What does PD-L1 positive or negative mean? (original) (raw)
Ribas and Hu-Lieskovan show that different processes may lead to the expression of PD-L1 on cancer cells, and each one of them may have a different meaning to interpret the results of clinical trials with anti–PD-1/L1 antibodies.
Abstract
Expression of the programmed death-1 (PD-1) ligand 1 (PD-L1) is used to select patients and analyze responses to anti–PD-1/L1 antibodies. The expression of PD-L1 is regulated in different ways, which leads to a different significance of its presence or absence. PD-L1 positivity may be a result of genetic events leading to constitutive PD-L1 expression on cancer cells or inducible PD-L1 expression on cancer cells and noncancer cells in response to a T cell infiltrate. A tumor may be PD-L1 negative because it has no T cell infiltrate, which may be reversed with an immune response. Finally, a tumor that is unable to express PD-L1 because of a genetic event will always be negative for PD-L1 on cancer cells.
Introduction
Immunotherapy with anti–programmed death-1 (PD-1) or anti–PD-1 ligand 1 (PD-L1) antibodies has been approved for the treatment of several cancers because of impressive durable responses; however, overall, only a small percentage of patients currently benefit from PD-1 blockade therapy alone (Topalian et al., 2012; Herbst et al., 2014; Powles et al., 2014; Ansell et al., 2015; Garon et al., 2015; Postow et al., 2015; Robert et al., 2015a,b; Weber et al., 2015; Nghiem et al., 2016; Ribas et al., 2016). The combination of anti–PD-1/L1 antibodies with other immune modulating agents seems to be more active, but it adds significant toxicities (Wolchok et al., 2013; Larkin et al., 2015; Postow et al., 2015), which may be unwarranted for patients who would respond to anti–PD-1/L1 alone or for patients whose tumors would not respond anyway to either approach. Toxicities of combined immunotherapies may be lower using different agents or with sequential therapy (Weber et al., 2016), but it would still be desirable to develop reliable biomarkers to predict response and select patients to single agent anti–PD-1/L1 treatments.
Tumor responses with anti–PD-1/L1 antibodies are not mediated by the antibody per se, but by tumor antigen–specific T cells that had been previously blocked by the PD-1–PD-L1 interaction (Pardoll, 2012; Tumeh et al., 2014). Based on the presence or absence of T cells and the expression of PD-L1 by cancer cells, a tumor can be categorized into four groups: (1) PD-L1 positive, T cell positive; (2) PD-L1 negative, T cell positive; (3) PD-L1 positive, T cell negative; and (4) PD-L1 negative, T cell negative (Sznol and Chen, 2013; Teng et al., 2015; Ock et al., 2016). As a positive PD-L1 expression can be a reactive process to a T cell response or regulated by cancer cell–intrinsic genetic or epigenetic events, it is becoming clear that the knowledge about PD-L1 expression needs to be put into the context of the presence or absence of a T cell infiltrate blocked by the PD-1 receptor engagement (Taube et al., 2012; Ribas, 2015). This knowledge would help in further interpreting the meaning of the assay results and providing insights in terms of the mechanisms of cancer escape from immune surveillance and potential for response to PD-1 blockade therapy.
Expression of PD-L1 on cells within a tumor has been used in multiple clinical trials and approved clinical indications for this purpose, with the thinking that positive PD-L1 expression in the tumors can select patients more likely to respond to these therapies (Topalian et al., 2012; Wolchok et al., 2013; Herbst et al., 2014; Garon et al., 2015; Reck et al., 2016). The caveat is that some patients who are tested positive for PD-L1 may not respond to the therapy, and more importantly some patients who are tested negative may still respond, making it an imperfect biomarker (Robert et al., 2015a; Ribas et al., 2016). In addition, questions have been raised about technical aspects of calling a positive or negative test for PD-L1. These included the specificity of several clones of anti–human PD-L1 antibodies for immunohistochemistry (IHC) and the artifacts that may be derived from different techniques for tissue fixation and antigen retrieval (Ribas and Tumeh, 2014; Ilie et al., 2016). Most of these technical issues have been alleviated with the standardization of the IHC assays. But even with standardized reagents, tissue processing, and assay performance, it has been challenging to turn the PD-L1 assay into a dichotomous result as there is no consensus of what is the relevant level of PD-L1 that separates positive from negative. As a result, the percent staining to call a positive PD-L1 in different reported series to date ranged from 1% to 50%, making it difficult to compare results across studies (Ilie et al., 2016; Reck et al., 2016). As the field advances, we will learn how results with different antibody clones and scoring algorithms compare with each other, but we will still be left with questions about the biological meaning of the results because different mechanisms leading to the presence or absence of PD-L1 expression are likely to have different implications for anti–PD-1/L1 therapy.
Mechanisms regulating PD-L1 expression
A tumor can be positive or negative for surface PD-L1 expression through several biological processes, thereby having different clinical significance. These can be categorized as (a) genetic mechanisms that lead to constitutive PD-L1 expression, (b) induced PD-L1 expression by the presence of T cells, (c) absence of T cells leading to no reactive PD-L1 expression, and (d) genetic events that preclude PD-L1 expression upon T cell infiltration. It is conceivable that the presence or absence of cancer cell surface PD-L1 may have different functional meaning depending on the underlying mechanism of expression.
Constitutive PD-L1 expression
A series of genetic mechanisms have been reported to lead to the constitutive expression of PD-L1 on cancer cells, but it has unclear relevance for response to anti–PD-1 therapy (Table 1). Constitutive oncogenic PD-L1 expression frequently results in high level, homogeneous PD-L1 staining on all the cancer cells (Fig. 1 a). PD-L1 has been reported to be constitutively expressed through the dysregulation of several oncogenic pathways, including genetic amplification of chromosome 9, which contains the locus of PD-L1, PD-L2, and the interferon receptor adapter JAK2 (termed PDJ amplicon; Green et al., 2010; Ansell et al., 2015; Rooney et al., 2015), PTEN deletions or PI3K/AKT mutations (Parsa et al., 2007; Lastwika et al., 2016), EGFR mutations (Akbay et al., 2013), MYC overexpression (Casey et al., 2016), CDK5 disruption (Dorand et al., 2016), or increase in PD-L1 transcripts stabilized by truncation of the 3′–untranslated region (UTR; Kataoka et al., 2016), among a rapidly growing list of genetic mechanisms of constitutive PD-L1 expression.
Table 1. Potential mechanisms for a tumor biopsy to be labeled as PD-L1 positive or negative.
| PD-L1 status and significance | Cell type/types being positive or negative for PD-L1 | Examples of mechanisms | References |
|---|---|---|---|
| Positive | |||
| Genetic process | Cancer cells | PDJ amplicon | Ansell et al., 2015 |
| MYC | Casey et al., 2016 | ||
| PDL1 3′-UTR disruption | Kataoka et al., 2016 | ||
| CDK5 disruption | Dorand et al., 2016 | ||
| Mutant EGFR | Akbay et al., 2013 | ||
| PTEN deletion | Parsa et al., 2007 | ||
| PI3K/AKT mutations | Lastwika et al., 2016 | ||
| Reactive to a T cell infiltrate | Cancer and immune cells/stromal cells in the tumor microenvironment | IFN-γ induced upon tumor antigen–specific T cell infiltration | Lee et al., 2006; Taube et al., 2012; Herbst et al., 2014; Tumeh et al., 2014 |
| Negative | |||
| Genetic process | Cancer cells | JAK1 or JAK2 truncating mutations | Shin, D.S., et al. 2015. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research. Abstr. 5013; Zaretsky et al., 2016 |
| Absence of a T cell infiltrate | Cancer and immune cells/stromal cells in the tumor microenvironment | No T cells in the tumor | Herbst et al., 2014; Tumeh et al., 2014 |
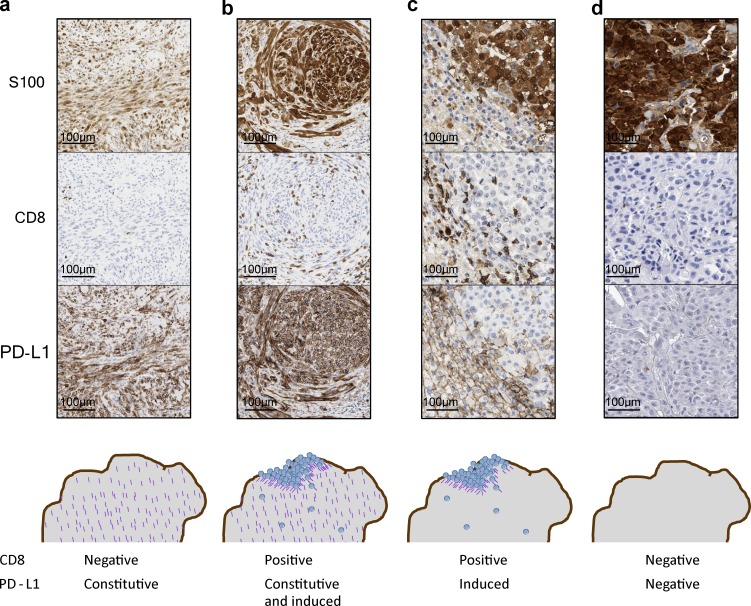
Figure 1.
Examples of different mechanisms leading to PD-L1 positivity or negativity. (a) Constitutive PD-L1 expression but no T cell infiltrate, resulting in constitutive PD-L1 expression in all cancer cells. (b) Constitutive PD-L1 expression with additional inducible expression by a T cell infiltrate, resulting in both constitutive and inducible PD-L1 expression in cancer cells. (c) Adaptive immune resistance, leading to reactive PD-L1 expression induced in cells that are at the site of a CD8+ T cell infiltrate. (d) PD-L1–negative tumor caused by absent T cell infiltration. By IHC, a tumor with JAK1/2 loss of function mutations and genetically negative for inducible PD-L1 would look similar without a CD8 T cell infiltrate (because of a lack of chemokine production in response to interferon-γ) and no PD-L1 expression in the tumor.
Constitutive expression of PD-L1 unquestionably leads to the tumor being scored as positive for PD-L1 by any detection method, but its significance in terms of the interaction with the immune system is unclear. Experimental approaches have reported that constitutive PD-L1 expression through MYC overexpression, CDK5 disruption, or increased PD-L1 mRNA expression from truncated 3′-UTR may have a main role in evading immune control by a developing cancer, thereby being important in immune surveillance but with unclear importance to response to PD-1 blockade therapy (Casey et al., 2016; Dorand et al., 2016; Kataoka et al., 2016). The frequent detection of the PDJ amplicon in the few malignant Reed-Stenberg cells in Hodgkin’s disease is correlated with the successful testing of the administration of anti–PD-1 therapy in patients with chemotherapy-refractory disease (Ansell et al., 2015), which would be an argument in favor of considering constitutive PD-L1 expression as a mechanism to select patients likely to respond to PD-1 blockade therapy. The significance for response to anti–PD-1/L1 therapy should still be related to the presence of antitumor T cells that is blocked by the constitutive PD-L1 expression (Fig. 1 a). Moreover, tumors with constitutive PD-L1 expression may have areas of further increased expression colocalized with a T cell infiltrate, reflective of an adaptive PD-L1 immune resistance (Fig. 1 b). Classic Hodgkin’s disease has a large lymphocytic infiltrate surrounding the few malignant Reed-Stenberg cells and PD-L1 expression on Reed-Stenberg cells through two mechanisms, constitutive and reactive to the brisk T cell infiltrate (Juszczynski et al., 2007).
Inducible PD-L1 expression
Surface expression of PD-L1 can be induced by both type I and II interferons, but it responds best to the type II interferon-γ (Liang et al., 2003; Loke and Allison, 2003; Blank et al., 2004; Lee et al., 2006). The interferon-inducible expression of PD-L1 is more common than the constitutive expression in most cancer histologies and can be detected as a patchy pattern of PD-L1 expression colocalized in T cell–rich areas of tumors, in particular at the invasive margin (Fig. 1 c; Taube et al., 2012; Tumeh et al., 2014). The PD-L1 adaptive expression is a consequence of the presence of tumor antigen–specific T cells that recognized the cancer cells leading to the production of interferon-γ. As interferon-γ would result in PD-L1 expression by any surrounding cell that has interferon receptors, it is frequent to observe PD-L1 expression on noncancer cells in the tumor microenvironment, such as myeloid-lineage cells (Tumeh et al., 2014) and on T cells (Herbst et al., 2014; McDermott et al., 2016), frequently at a level higher than the cancer cells themselves (McDermott et al., 2016). The physiological induction of PD-L1 by interferon-γ leads to evasion of a T cell response (Liang et al., 2003; Kim et al., 2005), termed adaptive immune resistance (Pardoll, 2012; Ribas, 2015), and it is in this setting that the significance of the PD-L1 expression is most logically related to a response to PD-1 blockade therapy (Tumeh et al., 2014; Ribas, 2015).
PD-L1–negative tumors without T cell infiltrates
Patients who do not respond to anti–PD-1 or anti–PD-L1 therapy most frequently have PD-L1–negative tumors, and analyses show that T cell infiltrates are infrequent in these cases (Herbst et al., 2014; Tumeh et al., 2014). The inducible expression of PD-L1 by tumor or immune infiltrating cells is a surrogate of the presence of activated T cells that recognize cancer antigens and produce interferon-γ. So it is logical that if these T cells are not there, cancer cells that do not constitutively express PD-L1 because of an oncogenic event would then be negative for its expression (Fig. 1 d). But in this case, the cancer cells have the potential to express PD-L1 if infiltrated by T cells that recognize cancer antigens and produce interferon-γ (Ribas, 2015). Therefore, this type of PD-L1–negative condition may be reversible with appropriate combinatorial therapies that would bring interferon-γ–producing T cells into the tumor, such as the combination of anti–PD-1 and anti–CTLA-4 (Larkin et al., 2015; Postow et al., 2015).
Genetic loss of function leading to PD-L1 negativity
As in the case of genetic events leading to the expression of PD-L1 on cancer cells irrespective of having a T cell infiltrate that induces it, it is possible that some cancer cells would have genetic events that lead to inability to express PD-L1. Reactive expression of PD-L1 is a favorable event for a cancer cell, as it can specifically inactivate the T cells that are attacking the cancer without inducing a systemic immune-suppressive state (Ribas, 2015). But as interferon-γ has a lot of other effects that may be detrimental to the cancer cell, it is possible that an immunoediting process may result in loss of signaling from the interferon-γ receptor, leading to inability to express multiple interferon-responsive genes, including PD-L1. This mechanism of cancer immunoediting, with loss of signaling through JAK1 or JAK2, has been described in mouse models and cell lines (Kaplan et al., 1998; Dunn et al., 2005). Its relevance to humans treated with anti–PD-1 therapy has been highlighted by the description of patients who developed acquired resistance to the therapy through new truncating mutations in JAK1 or JAK2 (Zaretsky et al., 2016). These were patients who responded to anti–PD-1 therapy and then relapsed many months to years later while on continued therapy. Comparison of baseline and relapsed tumors showed in two cases that JAK1 or JAK2 alleles had been mutated with a loss of function truncation event, whereas the other wild-type allele had been selectively lost. This was not part of a widespread series of such genetic changes, but instead was focused on these genes, suggesting a strong selective pressure to lose signaling from the interferon-γ receptor (Zaretsky et al., 2016). In addition to the loss of the adaptive immune response by not being able to express PD-L1, these tumor cells also became resistant to interferon-mediated killing, which also signals through the interferon-γ receptor. As loss of JAK1 function can lead to immunoediting in mouse models (Kaplan et al., 1998; Mazzolini et al., 2003; Dunn et al., 2005), it is possible that the same may happen in some patients who have PD-L1–negative tumors as a mechanism of innate resistance to anti–PD-1 therapies (Shin, D.S., et al. 2015. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research. Abstr. 5013; Shin et al., 2016). In this scenario, PD-L1 expression on cancer cells would not be corrected by combining with another therapy that could bring interferon-γ–producing T cells into the tumor, as the signaling circuit for inducible PD-L1 expression would be disabled immediately below the interferon-γ receptor.
Clinical significance of PD-L1–positive or –negative testing
In the absence of T cells, which are the effectors that kill cancer cells upon PD-1 blockade therapy, it is hard to imagine that PD-L1–positive tumors would respond to anti–PD-1 or anti–PD-L1 therapy. Therefore, the significance of PD-L1 positivity should be considered in the context of the presence of T cells that are producing interferon-γ, which is an indirect readout of T cells that have the adequate TCRs to recognize cognate tumor antigen expression on cancer cells. In this setting, the likelihood of responding to anti–PD-1 or anti–PD-L1 therapy should be high. But in cases with high-level constitutive PD-L1 expression with a complete absence of a T cell infiltrate and no areas of increased PD-L1 expression colocalized with T cells, then these are likely to be cancers that will not respond to therapy despite having the highest level of PD-L1 expression by IHC.
Patients with PD-L1–negative tumors may still respond to anti–PD-1 or anti–PD-L1 therapy if given a combinatorial treatment that is able to bring T cells into tumors. This is highlighted by the experience combining the anti–PD-1 nivolumab with the anti–CTLA-4 ipilimumab, where the benefit of the combined therapy is most noticeable in patients with baseline PD-L1–negative tumors (Wolchok et al., 2013; Larkin et al., 2015; Postow et al., 2015). This is likely because blocking CTLA-4 results in a marked diversification of the peripheral TCR repertoire (Cha et al., 2014; Robert et al., 2014) and increased T cell infiltration in tumors, even though a minority of patients have objective responses (Huang et al., 2011). It is reasonable to think that the T cell infiltrate induced by CTLA-4 blockade would result in reactive PD-L1 expression by cancer cells, and only when both immune checkpoints are blocked simultaneously would there be a high clinical response rate. Another scenario where patients with tumors scored as PD-L1–negative would respond to anti–PD-1/L1 therapy is that it was a false negative testing. There are multiple reasons for a false negative PD-L1 test, including analyzing archival samples where PD-L1 may have been degraded, having a small sample that does not include an area of invasive margin where T cells may have induced PD-L1 expression, or tumors with marked heterogeneity with different results in different metastatic sites. It would be preferable to always base the analysis of PD-L1 on a recent biopsy having collected a representative amount of a metastatic lesion.
Finally, the significance of a PD-L1–negative tumor would be very different in the case of a cancer that has a genetic event leading to inability to express PD-L1. It is unlikely that this genetic event would be truncations in PD-L1 itself, as this would not provide an advantage (and would be a liability) to the cancer cells. But lack of PD-L1 expression in response to interferon-γ through interferon receptor pathway mutations can be envisioned to lead to negative PD-L1 tumors that have the advantage of not responding to interferon-γ to increase antigen presentation, T cell attraction through chemokines, and antiproliferative effects of interferons (Zaretsky et al., 2016). These would be cases of an absolute negative PD-L1 tumor, which must be escaping immune surveillance through another checkpoint or immune escape mechanism. Therefore, it could not be corrected by bringing in interferon-γ–producing T cells and would be unlikely to respond even to combination immunotherapies.
Conclusions
Expression of PD-L1 is an important variable when analyzing results of therapy with anti–PD-1/L1 antibodies for cancer. PD-L1 positivity is only desirable in the context of treatment targeting the PD-1–PD-L1 interaction, as in the absence of this therapy it may be a mechanisms of immune escape that is only beneficial to the cancer cells. In the future, it is likely that rational decisions on the use of anti–PD-1/L1 antibody therapy alone or in combination will be based on the assessment of the presence or absence of tumor antigen–specific T cells that are inhibited by PD-L1 expression by cancer cells.
Acknowledgments
A. Ribas is supported by National Cancer Institute/National Institutes of Health grants R35 CA197633, P01 CA168585, 1U54 CA199090, and R01 CA170689, the Parker Institute for Cancer Immunotherapy, the Ressler Family Foundation, the Dr. Robert Vigen Memorial Fund, the Grimaldi Family Fund, the Samuels Family Fund, the Garcia-Corsini Family Fund, and Stand Up To Cancer – Cancer Research Institute (SU2C-CRI) Cancer Immunology Dream Team Translational Research Grant (SU2C-AACR-DT1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research (AACR). S. Hu-Lieskovan is supported by a Career Development Award from the American Society of Clinical Oncology (ASCO), a Tower Cancer Research Foundation Grant, a Dr. Charles Coltman Fellowship Award from The Hope Foundation, and a UCLA KL2 Award.
A. Ribas is a co-inventor in a patent regarding the prediction of responses to anti–PD-1 therapy based on analyses of CD8 and PD-L1 expression in tumors that is licensed to Acteris Inc. (South San Francisco, CA) and of an invention report on the role of JAK1/2 mutations in primary and acquired resistance to anti–PD-1 therapy. S. Hu-Lieskovan declares no competing financial interests in this work.
Footnotes
Abbreviations used:
IHC
immunohistochemistry
UTR
untranslated region
References
- Akbay E.A., Koyama S., Carretero J., Altabef A., Tchaicha J.H., Christensen C.L., Mikse O.R., Cherniack A.D., Beauchamp E.M., Pugh T.J., et al. . 2013. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 3:1355–1363. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell S.M., Lesokhin A.M., Borrello I., Halwani A., Scott E.C., Gutierrez M., Schuster S.J., Millenson M.M., Cattry D., Freeman G.J., et al. . 2015. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 372:311–319. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C., Brown I., Peterson A.C., Spiotto M., Iwai Y., Honjo T., and Gajewski T.F.. 2004. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 64:1140–1145. 10.1158/0008-5472.CAN-03-3259 [DOI] [PubMed] [Google Scholar]
- Casey S.C., Tong L., Li Y., Do R., Walz S., Fitzgerald K.N., Gouw A.M., Baylot V., Gütgemann I., Eilers M., and Felsher D.W.. 2016. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 352:227–231. 10.1126/science.aac9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha E., Klinger M., Hou Y., Cummings C., Ribas A., Faham M., and Fong L.. 2014. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 6:238ra70 10.1126/scitranslmed.3008211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorand R.D., Nthale J., Myers J.T., Barkauskas D.S., Avril S., Chirieleison S.M., Pareek T.K., Abbott D.W., Stearns D.S., Letterio J.J., et al. . 2016. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 353:399–403. 10.1126/science.aae0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G.P., Sheehan K.C., Old L.J., and Schreiber R.D.. 2005. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 65:3447–3453. 10.1158/0008-5472.CAN-04-4316 [DOI] [PubMed] [Google Scholar]
- Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. KEYNOTE-001 Investigators . 2015. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372:2018–2028. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- Green M.R., Monti S., Rodig S.J., Juszczynski P., Currie T., O’Donnell E., Chapuy B., Takeyama K., Neuberg D., Golub T.R., et al. . 2010. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 116:3268–3277. 10.1182/blood-2010-05-282780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N., et al. . 2014. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 515:563–567. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R.R., Jalil J., Economou J.S., Chmielowski B., Koya R.C., Mok S., Sazegar H., Seja E., Villanueva A., Gomez-Navarro J., et al. . 2011. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin. Cancer Res. 17:4101–4109. 10.1158/1078-0432.CCR-11-0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilie M., Hofman V., Dietel M., Soria J.C., and Hofman P.. 2016. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 468:511–525. 10.1007/s00428-016-1910-4 [DOI] [PubMed] [Google Scholar]
- Juszczynski P., Ouyang J., Monti S., Rodig S.J., Takeyama K., Abramson J., Chen W., Kutok J.L., Rabinovich G.A., and Shipp M.A.. 2007. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA. 104:13134–13139. 10.1073/pnas.0706017104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.H., Shankaran V., Dighe A.S., Stockert E., Aguet M., Old L.J., and Schreiber R.D.. 1998. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 95:7556–7561. 10.1073/pnas.95.13.7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Shiraishi Y., Takeda Y., Sakata S., Matsumoto M., Nagano S., Maeda T., Nagata Y., Kitanaka A., Mizuno S., et al. . 2016. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 534:402–406. 10.1038/nature18294 [DOI] [PubMed] [Google Scholar]
- Kim J., Myers A.C., Chen L., Pardoll D.M., Truong-Tran Q.A., Lane A.P., McDyer J.F., Fortuno L., and Schleimer R.P.. 2005. Constitutive and inducible expression of b7 family of ligands by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 33:280–289. 10.1165/rcmb.2004-0129OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. . 2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastwika K.J., Wilson W. III, Li Q.K., Norris J., Xu H., Ghazarian S.R., Kitagawa H., Kawabata S., Taube J.M., Yao S., et al. . 2016. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 76:227–238. 10.1158/0008-5472.CAN-14-3362 [DOI] [PubMed] [Google Scholar]
- Lee S.J., Jang B.C., Lee S.W., Yang Y.I., Suh S.I., Park Y.M., Oh S., Shin J.G., Yao S., Chen L., and Choi I.H.. 2006. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-γ-induced upregulation of B7-H1 (CD274). FEBS Lett. 580:755–762. 10.1016/j.febslet.2005.12.093 [DOI] [PubMed] [Google Scholar]
- Liang S.C., Latchman Y.E., Buhlmann J.E., Tomczak M.F., Horwitz B.H., Freeman G.J., and Sharpe A.H.. 2003. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 33:2706–2716. 10.1002/eji.200324228 [DOI] [PubMed] [Google Scholar]
- Loke P., and Allison J.P.. 2003. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA. 100:5336–5341. 10.1073/pnas.0931259100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini G., Narvaiza I., Martinez-Cruz L.A., Arina A., Barajas M., Galofré J.C., Qian C., Mato J.M., Prieto J., and Melero I.. 2003. Pancreatic cancer escape variants that evade immunogene therapy through loss of sensitivity to IFNγ-induced apoptosis. Gene Ther. 10:1067–1078. 10.1038/sj.gt.3301957 [DOI] [PubMed] [Google Scholar]
- McDermott D.F., Sosman J.A., Sznol M., Massard C., Gordon M.S., Hamid O., Powderly J.D., Infante J.R., Fassò M., Wang Y.V., et al. . 2016. Atezolizumab, an anti-Programmed Death-Ligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 34:833–842. 10.1200/JCO.2015.63.7421 [DOI] [PubMed] [Google Scholar]
- Nghiem P.T., Bhatia S., Lipson E.J., Kudchadkar R.R., Miller N.J., Annamalai L., Berry S., Chartash E.K., Daud A., Fling S.P., et al. . 2016. PD-1 blockade with Pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 374:2542–2552. 10.1056/NEJMoa1603702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ock C.Y., Keam B., Kim S., Lee J.S., Kim M., Kim T.M., Jeon Y.K., Kim D.W., Chung D.H., and Heo D.S.. 2016. Pan-Cancer Immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration. Clin. Cancer Res. 22:2261–2270. 10.1158/1078-0432.CCR-15-2834 [DOI] [PubMed] [Google Scholar]
- Pardoll D.M. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 12:252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa A.T., Waldron J.S., Panner A., Crane C.A., Parney I.F., Barry J.J., Cachola K.E., Murray J.C., Tihan T., Jensen M.C., et al. . 2007. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13:84–88. 10.1038/nm1517 [DOI] [PubMed] [Google Scholar]
- Postow M.A., Chesney J., Pavlick A.C., Robert C., Grossmann K., McDermott D., Linette G.P., Meyer N., Giguere J.K., Agarwala S.S., et al. . 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372:2006–2017. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C., Bellmunt J., Burris H.A., Petrylak D.P., Teng S.L., et al. . 2014. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 515:558–562. 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. KEYNOTE-024 Investigators . 2016. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375:1823–1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- Ribas A. 2015. Adaptive immune resistance: How cancer protects from immune attack. Cancer Discov. 5:915–919. 10.1158/2159-8290.CD-15-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., and Tumeh P.C.. 2014. The future of cancer therapy: selecting patients likely to respond to PD1/L1 blockade. Clin. Cancer Res. 20:4982–4984. 10.1158/1078-0432.CCR-14-0933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Hamid O., Daud A., Hodi F.S., Wolchok J.D., Kefford R., Joshua A.M., Patnaik A., Hwu W.J., Weber J.S., et al. . 2016. Association of Pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 315:1600–1609. 10.1001/jama.2016.4059 [DOI] [PubMed] [Google Scholar]
- Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. . 2015a Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372:320–330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. KEYNOTE-006 investigators . 2015b Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372:2521–2532. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- Robert L., Tsoi J., Wang X., Emerson R., Homet B., Chodon T., Mok S., Huang R.R., Cochran A.J., Comin-Anduix B., et al. . 2014. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin. Cancer Res. 20:2424–2432. 10.1158/1078-0432.CCR-13-2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney M.S., Shukla S.A., Wu C.J., Getz G., and Hacohen N.. 2015. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 160:48–61. 10.1016/j.cell.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.S., Zaretsky J.M., Escuin-Ordinas H., Garcia-Diaz A., Hu-Lieskovan S., Kalbasi A., Grasso C.S., Hugo W., Sandoval S., Torrejon D.Y., et al. . 2016. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 10.1158/2159-8290.CD-16-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznol M., and Chen L.. 2013. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin. Cancer Res. 19:1021–1034. 10.1158/1078-0432.CCR-12-2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J.M., Anders R.A., Young G.D., Xu H., Sharma R., McMiller T.L., Chen S., Klein A.P., Pardoll D.M., Topalian S.L., and Chen L.. 2012. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 4:127ra37 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng M.W., Ngiow S.F., Ribas A., and Smyth M.J.. 2015. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 75:2139–2145. 10.1158/0008-5472.CAN-15-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. . 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V., et al. . 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 515:568–571. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.S., D’Angelo S.P., Minor D., Hodi F.S., Gutzmer R., Neyns B., Hoeller C., Khushalani N.I., Miller W.H. Jr., Lao C.D., et al. . 2015. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16:375–384. 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- Weber J.S., Gibney G., Sullivan R.J., Sosman J.A., Slingluff C.L. Jr., Lawrence D.P., Logan T.F., Schuchter L.M., Nair S., Fecher L., et al. . 2016. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 17:943–955. 10.1016/S1470-2045(16)30126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M., Segal N.H., Ariyan C.E., Gordon R.A., Reed K., et al. . 2013. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369:122–133. 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., et al. . 2016. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375:819–829. 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]