Dose-dependent response of FGF-2 for lymphangiogenesis (original) (raw)
Abstract
Spatio-temporal studies on the growth of capillary blood vessels and capillary lymphatic vessels in tissue remodeling have suggested that lymphangiogenesis is angiogenesis-dependent. We revisited this concept by using fibroblast growth factor 2 (FGF-2) (80 ng) to stimulate the growth of both vessel types in the mouse cornea. When we lowered the dose of FGF-2 in the cornea 6.4-fold (12.5 ng), the primary response was lymphangiogenic. Further investigation revealed that vascular endothelial growth factor-C and -D are required for this apparent lymphangiogenic property of FGF-2, and when the small amount of accompanying angiogenesis was completely suppressed, lymphangiogenesis remained unaffected. Our findings demonstrate that there is a dose-dependent response of FGF-2 for lymphangiogenesis, and lymphangiogenesis can occur in the absence of a preexisting or developing vascular bed, i.e., in the absence of angiogenesis, in the mouse cornea.
Tissue remodeling, such as that which occurs in embryonic development (1), adult wound healing (2, 3), and female reproductive physiology (4, 5), is accompanied by the growth of capillary blood vessels (angiogenesis) as well as capillary lymphatic vessels (lymphangiogenesis). This coupling of angiogenesis and lymphangiogenesis illustrates an important function of the lymphatic vasculature, which is to maintain fluid homeostasis by collecting fluid that leaks from capillary blood vessels and returning it to the blood circulation (6).
In studies examining the relationship between growing blood and lymphatic vessels, angiogenesis has always preceded lymphangiogenesis. Spatio-temporal studies showed that new blood vessels were followed by lymphatic vessels and that the lymphatic vessels grew alongside new veins (2, 3). These observations and others have led to the belief that a preexisting blood vascular bed may be necessary to guide lymphangiogenesis.
Fibroblast growth factor 2 (FGF-2) and vascular endothelial growth factor (VEGF) -A, -C, and -D have been shown to induce both angiogenesis and lymphangiogenesis (7). When these factors are overexpressed in vivo, the vessels display morphological or functional abnormalities, e.g., VEGF overexpression results in malformed leaky vessels with irregular and large lumens, and VEGF-C and -D overexpression in skin causes hyperplastic lymphatic vessels and lymphedema (8–10). Interestingly, it has been shown that a local concentration of VEGF can determine whether newly forming blood vessels develop to be normal or abnormal (11).
In this study, we explored the relationship between lymphatic and blood vessel growth, asking specifically whether (i) lymphatic vessel growth requires a preexisting vascular matrix to guide its growth, and (ii) a pleiotropic factor, FGF-2, can gain specificity in inducing either angiogenesis or lymphangiogenesis.
The mammalian cornea is one of a few avascular tissues. Corneal lymphangiogenesis has been reported to occur in the midst of inflammatory angiogenesis (12–14). FGF-2 is a mediator of angiogenesis in inflammation and wound healing (15) and stimulates lymphatic endothelial cell (EC) proliferation and migration in vitro (16). Therefore, we reasoned that FGF-2 implanted in the mouse cornea would recapitulate the angiogenesis and lymphangiogenesis observed in corneal inflammation, but at the same time allow us to precisely control the dose of the cytokine. We found that lymphatic vessels are significantly more sensitive to FGF-2 than are blood vessels, and we defined a concentration of FGF-2 at which robust lymphangiogenesis was stimulated with minimal angiogenesis (17). Furthermore, we showed that the apparent lymphangiogenic property of FGF-2 was mediated by VEGF-C and -D, and that when the accompanying angiogenesis was completely suppressed, lymphangiogenesis was unaffected. The latter finding demonstrates that (i) lymphatic endothelium can grow independently of blood vessels and (ii) a pleiotropic factor, FGF-2, has a dose-dependent response to specifically stimulate lymphangiogenesis.
Materials and Methods
Corneal Lymphangiogenesis Assay. Male, 6- to 10-week-old, CB6F1 mice (Charles River Breeding Laboratories) and FVB _Tie2_-GFP mice (The Jackson Laboratory) were used. The intrastromal micropocket was created as described (18) with the following modifications: the pocket was started inferionasally, 1.2–1.4 mm away from the limbus. Pellets were made as described (18) with various amounts of FGF-2. After 7 days, lectin–FITC from Lycopersicon esculentum (Sigma) (3 μg/g of body weight) was injected intracardially and allowed to perfuse for 4 min. This step was omitted if the cornea was to be incubated later in anti-mouse CD34-FITC (BD Biosciences) (1:2,000) or anti-mouse CD45-FITC (BD Biosciences) (1:2,000). The cornea was dissected and fixed in acetone and incubated in anti-mouse platelet-endothelial cell adhesion molecule/phycoerythrin (PECAM-PE) (BD Biosciences) (1:500) in TNB blocking buffer (TSA Biotin System, NEN Life Science) with or without the other antibodies (above) for 24 h at 4°C. Corneas were digitally photographed (Spot Diagnostics) under fluorescent microscopy (Nikon). _Tie2_-GFP mice underwent pellet implantation as above. Seven days later, corneas were dissected, mounted in saline, and digitally photographed under a fluorescein filter. Corneas were then fixed in acetone and incubated for 24 h with anti-PECAM-PE, and rephotographed under a rhodamine filter. An alternative method to visualize corneal lymphatic vessels was to inject 2,000,000 molecular mass dextran conjugated to rhodamine (Molecular Probes) extravascularly into the corneal stroma adjacent to the pellet (19). The rhodamine-labeled dextran was visualized under a fluorescent dissecting microscope (Leica Microsystems).
Human Microvascular Blood Endothelial Cells (HMBEC). Commercial HMBEC (Cascade Biologics) were maintained according to the manufacturer's instructions in 5% CO2. HMBEC were labeled with anti-human VEGFR-3 antibody (20), detected with anti-mouse-FITC (Jackson ImmunoResearch), and analyzed by fluorescence-activated cell sorting (FACS) to detect possible contamination with lymphatic EC.
Human Microvascular Lymphatic Endothelial Cells (HMLEC). Human foreskins were sterilized in Betadine solution (Purdue), minced, and digested in collagenase. After incubation, collagenase was neutralized with 10% heat-inactivated BCS (HyClone) in DMEM (Invitrogen). A Teflon pestle (Wheaton) was used to extrude cells from the digested tissue fragments. The homogenate was filtered through a 100-μm cell strainer (BD Labware). The filtrate was centrifuged, and the pellet was resuspended in PBS (Sigma) with 0.1% BSA (Sigma). Cells were mixed with anti-human VEGF receptor 3 (VEGFR-3) antibody-coated magnetic beads according to manufacturer's instructions (Dynal). Cells were plated on a 1.5% gelatin (Difco) coated dish and grown in endothelial basal medium 2 (Biowhittaker) with 20% human serum (HS) (Irvine Scientific), 30% sarcoma 180-conditioned medium, 10 ng/ml FGF-2 (Scios Nova), 10 μg/ml heparin (Sigma), and 1% glutamine–penicillin–streptomycin. The day after plating, colonies containing 3–10 EC were isolated with cloning cylinders (Sigma), and expanded and characterized by FACS with anti-human VEGFR-3 antibody (20). For a more detailed description, see Supporting Text, which is published as supporting information on the PNAS web site.
Equilibrium Binding Assay. Equilibrium binding of 125I-FGF-2 was conducted with confluent HMLEC and HMBEC as described (21). 125I-FGF-2 was added at 0.28, 0.56, 1.4, 2.8, 4.2, 5.6, and 11.1 nM FGF-2 (5, 10, 25, 50, 75, 100, and 200 ng/ml, respectively).
EC Proliferation Assay. HMBEC and HMLEC were maintained in endothelial basal medium (EBM) with 20% HS, 30% tumor-conditioned medium, 1% glutamine–penicillin–streptomycin (GPS), 10 ng/ml FGF-2, and 10 μg/ml heparin for at least 3 days and then plated onto gelatinized 24-well plates in EBM with 20% HS and 1% GPS at 12,500 cells per well per 500 μl. The next day, cells were refed with or without FGF-2. After 72 h, cells were trypsinized and counted with a particle counter (Coulter).
EC Migration Assay. HMBEC and HMLEC migration assays were performed as described (22). Endothelial basal medium with 0, 5, 10, or 50 ng/ml FGF-2 was added to the bottom wells. As controls, we used medium alone or 10% human serum.
In Situ Hybridization. First-strand cDNA was generated from total RNA of adult mouse lung (RNeasy Mini kit, Qiagen, Valencia, CA) by using SuperScript II reverse transcriptase (Invitrogen) and random primers. An aliquot of the first-strand cDNA was then amplified by PCR with mouse VEGF-C forward primer 5′-TTG CTG TGC TTC TTG TCT CTG-3′ and reverse primer 5′-GTC TTC ATC CAG CTC CTT GTT-3′, and mouse VEGF-D forward primer 5′-TGT ATG GAG AAT GGG GAA TGG-3′ and reverse primer 5′-TGG GTT CCT GGA GGT AAG AGT-3′. PCR products were subcloned into pBluescript SK+/– (Statagene). In vitro transcription was performed with T3 and T7 RNA polymerase (Roche Diagnostics) and digoxigenin–RNA labeling mix (Roche Diagnostics). Riboprobes were quantified by UV spectrophotometry and used at 2 μg/ml. Nonradioactive in situ hybridization was carried out on paraffin-embedded sections as described (23).
Quantitative Real-Time RT-PCR. Total RNA was DNase-treated and column-purified (Qiagen). The RNA integrity was assessed by a microfluidics RNA 6000 Nano-Assay using a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA was transcribed by using a MultiScribe based reverse transcriptase reaction. The following primers and TaqMan probes were used for cDNA amplification: VEGF-A forward, 5′-TGTACCTCCACCATGCCAAGT-3′, and reverse, 5′-TGGAAGATGTCCACCAGGGT-3′; TqP: 5′-CCAGCGAAGCTACTGCCGTCCAATT-3′; VEGF-C forward, 5′-AGCTGAGGTTTTTCTCTTGTGATTTAA-3′, and reverse, 5′-TGATCACAGTGAGCTTTACCAATTG-3′; TqP, 5′-CCACTAAAAATATTGTTCCTGCATTCATTTTTATAGCA-3′; VEGF-D forward, 5′-TTGACCTAGTGTCATGGTAAAGC-3′, and reverse, 5′-TCAGTGAACTGGGGAATCAC-3′; and TqP, 5′-ACATTTCCATGCAATGGCG-3′.
Reactions were performed in duplicate by using a GeneAmp 5700 sequence detection system (Applied Biosystems). β2-microglobulin was used as an internal control for normalization.
Antagonization of VEGFR Ligands. The Fc portion of Ig and the soluble extracellular domains of VEGFR-1 (His-tagged) and VEGFR-2 (fused to murine Fc) were expressed in adenoviral vectors as described (24). A total of 109 plaque-forming units (pfu) were injected by tail vein 2–3 days before FGF-2 pellet implantation. Gene expression was confirmed by immunoblotting mouse sera as described (24). VEGFR-3 neutralizing antibody (Ebioscience), 600 μg, was injected i.p. 1 day before pellet implantation and every other day thereafter.
Results
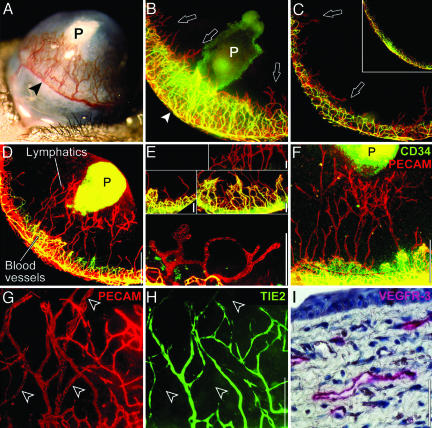
Low-Dose FGF-2 Selectively Stimulates Lymphangiogenesis. Corneal angiogenesis is stimulated by FGF-2 (typically 80 ng in mice) (Fig. 1_A_). To visualize corneal lymphatic vessels, we labeled blood vessels green by injecting i.v. FITC-conjugated lectin, then labeled all vessels red by incubating the whole cornea with PE-conjugated anti-PECAM. Upon merging the green and red fluorescent images, blood vessels appeared yellow and lymphatic vessels appeared red.
Fig. 1.
FGF-2 stimulates corneal lymphangiogenesis. (A) In the traditional corneal assay, 80 ng of FGF-2 (P) stimulates blood vessel growth from the peripheral limbal vasculature (arrowhead). (B) The traditional assay is viewed under fluorescent microscopy after labeling blood vessels yellow–green and lymphatic vessels red (arrowhead). Sucralfate in the FGF-2 pellet (P) autofluoresces green. (C) At the opposite end of the cornea, only lymphatic vessels (arrows) sprout. (Inset) Limbal vessels in the control cornea. (D) Lowering the dose of FGF-2 pellet to 12.5 ng (P) and moving it farther from the limbus results in less angiogenesis, but lymphatic vessels still reach the pellet. (E) Corneal lymphatic vessels were morphologically different from blood vessels. In addition, corneal lymphatic vessels did not express CD34 (F) or Tie2 (G and H, arrowheads) but did express VEGFR-3 (I). (Scale bars, 0.5 mm in B_–_D, 50 μmin E and I, and 200 μmin F_–_H.)
We observed intense angiogenesis confined to an area close to the pellet (Fig. 1_B_). Interestingly, new lymphatic sprouts were seen beyond the angiogenic front (arrows) and at the opposite end of the cornea farthest from the pellet (Fig. 1_C_, arrows). Therefore, we reduced the amount of FGF-2 in the pellet 6.4-fold to 12.5 ng and moved the pellet away from the limbus. With these modifications, significant lymphangiogenesis was stimulated with much reduced angiogenesis (Fig. 1_D_). A cornea with a control pellet devoid of FGF-2 (or a normal unmanipulated cornea) revealed normal quiescent limbal vasculature (Fig. 1_C_ Inset). These results suggest that the lymphangiogenic response to FGF-2 is more sensitive than that of the angiogenic response, and that FGF-2, a pleiotropic factor, can have different specificities at different concentrations.
Compared to corneal blood vessels, lymphatic vessels stained less intensely for PECAM, had wider lumens, were blind-ended, and occasionally formed “rotaries” (a circular configuration from which other sprouts emanate) (Fig. 1_E_). In contrast, the blood vessels formed characteristic arteriovenous loops and tight anastomotic networks (Fig. 1_E_). Corneal lymphatic vessels did not express CD34 (Fig. 1_F_), a marker of blood vascular endothelium (25). Furthermore, it has been published that lymphatic endothelium does not express Tie2 (26), a marker of blood vascular endothelium. Our data show that, in transgenic mice expressing GFP under control of the Tie2 promoter, blood vessels were GFP-positive and lymphatic vessels were GFP-negative (Fig. 1 G and H). Corneal lymphatic vessels expressed vascular endothelial growth factor receptor VEGFR-3 (Fig. 1_I_), a specific marker of normal lymphatic endothelium (27, 28). Moreover, these lymphatic vessels were detectable for a period of 1 year and absorbed extravascularly injected high-molecular mass dextran, suggesting that they were functional (data not shown).
FGF-2 Stimulates Proliferation and Migration of Blood Vascular and Lymphatic ECs Equally. The effects of FGF-2 in the cornea could be explained by at least two possibilities: (i) corneal lymphatic endothelium is more sensitive to direct effects of FGF-2 than blood vascular endothelium, and/or (ii) FGF-2 is acting through another cytokine such as VEGF-C or VEGF-D, both of which are known to be lymphangiogenic by virtue of affinity for VEGFR-3 (29, 30), which is crucial for lymphangiogenesis (31). Relevant to the second possibility, FGF-2 has been shown to induce VEGF-A (32) and more recently, VEGF-C (33).
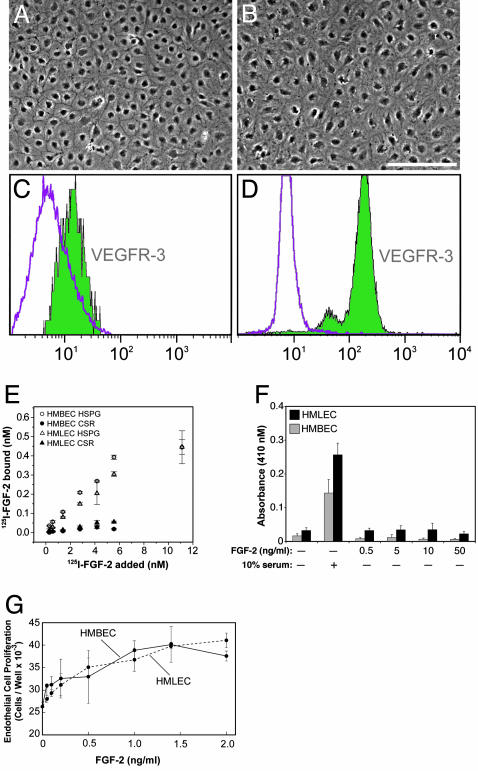
To test the first possibility, that FGF-2 has direct effects on endothelial cells, we first purified lymphatic endothelial cells by using VEGFR-3 antibody (Fig. 2 A_–_D). We then performed FGF-2 equilibrium-binding assays and FGF-2-stimulated proliferation and migration assays using human microvascular lymphatic EC and human microvascular blood vascular EC. We found that both EC types had similar affinities for FGF-2 and responded similarly to FGF-2 in vitro (Fig. 2 E_–_G). Another known direct effect of FGF-2, critical in cell invasion, is the activation of extracellular matrix proteases. Two families of proteases, serine proteases (plasmin) and matrix metalloproteinases (MMPs), have been shown to be most relevant in angiogenesis (34). Both lymphatic and blood vascular ECs have already been reported to produce plasminogen activator in vitro (34). We have now found that FGF-2 induced lymphangiogenesis and angiogenesis in the cornea are associated with MMP-2 and -9 up-regulation (Fig. 5, which is published as supporting information on the PNAS web site), suggesting that the protease profile is similar.
Fig. 2.
HMBEC and HMLEC respond similarly to FGF-2 in vitro. (A and B) Monolayers of pure HMBEC and pure HMLEC had cobblestone morphology, but HMLEC had more cytoplasm and less distinct intercellular borders. (C) Commercial HMBEC were shown to be free of HMLEC by FACS using anti-human VEGFR-3 antibody. The left peak represents HMBEC incubated only with anti-mouse-FITC, and the right peak which resides within the area of the left peak represents HMBEC incubated with anti-VEGFR-3 antibody. (D) The purity of isolated HMLEC was confirmed by FACS using anti-VEGFR-3 antibody. The left peak represents cells incubated only with anti-mouse-FITC, and the right peak represents cells positively labeled with anti-VEGFR-3 antibody. (E) Both cell types bound 125I-FGF-2 similarly. (F) Neither cell type migrated when stimulated with FGF-2 without serum. (G) Both cell types proliferated similarly in response to FGF-2. (Scale bar, 200 μm in A and B.) HSPG, heparan sulfate proteoglycan-bound fraction; CSR, cell surface receptor-bound fraction. Each point represents the mean ± SD.
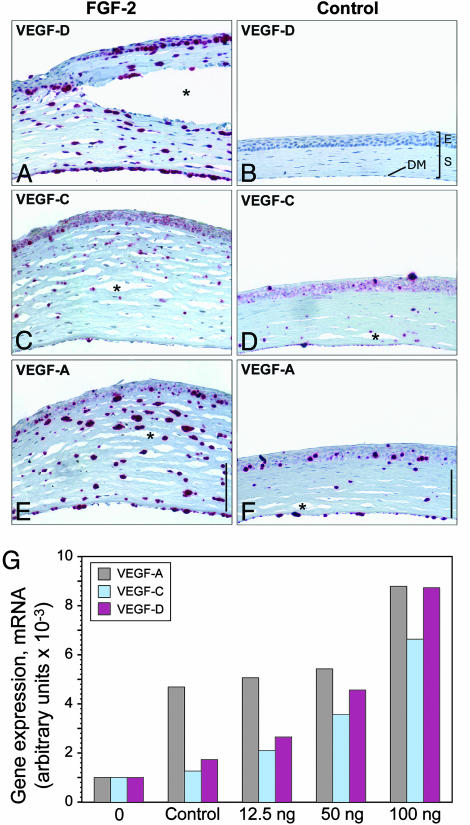
FGF-2 Increases Expression of VEGF-A, -C, and -D in Corneal Stromal Cells in Vivo. The second possibility, that FGF-2 is acting through another cytokine such as VEGF-C or VEGF-D, was pursued by examining for the presence of VEGF-A, -C, and -D in the cornea by in situ hybridization 3 days after FGF-2 (12.5 ng) pellet implantation. At this time, when corneal lymphangiogenesis is just beginning (data not shown), VEGF-A, -C, and -D were expressed in the stromal and anterior and posterior epithelial layers (Fig. 3 A_–_F). The corneal stroma after FGF-2 implantation was hypercellular and twice the normal thickness of the cornea with a control pellet (Fig. 6, which is published as supporting information on the PNAS web site). CD45-staining revealed many infiltrating leukocytes which can include dendritic cells, macrophages, granulocytes, and mononuclear cells (35) (Fig. 7 A_–_F, which is published as supporting information on the PNAS web site). Macrophages have been shown to be the source of VEGF-A, -C, and -D (36, 37). However, CD45-staining did not colocalize with VEGF-A or -D mRNA expression (Figs. 6 and 7 G_–_J), excluding leukocytes as a source of VEGF-A and -D (36). Purified corneal keratocytes expressed VEGF-A, -C, and -D (data not shown), suggesting that the sources of corneal VEGF-A and -D are corneal epithelial cells and may be keratocytes. In situ hybridization suggested that VEGF-A could be also expressed in lower levels in cornea with a control pellet. Real-time RT-PCR data revealed that a wound in the cornea can induce VEGF-A expression. This finding is consistent with the inflammatory response inducing VEGF-A expression (14). VEGF-D expression levels were slightly higher than VEGF-C mRNA levels and were expressed in a dose-dependent manner (Fig. 3_G_).
Fig. 3.
FGF-2 induces corneal VEGF-A, -C, and -D expression. (A, C, and E) In corneas containing FGF-2 (12.5 ng), in situ hybridization revealed prominent VEGF-D expression in anterior and posterior epithelial cells and stromal cells (A), VEGF-C expression was weak (C), and VEGF-A expression was prominent in the stromal layer (E). (B, D, and F) Corneas containing control pellets had minimal signal for all probes. (Scale bars, 100 μm in A_–_F.) Tissue separation (asterisk) was due to artifact. E, anterior epithelial layer; S, stromal layer; DM, Descemet's membrane and posterior epithelial layer. (G) Control pellets or pellets with varying amounts of FGF-2 (12.5, 50, and 100 ng) were implanted into corneas of mice. After 3 days, corneal stromas were harvested. The stroma from normal, unmanipulated corneas (0) was also included. Total RNA was transcribed to cDNA, and real-time RT-PCR was performed to quantitate expression levels for VEGF-A, -C, and -D. Arbitrary units represent normalization to β2-macroglobulin mRNA levels.
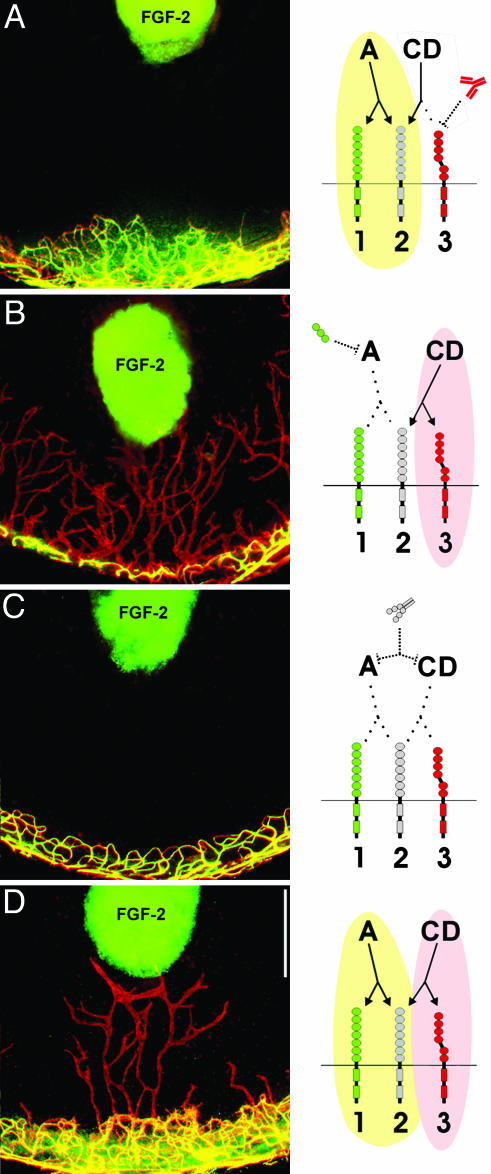
FGF-2-Stimulated Angiogenesis and Lymphangiogenesis Are Mediated by VEGF-A, VEGF-C, and VEGF-D, Respectively. To show that VEGF-C and -D were responsible for FGF-2-stimulated lymphangiogenesis, we blocked VEGF-C and -D with a neutralizing antibody against its receptor, VEGFR-3. When FGF-2 pellets (12.5 ng) were implanted in mice treated with VEGFR-3 neutralizing antibody, angiogenesis was not affected (Fig. 4_A_) because VEGFR-3 is not present on corneal blood vascular endothelium (data not shown). However, lymphangiogenesis was completely suppressed in the antibody-treated mice (Fig. 4_A_), suggesting that FGF-2-stimulated lymphangiogenesis in the cornea requires VEGF-D, and additionally, that FGF-2 at the dose of 12.5 ng has no direct effect on lymphangiogenesis.
Fig. 4.
FGF-2-stimulated lymphangiogenesis and angiogenesis independently require VEGF-A and VEGF-D. (A) VEGFR-3 neutralizing antibody treatment, which blocks VEGF-D binding to VEGFR-3, specifically suppressed lymphangiogenesis, whereas angiogenesis was unaffected. (B) Soluble VEGFR-1, which would deplete local VEGF-A, but not VEGF-D, specifically suppressed angiogenesis, whereas lymphangiogenesis was not affected. (C) Soluble VEGFR-2 completely suppressed both processes. (D) The Fc portion of Ig had no effect on FGF-2-stimulated angiogenesis and lymphangiogenesis. Drawings depict interactions between FGF-2-induced VEGF-A and VEGFR-1 and -2 (present on blood vascular endothelium) and between FGF-2-induced VEGF-D and VEGFR-3 (present on lymphatic endothelium). The interaction between VEGF-D and VEGFR-2 is unclear. Green, activation of angiogenesis; pink, activation of lymphangiogenesis. (Scale bar, 0.5 mm.)
To examine whether lymphangiogenesis can occur in the complete absence of angiogenesis, we specifically blocked angiogenesis. In situ hybridization had revealed that corneal VEGF-A was induced by FGF-2 (Fig. 2_E_). Therefore, we implanted FGF-2 pellets (12.5 ng) in mice inoculated with adenovirus expressing soluble VEGFR-1 that could sequester VEGF-A, but not VEGF-C and -D (30, 38). This virus resulted in complete suppression of angiogenesis (Fig. 4_B_). A previous report showed that, at a dose of 80 ng, FGF-2-stimulated angiogenesis is partially mediated by VEGF-A (32). Here it appears that at a dose of 12.5 ng, FGF-2-stimulated angiogenesis is completely mediated by VEGF-A. In contrast to the effect on angiogenesis, soluble VEGFR-1 treatment did not affect lymphangiogenesis (Fig. 4_B_). We then depleted VEGF-C, -D, and -A with the soluble form of VEGFR-2, which resulted in complete suppression of both lymphangiogenesis and angiogenesis (Fig. 4_C_). An adenovirus control expressing only the Fc portion of Ig had no effect on vessel growth (Fig. 4_D_).
Discussion
Previous work has shown that FGF-2, and VEGF-C and -D can induce both angiogenesis and lymphangiogenesis in the traditional 80-ng pellet corneal neovascularization assay (32, 33, 39, 40). In the cornea, 80-ng FGF-2 pellet-induced effects are partially mediated through VEGFs, but FGF-2 also has direct effects on endothelium (32). Our data reveal a previously undescribed aspect in the spectrum of FGF-2 activities. Although the effects of 12.5-ng FGF-2 pellet are mediated through different cytokines, i.e., VEGF-A, -C, and -D, the predominant result is lymphangiogenesis.
Several explanations may contribute to this lymphangiogenic phenotype. The corneal stroma is one of the few tissues that does not express heparan sulfate. Although heparan sulfate is critical for efficient binding and signaling of heparin-binding growth factors (HBGF), its presence in extracellular matrix can dramatically limit the diffusion and therefore, the availability of HBGFs, such as FGF-2 and VEGF-A (41). Corneal injury is known to activate stromal keratocytes to produce and secrete heparan sulfate proteoglycans (42). Therefore, VEGF-C and -D, which are not heparin-binding (43), may be diffusing farther and thereby promoting lymphangiogenesis over a larger area.
A second factor that might play a role in the preferential lymphangiogenic response to 12.5-ng FGF-2 pellet is that human VEGF-C and -D immature (unprocessed) forms have been shown to have high affinity to VEGFR-3 and low affinity to VEGFR-2 (30, 44). We were able to demonstrate that the VEGF-D generated in the cornea includes an unprocessed form (Fig. 8, which is published as supporting information on the PNAS web site). In fact, when we depleted VEGF-A by soluble VEGFR-1 or blocked the VEGFR-3 with a neutralizing antibody, angiogenesis did not occur in the presence of VEGF-C and -D, further suggesting that the immature forms might be involved. Mouse VEGF-D (mature and immature) does not bind VEGFR-2, and thus cannot induce angiogenesis through VEGFR-2 (45). However, soluble mouse VEGFR-2 inhibited VEGF-C- and -D-induced lymphangiogenesis. It is not clear whether the interaction between soluble VEGFR-2 and VEGF-C and -D is a general response to excess of soluble VEGFR-2.
Recent work has suggested that VEGF-A may not be restricted to angiogenic activity. Local delivery of adenoviral VEGF-A has been shown to induce lymphangiogenesis in the mouse ear.†† Although the expression of VEGFR-2 cannot be detected in the normal initial capillary lymphatic vessels in the skin (10, 46), VEGFR-2 might be up-regulated in lymphatic endothelium when VEGF-A is overexpressed. This phenomenon, i.e., VEGFR-2 up-regulation in lymphatic endothelium, has also been reported when the mature form of VEGF-C and -D were overexpressed (9, 10). However, our work shows that specificity of VEGF-A can be achieved. In our assay, VEGF-A was specifically angiogenic, because soluble VEGFR-1 abolished the minimal angiogenesis that occurred and had no effect on lymphangiogenesis.
It has been shown that VEGF-C can stimulate pure lymphangiogenesis (47) or lymphatic hyperplasia with or without lymphatic sprouting in some systems (9, 46). However, all of these studies have been done in matrices, such as skin and the chicken chorioallantoic membrane, which have tight vascular networks (47, 48), supporting a previous concept that preexisting blood vessels may have an important role in guiding the growth of lymphatic vessels. In the work presented here, we demonstrate that lymphatic endothelium has the ability to proliferate, migrate, and invade to form a new vessel in the absence of a preexisting vascular matrix. Furthermore, FGF-2 has a dose-dependent response to lymphangiogenesis, demonstrating that local FGF-2 concentration can selectively induce lymphangiogenesis. Our findings provide insight into how different growth factors might coordinately regulate angiogenesis and lymphangiogenesis. Our data provide a conceptual framework for further studies on the molecular mechanisms that govern the spatio-temporal relationship between lymphangiogenesis and angiogenesis.
Acknowledgments
We thank Drs. Carmen Barnes, Robert D'Amato, Deborah Freedman, and Sui Huang for helpful discussions. The excellent technical assistance of Ricky Sanchez is appreciated. VEGF-A plasmid and anti-human VEGFR-3 antibody were kindly provided by Dr. Kari Alitalo. This study was also supported by a sponsored research grant to Children's Hospital from EntreMed. This work was supported by the Daland Clinical Investigator Fellowship under the American Philosophical Society (to L.K.C.), a European Molecular Biology Organization fellowship and the Kopf Family Foundation (to A.K.), and the Swedish Medical Research Council, the Swedish Cancer Society, and the Children's Cancer Foundation of Sweden (to F.F.).
Abbreviations: FGF, fibroblast growth factor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; EC, endothelial cell; PECAM, platelet-endothelial cell adhesion molecule; PE, phycoerythrin; HMBEC, human microvascular blood endothelial cells; FACS, fluorescence-activated cell sorting; HMLEC, human microvascular lymphatic endothelial cells.
Footnotes
††
Nagy, J. A., Vasile, E., Brown, L. F., Manseau, E. J., Eckelhoefer, I. A., Bliss, S. H., Dvorak, A. M. & Dvorak, H. F. (2002) FASEB J. 16, A367 (abstr.).
References
- 1.Wilting, J., Neeff, H. & Christ, B. (1999) Cell Tissue Res. 297**,** 1–11. [DOI] [PubMed] [Google Scholar]
- 2.Clark, E. R. & Clark, E. L. (1932) Am. J. Anat. 51**,** 49–87. [Google Scholar]
- 3.Paavonen, K., Puolakkainen, P., Jussila, L., Jahkola, T. & Alitalo, K. (2000) Am. J. Pathol. 156**,** 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabian, G. (1978) Lymphology 11**,** 123–126. [PubMed] [Google Scholar]
- 5.Ichikawa, S., Uchino, S. & Hirata, Y. (1987) Lymphology 20**,** 73–83. [PubMed] [Google Scholar]
- 6.Guyton, A. C. (1986) in Textbook of Medical Physiology, ed. Dreibelbis, D. (Saunders, Philadelphia), p. 363.
- 7.Cao, R., Eriksson, A., Kubo, H., Alitalo, K., Cao, Y. & Thyberg, J. (2004) Circ. Res. 94**,** 664–670. [DOI] [PubMed] [Google Scholar]
- 8.Dor, Y., Djonov, V., Abramovitch, R., Itin, A., Fishman, G. I., Carmeliet, P., Goelman, G. & Keshet, E. (2002) EMBO J. 21**,** 1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeltsch, M., Kaipainen, A., Joukov, V., Meng, X., Lakso, M., Rauvala, H., Swartz, M., Fukumura, D., Jain, R. K. & Alitalo, K. (1997) Science 276**,** 1423–1425. [DOI] [PubMed] [Google Scholar]
- 10.Veikkola, T., Jussila, L., Makinen, T., Karpanen, T., Jeltsch, M., Petrova, T. V., Kubo, H., Thurston, G., McDonald, D. M., Achen, M. G., et al. (2001) EMBO J. 20**,** 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozawa, C. R., Banfi, A., Glazer, N. L., Thurston, G., Springer, M. L., Kraft, P. E., McDonald, D. M. & Blau, H. M. (2004) J. Clin. Invest. 113**,** 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collin, H. B. (1966) Invest. Ophthalmol. 5**,** 1–13. [Google Scholar]
- 13.Cursiefen, C., Schlotzer-Schrehardt, U., Kuchle, M., Sorokin, L., Breiteneder-Geleff, S., Alitalo, K. & Jackson, D. (2002) Invest. Ophthalmol. Vis. Sci. 43**,** 2127–2135. [PubMed] [Google Scholar]
- 14.Cursiefen, C., Chen, L., Borges, L. P., Jackson, D., Cao, J., Radziejewski, C., D'Amore, P. A., Dana, M. R., Wiegand, S. J. & Streilein, J. W. (2004) J. Clin. Invest. 113**,** 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer, A. J. & Clark, R. A. (1999) N. Engl. J. Med. 341**,** 738–746. [DOI] [PubMed] [Google Scholar]
- 16.Liu, N. F. & He, Q. L. (1997) Lymphology 30**,** 3–12. [PubMed] [Google Scholar]
- 17.Chang, L. K., Kaipainen, A. & Folkman, J. (2002) Ann. N.Y. Acad. Sci. 979**,** 1–10. [DOI] [PubMed] [Google Scholar]
- 18.Kenyon, B. M., Voest, E. E., Chen, C. C., Flynn, E., Folkman, J. & D'Amato, R. J. (1996) Invest. Ophthalmol. Vis. Sci. 37**,** 1625–1632. [PubMed] [Google Scholar]
- 19.Swartz, M. A., Berk, D. A. & Jain, R. K. (1996) Am. J. Physiol. 270**,** H324–H329. [DOI] [PubMed] [Google Scholar]
- 20.Jussila, L., Valtola, R., Partanen, T. A., Salven, P., Heikkila, P., Matikainen, M. T., Renkonen, R., Kaipainen, A., Detmar, M., Tschachler, E., et al. (1998) Cancer Res. 58**,** 1599–1604. [PubMed] [Google Scholar]
- 21.Fannon, M. & Nugent, M. A. (1996) J. Biol. Chem. 271**,** 17949–17956. [DOI] [PubMed] [Google Scholar]
- 22.Kisker, O., Becker, C. M., Prox, D., Fannon, M., D'Amato, R., Flynn, E., Fogler, W. E., Sim, B. K., Allred, E. N., Pirie-Shepherd, S. R. & Folkman, J. (2001) Cancer Res. 61**,** 7669–7674. [PubMed] [Google Scholar]
- 23.St. Croix, B., Rago, C., Velculescu, V., Traverso, G., Romans, K. E., Montgomery, E., Lal, A., Riggins, G. J., Lengauer, C., Vogelstein, B. & Kinzler, K. W. (2000) Science 289**,** 1197–1202. [DOI] [PubMed] [Google Scholar]
- 24.Kuo, C. J., Farnebo, F., Yu, E. Y., Christofferson, R., Swearingen, R. A., Carter, R., von Recum, H. A., Yuan, J., Kamihara, J., Flynn, E., et al. (2001) Proc. Natl. Acad. Sci. USA 98**,** 4605–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breiteneder-Geleff, S., Soleiman, A., Kowalski, H., Horvat, R., Amann, G., Kriehuber, E., Diem, K., Weninger, W., Tschachler, E., Alitalo, K. & Kerjaschki, D. (1999) Am. J. Pathol. 154**,** 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motoike, T., Loughna, S., Perens, E., Roman, B. L., Liao, W., Chau, T. C., Richardson, C. D., Kawate, T., Kuno, J., Weinstein, B. M., et al. (2000) Genesis 28**,** 75–81. [DOI] [PubMed] [Google Scholar]
- 27.Kaipainen, A., Korhonen, J., Mustonen, T., van Hinsbergh, V. W., Fang, G. H., Dumont, D., Breitman, M. & Alitalo, K. (1995) Proc. Natl. Acad. Sci. USA 92**,** 3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skobe, M., Hawighorst, T., Jackson, D. G., Prevo, R., Janes, L., Velasco, P., Riccardi, L., Alitalo, K., Claffey, K. & Detmar, M. (2001) Nat. Med. 7**,** 192–198. [DOI] [PubMed] [Google Scholar]
- 29.Joukov, V., Pajusola, K., Kaipainen, A., Chilov, D., Lahtinen, I., Kukk, E., Saksela, O., Kalkkinen, N. & Alitalo, K. (1996) EMBO J. 15**,** 290–298. [PMC free article] [PubMed] [Google Scholar]
- 30.Achen, M. G., Jeltsch, M., Kukk, E., Makinen, T., Vitali, A., Wilks, A. F., Alitalo, K. & Stacker, S. A. (1998) Proc. Natl. Acad. Sci. USA 95**,** 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karkkainen, M. J., Ferrell, R. E., Lawrence, E. C., Kimak, M. A., Levinson, K. L., McTigue, M. A., Alitalo, K. & Finegold, D. N. (2000) Nat. Genet. 25**,** 153–159. [DOI] [PubMed] [Google Scholar]
- 32.Seghezzi, G., Patel, S., Ren, C. J., Gualandris, A., Pintucci, G., Robbins, E. S., Shapiro, R. L., Galloway, A. C., Rifkin, D. B. & Mignatti, P. (1998) J. Cell. Biol. 141**,** 1659–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubo, H., Cao, R., Brakenhielm, E., Makinen, T., Cao, Y. & Alitalo, K. (2002) Proc. Natl. Acad. Sci. USA 99**,** 8868–8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepper, M. S. (2001) Arterioscler. Thromb. Vasc. Biol. 21**,** 1104–1117. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, M. L. (1989) Annu. Rev. Immunol. 7**,** 339–369. [DOI] [PubMed] [Google Scholar]
- 36.Schoppmann, S. F., Birner, P., Stockl, J., Kalt, R., Ullrich, R., Caucig, C., Kriehuber, E., Nagy, K., Alitalo, K. & Kerjaschki, D. (2002) Am. J. Pathol. 161**,** 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutanen, J., Leppanen, P., Tuomisto, T. T., Rissanen, T. T., Hiltunen, M. O., Vajanto, I., Niemi, M., Hakkinen, T., Karkola, K., Stacker, S. A., et al. (2003) Cardiovasc. Res. 59**,** 971–979. [DOI] [PubMed] [Google Scholar]
- 38.de Vries, C., Escobedo, J. A., Ueno, H., Houck, K., Ferrara, N. & Williams, L. T. (1992) Science 255**,** 989–991. [DOI] [PubMed] [Google Scholar]
- 39.Cao, Y., Linden, P., Farnebo, J., Cao, R., Eriksson, A., Kumar, V., Qi, J. H., Claesson-Welsh, L. & Alitalo, K. (1998) Proc. Natl. Acad. Sci. USA 95**,** 14389–14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marconcini, L., Marchio, S., Morbidelli, L., Cartocci, E., Albini, A., Ziche, M., Bussolino, F. & Oliviero, S. (1999) Proc. Natl. Acad. Sci. USA 96**,** 9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dowd, C. J., Cooney, C. L. & Nugent, M. A. (1999) J. Biol. Chem. 274**,** 5236–5244. [DOI] [PubMed] [Google Scholar]
- 42.Brown, C. T., Applebaum, E., Banwatt, R. & Trinkaus-Randall, V. (1995) J. Cell. Biochem. 59**,** 57–68. [DOI] [PubMed] [Google Scholar]
- 43.Veikkola, T. & Alitalo, K. (1999) Semin. Cancer Biol. 9**,** 211–220. [DOI] [PubMed] [Google Scholar]
- 44.Joukov, V., Sorsa, T., Kumar, V., Jeltsch, M., Claesson-Welsh, L., Cao, Y., Saksela, O., Kalkkinen, N. & Alitalo, K. (1997) EMBO J. 16**,** 3898–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baldwin, M. E., Catimel, B., Nice, E. C., Roufail, S., Hall, N. E., Stenvers, K. L., Karkkainen, M. J., Alitalo, K., Stacker, S. A. & Achen, M. G. (2001) J. Biol. Chem. 276**,** 19166–19171. [DOI] [PubMed] [Google Scholar]
- 46.Saaristo, A., Veikkola, T., Tammela, T., Enholm, B., Karkkainen, M. J., Pajusola, K., Bueler, H., Yla-Herttuala, S. & Alitalo, K. (2002) J. Exp. Med. 196**,** 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh, S. J., Jeltsch, M. M., Birkenhager, R., McCarthy, J. E., Weich, H. A., Christ, B., Alitalo, K. & Wilting, J. (1997) Dev. Biol. 188**,** 96–109. [DOI] [PubMed] [Google Scholar]
- 48.Podgrabinska, S., Braun, P., Velasco, P., Kloos, B., Pepper, M. S., Jackson, D. G. & Skobe, M. (2002) Proc. Natl. Acad. Sci. USA 99**,** 16069–16074. [DOI] [PMC free article] [PubMed] [Google Scholar]