Hypoxia inhibits primary cilia formation and reduces cell-mediated contraction in stress-deprived rat tail tendon fascicles (original) (raw)
Summary
Background
Hypoxia, which is associated with chronic tendinopathy, has recently been shown to decrease the mechanosensitivity of some cells. Therefore, the purpose of this study was to determine the effect of hypoxia on the formation of elongated primary cilia (a mechanosensing organelle of tendon cells) in vitro and to determine the effect of hypoxia on cell-mediated contraction of stress-deprived rat tail tendon fascicles (RTTfs).
Methods
Tendon cells isolated from RTTfs were cultured under normoxic (21% O2) or hypoxic (1% O2) conditions for 24 hours. The cells were then stained for tubulin and the number of cells with elongated cilia counted. RTTfs from 1-month-old male Sprague-Dawley rats were also cultured under hypoxic and normoxic conditions for three days and tendon length measured daily.
Results
A significant (p=0.002) decrease in the percent of elongated cilia was found in cells maintained in hypoxic conditions (54.1%±12.2) when compared in normoxic conditions (71.7%±6.32). RTTfs in hypoxia showed a significant decrease in the amount of contraction compared to RTTfs in normoxia after two (p=0.007) and three (p=0.001) days.
Conclusion
The decreased incidence of elongated primary cilia in a hypoxic environment, as well as the decreased mechanoresponsiveness of tendon cells under these conditions may relate to the inability of some cases of chronic tendinopathy to respond to strain-based rehabilitation modalities (i.e. eccentric loading).
Keywords: cilia, contraction, hypoxia, mechanotransduction, tendinopathy
Introduction
Various phases of hypoxic alterations of tenocytes have been found in ruptured tendons with degenerative tendinopathy1. Hypoxia-induced cell damage has been implicated as a potential mechanism in the progression of chronic tendinopathy2–4 with higher levels of hypoxic degeneration indicative of non-reparative, end stage pathology5. Studies using human tenocytes have also demonstrated that a hypoxic environment, depending on the magnitude and duration of exposure, is capable of disturbing the balance between reparative and degenerative changes in the extracellular matrix2, 6.
A critical mediator of the hypoxic response is the transcription factor hypoxic inducible factor 1α (HIF-1α)7. HIF-1α has been shown to negatively regulate skeletal mechanotransduction by decreasing the sensitivity of bone cells to mechanical signals8. The inability of some tendinopathy patients to respond to therapies designed to stimulate a mechanotransduction response (i.e. eccentric loading) may reflect a decrease in the mechanosensitivity of tendon cells secondary to a hypoxic environment9.
Primary cilia are mechanosensitive organelles that can detect mechanical environmental changes10 and are found in musculoskeletal tissue cell types, including tenocytes, osteocytes, and chondrocytes10–14. Passive cilia bending is required for mechanosensation of mechanical perturbations, with elongated cilia more sensitive to loading than shorter cilia15. A previous study on mesenchymal stem cells demonstrated a time dependent loss of elongated primary cilia in hypoxic conditions7. It is possible that tendon cells may also experience a hypoxia-induced loss of elongated cilia and subsequent loss of mechanosensitivity.
A hypoxic environment within tendons has been suggested to occur through mechanically induced collagen damage16. When collagen fibrils are damaged, or become lax, and lose the ability to bear load the tendon cells associated with the damaged collagen fibrils lose their cellular homeostatic tension17,18. The loss of cellular tensional homeostasis induces catabolic processes associated with tendinopathy17 and cilia elongation19,20. However, a cellular based contraction mechanism has been shown to recover tendon laxity and re-establish the cytoskeletal tensional homeostasis of these tendon cells20. This, in turn, allowed the tendon cells to recalibrate their catabolic gene expression and protein synthesis to its previous normal levels20.
A reduction in the mechanosensitivity of tendon cells exposed to hypoxic conditions could lead to a diminished cell-based contraction response of tendons and return to normal tensional homeostasis. Therefore, the purpose of the current study was to determine the effect of hypoxia on the formation of primary cilia, a mechanosensing organelle of tendon cells, in vitro and to determine the effect of hypoxia on cell-mediated contraction of stress-deprived rat tail tendon fascicles (RTTfs). We hypothesize that hypoxia will decrease the number of tendon cells expressing elongated primary cilia in vitro. In addition, we hypothesize that hypoxia will decrease the normal cell-induced tendon contraction that occurs with the loss of cytoskeletal tension.
Materials and methods
Institutional animal care and use approval was obtained prior to this study. This study was conducted ethically and in accordance with the international standards described by Padulo et al. in 201621.
The effect of hypoxia on elongated cilia formation
To determine the impact of hypoxia on the presence of elongated cilia, tendon cells were isolated from RTTfs of adult male Sprague-Dawley rats and cultured to the 3rd–4th passage. Each well of a 6-well tissue culture plate contained one cover glass on which tendon cells were cultured (37° F, 10% CO2) in supplemented Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Fisher Scientific) as previously described22 in the presence of normoxia (21% O2, 69% N2) or hypoxia (1% O2, 89% N2) for 24 hours (40,000 cells/well; n=7 plates/condition). After 24 hours, the media was removed and new media containing both Tubulin Tracker™ Green (250 nM), a cellular tubulin stain and Hoechst 33342 (5μg/ml), a nuclear stain (Thermo Fisher Scientific) was added to each well. The plates were incubated in the dark at 37° C, in their respective environmental oxygen conditions for 15 minutes. After incubation, the stain was removed and each cover-glass was rinsed twice with DMEM. Each cover-glass was mounted using ProLong® Gold (Thermo Fisher Scientific) and the cells were visualized using a Zeiss Axioplan2 microscope at 63x magnification. The presence or absence of elongated cilia were counted microscopically on 200 cells for each cover-glass for a total of 1200 cells per 6-well plate and 8400 cells per condition. The percent of cells with elongated cilia present on each cover-glass were averaged per plate. To determine if a significant difference in the presence of elongated cilia occurred between hypoxia and normoxia a paired t-test was performed with significance p≤0.05. All results are shown as mean ± standard deviation.
The effect of hypoxia on cell-based tendon contraction
To investigate the effect of hypoxia on cell-mediated tendon contraction RTTfs were removed from the tails of euthanized 1-month-old male Sprague-Dawley rats (_n_=6) and suspended vertically inside 15 ml conical centrifuge tubes containing supplemented DMEM20. RTTfs were cultured under either normoxic (21% oxygen) or hypoxic (1% oxygen) conditions for a total of 20 RTTfs/condition/rat for three days. Each RTTf was photographed daily to document length changes.
To determine if the low oxygen conditions were causing irreversible cell changes (i.e. cell death), following three days of hypoxia exposure, 10 of the RTTfs in hypoxia were moved into normoxia for an additional three days. On day six all RTTfs were photographed to document length changes due to the change in environmental oxygen conditions. RTTf contraction lengths were measured from calibrated photographs using Image-J software23. Measurements were standardized to a fixed scale present in each photo to account for any magnification effects. Tendon length was expressed as a percentage of day 0 length and results from differing environmental oxygen conditions were compared using multiple paired _t_-tests with a Bonferroni correction.
Results
The effect of hypoxia on elongated cilia formation
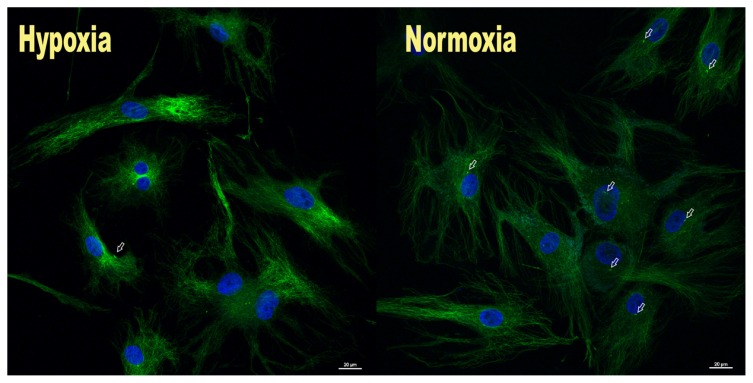
A significant (p=0.002) average decrease of 18.2%±9.34 was found in the number of elongated cilia present in those cells maintained in hypoxic conditions (54.1%±12.2) compared to cells in normoxic conditions (71.7% ± 6.32) (Fig. 1).
Figure 1.
Photomicrographs showing the number of elongated cilia (white arrows) significantly decreased in cells maintained in hypoxia (left) compared to cells in normoxia (right).
The effect of hypoxia on cell-based tendon contraction
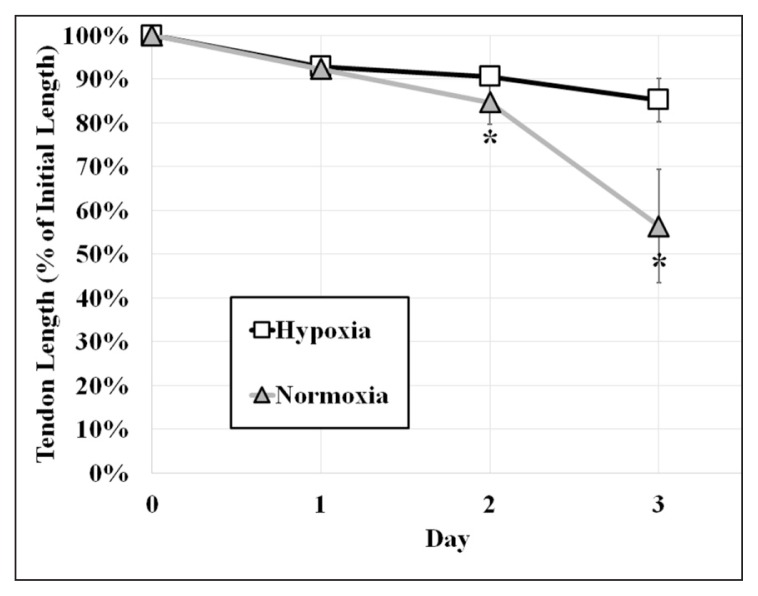
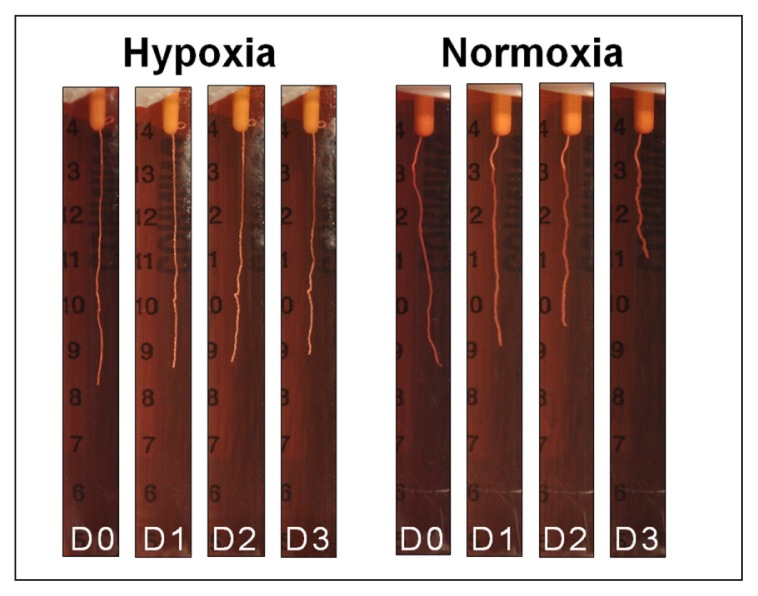
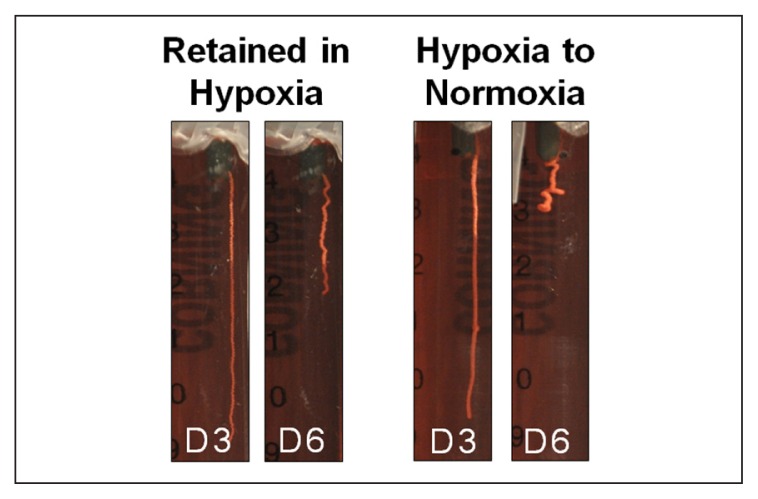
After 24 hours of incubation, RTTfs maintained under normoxic or hypoxic conditions demonstrated a similar (p=0.084), albeit small (8%±2 normoxia, 7%±2 hypoxia) decrease in their length. By day two, RTTfs under normoxic conditions showed a significant increase in the amount of contraction when compared to RTTfs under hypoxic conditions (85%±5 normoxia, 91%±2 hypoxia, p=0.007) (Figs. 2, 3). After three days, RTTfs under normoxic conditions were significantly shorter than RTTfs cultured under hypoxic conditions (56%±13 normoxia, 85%±5 hypoxia, p=0.001). Transferring RTTfs to normoxic conditions after three days of exposure to hypoxia resulted in significantly more contraction at day six than those RTTfs that remained in a hypoxic environment (12%±5 normoxia, 34%±7 hypoxia, p=0.008) (Fig. 4). This increase in contraction rate following exposure to normoxic conditions confirms that the decrease in tendon contraction seen under hypoxic conditions was not due to cell death.
Figure 2.
Graph showing tendons in hypoxia experienced a significant decrease in their amount of contraction (change in % of initial length) compared to those in normoxia at day 2 and day 3.
* Normoxia vs hypoxia. p<0.05
Figure 3.
Photographic series showing a noticeable decrease in the contraction of tendons at days 2–3 (D2–D3) when placed in hypoxia (left) compared to normoxia (right).
Figure 4.
Tendons that were transferred from hypoxic to normoxic conditions showed a significant increase in contraction compared to tendons that were retained in hypoxia.
Discussion
In the current study, cells maintained in hypoxic conditions were found to have a significant decrease in the number of elongated cilia present when compared to cells in normoxic conditions (Fig. 1). Two recent studies have also investigated the role of hypoxia on cilia prevalence7, 24. In one study, renal epithelial cells exposed to a chemically induced hypoxic environment simulated using cobalt chloride (CoCl2) resulted in an increase in the length of primary cilia, but with no change in percentage of cells with a cilium (~82%) compared to normoxia (~85%)24. However, this method of using CoCl2 to mimic the conditions of hypoxia may not have the same cellular effect as regulating the hypoxic enviroment through controlled oxygen levels25.
The results of the current study are similar to a previous study that also induced hypoxia through controlled oxygen levels in murine bone marrow-derived mesenchymal stem cells (MSC)7. A significant decrease in the number of cells with elongated cilia was observed with hypoxia (~20%) compared to normoxia (~70%)7. However, results from the MSC study showed a greater decrease in the percentages of cells expressing elongated cilia in hypoxic conditions (~20%) compared to the current study (54%)7. The reason for this discrepancy is unclear and may be due to the use of different cell types.
Cilia are believed to play an important role in maintaining tissue homeostasis14. The membrane of the cilia contains several cilia-specific receptors such as ion channels and signaling molecules that serve to receive signals from the environment in order to produce an appropriate response15. Primary cilia have been shown to transmit signals to the cytoskeleton and other cellular organelles that regulate the cells’ mechanoresponse in a manner dependent on the cilia length26. The elongation of the primary cilia has been shown to increase the mechanosensitivity of cells to signals and has been found as a biomarker of alterations in cellular homeostasis19,26,27. In the current study, cells under hypoxic conditions revealed a significantly lower percentage of elongated cilia than those seen in normoxia. These results suggest that with a lower percentage of elongated cilia that are perceptive to mechanotransduction signals, a decreased cellular response to alterations in mechanical loading will occur with hypoxia.
In the present study, the freely contracting RTTFs maintained in a hypoxic environment showed a significance decrease in the amount of contraction compared to tendons cultured in normoxic conditions at both two and three days (Figs. 2, 3). Cell mediated contraction may contribute to the recovery of tendon laxity caused by injury17, surgical manipulation28, or repetitive exercise29. Alterations in the normal, residual tension of the extracellular matrix in tendons, such as laxity, disrupt the cell tensional homeostasis and have been demonstrated to result in an up regulation of catabolic gene expression and protein synthesis in tendon cells17. Prolonged catabolic degradation leads to degeneration of the material properties of the tendon18. Cell mediated contraction in tendons that are initially lax but fixed between two points has been shown to regain cellular homeostatic tension and inhibit collagenase protein synthesis20. Thus a hypoxic induced decrease in tendon cell-mediated contraction may delay or inhibit the ability of the tendon to regain cellular homeostasis and thus prolong the catabolic degradation that may lead to tissue degeneration.
In the current study, tendons that were exposed to hypoxia for three days and then moved to normoxia for an additional three days demonstrated significantly more contraction at day six than those that remained in hypoxia for the full six days (Fig. 4). The increase in contraction with the return to normoxic conditions suggests that this decrease in mechanoresponsiveness to hypoxia was not due to cell death, but rather to the ability of the cell to respond to its decreased oxygen environment. The ability of cells to return to a normal contraction response within three days following three days of exposure to hypoxia also suggests that in the short term the diminished cellular response to hypoxia is reversible.
As a limitation of the current study, hypoxia was viewed as an isolated event rather than a sequence of events leading up to the development of a hypoxic environment in the tissue. A potential event leading to a hypoxic environment is damage to the extracellular matrices and surrounding vasculature, consequently diminishing the supply of nutrients to the tissue4. The current study utilizes an in vitro and an in situ model system at different time points to analyze the role of hypoxia on cellular mechanoresponsiveness. The decrease in elongated cilia prevalence that is shown to occur in vitro at 24 hours is assumed to occur in situ and remain in effect over time. Although previous studies analyzed the prevalence and/or length of cilia in situ11, the current study utilized tendon cells in monolayer to examine the effect of hypoxia on elongated cilia prevalence. This allowed for a greater numbers of cells (8400 cells/condition) to be examined than in previous in situ studies (90 cells/condition)11. Furthermore, previous research in mesenchymal stem cells demonstrated a continued decrease in the prevalence of elongated cilia after 2 days in hypoxia7. Therefore, although it appears from previous research that the decrease in elongated cilia prevalence with hypoxia would occur both in situ and over time, this requires further demonstration. The current study also focuses primarily on the alterations occurring to the cells primary cilia as a source of mechanosensation. However, other cellular changes take place in a hypoxic environment that may play a role in mechanotransduction such as a reduced cellular generation of ATP, failure of energy dependent systems within the cell such as ion pumps, depletion of glycogen stores, lowered pH of the intracellular environment, and a reduction in the synthesis of proteins30. Although the current study only investigated a representative mechanosensory pathway and cellular response to hypoxia, additional cellular responses may also play a role in the mechanosensation of cells under a hypoxic environment.
Primary cilia are important mechanosensing organelles in tendon cells and are thought to play a key role in maintaining tendon cell homeostasis27. The decreased incidence of elongated primary cilia in a hypoxic environment, as well as the decreased mechanoresponsiveness of tendon cells under these conditions may relate to the inability of some cases of chronic tendinopathy to respond to strain-based rehabilitation modalities (i.e. eccentric loading).
Footnotes
Conflicts of interests
The Authors declare that they have no competing interests.
References
- 1.Jozsa L, Balint BJ, Reffy A, Demel Z. Hypoxic Alterations of Tenocytes in Degenerative Tendinopathy. Arch Orthop Trauma Surg. 1982;99:243–246. doi: 10.1007/BF00381401. [DOI] [PubMed] [Google Scholar]
- 2.Benson RT, McDonnell SM, Knowles HJ, Rees JL, Carr AJ, Hulley PA. Tendinopathy And Tears Of The Rotator Cuff Are Associated With Hypoxia And Apoptosis. J Bone Joint Surg Br. 2010;92:448–453. doi: 10.1302/0301-620X.92B3.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millar NL, Reilly JH, Kerr SC, et al. Hypoxia: A Critical Regulator of Early Human Tendinopathy. Ann Rheum Dis. 2012;71:302–310. doi: 10.1136/ard.2011.154229. [DOI] [PubMed] [Google Scholar]
- 4.Kannus P, Natri A. Etiology and pathophysiology of tendon ruptures in sports. Scand J Med Sci Sports. 1997;7:107–112. doi: 10.1111/j.1600-0838.1997.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 5.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 6.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 7.Proulx-Bonneau S, Annabi B. The primary cilium as a biomarker in the hypoxic adaptation of bone marrow-derived mesenchymal stromal cells: a role for the secreted frizzled-related proteins. Biomark Insights. 2011;6:107–118. doi: 10.4137/BMI.S8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riddle RC, Leslie JM, Gross TS, Clemens TL. Hypoxia-induced Factor-1α Protein Negatively Regulates Load-induced Bone Formation. J Biol Chem. 2011;286:44449–44456. doi: 10.1074/jbc.M111.276683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purdam CR, Jonsson P, Alfredson H, Lorentzon R, Cook JL, Khan KM. A pilot study of the eccentric decline squat in the management of painful chronic patellar tendinopathy. Br J Sports Med. 2004;38:395–397. doi: 10.1136/bjsm.2003.000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Z, Leong DJ, Zhuo Z, et al. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and down regulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthritis Cartilage. 2015;15:1401–1406. doi: 10.1016/j.joca.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Gardner K, Arnoczky SP, Lavagnino M. Effect of in vitro stress-deprivation and cyclic loading on the length of tendon cell cilia in situ. J Orthop Res. 2011;29:582–587. doi: 10.1002/jor.21271. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly E, Williams R, Farnum C. The primary cilium of connective tissue cells imaging by multiphoton microscopy. Anat Rec. 2008;291:1062–1073. doi: 10.1002/ar.20665. [DOI] [PubMed] [Google Scholar]
- 13.Jensen CG, Poole CA, McGlashan SR, et al. Ultrastructural, tomographic, and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28:101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CT, Castillo AB, Brugmann SA, Helms JA, Jacobs CR, Stearns T. Primary cilia: cellular sensors for the skeleton. Anat Rec (Hoboken) 2008;291:1074–1078. doi: 10.1002/ar.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 16.Pufe T, Peterson WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15:211–222. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 17.Lavagnino M, Arnoczky SP, Egerbacher M, Gardner KL, Burns ME. Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J Biomech. 2006;39:2355–2362. doi: 10.1016/j.jbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavagnino M, Gardner K, Sedlak AM, Arnoczky SP. Tendon cell ciliary length as a biomarker of in situ cytoskeletal tensional homeostasis. Muscle Ligament Tendon J. 2013;3:118–121. [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner K, Lavagnino M, Egerbacher M, Arnoczky SP. Re-establishment of Cytoskeletal Tensional Homeostasis in Lax Tendons Occurs Through an Actin-mediated Cellular Contraction of the Extracellular Matrix. J Orthop Res. 2012;30:1695–1701. doi: 10.1002/jor.22131. [DOI] [PubMed] [Google Scholar]
- 21.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research: 2016 update. MLTJ. 2016;6( 1):1–5. doi: 10.11138/mltj/2016.6.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavagnino M, Gardner KL, Arnoczky SP. High magnitude, in vitro, biaxial, cyclic tensile strain induces actin depolymerization in tendon cells. Muscles Ligaments Tendons J. 2015;5:124–128. [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to Image J: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verghese E, Zhuang J, Saiti D, Ricardo SD, Deane JA. In vitro investigation of renal epithelial injury suggests that primary cilium length is regulated by hypoxia-inducible mechanisms. Cell Biol Int. 2011;35:909–913. doi: 10.1042/CBI20090154. [DOI] [PubMed] [Google Scholar]
- 25.Tsui L, Fong T, Wang I. The effect of 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) on cell viability under hypoxia. Mol Vis. 2013:2260–2273. [PMC free article] [PubMed] [Google Scholar]
- 26.Khayyeri H, Barreto S, Lacroix D. Primary cilia mechanics affects cell mechanosensation: A computational study. J Theor Biol. 2015;379:38–46. doi: 10.1016/j.jtbi.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 27.Gardner K, Arnoczky SP, Lavagnino M. Effect of in vitro stress-deprivation and cyclic loading on the length of tendon cell cilia in situ. J Orthop Res. 2011;29:582–587. doi: 10.1002/jor.21271. [DOI] [PubMed] [Google Scholar]
- 28.King GJ, Edwards P, Brant RF, Shrive NG, Frank CB. Intraoperative graft tensioning alters viscoelastic but not failure behaviours of rabbit medial collateral ligament autografts. J Orthop Res. 1995;13:915–922. doi: 10.1002/jor.1100130616. [DOI] [PubMed] [Google Scholar]
- 29.Freedman BR, Zuskov A, Sarver JJ, Buckley MR, Soslowsky LJ. Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage. J Orthop Res. 2015;33:904–910. doi: 10.1002/jor.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V, Abbas AK, Aster JC. Cell injury, cell death, and adaptations. In: Kumar V, Abbas AK, Aster JC, editors. Robbins Basic Pathology. 9th ed. Philadelphia, PA: Saunders; 2012. [Google Scholar]