The Transition from Proliferation to Differentiation Is Delayed in Satellite Cells from Mice Lacking MyoD (original) (raw)
. Author manuscript; available in PMC: 2016 Sep 19.
Published in final edited form as: Dev Biol. 1999 Jun 15;210(2):440–455. doi: 10.1006/dbio.1999.9284
Abstract
Satellite cells from adult rat muscle coexpress proliferating cell nuclear antigen and MyoD upon entry into the cell cycle, suggesting that MyoD plays a role during the recruitment of satellite cells. Moreover, the finding that muscle regeneration is compromised in MyoD−/− mice, has provided evidence for the role of MyoD during myogenesis in adult muscle. In order to gain further insight into the role of MyoD during myogenesis in the adult, we compared satellite cells from MyoD−/− and wildtype mice as they progress through myogenesis in single-myofiber cultures and in tissue-dissociated cell cultures (primary cultures). Satellite cells undergoing proliferation and differentiation were traced immunohistochemically using antibodies against various regulatory proteins. In addition, an antibody against the mitogen-activated protein kinases ERK1 and ERK2 was used to localize the cytoplasm of the fiber-associated satellite cells regardless of their ability to express specific myogenic regulatory factor proteins. We show that during the initial days in culture the myofibers isolated from both the MyoD−/− and the wildtype mice contain the same number of proliferating, ERK+ satellite cells. However, the MyoD−/− satellite cells continue to proliferate and only a very small number of cells transit into the myogenin+ state, whereas the wildtype cells exit the proliferative compartment and enter the myogenin+ stage. Analyzing tissue-dissociated cultures of MyoD−/− satellite cells, we identified numerous cells whose nuclei were positive for the Myf5 protein. In contrast, quantification of Myf5+ cells in the wildtype cultures was difficult due to the low level of Myf5 protein present. The Myf5+ cells in the MyoD−/− cultures were often positive for desmin, similar to the MyoD+ cells in the wildtype cultures. Myogenin+ cells were identified in the MyoD−/− primary cultures, but their appearance was delayed compared to the wildtype cells. These “delayed” myogenin+ cells can express other differentiation markers such as MEF2A and cyclin D3 and fuse into myotubes. Taken together, our studies suggest that the presence of MyoD is critical for the normal progression of satellite cells into the myogenin+, differentiative state. It is further proposed that the Myf5+/MyoD− phenotype may represent the myogenic stem cell compartment which is capable of maintaining the myogenic precursor pool in the adult muscle.
INTRODUCTION
Satellite cells, the myogenic precursors in postnatal and adult skeletal muscle, are located between the basement membrane and the plasma membrane of myofibers in growing and mature muscle (Mauro, 1961; Bischoff 1989; Yablonka-Reuveni, 1995). At least some of the satellite cells are mitotically active in the growing muscle, contributing myonuclei to the enlarging fibers (Moss and Leblond, 1971). As muscle matures, the addition of myofiber nuclei ceases and the satellite cells become mitotically quiescent (Schultz et al., 1978). These quiescent myogenic precursors can become mitotically active in response to various muscle stresses and their progeny can fuse into preexisting fibers or form new myofibers (reviewed in Grounds and Yablonka-Reuveni, 1993; Schultz and McCormick, 1994). Overt muscle injury is not the only condition that leads to satellite cell proliferation. Recruitment of these precursors occurs in response to more subtle stresses such as stretch, exercise, and muscle hypertrophy (Appell et al., 1988; Snow, 1990; Winchester et al., 1991; Schultz and McCormick, 1994).
Following their activation in vivo, satellite cells enter a program which involves the expression of the myogenic regulatory factors (MRFs) (Grounds et al., 1992; Füchtbauer and Westphal, 1992; Koishi et al., 1995; Anderson et al., 1998; McIntosh et al., 1998). These MRFs form the basic-helix-loop-helix family of myogenic transcription factors, which consists of MyoD, Myf5, myogenin, and MRF4, and is thought to be involved in the specification of the skeletal myogenic lineage during embryogenesis. MyoD and Myf5 are expressed earlier during muscle development and are involved in the determination of the myogenic lineage. Myogenin and MRF4 are expressed later as myoblasts progress through differentiation and are likely acting as differentiation factors (reviewed in Megeney and Rudnicki, 1995; Yun and Wold, 1996; Buckingham et al., 1998).
The MRFs are also detected in cultures of satellite cells and cell lines derived from these precursors (Wright et al., 1989; Hinterberger et al., 1991; T. H. Smith et al., 1993; C. K. Smith et al., 1994; Maley et al., 1994; Yablonka-Reuveni and Rivera, 1994, 1997a). The expression of MRFs by cells already committed to the muscle lineage likely reflects the role of MRFs in the transition from proliferation to differentiation (reviewed in Olson, 1992, 1993; Weintraub, 1993). Indeed, following their isolation and culturing, quiescent satellite cells enter the cell cycle and express MyoD concomitantly with cell proliferation (Yablonka-Reuveni and Rivera, 1994; Yablonka-Reuveni et al., 1999). Myogenin expression lags behind MyoD in satellite cell cultures and correlates with cell cycle withdrawal and transition into differentiation (C. K. Smith et al., 1994; Yablonka-Reuveni and Rivera, 1994; Yablonka-Reuveni et al., 1999). MRF transcript analysis in single cells has suggested that satellite cells may first express either MyoD or Myf5 and, subsequently, will coexpress both MyoD and Myf5 followed by myogenin and MRF4 expression (Cornelison and Wold, 1997).
The finding that MyoD protein is expressed concomitantly with proliferating cell nuclear antigen (PCNA) following activation of rat satellite cells in single fiber cultures has suggested a possible role for MyoD during satellite cell recruitment (Yablonka-Reuveni and Rivera, 1994; Yablonka-Reuveni et al., 1999). Moreover, the discovery that the muscle of the MyoD−/− mutant mouse was severely deficient in regenerative capacity following injury has provided evidence for a possible role of MyoD in the adult muscle (Megeney et al., 1996; McIntosh et al., 1998). The in vivo studies with the MyoD-deficient mouse led to the proposal that in the absence of MyoD the adult myogenic precursors (satellite cells) undergo enhanced self-renewal rather then give rise to progeny which are able to undergo differentiation (Megeney et al., 1996).
The present study was carried out to gain further insight into the role of MyoD during myogenesis of satellite cells. We compared the capacity of satellite cells from MyoD−/− and wildtype mice to progress through myogenesis in two different culture models: isolated myofibers and isolated satellite cells. The isolated myofibers retain their few satellite cells in the original site underneath the basement membrane, allowing analysis of satellite cells in their native position by the myofiber without the complexity of the intact tissue (Bischoff, 1986, 1989; Yablonka-Reuveni and Rivera, 1994, 1997b; Yablonka-Reuveni et al., 1999). This association between the satellite cells and the myofiber basement and plasma membranes is potentially important for maintaining the satellite cells in a quiescent or proliferative state (Bischoff, 1990a,b). Additionally, the myofibers can potentially serve as a paracrine source for various growth-regulating agents required by the satellite cells. Satellite cell dynamics in the isolated fiber system may model myogenesis in an environment in which the myofiber architecture is preserved, such as muscle growth and hypertrophy. In contrast, in cultures of tissue-dissociated cells (i.e., primary cultures), the dynamics of myogenesis may more closely model events following overt muscle trauma in which new myofibers are formed.
To study the mouse myofibers, we resorted to the same approach described for the rat system in which we showed that antibodies against PCNA, MyoD, or myogenin recognized a small number of nuclei associated with the myofibers; these nuclei were proven to be nuclei of satellite cells and were not merely myofiber nuclei (Yablonka-Reuveni and Rivera, 1994, 1997b). Tracing of the rat satellite cell cytoplasm regardless of its state along the myogenic program was additionally achieved by immunostaining of the fiber cultures with an antibody against the extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Yablonka-Reuveni et al., 1999). ERK1/2 are members of the mitogen-activated protein kinase superfamily, which is involved in the transmission of extracellular signals to their intracellular targets. While activated ERK1/2 are dually phosphorylated on specific tyrosine and threonine residues, the cells may also contain nonphosphorylated and singly phosphorylated ERK1/ERK2 (for reviews see Robinson and Cobb, 1997; Lewis et al., 1998). The antibody against ERK1/2 used for tracing satellite cells recognizes both the phosphorylated and the nonphosphorylated forms (Seger et al., 1994; Yablonka-Reuveni et al., 1999). The proliferation of the MyoD−/− and wildtype satellite cells in mouse myofiber cultures was further compared upon the addition of fibro-blast growth factor 2 (FGF2, basic FGF). In the rat myofiber model, the addition of FGF2 enhances the number of proliferating (PCNA+/MyoD+) satellite cells and leads to a subsequent increase in the number of differentiating (myogenin+) cells (Bischoff, 1989; Yablonka-Reuveni and Rivera, 1994, 1997b; Yablonka-Reuveni et al., 1999). The progression of myogenesis in the primary cultures of MyoD−/− and wildtype mouse satellite cells was also analyzed employing immunocytochemistry as previously described for the mouse-derived C2 cells (Yablonka-Reuveni and Rivera, 1997a).
We show that satellite cells lacking MyoD remain in the proliferative state for an extended amount of time and that their transition into the myogenin+ state is suppressed. In addition, the progeny of the cultured MyoD−/− satellite cells show far more Myf5+ cells than those of the wildtype cells. While the transition to the myogenin+ state is suppressed in the MyoD−/− cultures, the myogenin+ cells that appear in these cultures express various proteins whose presence correlates with muscle differentiation. Taken together, our studies suggest that the expression of MyoD is critical for the normal progression of satellite cells into the differentiating state. The delayed emergence of myogenin+ cells seen in cultures of satellite cells lacking MyoD likely contributes to the impaired regeneration of MyoD−/− muscles seen in vivo. It remains to be determined whether it is the lack of MyoD and/or the persistent nuclear expression of Myf5 that alters the differentiation capacity of the MyoD−/− satellite cells.
METHODS
Animals
Balb/C mice (purchased from B & K Universal, Kent, WA) and MyoD−/− mice (Rudnicki et al., 1992) were used throughout the study. Cultures were prepared from muscles of 6- to 10-week-old mice. Cohorts of either all males or all females were used in individual experiments and gender-related differences were not observed. The Balb/C strain was used as wildtype control in the study because the MyoD−/− mice are in a Balb/C-enriched genetic background (over three back crosses).
Cultures of Isolated Mouse Myofibers
Single muscle fibers with associated satellite cells were prepared from the flexor digitorum brevis (FDB) muscle of mouse hindfoot in a manner similar to that previously described for “young adult” rats (Yablonka-Reuveni and Rivera, 1994; Yablonka-Reuveni et al., 1999), except that all procedures used DMEM (Gibco BRL, Gaithersburg, MD) instead of MEM. Typically, muscles from both hindfeet of three to six mice were used for each preparation. FDB muscles, freed from outer connective tissue, were immersed in 0.2% collagenase type 1 (Sigma, St. Louis, MO) and incubated at 37.5°C for 2.5 h. Single fibers were released from the collagenase-treated muscles by gentle trituration of the tissue for about 5 min and further purified by settling three times through DMEM containing 10% horse serum. The final fiber sediment in residual medium was dispensed as 50-_μ_l aliquots into 35-mm tissue culture plates coated with 0.12 ml of isotonic Vitrogen 100 solution (stock concentration at 2.9 mg/ml; Celtrix Laboratories, Palo Alto, CA). Fiber cultures were preincubated for 20 min at 37.5°C in humidified air containing 5% CO2 to allow formation of Vitrogen gel and adherence of fibers to the Vitrogen. Following the initial incubation, cultures received 1 ml of basal medium with or without FGF2 at 2 ng/ml and incubation continued under the same conditions as for the preincubation replacing the medium (±FGF2) daily. The basal medium consisted of DMEM containing 20% Controlled Process Serum Replacement (CPSR2; Sigma), 1% horse serum (Sigma), and antibiotics (Yablonka-Reuveni and Rivera, 1997a). FGF2 (human recombinant, kindly provided by Dr. S. Hauschka, University of Washington) was added to enhance the number of satellite cells undergoing myogenesis. As shown in the results, only a small number of satellite cells can be detected in Balb/C fibers without the addition of FGF2.
Cultures of Tissue-Dissociated Mouse Satellite Cells
Satellite cells were isolated from several hindlimb muscles (see details in tables and figure legends) and from the diaphragm muscle. Muscles were typically collected from the same mice used for preparing cultures of single myofibers. Each batch of specific muscle type was processed separately. Muscles, cleaned from the surrounding connective tissue, were minced into small fragments and incubated with collagenase using the same conditions as for fiber isolation. The collagenase-treated preparations were subsequently triturated and the resulting suspensions were filtered through a double layer of lens tissue. Cells were harvested by centrifugation (300_g,_ 10 min), resuspended in DMEM containing 10% horse serum, filtered again through lens tissue, and collected again by centrifugation. This repetitive filtering and centrifugation removed most of the muscle debris which is generated during the enzymatic dissociation of satellite cells. The final cell pellet was resuspended in growth medium and cultured in 35-mm plates precoated with 2% gelatin. The growth medium, which was changed every 2–3 days, consisted of DMEM containing 25% fetal calf serum (Sigma), 10% horse serum (Sigma), 1% chicken embryo extract, and antibiotics (Yablonka-Reuveni and Rivera, 1997a). Cultures from the diaphragm and the quadriceps femoris muscles (which yielded more cells than other muscles) were initiated at 2 × 105 cells per plate. Cells isolated from other hindlimb muscles were seeded at 104 to 5 × 104 cells per plate.
Immunolabeling and Counterstaining of Nuclei
Indirect immunofluorescence of methanol-fixed cultures and staining of nuclei with DAPI were performed as previously described (Yablonka-Reuveni and Rivera, 1994, 1997a; Yablonka-Reuveni et al., 1999). Secondary antibodies were from Organon-Technika Cappel (Downington, PA). A fluorescein-conjugated goat anti-mouse IgG was used for the mouse monoclonal primary antibodies and rhodamine-conjugated goat anti-rabbit IgG was used for the rabbit polyclonal primary antibodies.
Primary Antibodies
All primary antibodies used in the present study (excluding the anti-Myf5 and the anti-cyclin D3) have been described in earlier studies (Yablonka-Reuveni and Rivera, 1994, 1997a,b; Yablonka-Reuveni et al., 1999). The following primary antibodies were used.
Anti-ERKs (pAb)
The polyclonal antibody against the mitogen-activated protein kinases ERK1 and ERK2 was raised in rabbit immunized with a peptide representing residues 307–327 of the ERK gene product. The peptide sequence is conserved in both ERK1 and ERK2, and the antibody recognizes both ERKs (phosphorylated and nonphosphorylated) with the same sensitivity. The antibody was originally produced by Drs. R. Seger and E. Krebs and is characterized in Gause et al. (1993) and in Seger et al. (1994). The use of this antibody to trace satellite cells in isolated rat fibers has been described in detail in Yablonka-Reuveni et al. (1999). We showed in the latter paper that ERK1 and ERK2 (44 and 42 kDa, respectively) and their slower-migrating phosphorylated forms have the same molecular weights when extracts of rat and mouse myogenic cultures were compared by immunoblotting. The antibody used for the current study was a gift from Drs. J. Campbell and E. Krebs.
Anti-Myf5 (pAb)
Two polyclonal antibodies against Myf5 were used. The antibodies were raised in rabbits immunized with either COOH-terminal domain or NH2-terminal domain peptides of rodent Myf5 (M. Primig and M. Buckingham, unpublished work). Immunofluorescence and immunoblotting of C2 mouse myoblasts with these anti-Myf5 antibodies were published (Aurade et al., 1997; Lindon et al., 1998; Carnac et al., 1998; Kitzmann et al., 1998). The C- and N-terminal-domain antibodies provided identical results when tested in myogenic cultures. Both antibodies were inappropriate for tracing Myf5+ cells in muscle sections or in fiber cultures due to some reactivity with the myofiber cytoplasm which prevented a clear distinction of Myf5+ nuclei. A similar reactivity with cultured myotubes was also described in Kitzmann et al. (1998) and was shown to be nonspecific. Our characterization of the antibodies further revealed that only myogenic cultures which express Myf5 mRNA react with the antibodies and show Myf5+ nuclei. These included cultures of the rat-derived L6 and L8 myogenic lines (Yaffe, 1968; Yaffe and Saxel, 1977a; obtained from the American Type Culture Collection (ATCC), Rockville, MD) and of the mouse myogenic C2 line (Yaffe and Saxel, 1977b; Yablonka-Reuveni and Rivera, 1997a). Cultures of the rat-derived line H9c2 (Kimes and Brandt, 1976; obtained from ATCC), which express mRNA for MyoD, myogenin, and MRF4 but not for Myf5, did not react with the antibodies (D. Graves, P. Natanson, and Z. Yablonka-Reuveni, unpublished results). For the studies described in the paper we mostly used the antibody against the NH2-terminal domain.
Anti-PCNA (mAb 19F4)
A mouse monoclonal antibody against PCNA was from Boehringer Mannheim (Indianapolis, IN).
Anti-MyoD (mAb 5.8A)
A mouse monoclonal antibody against murine MyoD (IgG fraction) was developed and kindly provided by Drs. P. Houghton and P. Dias (St. Jude Children's Research Hospital, Memphis, TN) (Dias et al., 1992).
Anti-MyoD (pAb)
A rabbit polyclonal antibody against rodent MyoD was prepared and provided by Dr. S. Alemá (Institute of Cell Biology, CNR, Rome, Italy). We described in previous studies additional characterizations of this antibody (Yablonka-Reuveni and Rivera, 1994; Anderson et al., 1998). Double immunofluorescence of myogenic cells with the monoclonal and polyclonal antibodies against MyoD showed that the two antibodies have the same staining pattern and neither antibody reacted with myogenic cultures from MyoD−/− mice.
Anti-myogenin (mAb F5D)
A mouse monoclonal antibody against rodent myogenin was used in a hybridoma supernatant form. The F5D hybridoma was developed and kindly provided by Dr. W. Wright, University of Texas.
Anti-MEF2A (pAb)
A rabbit polyclonal antibody against MEF2A was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The use of this antibody to detect MEF2A in extracts of C2 cultures via immunoblotting was described in Molkentin et al. (1996). Using immunofluorescence we showed that in C2 cultures the anti-MEF2A stains nuclei of mononucleated cells positive for myogenin and of all nuclei in myotubes (Yablonka-Reuveni and Rivera, 1997a).
Anti-cyclin D3 (pAb)
A rabbit polyclonal antibody against cyclin D3 was purchased from Santa Cruz Biotechnology. Immunoblotting of extracts of smooth and skeletal muscle cultures from rat and mouse demonstrated a single protein band (unpublished results).
Anti-desmin (mAb D3)
A mouse monoclonal antibody against desmin (mAb D3, hybridoma supernatant) was originally developed by Danto and Fischman (1984) and was obtained from the Developmental Studies Hybridoma Bank (University of Iowa). Although originally made against chicken desmin, studies of various laboratories documented the specificity of the antibody for mouse and rat desmin as well (Allen et al., 1991; Yablonka-Reuveni and Rivera, 1997a).
Counting Positive Cells in Isolated Myofibers and in Primary Cultures
Myofiber cultures were monitored for the number of fiber-associated cells using two parallel 35-mm plates for each time point of an individual experiment. Immunopositive cells were scored as the number of positives on each individual fiber, analyzing 30 fibers per plate. The total number of positive cells in 30 fibers was then averaged for the duplicate plates. This value is eventually expressed per 10 fibers as shown in the figures. To analyze primary cultures, arbitrary fields in each culture were scored for total cell numbers (all DAPI-stained nuclei present), the number of cells stained with the antibodies examined, and the number of nuclei within myotubes. Depending on the density of the cultures, 20 to 50 fields were monitored per 35-mm culture plate. The results shown in the tables are of single experiments, analyzing single plates per time point due to the limitation in the number of cells that can be isolated from individual muscles. All experiments were repeated several times. In all studies we used a Nikon Optiphot 2 fluorescence microscope.
RESULTS
Immunoreactivity and Quantification of Satellite Cells in Mouse Myofiber Cultures
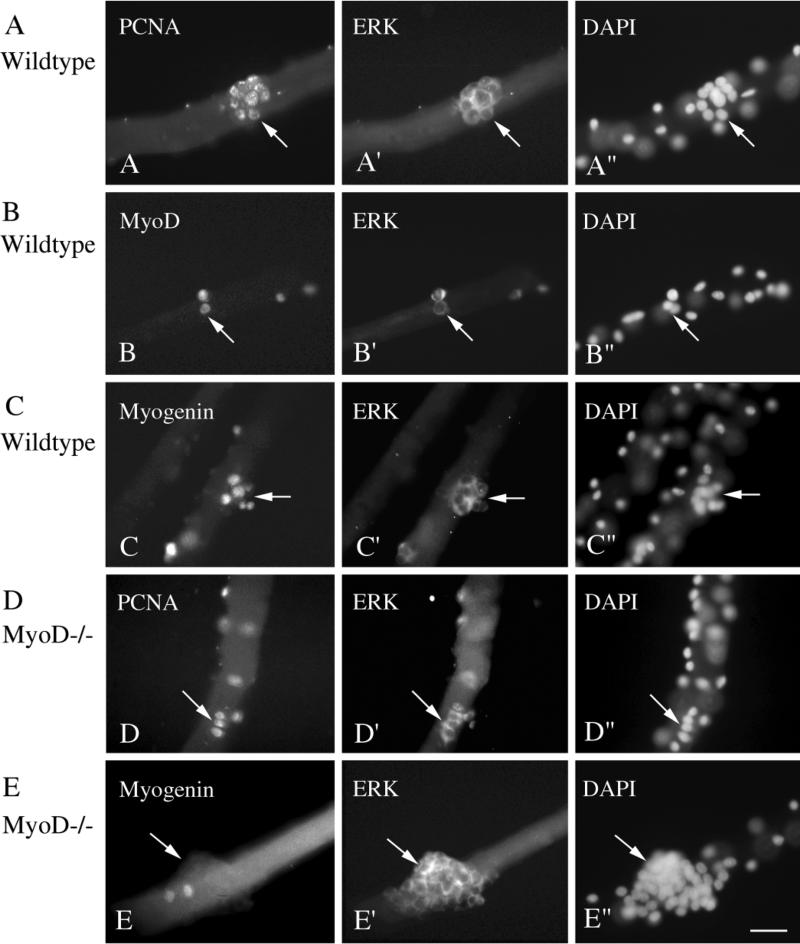
Figure 1 depicts micrographs of fiber cultures from Balb/C mice stained via double immunofluorescence with the antibodies against ERK1/ERK2 along with the antibodies against PCNA (Figs. 1A and 1A′), MyoD (Figs. 1B and 1B′), or myogenin (Figs. 1C and 1C′). Cultures were also stained with DAPI in order to trace the nuclei within the myofiber and the nuclei of the fiber-associated satellite cells (Figs. 1A″, 1B″, and 1C″). Nuclei positive for PCNA, MyoD, or myogenin were always colocalized with ERK+ cells. Immunohistochemical analysis of myofibers from the MyoD−/− mice detected nuclei positive for PCNA or myogenin, colocalized to ERK-positive cells (Figs. 1D, 1D′, 1E, and 1E′), but MyoD immunostaining was not found in the cultures. The lack of MyoD immunostaining was confirmed with both the monoclonal and the polyclonal antibody. For both Balb/C and MyoD−/− myofiber cultures, we cannot rule out the possibility that the cytoplasm of the myofiber itself is also positive for ERK. Nevertheless, the staining of the satellite cells with the anti-ERK is far more intense than that of the fiber cytoplasm, providing a means for cell tracing.
FIG. 1.
Micrographs of myofiber cultures from control wildtype mice (A, B, and C) and MyoD−/− mice (D and E) reacted via double immunofluorescence with monoclonal antibodies against the nuclear antigen PCNA, MyoD, or myogenin along with the polyclonal antibody against ERK1/ERK2. All micrographs depict cultures that were maintained in basal medium + FGF2 at 2 ng/ml. (A and A′) A day 4 culture reacted with anti-PCNA and anti-ERK. (B and B′) A day 3 culture reacted with anti-MyoD and anti-ERK. (C and C′) A day 4 culture reacted with anti-myogenin and anti-ERK. (D and D′) A day 3 culture reacted with anti-PCNA and anti-ERK. (E and E′) A day 5 culture reacted with anti-myogenin and anti-ERK. (A″, B″, C″, D″, and E″) Parallel micrographs of DAPI stain which highlight all myofiber and satellite cell nuclei. For each antibody combination, reactivity with the monoclonal antibody was visualized with a fluorescein-labeled secondary antibody and reactivity with the anti-ERK antibody was visualized rhodamine-labeled secondary antibody. Arrows in parallel images point to the same locations of cells as identified by immunostaining and by DAPI. Note that not all positive nuclei or cells on the myofibers are in the same focal plane. Bar, 34 _μ_m.
The immunofluorescence analysis often detected clustered cells in fibers from both the wildtype and the MyoD−/− mice. These clusters varied from small ones containing about 5–10 cells (Figs. 1A and 1C) to clusters containing numerous cells (Fig. 1E). These larger clusters were found only in the MyoD−/− fibers. Single cells and groups of 2–4 cells were also detected in both wildtype and MyoD−/− fibers (Figs. 1B and 1D). In wildtype fibers, nearly all cells within a cluster were positive for PCNA, MyoD, or myogenin (Figs. 1A, 1B, and 1C). Cell clusters in the MyoD−/− fibers were positive for PCNA, but most often were negative for myogenin even after 5–10 days in culture. Infrequently, some myogenin+ cells were identified in cell clusters that were nearly all myogenin-negative (Fig. 1E).
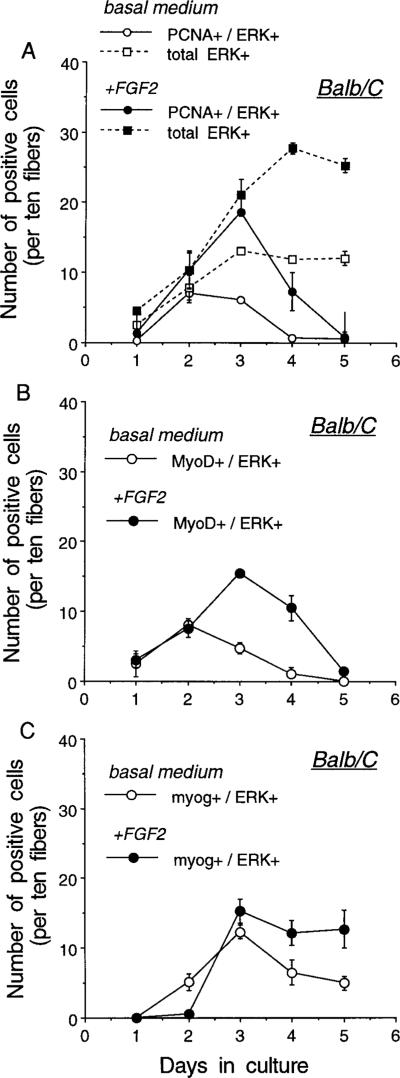
Quantification of fiber-associated cells positive for ERK, PCNA, MyoD, or myogenin in myofiber cultures from Balb/C and MyoD−/− mice is shown in Figs. 2 and 3, respectively. Studies were conducted with cultures maintained in basal medium ± FGF2 at 2 ng/ml. FGF2 was added to the myofiber cultures to increase the number of cells available for the analysis, since the number of satellite cells undergoing myogenesis in Balb/C cultures is far lower without the addition of FGF2 (Fig. 2). In addition, the FGF-treated myofiber cultures from Balb/C mice were used as controls for determining whether proliferation of satellite cells from the MyoD−/− mice can be influenced by FGF2 (see further details below in connection with Fig. 3). Figure 2 demonstrates that satellite cells undergoing myogenesis in myofibers from the Balb/C mice are first positive for PCNA and MyoD and subsequently become positive for myogenin. All PCNA+, MyoD+, and myogenin+ nuclei localized to ERK+ cells. For all three antibody pairs used, the total number of ERK+ cells for control and FGF2-treated cultures was similar to that shown in Fig. 2A. Kinetics of satellite cell proliferation and differentiation and an FGF2 effect similar to those seen with Balb/C myofiber cultures were also documented for cultured myofibers from other control mice of varying ages (data not shown). These included fibers isolated from younger C57Bl/10ScSn mice (6- to 10-week-old; The Jackson Laboratory, Bar Harbor, ME) and from older C57Bl/6 × DBA/2 mice (F1, 7- and 34-month-old; a gift from Dr. Norman Wolf, University of Washington).
FIG. 2.
Temporal appearance of cells or nuclei positive for ERK1/ERK2 and PCNA (A), MyoD (B), and myogenin (C) in cultures of myofibers isolated from Balb/C mice. Cultures were maintained in basal medium ± FGF2 at 2 ng/ml and the medium was changed daily. Plates were collected every 24 h and reacted via double immunofluorescence with the polyclonal antibody against ERK1/ERK2 in combination with the monoclonal antibodies against PCNA, MyoD, and myogenin. Immunostaining was performed as detailed in the legend to Fig. 1. Two parallel 35-mm plates were analyzed for each time point. For each panel, the total number of ERK+ cells and the number of doubly positive cells (PCNA+/ERK+, MyoD+/ERK+, myogenin+/ERK+) were determined. Nuclei positive for PCNA, MyoD, or myogenin which were not within ERK+ cells were never detected. The total number of ERK+ cells, shown in A, was similar for all three panels. Cells were scored as the number of positives on each individual fiber, analyzing 30 fibers per plate. Total positives were then averaged for duplicate plates and this value was eventually expressed per 10 fibers as indicated on the y axis. The error bar depicts the range of the variation between the duplicate plates of individual experiments.
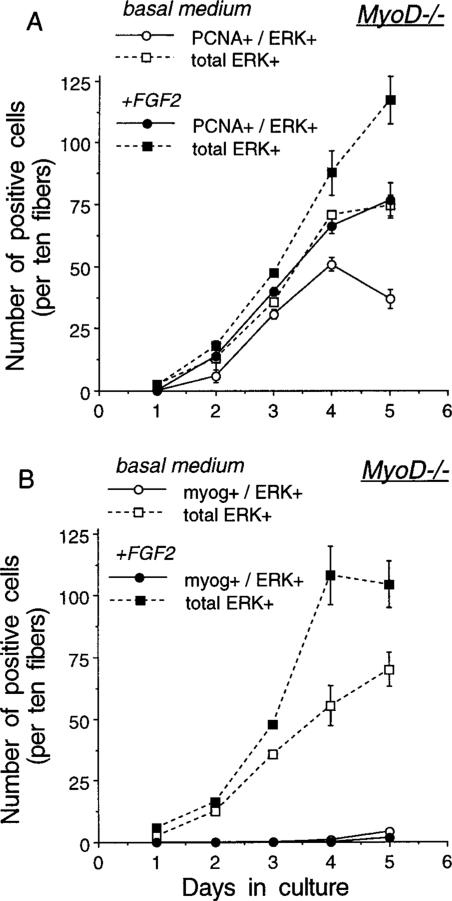
FIG. 3.
Temporal appearance of cells or nuclei positive for ERK1/ERK2 and PCNA (A) and ERK1/ERK2 and myogenin (B) in cultures of myofibers isolated from MyoD−/− mice. Cultures were maintained in basal medium (±FGF2 at 2 ng/ml) and the medium was changed daily. Plates were collected every 24 h and reacted via double immunofluorescence with the polyclonal antibody against ERK1/ERK2 in combination with the monoclonal antibodies against PCNA and myogenin. Immunostaining was performed as detailed in the legend to Fig. 1. Two parallel 35-mm plates were analyzed for each time point. For each panel, the total number of ERK+ cells and the number of doubly positive cells (PCNA+/ERK+ or myogenin+/ERK+) were determined. Nuclei positive for PCNA or myogenin which were not within ERK+ cells were never detected. Also, immunostaining with the antibody against MyoD could not detect immunostained nuclei or cells. Cells were scored as the number of positives on each individual fiber, analyzing 30 fibers per plate. Total positives were then averaged for duplicate plates and this value was eventually expressed per 10 fibers as indicated on the y axis. The error bar depicts the range of the variation between the duplicate plates of individual experiments.
Figure 3 summarizes the data about fiber-associated cells in myofiber cultures from the MyoD−/− mice. As in the Balb/C cultures, the cells in the MyoD−/− cultures can be monitored with the antibodies against PCNA or ERK (Fig. 3A). These MyoD−/− fiber-associated cells remained proliferative for longer time in culture (i.e., through days 4–5) compared to the Balb/C satellite cells whose proliferation declined following 3 days in culture. Consequently, the number of satellite cells is far higher in the MyoD−/− cultures. It is notable that FGF2 contributes to an increased number of PCNA+/ERK+ or total ERK+ cells in the MyoD−/− cultures, but the effect was subtle by day 3 in culture and became apparent only by day 4. In contrast, the effect of FGF2 on increasing the number of Balb/C satellite cells was distinctive by day 3 in culture (compare Figs. 2A and 3A). Also, in contrast to the Balb/C cultures, only a minimal number of the ERK+ cells were positive for myogenin in the MyoD−/− myofibers and these infrequent myogenin+ cells were not detected before day 4 (Fig. 3B). MyoD−/− fibers cultured for up to 9 days did not demonstrate a robust increase in the number of myogenin+ cells either. For example, the numbers of positive cells (expressed per 10 fibers/plate) for day 6, 7, 8, and 9 cultures maintained in medium lacking FGF2 were 8, 4, 8, and 2, respectively, for myogenin+ cells and 96, 95, 88, and 75, respectively, for ERK+ cells. A similar range in the frequencies of myogenin+ cells was seen in parallel cultures receiving FGF2.
MyoD−/− muscles are thought to express a higher level of Myf5 transcripts than wildtype muscle (Rudnicki et al., 1992; Kablar et al., 1997). It was therefore intriguing to compare Myf5 protein expression in myofiber cultures from Balb/C and MyoD−/− mice. However, since both the C- and the N-terminal domain antibodies against Myf5 reacted with the entire myofiber, reliable counting of Myf5+ nuclei using immunofluorescence was not possible. As discussed below, we were able to overcome this difficulty and analyze Myf5 protein expression in cultures of tissue-dissociated satellite cells.
Expression of Desmin, MyoD, Myf5, and Myogenin Proteins in Cultures of Tissue-Dissociated Mouse Satellite Cells
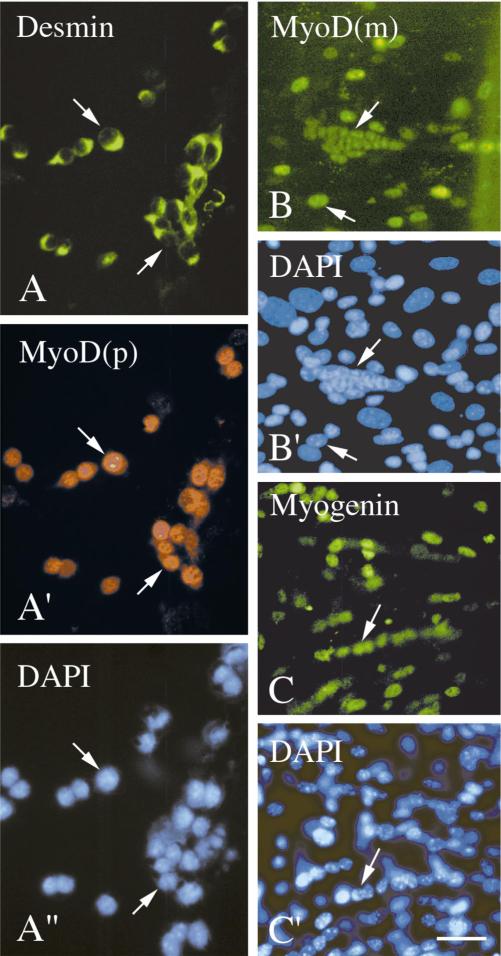
Figure 4 depicts immunofluorescent micrographs of cultured satellite cells which were isolated from Balb/C mice and stained with various antibodies. MyoD+ cells were already detected during early days in culture; these cells were also positive for desmin (Fig. 4A, day 4 culture), which is in agreement with the earlier finding that proliferating mouse myoblasts express desmin (Kaufman and Foster, 1988). Myogenin+ cells were not detected at this early time point. More advanced cultures showed both MyoD+ and myogenin+ cells (Figs. 4B and 4C, 10 and 8 days in culture, respectively). The quantification of MyoD+ and myogenin+ cells in myogenic cultures prepared from the adult diaphragm muscle of Balb/C mice is summarized in Table 1. By the 8th day in culture there is a robust increase in the number and frequency of the myogenin+ cells followed by an increase by day 11 in the number of cells which have fused into myotubes. A similar myogenic progression was detected in cultures from various limb muscles. However, the diaphragm muscle provided more satellite cells and thereby enabled a more detailed analysis. Immunostaining of cultures from diaphragm and various limb muscles with the antibodies against Myf5 revealed primarily cells that did not stain for Myf5 or whose immunostaining was questionable. Occasional cells showed some cytoplasmic stain, speckled nuclear stain, and/or uniform but faint nuclear stain. Altogether, compared to the unequivocal nuclear immunostaining of the cells for MyoD or myogenin, the immunosignal produced by potential Myf5+ cells in the wildtype cultures was unreliable for the quantification of positive cells.
FIG. 4.
Immunofluorescent micrographs of cultured satellite cells isolated from different muscles of Balb/C mice. Cultures were maintained in serum-rich growth medium and collected at different time points for immunocytochemistry. (A and A′) A day 4 culture from the soleus muscle reacted with a monoclonal antibody against desmin and a polyclonal antibody against MyoD, respectively. (B) A day 10 culture from the diaphragm muscle reacted with the monoclonal antibody against MyoD. (C) A day 8 culture from the diaphragm muscle reacted with the monoclonal antibody against myogenin. Reactivity with the monoclonal antibodies was visualized with a fluorescein-labeled secondary antibody and reactivity with the polyclonal antibody was visualized with a rhodamine-labeled secondary antibody. (A″, B′, C′) DAPI counterstains of the fields shown in A/A′, B, and C, respectively. Arrows mark the same positions of cells or myotubes in parallel panels. Bar, 34 _μ_m.
TABLE 1.
The Number of MyoD+ and Myogenin+ Cells in Culture of Satellite Cells Isolated from Balb/C Mice
| Source of muscle | Days in culture | Total number of cells analyzed | Nuclei in myotubes | MyoD+ mononucleated cells | Myogenin+ mononucleated cells | |
|---|---|---|---|---|---|---|
| No. | (%)b | No. | (%) | No. | (%) | |
| Diaphragm | 4 | 236 | 0 | 11 | (4.6) | — |
| 5 | 516 | 0 | 101 | (19.5) | — | |
| 5 | 357 | 0 | — | 0 | ||
| 7 | 733 | 6 | (0.8) | 162 | (22.1) | — |
| 7 | 469 | 0 | — | 2 | (0.4) | |
| 8 | 1853 | 15 | (0.8) | — | 476 | (25.6) |
| 11 | 1715 | 223 | (13.0) | 530 | (30.9) | — |
| 11 | 1151 | 70 | (6.0) | — | 283 | (24.5) |
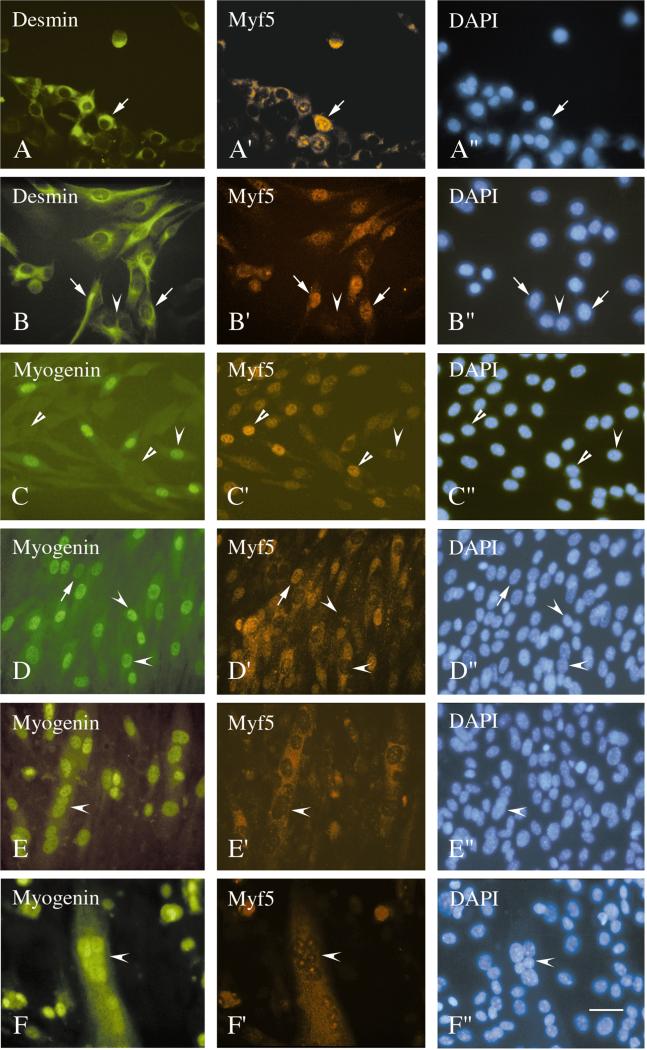
Figure 5 depicts micrographs of cultured satellite cells that were isolated from the MyoD−/− mice and stained with antibodies. MyoD+ cells were not detected with either the polyclonal or the monoclonal antibody. Desmin+ cells were detected in cultures from the diaphragm and various limb muscles. The immunostaining intensity for desmin varied in the MyoD−/− cultures, ranging from intensely stained cells as in the wildtype cultures to faintly stained cells. Because of this staining variation we did not attempt to quantify the desmin+ cells in the MyoD−/− cultures. Nevertheless, focusing on the cells which were clearly desmin+, it was notable that with time in culture, their nuclei became distinctly positive for Myf5 (Figs. 5A and 5B). Double staining with the antibodies against myogenin and Myf5 detected both Myf5+ and myogenin+ mononucleated cells in the same cultures, although the cells were typically not positive for the two proteins together (Figs. 5C and 5D). In addition to the nuclear Myf5, there was also a cytoplasmic stain with the anti-Myf5 antibody which was especially distinct during early days in culture when the cells were compact (Fig. 5A). A similar cytoplasmic stain was also seen in C2 cultures with a different Myf5 antibody (Yablonka-Reuveni and Rivera, 1997a). The nature of this cytoplasmic stain is unclear and might perhaps reflect the presence of authentic Myf5 protein in the cytoplasm. In the present study only the cells that demonstrated nuclear Myf5 immunostaining were considered positive and included in the counts of Myf5+ cells. The quantification of Myf5+ and myogenin+ cells in cultures isolated from the adult diaphragm and quadriceps muscles of MyoD−/− mice is provided in Table 2. The data show that myogenin+ cells can be detected in the MyoD−/− cultures but the appearance of the cells is delayed by about 2 days comparing the diaphragm cultures from Balb/C and MyoD−/− mice (Tables 1 and 2). Myotubes were also detected in the cultures from the MyoD−/− diaphragm and limb muscles and nuclei within these myotubes were consistently positive for myogenin but negative for Myf5 (Figs. 5E and 5F). However, the number of nuclei fusing into myotubes was lower in cultures from MyoD−/− muscle compared to Balb/C diaphragm cultures.
FIG. 5.
Immunofluorescent micrographs of cultured satellite cells isolated from different muscles of MyoD−/− mice. Cultures were maintained in serum-rich growth medium and collected at different time points. Cultures were reacted via double immunofluorescence with the polyclonal antibody against Myf5 in combination with the monoclonal antibodies against desmin (A/A′ and B/B′) or myogenin (C/C′, D/D′, E/E′, F/F′). Reactivity with the monoclonal antibodies was visualized with a fluorescein-labeled secondary antibody and reactivity with the polyclonal antibody was visualized with a rhodamine-labeled secondary antibody. The DAPI counterstain of nuclei in the fields shown in A/A′ through F/F′ are depicted in A″ through F″, respectively. (A) A day 5 culture from the soleus muscle. (B) A day 7 culture from the quadriceps muscle. (C, D) Day 10 cultures from the quadriceps muscle. (E, F) Day 10 cultures from the diaphragm muscle. Arrows in parallel micrographs mark the positions of cells or myotubes which are positive for both antibodies examined, concave arrowheads mark the positions of cells whose nuclei are positive for myogenin but negative for Myf5, and straight arrowheads mark the positions of cells whose nuclei are negative for myogenin but positive for Myf5. Bar, 34 _μ_m.
TABLE 2.
The Number of Myf5+ and Myogenin+ Cells in Satellite Cell Cultures Isolated from MyoD−/− Mice_a_
| Source of muscle | Days in culture | Total number of cells analyzed | Nuclei in myotubes | All Myf5+ cells | All myog+ cells | Myf5+/myog+ cells | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | (%)b | No. | (%) | No. | (%) | No. | (%) | ||
| Diaphragm | 4 | 282 | 0 | 12 | (4.2) | 0 | 0 | ||
| 5 | 231 | 0 | 4 | (1.7) | 0 | 0 | |||
| 6 | 274 | 0 | 15 | (5.4) | ND_c_ | ND | |||
| 7 | 852 | 0 | 201 | (23.5) | 2 | (0.2) | 1 | (0.1) | |
| 8 | 1714 | 0 | 253 | (14.7) | 1 | (0.05) | 0 | ||
| 9 | 1128 | 0 | 355 | (31.4) | 10 | (0.8) | 5 | (0.4) | |
| 10 | 1669 | 13 | (0.7) | 398 | (23.8) | 106 | (6.3) | 23 | (1.3) |
| 11 | 2730 | 49 | (1.7) | 201 | (7.3) | 337 | (12.3) | 3 | (0.1) |
| Quadriceps | 5 | 375 | 0 | 30 | (8.0) | 0 | 0 | ||
| 6 | 805 | 0 | 134 | (16.6) | 0 | 0 | |||
| 7 | 659 | 0 | 101 | (15.3) | 0 | 0 | |||
| 8 | 1368 | 0 | 294 | (21.4) | 1 | (0.07) | 0 | ||
| 9 | 2273 | 0 | 514 | (22.6) | 52 | (2.2) | 18 | (0.7) | |
| 10 | 2234 | 0 | 462 | (20.6) | 25 | (1.1) | 11 | (0.4) |
Expression of Cyclin D3 and MEF2A by Differentiating Myoblasts in Satellite Cell Cultures from MyoD−/− Mice
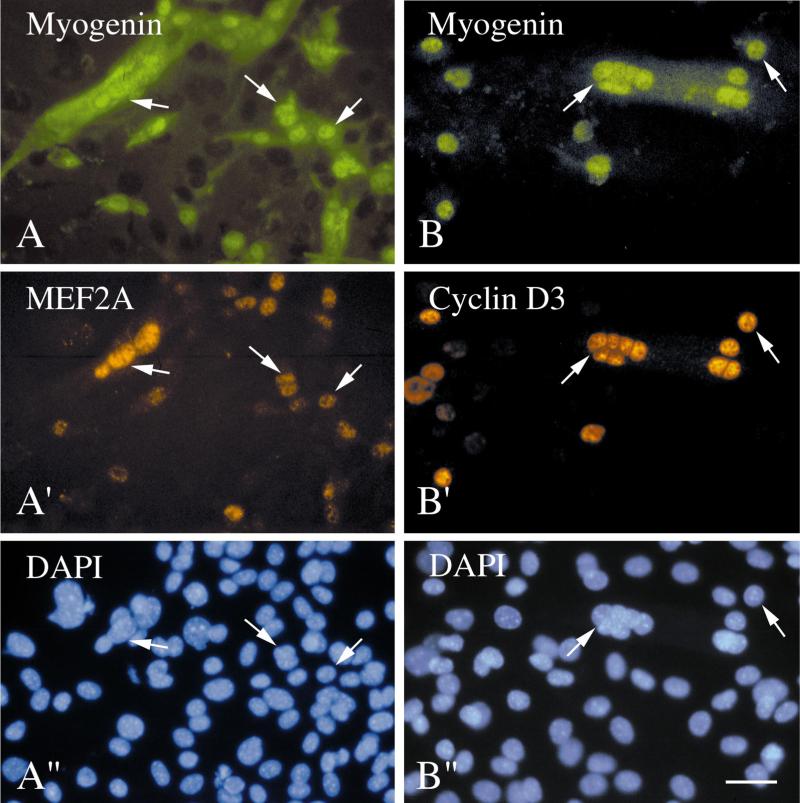
We were interested in determining if the myogenin+ cells in the MyoD−/− cultures were able to express other regulatory proteins expressed by differentiating wildtype myoblasts. We examined the expression of the nuclear proteins MEF2A and cyclin D3. MEF2A is a member of the MEF2 family of transcription factors, which are thought to act cooperatively with the MRF family during differentiation and activation of structural muscle genes (Molkentin and Olson, 1996). We showed that MEF2A is expressed in myogenin+ cells after the onset of myogenin expression in C2 myoblasts (Yablonka-Reuveni and Rivera, 1997a). Cyclin D3 protein, which is apparently a positive regulator of cell cycle progression in various mammalian systems, is expressed by differentiated (and not by proliferating) myoblasts and is thought to have a regulatory function during terminal differentiation (Kiess et al., 1995a). The correlation between differentiation and cyclin D3 protein expression was demonstrated in various rodent myogenic cell lines and agents promoting proliferation of myoblasts repressed the expression of cyclin D3 mRNA (Rao and Kohtz, 1995; Kiess et al., 1995b). In the present analysis of isolated satellite cells, we found that MEF2A and cyclin D3 are expressed by the nuclei of myogenin+ cells and myotubes in wildtype cultures (data not shown) and in MyoD−/− cultures (Fig. 6). Although the exact number of positive cells was not quantified, we concluded that most of the myogenin+ mononucleated cells and all the nuclei within myotubes were positive for MEF2A and cyclin D3. This finding indicates that although delayed in their appearance, the myogenin+ cells can express other regulatory proteins associated with myogenic differentiation. In some of the immunostaining investigations, after double staining for myogenin and MEF2A, the MyoD−/− cultures were restained for desmin. Similar to what has been observed in many species (reviewed in Yablonka-Reuveni and Nameroff, 1990), desmin was found to be present in differentiating myoblasts and myotubes in the MyoD−/− cultures (Fig. 6A).
FIG. 6.
Immunofluorescent micrographs of cultured satellite cells isolated from the diaphragm muscle of MyoD−/− mice and stained with the monoclonal antibody against myogenin together with the polyclonal antibodies against the differentiation markers MEF2A (A and A′) and cyclin D3 (B and B′). Reactivity with the monoclonal and polyclonal antibodies was visualized with a fluorescein-labeled and a rhodamine-labeled secondary antibody, respectively. The culture shown in A was also stained with the monoclonal antibody against desmin after monitoring the degree of immunostaining with the anti-myogenin and anti-MEF2A. Desmin immunoreactivity was detected by exposing the culture again to the fluorescein-labeled secondary. This staining with the desmin antibody produced the cytoplasmic signal shown by the myogenin+ cells and myotubes. The DAPI counterstain of nuclei in the fields shown in A/A′ and B/B′ are depicted in A″ and B″, respectively. Cells were cultured in serum-rich growth medium for 10 (A) and 17 days (B). Bar, 34 _μ_m.
DISCUSSION
In the present study we analyzed proliferation and differentiation of satellite cells from wildtype and MyoD−/− mutant mice. The use of the isolated myofiber cultures enabled us to distinguish satellite cells by their association with the myofibers. In these fiber cultures, the wildtype cells rapidly transit from proliferation to differentiation. The cells are first positive for both PCNA and MyoD and subsequently become positive for myogenin. The counterstaining of the cells with the antibody against ERK1/2 suggests that all myogenic cells in wildtype fibers transit from the PCNA+/MyoD+ state to the myogenin+ state. In contrast, satellite cells in fiber cultures from MyoD−/− mice give rise to a higher number of satellite cell progeny due to extended time in the proliferative state. Only a small number of these MyoD−/− cells transit into the myogenin+ state throughout the period of time in culture (0–9 days) that was examined. We did not extend the fiber analysis beyond this period because the fibers eventually disintegrate. Nevertheless, the myofiber cell tracings indicated that the emergence of the myogenin+ cells was delayed in MyoD−/− cultures compared to the wildtype cultures.
Analysis of cultures of tissue-dissociated satellite cells provided further insight into the differences between the progeny of satellite cells from wildtype and MyoD−/− mutant mice. Only a small number of the wildtype cells contained Myf5+ nuclei and the level of the protein seems to be low as estimated by the intensity of the immunosignal. In contrast, cultured satellite cells from the MyoD−/− mice gave rise to many cells whose nuclei were distinctly positive for Myf5. In these MyoD−/− cultures we also detected a delayed appearance of myogenin+ cells, which can fuse into myotubes and express other regulatory proteins associated with differentiation such as MEF2A and cyclin D3. Our finding that the progeny of the MyoD−/− satellite cells can express desmin protein, the expression of which we detected in both single cells and myotubes, contrasts with the findings of Sabourin et al. (1999) who failed to detect desmin in cultures of MyoD−/− satellite cells. As mentioned under Results, the intensity of desmin immunostaining varied within the MyoD-deficient cells and it is possible that MyoD−/− satellite cells express desmin at a lower level than wildtype cells. It is therefore conceivable that the antibody used in the present study is more sensitive for desmin detection than the antibody used in the study of Sabourin et al.
Primary cultures of tissue-dissociated satellite cells contain some nonmyogenic cells in addition to the myogenic precursors. Therefore, at this stage we cannot answer if all the progeny of the MyoD−/− satellite cells are of an identical Myf5+ phenotype. A future clonal analysis of individually cultured cells can potentially address this issue. We attempted to distinguish the myogenic cells by immunostaining the cultures with an antibody against c-met, the transmembrane receptor for hepatocyte growth factor. Such an anti-c-met antibody was shown to detect satellite cells in mouse myofiber cultures (Cornelison and Wold, 1997). This observation together with the reports on the ability of HGF to support proliferation and migration of satellite cells (Allen et al., 1995; Bischoff, 1997; Yablonka-Reuveni et al., 1999) have led to a commonly accepted convention that c-met is a specific marker for myogenic cells. We found the sensitivity of the antibody against c-met to be inappropriate for distinguishing and quantifying myogenic cells in cultures of dissociated satellite cells. A recent study indeed demonstrated only a very weak immunosignal when myogenic cells in tissue sections were traced with the c-met antibody (Anderson, 1998). Nevertheless, we can draw firm conclusions regarding the kinetics of appearance of MyoD+ and Myf5+ in the wildtype and MyoD−/− cultures. The data in Tables 1 and 2 clearly indicate that the absolute numbers and the frequency of the MyoD+ cells (in wildtype cultures) or Myf5+ cells (in MyoD−/− cultures) reached similar values by culture day 7 before the robust increase in myogenin+ cells. The data in Tables 1 and 2 further indicate that the emergence of myogenin+ cells was delayed in the MyoD−/− cultures. The wildtype diaphragm cultures contained numerous myogenin+ cells by day 8, whereas the mutant diaphragm cultures showed a major increase in myogenin+ cells only by day 10.
The MyoD−/− myofibers did not demonstrate a major increase in myogenin+ cells when analyzed throughout 9 days following culturing. We have not examined fiber cultures past this time period as the fibers eventually disintegrate, impairing the analysis of fiber-associated cells during late time points. Nevertheless, the myofiber cell tracings indicated that some myogenin+ cells were ultimately present in MyoD−/− myofibers and their emergence was delayed compared to myogenin+ cells in wildtype fiber cultures. It is notable that the delays in the appearance of myogenin+ cells in myofiber cultures and in satellite cells cultures from the MyoD−/− mice were of a similar time length.
Embryos of MyoD−/− mice are capable of forming muscle but display a delayed development of hypaxial (limb and abdominal wall) musculature and expressed about fourfold higher levels of Myf5 (Rudnicki et al., 1992; Kablar et al., 1997). Embryos of the Myf5−/− mice also develop their muscles but the development of the epaxial (paraspinal and intercostal) musculature is markedly delayed in these embryos (Braun et al., 1994; Kablar et al., 1997). Taken together, the studies of muscle development have suggested that embryogenesis of skeletal muscle can progress when only one of the two “early” myogenic regulatory factors are present, although the myogenic programs in the absence of MyoD or Myf5 are modified compared to the wildtype. Furthermore, different signaling programs are thought to be involved in the initiation of Myf5- and MyoD-dependent pathways during development (Tajbakhsh et al., 1998). In view of these studies of muscle embryogenesis, it is conceivable that Myf5 and MyoD might have different roles during myogenesis of satellite cells. However, Myf5−/− mice die at birth due to respiratory insufficiency associated with the absence of the major distal part of the ribs (Braun et al., 1992), preventing at present a direct comparison of satellite cells from mice deficient in MyoD or Myf5.
Despite the unavailability of an in vivo model to test the functional role of Myf5 in the postnatal environment, some proposals regarding the role of Myf5 can be put forward when our observations are considered together with findings of other investigators. The temporal kinetics of MyoD+/myogenin−, MyoD+/myogenin+, and MyoD−/myogenin+ cells in myogenic cultures and clones of cells with a “normal” MyoD phenotype have indicated that the MyoD+ cells give rise to myogenin+ cells (Yablonka-Reuveni and Rivera, 1994, 1997a). Likewise, the identification of rare cells which are positive for both Myf5 and myogenin in cultures from the MyoD−/− mice suggests that the Myf5+ cells can give rise to myogenin+ cells. However, the finding that in the MyoD−/− cultures most of the myogenin+ nuclei in single cells and all nuclei in myotubes did not costain for Myf5 indicates that Myf5 protein expression declines as the cells express myogenin. A similar downregulation of Myf5 protein at the onset of differentiation, when cells become positive for myogenin, was reported by Lindon et al. (1998) and by Kitzmann et al. (1998) in their studies of the mouse-derived C2 cell line. On the other hand, MyoD protein can continue to be expressed in C2 cultures by differentiating myogenin+ cells (Yablonka-Reuveni and Rivera, 1997a). The timing in the decline of Myf5 when myogenin+ cells emerge suggests that differentiation of myoblasts may require the down-regulation of Myf5.
Similar to the finding reported in the present study with wildtype mouse satellite cells, we showed that cultures of dissociated rat satellite cells contain only a low number of Myf5+ cells and a far higher number of MyoD+ cells which rapidly enter the myogenin+ state (unpublished results). Furthermore, we showed that the MyoD+ cells in rat myofiber cultures rapidly transit from proliferation to differentiation (Yablonka-Reuveni et al., 1999). In contrast, the rat myogenic cell lines L6 and L8, which were isolated from newborn rat thigh muscle (Yaffe, 1968; Yaffe and Saxel, 1977), were reported to express Myf5 but not MyoD (Braun et al., 1989). We recently detected the expression of MyoD mRNA and protein at a very low level in L6 and L8 cultures but Myf5+ cells were the prevalent cells with the expression of Myf5 preceding myogenin; also, the expression of Myf5 and myogenin proteins was temporally exclusive within individual cells (D. Graves, M. Primig, and Z. Yablonka-Reuveni, unpublished results). Hence, the study of rat primary cultures and cell lines and the study of wildtype and MyoD−/− mouse satellite cells have yielded similar conclusions regarding the relationship between the expression of MyoD, Myf5, and myogenin. Typically, Myf5 expression is elevated when MyoD expression is reduced and the expression of Myf5 precedes myogenin. Also, myoblasts deficient in MyoD remain proliferative for a longer time while myoblasts capable of expressing MyoD rapidly transit from proliferation to differentiation. It is thus attractive to propose that overexpression of Myf5, combined with little (or no) expression of MyoD, is the hallmark of the self-renewed myogenic stem cells. In vivo, such cells may undergo asymmetric division, maintaining the pool of stem cells, and give rise to progeny that can enter the MyoD-to-myogenin sequence. The cells in the MyoD-to-myogenin pathway might constitute a population of satellite cells that is readily available for muscle growth and repair. The concept of asymmetric cell division for maintaining in the growing rat a pool of proliferating satellite cells along with their differentiating progeny was initially proposed by Moss and Leblond (1971). Based on his studies of satellite cell proliferative dynamics in growing rats, Schultz (1996) proposed asymmetric division as a mechanism which produces true myogenic stem cells along with myoblasts which go through a minimal number of replications and are readily available for fusion into the growing myofibers. A proposed role of MyoD in directing a rapid transition from proliferation to differentiation fits well with the studies which showed that when present in its active form, MyoD leads to withdrawal of myoblasts from the cell cycle (Halevy et al., 1995) and with a recent study which showed that the reintroduction of MyoD expression to progeny of MyoD−/− satellite cells rescues the differentiation pathway (Sabourin et al., 1999). In contrast, the satellite cells which express high levels of Myf5 might be able to escape MyoD expression (and subsequent rapid differentiation), maintaining a pool of stem cells ready, when necessary, to provide rapidly differentiating cells. Muscle regeneration in MyoD-deficient mice might be impaired because of an imbalance between the stem cells and the cells which enter the rapid proliferation–differentiation pathway.
ACKNOWLEDGMENTS
This work was supported in parts by grants to Z.Y.-R. from the Cooperative State Research Service—U.S. Department of Agriculture (Agreement 95-37206-2356) and the National Institutes of Health (AG13798) and by grants to M.A.R. from the National Institutes of Health and the Muscular Dystrophy Association. We thank Maria Elias for helpful comments on the manuscript. We are also grateful to many colleagues for their gifts of valuable reagents: Drs. J. Campbell and E. Krebs (anti-ERK1/ERK2), Dr. S. Alemá (anti-MyoD, polyclonal), Drs. P. Houghton and P. Dias (anti-MyoD, monoclonal), Dr. W. Wright (anti-myogenin), Dr. S. Hauschka (FGF2), and Dr. N. Wolf (7- and 34-month old mice from the Center for Aging and Caloric Restricted Animals, National Institute of Aging). The monoclonal antibody against desmin (D3) was originally developed in Dr. D. A. Fischman's laboratory and was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine (Baltimore, MD), and the Department of Biology, University of Iowa (Iowa City, IA), under Contract NO1-HD-6-2915 from the NICHD.
REFERENCES
- Allen RE, Shannon SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell. Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Anderson JE. Studies of the dynamics of skeletal muscle regeneration: The mouse came back! Biochem. Cell Biol. 1998;76:13–26. [PubMed] [Google Scholar]
- Anderson JE, McIntosh LM, Moor AN, Yablonka-Reuveni Z. Levels of MyoD protein expression following injury of mdx and normal limb muscle are modified by thyroid hormone. J. Histochem. Cytochem. 1997;46:59–67. doi: 10.1177/002215549804600108. [DOI] [PubMed] [Google Scholar]
- Aurade F, Pfarr CM, Lindon C, Garcia A, Primig M, Montarras D, Pinset C. The glucocorticoid receptor and AP-1 are involved in a positive regulation of the muscle regulatory gene myf5 in cultured myoblasts. J. Cell Sci. 1997;110:2771–2779. doi: 10.1242/jcs.110.22.2771. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Proliferation of muscle satellite cells in intact myofibers in culture. Dev. Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Analysis of muscle regeneration using single myofibers in culture. Med. Sci. Sports Exerc. 1989;21:S164–S172. [PubMed] [Google Scholar]
- Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development. 1990a;109:943–952. doi: 10.1242/dev.109.4.943. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Cell cycle commitment of rat muscle satellite cells. J. Cell Biol. 1990b;111:201–207. doi: 10.1083/jcb.111.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T, Bober E, Rudnicki MA, Jaenisch R, Arnold HH. MyoD expression marks the onset of skeletal myogenesis in Myf-5 mutant mice. Development. 1994;120:3083–3092. doi: 10.1242/dev.120.11.3083. [DOI] [PubMed] [Google Scholar]
- Braun T, Rudnicki MA, Arnold HH, Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71:369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Kelly R, Tajbakhsh S, Zammit P. The formation and maturation of skeletal muscle in the mouse: The myosin MLC1F/3F gene as a molecular model. Acta Physiol. Scand. 1998;163:S3–S5. doi: 10.1046/j.1365-201X.1998.1630s30S3.x. [DOI] [PubMed] [Google Scholar]
- Carnac G, Primig M, Kitzmann M, Chafey P, Tuil D, Lamb N, Fernandez A. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell. 1998;9:1891–1902. doi: 10.1091/mbc.9.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison DDW, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Danto SI, Fischman DA. Immunohistochemical analysis of intermediate filaments in embryonic heart cells with monoclonal antibodies to desmin. J. Cell Biol. 1984;98:2179–2191. doi: 10.1083/jcb.98.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias P, Parham DM, Shapiro DN, Tapscott SJ, Houghton PJ. Monoclonal antibodies to the myogenic regulatory protein MyoD1: Epitope mapping and diagnostic utility. Cancer Res. 1992;52:6431–6439. [PubMed] [Google Scholar]
- Füchtbauer E-M, Westphal H. MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev. Dyn. 1992;193:34–39. doi: 10.1002/aja.1001930106. [DOI] [PubMed] [Google Scholar]
- Gause KC, Homma MK, Licciardi KA, Seger R, Ahn NG, Peterson MJ, Krebs EG, Meier KE. Effects of phorbol ester on mitogen-activated protein kinase kinase activity in wild-type and phorbol ester-resistant EL4 thymoma cells. J. Biol. Chem. 1993;268:16124–16129. [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. Molecular and cellular biology of muscle regeneration. In: Partridge T, editor. Molecular and Cell Biology of Muscular Dystrophy. Chapman & Hall; London: 1993. pp. 210–256. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Hinterberger TJ, Sassoon DA, Rhodes SJ, Konieczny SF. Expression of the muscle regulatory MRF4 during somite and skeletal myofiber development. Dev. Biol. 1991;147:144–156. doi: 10.1016/s0012-1606(05)80014-4. [DOI] [PubMed] [Google Scholar]
- Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA. MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development. 1997;124:4729–4738. doi: 10.1242/dev.124.23.4729. [DOI] [PubMed] [Google Scholar]
- Kami K, Noguchi K, Senba E. Localization of myogenin, c-fos, c-jun, and muscle-specific gene mRNA in regenerating rat skeletal muscle. Cell Tissue Res. 1995;280:11–19. doi: 10.1007/BF00304506. [DOI] [PubMed] [Google Scholar]
- Kaufman SJ, Foster RF. Replicating myoblasts express a muscle-specific phenotype. Proc. Natl. Acad. Sci. USA. 1988;185:9606–9610. doi: 10.1073/pnas.85.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp. Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- Kiess M, Gill RM, Hamel PA. Expression and activity of the retinoblastoma protein (pRB)-family proteins, p107 and p130, during L6 myoblast differentiation. Cell Growth Differ. 1995a;6:1287–1298. [PubMed] [Google Scholar]
- Kiess M, Gill RM, Hamel PA. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation of myoblasts into quiescent myotubes. Oncogene. 1995b;10:159–166. [PubMed] [Google Scholar]
- Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJC, Ferandez A. The muscle regulatory factors MyoD and Myf-5 undergo distinct cell cycle-specific expression in muscle cells. J. Cell Biol. 1998;142:1447–1459. doi: 10.1083/jcb.142.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koishi K, Zhang M, McLennan IS, Harris AJ. MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev. Dyn. 1995;202:244–254. doi: 10.1002/aja.1002020304. [DOI] [PubMed] [Google Scholar]
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinases cascades. Adv. Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Lindon C, Montarras D, Pinset C. Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J. Cell Biol. 1998;140:111–118. doi: 10.1083/jcb.140.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley MAL, Fan Y, Beilharz MW, Grounds MD. Intrinsic differences in MyoD and myogenin expression between primary cultures of SLJ/J and BALB/C skeletal muscle. Exp. Cell Res. 1994;211:99–107. doi: 10.1006/excr.1994.1064. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh LM, Garrett KL, Megeney L, Rudnicki MA, Anderson JE. Regeneration and myogenic cell prolifera tion correlate with taurine levels in dystrophin- and MyoD-deficient muscles. Anat. Rec. 1998;252:311–324. doi: 10.1002/(SICI)1097-0185(199810)252:2<311::AID-AR17>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Rudnicki MA. Determination versus differentiation and the MyoD family of transcription factors. Biochem. Cell Biol. 1995;73:723–732. doi: 10.1139/o95-080. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as a source of nuclei in muscles of growing rats. Anat. Rec. 1971;170:421–436. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev. Biol. 1992;154:216–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olson EN. Signal transduction pathways that regulate skeletal muscle gene expression. Mol. Endocrinol. 1993;7:1369–1378. doi: 10.1210/mend.7.11.8114752. [DOI] [PubMed] [Google Scholar]
- Rao SS, Kohtz DS. Positive and negative regulation of D-type cyclin expression in skeletal myoblasts by basic fibroblast growth factor and transforming growth factor beta. A role for cyclin D1 in control of myoblast differentiation. J. Biol. Chem. 1995;270:4093–4100. doi: 10.1074/jbc.270.8.4093. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. Reduced differentiation potential of primary MyoD–/– myogenic cells derived from adult skeletal muscle. J. Cell Biol. 1999;144:631–643. doi: 10.1083/jcb.144.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: An EM and radioautographic study. J. Exp. Zool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Seger R, Seger D, Reszka AA, Munar ES, Eldar-Finkelman H, Dobrowolska G, Jensen AM, Campbell JS, Fischer EH, Krebs EG. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J. Biol. Chem. 1994;269:25699–25709. [PubMed] [Google Scholar]
- Smith CK, II, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J. Cell. Physiol. 1994;159:379–385. doi: 10.1002/jcp.1041590222. [DOI] [PubMed] [Google Scholar]
- Smith TH, Block NE, Rhodes SJ, Konieczny SF, Miller JB. A unique pattern of expression of the four muscle regulatory factor proteins distinguishes somitic from embryonic, fetal and newborn mouse myogenic cells. Development. 1993;117:1125–1133. doi: 10.1242/dev.117.3.1125. [DOI] [PubMed] [Google Scholar]
- Snow MH. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat. Rec. 1990;227:437–446. doi: 10.1002/ar.1092270407. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Borello U, Vivarelli E, Kelly R, Duprez D, Buckingham M, Cossu G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: Redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Winchester PK, Davis ME, Alway SE, Gonyea WJ. Satellite cell activation in the stretch-enlarged anterior latissimus dorsi muscle of the adult quail. Am J. Physiol. 1991;260:C206–C212. doi: 10.1152/ajpcell.1991.260.2.C206. (Cell Physiol. 29) [DOI] [PubMed] [Google Scholar]
- Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Development and postnatal regulation of adult myoblasts. Microsc. Res. Tech. 1995;30:366–380. doi: 10.1002/jemt.1070300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Nameroff M. Temporal differences in desmin expression between myoblasts from embryonic and adult chicken skeletal muscle. Differentiation. 1990;45:21–28. doi: 10.1111/j.1432-0436.1990.tb00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Influence of PDGF-BB on proliferation and transition through the MyoD– myogenin–MEF2A expression program during myogenesis in mouse C2 myoblasts. Growth Factors. 1997a;15:1–25. doi: 10.3109/08977199709002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Proliferative dynamics and the role of FGF2 during myogenesis of rat satellite cells on isolated fibers. Basic Appl. Myol. 1997b;7:189–202. [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seger R, Rivera AJ. Fibro-blast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J. Histochem. Cytochem. 1999;47:23–42. doi: 10.1177/002215549904700104. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc. Natl. Acad. Sci. USA. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977a;7:159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977b;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yun K, Wold B. Skeletal muscle determination and differentiation: Story of a core regulatory network and its context. Curr. Opin. Cell Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]