Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus (original) (raw)
Abstract
The ongoing Zika virus (ZIKV) outbreaks have raised global concerns due to its unexpected clinical manifestations. Antiviral development is of high priority in response to the ZIKV emergency. In this study, we report that an adenosine analog NITD008 has potent in vitro and in vivo antiviral activity against ZIKV. The compound can effectively inhibit the historical and contemporary ZIKV strains in cultures as well as significantly reduce viremia and prevent mortality in A129 mice. Our results have demonstrated that NITD008 is potent inhibitor of ZIKV and can be used as reference inhibitor for future ZIKV antiviral drug screen and discovery.
Keywords: adenosine analog, antiviral, mouse model, Zika virus
Zika virus (ZIKV) used to be an obscure member of Flavivirus genus within the family of Flaviviridae. It was firstly isolated in 1947 from a febrile rhesus macaque in Zika forest, Uganda. For the first 60 years after its discovery, ZIKV caused only sporadic human cases with mild symptoms in African and Asia. However, since 2007, large ZIKV outbreaks emerged in the Oceania and then expanded into the Americas. More concerning is the microcephaly cases in babies and neurological disorders in adults associated with the recent ZIKV outbreaks. With accumulating evidence from epidemiological and experimental investigations [1, 2], the World Health Organization has declared current ZIKV epidemics a public health emergency of international concern. Currently, active ZIKV transmission has been documented in a total of 66 countries and territories, and over 2.17 billion people live in areas that could be affected by ZIKV infection [3]. Besides ZIKV, many other Flaviviruses are important human pathogens of global public health concern, such as dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus, Yellow fever virus, and tick-borne encephalitis virus.
Due to cross-reactivity of antibodies between DENV and ZIKV, a recent study suggests that DENV serocross-reactivity drives antibody-dependent enhancement of infection with ZIKV [4]. This finding has posed challenges for ZIKV vaccine development. Therefore, discovery of anti-ZIKV drugs should be of high priority in response to ZIKV emergency. Similar to other Flaviviruses, ZIKV is an enveloped virus containing a single-stranded, positive-sense ribonucleic acid (RNA) genome of approximately 11 000 nucleotides in length. The ZIKV genome encodes a single polyprotein that is cleaved co- and posttranslationally by host and viral proteases into 3 structural proteins (C, prM, and E) and 7 nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). Zika virus shares similar genome organization and replication scheme to other Flaviviruses. Consequently, previous knowledge about other Flaviviruses, in particular DENV and WNV, could provide useful insight and direction in ZIKV research and drug discovery.
Current Flavivirus drug targets include the fusogenic activity of E protein, the protease and helicase activity of NS3, and the RNA-dependent RNA polymerase and methyltransferase activities of NS5. Nucleoside analogs targeting viral polymerases have been widely used in clinics and yielded efficacious therapies against several viruses, including hepatitis B virus, human immunodeficiency virus, and hepatitis C virus. We previously reported that an adenosine nucleoside analog inhibitor (NITD008) exhibited potent antiviral activity against DENV in vitro and in vivo [5]. NITD008 could inhibit replication of several other Flaviviruses and enterovirus [6–8]. In the present study, we characterize the in vitro and in vivo antiviral activity of NITD008 against historical and contemporary ZIKV strains.
METHODS
Vero (African green monkey kidney) and BHK-21 (baby hamster kidney) cells were incubated at 37°C in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. C6/36 (Aedes albopictus mosquito) cells were maintained at 28°C in DMEM supplemented with 10% FBS. NITD008 was kindly provided by the Novartis Institute for Infectious Diseases, Singapore. For cell culture experiments, NITD008 was initially dissolved in 100% dimethyl sulfoxide (DMSO). The compound was diluted to the desired concentrations in 0.5% DMSO.
Two ZIKV strains were used in this study. The GZ01/2016 strain (GenBank number KU740184) was isolated from a Chinese patient returned from Venezuela in 2016, and it has >99.9% amino acid identity to the strain that is currently circulating in Latin Americas [9]. The FSS13025/2010 strain (GenBank number KU955593.1) was isolated in 2010 in Cambodia [10], and it is represented as a historical ZIKV strain. Both strains are classified within the Asian lineage based on phylogenetic analysis [11]. Viral stocks were prepared in C6/36 cells and titrated in BHK-21 cell by plaque-forming assay, as previously described [10], and stored as aliquots at −80°C until use. Studies with infectious ZIKV were conducted under biosafety level 2 facilities at Beijing Institute of Microbiology and Epidemiology.
The in vitro antiviral activity of NITD008 was assayed by a standard viral titer reduction assay. In brief, Vero cells were infected with 2 ZIKV strains (GZ01/2016 and FSS13025/2010) at a multiplicity of infection of 0.1 and then treated with 5-fold serial dilutions of NITD008. Cell cultures were collected at 48 hours postinfection (p.i.), and viral titers in supernatants were quantified by plaque assay in BHK-21 cells. The incubations for each concentration treatment were performed in triplicate. The vehicle with final concentration of 0.5% dimethyl sulfoxide (DMSO) was set as mock treatment. The 50% effective concentration (EC50) was calculated accordingly. In addition, viral RNA in cell lysates were assayed using ZIKV-specific reverse-transcription quantitative polymerase chain reaction (RT-qPCR) as previously described [12]. In brief, RNAs were extracted from infected cells with PureLink RNA Mini Kit (Life Technologies) according to the manufacturer's recommendation and detected by RT-qPCR with the One-Step PrimeScript RT-PCR Kit (Takara, Japan) using ZIKV-specific primers. The EC50 values were calculated by fitting the dose-response curves, and error bars represented data from 3 independent experiments. Standard RT-PCR and genome sequencing of progeny ZIKV were performed to identify any adaptive mutations.
The in vivo antiviral activity of NITD008 was assayed with the newly described A129 mice model that lacks type I interferon signaling [13]. The experimental protocols were approved by the Animal Experiment Committee of Laboratory Animal Center (Academy of Military Medical Sciences, China) (IACUC-13-2016-001). In brief, a group of 4-week-old A129 mice (n = 8 per group) were infected intraperitoneally with 105 plaque-forming units of ZIKV strain GZ01/2016. The infected mice were orally administered with 100 µL NITD008 at 50 mg/kg body weight at 4, 24, 48, 72, and 96 hours p.i. The same volume of 0.5% DMSO was used for mock treatment. The animals were observed daily for signs of illness and mortality; meanwhile, the animals were bled via tail vein for 3 consecutive days, and 50 μL of sera was used to measure viremia by RT-qPCR as described above.
For survival analyses, GraphPad Prism 5.0 was used to perform Kaplan-Meier log-rank test to compare curves. Viral RNA loads in serum on day 1, 2, and 3 p.i. were determined by RT-qPCR, and statistical analysis was performed using the unpaired, 2-tailed t test. Differences of P < .05 were considered significant.
RESULTS
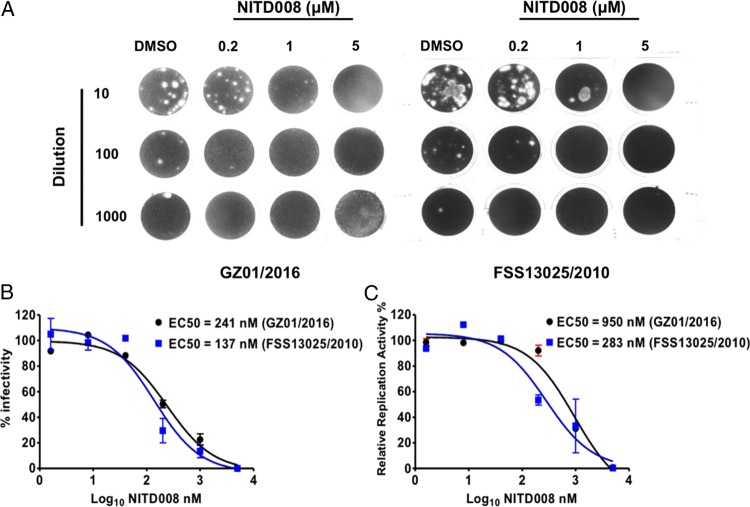
Similar to other Flaviviruses, ZIKV replicates efficiently in Vero cells with typical cytopathic effects (CPEs). In the present study, we first evaluated the antiviral activity of NITD008 in Vero cells against 2 ZIKV strains. The compound exhibited dose-dependent inhibition of CPEs in Vero cells, and a standard plaque reduction assay showed that treatment with different concentrations of NITD008 decreased the plaque numbers (Figure 1A). As shown in Figure 1B, NITD008 effectively inhibited the progeny viral titers of 2 ZIKV strains, and the EC50 values of NITD008 against GZ01/2016 and FSS13025/2010 were calculated to be 241 nM and 137 nM, respectively (Figure 1B). At 5 μM concentration, NITD008 reduced the viral titers of GZ01/2016 and FSS13025/2010 by up to 400- and 1000-fold, respectively. The RT-qPCR assay of the culture lysates also indicated that NITD008 could efficiently inhibit viral genome RNA replication, and the EC50 values were calculated to be 950 and 283 nM against GZ01/2016 and FSS13025/2010, respectively (Figure 1C).
Figure 1.
In vitro and antiviral activity of NITD008 against Zika virus (ZIKV). Vero cells were infected with the indicated ZIKV stains at a multiplicity of infection of 0.1 followed by the treatment with the indicated concentrations of NITD008. Supernatants of the infected cells were collected and measured for viral titers using plaque assays on BHK-21 cell (A). The 50% effective concentration (EC50) values were calculated by fitting the dose-response curves from a viral titer reduction assay (B) and reverse-transcription quantitative polymerase chain reaction assay (C), and the error bars represented data from 3 independent experiments.
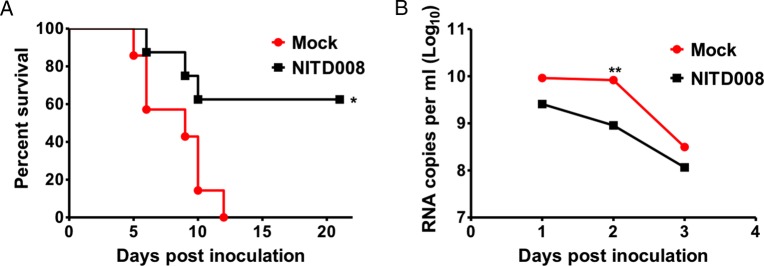
Next, we assessed the in vivo therapeutic effect of NITD008 in the A129 mouse model of ZIKV infection [12] using ZIKV strain GZ01/2016. As shown in Figure 2A, all of the ZIKV-inoculated animals received mock treatment displayed a 100% mortality rate within 12 days. All infected mice began to lose their starting body weight on day 5 p.i., developed neurological symptoms (including hind limb weakness, paralysis), and finally succumbed to infection. The duration of viremia was 7 days, and the peak viremia was observed on day 2 p.i. In contrast, treatment with 50 mg/kg NITD008 protected 50% of the infected mice from death, and the survival mice did not develop any neurological symptoms. As shown in Figure 2B, NITD008 treatment significantly decreased the mean peak viremia by 2.6-fold on day 2 p.i. (P < .05) compared with the mock treatment group. Taken together, these in vitro and in vivo results show that NITD008 is a potent inhibitor of various ZIKV strains.
Figure 2.
The therapeutic effects of NITD008 against Zika virus (ZIKV) challenge in A129 mice. Groups of 4-week-old A129 mice (n = 8) intraperitoneally challenged with 105 plaque-forming units of ZIKV strains GZ01/2016 were orally administered with NITD008 at 50 mg/kg body weight for 5 days. Percentage survival between ZIKV-infected mice treated with mock or NITD008 was compared using the log-rank test (A). Viral ribonucleic acid loads in serum at day 1, 2, and 3 postinfection were determined by reverse-transcription quantitative polymerase chain reaction (B), and statistical analysis was performed using the unpaired, 2-tailed _t_-test. Differences of P < .05 were considered significant.
DISCUSSION
The continuing outbreaks of ZIKV in Caribbean and the Americas underscore the urgent need for effective antivirals. To date, only a few compounds were reported to have anti-ZIKV activity [14, 15]. In this study, we found that the pan-Flavivirus adenosine analog NITD008 could effectively inhibit both historical and contemporary ZIKV strains. The EC50 value against ZIKV was similar to those against DENV, WNV, and other Flaviviruses [5, 7, 8]. NITD008 is an adenosine nucleoside analog that contains a carbon substitution for N-7 of the purine and an acetylene at the 2′ position of ribose [5]; the triphosphate form of NITD008 competes with natural adenosine triphosphate substrates to incorporate into the growing RNA chain and, upon incorporation, terminates RNA elongation. Previous studies have demonstrated that continuous culturing of DENV or WNV on BHK-21 or Vero cells in presence of NITD008 did not result in the emergence of drug-resistant viruses [5]. In line with these findings, genome sequencing of the progeny ZIKV (recovered from the A129 mouse experiment) failed to identify any adaptive mutations in response to NITD008 treatment in our study. Our results also indicated that the EC50 of NITD008 was slightly different between the contemporary strain GZ01/2016 and the historical strain FSS13025/2010. Bioinformatics analysis has revealed the continuing evolving of ZIKV strains [11], and whether the mutations on the NS5 polymerase between these strains attribute to the phenotype deserves further investigation.
It is remarkable that NITD008 treatment significantly reduced viremia and prevented death from the American ZIKV strain challenge. This result warrants further characterization and evaluation of the compound for potential development. We have previously shown that a pan-Flavivirus neutralization monoclonal antibody 2A10G6 conferred partial protection against lethal ZIKV infection in A129 model [12]. Zmurko et al [14] have recently reported that a viral polymerase inhibitor 7DMA reduced viremia and delayed virus-induced morbidity and mortality in AG129 mice. Although the therapeutic effects of these candidates look promising, more studies are needed to develop these inhibitors for ZIKV therapeutics [16].
CONCLUSIONS
In summary, we have provided conclusive evidence that NITD008 is an effective antiviral compound that protects mice from lethal ZIKV challenge. NITD008 could serve as a reference inhibitor for future drug screen and discovery. In addition, our study demonstrates that the A129 mouse model could be used for testing the in vivo efficacy of ZIKV inhibitors.
Acknowledgments
Financial support. This work was supported by the State Key Laboratory of Pathogen and Biosecurity (SKLPBS1601), the Guangzhou Science and Technology Program for Public Wellbeing (2014Y2-00185, No. 2014Y2-00550), and the National Natural Science Foundation (NSFC) of China (No. 31470265). C.-F. Q. was supported by the Excellent Young Scientist Program from the NSFC of China (No. 81522025) and the Newton Advanced Fellowship from the Academy of Medical Sciences, UK and the NSFC of China (No. 81661130162). PYS's group is supported by NIH grant (AI087856).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Faria NR, Azevedo Rdo S, Kraemer MU et al. . Zika virus in the Americas: early epidemiological and genetic findings. Science 2016; 352:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, Xu D, Ye Q et al. . Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 2016; 19:120–6. [DOI] [PubMed] [Google Scholar]
- 3.Messina JP, Kraemer MU, Brady OJ et al. . Mapping global environmental suitability for Zika virus. ELife 2016; pii:e15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejnirattisai W, Supasa P, Wongwiwat W et al. . Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat Immunol 2016; 17:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Z, Chen YL, Schul W et al. . An adenosine nucleoside inhibitor of dengue virus. Proc Natl Acad Sci U S A 2009; 106:20435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng CL, Yeo H, Ye HQ et al. . Inhibition of enterovirus 71 by adenosine analog NITD008. J Virol 2014; 88:11915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo MK, Shi PY, Chen YL et al. . In vitro antiviral activity of adenosine analog NITD008 against tick-borne flaviviruses. Antiviral Res 2016; 130:46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qing J, Luo R, Wang Y et al. . Resistance analysis and characterization of NITD008 as an adenosine analog inhibitor against hepatitis C virus. Antiviral Res 2016; 126:43–54. [DOI] [PubMed] [Google Scholar]
- 9.Zhang FC, Li XF, Deng YQ et al. . Excretion of infectious Zika virus in urine. Lancet Infect Dis 2016; 16:641–2. [DOI] [PubMed] [Google Scholar]
- 10.Shan C, Xie X, Muruato AE et al. . An Infectious cDNA clone of Zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe 2016; 19:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye Q, Liu ZY, Han JF et al. . Genomic characterization and phylogenetic analysis of Zika virus circulating in the Americas. Infect Genet Evol 2016; 43:43–9. [DOI] [PubMed] [Google Scholar]
- 12.Dai L, Song J, Lu X et al. . Structures of the Zika virus envelope protein and its complex with a Flavivirus broadly protective antibody. Cell Host Microbe 2016; 19:696–704. [DOI] [PubMed] [Google Scholar]
- 13.Lazear HM, Govero J, Smith AM et al. . A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016; 19:720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zmurko J, Marques RE, Schols D et al. . The viral polymerase inhibitor 7-deaza-2’-C-methyladenosine is a potent inhibitor of in vitro Zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl Trop Dis 2016; 10:e0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyer L, Nencka R, Huvarova I et al. . Nucleoside inhibitors of Zika virus. J Infect Dis 2016; 214:704–11. [DOI] [PubMed] [Google Scholar]
- 16.Weaver SC, Costa F, Garcia-Blanco MA et al. . Zika virus: History, emergence, biology, and prospects for control. Antiviral Res 2016; 130:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]