MOLECULAR PATHWAYS: JAK/STAT PATHWAY: MUTATIONS, INHIBITORS, AND RESISTANCE (original) (raw)
. Author manuscript; available in PMC: 2016 Sep 13.
Abstract
Aberrant activation of the JAK/STAT pathway has been reported in a variety of disease states, including inflammatory conditions, hematologic malignancies, and solid tumors. For instance, a large proportion of patients with myeloproliferative neoplasms (MPNs) carry the acquired gain-of-function JAK2 V617F somatic mutation. This knowledge has dramatically improved our understanding of the pathogenesis of MPNs and it has facilitated the development of therapeutics capable of suppressing the constitutive activation of the JAK/STAT pathway, now recognized as a common underlying biological abnormality in MPNs. Ruxolitinib is an oral JAK1 and JAK2 inhibitor that has recently been approved for the treatment of myelofibrosis and has been tested against other hematologic malignancies. A series of agents with different specificities against different members of the JAK family of proteins is currently undergoing evaluation in clinical trials for patients with MPNs, lymphoma, and solid tumors such as breast or pancreatic cancer. Despite their significant clinical activity exhibited in myelofibrosis, some patients fail to respond or progress during JAK kinase inhibitor therapy. Recent reports have shed light into the mechanisms of resistance to JAK kinase inhibitor therapy. Several approaches hold promise to overcome such resistance.
Keywords: JAK2, STAT, myeloproliferative neoplasias, resistance, ruxolitinib
Background
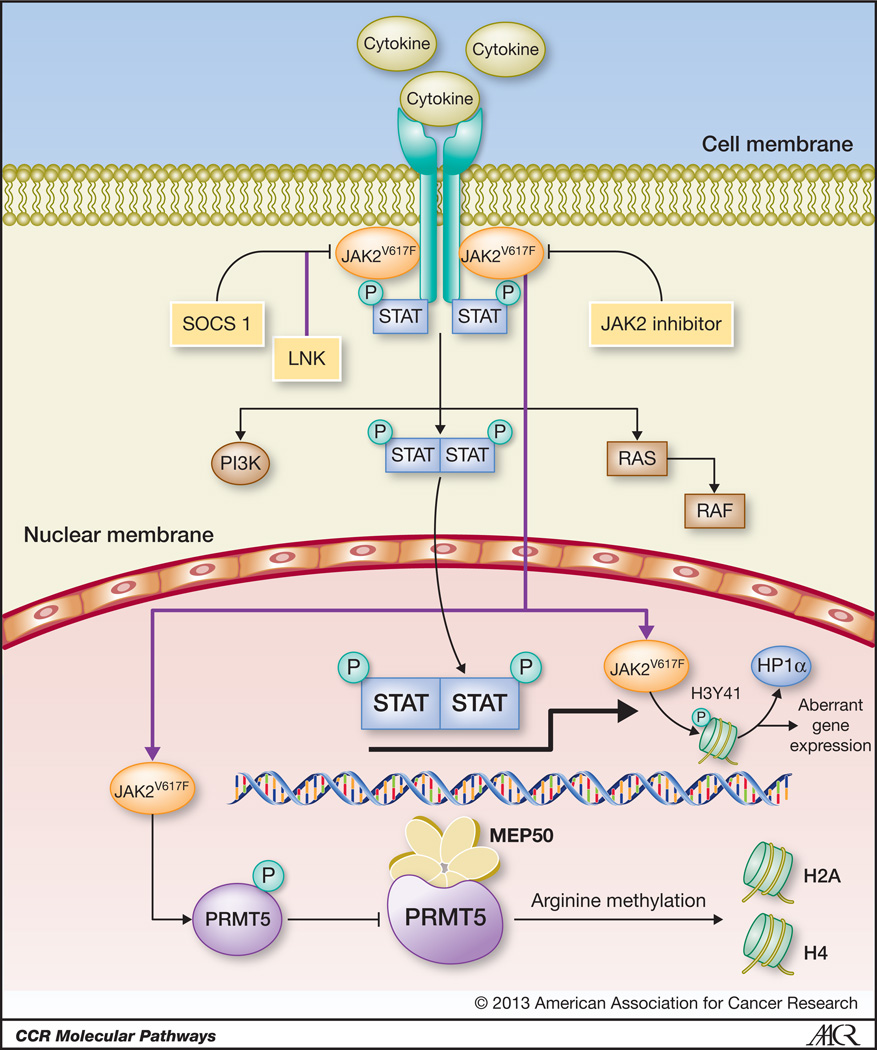
The JAK-STAT pathway is critical in normal hematopoiesis. The JAK family of kinases includes JAK1, JAK2, JAK3, and TYK2. Homozygous germline deletion of JAK2 alleles in mice results in embryonic lethality due to ineffective erythropoiesis.1,2 JAK kinases are activated through tyrosine-phosphorylation of the cytoplasmic domains of cytokine receptors, upon cytokine binding.3 Erythropoietin, thrombopoietin, GM-CSF, IL-3 and IL-5, among others signal through JAK2 whilst IL-6, IL-10, IL-11, IL-19, IL-20, IL-22, and interferon (IFN)-γ signal through both JAK1 and JAK2. JAK2 activation promotes recruitment to the receptor complex of the transcription factors signal transducer and activator of transcription (STAT)3 and STAT5.3 JAK2-mediated STAT phosphorylation leads to the formation of stable homodimers and heterodimers, which leads to their nuclear translocation (Figure 1). Once in the nucleus, STAT molecules bind specific promoter DNA sequences that result in the transcription of genes that regulate cell proliferation, differentiation, and apoptosis (e.g., Bcl-xL, cyclin D1, and PIM1).3,4
FIGURE 1. JAK/STAT pathway in myeloproliferative neoplasms.
Upon cytokine binding, JAK2 molecules are recruited and activated by cytokine receptors, which results in phosphorylation of downstream signaling pathways such as PI3K, RAS, and STAT3/5. STAT heterodimers and homodimers translocate to the nucleus and bind cognate DNA sequences at the promoter regions of genes involved in proliferation and apoptosis. In the presence of JAK2V617F mutations, the JAK/STAT pathway is constitutive activated. JAK2 inhibitors abrogate the JAK/STAT pathway through the inhibition of the kinase activity of JAK2V617F kinase. The activity of the JAK2/STAT pathway is negatively regulated by SOCS1 and LNK. Recently, JAK2 and JAK2V617F kinase have been shown to localize to the nucleus of hematopoietic precursors where it phosphorylates histone H3 (H3Y41), thus preventing its binding to the repressor heterochromatin protein 1 alpha (HP1α). The latter results in increased expression of the oncogene lmo2. These effects are reversible upon exposure to JAK2 inhibitors. JAK2V617F also binds and phosphorylates the arginine methyltransferase PRMT5, which hampers its interaction with methylosome protein 50 (MEP50), thus decreasing global arginine methylation of histones H2A and H4. The figure is modified from Quintás-Cardama, et al (reference #59)
The myeloproliferative neoplasms (MPNs) polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (MF) are clonal malignancies that arise from hematopoietic stem or progenitor cells and are characterized by unchecked proliferation of terminally differentiated myeloid cells.5 Despite certain idiosyncratic features, there are remarkable phenotypic and clinical commonalities to these MPNs, such as their proclivity to develop thrombotic and hemorrhagic complications and to progress to acute myeloid leukemia (AML).6–9 The molecular pathogenesis of MPNs remained elusive for decades, which has proven detrimental for the development of effective therapies, particularly for primary MF, an MPN associated with high morbidity and mortality. In fact, none of the standard approaches for the treatment of MPNs (e.g. hydroxyurea, growth factors, splenectomy) has been shown to improve the survival of patients with PMF, which is estimated to be only about 5 years.10 Allogeneic stem cell transplantation has proven the only curative strategy in MF but at the expense of a very high morbidity and mortality.11,12 A nexus linking PV, ET, and MF was revealed in 2005 with the discovery of a recurrent somatic point mutation in the pseudokinase domain of the Janus Kinase 2 (JAK2) gene, which is present in a large proportion of patients with these MPNs. In addition, overactivation of the JAK/STAT pathway with or without JAK protein mutations have been reported in subsets of patients with certain solid tumors and hematological malignancies. Somatic mutations in the JAK3 gene, including _JAK3_A572V, _JAK3_V722I, and _JAK3_P132T, and chimeric fusion transcripts involving JAK2, such as ETV6-JAK2, PCM1-JAK2, TEL-JAK2, and PCR-JAK2, have been reported in acute lymphoblastic (ALL) and acute myeloid leukemias (AML),13,14 as well as in multiple myeloma and non-Hodgkin’s lymphoma (NHL).15 JAK2R683 mutations have been reported in 7% of patients with high-risk B-cell ALL an in 25% of cases of ALL associated with Down syndrome.16–18 Overall, mutated JAK1, JAK2, and JAK3 proteins have been reported in approximately 10% of children with high-risk Philadelphia chromosome negative ALL,16 and anecdotally in AML.19–22 Activation of the JAK-STAT pathway activation is also present in chronic myeloid leukemia, where inhibition of JAK2-mediated extrinsic survival signals restores sensitivity to BCR-ABL1 kinase inhibitors.23 JAK2 mutations have not been reported in NHL but the JAK/STAT pathway is frequently activated through JAK2 amplification via 9p24 copy number gain, which has been reported in 30–50% of cases of Hodgkin’s lymphoma and primary mediastinal B-cell lymphoma.24–26 In solid tumors, persistent phosphorylation of STAT1, STAT3, and STAT5 have been demonstrated in breast, lung, and head and neck cancers, mediated by an increase in cytokine levels, in both an autocrine and paracrine manner, and by enhanced expression of cytokine receptors.27
Molecular biology of the JAK/STAT pathway
In 2005, a gain-of-function acquired somatic mutation was described in the JAK2 gene in a significant proportion of patients with MPNs.28–32 JAK2V617F mutation arises from a single base G→T transversion in the pseudokinase domain of JAK2, resulting in a valine-to-phenylalanine substitution at codon 617 that putatively disrupts the autoinhibitory activity of the pseudokinase domain (JH2), thus constitutively activating the kinase domain (JH1) of JAK2.33 As a consequence, hematopoietic cells carrying the JAK2V617F mutation exhibit cytokine hypersensitivity and cytokine-independent growth.34 JAK2V617F is present in 50%–60% of patients with PMF or ET, and in over 95% of those with PV.28,31 While most bone marrow erythroid colonies obtained from patients with ET bear JAK2 alleles that are either wild-type or JAK2V617F heterozygous,3,30,31 virtually all patients with PV carry JAK2V617F homozygous erythroid colonies as a result of uniparental disomy at the JAK2 locus.3,30,31 Several somatic gain-of-function mutations at exon 12 of JAK2 have been described in patients with PV without the JAK2V617F mutation.35–38 Therefore, JAK2 mutations are present virtually in all patients with PV. In addition, somatic mutations at exon 10 of the MPL gene, which encodes the transmembrane–juxtamembrane junction of MPL (W515L/K/A), are present in approximately 5% of patients with ET or MF, resulting in downstream signaling activation similar to that mediated by JAK2V617F.39–42
LNK, a negative regulator of JAK-STAT signaling, has been found mutated in a subset of patients with MPNs, providing a mechanism of JAK-STAT activation in patients carrying wild-type JAK2 alleles.43 The presence of the JAK2V617F mutation in CD34+CD38− hematopoietic stem cells in all mature blood cell lineages of patients with MPNs,44–46 disrupts the autoregulatory activity of JH2, resulting in constitutive activation of the JAK2/STAT pathway and cell growth in the absence of cytokine stimulation.29,31 JAK2V617F also activates the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target or rapamycin (mTOR)/forkhead transcription factors (FoxO) signaling proteins as well as the Ras pathway that promote survival and proliferation, thereby preventing apoptosis of hematopoietic progenitor cells.3 Furthermore, enforced expression of JAK2V617F in human hematopoietic stem cells and myeloid progenitors steers differentiation towards the erythroid lineage, which is accompanied by decreased expression of PU.1 and enhanced expression and phosphorylation of GATA-1.47–49 JAK2 signaling is negatively regulated by suppressor of cytokine signaling (SOCS) proteins, most importantly SOCS1. JAK2 inhibitor treatment,50,51 or overexpression of a dominant negative form of STAT5 abrogates the growth of PV erythroid progenitors in vitro,52 thus implicating JAK2 in the pathogenesis of MPNs and providing the rationale to use JAK2 inhibitors for the treatment of patients with MPNs.
The role of JAK2V617F in the pathogenesis of MPNs has been validated in transgenic mouse models of JAK2V617F-driven disease in which low levels of JAK2V617F rendered an ET-like phenotype, whereas high levels were associated with a PV-like phenotype.53,54 A mouse model in which JAK2V617F is expressed from its endogenous promoter, displays a phenotype resembling human PV and it is transplantable to recipient mice,55 indicating that the resulting _JAK2V617F_–induced MPN is cell autonomous in nature. In this model, JAK2 inhibitor therapy ameliorated the phenotype but failed to fully eradicate MPN-initiating cells.55 To definitely confirm these results, a similar knock-in approach in a different mouse model produced a phenotype characterized by erythrocytosis, leukocytosis, thrombocytosis, splenomegaly, reduced serum erythropoietin, and erythropoietin-independent erythroid colonies in both heterozygous and homozygous mice for the mutation, although most significantly in the latter.56
STAT-independent JAK2 oncogenic signaling
In addition to modulating cytokine-mediated signaling via activation of STAT transcription factors, JAK2 kinase also renders oncogenic effects through epigenomic alterations (Figure 1). Wild-type JAK2 as well as JAK2V617F proteins have been found in the cytoplasm and the nucleus of human leukemic cell lines and primary CD34+ hematopoietic progenitors.57 In the nucleus, JAK2 phosphorylates histone H3 at tyrosine 41 (H3Y41). The levels of phosphorylated H3Y41 correlate with JAK2 activity in vivo. Notably, JAK2 appears to be the only kinase responsible for H3Y41 phosphorylation as treatment with JAK2 inhibitors such as TG101209 or AT9283 abrogates H3Y41 phosphorylation in the nucleus.57 In Drosophila, JAK2 kinase activation disrupts the binding of the transcriptional repressor heterochromatin protein 1 alpha (HP1α) from chromatin. Interestingly, the affinity of HP1α for histone H3 is dependent on the phosphorylation status of H3Y41. H3Y41 phosphorylation decreases the affinity of H3 to HP1α. JAK2 inhibitors abrogate H3Y41 phosphorylation and enhance chromatin-bound HP1α in cells, thus repressing HP1α–regulated genes. Most JAK2-regulated genes do not contain a predicted STAT5 binding site, suggesting that these genes are regulated by signals other than JAK2–STAT5 pathway.57 One such gene is lmo2, which is involved in normal hematopoiesis and in leukemogenesis. JAK2 inhibitors decrease H3Y41 phosphorylation and promote HP1α binding at the lmo2 transcriptional start site, which results in downregulation of lmo2 expression. Therefore, the JAK2-H3Y41-HP1α pathway interconnects JAK2 kinase activity, histone phosphorylation, aberrant gene expression and genome instability.
Recently, JAK2V617F was found to bind and phosphorylate the protein arginine methyltransferase 5 (PRMT5) much more efficiently than JAK2.58 Such modification impairs the activity of PRMT5 and impedes its interaction with MEP50 (methylosome protein 50). The net result is a marked decrease in global arginine methylation of histones H2A and H4, both targets of MEP50. The gain-of-function activities described above represent non-canonical functions of JAK2V617F that contribute to tumorigenesis through epigenomic alterations.58
Clinical-Translational Advances
Clinical trials with JAK tyrosine kinase inhibitors
Various JAK2 inhibitors have been tested in clinical trials for patients with intermediate or high-risk MF. An account of the in vitro activity of the ones further in clinical development is presented in Table 1.59 Ruxolitinib potently inhibits the phosphorylation of JAK1, JAK2, JAK2V617F, STAT5, and ERK1/2 in vitro, which is coupled with induction of apoptosis.60 In a phase I/II study in 153 patients with MF, the dose-limiting toxicity (DLT) was grade 4 thrombocytopenia.61 Ruxolitinib markedly reduced the levels of multiple fibrogenic and pro-inflammatory cytokines (e.g. IL-6, IL-8, TNF-alpha) regardless of JAK2 mutational status, suggesting that the clinical activity of ruxolitinib may be partly due to its JAK1 inhibitory activity.61 In the phase III study COMFORT-I, patients with MF were randomized to placebo (_n_=154) or ruxolitinib (_n_=155). The primary endpoint, ≥35% volumetric reduction of the spleen at week 24, occurred in 41.9% vs 0.7% (p<0.001) of patients receiving ruxolitinib or placebo, respectively.62 Similarly, ruxolitinib improved total symptom score by at least 50% in 45.9% vs 5.3% with placebo.62 After a median follow-up of 24 months, fewer deaths were observed in the ruxolitinib arm (27 vs. 41 for placebo; _p_=0.028).63 The most common grade 3–4 adverse events with ruxolitinib were anemia (45.2% vs. 19.2% with placebo) and thrombocytopenia (12.9% vs. 1.3% with placebo).62 COMFORT-II randomized patients with intermediate or high-risk MF 2:1 to ruxolitinib (n=146) or best available therapy (BAT; n=73).64 Reduction of spleen volume ≥35% at 48 weeks occurred in 28.5% of patients with ruxolitinib and 0% in those receiving BAT (p<0.001). After a median follow-up of 28 months, the percentage of deaths was lower in the ruxolitinib arm (14% vs. 22% for BAT; _p_=0.041).65 Since November 2011, ruxolitinib is approved by the FDA for treating intermediate- and high-risk MF based on the results of the COMFORT trials.
TABLE 1.
Activity of selected JAK kinase inhibitors currently being evaluated in clinical trials.
| Compound | IC50 (nanomolar) | Stage of Development | Reference | |||
|---|---|---|---|---|---|---|
| JAK1 | JAK2 | JAK3 | TYK2 | |||
| Ruxolitinib | 3.3 | 2.8 | 428 | 19 | Approved inmyelofibrosis, Phase IIIin polycythemia Vera,Phase II in pancreaticcancer, breast cancer,and acute leukemia | 50,61 |
| SAR302503 | 105 | 3 | 1002 | 405 | Phase III inmyelofibrosis, Phase I insolid tumors | 79 |
| Pacritinib(SB1518) | 23 | 23 | 56 | 2 | Phase I in hematologicmalignancies, Phase I inlymphoma, Phase II inmyelofibrosis | 80,72 |
| CYT387 | 11 | 18 | 155 | NA | Phase II | 81,82 |
| AZD1480 | 1.3 | <0.4 | 3.9 | NA | Phase I in solid tumors,Phase II in myelofibrosis | 83 |
| Tasocitinib1,2 | 1.7 | 1.8 | 0.75 | 260 | Approved in rheumatoidarthritis** | 84 |
| INCB0280502 | 5.9 | 5.7 | 560 | 53 | Phase II in rheumatoidarthritis** | 85 |
SAR302503 (formerly TG101348) inhibits JAK2 and JAK2V617F preferentially over other members of the JAK family of kinases, and only 3 (JAK2, FLT3, and RET) among 223 kinases tested were inhibited by SAR302503 with an IC50 <50nM.51 In a phase I study,66 the maximum tolerated dose (MTD) of SAR302503 was 680 mg/d. Grade 3/4 anemia, thrombocytopenia, and neutropenia occurred in 35%, 24%, and 10%, respectively. After 12 cycles, 47% of patients in the MTD cohort achieved ≥50% decrease in splenomegaly sustained for ≥8 weeks.66,67 In a phase II study patients were randomized to SAR302503 at 300mg, 400mg, or 500mg daily. Reduction of spleen volume ≥35% at the end of cycle 3 were dose dependent: 30%, 50%, and 64% for the 300mg, 400mg, and 500mg daily groups, respectively, which correlated with inhibition of STAT3 phosphorylation.68 A phase III placebo-controlled study of SAR302503 in MF is underway.
CYT387 is a JAK1/2 inhibitor that is being studied in a phase I/II study that includes 166 patients with MF in the core portion of the study and 120 patients in the multicenter extension portion.69 The MTD was determined to be 300mg/day and the DLT was reached at 400mg/day.70 After a median follow-up of 16.9 months, 13% of patients in the core study had an increase in hemoglobin level of at least 2g/dL and 37% had durable reduction in spleen length by palpation of at least 50%. Based on these encouraging results the dose of 300mg/daily was selected for a phase III study that will be conducted as a strategy for the approval of this agent.
Pacritinib is a selective JAK2 inhibitor that has been tested in a phase II study involving 34 patients with MF. Eleven (32%) patients had ≥35% reduction in spleen volume at 24 weeks.71 The most frequent toxicity was gastrointestinal, although it was generally mild and manageable, without significant myelosuppresion.71 Two patients had clinical improvement of hemoglobin and a significant proportion of patients had improvement of constitutional symptoms. A phase III study of pacritinib for patients with low platelets is being planned. Of note, pacritinib has been recently tested in a phase I study in 34 patients with relapsed/refractory Hodgkin’s lymphoma or NHL. Pacritinib was well tolerated and the MTD was not reached. Three patients had partial response and 15 had stable disease.72
Overcoming JAK2 inhibitor resistance
While JAK2 inhibitors have proven effective in patients with MPNs, a fraction of them will have suboptimal responses and overall none will experience significant reductions in JAK2V617F allele burden, indicating persistence of the malignant clones. It has been recently shown that such resistance to JAK2 inhibitor therapy is not mediated by secondary mutations in the JAK2V617F kinase. Rather, it is the consequence of the reactivation of the JAK/STAT pathway via heterodimerization of activated JAK2 and JAK1 or TYK2 that promotes resistance to JAK2 inhibitor-induced apoptosis.73 Chronic JAK2 inhibitor therapy results in stabilization of activated JAK2 and an increase in JAK2 mRNA expression, which facilitates the formation of heterodimers. Notably, removal of JAK2 inhibitor treatment results in resistant JAK2V617F-positive cell resensitization, suggesting that patients resistant to JAK2 inhibitors might respond to retreatment with the same JAK2 inhibitor or with others after a period of therapy discontinuation.
It has been shown that Hsp90 inhibitors or histone deacetylase inhibitors (HDACi) promote JAK2 degradation, which suggests a potential role for these agents in the treatment of JAK2 inhibitor resistant MPNs.74,75 Treatment of cells with persistent JAK2 inhibitor-induced JAK/STAT pathway activation with the Hsp90 inhibitor PU-H71 resulted in JAK2 degradation and decreased activation of the JAK/STAT pathway. The combination of panobinostat, which is known to inhibit the chaperone function of Hsp90 and promote proteasomal degradation of JAK2V617F, with the JAK2 inhibitor TG101209 synergistically induced apoptosis of HEL and Ba/F3-JAK2V617F cells and exerted greater cytotoxicity against primary CD34+ MPN cells than normal CD34+ hematopoietic progenitor cells.74 Murine models suggest that the combination of a HDACi such as panobinostat with ruxolitinib exhibits markedly improved anticancer activity compared with either agent alone in a JAK2V617F bone marrow transplantation mouse model of MPN.76
An alternative strategy to eliminate resistance cells is the use of type II JAK2 inhibitors such as BBT-594, which unlike available JAK2 inhibitors, retain the ability to bind the inactive conformation of JAK2 and inhibit signaling emanating from mutant JAK2 in persistent JAK2V617F-positive cells.
In addition to cell autonomous mechanisms of resistance, extrinsic humoral factors secreted by the bone marrow microenvironment have been shown to protect MPN clones from JAK2 inhibitor therapy. We have recently shown the potent growth suppression exerted by the JAK2 inhibitor atiprimod on murine FDCP-EpoRV617F and JAK2V617F-positive human SET-2 cells while causing minimal effects on stromal cells.77 However, culture of JAK2V617F-positive cells on monolayers of stromal cells markedly impaired the ability of atiprimod to inhibit the phosphorylation of the JAK2/STAT3/5 pathway and the proliferation of JAK2V617F-positive cells. These effects are not due to direct interactions between the malignant clones and the marrow stroma. Rather, they are mediated by a network of cytokines secreted by the stromal cells such as IL-6, FGF, and IP-10.77 Blocking such cytokines with specific neutralizing antibodies restored JAK2 inhibitor sensitivity, thus demonstrating the importance of non-cell autonomous mechanisms of resistance against JAK2 inhibitors and the therapeutic potential of strategies targeting the bone marrow niche in MPNs.
Conclusions
Our understanding of the pathogenesis of MPNs has markedly improved in recent years. The critical importance of the JAK2V617F mutation has been validated in vitro as well as in vivo by means of murine models of MPNs driven by JAK2V617F. In addition, several novel mutations in other genes have since been described in patients with MPNs, including TET2 mutations, which can appear before the acquisition of JAK2V617F, or IKZF1, EZH2, and ASXL1, which appear to contribute to leukemic transformation.78
In spite of this wealth of information, several questions remain to be answered. First, the precise role of other mutated alleles in the pathogenesis of MPNs and potentially in the resistance to JAK kinase inhibitor therapy remains unknown. Second, mounting data indicate that mutant JAK2 induces epigenetic deregulation, which coupled with the fact that some of the mutant alleles found in MPNs outside of the JAK2 locus encode important epigenetic regulators, suggests the possibility that therapeutic epigenetic modifiers might play a role in the management of these malignancies. Third, available evidence indicates that JAK2 inhibition produces very modest effects on JAK2V617F allele burden, cytopenias, and bone marrow fibrosis, which suggests that such approach alone cannot significantly correct the malignant phenotype observed in patients with MF. Fourth, the therapeutic role of JAK1 inhibition versus that of JAK2 inhibition in MF is unclear. Ongoing studies with selective JAK1 inhibitors in this context are eagerly awaited. Finally, it remains to be seen whether the synergism observed in vitro between JAK2 inhibitors and other therapeutics will translate into efficacious therapeutic strategies in patients exhibiting resistance to JAK2 inhibitor therapy and whether the treatment paradigm of JAK2 inhibition can be successfully extended to other malignancies, hematological or otherwise, with deregulation of the JAK/STAT pathway.
Acknowledgments
SV received research support for conduct of clinical studies with JAK inhibitors from Incyte, Astrazeneca, SBIO, Lilly Oncology, Exelixis, NS Pharma, Bristol Myers Squibb, YM Biosciences, and Cephalon. Supported in part by P30 CA016672.
References
- 1.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 2.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 4.Silva M, Richard C, Benito A, Sanz C, Olalla I, Fernández-Luna JL. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N Engl J Med. 1998;338:564–571. doi: 10.1056/NEJM199802263380902. [DOI] [PubMed] [Google Scholar]
- 5.Spivak JL. The chronic myeloproliferative disorders: clonality and clinical heterogeneity. Semin Hematol. 2004;41:1–5. doi: 10.1053/j.seminhematol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100:4272–4290. doi: 10.1182/blood-2001-12-0349. [DOI] [PubMed] [Google Scholar]
- 7.Beer PA, Green AR. Pathogenesis and management of essential thrombocythemia. Hematology Am Soc Hematol Educ Program. 2009:621–628. doi: 10.1182/asheducation-2009.1.621. [DOI] [PubMed] [Google Scholar]
- 8.Finazzi G, Barbui T. How I treat patients with polycythemia vera. Blood. 2007;109:5104–5111. doi: 10.1182/blood-2006-12-038968. [DOI] [PubMed] [Google Scholar]
- 9.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 10.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 45:458–463. doi: 10.1038/bmt.2009.188. [DOI] [PubMed] [Google Scholar]
- 12.Stewart WA, Pearce R, Kirkland KE, Bloor A, Thomson K, Apperley J, et al. The role of allogeneic SCT in primary myelofibrosis: a British Society for Blood and Marrow Transplantation study. Bone Marrow Transplant. doi: 10.1038/bmt.2010.14. [DOI] [PubMed] [Google Scholar]
- 13.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffé M, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 14.Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J, Philip P, et al. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- 15.Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000;95:19–29. [PubMed] [Google Scholar]
- 16.Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bercovich D, Ganmore I, Scott LM, Wainreb G, Birger Y, Elimelech A, et al. Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet. 2008;372:1484–1492. doi: 10.1016/S0140-6736(08)61341-0. [DOI] [PubMed] [Google Scholar]
- 18.Kearney L, Gonzalez De Castro D, Yeung J, Procter J, Horsley SW, Eguchi-Ishimae M, et al. Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood. 2009;113:646–648. doi: 10.1182/blood-2008-08-170928. [DOI] [PubMed] [Google Scholar]
- 19.Xiang Z, Zhao Y, Mitaksov V, Kasai Y, Molitoris A, Ries RE, et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood. 2008;111:4809–4812. doi: 10.1182/blood-2007-05-090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M, et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10:65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Kim YG, Soung YH, Han KJ, Kim SY, Rhim HS, et al. The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene. 2006;25:1434–1436. doi: 10.1038/sj.onc.1209163. [DOI] [PubMed] [Google Scholar]
- 22.Jelinek J, Oki Y, Gharibyan V, Bueso-Ramos C, Prchal JT, Verstovsek S, et al. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106:3370–3373. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traer E, MacKenzie R, Snead J, Agarwal A, Eiring AM, O'Hare T, et al. Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia. 26:1140–1143. doi: 10.1038/leu.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joos S, Kupper M, Ohl S, von Bonin F, Mechtersheimer G, Bentz M, et al. Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Res. 2000;60:549–552. [PubMed] [Google Scholar]
- 25.Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 29.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 30.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 31.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan KJ, Gilliland DG. A role for JAK2 mutations in myeloproliferative diseases. Annu Rev Med. 2008;59:213–222. doi: 10.1146/annurev.med.59.061506.154159. [DOI] [PubMed] [Google Scholar]
- 35.Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pietra D, Li S, Brisci A, Rumi E, Theocharides A, Ferrari M, et al. Somatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disorders. Blood. 2008;111:1686–1689. doi: 10.1182/blood-2007-07-101576. [DOI] [PubMed] [Google Scholar]
- 37.Pardanani A, Lasho TL, Finke C, Hanson CA, Tefferi Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia. 2007;21:1960–1963. doi: 10.1038/sj.leu.2404810. [DOI] [PubMed] [Google Scholar]
- 38.Wang YL, Vandris K, Jones A, Cross NC, Christos P, Adriano F, et al. JAK2 Mutations are present in all cases of polycythemia vera. Leukemia. 2008;22:1289. doi: 10.1038/sj.leu.2405047. [DOI] [PubMed] [Google Scholar]
- 39.Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 41.Vannucchi AM, Antonioli E, Guglielmelli P, Pancrazzi A, Guerini V, Barosi G, et al. Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood. 2008;112:844–847. doi: 10.1182/blood-2008-01-135897. [DOI] [PubMed] [Google Scholar]
- 42.Beer PA, Campbell PJ, Scott LM, Bench AJ, Erber WN, Bareford D, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008;112:141–149. doi: 10.1182/blood-2008-01-131664. [DOI] [PubMed] [Google Scholar]
- 43.Bersenev A, Wu C, Balcerek J, Jing J, Kundu M, Blobel GA, et al. Lnk constrains myeloproliferative diseases in mice. J Clin Invest. 120:2058–2069. doi: 10.1172/JCI42032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jamieson CH, Gotlib J, Durocher JA, Chao MP, Mariappan MR, Lay M, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc Natl Acad Sci U S A. 2006;103:6224–6229. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishii T, Bruno E, Hoffman R, Xu Me. Involvement of various hematopoietic-cell lineages by the JAK2V617F mutation in polycythemia vera. Blood. 2006;108:3128–3134. doi: 10.1182/blood-2006-04-017392. [DOI] [PubMed] [Google Scholar]
- 46.Delhommeau F, Dupont S, Tonetti C, Masse A, Godin I, Le Couedic JP, et al. Evidence that the JAK2 G1849T (V617F) mutation occurs in a lymphomyeloid progenitor in polycythemia vera and idiopathic myelofibrosis. Blood. 2007;109:71–77. doi: 10.1182/blood-2006-03-007146. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zamegar S, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci U S A. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jamieson CH, Barroga CF, Vainchenker WP. Miscreant myeloproliferative disorder stem cells. Leukemia. 2008;22:2011–2019. doi: 10.1038/leu.2008.290. [DOI] [PubMed] [Google Scholar]
- 49.Kota J, Caceres N, Constantinescu SN. Aberrant signal transduction pathways in myeloproliferative neoplasms. Leukemia. 2008;22:1828–1840. doi: 10.1038/leu.2008.236. [DOI] [PubMed] [Google Scholar]
- 50.Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 115:3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wernig G, Kharas MG, Okabe R, Moore SA, Leeman DS, Cullen DE, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Ugo V, Marzac C, Teyssandier I, Larbret F, Lecluse Y, Debili N, et al. Multiple signaling pathways are involved in erythropoietin-independent differentiation of erythroid progenitors in polycythemia vera. Exp Hematol. 2004;32:179–187. doi: 10.1016/j.exphem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dimhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 54.Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 17:584–596. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 115:3589–3597. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu F, Zhao X, Perna F, Koppikar P, Abdel-Wahab O, Harr MW, et al. JAK2 V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell. 2011;19:283–294. doi: 10.1016/j.ccr.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quintas-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10:127–140. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]
- 60.Nussenzveig RH, Cortes J, Sever M, Quintas-Cardama A, Ault P, Manshouri T, et al. Imatinib mesylate therapy for polycythemia vera: final result of a phase II study initiated in 2001. Int J Hematol. 2009;90:58–63. doi: 10.1007/s12185-009-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verstovsek S, Kantarjian H, Mesa R, Pardanani AD, Cortes-Franco J, et al. Safety and efficacy of a JAK1 & JAK2 inhibitor, INCB018424, in myelofibrosis. N Engl J Med Epub ahead of print. 2010 doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verstovsek S, Mesa RA, Gotlib J, Levy R, Gupta V, DiPersio JF, et al. Long-Term Outcome of Ruxolitinib Treatment in Patients with Myelofibrosis: Durable Reductions in Spleen Volume, Improvements in Quality of Life, and Overall Survival Advantage in COMFORT-I. Blood. 2012;120 (abstract 800) [Google Scholar]
- 64.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 65.Cervantes F, Kiladjian JJ, Niederwieser D, Sirulnik A, Stalbovskaya V, McQuitty M, et al. Long-Term Safety, Efficacy, and Survival Findings From Comfort-II, a Phase 3 Study Comparing Ruxolitinib with Best Available Therapy (BAT) for the Treatment of Myelofibrosis (MF) Blood. 2012;120 (abstract 800) [Google Scholar]
- 66.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone R, et al. SAR302503: interim safety, efficacy and long-term impact on JAK2 V617F allele burden in a phase I/II study in patients with myelofibrosis. Blood. 2011;118 abstract 3838. [Google Scholar]
- 68.Talpaz M, Jamieson C, Gabrail NY, Lebedinsky C, Neumann F, Gao G, et al. A phase II randomized dose-ranging study of the JAK2-selective inhibitor SAR302503 in patients with intermediate-2 or high-risk primary myelofibrosis (MF), post-polycythemia vera MF or post-essential thrombocythemia MF. Blood. 2012;120 (abstract 2837) [Google Scholar]
- 69.Pardanani A, Gotlib J, Gupta V, Roberts AW, Wadleigh M, Sirhan S, et al. Phase I/II Study of CYT387, a JAK1/JAK2 Inhibitor for the Treatment of Myelofibrosis. Blood. 2012;120 (abstract 178) [Google Scholar]
- 70.Pardanani A, George G, Lasho T, Hogan WJ, Litzow MR, Begna K, et al. A phase I/II study of CYT387, an oral JAK-1/2 inhibitor, in myelofibrosis: significant response rates in anemia, splenomegaly, and constitutional symptoms. Blood. 2010;116 abstract 460. [Google Scholar]
- 71.Komrokji R, Wadleigh M, Seymour JF, Roberts AW, To LB, Zhu HJ, et al. Results of a phase 2 study of pacritinib (SB1518), a novel oral JAK2 inhibitor, in patients with primary, post-polycythemia vera, and post-essential thrombocythemia myelofibrosis. Blood. 2011;118 abstract 282. [Google Scholar]
- 72.Younes A, Romaguera J, Fanale M, McLaughlin P, Hagemeister F, Copeland A, et al. Phase I Study of a Novel Oral Janus Kinase 2 Inhibitor, SB1518, in Patients With Relapsed Lymphoma: Evidence of Clinical and Biologic Activity in Multiple Lymphoma Subtypes. J Clin Oncol. 30:4161–4167. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489:155–159. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Fiskus W, Chong DG, Buckley KM, Natarajan K, Rao R, et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009;114:5024–5033. doi: 10.1182/blood-2009-05-222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerini V, Barbui V, Spinelli O, Salvi A, Dellacasa C, Carobbio A, et al. The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2V617F. Leukemia. 2009;22:740–747. doi: 10.1038/sj.leu.2405049. [DOI] [PubMed] [Google Scholar]
- 76.Baffert F, Evrot E, Ebel N, Roelli C, Andraos R, Qian Z, et al. Improved Efficacy Upon Combined JAK1/2 and Pan-Deacetylase Inhibition Using Ruxolitinib (INC424) and Panobinostat (LBH589) in Preclinical Mouse Models of JAK2V617F-Driven Disease. Blood. 2011;118 (abstract 798) [Google Scholar]
- 77.Manshouri T, Estrov Z, Quintas-Cardama A, Burger J, Zhang Y, Livun A, et al. Bone marrow stroma-secreted cytokines protect JAK2(V617F)-mutated cells from the effects of a JAK2 inhibitor. Cancer Res. 2011;71:3831–3840. doi: 10.1158/0008-5472.CAN-10-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 24:1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pardanani A, Gotlib J, Jamieson C, Cortes J, Talpaz M, Stone R, et al. A phase I study of TG101348, a selective JAK2 inhibitor, in myelofibrosis: clinical response is accompanied by significant reduction in JAK2V617F allele burden. Blood. 2009;114 (Abstract 755) [Google Scholar]
- 80.Verstovsek S, Odenike O, Scott B, Estrov Z, Cortes j, Thomas DA, et al. Phase I Dose-Escalation Trial of SB1518, a Novel JAK2/FLT3 Inhibitor, in Acute and Chronic Myeloid Diseases, Including Primary or Post-Essential Thrombocythemia/ Polycythemia Vera Myelofibrosis. Blood. 2009;114 (abstract 3905) [Google Scholar]
- 81.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23:1441–1445. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 82.Tyner JW, Bumm TG, Deininger J, Wood L, Aichberger KJ, Loriaux MM, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 115:5232–5240. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang JK, Ghoreschi K, Deflorian F, Chen Z, Perreira M, Pesu M, et al. Examining the chirality, conformation and selective kinase inhibition of 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperi din-1-yl)-3-oxopropanenitrile (CP-690,550) J Med Chem. 2008;51:8012–8018. doi: 10.1021/jm801142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 184:5298–5307. doi: 10.4049/jimmunol.0902819. [DOI] [PubMed] [Google Scholar]