The urgent need to recover MHC class I in cancers for effective immunotherapy (original) (raw)
Highlights
- •
Tumor immune escape compromises the efficacy of cancer immunotherapy. - •
Loss of MHC class I expression is a frequent event in cancer cells. - •
Three tumor phenotypes determine cancer fate: escape, rejection and dormancy. - •
Recovery of MHC class I expression is required to improve cancer immunotherapy.
Abstract
Immune escape strategies aimed to avoid T-cell recognition, including the loss of tumor MHC class I expression, are commonly found in malignant cells. Tumor immune escape has proven to have a negative effect on the clinical outcome of cancer immunotherapy, including treatment with antibodies blocking immune checkpoint molecules. Hence, there is an urgent need to develop novel approaches to overcome tumor immune evasion. MHC class I antigen presentation is often affected in human cancers and the capacity to induce upregulation of MHC class I cell surface expression is a critical step in the induction of tumor rejection. This review focuses on characterization of rejection, escape, and dormant profiles of tumors and its microenvironment with a special emphasis on the tumor MHC class I expression. We also discuss possible approaches to recover MHC class I expression on tumor cells harboring reversible/‘soft’ or irreversible/‘hard’ genetic lesions. Such MHC class I recovery approaches might well synergize with complementary forms of immunotherapy.
Current Opinion in Immunology 2016, 39:44–51
This review comes from a themed issue on Tumour immunology
Edited by Sjoerd H van der Burg and Francesco Marincola
For a complete overview see the Issue and the Editorial
Available online 18th January 2016
http://dx.doi.org/10.1016/j.coi.2015.12.007
0952-7915/© 2016 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Cancer immunotherapy in humans has historically used a variety of products that boost T lymphocyte responses, such as IL-2 and IFN-α in melanoma and renal cell carcinoma and bacterial products as BCG in bladder cancer therapy [1, 2, 3]. More recently, antigenic tumor peptides or dendritic cells loaded with shared peptides have been introduced to the clinic [4, 5]. These therapies created great expectations among clinical oncologist because they could activate specific anti-tumor T-cell immunity. However, the observed tumor regressions were below expectations [6]. The absence or downregulation of tumor MHC class I (MHC-I) molecules could be one of possible explanations for these disappointing results, since MHC-I expression on cancer cells is required for detection and destruction by T-cells [7, 8]. MHC-I loss or dowregulation is a major tumor escape mechanism from T lymphocytes described in human tumors of different origin [9, 10, 11, 12]. The HLA evaluation in human tumor tissues needs a complex approach since HLA class I (HLA-I) heavy chains are highly polymorphic and requires analysis of the expression of six HLA-I alleles on tumor cell surface which differ among cancer patients [13]. It is obvious that the information about tumor HLA expression mostly comes from the analysis of progressing tumors, which have already developed escape strategies. In contrast, the tumor rejection profile is difficult to study since such regressing lesions either disappear in a short period of time or progress while acquiring the immunoedited escape phenotype [14]. There are also evidences that some tumor cells can survive in the host in a ‘dormant state’ for long periods of time without being detected. These dormant tumor cells ‘awake’ in immune-compromised environments, especially when CD4+ and CD8+ lymphocytes are not present or their numbers are heavily reduced [15••, 16].
The intimate interaction of MHC class I expression by tumors and the T-cell immune pressure
One of the major problems facing any type of cancer treatment is the extensive heterogeneity of primary tumors, which arises as a result of genetic and epigenetic alterations at a clonal level [17••, 18]. In a mouse model of 3-methyl-cholantrene-induced fibrosarcoma we observed that primary tumor clone diversity is characterized by different expression patterns of MHC-I genes and molecules [19]. This explosion of diversity can be described as a ‘big bang’ because of the large variety of different tumor cells with different genotypes and phenotypes, and because it can be detected few weeks after the injection of the chemical carcinogen. Genetic alterations in any particular marker creating this heterogeneity is probably a random process, but the interaction with the host immune system determines the capacity of a given tumor cell clone to survive and disseminate. Therefore, a process of ‘selection’, especially due to T-cell immune pressure on MHC-I deficient tumor variants, might represent a natural process.
We and other groups have evidence that this strong selection process mediated by the interaction of MHC-I and CD8+ T-cells in primary tumors is taking place during the early stages of tumor development leading to either tumor rejection or immune escape via immunoediting [19, 20]. Tumors are predominantly MHC-I positive at early stages. The specific antitumor CD8+ T-cells attack is progressively killing MHC-I positive cells and selecting MHC negative ones (Figure 1). The MHC-I heterogeneity can be observed in many tumors at these early stages. Finally, the T cell immunoediting leaves tumors homogeneously deficient or completely negative for MHC-I expression [20, 21]. A clinical example of T-cell mediated immunoselection of MHC-I negative tumor cells came from the study of melanoma lesions derived from a single patient during the course of cancer progression. A point mutation present in the codon 67 of the beta 2-microglobulin gene in HLA-I negative melanoma cells from the heterogeneous primary tumor was found 10 months later in an uniformly HLA-I-negative metastatic lesion, strongly suggesting an active T-cell immunoselection of MHC-I negative melanoma cells [21].
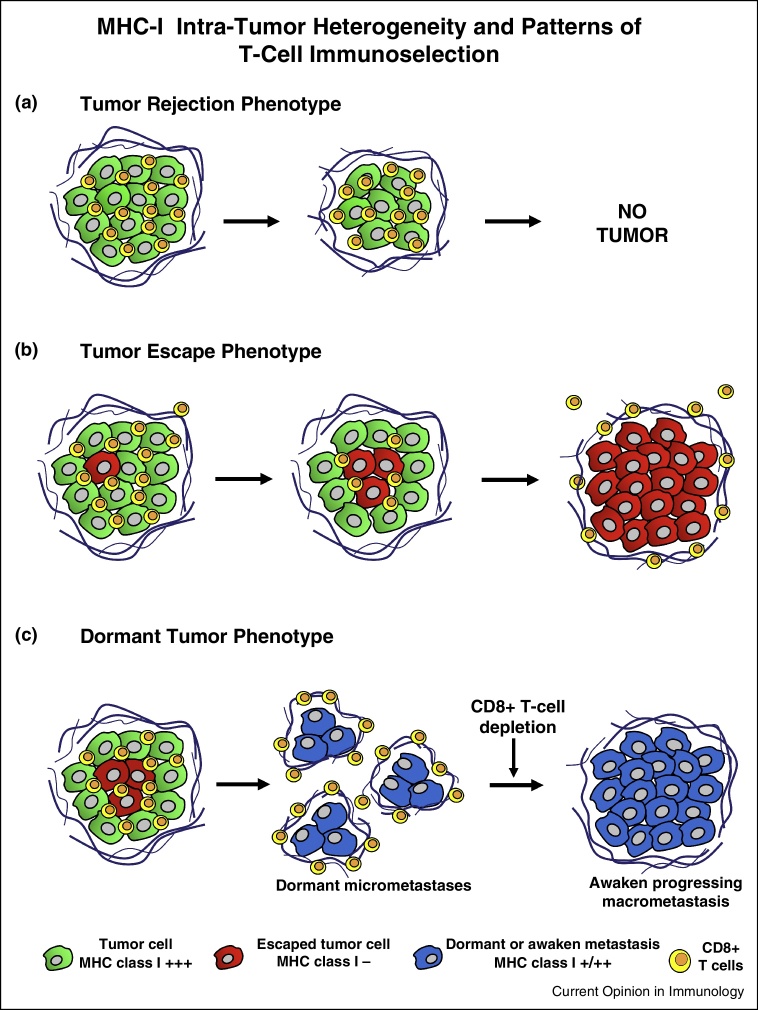
Figure 1.
Schematic representation of the evolution of different tumor phenotypes during cancer progression. (a) Tumor rejection phenotype — tumor cells expressing MHC class I molecules (green) surrounded by CD3+, CD4+ and CD8+ tumor infiltrating lymphocytes (TILs) inside the tumor. The tumor is rejected and is there is no clinical evidence of the tumor in the majority of cases. (b) Tumor escape phenotype — MHC class I negative tumor cell variants appear. Tumor is heterogeneous containing cells both positive and negative for MHC class I expression. There are infiltrating CD4+ and CD8+ T-lymphocytes at early stages. MHC class I negative cells (red) escape from anti-tumor CD8+ T-cells, and tumor is now composed of only MHC class I negative cells (red). Tumor-specific T-cells remains now at the peritumoral area (stroma) and do not infiltrate tumor mass. (c) Tumor dormant phenotype — MHC class I negative cells within the primary tumor develop unnoticed micrometastases, which maintain an immunological equilibrium. They can survive in a dormant stage for long periods of time. Depletion of CD8, CD4 and asialo GM1 positive cells awakes these cells to progression producing detectable macrometastasis. Interestingly, awaken dormant cells are now positive for MHC class I.
In a mouse cancer model, a very clear example of T cell mediated immunoselection came from the assessment of H-2 expression in metastatic lung colonies obtained from an H-2 negative tumor cell clone growing in immunocompetent and in immunodeficient mice [22, 23]. Lung metastatic colonies growing in immunocompetent syngeneic mice were H-2 negative. In contrast, colonies growing in immunodeficient mice lacking T-cells were H-2 positive. The mechanism responsible for the H-2 downregulation in this tumor clone was reversible (‘soft’) since the expression of H-2 antigens could be recovered by IFN-γ [22, 23]. We also observed that MHC-I positive tumor clones are highly immunogenic, while MHC-I negative variants have low immunogenicity. Nevertheless, when the number of locally injected tumor cells from MHC-I positive clone is considerably large, they generate local tumors and develop a high number of distant metastases, because are able to overcome T-cell responses and induce immunosuppression [22]. Importantly, MHC-I positive lung metastases in this model could be completely eradicated by immunotherapy [24]. In contrast, MHC-I negative tumor clones have none or low metastatic capacity. They generate dormant micrometastases with increased MHC-I expression, which are capable of inducing T-cell immune response (Figure 1) [15••].
Tumor rejection profile
There are only few reports describing the MHC-I expression patterns in tumors undergoing rejection in humans. This rejection can sometimes be induced using different protocols of immunotherapy, including treatment with IL-2, BCG, IFN-α, autologous tumor vaccine, peptide vaccination or transfer of autologous anti-tumor CD8+ T-cells, and is associated with the expression of high levels of HLA-I molecules in tumor cells [20]. It is difficult to obtain regressing malignant lesions, since there are no clinical indications for surgical removal or biopsy. In this situation, we rely only on the evaluation of systemic CD8+ T-cell responses and intratumoral infiltration [25]. Nevertheless, we had an opportunity to carefully analyze regressing and progressing subcutaneous melanoma lesions in two ‘mixed responder’ patients after autologous vaccination [14, 20]. We observed a massive intratumoral infiltration of CD4+ and CD8+ lymphocytes (TILs) within the regressing melanoma lesions with a positive correlation of high MHC-I levels. In contrast, the lack of tumor HLA-I expression in progressing lesions correlated with absence of TILs with mostly peritumoral infiltration patterns [14, 20]. These two opposite patterns in distinct lesions of the same patient reflect different phenotypes of tumor microenvironment, namely tumor rejection and tumor escape (Figure 1). Tumor regression was also associated with increased transcriptional upregulation of HLA and interferon stimulation pathway genes pointing to an enhanced antigen presentation capability of tumor cells [14]. This histological and molecular signature of tumor rejection mediated by CD8+ T-cells seems to be similar to that found in allograft rejection and graft versus host disease suggesting an existence of an immunological circuitry of rejection [26].
High degree of tumor infiltration with T-cells is considered to be a good prognostic factor [27] and has been included into a new tumor immunological grading system called ‘immunoscore’ [28]. We have previously observed in various types of cancers that the HLA-I negative tumors lack TILs. In contrast, HLA-I positive tumors are characterized by high degree of intratumoral infiltration with CD8+ T cells [14, 20]. The status of intratumoral infiltration, perhaps, reflects the stage of cancer immune escape during natural cancer progression. At early stages there are more HLA-positive tumor cells and many TILs, while at more advanced stages tumor contains more HLA-negative escape variants and T-cells are restrained in the peritumoral area (Figure 1).
Tumor HLA-I expression patterns have been discussed at the 12th International Histocompatibility Workshop (Paris, France, June 9–12, 1996). Tumors sections were classified as HLA-I negative (<25% tumor cells stained), heterogeneous (between 25% and 75% tumor cells labeled) and positive (>75% tumor cells stained) [13, 29]. It would be interesting to determine whether the lack of HLA-I is the cause of poor T-cell recruitment into the tumor or, the other way around, the local infiltrating T cells mediate high HLA-I expression by producing IFN-γ. A recent report indicated that the latter option could be valid, at least in sarcomas [30]. In this context, the broadened melanoma-reactive CD8+ T cell responses reported after anti-CTLA-4 therapy in melanoma [31•, 32, 33••] could be associated with the upregulation of MHC expression and could lead to the presentation of a variety of previously hidden tumor specific peptides, which subsequently activate a pre-existing T-cell pool. Similar events were previously reported in clinical trials using peptide-based immunotherapy and were defined as ‘epitope spreading’ [25, 34].
Tumor escape profile
It is well established that tumor immune escape is associated with MHC-I downregulation, as seen in different human and experimental tumors and reviewed in many previous reports [8, 10, 12, 35]. Tumors with this profile can be derived from established progressed tumors after they had escaped T-cell mediated immunosurveillance [11]. A tumor derived from an HLA-I positive epithelium can lose totally or partially the expression of class I molecules [9]. The total percentage of various types of HLA-I loss, including total loss, haplotype loss, or allelic loss, ranges from 65 to 90%, depending on the type of cancer [9, 35, 36].
Another evidence of tumor escape and the resistance to T cell immunity caused by MHC-I down-regulation has been corroborated by a cancer in a small mammal, the Tasmanian Devil. A facial tumor in the Tasmanian Devil silenced the genes for antigen presentation at the epigenetic level and thereby created an infectious cancer that is transmissible to histo-incompatible companions [37, 38•, 39]. This curious case of a transmissible tumor clearly emphasizes the relevance of MHC loss for immune escape of tumors. The clear impression in the field is that most MHC-I defects in human cancers belong to the category of ‘soft’-wired lesions [40••, 41, 42, 43]. Consequently, this type of immuno-editing can be counteracted by clever therapeutic targeting, such as activation of the interferon signaling pathway in cancers or intervention with HDAC inhibitors [44].
Tumor dormancy profile
Clinical and experimental evidence indicate that the immune system can maintain cancer cells in a dormant state [15••, 16]. Metastatic tumor cells can remain in a state of equilibrium with the immune system for long periods of time during which metastatic colonies do not progress and the cellular immune response does not reject the tumor. It resembles the symbiotic co-existence we see in different species when each partner benefits from another in a particular ‘status quo’ of no aggression. Despite clinical reports suggesting that immunosuppression is associated in humans with clinical appearance of metastatic colonies [45], the profile of the dormant microenvironment is not precisely known. We have developed a mouse tumor model (GR9) in which several metastatic tumor nodules were kept in a permanent state of immune-mediated dormancy in an immunocompetent host [15••]. Interestingly, when the mice were depleted of CD8-T lymphocytes the colonies started to growth resulting in overt metastases. Moreover, the same tumor clone produced overt pulmonary metastases in nude mice. Tumor cells capable of generating these dormant metastatic colonies are very exceptional; they were completely negative for MHC-I, but the dormant micrometastases recovered MHC-I surface expression (Figure 1) [15••]. These results suggested that MHC-I surface expression and CD8+ T lymphocytes play an important role in immune-mediated dormancy [46] (Figure 1).
Antitumor strategies could be directed to harness the immune response to maintain cancer cells in a permanent dormant state or to favor a complete tumor rejection. In the GR9 mouse tumor model, immunotherapy turned a highly metastatic tumor clone into dormant micrometastases ([24] and Garcia Lora et al., unpublished observations). Upregulating MHC-I expression on tumor cells by cytokines, by increasing FHIT gene expression [47], by blockade of the immune-checkpoint inhibitors, by suppression of T regulatory cells or myeloid suppressor cells could lead to activation of anti-tumor T lymphocyte responses [44]. An attractive strategy for the restoration of MHC-I expression is by epigenetic modifiers, like inhibitors of DNA methyltransferase (DNMT) or histone deacetylase (HDAC). In several recent papers, such regulation at the epigenetic level was shown to be able to synergize with immunotherapy for the eradication of mouse tumor models [48, 49, 50•]. Interestingly, IFN-γ-induced restoration of ‘soft’ lesions of MHC-I, one of the most powerful inducers of this gene, was shown to partly mediate its effect by inducing demethylation of antigen-processing machinery related genes, including the TAP genes and LMP-2 [51].
How to deal with ‘hard’ lesions? Gene therapy and alternative lymphocytes
Targeting the tumor escape phenotype is one of the major tasks of the present and future cancer therapies [44]. In the examples referred to above, the molecular mechanism responsible for the HLA-I downregulation is reversible or ‘soft’ [40••, 52]. In contrast, when the genes of HLA or beta 2-microglobulin are corrupted due to mutations or deletions resulting in loss of heterozygosity (LOH) at chromosomes 6 or 15, the HLA-I loss is irreversible due to these ‘hard’ lesions [53, 54, 55]. In this case, tumor cells cannot recover the antigen presentation capacity and the tumor microenvironment retains tumor escape phenotype favoring cancer progression. ‘Hard’ HLA-I aberrations in tumors (LOH in chromosomes 6 or 15 and beta 2-microglobulin mutations) are in the range of 30–40% of human cancers [55, 56]. In order to restore HLA-I expression in human tumors with ‘hard’ lesions we have made a recombinant adenovirus carrying beta 2-microglobulin gene and demonstrated a recovery of HLA-I expression on tumor cells deficient in beta 2-microglobulin. This HLA reconstitution also recovered tumor cell destruction by peptide specific CD8+ T-cells in HLA-restricted manner [57, 58, 59].
Natural killer (NK) cells provide a natural barrier against MHC-I negative tumors and are, therefore, interesting immune effectors to exploit in the treatment of immune-escaped tumors with ‘hard’ lesions. However, there is no clear evidence suggesting that NK cells selectively infiltrate MHC-I negative tumor tissues. Tumor-infiltrating NK cells might harbor an anergic phenotype in MHC-I low tumors, in contrast to MHC-I-positive tumors [60]. This anergic state was reversed with IL-12/IL-18 treatment and was even further enhanced by an improved form of IL-2, leading to NK-dependent tumor control. Another group showed that transfer of in vitro pre-activated NK cells, in combination with body irradiation, was effective in eradication of tumors with ‘hard’ genetic lesion in the MHC-I pathway [61]. Interestingly, addition of HDAC inhibitors can increase the susceptibility of cancer cells for NK cells by upregulation of the NKG2D-activating ligand MICA [62, 63]. In addition to upregulation of its ligands, the NKG2D receptor was also upregulated, which led to a further enhanced cytotoxicity of tumor cells [62]. However, caution is needed, as tumor cells treated with HDAC inhibitors might reduce their levels of other activating ligands, as shown for B7-H6, which stimulates NK cells via the NKp30 receptor [64].
Some years ago, our group identified a novel group of CD8+ T cells which specifically recognize cells with low MHC-I expression due to a defect in the peptide transporter TAP [65••, 66]. These T cells recognize an alternative peptide repertoire on immunoescaped tumor cells. We named these peptides TEIPP, for ‘T cell epitopes associated with impaired peptide processing’ and they emerge in the residual MHC-I molecules as a result of alternative antigen processing pathways [67, 68]. We showed that the prototypic TEIPP epitope, derived from the housekeeping protein Trh4, is processed by signal peptide peptidase and is, therefore, processed independently of TAP or the proteasome [69]. In a novel TCR transgenic mouse model based on the Trh4-specific CD8+ T cell clone, we observed an efficient thymic selection of these T cells, indicating that the TEIPP T cell repertoire is not affected by central tolerance [70••]. In addition, the TEIPP T cells were effective in tumor control of the TAP-deficient RMA-S tumor. We anticipate that this CD8+ T cell subset can be exploited for treatment of immune-escaped tumors.
Conclusions
We have defined the following three major tumor phenotypes relevant for tumor-host interactions and anti-tumor immunity: rejection, escape and dormancy. We highlighted the key role of tumor MHC expression that influences the degree and composition the immune cellular infiltration. This type of cellular immune response markedly determines the prognosis and clinical outcome in different types of malignancies. There is accumulating evidence suggesting that the efficacy of traditional (IL2, BCG, peptides, etc.) and newly developed immunotherapy (‘immune checkpoint’ blocking antibodies) depends on the expression levels of MHC-I on tumors cells [49]. ‘Soft’ MHC-I molecular lesions can be recovered by a variety of interventions that modify tumor microenvironment in such a way that Th1 type cytokines are released. ‘Hard’ MHC molecular lesions can only be corrected by transferring the appropriate wild type MHC-I or beta 2-microglobulin gene, or by the application of natural killer cells and TEIPP-specific T cells. Hence, identification of molecular aberrations responsible for altered tumor MHC-I expression, as well as monitoring the evolution of this expression during the course of treatment becomes essential for the success of T-cell mediated cancer immunotherapy and for the development of novel complementary approaches for MHC-I upregulation. We are undoubtedly oversimplifying the enormous complexity of the tumor microenvironment but future findings of key molecules and/or cells capable of overriding the immune escape routes used by tumor cells will certainly help in inducing durable tumor rejection. Among them, MHC re-expression is a major target for future studies.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
- • of special interest
- •• of outstanding interest
Acknowledgements
We would like to thank Dr M Bernal who has helped us in preparing the figure for the manuscript. This work was supported by grants co-financed by FEDER funds (EU) from the Instituto de Salud Carlos III (CP03/0111, PI12/02031, PI 08/1265, PI 11/01022, PI11/01386, PI14/01978, PI15/00528, RETIC RD 06/020, RD09/0076/00165, PT13/0010/0039), Junta de Andalucía in Spain (Group CTS-143, and CTS-695, CTS-3952, CVI-4740 and PI 09/0382 grant), Worldwide Cancer Research 15-1166 grant, and by Dutch Cancer Society (UL 2010-4785, TvH).
Contributor Information
Federico Garrido, Email: federico.garrido.sspa@juntadeandalucia.es.
Thorbald van Hall, Email: T.van_Hall@lumc.nl.
References
- 1.Rosenberg S.A., Lotze M.T., Muul L.M. A progress report on the treatment of 157 patients with advance cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 2.Morales A., Eidinger D., Bruce A.W. Intracavitary Bacillus Calmette Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 3.Askeland E.J., Newton M.R., O'Donnell M.A., Luo Y. Bladder cancer immunotherapy: BCG and beyond. Adv Urol. 2012 doi: 10.1155/2012/181987. article 181987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchand M., Van Baren N., Weynan P., Brichard V., Dreno B., Tessier M.-H., Rankin E., Parmiani G., Arienti F., Humblet Y. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Nestle F.O., Alijagic S., Gilliet M., Sun Y., Grabbe S., Dummer R., Burg G., Shadendorf D. Vaccination of melanoma patients with peptide or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg S.A., Sherry R.M., Morton K.E., Scharfman W.J., Yang J.C., Topalian S.L., Royal R.E., Kammula U., Restifo N.P., Hughes M.S. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 7.Festenstein H., Garrido F. MHC antigens and malignancy. Nature. 1986;322:502–503. doi: 10.1038/322502a0. [DOI] [PubMed] [Google Scholar]
- 8.Garrido F., Cabrera T., Concha A., Glew S., Ruiz-Cabello F., Stern P.L. Natural history of HLA expression during tumor development. Immunol Today. 1993;14:491–499. doi: 10.1016/0167-5699(93)90264-L. [DOI] [PubMed] [Google Scholar]
- 9.Garrido F., Ruiz-Cabello F., Cabrera T., Perez-Villar J.J., Lopez-Botet M., Duggan-Keen M., Stern P.L. Implications for immunosurveillance of altered. HLA class I phenotypes in human tumors. Immunol Today. 1997;18:89–95. doi: 10.1016/s0167-5699(96)10075-x. [DOI] [PubMed] [Google Scholar]
- 10.Marincola F.M., Jafee E.M., Hicklin D.J., Ferrone S. Escape of human solid tumors from T cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 11.Garrido F., Algarra I. MHC antigens and tumor escape from immune surveillance. Adv Cancer Res. 2001;83:117–158. doi: 10.1016/s0065-230x(01)83005-0. [DOI] [PubMed] [Google Scholar]
- 12.Seliger B., Cabrera T., Garrido F., Ferrone S. HLA class I antigen abnormalities and immune escape by malignant cells. Semin Cancer Biol. 2002;12:3–13. doi: 10.1006/scbi.2001.0404. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera T., Lopez-Nevot M.A., Gaforio J.J., Ruiz-Cabello F., Garrido F. Analysis of HLA expression in human tumor tissues. Cancer Immunol Immunother. 2003;52:1–9. doi: 10.1007/s00262-002-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carretero R., Wang E., Rodriguez A.I., Reinboth J., Ascierto M.L., Engle A.M., Liu H., Camacho F., Marincola F.M., Garrido F., Cabrera T. Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes. Int J Cancer. 2012;131:387–395. doi: 10.1002/ijc.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Romero I., Garrido C., Algarra I., Collado A., Garrido F., Garcia-Lora A.M. T lymphocytes restrain spontaneous metastases in permanent dormancy. Cancer Res. 2014;74:1958–1968. doi: 10.1158/0008-5472.CAN-13-2084. [DOI] [PubMed] [Google Scholar]; This paper shows that immune system may control metastatic cells in immunological dormancy. The CD8+ T cell-mediated immune response generated against tumor maintains spontaneous metastasis in latency throughout the life of the animals. Depletion of host CD8+ T cells awakens dormant disseminated spontaneous metastatic cells.
- 16.Koebel C.M., Vermi W., Swann J.B., Zerafa N., Rodig S.J., Old L.J., Smyth M.J., Schreiber R.D. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 17••.Fisher R., Puztai I., Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108:479–485. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this interesting focused mini-review the authors discuss the current clinical and experimental evidence for intra-tumor heterogeneity, its spectrum and its relevance to cancer therapeutics.
- 18.Gerlinger M., Rowan A.J., Horswell S. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido F., Romero I., Aptsiauri N., Garcia-Lora A.M. Generation of MHC class I diversity in primary tumors and selection of the malignant phenotype. Int J Cancer. 2016;138:271–280. doi: 10.1002/ijc.29375. [DOI] [PubMed] [Google Scholar]
- 20.Carretero R., Romero J.M., Ruiz-Cabello F., Maleno I., Rodriguez F., Camacho F.M., Real L.M., Garrido F., Cabrera T. Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics. 2008;60:439–447. doi: 10.1007/s00251-008-0303-5. [DOI] [PubMed] [Google Scholar]
- 21.del Campo A.B., Kyte J.A., Carretero J., Zinchencko S., Méndez R., González-Aseguinolaza G., RuizCabello F., Aamdal S., Gaudernack G. Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. Int J Cancer. 2014;134:102–113. doi: 10.1002/ijc.28338. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Lora A., Algarra I., Gafori J.J., Ruiz-Cabello F., Garrido F. Immunoselection by T lymphocytes generates repeated MHC class I-deficient metastatic tumor variants. Int J Cancer. 2001;91:109–119. doi: 10.1002/1097-0215(20010101)91:1<109::aid-ijc1017>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Lora A., Martinez M., Algarra I., Gaforio J.J., Garrido F. MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components. Int J Cancer. 2003;106:521–527. doi: 10.1002/ijc.11241. [DOI] [PubMed] [Google Scholar]
- 24.Garrido C., Romero I., Berruguilla E., Cancela B., Algarra I., Collado A., Garcia-Lora A.M., Garrido F. Immunotherapy eradicates metastases with reversible defects in MHC class I expression. Cancer Immunol Immunother. 2011;60:1257–1268. doi: 10.1007/s00262-011-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero P., Coulie P. Adaptive T-cell immunity and tumor antigen recognition. In: Rees R.C., editor. Tumor Immunology and Immunotherapy. Oxford University Press; 2014. pp. 1–14. [Google Scholar]
- 26.Wang E., Worschech A., Marincola F.M. The immunological constant of rejection. Trends Immunol. 2008;29:256–262. doi: 10.1016/j.it.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Ryschich E., Nötzel T., Hinz U., Autschbach F., Ferguson J., Simon I., Weitz J., Fröhlich B., Klar E., Büchler M.W., Schmidt J. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res. 2005;11(Pt 1):498–504. [PubMed] [Google Scholar]
- 28.Pages F., Kirilovsky A., Mlecnik B., Asslaber M., Tosolini M., Bindea G., Lagorce C., Wind P., Marliot F., Bruneval P. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 29.Garrido F., Cabrera T., Accola R.S., Bensa J.C., Wodmer W., Dohr G., Drenou B., Drouet M., Fauchet R., Ferrara G.B. HLA and cancer. In: Charron D., editor. vol. 1. EDK; 1997. pp. 445–452. (HLA Genetic diversity of HLA. Functional and Medical Implications). [Google Scholar]
- 30.Rusakiewicz S., Semeraro M., Sarabi M., Desbois M., Locher C., Mendez R., Vimond N., Concha A., Garrido F., Isambert N. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73:3499–3510. doi: 10.1158/0008-5472.CAN-13-0371. [DOI] [PubMed] [Google Scholar]
- 31•.Kvistborg P., Philips D., Kelderman S., Hageman L., Ottensmeier C., Joseph-Pietras D., Welters M., van de Burg S., Kapiteijn E., Michielin O. Anti CTL-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:1–9. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]; This paper provides strong evidence for anti-CTLA-4 therapy-enhanced T cell priming as a component of the clinical mode of action. Comparison of pre-treatment and post-treatment T cell melanoma peptide-specific reactivity in peripheral blood mononuclear cell samples from melanoma patients demonstrated that the anti-CTLA-4 treatment induces a significant increase in the number of melanoma-specific CD8 T cell responses, but does not boost the pre-existing virus-specific and melanoma-specific T cell.
- 32.Robert L., Tsoi J., Wang X., Emerson R., Homet B., Chodon T., Mok S., Huang R.R., Cochran A., Comin-Anduix B. CTLA4 blockade broadens the peripheral T cell receptor repertoire. Clin Cancer Res. 2014;20:2424–2432. doi: 10.1158/1078-0432.CCR-13-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Buferne M., Chasson L., Grange M., Mas A., Arnoux F., Bertuzzi M., Naquet P., Leserman L., Schmitt-Verhulst A.M., Auphan-Anezin N. IFN-γ producing CD8+ cells modified to resist major immune checkpoints induce regression of MHC class I-deficient melanomas. Oncoimmunology. 2015;4:e974759. doi: 10.4161/2162402X.2014.974959. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reveals that regression of experimental melanoma with reduced MHC-I expression can be achieved by CD8+ T cells transfected with STAT5CA, which produce IFNγ and subsequently increase tumor MHC-I. These CD8+ T cells also express PD-1 and upregulate PDL-1 on melanoma cells. Despite upregulation of this immunosuppressive pathway, efficient IFN-γ production in the melanoma microenvironment was found associated with resistance of STAT5CA-expressing CD8+ T cells to inhibition both by PD-1/PDL-1 engagement.
- 34.Corbiere V., Chapiro J., Stroobant V., Ma W., Lurquin C. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011;71:1253–1262. doi: 10.1158/0008-5472.CAN-10-2693. [DOI] [PubMed] [Google Scholar]
- 35.Garrido F., Klein E. MHC antigen expression. I. Human tumors. In: Klein E., Garrido F., editors. vol 2-1. W.B. Saunders Scientific Publications; 1991. pp. 1–2. (Seminars in Cancer Biology). [Google Scholar]
- 36.Koopman L.A., Corver W.E., Van del Slik A.R., Giphart M.J., Fleuren G.J. Multiple genetic alterations cause frequent and heterogeneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J Exp Med. 2000;191:961–976. doi: 10.1084/jem.191.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murchison E.P., Schulz-Trieglaff O.B., Ning Z., Alexandrov L.B., Bauer M.J., Fu B., Hims M., Ding Z., Ivakhno S., Stewart C. Genome sequencing and analysis of the Tasmanian devil and its transmissible cancer. Cell. 2012;148:780–791. doi: 10.1016/j.cell.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Siddle H.V., Kreiss A., Tovar C., Yuen C.K., Cheng Y., Belov K., Swift K., Pearse A.M., Hamede R., Jones M.E. Reversible epigenetic down-regulation of MHC molecules by devil facial tumor disease illustrates immune escape by a contagious cancer. Proc Natl Acad Sci U S A. 2013;110:5103–5108. doi: 10.1073/pnas.1219920110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper explains the mechanism of tumor immune escape associated with epigenetic down-regulation of MHC molecules in a rare contagious animal cancer called Devil facial tumor disease (DFTD), which causes 100% mortality among infected animals. The authors propose that, IFN-γ and MHC-positive or epigenetically modified DFTD cells may provide a vaccine to this disease.
- 39.Woods G.M., Howson L.J., Brown G.K., Tovar C., Kreiss A., Corcoran L.M., Lyons A.B. Immunology of a transmissible cancer spreading among Tasmanian Devils. J Immunol. 2015;195:23–29. doi: 10.4049/jimmunol.1500131. [DOI] [PubMed] [Google Scholar]
- 40••.Garrido F., Cabrera T., Aptsiauri N: ‘Hard’ and ‘soft’ lesions underlying the HLA Class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–256. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]; This paper summarizes the accumulating clinical and experimental evidences indicating that the nature of the preexisting tumor MHC-I alterations determines the metastatic potential of a malignancy and the final outcome of cancer immunotherapy. Reversible tumor MHC-I alterations (‘soft’ lesions) can be recovered leading to the activation of T cell-mediated responses and tumor regression. However, structural defects (‘hard’ lesion) provide an escape route associated with metastatic progression or tumor recurrence.
- 41.Seliger B. Novel insights into the molecular mechanisms of HLA class I abnormalities. Cancer Immunol Immunother. 2012;61:249–254. doi: 10.1007/s00262-011-1153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seliger B. B7-H abnormalities in melanoma and clinical relevance. Methods Mol Biol. 2014;1102:367–380. doi: 10.1007/978-1-62703-727-3_19. [DOI] [PubMed] [Google Scholar]
- 43.Garrido G., Rabasa A., Garrido C., López A., Chao L., García-Lora A.M., Garrido F., Fernández L.E., Sánchez B. Preclinical modeling of EGFR-specific antibody resistance: oncogenic and immune-associated escape mechanisms. Oncogene. 2014;33:3129–3139. doi: 10.1038/onc.2013.288. [DOI] [PubMed] [Google Scholar]
- 44.Lampen M., van Hall T. Strategies to counteract MHC-I defects in tumors. Curr Opin Immunol. 2011;23:293–298. doi: 10.1016/j.coi.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Cozar J.M., Aptsiauri N., Tallada M., Garrido F., Ruiz-Cabello F. Late pulmonary metastases of renal cell carcinoma immediately after post-transplantation immunosuppressive treatment: a case report. J Med Case Rep. 2008;2:111. doi: 10.1186/1752-1947-2-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero I., Garrido F., Garcia-Lora A.M. Metastases in immune-mediated dormancy: a new opportunity for targeting cancer. Cancer Res. 2014;74:6750–6757. doi: 10.1158/0008-5472.CAN-14-2406. [DOI] [PubMed] [Google Scholar]
- 47.Romero I., Martinez M., Garrido C., Collado A., Algarra I., Garrido F., Garcia-Lora A.M. The tumor suppressor Fhit positively regulates MHC class I expression on cancer cells. J Pathol. 2012;227:367–379. doi: 10.1002/path.4029. [DOI] [PubMed] [Google Scholar]
- 48.Símová J1, Polláková V., Indrová M., Mikyšková R., Bieblová J., Stěpánek I., Bubeník J., Reiniš M. Immunotherapy augments the effect of 5-azacytidine on HPV16-associated tumors with different MHC class I-expression status. Br J Cancer. 2011;105:1533–1541. doi: 10.1038/bjc.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park J., Thomas S., Munster P.N. Epigenetic modulation with histone deacetylase inhibitors in combination with immunotherapy. Epigenomics. 2015;7:641–652. doi: 10.2217/epi.15.16. [DOI] [PubMed] [Google Scholar]
- 50•.Kim K., Skora A.D., Li Z., Liu Q., Tam A.J., Blosser R.L., Diaz L.A., Jr., Papadopoulos N., Kinzler K.W., Vogelstein B. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111:11774–11779. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report that mouse cancers resistant to immune checkpoint blockade can be eradicated by epigenetic modulators targeting MDSCs. Only combined treatment using epigenetic-modulating drugs and checkpoint inhibitors cured more than 80% of the CT26 or 4T1 tumor-bearing mice.
- 51.Vlková V., Štěpánek I., Hrušková V., Šenigl F., Mayerová V., Šrámek M., Šímová J., Bieblová J., Indrová M., Hejhal T. Epigenetic regulations in the IFNγ signalling pathway: IFN-γ-mediated MHC class I upregulation on tumor cells is associated with DNA demethylation of antigen-presenting machinery genes. Oncotarget. 2014;5:6923–6935. doi: 10.18632/oncotarget.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aptsiauri N., Garcia-Lora A., Garrido F. ‘Hard’ and ‘soft’ loss of MHC class I expression in cancer cells. In: Rees R.C., editor. Tumor Immunology and Immunotherapy. Oxford University Press; 2014. pp. 63–78. [Google Scholar]
- 53.Maleno I., Cabrera C.M., Cabrera T., Paco L., Lopez-Nevot M.A., Collado A., Ferron A., Garrido F. Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics. 2004;56:244–253. doi: 10.1007/s00251-004-0692-z. [DOI] [PubMed] [Google Scholar]
- 54.Kloor M., Michel S., von Knebel Doeberitz M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer. 2010;127:1001–1010. doi: 10.1002/ijc.25283. [DOI] [PubMed] [Google Scholar]
- 55.Bernal M., Ruiz-Cabello F., Concha A., Paschen A., Garrido F. Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother. 2012;61:1359–1371. doi: 10.1007/s00262-012-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maleno I., Aptsiauri N., Cabrera T., Gallego A., Paschen A., López-Nevot M.A., Garrido F. Frequent loss of heterozygosity in the β2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics. 2011;63:65–71. doi: 10.1007/s00251-010-0494-4. [DOI] [PubMed] [Google Scholar]
- 57.del Campo A., Aptsiauri N., Mendez R., Zinchenko S., Vales A., Paschen A., Ward S., Ruiz-Cabello F., González-Aseguinolaza G., Garrido F. Efficient recovery of HLA class I expression in human tumor cells after beta2-microglobulin gene transfer using adenoviral vector: implications for cancer immunotherapy. Scand J Immunol. 2009;70:125–135. doi: 10.1111/j.1365-3083.2009.02276.x. [DOI] [PubMed] [Google Scholar]
- 58.del Campo Ana B., Carretero J., Aptsiauri N., Garrido F. Targeting tumor HLA class I expression to increase tumor immunogenicity. Tissue Antigens. 2012;79:147–154. doi: 10.1111/j.1399-0039.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 59.del Campo A.B., Carretero J., Muñoz J.A., Zinchenko S., Ruiz-Cabello F., González-Aseguinolaza G., Garrido F., Aptsiauri N. Adenovirus expressing β2-microglobulin recovers HLA class I expression and antitumor immunity by increasing T-cell recognition. Cancer Gene Ther. 2014;21:317–332. doi: 10.1038/cgt.2014.32. [DOI] [PubMed] [Google Scholar]
- 60.Ardolino M., Azimi C.S., Iannello A., Trevino T.N., Horan L., Zhang L., Deng W., Ring A.M., Fischer S., Garcia K.C. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest. 2014;124:4781–4794. doi: 10.1172/JCI74337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ni J., Miller M., Stojanovic A., Garbi N., Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang H., Lan P., Hou Z., Guan Y., Zhang J., Xu W., Tian Z., Zhang C. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. Br J Cancer. 2015;112:112–121. doi: 10.1038/bjc.2014.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu S., Denman C.J., Cobanoglu Z.S., Kiany S., Lau C.C., Gottschalk S.M., Hughes D.P., Kleinerman E.S., Lee D.A. The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharm Res. 2015;32:779–792. doi: 10.1007/s11095-013-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiegler N., Textor S., Arnold A., Rölle A., Oehme I., Breuhahn K., Moldenhauer G., Witzens-Harig M., Cerwenka A. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood. 2013;122:684–693. doi: 10.1182/blood-2013-02-482513. [DOI] [PubMed] [Google Scholar]
- 65••.van Hall T., Wolpert E.Z., van Veelen P., Laban S., van der Veer M., Roseboom M., Bres S., Grufman P., de Ru A., Meiring H. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med. 2006;12:417–424. doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]; This paper demonstrates the existence of a unique category of CTLs that can prevent immune escape of tumor cells with defects in MHC-I restricted antigen presentation. These CTLs target an alternative repertoire of peptide epitopes that emerge in MHC-I at the surface of cells with impaired function of TAP, tapasin or the proteasome. These peptides, although derived from self-antigens, are not presented by normal cells and act as immunogenic neoantigens.
- 66.Seidel U.J., Oliveira C.C., Lampen M.H., van Hall T. A novel category of antigens enabling CTL immunity to tumor escape variants: Cinderella antigens. Cancer Immunol Immunother. 2012;61:119–125. doi: 10.1007/s00262-011-1160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliveira C.C., van Hall T. Importance of TAP-independent processing pathways. Mol Immunol. 2013;55:113–116. doi: 10.1016/j.molimm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira C.C., van Hall T. Alternative antigen processing for MHC class I: multiple roads lead to Rome. Front Immunol. 2015;6:298. doi: 10.3389/fimmu.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliveira CC1, Querido B., Sluijter M., de Groot A.F., van der Zee R., Rabelink M.J., Hoeben R.C., Ossendorp F., van der Burg S.H., van Hall T. New role of signal peptide peptidase to liberate C-terminal peptides for MHC class I presentation. J Immunol. 2013;191:4020–4028. doi: 10.4049/jimmunol.1301496. [DOI] [PubMed] [Google Scholar]
- 70••.Doorduijn E.M., Sluijter M., Querido B.J., Oliveira C.C., Achour A., Ossendorp F., van der Burg S.H., van Hall T. TAP-independent self-peptides enhance T cell recognition of immune-escaped tumors. J Clin Invest. 2016;126 doi: 10.1172/JCI83671. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this paper, it is shown that TEIPP T cells undergo normal thymic selection and have no signs of central or peripheral tolerance. In addition, the cells show good recognition and killing of TAP-deficient cells both in vitro and in vivo.