The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies – The Cardiac Rehabilitation Outcome Study (CROS) (original) (raw)
Abstract
Background
The prognostic effect of multi-component cardiac rehabilitation (CR) in the modern era of statins and acute revascularisation remains controversial. Focusing on actual clinical practice, the aim was to evaluate the effect of CR on total mortality and other clinical endpoints after an acute coronary event.
Design
Structured review and meta-analysis.
Methods
Randomised controlled trials (RCTs), retrospective controlled cohort studies (rCCSs) and prospective controlled cohort studies (pCCSs) evaluating patients after acute coronary syndrome (ACS), coronary artery bypass grafting (CABG) or mixed populations with coronary artery disease (CAD) were included, provided the index event was in 1995 or later.
Results
Out of n = 18,534 abstracts, 25 studies were identified for final evaluation (RCT: n = 1; pCCS: n = 7; rCCS: n = 17), including n = 219,702 patients (after ACS: n = 46,338; after CABG: n = 14,583; mixed populations: n = 158,781; mean follow-up: 40 months). Heterogeneity in design, biometrical assessment of results and potential confounders was evident. CCSs evaluating ACS patients showed a significantly reduced mortality for CR participants (pCCS: hazard ratio (HR) 0.37, 95% confidence interval (CI) 0.20–0.69; rCCS: HR 0.64, 95% CI 0.49–0.84; odds ratio 0.20, 95% CI 0.08–0.48), but the single RCT fulfilling Cardiac Rehabilitation Outcome Study (CROS) inclusion criteria showed neutral results. CR participation was also associated with reduced mortality after CABG (rCCS: HR 0.62, 95% CI 0.54–0.70) and in mixed CAD populations.
Conclusions
CR participation after ACS and CABG is associated with reduced mortality even in the modern era of CAD treatment. However, the heterogeneity of study designs and CR programmes highlights the need for defining internationally accepted standards in CR delivery and scientific evaluation.
Keywords: Rehabilitation, acute coronary syndrome, coronary bypass grafting, coronary artery disease, mortality, hospital readmission
Introduction
Although several recent studies, meta-analyses1–11 and recommendations of national and international guidelines12,13 suggest a beneficial effect of cardiac rehabilitation (CR) in patients with coronary artery disease (CAD), considerable scientific doubt is still apparent for the following reasons:
- The type of CR offered varies considerably between and within the countries with respect to content, duration, intensity and volume, and worldwide there are no accepted minimal standards for judging the quality of CR delivery, thereby leaving doubt as to the effectiveness of CR as delivered in routine clinical practice.14,15
- Developments within the past 20 years, including interventional therapies, surgery and medications, have had a large impact on the quality of care delivered to patients who are participating in modern CR.16,17 On this basis, older studies evaluating the effect of CR are no longer suitable for estimating CR effectiveness.
- In some countries, high levels of CR participation supported by government policy, health insurance, pension funds and ethical criteria make it virtually impossible to randomise patients out of CR, and large prospective randomised trials on CR efficacy with experimental and highly reproducible designs are scarce.18–20 However, alternative robust research designs using routine clinical data captured through cohort studies, observational studies and registries have been published with findings that are worthy of consideration.3,4–9,21
For these reasons, the present study sought to assess the actual evidence of CR’s effectiveness by focusing on CAD patients after a recent cardiac event (acute coronary syndrome (ACS), coronary artery bypass grafting (CABG) or mixed populations also including patients with stable CAD) and treated in the era of acute revascularisation during ACS and routine medication with statins. Furthermore, in order to better reflect clinical practice, apart from randomised controlled trials (RCTs), controlled cohort studies (CCSs) were also included in the meta-analysis.
Methods
This systematic review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statement (see also Supplemental Material, Table SM 5).22,23 The study protocol was prospectively published in PROSPERO International prospective register of systematic reviews (University of York, Centre for Reviews and Dissemination) and verified as original (CRD42014007084).
Study eligibility criteria
The study selection criteria (populations, interventions, controls, outcomes and designs) are outlined in detail in Table 1. Three groups of patients were defined:
- patients after hospitalisation for ACS, including ST-elevation myocardial infarction (STEMI), non-STEMI (NSTEMI) or unstable angina pectoris (UAP);
- patients after hospitalisation for CABG;
- mixed populations including patients after ACS and/or after CABG as a basic requirement, but also including patients with chronic stable CAD with or without elective percutaneous coronary intervention (PCI).
Table 1.
Cardiac Rehabilitation Outcome Study inclusion criteria.
| Population | ||
|---|---|---|
| After ACS | After CABG | Mixed population |
| Age | No restriction | |
| Time of index events | 1995 or later* | |
| Minimal standards of acute treatment | In-hospital standard therapy according to actual guidelines | |
| Intervention | ||
| Multi-component CR | ||
| Start | No later than 3 months after hospital discharge | |
| Supervision | CR must be under supervision and responsibility of a rehabilitation centre (centre-based CR) | |
| Definition of ‘multi-component’ | CR including supervised and structured physical exercise at least twice a week as basic requirement plus at least one, preferably more, of the following components: information, motivational techniques, education, psychological support and interventions, social and vocational support | |
| CR setting | In-patient, out-patient or mixed. Tele-rehabilitation will be included as long as the major part of CR sessions is centre-based and all other predefined criteria are fulfilled | |
| Control | ||
| Usual care | ||
| Definition | Patients with index event, but not participating in CR Patients of the control group may be supervised by general practitioners and/or resident cardiologists. They also may participate in non-structured and non-supervised exercise programmes outside of a CR programme | |
| Outcomes; clinical course after the index event | ||
| Primary outcome | (1) Total mortality | |
| Secondary outcomes | (2) Cardiovascular mortality (3) Major cardiovascular and cerebrovascular events (MACCE = combined endpoint of death, non-fatal myocardial infarction and non-fatal stroke) (4) Non-fatal myocardial infarction (5) Non-fatal stroke (6) Hospital readmission for any reason (7) Unplanned hospital readmission for any cardiovascular event (8) Unplanned coronary revascularization (9) Cardiovascular mortality + admission for any cardiovascular event (10) All combined endpoints including fatal and non-fatal events not predefined (amendment by the CROS steering committee, 18 January 2015) | |
| Observation period | 6 months or more after hospital discharge | |
| Study designs and biometry | ||
| Study designs included | Randomised controlled trials; prospective and retrospective cohort studies with a control group | |
| Biometry | Cohort studies must provide a description of data sources, should have used methods to reduce risk of selection bias (e.g. linear regression analysis and propensity score methods) and should provide information on dealing with patients lost at follow-up and missing data |
To guarantee current CAD treatment standards (operationally defined by the Cardiac Rehabilitation Outcome Study (CROS) as revascularisation for acute myocardial infarction (AMI) and routine use of statins), only studies that recruited patients in 1995 or later were included. Total mortality was the primary endpoint. Predefined secondary endpoints are outlined in Table 1 and primarily include non-fatal cardiovascular events, hospital readmissions and mixed endpoints.
Search methods and identification of studies
Highly sensitive search strategies were developed by a graduate information scientist (MIM) for seven databases in order to identify two types of studies: RCTs and CCSs, regardless of the studies’ current status (published, unpublished, finished or ongoing). For developing the search strategy, candidate terms were identified (text words and controlled vocabulary) by using a multi-stranded approach. Known key literature and the publications included in two systematic reviews on the same topic were assessed.24,25 Fifty abstracts retrieved from PubMed using the Medical Subject Heading (MeSH) ‘myocardial infarction/rehabilitation’ were evaluated. All MeSH terms belonging to ‘heart diseases ‘and ‘rehabilitation ‘were reviewed. Afterwards, search blocks on two concepts were built: ‘myocardial infarction ‘and ‘coronary bypass’ for the population of interest, and ‘rehabilitation’ as the intervention under evaluation. These were then combined with validated methodological search filters for the two included study types.
The search strategy was elaborated for PubMed and subsequently peer-reviewed by an independent, external information specialist (Margaret Sampson, Children's Hospital of Eastern Ontario, USA). After revisions resulting from this quality assurance process, the strategy was adapted to the specific requirements of each database (syntax, search options and controlled vocabulary). If validated search filters were not available, filters were developed for databases where filtering seemed reasonable.
Starting with the year 1995, the following bibliographic databases were used with no restriction on language: PubMed, Embase, Cochrane Central Register of Controlled Trials, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS) and Center for International Rehabilitation Research Information and Exchange (CIRRIE). Additionally, unpublished or ongoing studies were searched using the World Health Organization’s International Clinical Trials Registry Platform (ICTRP), a meta-register of trials including 16 primary trial registers of different countries. The search was originally run in December 2013, and thereafter updated in April 2015 and again in 22 December 2015. The details of all search strategies are documented in the Supplemental Material (Table SM 1). The only difference between the protocol and this review was the exclusion of the databases Current Contents Medicine (CC MED) and Web of Science due to the limited benefits they were judged to provide.
Study selection
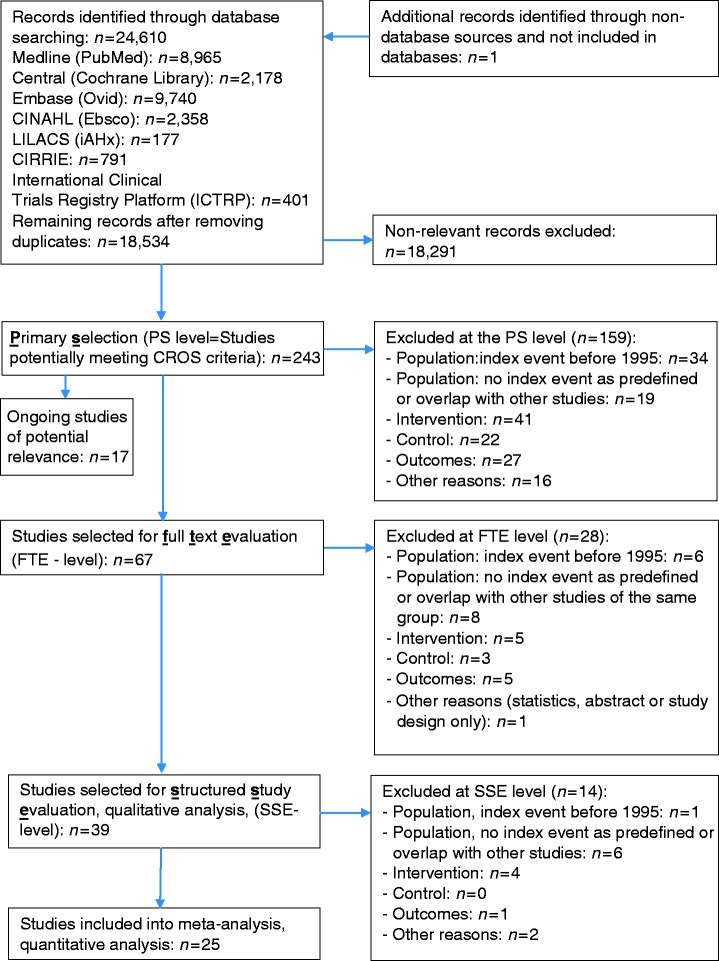
The selection process is outlined in Figure 1. All references (titles plus abstracts) were independently evaluated by three members of the CROS study group (BR, CHD and PD, the ‘reference selection board’) using an algorithm that guaranteed the independent evaluation of each title by at least two of these experts. In addition, the references of recent meta-analyses and potentially eligible studies were screened. This primary selection (PS) process was finalised by consensus within the reference selection board, resulting in n = 243 abstracts of potential interest. By re-evaluating these abstracts, n = 67 publications were selected for full-text evaluation, resulting in n = 39 publications being selected for a structured study evaluation (SSE). SSE was performed and consented within an extended reference selection board (BR, CHD, PD, AS and HV), including two biometricians (DS and KJ). In four publications, descriptions of the CR characteristics remained incomplete despite contacting the authors for clarification (see Tables 2 and 4a). Incomplete description of CR characteristics did not lead to study exclusion by decision of the reference selection board, provided the other inclusion criteria were fulfilled. On the basis of the SSE process, 25 studies remained for meta-analysis. The primary reasons for study exclusion at the PS level are given in Supplementary material Table SM 2. Table SM 2 also includes studies of potential interest that were not published at the closure of the CROS literature search.
Figure 1.
Study selection flow chart.
CINAHL: Cumulative Index to Nursing and Allied Health Literature; LILACS: Literatura Latino-Americana e do Caribe em Ciências da Saúde; CIRRIE: Center for International Rehabilitation Research Information and Exchange; PS: primary selection of extracted studies; FTE: full-text evaluation; SSE: structured study evaluation and quality analysis according to the checklist of methodological issues on non-randomized studies; ICTRP: International Clinical Trials Registry Platform.26
Table 2.
Studies selected for quantitative analysis; baseline study characteristics and overall results.
| Study, year, country | Study design | Population: a. Data sources b. Number of included participants (N) c. Index events d. Inclusion period e. Other inclusion criteria and characteristics f. Age (y, mean ± SD or as stated) g. Gender (male, %) | Intervention: a. Number (n) b. Structured and multi-component CR (SMC-CR)? c. Start after index event d. Duration (time period and/or total number of CR sessions) e. Frequency (CR exercise sessions per wk) f. CR setting | Control: a. Number (n) b. Treatment, characteristics | Outcome: a. Follow-up period b. Outcomes according to the CROS criteria (numbers according to Table 1) c. Other outcomes | Overall results with respect to endpoints 1-10 as defined by CROS(definitions of numbers and correspondent endpoints are given in Table 1) | Remarks |
|---|---|---|---|---|---|---|---|
| Boulay et al., 2004,36 Canada | p/rCCS | a. Institutional b. n = 128 c. AMI d. Probably after 1995 e. Aged ≤ 75 y, EF >35%, first ischaemic event f. 53.8 ± 9.9 (CR+, phase II) 54.3 ± 10.3 (CR+, phase II + III) 56.5 ± 9.7 (no CR) g. 86.5 (CR+, phase II) 78.4 (CR+, phase II + III) 77.8 (no CR) | a. n = 37 (phase II) n = 37 (phase II + III) b. SMC-CR c. ≤1 wk after discharge (phase II) d. 12 wk (phase II) At least 9 mo (phase III) e. n = 2 f. Out-patient (phase II, III) | a. n = 54 b. UC, AMI within 1 y before start of the study | a. 1 y post-AMI b. (4), (7) c. Number of emergency room visits for chest pain or suspicion for cardiac-related symptoms, recurrences of fatal and non-fatal AMI, duration of hospital stay | Event rate (%) Endpoint 7: No CR: 37 CR+ phase II: 29.7 CR+ phase II + III: 16.2 p < 0.05 Endpoint 4: Control: 5.6 CR phase II: 0 CR phase II + III: 2.7 p < 0.05 | – Different time periods for CR and control group (prospective and retrospective evaluation) – Inclusion period confirmed by authors |
| Norris et al. 2004,5 Canada | rCCS | a. Data linkage: Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) with the Northern Alberta Cardiac Rehabilitation Program (NACRP) b. n = 5081 c. Mixed population: catheterisation for AP and ACS, followed by PCI, CABG or medical therapy d. January 1995–December 1999 e. ≥6 mo survival after index event f. 60.8 (CR+) 64.2 (no CR) g. 80.7 (CR+) 75.2 (no CR) | a. n = 1470 b. SMC-CR c. 88.65 ± 78.09 d Mdn 54 d (information by author) d. 12 wk (information by author) e. n = 2–3 (information by author) f. Out-patient | a. n = 3,611 b. UC | a. 1, 2, 6 y b. (1) c. – | HR (95% CI) Endpoint 1: 0.79 (0.64–0.98) in favour of CR+ p = 0.036 | – Description of CR obtained by author |
| Kutner et al. 2006,37 USA | rCCS | a. United States Renal Data System (USRDS) b. n = 6215 n = 1855 aged <65 y n = 4353 aged >65 y n = 7 lost at follow-up c. CABG d. 1 January 1998–31 December 2002 e. HD patients surviving ≥90 d post-surgery f. 67.9 ± 10.3 (total) g. 61.4 (total) | a. n = 193 (10.4% of the population <65 y) n = 431 (9.9% of the population >65 y) b. Not clear, includes physical exercise supervised or not supervised c .88 ± 100 d d. Total: 36 CR sessions within 12 wk e. n = 3 f. Out-patient | a. n = 5581 b. UC | a. Up to 6 y b. (1), (2) c. – | HR (95% CI) Endpoint 1: 0.65 (0.56–0.76) in favour to CR+ p < 0.001 Endpoint 2: 0.64 (0.51–0.81) in favour of CR+ p < 0.001 | – Description of CR incomplete – Multi-component CR as defined by CROS not witnessed – Author contacted but no reply |
| Milani et al. 2007,33 USA | rCCS | a. Ochsner Medical Center, New Orleans b. n = 701 c. Coronary events, including AMI (39%), CABG (35%), PCI (44%) d. January 2000–July 2005 e. Including depressive patients f. 64 ± 11 (total) g. 72 (total) | a. n = 522 b. SMC-CR c. 2–6 wk after index event d. 12 wk, total: 36 sessions e. n = 3 f. Out-patient | a. n = 179 b. UC after non completion of 2 wks CR (<5 sessions) | a. 1296 ± 551 d (range: 109–2,188 d) b. (1) c. Cardiovascular risk factors, psychological parameters, quality of life | Event rate (% CR+/no CR) Endpoint 1: 8/30 p = 0.0005 (subgroup of depressed patients) | – No mortality data from the whole study group (with and without depression) available – Contact to author not successful |
| Nielsen et al., 2008, 38 Denmark | rCCS | a. Coronary care unit at Aarhus Sygehus, Municipality of Aarhus cohort, Denmark, aged 30–69 y b. n = 200 c. AMI d. 1 April 2000–31 March 2002 e. ≥30 d survival after AMI f. Mdn 59.8 (CR+) Mdn 59.7 (no CR) g. 71.5 (CR+), na (no CR) | a. n = 145 b. SMC-CR c. 1–2 wk after hospital admission d. 6 wk (phase II) e. n = 2 exercise sessions + education, lifestyle and psychosocial support f. Out-patient | a. n = 55 b. CR non-attenders, UC | a. 1 and 2 y b. (1), (4) c. – | Event rate (% CR+/no CR) Endpoint 1 after 1 y: 2.1/14.5, p = 0.001 Endpoint 1 after 2 y: 2.8/21.8, p = 0.0001 Endpoint 4 after 1 y: 22.1/10.9, p = 0.07 | |
| Alter et al. 2009,6 Canada | rCCS | a. Data linkage: Toronto Rehabilitation Institutes, Clinical Registry (UNIX platform), Canadian Institute of Health Information Discharge Abstract Database (DAD), Ontario Health Insurance Plan, and Registered Persons Database b. n = 4084 c. Primary index event ACS (97.7%), CHF and others (2.3%) d. 6 January 1999–10 December 2003 e. Death or readmissions within 1 y after index event were excluded f. 59.4 ± 10 g. 87.4 | a. n = 2,042 b. SMC-CR c. 89 d average d. 12 mo, total: 26–36 sessions e. n = 1 on-site exercise session + monitored home-based sessions and education f. Out-patient | a. n = 2,042 b. CR non-attenders matched for index events, medical history, age, gender, socioeconomic status, geographical region; UC | a. 2 y + 5.2 y (mean) (4.0–6.6) y b. (1) (ITT analysis) c. Effect of CR in various subgroups; effect of CR completion and non-completion | HR (95% CI) Endpoint 1: Total: 0.47 (0.32–0.68); p < 0.001 ≤65 y: 0.59 (0.35–0.97); p = 0.04 ≥66 y: 0.31 (0.17–0.56); p < 0.001 high risk: 0.57 (0.36–0.90); p = 0.02 low risk: 0.57 (0.17–1.95); p = 0.31 CR non-completers: 0.71 (0.29–1.71), p = 0.41 CR completers: 0.28 (0.13–0.60), p < 0.001 (below 1.00 is in favour of CR+) | – Follow-up started 1 y after index event |
| Hansen et al. 2009,34 Belgium | pCCS | a. Hospital files and general practitioners b. n = 238 c. Successful CABG d. January 1998–October 2002 e. Blanking period: 4 wk post-CABG, exclusion: symptomatic patients, comorbidity of prognostic relevance f. 65.0 ± 9.0 (CR+) 66.2 ± 8.3 (no CR) g. 69.8 (CR+) 67.7 (no CR) | a. n = 149 b. SMC-CR c. 1–2 wk after discharge d. 3 mo, total ≥24 sessions e. n = 3 + psychological/ educational interventions f. Out-patient | a. n = 89 b. UC | a. 2 y b. (1), (4), (8), (10) c. – | Event rate (% CR+/no CR) Endpoint 1: 0.7/5.4, p < 0.05 Endpoint 4: 0.0/3.2, p < 0.05 Endpoint 8: With PCI: 4.0/6.5 With CABG: 0.0/0.7 Endpoint 10: 4.7/14.0 | – Potential selection bias by using 2 medical centres offering CR or no inclusion period from information of the author |
| Suaya et al. 2009,4 USA | rCCS | a. Data linkage: Medicare’s National Claims History File, Medicare’s master enrolment database, American Hospital Association b. n = 601,099 n = 70,040 matched pairs c. Mixed population: AMI (37.1%), CABG (35.4%), PCI (21.0%), others d. Through 1997 e. age ≥65 y, hospital stay ≤30 d, surviving ≥30 d after discharge f. 6574 y: 65.2% 7584 y: 32.7% ≥85 y: 2.1% g. 63.6 | a. n = 70,040 b. SMC-CR c. Not reported d. Average: 24 CR sessions Low CR users: 1–24 sessions High CR users: ≥ 25 sessions e. Not reported f. Out-patient | a. n = 70,040 b. Non-users of CR matched on AMI, PCI and CABG and demographics | a. 1 + 5 y after discharge from index hospitalisation b. (1) c. – | Event rate (% CR+/no CR) Endpoint 1 after 1 y: Propensity-based matching: 2.2/5.3 Regression modelling: 4.8/10.9 Endpoint 1 after 5 y: Propensity-based matching: 16.3/24.6 Regression modelling: 28.1/38.0 p < 0.0001 for all | – Description of CR is limited to the ‘use of CR services defined by Medicare reimbursement for at least 1 CR session within 1 y of follow-up’ – CR content is not reported in publication but known as multi-component through official Medicare sites: www.massgeneral.org; http://www.massgeneral.org/heartcenter/cardiac_rehab_program.aspx |
| Jünger et al. 2010,39 Germany | rCCS | a. Acute Coronary Syndrome Registry (ACOS), including 155 hospitals in Germany b. STEMI, n = 2432 NSTEMI, n = 2115 c. STEMI, NSTEMI d. June 2000–December 2002 e. Alive at hospital discharge f. Mdn: STEMI 63.2 (CR+) 70.0 (no CR) NSTEMI 66.3 (CR+) 71.3 (no CR) g. STEMI 73.6 (CR+); 70.0 (no CR) NSTEMI 71.5 (CR+); 63.6 (no CR) | a. STEMI n = 1649 NSTEMI n = 1107 b. SMC-CR c. ≤2 wk after hospital discharge d. 3–4 wk e. ≥5 exercise sessions per wk + education, motivation, psychosocial support f. In-patient | a. STEMI n = 783 NSTEMI n = 1008 b. UC (general practitioner, control by cardiologists) | a. 1 y b. (1), (3), (10) c. – | OR (95% CI) Endpoint 1: STEMI: 0.41 (0.28–0.60) NSTEMI: 0.53 (0.38–0.76) Endpoint 3: STEMI: 0.66 (0.49–0.89) NSTEMI: 0.73 (0.55–0.98) Endpoint 10: STEMI: 0.58 (0.42–0.79) NSTEMI: 0.71 (0.53–0.97) p < 0.001 for all calculations | – CR controlled by German pension funds; the numbers of exercise sessions represent a minimum – Evaluation of deceased patients: retrospective questionnaires and/or telephone calls for assessment of CR participation with help of relatives, not verified by medical records – High risk of selection bias |
| Goel et al. 2011,2 USA | rCCS | a. Mayo Clinic PCI registry (Rochester area, Olmsted County) + database of the Mayo Clinic CR programme b. n = 2395 n = 719 matched pairs c. PCI (elective, urgent or emergency due to ACS) d. 1 January 1994–30 June 2008 e. – f. 62.5 ± 11.7 (CR + ) 66.8 ± 13.5 (no CR) g. 72 (CR+) 66 (no CR) | a. n = 964 (entire cohort) n = 719 (matched pairs) b. SMC-CR c. Within 3 mo after index event d. Total: Mdn 13 sessions e. Not reported f. Out-patient | a. n = 1431 (entire cohort) n = 719 (matched pairs) b. UC | a. Mdn 6.3 y b. (1), (2), (4), (8), (10) c. – | HR (95% CI) Propensity score stratification: Endpoint 1: 0.53 (0.42–0.67) p < 0.001 Endpoint 2: 0.61 (0.41–0.91) p < 0.016 Endpoint 4: 1.07 (0.85–1.36) p < 0.56 Endpoint 8: 1.06 (0.90–1.25) p = 0.47 Endpoint 10: death, AMI, PCI, CABG: 0.85 (0.74–0.98) p = 0.022 Matched groups analysis: Endpoint 1: 0.54 (0.41–0.71) p < 0.001 Endpoint 2: 0.69 (0.44–1.07) p = 0.095 Endpoint 4: 1.11 (0.84–1.45) p = 0.47 Endpoint 8: 1.16 (0.96–1.39) p = 0.13 Endpoint 10: death, AMI, PCI, CABG: 0.92 (0.78–1.07) p = 0.28 | – Study includes a small sample of patients in 1994 – Mixed population including stable CAD patients – No detailed description of CR, but SMC-CR confirmed by author – Per definition in the study, CR could be of low volume – ‘Repeat PCI/CABG’ as calculated in the study was regarded as CROS endpoint 8 |
| Kim et al. 2011,31 Korea | pCCS | a. Sanggye Paik Hospital, Seoul, Korea b. n = 141 c. AMI d. January 2006–December 2007 e. PCI or CABG, exclusion: stroke, cancer, neuro-musculoskeletal symptoms f. 61.9 ± 10.7 (CR + ) 64.5 ± 12.8 (no CR) g. 71 (CR+) 83 (no CR) | a. n = 69 b. SMC-CR c. Not reported d. 6–8 wk, hospital monitored, followed by monitored home based exercise e. Not reported f. Out-patient | a. n = 72 b. UC | a. 1 y b. (1), (6), (8), (10) c. – | Event rate (% CR+/no CR) Endpoint 1: 1.4/1.04, p = 0.95 Endpoint 6: 0.0/3.0, p = 0.49 Endpoint 8: 6.0/10.0, p = 0.53 Endpoint 10: 10.0/24.0, p = 0.033 | – Endpoint 10 was defined as ‘recurrence’, which was a composite of re-hospitalisation, re-ACS, coronary angiography, PCI, CABG and death – Start after index event and CR exercise frequency not reported – Contact to author not successful |
| Schwaab et al. 2011,32 Germany | rCCS | a. Secondary selection of participants from the TeleGuard trial,40 b. n = 1474 c. Mixed population (AMI, stable AP, elective or emergency PCI, CABG) d. 2001–2004 e. Participation in the TeleGuard trial f. 64.1 ± 9.6 (CR+) 62.2 ± 10.3 (no CR) g. 73.7 (CR+) 76.9 (no CR) | a. n = 794 b. SMC-CR c. ≤2 wk after hospital discharge d. 3–4 wk e. >5 exercise sessions per wk + education, psychosocial support f. In-patient (majority) | a. n = 679 b. UC | a. 1 y upon CR start b. PEP: (10) SEPs: (1), (4), (6), (8) c. – | Event rate (% CR+/no CR) Endpoint 1: 2.1/2.4, p = 0.014 Endpoint 4: 1.8/3.8, p = 0.015 Endpoint 6: 31.8/38.0, p = 0.013 OR (95% CI) Endpoint 10: 0.73 (0.59–0.91) p = 0.005 in favour of CR+ | – Exercise frequency is not reported but CR follows regulations of German pension funds (numbers represent a minimum as confirmed by author) – Self-reported CR participation, not verified – Potential selection bias due to 56.4% CABG patients in the CR+ group vs. only 27.9% CABG patients in the control group (‘no CR’) – Suspicion of under-representation of NSTEMI patients in both groups |
| Martin et al. 2012,7 Canada | pCCS | a. Data linkage: Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH), Cardiac Wellness Institute of Calgary (CWIC) inpatient and emergency databases; Canada b. n = 5886 c. Population (ACS + stable AP, others) d. 1 July 1996–31 January 2009 e. Exclusion: aged <18 y, no official health number, surviving <6 m after index event f. 60.1 (CR+) 61.1 (no CR) g. 83.8 (CR+) 74.7 (no CR) | a. n = 2900 (entire population) n = 2256 (matched pairs) b. SMC-CR c. 105.8 d (mean from referral to CR enrolment) d. 12 wk, total: 21.9 ± 10.2 sessions e. n = 2–3 supervised exercise session per wk + resistance training + non-supervised sessions at home f. Out-patient | a. n = 2986 (entire population) n = 2256 (matched pairs) b. No CR and non-completers of CR; UC | a. Up to 14 y b. (1), (6), (7) c. Emergency room visits without hospitalisation | HR (95% CI) Endpoint 1: Adjusted: 0.59 (0.49–0.70) Propensity matched: 0.67 (0.54–0.81) Endpoint 6: CR+ completion: 0.77 (0.71–0.84) CR non- completers: 1.30 (1.13–1.49) Endpoint 7: CR+ completion: 0.68 (0.55–0.83) CR non- completers: 0.87 (0.64–1.19) | – Information on CR content not included in publication but obtained from author |
| West et al. 2012,20 UK | pRCT | a. Multicentre based b. n = 1813 c. AMI d. August 1997–April 2000 e. Discharged home within 28 d f. 64.2 ± 11.2 (CR+) 64.7 ± 10.9 (no CR) g. 72.6 (CR+) 74.4 (no CR) | a. n = 903 b. SMC-CR c. Not reported d. Mean: 20 h within 6–8 wk e. n = 1–2 per wk f. Out-patient | a. n = 910 b. UC | a. 1 y, 2 y until 7–9 y b. (1), (4), (5), (7), (10) c. Quality of life (SF36), lifestyle | RR (95% CI) Endpoint 1 after 1 y: 1.16 (0.79–1.69) Endpoint 1 after 2 y: 0.98 (0.74–1.30) Endpoint 1 after 7–9 y: 0.99 (0.85–1.15) Endpoint 10 after 1 y: 0.96 (0.88–1.07) Endpoints 4, 5, 7: no differences between CR and control | – High risk of under-powering – Early closure of enrolment due to limited funding: from an anticipated total of 6000 patients only 1813 patients were included in the study |
| Beauchamp et al. 2013,41 Australia | rCCS | a. A sample of participants of an earlier study42 b. n = 544 c. Mixed population: AMI, CABG and PCI d. 1996–1997 e. Survival within 1 y after index event f. 60.9 ± 10.1 (CR + ) 64.2 ± 12.3 (no CR) g. 77 (CR+) 69 (no CR) | a. n = 281 b. SMC-CR c. Not reported d. Total: 6–12 CR sessions (each session: 1 h exercise + 1 h education) e. Not reported f. Out-patient | a. n = 263 b. UC | a. 14 y b. (1) c. – | HR (95% CI) Endpoint 1: 1.58 (1.16–2.15) p = 0.004 in favour of CR+ | – Mortality was ascertained through linkage to the Australian National Death Index – No external validation of clinical characteristics – CR duration and frequency of sessions not reported |
| Lee et al. 2013,43 Korea | pCCS | a. Sanggye Paik Hospital, Seoul, Korea b. n = 74 c. AMI after successful PCI with drug-eluting stent d. November 2007–May 2009 e. Age 50–75 y excluded if prior revascularisation, cardiovascular or other comorbidities f. 58.8 ± 10.8 (CR+) 60.3 ± 8.7 (no CR) g. 81.8 (CR+) 83.8 (no CR) | a. n = 37 b. Not reported c. Within 4 wk d. 6 wk including structured and supervised exercise, followed by community-based and self-managed exercise (total 9 mo) e. n = 3 per wk f. Out-patient | a. n = 37 (similar age as CR+) b. UC | a. 9 mo b. (2), (4), (10) c. Coronary restenosis as PEP | Event quantity (n CR+/no CR) Endpoint 2: 0/1, p = 0.33 Endpoint 4: 0/0 Endpoint 10: 1/6, p = 0.20 | – Multi-component CR not reported in detail – Small numbers of study participants |
| Marzolini et al. 2013,44 Canada | pCCS | a. Secondary analysis of CR CARE survey comparing CR participation by referral strategy (medically stable patients from 11 hospitals between Windsor, Sudbury, Ottawa, Ontario)45; linkage to medical charts and administrative data bases b. n = 851 c. ACS d. 2006–2008 e. Musculoskeletal comorbidities f. 64.8 ± 9.7 (CR + ) 68.1 ± 10.6 (no CR) g. 78.1 (CR+) 64.7 (no CR) | a. n = 424 b. SMC c. Data not available d. Data not available e. Data not available f. Out-patient | a. n = 427 b. UC | a. Mdn: 2.7 y b. (1), (10) c. – | HR (95% CI) Endpoint 1: 3.91 (1.23–12.36) in favour of CR+ Endpoint 10: no significant differences | – Self-reported CR participation – Information on CR content given by author; data on CR start, duration and intensity are not available |
| Pack et al. 2013,21 USA | rCCS | a. Database of the Division of Cardiovascular Surgery, Mayo Clinic, Rochester, including consecutive residents of Olmstedt County b. n = 846 c. CABG d. January 1996–December 2007 e. Exclusion if combined procedure or discharged to a long-term facility f. 64.4 ± 10.3 (CR+) 68.3 ± 11.0 (no CR) g. 78 (CR+) 73 (no CR) | a. n = 582 b. SMC-CR c. Majority within 1 mo Mdn: 10 d d. Mdn: 55 d Total: Mdn 14 sessions e. n = 3 exercise sessions (30–45 min each) + encouragement to exercise for 30 min/d on ‘non-CR’ days f. Out-patient | a. n = 264 b. UC | a. 9.0 ± 3.7 y b. (1) c. – | HR (95% CI) Endpoint 1: 0.54 (0.40–0.74) p < 0.001 in favour of CR+ | – CR attendance was ascertained by Mayo Clinic database – Patients were considered to have participated in CR if they attended at least 1 out-patient session within 6 mo of the index CABG surgery |
| Coll-Fernández et al. 2014,46 Spain | pCCS | a. Risk Factors and Arterial Disease (FRENA) registry, Spain47 b. n = 1043 c. AMI d. May 2003–August 2012 e. Patients with a first AMI occurring <3 mo prior to enrolment were considered f. 56.0 ± 10.0 (CR+) 67.0 ± 13.0 (no CR) g. 90 (CR+) 71 (no CR) | a. n = 521 b. Based on international clinical practice guidelines, but no standardised protocol for all hospitals c. <3 mo after AMI d. Not reported e. Not reported f. Out-patient | a. n = 522 b. UC | a. Mean: 18 mo b. (1), (10) c. – | HR (95% CI) Endpoint 1: 0.08 (0.01–0.63) p = 0.16 Endpoint 10: 0.65 (0.30–1.42) p = 0.28 | – Part of the information with respect to study design was obtained from author |
| Prince et al. 2014,48 USA | rCCS | a. Montefiore Medical Center, New York b. n = 822 c. Mixed population (AMI, CAD, CHF, stable AP, valvular heart disease) d. 1 May 2001–31 January 2011 e. – f. 61.6 ± 10.8 (CR+) 61.6 ± 12.6 (no CR) g. 63.1 (CR+) 58.1 (no CR) | a. n = 488 b. Not reported c. Not reported d. Not reported e. Total (mean ± SD): 21.6 ± 13.5 f. Out-patient | a. n = 334 b. UC | a. Up to 14 y b. (1) c. Predictors of CR initiation, adherence and completion | Endpoint 1: in favour of CR + , p = 0.0022 | – Description of CR incomplete; SMC-CR therefore not witnessed – Duration of follow-up not precisely defined – Steps to reduce selection bias between CR+ and no CR are unclear |
| Rauch et al. 2014,8 Germany | pCCS | a. OMEGA trial data base49 b. n = 3560 c. AMI d. October 2003–June 2007 e. >3 mo survival after index event f. Mdn: 62 (CR+) 69 (no CR) g. 76.4 (CR+) 71.1 (no CR) | a. n = 2513 b. SMC-CR c. ≤2 wk after hospital discharge (according to the German CR system, but not witnessed by OMEGA database) d. 3–4 wk e. ≥5 exercise sessions + education, motivation, psychosocial support f. In-patient (vast majority) | a. n = 1047 b. UC | a. 4–12 mo after index event b. (1), (2), (3), (4), (5), (6), (8) c. PCI/CABG, heart failure, medication, laboratory tests | OR (95% CI) Endpoint 1: 0.46 (0.27–0.77) in favour of CR + Endpoint 2: 0.43 (0.23–0.79) in favour of CR + Endpoint 3: 0.53 (0.38–0.75) in favour of CR+ Endpoint 4: 0.72 (0.43–1.21) Endpoint 5: 0.35 (0.15–0.84) in favour of CR+ Endpoint 6: 0.96 (0.81–1.13) Endpoint 8: 1.00 (0.78–1.27) | – CR content and volume controlled by German pension funds – Self-reported CR participation by predefined structured interviews |
| Goel K et al. 2015,3 USA | rCCS | a. Institutional, Mayo Clinic, Rochester Minnesota b. n = 201 c. CABG + heart valve surgery d. 1996–2007 e. Olmsted country residents, aged ≥18 y, discharged alive f. 71.5 ± 9.0 (CR+) 73.8 ± 12.0 (no CR) g. 78 (CR+) 57 (no CR) | a. n = 94 b. SMC-CR c. Not reported d. 12 wk (phase II), in addition, phase III recommended Total: Mdn 13 e. n = 1–3 per wk f. Out-patient | a. n = 107 b. UC | a. 6.8 ± 2.8 y b. (1) c. – | HR (95% CI) Endpoint 1: 0.48 (0.27–0.83) p = 0.009 in favour of CR+, adjusted for propensity scores and mortality risk factors | |
| De Vries et al. 2015,30 The Netherlands | rCCS | a. Institutional, Dutch health insurance firm, Achmea Zorg en Gezondheid b. n = 35,919 c. ACS, and/or PCI, CABG and/or valve surgery d. 1 January 2007–1 June 2010 e. Alive + insured 365 days before and 180 d after event f. 63.4 ± 10.8 (CR+) 68.1 ± 13.2 (no CR) g. 75 (CR+) 58 (no CR) | a. n = 11,014 b. SMC-CR c. Within 180 d after index event day. 6–12 wk e. n = 2.3 exercise sessions per wk + education, psychology, social support, physiotherapy according to Dutch guidelines f. Out-patient | a. n = 24,905 b. UC | a. 4 y b. (1) c. – | HR (±95% CI) Endpoint 1: Total population: 0.65 (0.56–0.77) p < 0.01 in favour of CR+, adjusted for propensity scores and mortality risk factors Subpopulations: CABG/valve surgery: 0.55 (0.42–0.74) p < 0.01 ACS: 0.68 (0.57–0.82) p < 0.01 | – Extensive management of confounding by automated variable selection out of 919 potential confounders |
| Meurs et al. 2015,50 The Netherlands | rCCS | a. Secondary selection out of two studies: DepreMI, MIND-IT51,52 b. n = 1702 c. After AMI with or without depression d. September 1997–September 2000; September 1999–November 2002 e. None f. 57 ± 10 (CR+) 65 ± 11 (no CR) g. 83 (CR+) 75 (no CR) | a. n = 878 b. SMC-CR c. Mean 63 d after AMI d. 9 wk average e. n = 2.2 ± 1.6 exercise sessions per wk f. Out-patient | a. n = 824 | a. 6 mo (mean) b. (1), (6) c. – | HR (±95% CI) Endpoint 1: Total population: 0.83 (0.54–1.30) p = 0.41 Non-depressed patients: 1.09 (0.63–1.89) p = 0.74 Depressed patients: 0.48 (0.28–0.84) p = 0.01 HR below 1.0 is in favour of CR+ | – Information of CR content, duration and intensity obtained from author by request |
| Schlitt et al. 2015,53 Germany | rCCS | a. Secondary analysis of two RCTs with other primary objectives54 b. n = 1798 c. Mixed population: stable CAD, ACS, CABG, heart failure others d. 2007–2011; 2007–2009 e. >18 y, life expectancy >12 mo | a. n = 552 b. SMC-CR c. Within 180 d after index event as outlined in publication; within 1 mo after index event like ACS or CABG according rules of German authorities d. Not reported: 3–4 wk according rules of German authorities e. Not reported: >5 exercise sessions per week to be supposed f. In-patient (majority) and out-patient | a. n = 1246 b. UC | a. 136 ± 71 wk b. (1) c. – | HR (±95% CI) Endpoint 1: 0.067 (0.025–0.180) p < 0.001 | – High risk of selection bias, as study is a secondary evaluation of two RCTs with other objectives63,64 – CR not described in detail within the publication but following minimal standards given by German pension funds and confirmed by author |
Table 4a.
Quality evaluation of cohort studies included into meta-analysis.26,35
Study evaluation process
The study evaluation included design, data sources, information on populations, interventions, controls, calculation and presentation of outcomes and handling of bias. For RCTs, the Cochrane risk of bias table (http://tech.cochrane.org/revman/download) was used, and for the CCSs, the checklists of methodological issues on non-randomised studies26 and the Newcastle–Ottawa Scale (NOS) were used.27 In order to facilitate the study evaluation with respect to the management of confounding, n = 8 potential confounders were prespecified, including age, gender, smoker, diabetes, history of stroke, history of AMI, reduced left ventricular ejection fraction and acute or early PCI during AMI.
Data extraction
The following data were extracted from the studies that were selected for meta-analysis: name of first author, year of publication, study location (country), study design, data source, number of participants, population (AMI, CABG or mixed), inclusion period, exclusion criteria, mean follow-up time, mean age of participants, gender, intervention characteristics, control characteristics, reported outcomes, information on outcomes, data on outcomes and covariates included in the adjusted models.
Statistical analysis
Analyses were separately performed with regards to population (ACS, CABG or mixed) and study design (prospective RCT or prospective or retrospective cohort study). For time-to-event outcomes, the hazard ratio (HR) with its 95% confidence interval (CI) was chosen as the effect measure. If possible, log HRs and their standard errors were extracted directly, preferably from an adjusted model and matched-group analysis. If these were not reported but adequate univariate analyses were available, an indirect estimation method was used.28,29 In some publications, an odds ratio (OR) or only absolute event numbers were reported. Therefore, in this review, studies calculating HRs or ORs were separately pooled and presented.28 For dichotomous outcomes, the OR with its 95% CI was used as the effect measure. If necessary, the treatment effect was recalculated in order to be in the same direction, with HR or OR >1.0 indicating a higher event risk for patients participating in CR. HRs were combined using the generic inverse-variance method. ORs were pooled using the Mantel–Haenszel method or the generic inverse-variance method. The latter was only used when at least one study reported an adjusted OR and no absolute event numbers were given. Random-effects models were used to calculate overall effect estimates and confidence intervals, as heterogeneity between the ‘true’ effects of different rehabilitation programmes that were evaluated in the studies was assumed.
All of the results were checked for statistical heterogeneity by _I_2 statistics with 0–30% representing no or only small heterogeneity, 30–60% representing moderate heterogeneity, 50–90% representing substantial heterogeneity and 75–100% representing considerable heterogeneity.29 Due to the heterogeneous study designs (rCCSs, pCCSs and RCTs) and statistical analysis methods (calculating either HR or OR), the number of studies per single meta-analysis was low. A statistical evaluation of potential publication bias based on funnel plot asymmetry could therefore not be performed.29 Nevertheless, sensitivity analyses have been performed with respect to extracted results of alternative analysis techniques (e.g. independent groups instead of matched groups) and with respect to study quality (Table SM 4, Supplemental Material)).
Some deviations from the review protocol published in PROSPERO have to be reported. ORs instead of risk ratios were used as effect measures for dichotomous outcomes because, in some studies, adjusted ORs and no absolute event numbers were reported. Due to the small number of studies, a subgroup analysis, as originally planned, was not performed. R version 3.2.2 (R Foundation for Statistical Computing, 2015) and the R meta package version 4.3-2 (developed by Guido Schwarzer) were used for statistical analyses.
Results
Study characteristics
Study characteristics (design, population, interventions, controls and primary results) are given in Table 2. With respect to the design, only one RCT (n = 1813 patients) fulfilled the CROS criteria. In addition, 17 rCCSs (n = 206,096 patients) and seven pCCSs (n = 12,193 patients) were included. The populations predefined in CROS were distributed as follows: after ACS, n = 12 studies (n = 46,338 patients); after CABG, n = 5 studies (n = 14,583 patients); and mixed populations, n = 9 studies (n = 158,781 patients). The CR setting was ‘out-patient’ in most studies (n = 21) and predominantly ‘in-patient’ (including a variable part of “out-patient” CR) in the four studies from Germany. CR duration varied from 3–4 weeks up to 12 months, and CR intensity varied from two up to more than five exercise sessions per week plus sessions for motivation, information, education and psychosocial interventions, with variable intensities and combinations.
Meta-analysis
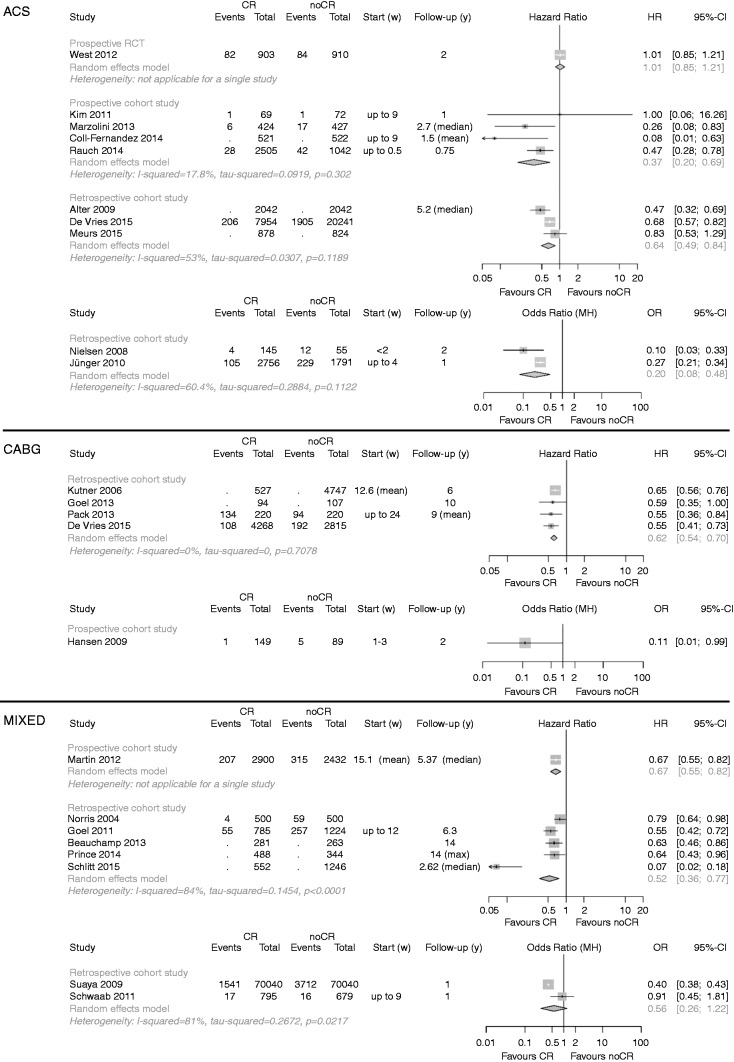
A summary of the clinical outcomes is given in Table 3. The primary endpoint ‘total mortality’ was evaluated in n = 22 studies, one of them evaluating both mortality after ACS and after CABG (Figure 2).30 Participation in CR was associated with significantly reduced mortality in all but three studies.20,31,32 In another study, total mortality after AMI was reduced only in depressed patients.33
Table 3.
Summary of results.
| Outcome | Population (number of studies) | Design (number of studies) | Events/number of patients (CR) | Events/number of patients (control) | HR (95% CI) | OR (95% CI); pooling method | Heterogeneity: I2; tau2; p-value |
|---|---|---|---|---|---|---|---|
| Total mortality | ACS (10) | rCCS (3) | NO/10,874 | NO/23,107 | 0.64 (0.49–0.84) | 53%; 0.031 p = 0.12 | |
| rCCS (2) | 109/2901 | 241/1846 | 0.20 (0.08–0.48); MH | 77.7%; 0.615 p = 0.03 | |||
| pCCS (4) | NO/3519 | NO/1993 | 0.37 (0.20–0.69) | 17.8%; 0.092 p = 0.30 | |||
| RCT (1) | 82/903 | 84/910 | 1.01 (0.85–1.21) | NA | |||
| CABG (5) | rCCS (4) | NO/5109 | NO/5889 | 0.62 (0.54–0.70) | 0.0%; 0.0 p = 0.71 | ||
| pCCS (1) | 1/149 | 5/89 | 0.11 (0.01–0.99); MH | NA | |||
| Mixed (8) | rCCS (5) | NO/2606 | NO/3577 | 0.52 (0.36–0.77) | 84%; 0.145 p < 0.0001 | ||
| rCCS (2) | 1558/70,835 | 3728/70,719 | 0.56 (0.26–1.22); MH | 81.0%; 0.267 p = 0.02 | |||
| pCCS (1) | 207/2900 | 315/2432 | 0.67 (0.55–0.82) | NA | |||
| Cardiovascular mortality | ACS (2) | pCCS (1) | 18/2505 | 32/1042 | 0.44 (0.24–0.82) | NA | |
| pCCS (1) | 0/37 | 1/37 | 0.32 (0.01–8.22); IV | NA | |||
| CABG (1) | rCCS (1) | NO/527 | NO/4747 | 0.64 (0.51–0.81) | NA | ||
| Mixed (1) | rCCS (1) | 34/719 | 46/719 | 0.67 (0.44–0.103) | NA | ||
| MACCE | ACS (2) | rCCS (1) | 212/2756 | 281/1791 | 0.39 (0.28–0.53); IV | NA | |
| pCCS (1) | 81/2376 | 81/971 | 0.55 (0.39–0.77) | NA | |||
| Mixed (1) | rCCS (1) | 158/785 | 206/1224 | 0.85 (0.74–0.98) | NA | ||
| Non-fatal myocardial infarction | ACS (3) | pCCS (1) | 0/37 | 0/37 | 1.0 (0.02–51.73); MH | NA | |
| pCCS (1) | 43/2362 | 27/946 | 0.75 (0.45–1.26) | NA | |||
| RCT (1) | 7/162 | 8/115 | 0.60 (0.21–1.72); MH | NA | |||
| CABG (1) | pCCS (1) | 3/343 | 13/334 | 0.22 (0.06–0.77); MH | NA | ||
| Mixed (2) | rCCS (1) | NO/785 | NO/1224 | 1.01 (0.74–1.37) | NA | ||
| rCCS (1) | 14/795 | 26/679 | 0.45 (0.23–0.87); MH | NA | |||
| Non-fatal stroke | ACS (2) | pCCS (1) | 10/2364 | 13/954 | 0.35 (0.14–0.85) | NA | |
| RCT (1) | 0/162 | 1/115 | 0.23 (0.01–5.81); IV | NA | |||
| Hospital readmission for any reason | ACS (2) | pCCS (2) | 794/2447 | 351/1035 | 0.73 (0.23–2.34); IV | 35.2%, 0.426 p = 0.21 | |
| Unplanned readmission for any cardiovascular event | ACS (2) | pCCS (1) | 17/74 | 20/54 | 0.51 (0.23–1.10); MH | NA | |
| RCT (1) | 23/162 | 16/115 | 1.02 (0.51–2.04); MH | NA | |||
| Mixed (1) | pCCS (1) | 32/2900 | 109/2432 | 0.68 (0.55–0.84) | NA | ||
| Unplanned coronary revascularisation | ACS (1) | pCCS (1) | 4/69 | 7/72 | 0.57 (0.16–2.05); MH | NA | |
| CABG (1) | pCCS (1) | 44/343 | 49/334 | 0.86 (0.55–1.33); MH | NA | ||
| Cardiovascular mortality and readmission | ACS (1) | pCCS (1) | 0/74 | 4/54 | 0.08 (0.00–1.43); MH | NA | |
| Combined endpoints | ACS (6) | pCCS (1) | NO/521 | NO/522 | 0.65 (0.3–1.41) | NA | |
| rCCS (1) | 101/2756 | 119/1791 | 0.64 (0.28–1.46); MH | NA | |||
| pCCA (3) | 41/530 | 67/536 | 0.50 (0.24–1.02); MH | 42.1%; 0.176 p = 0.18 | |||
| RCT (1) | 24/162 | 25/115 | 0.63 (0.34–1.15); MH | NA | |||
| Mixed (1) | rCCS (1) | NO/785 | NO/1224 | 0.77 (0.65–0.91) | NA |
Figure 2.
Analysis of total mortality. Forest plots presenting the evaluation of the endpoint ‘total mortality’.
HR: hazard ratio; OR: Odds ratio; MH: Mantel–Haenszel pooling method; CR: cardiac rehabilitation; No CR: no cardiac rehabilitation (control); CI: confidence interval; Events: number of events in the evaluated group; Total: number of patients in the evaluated group; Start (w): start of cardiac rehabilitation after hospital discharge in weeks; Follow-up: follow-up in years.
After ACS, mortality was reduced in all pCCSs by a factor of 0.37 for patients participating in CR (n = 4 studies; HR 0.37, 95% CI 0.20–0.69), and heterogeneity was low (_I_2 = 17.8%). Similar results were obtained in the rCCSs, but heterogeneity was moderate to substantial. Sensitivity analyses did not change the results. The single RCT meeting the CROS inclusion criteria yielded a neutral result.20
After CABG, all rCCSs consistently showed reduced mortality in patients participating in CR (HR 0.62, 95% CI 0.54-0.70), and heterogeneity was absent (_I_2 = 0%). One additional pCCS supported this result.34 Using independent groups instead of matched groups in the study of Goel et al. did not change the results substantially (HR 0.56, 95% CI 0.45–0.69).3
In ‘mixed populations’, CR participation was associated with a significant mortality reduction on the basis of n = 5 rCCSs and n = 1 pCCS. The analysis of the two rCCSs using ORs yielded a neutral result (OR 0.56, 95% CI 0.26–1.22), but heterogeneity was high (_I_2 = 81%). While the study of Suaya et al. showed a significant mortality reduction (OR 0.42, 95% CI 0.40–0.45),4 the results of Schwaab et al. were neutral (OR 0.91, 95% CI 0.45–1.81).32 Sensitivity analyses did not change the overall results.
Regarding the endpoints ‘cardiovascular mortality’ (n = 4 studies) and ‘major cardiovascular and cerebrovascular events (MACCE)’ (n = 3 studies), only single studies with different populations and designs could be identified, showing a trend in favour to CR participation. The outcomes ‘non-fatal myocardial infarction’ (total n = 6 studies) and ‘non-fatal stroke’ (total n = 2 studies) did not show any trends, and again all selected studies had different designs and populations.
Hospital readmission was investigated under various conditions (endpoints 6–9) by n = 6 studies with different designs. A consistent and clear effect of CR on hospital readmissions could not be observed after ACS, after CABG or in mixed populations.
In n = 7 studies, combined endpoints with various components were evaluated without any clear effect of CR participation. Again, these studies differed with respect to design and study population.
Quality evaluation of the studies
The quality of the cohort studies was assessed using the NOS and the checklists of methodological issues in non-randomised studies criteria.26,27,35 The sum of positive adjudications estimated by NOS is given in Table 4a (for details, see Table SM 2, supplemental material). Four out of 24 studies were adjudicated to have 5 points or less. Limitations have been adjudicated with respect to representativeness (n = 6), comparability of the cohorts (n = 3), adequacy of follow-up (n = 5) and the assessment of outcomes (n = 2).
On the basis of the checklist of methodological issues in non-randomized studies, the following characteristics were obtained: n = 3 studies gained their results by secondary analysis of other clinical studies with different original objectives. In n = 2 studies, there were either time or location differences between the study groups. Health care decision makers and patient preferences had potential influences on group formation in most studies. Moreover, the existence of study protocols was unclear in most studies, and a consort flow diagram was presented only in six out of 24 cohort studies. Management of confounding was not reported in n = 2 studies, whereas the description of potential confounding domains was unclear or not reported in n = 12 studies. Predefinition and calculation of confounding domains as prespecified by CROS (see ‘Methods’ section) were performed to various degrees, reflecting all eight predefined items in n = 4 studies. In contrast, n = 6 studies considered only three items, or even fewer. Adjustment for confounding was performed in n = 21 CCSs, with n = 3 studies not applying adequate biometrical methods.
In the only RCT meeting the CROS inclusion criteria, a high risk of under-powering has to be assumed (Table 4b).20
Table 4b.
Quality evaluation of randomised controlled trials included into meta-analysis (according to the Cochrane risk of bias table; study evaluated: West et al.20).
| Risk | Adjudication | Comments |
|---|---|---|
| Under-powering | High risk | Low recruitment (22.5% cardiac rehabilitation arm; 22.7% control arm) |
| Selection bias | Unclear risk | Study participation influenced by patient preferences |
| Random sequence selection bias | Unclear risk | Random sequence generation is not reported |
| Allocation concealment | Low risk | Per-protocol centrally organised randomisation and blinded with respect to baseline characteristics |
| Confounding variables | Unclear risk | – |
| Performance bias | Low risk | Confirmation of exposure sufficient |
| Detection bias | Low risk | Cardiac rehabilitation status has been blinded before outcome assessment |
| Attrition bias (incomplete outcome data) | Low risk | Follow-up reporting was completed in 95% of surviving patients |
| Groups balanced at baseline | Yes | – |
| Groups not receiving the same baseline treatment | Unclear risk | Baseline treatment with respect to medication and medical supervision has to be assumed; control groups may also have received lifestyle support to a variable extent |
| Intention-to-treat analysis | Yes | – |
| Reporting bias | Low | – |
Discussion
CROS is the first review and meta-analysis evaluating the prognostic effect of structured and multi-component CR exclusively in the era of statins and early interventional revascularisation for acute coronary events. Moreover, by systematically evaluating large CCSs, CROS makes an important independent contribution that more closely reflects the conditions in routine clinical practice. Previous systematic reviews have, in the pursuit of increased validity, exclusively included RCTs irrespective of publication date, with almost half of the studies having been performed in the pre-statin era.1,25 During this earlier period, treatment and medications were very different compared to clinical practice from 1995 onwards, and the impact of CR participation on the long-term clinical course could potentially have been attenuated through modern treatment options.
The major finding of CROS is that CR in the modern era of cardiology is associated with significantly reduced total mortality after ACS and after CABG (Table 3 and Figure 2). However, in the population after ACS, this positive result of CCSs does not concur with the only RCT included, which showed a neutral result (RAMIT).20 However, the RAMIT sample size represented, at best, 23% of the original predefined sample in each trial arm. This issue of poor recruitment does not explain the differences in findings, but it does indicate that the results from RAMIT may not be generalisable to a wider population. Plausible reasons for the neutral result in RAMIT may include super-selection of patients ready to participate in a RCT and a variable dose of CR compared to other trials.8,9,21,30,36
It may be criticised that within CROS, only one RCT was included. However, this was the result of a rigorous and targeted application of predefined selection criteria (e.g. population, timing and type of CR) (Table 1). The latest Cochrane review exclusively including RCTs also did not show a reduction of total mortality in the subgroup of studies published after 1995. However, in the same review, cardiovascular mortality was significantly reduced in both time periods, before and after 1995.1 The variation in mode of mortality benefit between CROS (total mortality) and the Cochrane review (cardiac mortality) is not clarified, but may be the result of differences in populations under investigation and the type of CR delivered; for instance, ‘exercise-only’ interventions being part of the Cochrane analysis versus ‘multi-component’ CR being exclusively evaluated in CROS. Such differences in outcome from two recent meta-analyses highlight the ongoing need for well-designed studies with specified minimal standards in CR delivery and study reporting. Moreover, these problems underscore the need of both RCTs to prove efficacy under controlled (experimental) conditions and controlled and well-designed observational studies in order to prove the effectiveness of such complex clinical interventions as CR in clinical practice.
As structured and supervised exercise during CR has been a precondition for studies to be included in CROS, this may be regarded as the major mechanism contributing to mortality reduction. However, medical supervision, motivation, education and increased adherence to secondary prevention medication as shown in some included studies may also have contributed to the positive results.
No clear CR effect could be demonstrated with respect to non-fatal re-infarction and hospital readmissions (Table 3). One explanation for this could be that CR participation shifts a number of potentially ‘fatal re-infarctions’ to ‘non-fatal’ events, thereby reducing mortality, but not the rate of non-fatal re-infarctions. ‘Hospital readmission’ by definition is a weak clinical endpoint, as it is exposed to a variety of effectors and potential confounders (e.g. routine control coronary angiography in some areas, not necessarily reflecting the individual's health condition, availability of ambulatory cardiologists, psychosocial confounders, etc.). The results with respect to the remaining secondary endpoints are based on a single study or a low number of studies, therefore not allowing us to derive sufficiently evidence-based conclusions (Table 3).
In summary, from the presented results, it can be concluded that in the modern era of cardiology, multi-component CR remains an important and effective therapeutic intervention for reducing the risk of the premature death of CAD patients, especially after an acute event. CR therefore should be recommended as a core part of clinical practice after ACS or following CABG.
Limitations and strengths
Some aspects and limitations have to be considered.
- Search strategy: while validated methodological search filters for RCTs exist, we were not aware of any validated methodological filters for cohort studies. Therefore, for cohort studies, the search filters used have not been validated so far.
- Study quality: for a final and conclusive estimation of the presented outcomes, the quality evaluation of the studies included is a basic requirement. However, the transferability of some predefined evaluation items of the methodological checklist for reviewing non-randomised trials was hampered, mainly due to the limited presentation of study protocol details in several studies. Limitations of the studies include the processes for group formation, information on study protocols and CR content, missing consort flow diagrams and management of confounding at the design stage (Tables 4a,b). The application of the NOS did not add significantly more information; rather, it confirmed the limitations of some of the studies (Tables 4a,b and SM3 in supplemental materials).
Heterogeneity of included studies: the CCSs included in CROS exhibited large heterogeneity due to them being prospective or retrospective and – as exemplified by nine studies – predominantly evaluating mixed populations, including patients after ACS and CABG, but also stable CAD patients in considerably varying proportions. Heterogeneity was also noted with respect to CR duration, intensity and volume (Table 2). Whereas the endpoint of ‘total mortality’ was evaluated in n = 22 studies (88%), the distribution and combination of secondary endpoints differed in every study, as did the composite endpoints under investigation with respect to their single components. Finally, a large variation was found with respect to the statistical methods applied in order to reduce confounding and the potential confounders included in the calculations (Tables 4a,b).
Heterogeneity with respect to study designs and statistical methods limits the validity of additional detailed analysis, hence our main task was to provide least biased and conservative effect estimates. Therefore, neither different types of effect estimates nor different study types were pooled together, meaning that only data based on adjusted models and matched-group analyses were used for the primary analysis. The heterogeneity of the studies therefore resulted in small numbers of studies per single meta-analysis, and evaluation of potential publication bias by funnel plots was not possible (see the ‘Methods’ section).
Heterogeneity, on the other hand, may also reflect the reality of routine clinical practice, which is known to vary between countries. This includes health care systems with different modalities of delivering CR and different conditions for gaining clinical outcome data for scientific evaluations. As these social, health economic and political preconditions cannot be changed, clinical science should try to balance and compensate for these factors by defining common international modalities for study designs that are appropriate for the investigation of multi-factorial health care interventions such as CR.
Conversely, the similarity of clinical results, such as the reduction of mortality in CAD patients associated with CR participation despite heterogeneous preconditions, could also reflect the robustness of the clinical CR effect. Against this background, the criteria for multi-component CR as defined for inclusion in CROS could, as a first step, become the minimal requirements (or standards) for successful CR. These standards should consist of early CR referral after an acute event and structured and supervised exercise at least twice a week, with additional education sessions and psychosocial interventions, all delivered by a multi-disciplinary team of skilled health professionals.
Conclusions
From the basis of 24 CCSs including 217,889 patients and reflecting routine clinical care in nine countries worldwide, participation in structured multi-component CR is associated with reduced mortality after an acute coronary event, even in the era of statins and acute revascularisations. In order to achieve high-quality evidence, internationally accepted minimal standards for the planning, performing and presenting of CCSs are warranted.
Supplementary Material
Supplementary material
Acknowledgements
We thank Margaret Sampson (Children's Hospital of Eastern Ontario) for her peer review of the MEDLINE search strategy. We also thank Thomas Werner Holzinger for supporting the scientific group during the process of study evaluation.
EAPC Cardiac Rehabilitation Section, nucleus members:
- – Patrick Doherty, Dep. of Health Sciences, University of York, Heslington, York, UK
- – Constantinos H Davos, Cardiovascular Research Laboratory, Biomedical Research Foundation, Academy of Athens, Athens, Greece
- – Ana Abreu, Dept. Hospital Santa Marta, Lisbon, Portugal
- – Jean-Paul Schmid, Department of Cardiology Spital Tiefenau, Bern, Switzerland
- – Marco Ambrosetti, Cardiovascular Rehabilitation Unit, ‘Le Terrazze’ Clinic, Cunardo, Italy
- – Romualdo Belardinelli, Cardiac Rehabilitation & Prevention, Lancisi Heart Inst. – Azienda Ospedali Riuniti, Ancona, Italy
- – Ugo Corra, Cardiology Div., Salvatore Maugeri Foundation, IRCCS, Scientific Institute of Veruno, Veruno, Italy
- – Margaret Cupples, Dept. of General Practice, UKCRC Centre of Excellence for Public Health Research, Queens University, Belfast, Northern Ireland, UK
- – Stefan Höfer, Innsbruck Medical University, Austria
- – Marie-Christine Iliou, Cardiac Rehabilitation and Secondary Prevention, Corentin Celton Hospital, APHP, Paris, France
- – Carlo Vigorito, Internal Medicine and Cardiac Rehabilitation, Dept. Translational Medical Sciences, University of Naples Federico II, Italy
- – Heinz Völler, Centre of Rehabilitation Research, University of Potsdam, Germany
Author contribution
All authors participated in designing the study, generating hypotheses, interpreting data and critically reviewing the report. The special responsibilities were as follows: initiation, organisation and leading of the project: Bernhard Rauch, Patrick Doherty, Constantinos H. Davos, Jean-Paul Schmid and Heinz Völler; literature search and search strategies: Maria-Inti Metzendorf and Bernhard Rauch; study selection: Constantinos H Davos, Patrick Doherty and Bernhard Rauch; study evaluation: Daniel Saure, Constantinos H Davos, Patrick Doherty, Annett Salzwedel, Bernhard Rauch, Heinz Völler and Katrin Jensen; statistical and biometrical analyses: Daniel Saure and Katrin Jensen; writing: Bernhard Rauch, Constantinos H Davos, Patrick Doherty, Daniel Saure, Maria-Inti Metzendorf and Katrin Jensen; internal reviewing: Jean-Paul Schmid, Heinz Völler, Annett Salzwedel and the members of the nucleus of the cardiac rehabilitation section of the European Association of Preventive Cardiology (EAPC).
Systematic review registration
PROSPERO international prospective register of systematic reviews: http://www.crd.york.ac.uk/prospero/review_print.asp?RecordID=7084&UserID=5736. Prospero registration number: CRD42014007084.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Pfizer AG Switzerland (unrestricted grant), Deutsche Herzstiftung e.V. (German Heart Foundation), Deutsche Gesellschaft für Prävention und Rehabilitation von Herz-Kreislauferkrankungen e.V. (DGPR; German Society of Cardiovascular Prevention and Cardiac Rehabilitation). The sponsors did not have any influence on study initiation, conducting and reporting.
References
- 1.Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease. J Am Coll Cardiol 2016; 67(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 2.Goel K, Lennon RJ, Tilbury RT, et al. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011; 123(21): 2344–2352. [DOI] [PubMed] [Google Scholar]
- 3.Goel K, Pack QR, Lahr B, et al. Cardiac rehabilitation is associated with reduced long-term mortality in patients undergoing combined heart valve and CABG surgery. Eur J Prev Cardiol 2015; 22(2): 159–168. [DOI] [PubMed] [Google Scholar]
- 4.Suaya JA, Stason WB, Ades PA, et al. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1): 25–33. [DOI] [PubMed] [Google Scholar]
- 5.Norris CM, Jensen LA, Galbraith PD, et al. Referral rate and outcomes of cardiac rehabilitation after cardiac catheterization in a large Canadian city. J Cardiopulm Rehabil 2004; 24: 392–400. [DOI] [PubMed] [Google Scholar]
- 6.Alter DA, Oh PI, Chong A. Relationship between cardiac rehabilitation and survival after acute cardiac hospitalization within a universal health care system. Eur J Cardiovasc Prev Rehabil 2009; 16(1): 102–113. [DOI] [PubMed] [Google Scholar]
- 7.Martin BJ, Hauer T, Arena R, et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation 2012; 126(6): 677–687. [DOI] [PubMed] [Google Scholar]
- 8.Rauch B, Riemer T, Schwaab B, et al. Short-term comprehensive cardiac rehabilitation after AMI is associated with reduced 1-year mortality: results from the OMEGA study. Eur J Prev Cardiol 2014; 21: 1060–1069. [DOI] [PubMed] [Google Scholar]
- 9.Giannuzzi P, Temporelli PL, Marchioli R, et al. Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Arch Intern Med 2008; 168(20): 2194–2204. [DOI] [PubMed] [Google Scholar]
- 10.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J 2011; 162(4): 571–584. [DOI] [PubMed] [Google Scholar]
- 11.Janssen V, De Gucht V, Dusseldorp E, et al. Lifestyle modification programmes for patients with coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol 2013; 20(4): 620–640. [DOI] [PubMed] [Google Scholar]
- 12.Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011; 124: 2458–2473. [DOI] [PubMed] [Google Scholar]
- 13.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2016; 37(29): 2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjarnason-Wehrens B, McGee H, Zwisler AD, et al. Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil 2010; 17: 410–418. [DOI] [PubMed] [Google Scholar]
- 15.Zwisler AD, Bjarnason-Wehrens B, McGee H, et al. Can level of education, accreditation and use of databases in cardiac rehabilitation be improved? Results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil 2012; 19: 143–150. [DOI] [PubMed] [Google Scholar]
- 16.Johannesson M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. Scandinavian Simvastatin Survival Study Group. N Engl J Med 1997; 336(5): 332–336. [DOI] [PubMed] [Google Scholar]
- 17.Montalescot G, Andersen HR, Antoniucci D, et al. Recommendations on percutaneous coronary intervention for the reperfusion of acute ST elevation myocardial infarction. Heart 2004; 90(6): e37–e37. [PMC free article] [PubMed] [Google Scholar]
- 18.Karoff M, Held K, Bjarnason-Wehrens B. Cardiac rehabilitation in Germany. Eur J Cardiovasc Prev Rehabil 2007; 14(1): 18–27. [DOI] [PubMed] [Google Scholar]
- 19.Zwisler AD, Soja AM, Rasmussen S, et al. Hospital-based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12-month results of a randomized clinical trial. Am Heart J 2008; 155(6): 1106–1113. [DOI] [PubMed] [Google Scholar]
- 20.West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012; 98(8): 637–644. [DOI] [PubMed] [Google Scholar]
- 21.Pack QR, Goel K, Lahr BD, et al. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation 2013; 128(6): 590–597. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology – a proposal of reporting. JAMA 2000; 283(15): 2008–2012. [DOI] [PubMed] [Google Scholar]
- 24.Cole JA, Smith SM, Hart N, et al. Systematic review of the effect of diet and exercise lifestyle interventions in the secondary prevention of coronary heart disease. Cardiol Res Pract 2011; 2011: 232–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2011; 7: CD001800–CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells GA, Shea B, Higgins JP, et al. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res Synth Methods 2013; 4(1): 63–77. [DOI] [PubMed] [Google Scholar]
- 27.Wells GA, Shea B, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2008).
- 28.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol 2002; 55(9): 893–899. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Green SE. Cochrane Handbook for Systematic Reviews of Interventions, Chichester, UK: John Wiley & Sons, Ltd, 2011. [Google Scholar]
- 30.De Vries H, Kemps HMC, Van Engen Verheul MM, et al. Cardiac rehabilitation and survival in a large representative community cohort of Dutch patients. Eur Heart J 2015; 36(24): 1519–1528. [DOI] [PubMed] [Google Scholar]
- 31.Kim C, Kim DY, Moon CJ. Prognostic influences of cardiac rehabilitation in Korean acute myocardial infarction patients. Ann Rehabil Med 2011; 35(3): 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwaab B, Waldmann A, Katalinic A, et al. In-patient cardiac rehabilitation versus medical care – a prospective multicentre controlled 12 months follow-up in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil 2011; 18(4): 581–586. [DOI] [PubMed] [Google Scholar]
- 33.Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med 2007; 120(9): 799–806. [DOI] [PubMed] [Google Scholar]
- 34.Hansen D, Dendale P, Leenders M, et al. Reduction of cardiovascular event rate: different effects of cardiac rehabilitation in CABG and PCI patients. Acta Cardiol 2009; 64(5): 639–644. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Ramsay C, Reeves BC, et al. Issues relating to study design and risk of bias when including non-randomized studies in systematic reviews on the effects of interventions. Res Synth Methods 2013; 4(1): 12–25. [DOI] [PubMed] [Google Scholar]
- 36.Boulay P, Prud’homme D. Health-care consumption and recurrent myocardial infarction after 1 year of conventional treatment versus short- and long-term cardiac rehabilitation. Prev Med 2004; 38(5): 586–593. [DOI] [PubMed] [Google Scholar]
- 37.Kutner NG, Zhang R, Huang Y, et al. Cardiac rehabilitation and survival of dialysis patients after coronary bypass. J Am Soc Nephrol 2006; 17(4): 1175–1180. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen KM, Faergeman O, Foldspang A, et al. Cardiac rehabilitation: health characteristics and socio-economic status among those who do not attend. Eur J Public Health 2008; 18(5): 479–483. [DOI] [PubMed] [Google Scholar]
- 39.Junger C, Rauch B, Schneider S, et al. Effect of early short-term cardiac rehabilitation after acute ST-elevation and non-ST-elevation myocardial infarction on 1-year mortality. Curr Med Res Opin 2010; 26(4): 803–811. [DOI] [PubMed] [Google Scholar]
- 40.Waldmann A, Katalinic A, Schwaab B, et al. The TeleGuard trial of additional telemedicine care in CAD patients. Morbidity and mortality after 12 months. J Telemed Telecare 2008; 14: 22–26. [DOI] [PubMed] [Google Scholar]
- 41.Beauchamp A, Worcester M, Ng A, et al. Attendance at cardiac rehabilitation is associated with lower all-cause mortality after 14 years of follow-up. Heart 2013; 99(9): 620–625. [DOI] [PubMed] [Google Scholar]
- 42.Worcester MU, Murphy BM, Mee VK, et al. Cardiac rehabilitation programmes: predictors of non-attendance and drop-out. Eur J Cardiovasc Prev Rehabil 2004; 11: 328–335. [DOI] [PubMed] [Google Scholar]
- 43.Lee HY, Kim JH, Kim BO, et al. Regular exercise training reduces coronary restenosis after percutaneous coronary intervention in patients with acute myocardial infarction. Int J Cardiol 2013; 167(6): 2617–2622. [DOI] [PubMed] [Google Scholar]
- 44.Marzolini S, Leung YW, Alter DA, et al. Outcomes associated with cardiac rehabilitation participation in patients with musculoskeletal comorbidities. Eur J Phys Rehabil Med 2013; 49: 775–783. [PubMed] [Google Scholar]
- 45.Grace SL, Russell KL, Reid RD, et al. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3): 235–241. [DOI] [PubMed] [Google Scholar]
- 46.Coll-Fernandez R, Coll R, Pascual T, et al. Cardiac rehabilitation and outcome in stable outpatients with recent myocardial infarction. Arch Phys Med Rehabil 2014; 95: 322–329. [DOI] [PubMed] [Google Scholar]
- 47.Barba R, Bisbe J, Pedrajas JN, et al. Body mass index and outcome in patients with coronary, cerebrovascular, or peripheral artery disease: findings from the FRENA registry. Eur J Cardiovasc Prev Rehabil 2009; 16: 457–463. [DOI] [PubMed] [Google Scholar]
- 48.Prince DZ, Sobolev M, Gao J, et al. Racial disparities in cardiac rehabilitation initiation and the effect on survival. PM R 2014; 6(6): 486–492. [DOI] [PubMed] [Google Scholar]
- 49.Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010; 122: 2152–2129. [DOI] [PubMed] [Google Scholar]
- 50.Meurs M, Burger H, van Riezen J, et al. The association between cardiac rehabilitation and mortality risk for myocardial infarction patients with and without depressive symptoms. J Affect Disord 2015; 188: 278–283. [DOI] [PubMed] [Google Scholar]
- 51.Spijkerman TA, van den Brink RHS, May JF, et al. Decreased impact of post-myocardial infarction depression on cardiac prognosis? J Psychosom Res 2006; 61: 493–499. [DOI] [PubMed] [Google Scholar]
- 52.van den Brink RHS, Van Melle JP, Honig A, et al. Treatment of depression after myocardial infarction and the effects on cardiac prognosis and quality of life: Rationale and outline of the Myocardial INfarction and Depression-Intervention Trial (MIND-IT). Am Heart J 2002; 144: 219–225. [PubMed] [Google Scholar]
- 53.Schlitt A, Wischmann P, Wienke A, et al. Rehabilitation in patients with coronary heart disease. Dtsch Arztebl Int 2015; 112: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulz S, Schlitt A, Lutze A, et al. The importance of genetic variants in TNF alpha for periodontal disease in a cohort of coronary patients. J Clin Periodontol 2012; 39: 699–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material