Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain–Barré syndrome (original) (raw)
Abstract
Molecular mimicry between microbial and self-components is postulated as the mechanism that accounts for the antigen and tissue specificity of immune responses in postinfectious autoimmune diseases. Little direct evidence exists, and research in this area has focused principally on T cell-mediated, antipeptide responses, rather than on humoral responses to carbohydrate structures. Guillain–Barré syndrome, the most frequent cause of acute neuromuscular paralysis, occurs 1–2 wk after various infections, in particular, Campylobacter jejuni enteritis. Carbohydrate mimicry [Galβ1–3GalNAcβ1–4(NeuAcα2–3)Galβ1-] between the bacterial lipooligosaccharide and human GM1 ganglioside is seen as having relevance to the pathogenesis of Guillain–Barré syndrome, and conclusive evidence is reported here. On sensitization with C. jejuni lipooligosaccharide, rabbits developed anti-GM1 IgG antibody and flaccid limb weakness. Paralyzed rabbits had pathological changes in their peripheral nerves identical with those present in Guillain–Barré syndrome. Immunization of mice with the lipooligosaccharide generated a mAb that reacted with GM1 and bound to human peripheral nerves. The mAb and anti-GM1 IgG from patients with Guillain–Barré syndrome did not induce paralysis but blocked muscle action potentials in a muscle–spinal cord coculture, indicating that anti-GM1 antibody can cause muscle weakness. These findings show that carbohydrate mimicry is an important cause of autoimmune neuropathy.
Molecular mimicry is one mechanism by which infectious agents may trigger an immune response against autoantigens. Many reports have presented findings consistent with the mimicry hypothesis, but none have convincingly demonstrated that mimicry is an important mechanism in the development of autoimmune disease in humans (1). Although several examples of molecular mimicry between microbial and self-components are known, in most cases the epidemiological relationship between autoimmune disease and microbial infection has not been established. In other cases, moreover, no replicas of human autoimmune disease have been obtained by immunizing with the mimic of an infectious agent. Replicas associated with definite, epidemiological evidence of microbial infection are required to test the molecular mimicry theory of the development of autoimmune diseases.
Guillain–Barré syndrome (GBS), the prototype of postinfectious autoimmune diseases, ranks as the most frequent cause of acute flaccid paralysis (2). The Gram-negative bacterium Campylobacter jejuni, a leading cause of acute gastroenteritis in humans, is the most frequent antecedent pathogen. Epidemiological studies, which established the relationship between GBS and antecedent C. jejuni infection, showed that one-fourth to one-third of GBS patients develop the syndrome after being infected. GBS was considered a demyelinating disease of the peripheral nerves, but the existence of primary “axonal GBS” has been confirmed and is now widely recognized (3, 4). Ganglioside GM1 is an autoantigen for IgG Abs in patients with axonal GBS subsequent to C. jejuni enteritis (2, 5). C. jejuni strains isolated from such patients have a lipooligosaccharide (LOS) with a GM1-like structure (2, 6).
To verify that molecular mimicry between an environmental agent and the peripheral nerves causes GBS, we sensitized animals with C. jejuni LOS and produced a replica of human GBS, generated anti-GM1 mAb by immunization with the LOS, and determined the distribution of GM1 in human spinal nerve roots. As further proof that an autoimmune reaction causes neuromuscular disease, we showed that anti-GM1 mAb blocked muscle action potentials in a muscle–spinal cord coculture.
Methods
Preparation of C. jejuni LOS. The GM1-like LOS (Fig. 1_a_) was prepared from the C. jejuni strain (CF 90-26) isolated from a GBS patient (6) as described (7) with minor modifications. A 5-g sample of freeze-dried bacteria was suspended in 25 ml of 50 mM PBS (pH 7.0) containing 5 mM EDTA. The suspension was stirred by a shearing mixer and ultra-high-speed homogenizer (Physcotron, Microtec Nition, Chiba, Japan), after which 100 mg of hen egg lysozyme (Worthington) was added, and the whole stirred overnight at 4°C. The suspension was kept at 37°C for 20 min and then stirred again as above. The volume of the suspension was increased to 100 ml with 50 mM PBS (pH 7.0) combined with 20 mM MgCl2, after which 100 μg each of ribonuclease A and DNase I (Worthington) was added. The suspension was incubated for 60 min at 37°C and then for 60 min at 60°C. After being stirred in the shearing mixer and homogenizer, the suspension was kept in a 70°C water bath for 10 min. An equal volume of 90% phenol that had been heated to 70°C was added, and the whole was homogenized for 5 min. The homogenate was rapidly cooled in an ice-water bath for 15 min and then centrifuged at 2,000 × g. The upper aqueous layer was removed, dialyzed against distilled water for 3 d, and then extensively centrifuged (105,000 × g for 16 h). The gel-like pellet obtained as the C. jejuni LOS was freeze-dried until used.
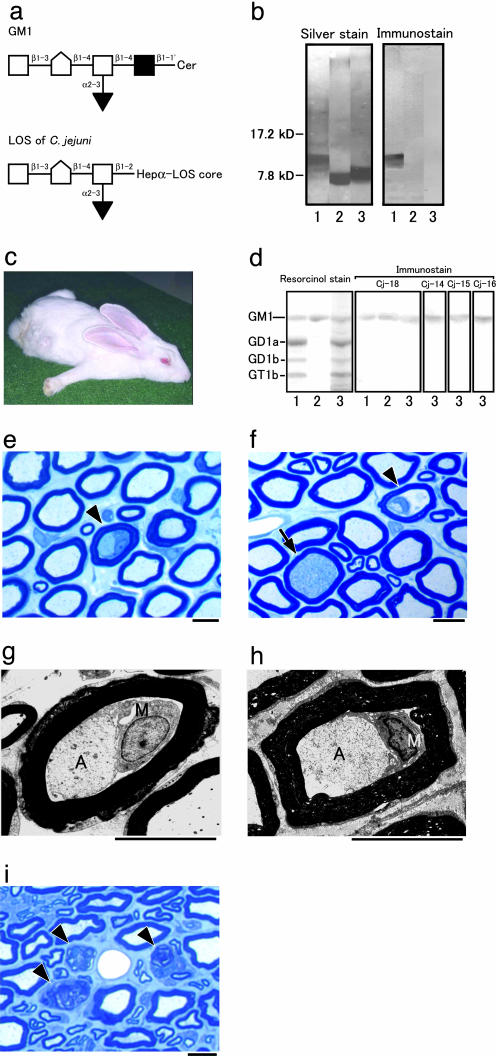
Fig. 1.
Rabbit GBS model sensitized with C. jejuni LOS. (a) Carbohydrate mimicry of GM1 ganglioside by the LOS of C. jejuni (CF 90-26) from a GBS patient. GM1 is located in the nerve cell membrane. The LOS that mimics GM1 is in the outer part of the cell wall of C. jejuni. □, Galactose; [hwyschool], _N_-acetylgalactosamine; ▪, glucose; ▾, _N_-acetylneuraminic acid; Cer, ceramide; Hep, heptose. (b) Presence or absence of the GM1 epitope in bacterial LOS-immunized rabbits. Cholera toxin B subunit reacts with the C. jejuni LOS (lane 1) but not with E. coli K12 LOS (lane 2) or S. minnesota R595 LOS (lane 3). (c) Rabbit with flaccid limb weakness induced by sensitization with C. jejuni LOS. Rabbit Cj-18 lays splayed out, all extremities extended, head on the floor, instead of sitting upright in the usual compact, hunched posture. (d) Anti-ganglioside Ab from rabbits that developed limb weakness after sensitization with C. jejuni LOS. Of the bovine brain gangliosides, plasma IgG from rabbit Cj-18 binds to GM1 (lane 1), isolated GM1 from bovine brain (lane 2), and GM1 from rabbit peripheral nerve (lane 3). The IgGs are from rabbits Cj-14, Cj-15, and Cj-16, and the GM1 is from rabbit peripheral nerve (lane 3). (e_–_h) Macrophages in nerve fibers. Shown are cross sections of the cauda equina from rabbit Cj-18. (e and f) Toluidine blue stain. Macrophages are present in the nerve fibers (arrowheads). The initial degenerated axon stage also is shown (arrow in f). Demyelination and remyelination are rare, and no inflammatory cells exist in the endoneurium. (g and h) Electron micrographs of nerve fibers with macrophage infiltration. The nerve fiber in g is the same as in e. Macrophages (M) occupy the periaxonal space between the atrophic axons (A) and the surrounding myelin sheaths, which appear almost normal. (Scale bars = 10 μm.) (i) Wallerian-like degeneration of nerve fibers. Shown are cross sections of the sciatic nerve from rabbit Cj-14 killed 39 days after onset. Toluidine blue stain was used. Myelin ovoids produced by Wallerian-like degeneration of the myelinated fibers are present (arrowheads). (Scale bar = 10 μm.)
Western Blotting. Escherichia coli K12, D31m4 (Re) LOS and Salmonella minnesota R595 (Re) LOS were purchased from List Biological Laboratories (Campbell, CA). A 2-μg portion of each bacterial LOS was separated on a 15% tricine-SDS-polyacrylamide gel (SPU-15S series, Atto Corporation, Tokyo) (8), and each was blotted on a polyvinylidene difluoride membrane (Atto Corporation), which then was incubated for 2 h at 4°C with the peroxidase-conjugated cholera toxin B subunit (List Biological Laboratories). Binding was made visible with 4-chloro-1-naphtol.
Rabbit Immunization. Male Japanese white rabbits (Kbs:JW), weighing 2.0–2.5 kg, obtained from Oriental Bioservice Kanto (Ibaraki, Japan), were immunized as described (9) with minor modifications. A 2.5- or 10-mg portion of the C. jejuni LOS was dissolved in 0.5 ml of keyhole lympet hemocyanin (KLH) (2 mg/ml; Sigma) in PBS, after which 0.5 ml of complete Freund's adjuvant (CFA) (Sigma) was added, and the mixture was emulsified. A 1-ml sample of the C. jejuni LOS emulsion was injected s.c. in the back at 3-wk intervals until limb weakness developed or 12 mo had passed since the first inoculation. Control rabbits were injected under the same protocol with 10 mg of E. coli LOS or of S. minnesota LOS or without the LOS. The rabbits were checked daily for clinical signs, and plasma samples taken weekly by ear vein puncture.
TLC Immunostaining and ELISA. Total gangliosides were extracted from the sciatic nerves of normal rabbits and humans, and TLC immunostaining was done as described (9). In brief, the peripheral nerve gangliosides, authentic GM1, bovine brain ganglioside mixture (Cronassial; Fidia, Padova, Italy), and each LOS were layered on TLC plates. The plates were developed then overlaid with plasma from the rabbits or from a GBS patient from whom C. jejuni (CF 90-26) had been isolated and the anti-GM1 mAb. Last, they were incubated with peroxidase-conjugated anti-rabbit (Nordic, Tilburg, The Netherlands), anti-human (Dako, Glostrup, Denmark), or anti-mouse IgG Abs. Rabbit anti-GM1 Ab titers were tested by ELISA as described (9).
Pathological and Immunohistochemical Studies of Rabbit Peripheral Nerves. The lumbar spinal nerve root, cauda equina, and sciatic nerve specimens were evaluated pathologically, as described (9). For electron microscopy, ultrathin sections cut from tissue fragments embedded in Epon 812 resin were stained with uranyl acetate and lead citrate and then examined in a Hitachi-7100 electron microscope (Hitachi, Tokyo). In the immunohistochemical study, the nerve root, cauda equina, sciatic nerve, and tibial nerve specimens were stained with peroxidase-conjugated protein G (Sigma) as reported (9).
Generation of Anti-GM1 mAb. Mice lacking the functional gene for β1,4-_N_-acetylgalactosaminyltransferase (GM2/GD2 synthase; EC 2.4.1.92) were raised, and their genotypes were determined as described (10). Those mice did not express complex gangliosides including GM1. They were immunized i.p. four times at 2-wk intervals with 50 μg of C. jejuni LOS dissolved in 50 μl of KLH solution and then mixed with 50 μl of CFA. Three days after the final immunization with 50 μg of LOS in 50 μl of PBS, splenocytes were fused with P3/NS1/1-Ag4-1 myeloma cells (Health Science Research Resources Bank, Osaka). Cloning was done with a hybridoma-cloning kit (ClonaCell-HY; StemCell Technologies, Vancouver, BC, Canada). Culture supernatants underwent an ELISA (9) with GM1 (Sigma) and anti-mouse IgG Ab (Sigma) to obtain hybridomas that secrete anti-GM1 IgG mAb. The mAb isotype produced by the hybridomas was tested with a mouse monoclonal isotyping kit (Amersham Pharmacia). The mAb produced with an i-MAb mAb production kit (Diagnostic Chemicals, Charlottetown, PE, Canada) was purified by protein G affinity chromatography according to the manufacturer's instructions (Amersham Pharmacia).
Immunohistochemistry of Human Spinal Nerve Roots. Anti-GM1 IgG from a GBS patient (Patient 1, ref. 5) was purified by affinity chromatography (11) then biotinylated with a FluoReporter MiniBiotin-XX Protein Labeling Kit (F-6347) (Molecular Probes). Spinal nerve roots without pathological changes were obtained at autopsy from an 82-year-old woman who died of bronchopneumonia. In the immunohistochemistry study, cryostat sections (6 μm thick) from snap-frozen nerve tissues were fixed with cold acetone and immunostained first with the biotinylated human anti-GM1 IgG then the avidin-biotinperoxidase complex (ABC) in a Vectastain ABC kit (Vector Laboratories). In mouse anti-GM1 mAb, biotinylated horse anti-mouse IgG (Vector Laboratories) was the secondary Ab. Diaminobenzidine was the chromogen. After immunostaining, sections were counterstained with hematoxylin. For the negative controls, the first Abs were omitted or replaced with normal sera.
For immunoelectron microscopy, nerve roots obtained at autopsy were fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.4), dehydrated in a graded dimethylformamide series at –25°C, and embedded in LR White resin (London Resin, Berkshire, U.K.). Ultrathin sections were cut and mounted on nickel grids. After incubation with 10% normal goat serum for 10 min, the sections were incubated overnight at 4°C with the mouse anti-GM1 mAb. After being washed with PBS, the sections were incubated for 30 min at room temperature with goat anti-mouse IgG conjugated to 15-nm gold particles (British BioCell International, Cardiff, U.K.). They then were washed with PBS and incubated with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). After being washed with distilled water, the sections were stained with uranyl acetate and lead citrate and examined in a Hitachi H-7100 electron microscope. For the negative controls, the primary Ab was replaced with normal mouse serum.
Muscle Action Potentials in a Rat Muscle–Spinal Cord Coculture. This was done as described (12). After 1 wk of coculture, the innervated muscle specimens were placed in an experimental chamber (35-mm Petri dish) on the stage of an inverted light microscope (IX-70; Olympus, Tokyo). The chamber (volume, 1 ml) was perfused continually with culture medium at the rate of 2–3 ml/min. Glass microelectrodes filled with 3 M KCl that had a tip resistance of 80–120 MΩ were used to record spontaneous muscle action potentials. Ten microliters of anti-GM1 mAb (GB2, 1 mg/ml), mouse IgG2bκ (MOPC-141, 1 mg/ml; Cappel, Aurora, OH), sera samples from patients who had GBS with anti-GM1 IgG, myasthenia gravis, or amyotrophic lateral sclerosis, and from normal controls with and without complement inactivation, or purified anti-GM1 IgG were added in a bath application. Electrical activity recorded by electrodes connected to a microelectrode amplifier (MEZ-8301; Nihon Kohden, Tokyo) was displayed on an oscilloscope (VC-11; Nihon Kohden). Data were transferred to a computer through an A-D converter (Digidata-1200 Interface; Axon Instruments, Union City, CA) and stored. The spontaneous action potential was low-pass filtered at 1 kHz. All experiments were performed at 33 ± 1°C.
Passive Transfer in Mice. Male C57BL/6 mice (12 wk of age; SLC, Hamamatsu, Japan) were injected intravenously with 1 mg of the anti-GM1 mAb or 10 mg of the purified human anti-GM1 IgG. The mice were checked daily for clinical sings. Sera and nerve tissue were obtained 2 wk after the injection. Mouse and human anti-GM1 Ab titers were tested by ELISA, and the cauda equina was stained with peroxidase-conjugated protein G (Sigma) as described (9).
Results
Induction of Paralyzed Rabbits. None of the 10 controls inoculated only with KLH and CFA every 3 wk showed limb weakness until 12 mo after the first inoculation, whereas 4 of the 10 rabbits immunized with 2.5 mg of C. jejuni LOS, KLH, and CFA developed limb weakness sooner. Two rabbits (Cj-9 and Cj-6) developed flaccid paresis of the hind limbs 215 and 216 d after the initial inoculation and tetraparesis the next day. They were unable to lift their heads and bodies, respectively, 5 and 4 d after onset. Two other rabbits (Cj-1 and Cj-4) developed tetraparesis 133 and 329 d after the initial inoculation, but weakness was mild during the course of the illness.
The cholera toxin B subunit (a specific ligand for GM1-oligosaccharide) bound strongly to the C. jejuni LOS, but did not react with E. coli K12 LOS or S. minnesota R595 LOS (Fig. 1_b_). None of the control rabbits inoculated with 10 mg of E. coli LOS (n = 5) or S. minnesota LOS (n = 5) showed limb weakness until 12 mo after the first inoculation. In contrast, all eight rabbits immunized with 10 mg of the C. jejuni LOS, KLH, and CFA developed limb weakness earlier. One rabbit (Cj-18) developed flaccid paresis of the hind limbs 64 d after the initial inoculation and tetraparesis 7 d after its onset (Fig. 1_c_ and Movie 1, which is published as supporting information on the PNAS web site). The others (Cj-11 to Cj-17) developed tetraparesis 227, 47, 176, 172, 40, 64, and 128 d, respectively, after the initial inoculation. Quadriparesis and respiratory paresis developed and progressed rapidly in three rabbits (Cj-11, Cj-13, and Cj-17), causing death 2, 15, and 1 d, respectively, after onset of limb weakness. One rabbit (Cj-15), however, had a monophasic course like that of patients with GBS. After onset, limb weakness worsened for 8 d, reached a plateau, and then lessened from day 16.
Induction of Anti-GM1 Antibody. TLC with immunostaining showed that, as to rabbit peripheral nerve gangliosides, plasma IgG Abs from the paralyzed rabbits reacted strongly with the GM1 (Fig. 1_d_) and with the C. jejuni LOS (data not shown), whereas neither E. coli LOS nor S. minnesota LOS induced anti-GM1 Abs in the rabbits. The IgG Abs from the paralyzed rabbits did not react with GD1a, GD1b, or GT1b (Fig. 1_d_). Anti-GM1 IgM antibodies were detected in the eight paralyzed rabbits 2–4 wk after their first sensitization with C. jejuni LOS. Anti-GM1 IgG antibodies were detected in these rabbits 4–6wk after their first inoculation, and the titers gradually increased by repeated immunizations. Six of the eight rabbits developed flaccid paresis within 3 wk (median, 1 wk) after the peak anti-GM1 IgG titer was reached (range, 2,000–32,000; median, 8,000), but the titers did not correlate with the severity.
Pathological Findings in Diseased Rabbits. The spinal nerve roots of rabbit Cj-18 killed 11 d after onset had few nerve fibers showing Wallerian-like degeneration, whereas the nerve roots showed occasional macrophages within the periaxonal spaces surrounded by almost intact myelin sheaths (Fig. 1 e_–_h). Axons of these nerve fibers had various degrees of degeneration. Sciatic nerve specimens from the paralyzed rabbits showed more severe Wallerian-like degeneration than did the proximal nerve roots (Fig. 1_i_). Demyelination and remyelination were rare, and no inflammatory cells were present in the endoneurium. Furthermore, protein G bound selectively to some axons in the cauda equina of rabbit Cj-12, indicating that IgG deposits were on those axons (data not shown). In contrast, no significant changes were found in the brains or spinal cords of any of the paralyzed rabbits.
Characteristics of mAb GB2. A clone with anti-GM1 IgG activity (GB2), subclass IgG2bκ, was obtained. TLC immunostaining showed it reacted strongly with the GM1 in a bovine brain ganglioside mixture that included GM1, GD1a, GD1b, and GT1b (Fig. 2_a_) and with the C. jejuni LOS (Fig. 2_b_). An ELISA showed that GB2 reacted with GM1 but not with gangliosides GM2, GD2, GalNAc-GD1a, GT1a, or GQ1b, evidence of its highly restricted binding specificity for GM1. Anti-GM1 mAb was therefore used in our study. TLC immunostaining confirmed that our anti-GM1 mAb reacted strongly with the GM1 in human peripheral nerve gangliosides (Fig. 2_a_).
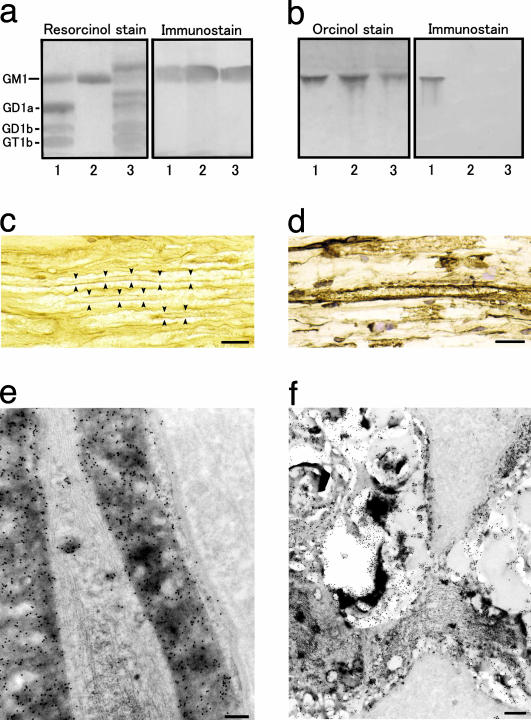
Fig. 2.
Immunoreactivity of anti-GM1 mAb. (a and b) Binding specificity of the mAb (GB2) generated by C. jejuni LOS. (a) Of the bovine brain gangliosides, it recognizes GM1 (lane 1), the GM1 isolated from bovine brain (lane 2), and the GM1 of human peripheral nerve (lane 3). (b) The mAb reacts with the C. jejuni LOS (lane 1) but not with E. coli K12 LOS (lane 2) or S. minnesota R595 LOS (lane 3). (c_–_f) GM1 immunoreactivity in the anterior nerve roots of the human lumbar cord with the biotin-conjugated anti-GM1 IgG from a GBS patient (c) and the anti-GM1 mAb (d_–_f). (c and d) Labeling is present in the myelin sheaths of both large and small myelinated axons. (e) GM1 antigenicity is localized on the myelin lamellae, plasma membrane of the outer Schwann cell process, the basal lamina, and the mitochondria and some vacuoles of an axon. (f) Labeling also is present on Schwann cell processes surrounding the nodal region of an axon. (c and d) The immunoperoxidase method was used. (e and f) The immunogold method is indicated by 15-nm gold particles. (Scale bars = 30 μmin c and d, 1 μmin e, and 2 μmin f.)
Localization of Anti-GM1 Ab Binding in Human Spinal Nerve Roots. The immunohistochemistry of the anterior roots of the human spinal cord showed that the anti-GM1 IgG from the patient with GBS subsequent to C. jejuni enteritis reacted with the myelin sheaths of both large and small myelinated fibers (Fig. 2_c_). Fine dot-like staining of the axons also was detectable. Immunostaining with mAb gave similar results (Fig. 2_d_) and visibly labeled Schwann cell cytoplasms. Fibroblasts or collagen fibrils were negative for GM1. No immunostaining occurred when the first Ab was omitted or replaced with normal mouse serum. Immunoelectron microscopy with mAb confirmed the presence of GM1 antigenicity on the myelin lamellae on the outermost cell membrane of Schwann cell processes and on the basal lamina.
Labeling was present along the axolemmas of axons and on occasional vesicles and mitochondria in the axoplasm (Fig. 2_e_). Labeling of Schwann cell processes around unmyelinated axons also was visible. At the nodes of Ranvier immunoreactivity occurred on Schwann cell processes covering the nodal regions of axons (Fig. 2_f_). No immunostaining took place when the first Ab was replaced with normal mouse serum.
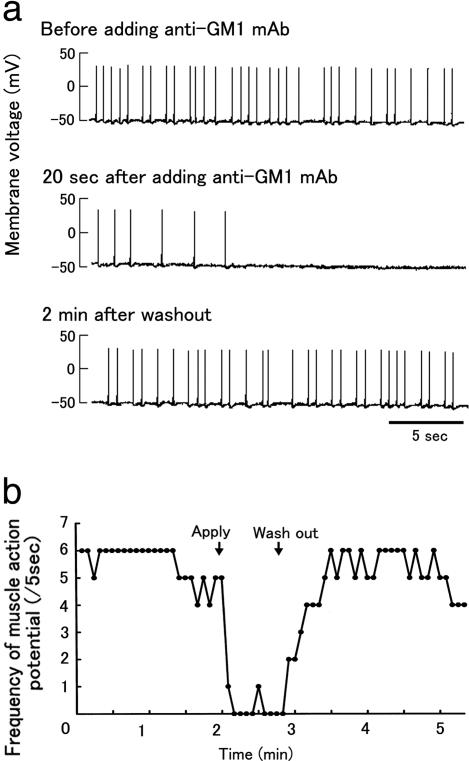
Biologic Activity of Anti-GM1 Ab in Muscle–Spinal Cord Cocultures. After 1 wk of coculture, asynchronous contractions of several individual muscle fibers had occurred on formation of neuromuscular junctions (Fig. 3_a_ and Movie 2, which is published as supporting information on the PNAS web site). Muscle action potential frequency was 1.3 ± 0.54 per s and amplitude was 75.2 ± 6.4 mV (n = 6). Anti-GM1 mAb prolonged the muscle action potential intervals at the neuromuscular junctions, beyond which potentials were inhibited (Fig. 3 and Movie 3, which is published as supporting information on the PNAS web site). This inhibition did not occur with the isotype-matched control Ab. The blockade of muscle action potentials by anti-GM1 mAb was fully reversed after a wash with the culture medium (Fig. 3). Sera from 11 patients who had GBS associated with anti-GM1 IgG after C. jejuni enteritis and from seven patients with myasthenia gravis also blocked the muscle action potentials, whereas sera from seven patients with amyotrophic lateral sclerosis and seven healthy control subjects did not. GBS sera with complement inactivation inhibited potentials, and purified anti-GM1 IgG from the GBS patient (patient 1, ref. 5) also blocked them.
Fig. 3.
Biologic activity of anti-GM1 mAb. (a) Blockade of muscle action potentials by anti-GM1 mAb in muscle–spinal cord cocultured cells. (b) Time course of inhibition of spontaneous muscle action potentials by anti-GM1 mAb recovery after washout. The number of potentials was measured every 5 s. Arrows show times of the anti-GM1 mAb addition and exchange of the bath solution.
Passive Transfer in Mice. None of the mice injected with mouse (n = 5) and human (n = 5) anti-GM1 IgG showed limb weakness until 2 wk after the i.v. injection. Mouse and human anti-GM1, respectively, were detected in their sera obtained at the time of killing. No protein G bound to axons in the cauda equina of the mice, indicating that no IgG deposits were present on those axons.
Discussion
If molecular mimicry by an infectious agent is the trigger of an autoimmune response leading to an autoimmune disease, several conditions must be satisfied: (i) the pathogen must be associated with the onset of the symptoms or disease in a convincing number of cases; (ii) a clinically detectable immune response to the pathogen must be demonstrable, at least at disease onset, which response must be shown to cross-react with host antigens of the affected tissues; and (iii) the pathology of the disease must be consistent with the immune response (13). As discussed below, these conditions have been established for GBS subsequent to C. jejuni infection, proof that GM1 mimicry by C. jejuni does cause GBS. Many C. jejuni strains actually have been isolated from GBS patients at the onset of limb weakness (14). Patients with axonal GBS subsequent to C. jejuni enteritis have anti-GM1 IgG, and the titers peak early in the disease, followed by a gradual decline (5). C. jejuni LOSs from GBS isolates have a tetrasaccharide structure consistent with GM1 mimicry (2, 6).
Several research groups have failed to induce neuropathy by sensitization with C. jejuni LOS with GM1 epitope. Rats immunized with C. jejuni LOS only showed an IgM response to GM1 (15). Anti-GM1 IgG has been induced in rabbits by sensitization with the LOS from the C. jejuni reference strain obtained from a patient with enteritis and the LOSs from GBS-associated strains, but no muscle weakness was observed (16, 17): The New Zealand White rabbits were immunized with a smaller amount of LOS and no KLH, and the observation period may have been too short. Because induction rates of the GM1-inoculated GBS model depend on species or breed susceptibility and the immunization procedure used (18), Japanese white rabbits were injected repeatedly with KLH and 2.5 mg of C. jejuni LOS bearing a GM1-like structure. Four of the 10 rabbits developed weakness that was associated with anti-GM1 IgG. Next, a larger amount of the LOS was injected. All eight rabbits immunized with 10 mg of the LOS developed limb weakness, and muscle weakness progressed rapidly. One rabbit had a monophasic course similar to the clinical course of patients with GBS. GM1 is expressed on rabbit peripheral nerve axons (9), and all the paralyzed rabbits had IgG Abs to GM1 in their peripheral nerve gangliosides. Macrophages within the periaxonal space surrounded by an intact myelin sheath are the pathological substrate of axonal GBS (3). This finding was confirmed in the nerve roots of rabbit Cj-18. Sciatic nerve specimens from the paralyzed rabbits showed Wallerian-like degeneration. Demyelination and remyelination were rare, and no T cell infiltration occurred. IgG was deposited on some axons in the cauda equina of rabbit Cj-12. These findings, compatible with the features of human axonal GBS (3, 4), provide evidence that the rabbits inoculated with C. jejuni LOS constitute a valid GBS model. This definitive replica of a human autoimmune disease produced by immunizing with the mimic of the infectious agent associated with epidemiological evidence of microbial infection has not been reported previously.
Whereas most GBS patients develop limb weakness within 3 wk after C. jejuni infection, the paralyzed rabbits required several inoculations with GM1-like LOS and with GM1 (9). In contrast, the anti-GM1 IgG titers correlated with the disease onset in the inoculated rabbits and in the GBS patients (5). The inflammatory response in GBS may be triggered by either activation of complement or leukocytes. The induction of inflammation is triggered by the concerted action of complement and FcγR (19). Anti-GM1 IgG from GBS patients induces leukocyte effector functions such as degranulation and phagocytosis (20). We have shown in the GM1-inoculated rabbits that anti-GM1 IgG efficiently induces such inflammatory reactions at the onset, whereas it dose not before the onset (W.-L. van der Pol and N.Y., unpublished data). These findings suggest that a large amount of anti-GM1 IgG associated with the effector functions is required for the development of limb weakness. The pathogenic antibody could be induced easily in the patients with GBS subsequent to C. jejuni infection but not in the rabbits immunized by GM1 and C. jejuni LOS, probably because of their diversity of the anti-GM1 response. The reason should be elucidated by further studies.
LOS is composed of oligosaccharide and lipid A. Several lines of evidence support an GM1 oligosaccharide rather than lipid A structure for the C. jejuni LOS that has importance in the development of GBS. E. coli K12 LOS and S. minnesota R595 LOS do not carry the GM1 epitope. Sensitization of those LOSs that carry lipid A did not induce anti-GM1 Abs in the rabbits, evidence that anti-GM1 IgG is not a result of polyclonal B cell stimulation. None of the rabbits developed limb weakness. In contrast, a GBS model has been established that uses inoculation with GM1, which carries a ceramide moiety but not lipid A (9). Sensitization with GM1 induced anti-GM1 IgG in rabbits, who developed limb weakness. The pathology is identical with that of human axonal GBS and with that of paralyzed rabbits inoculated with C. jejuni LOS. IgG Ab to GQ1b also is associated with Fisher syndrome, which is characterized by ophthalmoplegia, ataxia, and areflexia (21). That syndrome is considered a variant of GBS because some patients develop GBS during the clinical course of Fisher syndrome, but patients with Fisher syndrome typically do not develop limb weakness or have anti-GM1 IgG. C. jejuni isolates from Fisher syndrome have an LOS with GQ1b epitope (22). Five rabbits were sensitized with 10 mg of C. jejuni (CF 93–6) LOS bearing GQ1b epitope, but none of them developed limb weakness (N.Y., unpublished data). This indirect evidence shows that the nature of the GM1-oligosaccharide structure is important for the development of GBS.
We injected C. jejuni LOS into the mice lacking GM1 (immune naïve hosts) and obtained an IgG-class mAb that is highly specific for GM1. The anti-GM1 mAb bound to the GM1 of human peripheral nerve gangliosides on a TLC plate. A point worth noting is that GM1 is expressed in both the peripheral and central nervous systems but that sensitization with GM1 or the GM1-like LOS produces only peripheral neuropathy. This characteristic seems to occur because the blood–brain barrier that protects the brain and spinal cord is much tighter than the blood–nerve barrier. Electrophysiological, immunohistochemical, and pathological study findings show that the initial lesion in axonal GBS may be localized in the spinal nerve roots (23, 24). The reason may be that nerve roots are vulnerable because they lack a blood–nerve barrier. The cholera toxin B subunit stains both the nodes of Ranvier and paranodal Schwann cells in human spinal roots (25), which we confirmed with the anti-GM1 mAb and anti-GM1 IgG from a GBS patient. Similar immunohistochemical results with mAbs were obtained for spinal nerve roots in rabbits (data not shown). GM1 was expressed on axons of the anterior nerve roots in both humans and rabbits, which nerve roots seem to be the initial lesion in axonal GBS. The clinical, pathological, and immunological features of the paralyzed rabbits sensitized with the C. jejuni LOS were very similar to those of axonal GBS. We therefore speculate that the pathogenesis of axonal GBS subsequent to C. jejuni enteritis is as follows: (i) Infection by C. jejuni carrying a GM1-like LOS induces high production of anti-GM1 IgG in patients who have a particular immunogenetic background, (ii) the autoantibody binds to GM1 expressed on motor nerve axons in the spinal roots, and (iii) the deposited IgG recruits macrophages into the periaxonal space where they attack the axon and Wallerian-like degeneration occurs.
Limb weakness in some GBS patients is quickly cured by plasmapheresis, indicating that autoantibody causes physiological conduction failure in the motor nerves before any pathological change occurs (26). The nerve terminal lacks a blood–nerve barrier; therefore, Abs have easy access to neuronal membranes, the presumed sites of injury. In a mouse phrenic nerve–diaphragm preparation, the IgG from GBS patients produced neuromuscular blockade in a complement-independent manner (27). We added anti-GM1 mAb to rat muscle–spinal cord cocultures, and it blocked muscle action potentials at the neuromuscular junctions. This blockade was fully reversed after a wash with the culture medium, consistent with the clinical observation that in GBS plasma exchange induces rapid recovery. The anti-GM1 IgG present in GBS subsequent to C. jejuni enteritis also blocked muscle action potentials and was complement-independent. Although the blockade mechanism needs to be clarified, these findings are consistent with anti-GM1 IgG being biologically active and pathogenic and producing muscle weakness directly in patients with GBS. In mice, systemic injection of mouse or human anti-GM1 IgG did not induce paralysis and IgG binding to the peripheral nerves, although the amount of antibody injected and/or the duration of treatment might not be sufficient. Passive transfer of axonal degeneration in rat peripheral nerves, however, was produced by intraneural injection of human and rabbit anti-GM1 IgGs associated with complements (M. Kamijo and N.Y., unpublished data). These findings suggest that other factors such as tumor necrosis factor-α and IL-1β, which enhance leakage in the blood–nerve barrier (28), are required to induce clinical and pathological disease by the systemic injection. Circulating tumor necrosis factor-α and IL-1β increase in GBS patients (29), and this increase could occur also in our active immunization model using CFA.
In conclusion, this study has verified that molecular mimicry between an environmental agent and the peripheral nerves causes GBS. Molecular mimicry is an important mechanism in the development of human autoimmune diseases. Research on molecular mimicry and autoimmunity principally has focused on T cell-mediated, antipeptide responses, rather than on Ab responses to carbohydrate structures (1). Our findings show that carbohydrate mimicry between GM1 and the C. jejuni LOS induces the production of pathogenic autoantibodies and the development of GBS. This new concept that carbohydrate mimicry can cause an autoimmune disease provides a clue to the resolution of the pathogenesis of other immune-mediated diseases.
Supplementary Material
Supporting Movies
Acknowledgments
We thank S. Koike and K. Yamaguchi (Institute for Medical Science, Dokkyo University School of Medicine) for their technical assistance. This work was supported in part by a grant-in-aid for Scientific Research (B) (KAKENHI 14370210 to N.Y.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a Health Science Research grant (Research on Brain Science) from the Ministry of Health and Welfare of Japan; and Human Frontier Science Program Grant RGP 38/2003.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CFA, complete Freund's adjuvant; GBS, Guillain–Barré syndrome; KLH, keyhole lympet hemocyanin; LOS, lipooligosaccharide.
References
- 1.Marrack, P. J. & Kotzin, B. L. (2001) Nat. Med. 7**,** 899–905. [DOI] [PubMed] [Google Scholar]
- 2.Yuki, N. (2001) Lancet Infect. Dis. 1**,** 29–37. [DOI] [PubMed] [Google Scholar]
- 3.McKhann, G. M., Cornblath, D. R., Griffin, J. W., Ho, T. W., Li, C. Y., Jiang, Z., Wu, H. S., Zhaori, G., Liu, Y., Jou, L. P., et al. 1993. Ann. Neurol. 33**,** 333–342. [DOI] [PubMed] [Google Scholar]
- 4.Hafer-Macko, C., Hsieh, S.-T., Li, C. Y., Ho, T. W., Sheikh, K., Cornblath, D. R., McKhann, G. M., Asbury, A. K. & Griffin J. W. (1996) Ann. Neurol. 40**,** 635–644. [DOI] [PubMed] [Google Scholar]
- 5.Yuki, N., Yoshino, H., Sato, S. & Miyatake, T. (1990) Neurology 40**,** 1900–1902. [DOI] [PubMed] [Google Scholar]
- 6.Yuki, N., Taki, T., Inagaki, F., Kasama, T., Takahashi, M., Saito, K., Handa, S. & Miyatake, T. (1993) J. Exp. Med. 178**,** 1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, K. G. & Perry, M. B. (1976) Can. J. Microbiol. 22**,** 29–34. [DOI] [PubMed] [Google Scholar]
- 8.Schägger, H. & von Jagow, G. (1987) Anal. Biochem. 166**,** 368–379. [DOI] [PubMed] [Google Scholar]
- 9.Yuki, N., Yamada, M., Koga, M., Odaka, M., Susuki, K., Tagawa, Y., Ueda, S., Kasama, T., Ohnishi, A., Hayashi, S., et al. (2001) Ann. Neurol. 49**,** 712–720. [PubMed] [Google Scholar]
- 10.Takamiya, K., Yamamoto, A., Furukawa, K., Yamashiro, S., Shin, M., Okada, M., Fukumoto, S., Haraguchi, M., Takeda, N., Fujimura, K., et al. (1996) Proc. Natl. Acad. Sci. USA 93**,** 10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirabayashi, Y., Suzuki, T., Suzuki, Y., Taki, T., Matsumoto, M., Higashi, H. & Kato, S. (1983) J. Biochem. (Tokyo) 94**,** 327–330. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi, K., Utsunomiya, I., Ren, J., Yoshida, N., Aoyagi, H., Nakatani, Y., Ariga, T., Usuki, S., Yu, R. K. & Miyatake, T. (2004) Neurochem. Res. 29**,** 953–960. [DOI] [PubMed] [Google Scholar]
- 13.Davies, J. M. (1997) Immunol. Cell Biol. 75**,** 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuki, N., Takahashi, M., Tagawa, Y., Kashiwase, K., Tadokoro, K. & Saito, K. (1997) Ann. Neurol. 42**,** 28–33. [DOI] [PubMed] [Google Scholar]
- 15.Wirguin, I., Briani, C., Suturkova-Milosevic, L., Fisher, T., Della-Latta, P., Chalif, P. & Latov, N. (1997) J. Neuroimmunol. 78**,** 138–142. [DOI] [PubMed] [Google Scholar]
- 16.Ritter, G., Fortunato, S. R., Cohen, L., Noguchi, Y., Bernard, E. M., Stockert, E. & Old, L. J. (1996) Int. J. Cancer 66**,** 184–190. [DOI] [PubMed] [Google Scholar]
- 17.Ang, C. W., Endtz, H. P., Jacobs, B. C., Laman, J. D., de Klerk, M. A., van der Meché, F. G. & van Doorn, P. A. (2000) J. Neuroimmunol. 104**,** 133–138. [DOI] [PubMed] [Google Scholar]
- 18.Yuki, N., Mori, I. & Susuki, K. (2002) Ann. Neurol. 52**,** 128–129. [Google Scholar]
- 19.Ravetch, J. V. (2002) J. Clin. Invest. 110**,** 1759–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Sorge, N. M., van den Berg, L. H., Geleijns, K. P. W., van Strijp, J. A., Jacobs, B. C., van Doorn, P. A., Wokke, J. H. J., van de Winkel, J. G. J., Leusen, J. H. W. & van der Pol, W.-L. (2003) Ann. Neurol. 53**,** 570–579. [DOI] [PubMed] [Google Scholar]
- 21.Chiba, A., Kusunoki, S., Shimizu, T. & Kanazawa, I. (1992) Ann. Neurol. 31**,** 677–679. [DOI] [PubMed] [Google Scholar]
- 22.Yuki, N., Taki, T., Takahashi, M., Saito, K., Yoshino, H., Tai, T., Handa, S. & Miyatake, T. (1994) Ann. Neurol. 36**,** 791–793. [DOI] [PubMed] [Google Scholar]
- 23.Griffin, J. W., Li, C. Y., Macko, C., Ho, T. W., Hsieh, S.-T., Xue, P., Wang, F. A., Cornblath, D. R., McKhann, G. M. & Asbury, A. K. (1996) J. Neurocytol. 25**,** 33–51. [DOI] [PubMed] [Google Scholar]
- 24.Susuki, K., Nishimoto, Y., Yamada, M., Baba, M., Ueda, S., Hirata, K. & Yuki, N. (2003) Ann. Neurol. 54**,** 383–388. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh, K. A., Deerinck, T. J., Ellisman, M. H. & Griffin, J. W. (1999) Brain 122**,** 449–460. [DOI] [PubMed] [Google Scholar]
- 26.Kuwabara, S., Yuki, N., Koga, M., Hattori, T., Matsuura, D., Miyake, M. & Noda, M. (1998) Ann. Neurol. 44**,** 202–208. [DOI] [PubMed] [Google Scholar]
- 27.Buchwald, B., Toyka, K. V., Zielasek, J., Weishaupt, A., Schweiger, S. & Dudel, J. (1998) Ann. Neurol. 44**,** 913–922. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki, T., Kanda, T. & Mizusawa, H. (1999) J. Med. Dent. Sci. 46**,** 31–40. [PubMed] [Google Scholar]
- 29.Sharief, M. K., Ingram, D. A., Swash, M. & Thompson, E. J. (1999) Neurology 52**,** 1833–1838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Movies