and chronic liver diseases. Dig Liver Dis. 2011;43:89–95. doi: 10.1016/j.dld.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort study. J Clin Oncol. 2011;29:4096–4104. doi: 10.1200/JCO.2011.34.8268. [DOI] [PubMed] [Google Scholar]
- 48.Schoenfeld JD, Mauch PM, Das P, Silver B, Marcus KJ, Stevenson MA, et al. Lung malignancies after Hodgkin lymphoma: disease characteristics, detection methods and clinical outcome. Ann Oncol. 2012;23:1813–1818. doi: 10.1093/annonc/mdr551. [DOI] [PubMed] [Google Scholar]
- 49.Gould MK. Clinical practice. Lung-cancer screening with low-dose computed tomography. N Engl J Med. 2014;371:1813–1820. doi: 10.1056/NEJMcp1404071. [DOI] [PubMed] [Google Scholar]
- 50.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 51.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 52.Independent UKPoBCS. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 53.Paci E, Group EW. Summary of the evidence of breast cancer service screening outcomes in Europe and first estimate of the benefit and harm balance sheet. J Med Screen. 2012;19:5–13. doi: 10.1258/jms.2012.012077. [DOI] [PubMed] [Google Scholar]
- 54.Friedman DL, Rovo A, Leisenring W, Locasciulli A, Flowers ME, Tichelli A, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111:939–944. doi: 10.1182/blood-2007-07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Bohm-Velez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilck MB, Baden LR. Vaccination after stem cell transplant: a review of recent developments and implications for current practice. Curr Opin Infect Dis. 2008;21:399–408. doi: 10.1097/QCO.0b013e328307c7c5. [DOI] [PubMed] [Google Scholar]
- 57.Tedeschi SK, Savani BN, Jagasia M, Engelhardt B, Anasetti C, Barrett AJ, et al. Time to consider HPV vaccination after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1033–1036. doi: 10.1016/j.bbmt.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607–2618. doi: 10.1200/JCO.2012.48.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savani BN, Stratton P, Shenoy A, Kozanas E, Goodman S, Barrett AJ. Increased risk of cervical dysplasia in long-term survivors of allogeneic stem cell transplantation--implications for screening and HPV vaccination. Biol Blood Marrow Transplant. 2008;14:1072–1075. doi: 10.1016/j.bbmt.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen ML, Flowers L. Cervical cancer screening in immunocompromised women. Obstet Gynecol Clin North Am. 2013;40:339–357. doi: 10.1016/j.ogc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 62.Savani BN, Goodman S, Barrett AJ. Can routine posttransplant HPV vaccination prevent commonly occurring epithelial cancers after allogeneic stem cell transplantation? Clin Cancer Res. 2009;15:2219–2221. doi: 10.1158/1078-0432.CCR-08-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fournier A, Dossus L, Mesrine S, Vilier A, Boutron-Ruault MC, Clavel-Chapelon F, et al. Risks of endometrial cancer associated with different hormone replacement therapies in the E3N cohort, 1992–2008. Am J Epidemiol. 2014;180:508–517. doi: 10.1093/aje/kwu146. [DOI] [PubMed] [Google Scholar]
- 65.Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–1167. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- 66.Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–1170. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]
- 67.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol. 2011;117:742–746. doi: 10.1097/AOG.0b013e31821477db. [DOI] [PubMed] [Google Scholar]
- 68.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 69.Nelson AS, Ashton LJ, Vajdic CM, Le Marsney RE, Daniels B, Nivison-Smith I, et al. Second cancers and late mortality in Australian children treated by allogeneic HSCT for haematological malignancy. Leukemia. 2014;29:441–447. doi: 10.1038/leu.2014.203. [DOI] [PubMed] [Google Scholar]
- 70.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 71.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013;1:CD004720. doi: 10.1002/14651858.CD004720.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mannuel HD, Mitikiri N, Hussain A. Update on testicular germ cell tumors. Curr Opin Oncol. 2011;23:265–270. doi: 10.1097/CCO.0b013e32834579f0. [DOI] [PubMed] [Google Scholar]
- 74.Richiardi L, Bellocco R, Adami HO, Torrang A, Barlow L, Hakulinen T, et al. Testicular cancer incidence in eight northern European countries: secular and recent trends. Cancer Epidemiol Biomarkers Prev. 2004;13:2157–2166. [PubMed] [Google Scholar]
- 75.Raman JD, Nobert CF, Goldstein M. Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. J Urol. 2005;174:1819–1822. doi: 10.1097/01.ju.0000177491.98461.aa. discussion 1822. [DOI] [PubMed] [Google Scholar]
- 76.Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351–356. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hentrich MU, Brack NG, Schmid P, Schuster T, Clemm C, Hartenstein RC. Testicular germ cell tumors in patients with human immunodeficiency virus infection. Cancer. 1996;77:2109–2116. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2109::AID-CNCR22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 78.Lyter DW, Bryant J, Thackeray R, Rinaldo CR, Kingsley LA. Incidence of human immunodeficiency virus-related and nonrelated malignancies in a large cohort of homosexual men. J Clin Oncol. 1995;13:2540–2546. doi: 10.1200/JCO.1995.13.10.2540. [DOI] [PubMed] [Google Scholar]
- 79.Powles T, Bower M, Daugaard G, Shamash J, De Ruiter A, Johnson M, et al. Multicenter study of human immunodeficiency virus-related germ cell tumors. J Clin Oncol. 2003;21:1922–1927. doi: 10.1200/JCO.2003.09.107. [DOI] [PubMed] [Google Scholar]
- 80.Dieckmann KP, Hartmann JT, Classen J, Diederichs M, Pichlmeier U. Is increased body mass index associated with the incidence of testicular germ cell cancer? J Cancer Res Clin Oncol. 2009;135:731–738. doi: 10.1007/s00432-008-0504-1. [DOI] [PubMed] [Google Scholar]
- 81.Srivastava A, Kreiger N. Cigarette smoking and testicular cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:49–54. doi: 10.1158/1055-9965.epi-03-0133. [DOI] [PubMed] [Google Scholar]
- 82.Chien FL, Schwartz SM, Johnson RH. Increase in testicular germ cell tumor incidence among Hispanic adolescents and young adults in the United States. Cancer. 2014;120:2728–2734. doi: 10.1002/cncr.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmiegelow K, Levinsen MF, Attarbaschi A, Baruchel A, Devidas M, Escherich G, et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2469–2476. doi: 10.1200/JCO.2012.47.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olsen JH, Moller T, Anderson H, Langmark F, Sankila R, Tryggvadottir L, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst. 2009;101:806–813. doi: 10.1093/jnci/djp104. [DOI] [PubMed] [Google Scholar]
- 85.Cancer Incidence in Five Continents Time Trends. Lyon, France: Internatiional Agency for Research on Cancer; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials