Temporal Modulation of an Autoprotease Is Crucial for Replication and Pathogenicity of an RNA Virus (original) (raw)
Abstract
Pestiviruses belong to the family Flaviviridae, and their genome is a single-stranded RNA of positive polarity encoding one large polyprotein which is further processed into mature proteins. Noncytopathogenic (noncp) strains of the pestivirus bovine viral diarrhea virus (BVDV) can establish persistent infection. In persistently infected animals, noncp BVDVs occasionally acquire mutations in viral nonstructural protein 2 (NS2) that give rise to cytopathogenic (cp) BVDV variants, and, eventually, lead to the onset of lethal disease. A molecular marker of cp BVDV infection is a high-level expression of the replicative NS3 protease/helicase that together with NS2 is derived from NS2-3. Here, we present evidence for NS2-3 autoprocessing by a newly identified cysteine protease in NS2 that is distantly related to the NS2-3 autoprotease of hepatitis C and GB viruses. The vital role of this autoprotease in BVDV infection was established, implying an essential function for NS3 in pestiviral RNA replication which cannot be supplied by its NS2-3 precursor. Accordingly, and contrary to a current paradigm, we detected almost complete cleavage of NS2-3 in noncp BVDV at early hours of infection. At 6 to 9 h postinfection, NS2-3 autoprocessing diminished to barely detectable levels for noncp BVDV but decreased only moderately for cp BVDV. Viral RNA synthesis rates strictly correlated with different NS3 levels in noncp and cp BVDV-infected cells, implicating the NS2 autoprotease in RNA replication control. The biotype-specific modulation of NS2-3 autoprocessing indicates a crucial role of the NS2 autoprotease in the pathogenicity of BVDV.
Pestiviruses are animal pathogens that are recognized as a separate genus of the family Flaviviridae, which also includes the genera Flavivirus and Hepacivirus (hepatitis C viruses [HCV]), as well as the unassigned GB viruses (32). Pestiviruses are widely used as a surrogate model for studying HCV, which grows poorly in available cell culture systems. Persistent HCV infections are a major cause of liver cirrhosis and hepatocellular carcinoma in humans worldwide.
The pestiviral genome is a positive-stranded RNA of 12.3 kb. It is translated into a large polyprotein, which is cotranslationally and posttranslationally processed by viral and cellular proteases. The order of proteins in the polyprotein is NH2-Npro-C-Erns-E1-E2-p7-NS2-3-NS4A-NS4B-NS5A-NS5B-COOH. The autoprotease Npro generates its C terminus and the N terminus of the downstream core protein C. The proteolytic releases of the structural glycoproteins Erns (RNase secreted), E1, E2, and p7 are mediated by cellular signal peptidases. The nonstructural protein 4A (NS4A)-dependent chymotrypsin-like serine protease in NS3 mediates processing in the NS region downstream of NS3 (32). The mechanism of NS2-3 cleavage was hitherto unknown and is the subject of this study (see below).
The pestivirus bovine viral diarrhea virus (BVDV) can establish lifelong persistent infections in animals, which become the primary sources for the horizontal spread of the virus (3). One prerequisite of viral persistence is a diaplacental infection of a bovine fetus in conjunction with an acquired immunotolerance against the infecting virus. BVDV strains that cause persistent infections are noncytopathogenic (noncp) in cell culture. During persistence, noncp BVDV strains occasionally mutate into cytopathogenic (cp) BVDV strains, and together the two strains trigger the development of lethal mucosal disease in the infected animals (37). In contrast to their noncp parents, the cp BVDV strains are not able to establish persistent infection. Accordingly, BVDV pathogenesis as well as spread of the virus is determined by the viral biotype, cp or noncp.
The molecular mechanism of biotype control of BVDV is not yet understood, although the level of NS3 accumulation, involving NS2-3 cleavage in many BVDV isolates, seems to be of crucial importance (37). In noncp BVDV-infected cells, only uncleaved NS2-3 has been detected so far (37, 41). In contrast, all cp BVDV strains, which are derived from noncp parents through a mutation(s) in various regions of the viral genome, efficiently express NS3 (32, 37). For BVDV strain CP7, its cp biotype and associated efficient cleavage of NS2-3 were linked to the cp-specific insertion of a unique 9-amino-acid (9-aa) peptide into NS2 (36). When this insertion was reconstructed in the noncp BVDV background, it was sufficient to induce efficient NS2-3 processing (51). This processing does not involve proteolytic activity of the NS3 serine protease (28, 37, 51), and accordingly, the NS2-3 cleavage site differs significantly from sites processed by the NS3 protease (28, 32, 35).
NS2-3 processing in viruses of two other genera of the family Flaviviridae is based on different mechanisms. In the genus Flavivirus, the NS2B-dependent serine protease in NS3 mediates NS2-3 processing. In contrast, a viral cysteine autoprotease residing in NS2 and the N-terminal part of NS3 mediates NS2-3 cleavage of hepaciviruses (32). The hepaciviral NS2 seems to provide a cysteine-dependent proteolytic activity while the NS3 N-terminal domain, which encompasses an essential noncatalytic Zn-binding (ZnB) site, plays an accessory role (17, 19, 25). This role does not involve NS3-associated proteolytic activity, and accordingly, the hepaciviral NS2-3 cleavage site does not match the consensus of the NS3-processed cleavage sites (25).
In this report, combining the results of bioinformatics, molecular genetics, and biochemical analyses of BVDV, we present evidence that NS2 is an autoprotease. A model for pestivirus NS2-3 processing, resembling the mechanism employed by hepaciviruses, consistently explains the available and newly obtained data. We unravel modulation of NS2-3 processing over the course of BVDV infection with a profound biotype-specific amplitude and correlation with RNA synthesis. The NS2 protease is further shown to be crucial for both noncp and cp BVDV biotypes.
MATERIALS AND METHODS
Bioinformatics analysis.
Protein sequences were retrieved from GenBank. Amino acid sequence alignments were generated with ClustalX, version 1.81 (52), and MACAW (48) programs assisted by Blosum position-based weight matrices (24) and were processed for presentation with GeneDoc (38).
Cells and viruses.
Madin-Darby bovine kidney (MDBK) cells and BHK-21 cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum. Cells were maintained at 37°C and 5% CO2. Vaccinia virus modified virus Ankara (MVA)-T7pol (49) was generously provided by G. Sutter (GSF, Oberschleiβheim, Germany). BVDV strains CP7 and NCP7 were described previously (11).
BVDV infection.
Cells were infected with BVDV at a multiplicity of infection (MOI) of 10 for 1 h. For metabolic labeling, cells were incubated for 30 min with DMEM without cysteine and methionine (label medium) at 37°C prior to the addition of 1 ml of label medium containing 280 μCi of [35S]methionine-cysteine ([35S]-ProMix; Amersham Biosciences, Freiburg, Germany) to a 2-cm dish with 106 cells; protein expression was allowed to proceed for 1 h at 37°C.
In vitro transcription and electroporation.
The infectious cDNA clones of BVDV CP7 (pCP7-5A) (4) and NCP7 (pNCP7-5A) (5) have been described previously. After linearization of 3 μg of plasmid DNA with SmaI at the 3′ end of the cDNA, RNA was transcribed with the MAXIscript SP6 kit (Ambion, Huntingdon, United Kingdom) without DNase digestion according to the protocol of the manufacturer. All transcripts were prepared in parallel, and the amount of RNA was estimated by agarose gel electrophoresis. Similar amounts of each RNA (about one-third of each transcript) were used to electroporate one-third of the MDBK cells from a confluent 10-cm dish as previously described (50).
Expression plasmids.
pCITE (Novagen, Madison, Wis.) encompasses the internal ribosomal entry site of encephalomyocarditis virus downstream of the T7 RNA polymerase promoter. pC/E2-4A and pN/E2-4A have been described previously (45). Mutations were introduced by the QuikChange method (Stratagene, Heidelberg, Germany). The following constructs are based on pCITE and code for the indicated amino acids of BVDV CP7; the amino acid positions refer to those of BVDV strain SD-1 (13): for pC/E2-NS3/1645GST, amino acids (aa) 693 to 1645, followed by glutathione _S_-transferase (GST); for pflagNS2-3/1645GST, pflagNS2-3/1599GST, pflagNS2-3/1596GST, and pflagNS2-3/1595GST, aa 1137 to 1645, 1137 to 1599, 1137 to 1596, and 1137 to 1595, respectively, preceded by the peptide MDYKDDDDKL (including the flag epitope) and followed by GST; for p1210/NS2-3/1645GST and p1272/NS2-3/1645GST, aa 1210 to 1645 and 1272 to 1645, respectively, followed by GST.
Transient expression with the T7-vaccinia virus system.
BHK-21 cells (106 cells in a six-well dish) were infected with MVA-T7pol at an MOI of 5 in medium lacking fetal calf serum for 1 h at 37°C. For transfection of plasmid DNA (2 μg), Superfect was applied (QIAGEN, Hilden, Germany).
Radioactive labeling of proteins in BHK-21 cells.
At 2 h posttransfection of plasmid DNA, cells were incubated for 30 min in label medium (lacking cysteine and methionine) at 37°C. After addition of 1 ml of label medium containing 70 μCi of [35S]methionine-cysteine ([35S]-ProMix; Amersham Biosciences) to a 2-cm dish with 106 cells, protein expression was allowed to proceed for 4 h at 37°C. Cells were lysed in RIPA buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% [vol/vol] NP-40, 1% [wt/vol] deoxycholate, 0.1% [wt/vol] sodium dodecyl sulfate [SDS], and 0.5 mM PefablocSC [Merck, Darmstadt, Germany]).
Radioimmunoprecipitation (RIP).
Protein A-Sepharose (Sigma-Aldrich, Taufkirchen, Germany) and RIPA buffer were used for RIP (45).
Radiosequencing.
A pCITE-based plasmid, which encodes the flag epitope NS2, and 7 aa of NS3 of BVDV strain CP7 (aa 1137 to 1596) followed by the peptide Met-Leu-Thr-Met-Ala-Met and GST, was used for MVA-T7pol-based expression. For protein expression, 106 BHK-21 cells were used and labeled with 500 μCi of 35S-Met (Amersham Biosciences) for 4 h; the cell lysate was processed by RIP with anti-GST monoclonal antibody (MAb). Following SDS-polyacrylamide gel electrophoresis (PAGE) and transfer onto a Sequi-Blot polyvinylidene difluoride membrane (Bio-Rad, Munich, Germany), the protein was subjected to automated Edman degradation in an Applied Biosystems, Inc., model 473A protein sequencer, and the obtained fractions were analyzed with a β counter.
SDS-PAGE and immunoblotting.
Proteins were separated in polyacrylamide-Tricine gels (8, 10, or 12% polyacrylamide) (47). After SDS-PAGE, proteins were transferred onto a nitrocellulose membrane (Optitran BA-S83 reinforced NC; Schleicher & Schuell, Düren, Germany); the membrane was blocked with 3% (wt/vol) dried skim milk in phosphate-buffered saline with 0.05% (vol/vol) Tween 20. For antigen detection, peroxidase-coupled species-specific secondary antibodies and Renaissance Western Blot Chemiluminescence Reagent Plus (NEN Life Sciences, Boston, Mass.) were applied.
Quantification of NS2-3 cleavage efficiencies.
Radioactivity of proteins in the dried SDS-PAGE gels was determined by phosphorimaging. Cleavage efficiency was calculated as the quotient of the signals of free NS3 and total NS3 (free NS3 signals plus the NS2-3 signal, considering also the label in NS2); for evaluation of the protease mutants, the cleavage rate of wild-type (wt) CP7 was set to 100% (see Fig. 3).
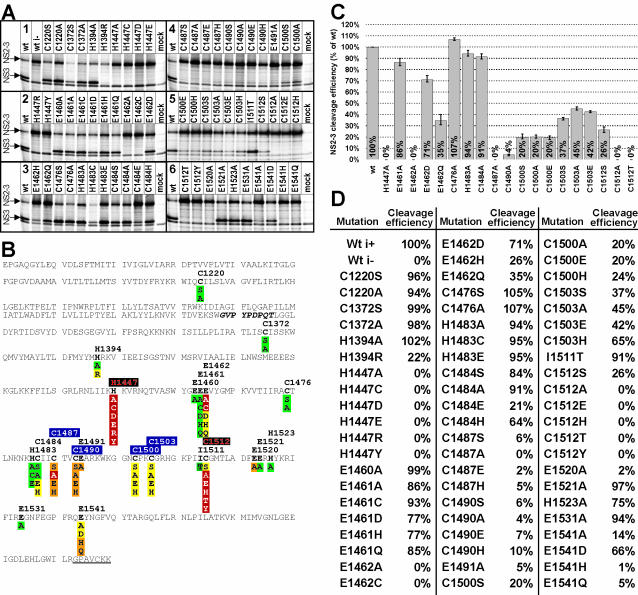
FIG. 3.
Effect of single-amino-acid exchanges in NS2 of cp BVDV strain CP7 on NS2-3 cleavage efficiency. Wt i− indicates wt CP7 with the cp-specific insertion deleted. The numbers indicate amino acid residues based on those of strain BVDV SD-1. (A) RIP analysis. Metabolically labeled NS2-3 was isolated from BHK-21 cells by RIP, application of an NS3-specific MAb, and further analysis by SDS-PAGE and phosphorimaging. Cleavage of CP7 wt NS2-3 was set at 100%. The order of the six blocks is indicated by numbers. (B) Summary of the results presented in panel A. Shown is the NS2 amino acid sequence, followed by seven amino acids of NS3; the NS3-derived amino acids are underlined. The indicated NS2-3 cleavage efficiency is based on a single- or triple-quantified experiment. Color code for cleavage (percentage of wt CP7): red, no NS3 visible; orange, <10%; yellow, 10 to 75%; green, >75%. The 9-aa insertion is indicated by italics. Putative functions of residues according to the experimental results are indicated by colors and shading of residue numbers: red characters on black background, catalytic; white characters on blue background, coordination of Zn2+ ion. (C) NS2-3 cleavage efficiency, based on the results of three quantified experiments. Error bars reach from the lowest to the highest values measured. (D) Quantification of NS2-3 cleavage efficiency based one or three (see panel C legend) independent experiments.
RNA preparation, gel electrophoresis, and Northern blotting.
Total RNA from MDBK cells was prepared with a NucleoSpin RNA II kit (Macherey & Nagel, Düren, Germany). For Northern blot analyses, 5 μg of each RNA was glyoxylated, separated on 1% agarose gels containing 3.7% formaldehyde, and transferred to Duralon-UV membranes (Stratagene). For hybridization, an [α-32P]dCTP-labeled cDNA probe encompassing nucleotides 5171 to 5888 of BVDV CP7 (51) was used. The probe, derived from a bovine cDNA encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH), had a length of about 300 bp and was the kind gift of C. Grassmann (Institut für Virologie, Giessen, Germany). Probes were labeled with a nick translation kit (Amersham Biosciences). Further details have been described previously (51).
Antibodies and antisera.
The antiflag tag and anti-GST MAbs were purchased from Sigma-Aldrich. For the detection of NS3, mouse MAb 8.12.7 (10) was used. Species-specific secondary antibodies were purchased from Dianova (Hamburg, Germany).
Metabolic labeling of viral RNA.
A total of 106 MDBK cells in a 2-cm dish were infected with BVDV CP7 or NCP7 at an MOI of 10. Prior to metabolic labeling for 4 h with 300 μCi of [32P]orthophosphate (Amersham Biosciences), cells were incubated for 30 min with phosphate-free DMEM (Sigma) containing 2 μg of dactinomycin (Sigma-Aldrich)/ml to inhibit RNA synthesis. Total cellular RNA was purified, and 5 μg of each RNA was separated by denaturing agarose gel electrophoresis. A full-length RNA of BVDV CP7 transcribed in the presence of [32P]UTP served as a size marker (data not shown). The gels were dried on Duralon-UV membranes (Stratagene) and analyzed by autoradiography and phosphorimaging.
Virus titration and immunofluorescence
End point titration was done with four replicates on MDBK cells, and the 50% tissue culture infective dose (TCID50) was determined (5). The intracellular synthesis of virus-specific proteins postinfection or posttransfection was monitored by indirect immunofluorescence (IF) analysis with MAb 8.12.7 directed against NS3 of BVDV (10) and a secondary cyanogen-3-labeled antibody as described previously (45).
RESULTS
Bioinformatics analysis predicts pestiviral NS2 to be a cysteine autoprotease distantly related to hepaciviral NS2-3 autoprotease.
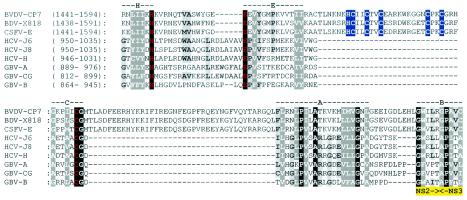
To gain a first insight into the mechanism of NS2-3 cleavage, pestiviral sequences were examined for elements associated with NS2-3 processing in the two other Flaviviridae genera (see the introduction). Our bioinformatics _Flaviviridae_-wide analysis of NS2-3 proteins predicts a multidomain organization for pestiviral NS2; the C-terminal-most domain of NS2 was found to be distantly related to NS2 of hepaciviruses and GB viruses (Fig. 1 and data not shown). Strikingly, the intergenus conservation in NS2 includes three residues, His, Glu, and Cys (Fig. 1); these residues are essential for NS2-3 autoprocessing in hepaciviruses and were implicated in catalysis of this cleavage (17, 19, 25). They correspond to H1447, E1462, and C1512 of noncp BVDV (Fig. 1). In addition, one Pro, one Ala, and three Gly residues are also conserved. The same linear arrangement of the catalytic triad was previously found in picornaviral cysteine proteases with chymotrypsin-like folds, which have otherwise no statistically significant similarity with NS2 (17).
FIG. 1.
The NS2 protease domain is conserved between hepaciviruses and pestiviruses. Fragments of polyproteins (locations are indicated in parentheses) including the C-terminal part of NS2 and five N-terminal amino acid residues of NS3 of a representative set of pestiviruses, hepaciviruses, and GB virus were aligned (see Materials and Methods). The alignment was subsequently manually adjusted to maximize similarity (gaps are indicated by dashes). The Gibbs sampler (29) of the MACAW workbench (48) was used to assess the similarity between pestivirus BVDV CP7 and three hepaciviruses that share <30% identical residues. Blocks A (1.4e−08) and B (5.1e−04) were statistically significant with the NS2-3 protein and the interblock A-NS3-catalytic-Ser searching spaces, respectively. Blocks H, E, and C are named after respective (putative) catalytic residues of NS2 (His, Glu, and Cys) and were recognized in hepaciviruses and, subsequently, in pestiviruses with the catalytic residues of HCV NS2 as anchors. Note that a region separating blocks C and A hosts cellular insertions of variable sizes in some cp BVDV isolates (37) (not shown) supporting this alignment. The position of the NS2-3 cleavage site is indicated below the alignment in yellow. Red characters, (putative) catalytic His, Glu, and Cys residues; blue background, putative Zn2+-coordinating residues of the ZnB site; black background, invariant residues; dark gray background, residues conserved to 100% (see similarity groups) or invariant residues in 80% of the positions; light gray background, residues conserved in not less than 60% of the positions. Amino acid similarity group members: D, N, Q, and E; K, R, and H; F, Y, and W; A and G; S and T; A, C, L, I, V, M, F, and Y. The experimental data (see Results) did not support the putative functions indicated for E1461 (red) and H1483 and C1484 (blue background). Virus names and respective NCBI protein identification numbers are as follows: BVDV-CP7, BVDV strain CP7 (1518836); BDV, border disease virus X818 (20198946); CSFV-E, classical swine fever virus strain Eystrup (12657942); GBV-A, GB virus A (1096574); GBV-B, GB virus B (9628102); GBV-CG, GB virus C-hepatitis G virus (4426796); HCV-J6, HCV isolate HC-J6 (221651); HCV-J8, HCV isolate HC-J8 (221609); HCV-H, HCV isolate H (329738). Numbers in parentheses in the alignment of BVDV-CP7 correspond to amino acid sequences of BVDV strain SD-1 (289507).
Upstream and downstream of the predicted catalytic C1512 residue, two unique sequence blocks are evident in pestiviral NS2. The upstream insertion of 30 aa includes five Cys and one His that are conserved among all known pestivirus isolates; these residues are organized in a linear fashion, suggesting the presence of a mononuclear ZnB site (12). Accordingly, two amino acid pairs may coordinate a Zn2+ ion: candidate members for the N-terminal pair are H1483 or C1484, and C1487, and C1490, while C1500 and C1503 may form the C-terminal pair (Fig. 1). This putative NS2 ZnB site of pestiviruses might take over the role of the NS3 ZnB site unique to hepaciviruses and essential for their NS2-3 autoprocessing (17, 19, 25). The downstream additional sequence block in pestiviral NS2 has a length of 41 aa and is enriched in hydrophilic residues. Finally, a striking conservation was observed between pestiviruses, hepaciviruses, and GB viruses in the region adjacent to and encompassing the C-terminal residues of NS2 and the first two residues of NS3 (Fig. 1, blocks A and B).
We concluded from this analysis that pestiviruses may employ a variant of the mechanism of NS2-3 autoprocessing conserved among hepaciviruses. Our model predicts NS2-3 cleavage by an NS2-associated NS3-independent cysteine autoprotease in pestiviruses.
BVDV CP7 NS2-3 processing proceeds at the conserved 1589Arg-Gly1590 bond and does not depend on NS3.
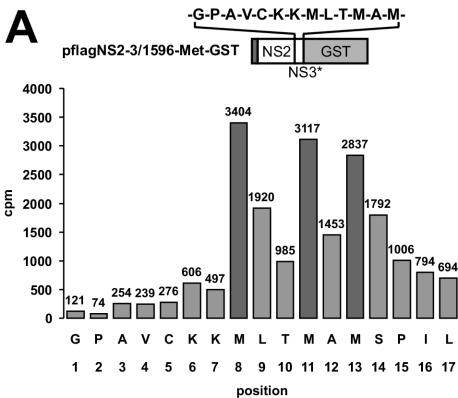
The model presented above was tested experimentally with the E2-NS4A polyprotein region encoded by cp BVDV strain CP7 (see the introduction). Our attempts to express this fragment in Escherichia coli failed, likely due to the toxicity of the highly hydrophobic NS2 (data not shown). Therefore, we switched to transient expression of this polyprotein fragment with the T7-vaccinia virus, which has already been proven to be well suited (51). For cp BVDV strain Oregon, the NS2-3 cleavage was previously shown to proceed at the conserved 1589Arg-Gly1590 junction (Fig. 1) (28). By N-terminal sequencing of NS3, we determined that BVDV CP7 NS2-3 processing occurs at the same position in our surrogate system (Fig. 2A). When either P1 or P1′ residues were replaced by Pro, NS2-3 cleavage was abolished (Fig. 2C).
FIG. 2.
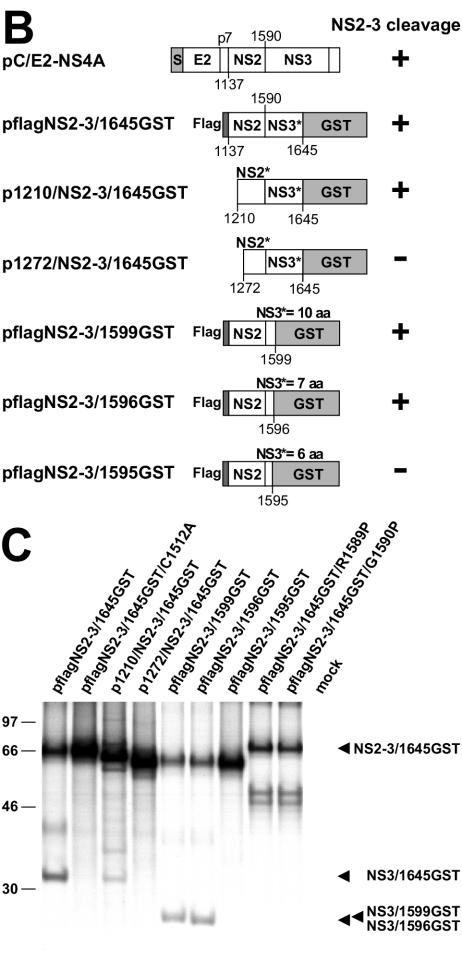
Determinants of NS2-3 processing. (A) Radiosequencing. The diagram depicts the amount of radioactivity released by each cycle of Edman degradation of the partially purified NS3/1596-Met-GST. The amino acid sequence encoded by flagNS2-3/1596-Met-GST in the region of the NS2-3 cleavage site was aligned to the fractions with respect to the methionine residues. The cDNA construct used for protein expression is shown above the diagram; the amino acid sequence downstream of aa 1589 is shown above pflagNS2-3/1596-Met-GST (the number corresponds to amino acid sequences in BVDV strain SD-1); the N-terminal 7 aa of NS3 are followed by a sequence that includes three methionine residues. (B) Scheme of the expression constructs. Bars symbolize proteins; numbers below the bars indicate the amino acid positions in the BVDV polyprotein (the numbers correspond to amino acids in BVDV strain SD-1): 1137, N terminus of NS2; 1590, N terminus of NS3. Truncated proteins are indicated by asterisks. S, signal peptide preceding E2; flag, flag epitope. (C) NS2-3 cleavage studied by transient expression in metabolically labeled BHK-21 cells; the transfected plasmids are indicated above the lanes. For RIP, a GST-specific antibody was applied, and precipitated proteins were analyzed by SDS-PAGE and autoradiography. Arrows indicate the positions of NS2-3/1645GST, NS3/1645GST, NS3/1599GST, and NS3/1596GST.
To determine a minimal, processing-competent part of NS2-3, a series of NS2-3 derivatives truncated from either the N or C terminus and fused with GST at the C terminus were tested (Fig. 2B). The NS2-3/NS3 derivatives were isolated by RIP with a GST-specific antibody and analyzed by SDS-PAGE (Fig. 2C). According to this analysis, NS2 followed by seven (or more) residues of NS3 was efficiently cleaved, while a construct encompassing 6 aa of NS3 showed no processing (Fig. 2C). The N-terminal truncation of NS2 by 73 aa significantly reduced NS2-3 processing, and a deletion of the N-terminal 135 aa abolished NS2-3 cleavage (Fig. 2C). These results extend and refine previous analyses with cp BVDV strain Oregon (28). According to our findings, only a minimal part of NS3 immediately adjacent to the cleavage site and virtually the entire NS2, including its hydrophobic part, are important for NS2-3 cleavage. These observations are in full agreement with the alignment model (Fig. 1).
The predicted active-site residues of the putative NS2 protease are essential for NS2-3 autoprocessing of cp BVDV CP7 in a surrogate system.
Next, we determined the effect of single-amino-acid substitutions in NS2 on NS2-3 cleavage efficiency. Nine putative active-site or ZnB residues plus 12 other (control) Cys, His, and Glu residues were replaced with different residues including Ala at each tested position, generating 64 mutants in total (Fig. 3). At 11 positions, the effect of the Ala substitution on NS2-3 cleavage was small or negligible (>75% of wt activity was retained); at 3 positions, one Glu and two Cys residues, the effect was moderate (at least 10% of wt activity was retained), and at 7 positions that included three Cys, one His, and three Glu residues the effect was most prominent (<10% of wt or no activity). These effects were further quantitatively verified in three independent analyses of 19 mutants with replacements at 11 selected positions, including putative catalytic residues His1447 and Cys1512, two Glu residues, and candidate His or Cys residues of the predicted ZnB site (Fig. 3C). Upon expression of E2-NS4A with several mutations (H1447A, H1447C, H1447D, H1447E, H1447R, and H1447Y; E1462A and E1462C; C1487A; C1512A, C1512E, C1512H, C1512T, and C1512Y), no NS3 could be detected; for all C1490 mutants, very inefficient NS2-3 cleavage was observed. Importantly, the predicted catalytic Cys1512 partially tolerated only a Ser substitution out of six replacements tested (Fig. 3); similar observations were reported for the Cys nucleophile of proven proteases (23, 30).
This mutagenesis analysis supports the relationship between hepaciviral and pestiviral NS2 (Fig. 1). It refines the pestiviral NS2 protease model and confirms the pivotal, putative catalytic role for His1447 and Cys1512 in the protease, and it favors Cys1487, Cys1490, Cys1500, and Cys1503 as the most plausible candidates for coordination of a Zn2+ ion in the putative ZnB site (Fig. 3).
The NS2 protease is vital for RNA replication of BVDV CP7.
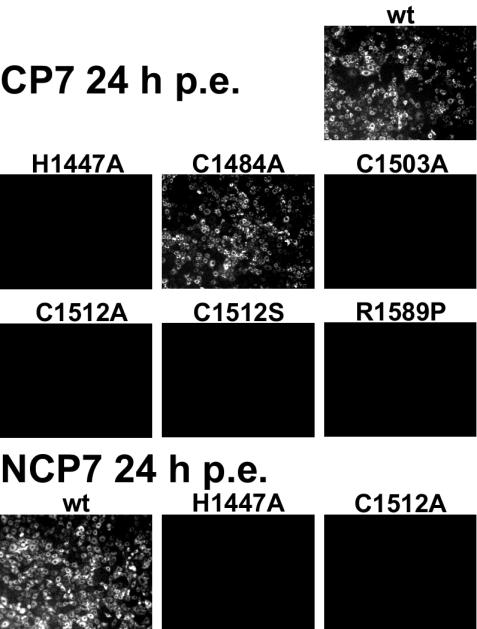
To determine the significance of the NS2 protease for pestiviral replication, three mutations at the catalytic His1447 and Cys1512 residues, two mutations at Cys residues located either in the putative ZnB site (Cys1503) or its vicinity (Cys1484), and a mutation at the P1 position of the NS2-3 cleavage site (Arg1589) were introduced into the BVDV CP7 genome. The effects of these mutations were monitored by IF analysis with an MAb specific for NS3 (also recognizing the NS3 moiety in NS2-3) at 24, 48 and 72 h postelectroporation (p.e.) of MDBK cells with cDNA transcripts. According to previous studies, the IF-mediated detection of NS3 is indicative of viral RNA replication (8, 34, 36). In addition, development of a cytopathic effect was monitored, and the titer of infectious progeny virus was determined in the cell supernatants harvested at 48 h p.e.
Most cells electroporated with CP7 wt RNA were positive for viral antigen at 24 h p.e. (Fig. 4). The cytopathic effect became clearly visible at 48 h p.e. (data not shown). The virus titer reached 1.1 × 106 TCID50 per ml in the cell supernatant at 48 h p.e. In contrast, no signs of virus replication were observed upon electroporation of transcripts encompassing active-site mutations C1512A and H1447A (no NS2-3 cleavage in the T7-vaccinia system) and C1512S (26% of wt NS2-3 cleavage) (Fig. 4 and data not shown).
FIG. 4.
IF analysis of MDBK cells 24 h p.e. with RNA transcribed from full-length cDNA clones of BVDV strains CP7 and NCP7. The wt and mutants indicated above the individual pictures were characterized. A representative part of each dish is shown; primary magnification, ×100.
All three mutants carrying replacements of residues other than the catalytic ones were replication competent, although the negative effect of the mutations on viral replication varied widely and was proportional to the reduction of NS2-3 cleavage observed in the T7-vaccinia system. Almost all cells electroporated with the C1484A transcript (91% of wt NS2-3 cleavage in vitro) were positive by the IF test at 24 h p.e. (Fig. 4), and virus progeny yielded 2.5 × 106 TCID50/ml at 48 h p.e. In one of three independent experiments with C1503A transcripts (45% of wt NS2-3 cleavage), few antigen-positive cells were observed at 24 h p.e. (data not shown). Interestingly, even the cleavage-impaired R1589P mutant proved to be quasi-infectious, using this term as defined by Gmyl et al. (16), as NS3 was detected in 20 to 40 cells per dish at 24 h p.e. (data not shown). In all three cDNA clones derived from independent reverse transcription-PCR analyses of the latter cells, a reversion of the mutated residue aa 1589 back to the wt Arg codon was observed. A possible contamination by wt CP7 virus was ruled out by the presence of a silent genetic marker in the revertant that was originally introduced along with the R1589P mutation (data not shown).
These results show that the predicted NS2 protease is essential for RNA replication of cp BVDV CP7 and support the catalytic role of residues His1447 and Cys1512. Moreover, this experiment strongly implied that uncleaved NS2-3 cannot functionally replace its cleavage products (see Discussion).
Evidence for the NS2 protease activity in noncp BVDV NCP7.
Cleavage of noncp BVDV NS2-3 has not yet been observed. Our model (Fig. 1), however, suggested that the NS2 autoprotease may be active in all pestiviruses, including noncp BVDV. Since this protease is vital for BVDV CP7, its inactivation is expected to be deleterious for BVDV NCP7 as well.
To verify the essentiality of the NS2 protease in the replication of noncp BVDV, Ala replacements of the catalytic His1447 and Cys1512 were introduced into the cDNA of BVDV NCP7 and mutated, and wt RNA transcripts were electroporated into MDBK cells. Upon electroporation of wt RNA, the IF assay indicated viral replication at 24, 48, and 72 h p.e. (Fig. 4 and data not shown) and virus progeny with a titer of 1.6 × 105 TCID50/ml were detected in the supernatant at 48 h p.e. In contrast, in cells electroporated with the mutated NCP7 transcripts no viral replication was detected at 24, 48, and 72 h p.e. (Fig. 4 and data not shown); no virus progeny were detected in the cell supernatants throughout the observation period (data not shown). These data strongly suggest that the NS2 protease is also essential for noncp BVDV.
NS2-3 autoprocessing in BVDV-infected cells is temporally downregulated with a biotype-specific amplitude.
The findings depicted above implicated an essential role of NS2-3 cleavage in the replication of noncp BVDV and challenged a present paradigm, according to which these viruses express no NS3 (37, 41). These observations prompted us to reevaluate the generation of NS3 in noncp BVDV-infected cells, including, in contrast to previous studies, that in the early hours postinfection (p.i.).
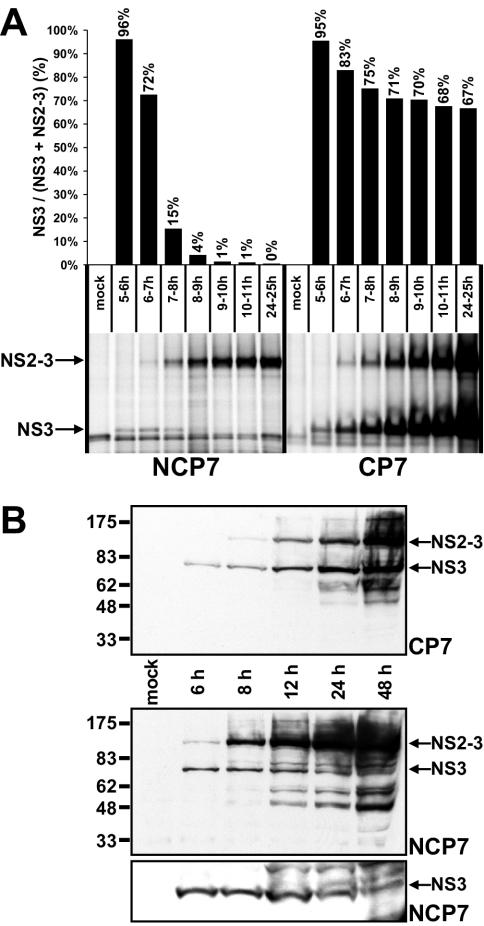
To investigate the kinetics of NS2-3 and NS3 appearance, cells infected by either BVDV CP7 or NCP7 with an MOI of 10 were metabolically labeled for 1-h periods from 5 to 10 h p.i. and, in addition, from 24 to 25 h p.i. To determine the NS3/(NS3 + NS2-3) ratio, both proteins were precipitated from the cell lysates by RIP with an NS3-specific MAb and further analyzed by SDS-PAGE followed by phosphorimaging (Fig. 5A). Remarkably, NS3 but almost no NS2-3 was detected in CP7-infected cells and, for the first time, in NCP7-infected cells labeled from 5 to 6 h p.i. The combined amounts of NS3 plus NS2-3 steadily increased throughout infection with biotype-specific cleavage kinetics of the precursor. In NCP7-infected cells, the rate of NS2-3 cleavage decreased sharply from highly efficient (96% at 5 to 6 h p.i.) to barely detectable (from 4% to below the limit of detection at 8 to 9 and 24 to 25 h p.i., respectively). In contrast for cp BVDV CP7, only a moderate decrease in the efficiency of NS2-3 processing from about 95% at 5 to 6 h p.i. to a stable high level of about 70% at 8 to 9 or 24 to 25 h p.i., respectively, was observed (Fig. 5A). This analysis identified a 3-h window between 6 and 9 h p.i. as the critical phase of infection when NS2-3 autoprocessing efficiency decreases; most importantly, downregulation of NS2-3 cleavage occurs with a biotype-specific amplitude.
FIG. 5.
NS2-3 cleavage in cells infected with noncp BVDV NCP7 and cp BVDV CP7. NS3 and NS2-3 are marked with arrows. (A) RIP analysis of MDBK cells infected with strain NCP7 (left) or CP7 (right) after metabolic labeling with [35S]methionine-cysteine for the indicated time periods p.i. The diagram above indicates the NS2-3 cleavage rate as [NS3/(NS3 + NS2-3)], with values in percentages; values were obtained by phosphorimager analysis. (B) Western blot analysis of MDBK cells infected with strain CP7 (top panel) or NCP7 (middle and bottom panels) with an NS3/NS2-3-specific MAb. The lysate separated in each lane represents about 5 × 104 cells (top and middle) or 5 × 105 cells (bottom). Lysates were prepared at the indicated time points p.i.
To determine the effect of the observed differences in kinetics of NS2-3 processing on accumulation of NS2-3 and NS3, cells harvested at different times postinfection were subjected to Western blot analysis. With CP7-infected cells, NS3 but no NS2-3 was detected throughout the first 6 h p.i. (Fig. 5B). The absolute amount of NS3 increased constantly through the observation period (48 h p.i.); after 8 h p.i., NS2-3 was always detected as well. The NS3/NS2-3 ratio slightly changed throughout the entire period in favor of uncleaved NS2-3 (Fig. 5B and data not shown).
In NCP7-infected cells, NS3 is more prevalent than NS2-3 at 6 h p.i. (Fig. 5B). However, the NS3/NS2-3 ratio is sharply reversed at 8 h p.i., and the prevalence of NS2-3 over NS3 increased further at later time points. To confirm the presence of NS3 beyond 12 h p.i., we analyzed approximately 10 times more cell lysate per sample by longer-run gels (Fig. 5B, compare the middle and bottom panels).
The results of the Western blot analysis supplement the kinetic data obtained for NS2-3 processing and imply that the NS3 turnover rate is slower than its synthesis rate. Collectively, these analyses reveal the presence of NS3 in both cp and noncp BVDV-infected cells and identify a temporal and biotype-specific downregulation of NS2-3 autoprocessing.
Synthesis of viral RNA is correlated with quantity of NS3 in cp and noncp BVDV-infected cells.
The vital character of NS2-3 processing and the detection of NS3 in cells infected with cp and noncp strains of BVDV suggest that reproduction of this virus requires functions provided by the cleavage product(s) of NS2-3, which are not supplied by this precursor. Viral RNA replication requires NS3 but neither NS2 nor NS2-3 (7). The profound and biotype-specific variation of the NS3 levels in the course of infection observed in our experiments therefore suggested that viral RNA synthesis may be modulated accordingly.
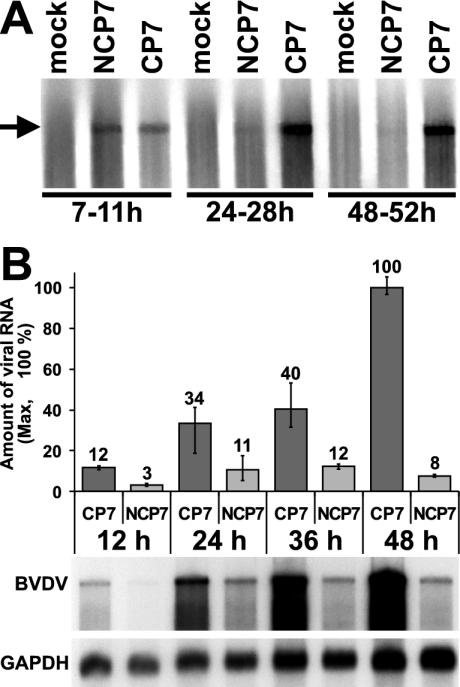
To verify this hypothesis, viral RNA synthesis was characterized in MDBK cells infected at an MOI of 10 with either CP7 or NCP7. Prior to metabolic labeling of the cells with [32P]orthophosphate for 4-h periods starting at 7, 24, and 48 h p.i., cellular RNA synthesis was inhibited by the addition of dactinomycin. Total intracellular RNA was prepared and analyzed by denaturing agarose gel electrophoresis, followed by phosphorimaging and autoradiography (Fig. 6A). Similar RNA synthesis rates were observed for CP7 and NCP7 between 7 and 11 h p.i. At the two later time points, RNA synthesis steadily declined to become barely detectable in NCP7-infected cells but increased in CP7-infected cells. Thus, biotype-specific RNA synthesis correlates with the dynamics of NS3 accumulation (Fig. 5B and 6A).
FIG. 6.
Intracellular accumulation of viral RNA. (A) Analysis of viral RNA synthesis in cells infected with BVDV strain NCP7 or CP7. Metabolically labeled RNA was purified and separated by denaturing agarose gel electrophoresis. Labeling periods in hours are specified below the lanes. The position of a BVDV full-length RNA transcript is indicated by an arrow. (B) (Top) Graph depicting the relative amounts of viral RNA. The amount of BVDV CP7 RNA measured at 48 h p.i. was set at 100%; bovine GAPDH RNA served to standardize the RNA amounts. Values were obtained by phosphorimager analysis. Error bars reach from the lowest to the highest value measured in three independent experiments. (Bottom) Northern blot analysis. Total RNA was prepared from MDBK cells infected with BVDV strains NCP7 or CP7 at the indicated time points p.i. Upon separation by denaturing agarose gel electrophoresis, RNA was blotted onto a membrane and hybridized in parallel against probes specific for BVDV or GAPDH. The entire experiment was done in triplicate, and the data shown is representative of all experiments.
Furthermore, we investigated the viral RNA levels in infected cells by Northern blotting. Total intracellular RNA was prepared from cells harvested at 12, 24, 36, and 48 h p.i. with BVDV strain CP7 or NCP7 at an MOI of 10. These RNAs were hybridized in the Northern blot analysis against 32P-labeled probes specific for BVDV RNA or bovine GAPDH mRNA, the latter serving as a loading control (Fig. 6B). Results of three independent experiments were quantified by phosphorimaging. Throughout the time period observed, the amount of intracellular viral RNA was found to be significantly (approximately 3 to 13 times) lower in the NCP7-infected cells than in CP7-infected cells (Fig. 6B). A three to fourfold increase in the amount of intracellular viral RNA was observed for the viruses of both biotypes between 12 to 24 h p.i. In NCP7-infected cells, the amount of intracellular viral RNA showed no further significant increase over the next 12 h and even decreased slightly thereafter. In contrast, in CP7-infected cells the amount of viral RNA further accumulated by a factor of about 3 between 24 and 48 h p.i. The largest difference in the amounts of intracellular viral RNA (about 13 times) between CP7- and NCP7-infected cells was observed at 48 h p.i. (Fig. 6B). This difference is apparently not reflected in the production of progeny virus (Fig. 7) which suggests that the RNA amount is not the limiting factor for virus production.
FIG. 7.
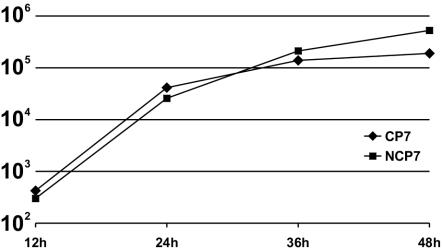
Growth kinetics for BVDV strains CP7 and NCP7. The graph shows mean values derived from three independent experiments. MDBK cells were infected at an MOI of 10. Culture supernatants were harvested at the indicated times p.i. Virus titers are given as log 50% tissue culture infective doses per milliliter. For cDNA-derived viruses, see previously published results (6).
DISCUSSION
In this report, we provide evidence for the NS2 autoprotease responsible for NS2-3 cleavage in BVDV and, by implication, other pestiviruses. Our analysis revealed biotype-specific, temporal modulation of NS2-3 autoprocessing identifying the NS2 autoprotease as a key factor in the control of the pathogenicity of BVDV.
Modular relationship of autoproteases in pestiviral NS2 and hepaciviral NS2-3.
The identification of the long-elusive protease responsible for NS2-3 cleavage was a crucial step toward unraveling the unprecedented modulation of proteolytic processing of BVDV replicase. Our bioinformatics-led mutagenesis analysis, which involved the characterization of 64 mutants in vitro and a dozen selected mutants in vivo, identified a cysteine autoprotease in the C-terminal domain of NS2. This protease is homologous to the proteolytic domain of the NS2-3 autoprotease of hepaciviruses. We confirmed that, like the catalytic Cys and His residues of the HCV NS2-3 protease (27), their counterparts in the BVDV NS2 are indeed essential both for the viability of viral biotypes and for NS2-3 processing in vitro.
Most interesting are the observations of the contrasting phenotypes of the active-site mutant C1512S, which is partially active in vitro but is lethal in vivo, and of the cleavage site mutant R1589P, which shows no detectable cleavage in vitro but is quasiinfectious in vivo. The former phenotype implies that the NS2-3 autoprocessing, like autocleavages in other virus systems (9, 22), may represent an essential rate-limiting aspect of RNA synthesis. Specifically, the mutant NS2 protease with the Ser nucleophile may function with slowed kinetics, severely affecting a downstream process(es) like membrane insertion and/or association, which is known to depend on NS2-3 cleavage in the HCV system (46). Consequently, these effects may interfere with the formation of a functional replication complex and block RNA synthesis and the generation of a (pseudo)revertant(s) entirely. In contrast, a much stronger inhibitory effect of the cleavage site mutation R1589P on NS2-3 cleavage may not prevent the otherwise-intact enzymatic domain from correctly processing the NS2-3 junctions in a few molecules with proper kinetics, resulting in the formation of functional replication complexes. The few complexes produced in this way may be sufficient for the generation of viable revertants (quasiinfectious virus).
Although the mutagenesis analysis supports the bioinformatics-based NS2 model, the identity of a possible third catalytic residue in the NS2 protease remains unresolved. NS2-3 processing in vitro is mildly sensitive to mutations of the Glu1462 residue and tolerates a range of replacements of Glu1461, which is absolutely conserved in NS2 of pestiviruses, hepaciviruses, and GB viruses (Fig. 1). Because of overall poor conservation, we cannot rule out that the generated alignment for the region around Glu1461-Glu1462 will be revised. The role of the Glu1461 counterpart in the HCV NS2-3 autoprotease has also not been firmly established, and future studies must address these issues.
The conservation of the NS2 protease domain in pestiviruses and hepaciviruses was an unexpected observation, since the other essential part of the NS2-3 autoprotease, which resides in the N-terminal part of NS3 and encompasses ∼180 aa in HCV (18, 24), was reduced to no more than 7 aa in BVDV (19, 25). The dependence of the HCV NS2-3 autoprotease on NS3 was previously linked to a noncatalytic ZnB site naturally engineered on the two-beta-barrel chymotrypsin-like fold (17). This ZnB site is located at the side opposite to the active-site cleft of the NS3 serine protease (26, 33). This site may be regarded as a peculiar variant of a Zn finger otherwise found only in 2A cysteine proteases of enteroviruses or rhinoviruses (40, 54) but not in the NS3 protease of pestiviruses. Strikingly, our experimentally backed NS2 alignment features a putative ZnB site, which is critical for NS2-3 autoprocessing and viral replication (Fig. 8), as part of a unique insertion in the pestiviral protease domain.
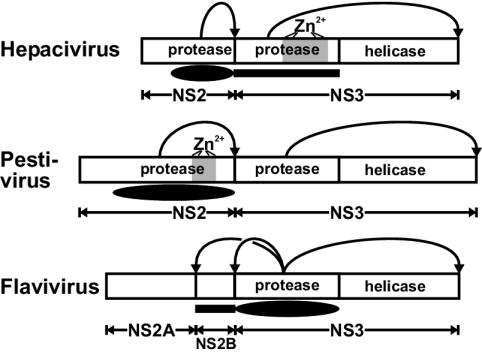
FIG. 8.
NS2-3 cleavage in three genera of the family Flaviviridae. Polyprotein fragments encompassing NS2-3 of the three Flaviviridae genera are depicted schematically. The locations of the helicase and protease domains are indicated; (putative) ZnB motifs (Zn2+) are shaded in gray. The positions of the minimal protease domains capable of cleaving the NS2-3 junction in vitro are shown below the polyproteins by filled ovals (catalytic domains) and rectangles (essential accessory domains). Curved arrows point to the cleavage sites processed by the respective protease.
To fully understand its function, the NS2 ZnB site must be characterized structurally and biochemically. In the meantime, the genus-specific structural requirements of the NS2-3 cleavage in pestiviruses and hepaciviruses could be reconciled as variations of a common mechanism. We suggest that NS2-3 autoprocessing is mediated by a protease associated with the NS2 domain and is assisted by a Zn2+-dependent structure supplied by either NS3 in hepaciviruses or NS2 in pestiviruses. In contrast, flaviviruses employ NS2B to assist the serine protease in NS3 in cleaving the NS2-3 junction (Fig. 8) (32). These genus-based variations in NS2-3 autoprocessing seem sensible from the evolutionary perspective, as hepaciviruses and pestiviruses form sister phylogenetic lineages within the Flaviviridae. This similarity in the mechanism of NS2-3 autoprocessing between BVDV and HCV further strengthens the validity of pestiviruses as a model system to study HCV replication.
Despite continuous efforts, evidence of NS2-3 processing in reticulocyte lysate, in E. coli, and in trans, which was reported for HCV (19, 39, 44), could not be demonstrated for BVDV CP7 or NCP7 (N. Tautz and A. E. Gorbalenya, unpublished data). Aside from purely technical issues, these differences may be genuine and may involve the hydrophobic N-terminal region of NS2, which is only moderately important for NS2-3 cleavage of HCV (39) but is an essential part of the BVDV NS2 protease (Fig. 2C).
Modulation of NS2-3 cleavage efficiency.
It was a paradigm that NS2-3 is cleaved only in cp but not in noncp BVDV-infected cells (10, 37). We were able to demonstrate efficient NS2-3 cleavage in noncp BVDV-infected cells at early time points not included in previous analyses. The observed profound downregulation of the NS2-3 cleavage as early as 9 h p.i. implies that between 6 and 9 h p.i., either a potent activator of the autoprocessing is depleted or an inhibitor of this process is induced or produced.
The CP7-specific insertion in the central region of NS2 obviously interferes with the downregulation of the autoprocessing. Nevertheless, we also observed a downregulation of cleavage in CP7-infected cells between 6 and 9 h p.i., although the resulting cleavage level of about 67% [NS3/(NS3 + NS2-3)] was still high. Interestingly, upon transient expression in BHK-21 cells, e.g., in the context of the E2-NS4A polyprotein fragment, cleavage of noncp BVDV-derived NS2-3 was not detected (36, 51; this report). Under the same conditions, NS2-3 of BVDV CP7 is cleaved, while not fully approximating the level observed in CP7-infected MDBK cells at 9 h p.i. and later. In this context, it is important to note that BHK-21 cells are not natural host cells for BVDV and do not support efficient RNA replication of noncp BVDV even though cell lines can be selected that allow a low-level replication (20). Less-efficient NS2-3 processing under the conditions used for transient expression may thus originate from a shortage of virus- or cell-derived cofactors or the presence of an inhibitor(s). Furthermore, the available data suggest that the 9-aa insertion in NS2 of CP7 switches on NS2-3 autoprocessing and makes it less sensitive to hypothetical cofactors present in BVDV-infected MDBK cells.
With respect to potential cofactors of the pestiviral NS2-3 processing, the identification of a cellular J-domain protein, which interacts with pestiviral NS2 and is capable of promoting NS2-3 cleavage in noncp BVDV-infected cells, may be relevant (45); a possible role of this protein in modulating the NS2 protease is currently under investigation (N. Tautz, unpublished data). Regardless of the identity of the factor, it is evident that BVDV NS2-3 autoprocessing is sensitive to the cellular environment and may be host restricted. The underlying mechanism is an important subject for future studies.
Regulatory effects of NS2-3 autoprocessing in the pestiviral life cycle.
Proteolytic processing of viral polyproteins is a major mechanism regulating the replication of positive-strand RNA viruses. For picornaviruses or the alphavirus Sindbis virus, a pivotal role of differential cleavages or a temporal regulation of certain processing steps on diverse aspects of the viral replication cycle has been established (21, 31, 43).
The temporal modulation of NS2-3 cleavage, especially in noncp BVDV-infected cells, provides a mechanism for regulating the ratio between NS2-3 and its processing products during the course of infection. Intriguingly, when compared to noncp BVDV strains, the high levels of NS3 expressed by cp BVDV correlate with an enhanced accumulation of intracellular viral RNA (6, 34, 53). This is in line with our observation that the intracellular concentration of NS3 strongly correlates with the efficiency of RNA replication (Fig. 5 and 6). NS3, which has protease, helicase, and NTPase activities, was previously shown to be essential for the replication of subgenomic pestiviral RNAs (replicons) encoding viral proteins NS3 to NS5B (7). Since these RNAs replicated efficiently, NS2 and NS2-3 do not play essential roles in positive- or negative-strand RNA synthesis (7). Our finding that NS3 cannot be functionally replaced by uncleaved NS2-3, as well as the strict correlation between NS3 level and efficiency of RNA replication, thus strongly suggest that NS3 but not NS2-3 is an essential component of the viral replication complex, further implicating the NS2 protease in replication control.
While cp BVDV strain CP7 generates NS3 by cleavage of NS2-3, the majority of cp BVDV strains express NS3 from a duplicated genomic region (37). In the respective polyproteins, cellular sequences like ubiquitin are often located directly upstream of NS3. They promote the generation of the authentic N terminus of NS3 by cellular proteases. It is not known whether these viruses also require an active NS2 protease for their replication. After infection with these cp BVDV strains, there is efficient generation of NS3 throughout infection, viral RNA accumulates to high concentrations, and the infected cells undergo apoptosis. Accordingly, efficient generation of NS3 generally correlates with an upregulated synthesis of viral RNA and the cp biotype of the virus. This is in line with our data that show that the concentration of NS3 appears to limit RNA replication of noncp BVDV, which may be crucial for its biotype.
A correlation between increased viral RNA synthesis and viral cytopathogenicity was also described for the picornavirus hepatitis A virus (references 18 and 55 and references therein) and the alphavirus Sindbis virus (1, 14, 15). For natural isolates of BVDV, enhanced viral RNA synthesis was described only for cp strains (6, 34, 53); the only exception to this trend is a cp BVDV-derived noncp virus isolated under selection in tissue culture (42). Although this virus showed high levels of NS3 expression and RNA replication, it exhibited the noncp phenotype. This last observation indicates that the mechanism of cytopathogenicity in vitro is complex and needs further study.
With respect to the viral life cycle, effective processing of NS2-3 shortly after infection appears to be required for efficient RNA replication. At about 8 to 10 h p.i., the production and secretion of newly generated infectious BVDV progeny begins (data not shown). At that time, NS2-3 cleavage efficiency drops sharply, leading to the accumulation of unprocessed NS2-3. It has been observed that uncleaved NS2-3 is essential for the generation of infectious progeny virus (2; Tautz, unpublished). Accordingly, temporal regulation of NS2-3 autoprocessing is most likely necessary for the switch between the phase of highly active viral RNA replication and virus morphogenesis.
NS2-3 cleavage and outcome of infection.
BVDV represents a model system for persistent infections in mammals. noncp BVDV strains have a high prevalence in cattle and persist in a low percentage of cattle worldwide, accompanied by continuous shedding of infectious virus (3). In contrast, cp BVDV strains are unable to establish persistent infections and are therefore almost exclusively isolated from sporadic cases of animals with mucosal disease. In cattle persistently infected with noncp BVDV, the emergence of a cp BVDV variant, like BVDV CP7 analyzed in detail in this study, leads to the onset of lethal disease. The viral biotype is thus a crucial determinant for the pathogenicity of BVDV and the spread of the virus in its host population. In this context, our study strongly suggests that the observed temporal modulation of NS2-3 autoprocessing may be central for the adaptation of BVDV to its animal host. Restriction of this process, as shown here for cp BVDV CP7, is correlated with viral cytopathogenicity and the conversion of persistent infection into lethal disease. The results obtained in the present study therefore contribute to the understanding of the molecular basis for viral persistence and progression to disease.
Acknowledgments
We thank S. Jacobi for excellent technical assistance. We are grateful to M. Ziess and C. Birghan for their contributions in the initial phase of the project and thank J. Ziehbur (Institut für Virologie und Immunologie, Universität Würzburg) for his support in the radiosequencing of proteins. A.E.G. gives special thanks to The Netherlands Organization for Scientific Research (NWO) for support at the initial stage of the project and Willy Spaan (Leiden University) for encouragement.
This study was supported by SFB 535 Invasionsmechanismen und Replikationsstrategien von Krankheitserregern (T.L.) and Graduiertenkolleg 455 Molekulare Veterinärmedizin (A.M.) of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Agapov, E. V., I. Frolov, B. D. Lindenbach, B. M. Pragai, S. Schlesinger, and C. M. Rice. 1998. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc. Natl. Acad. Sci. USA 95**:**12989-12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agapov, E. V., C. L. Murray, I. Frolov, L. Qu, T. M. Myers, and C. M. Rice. 2004. Uncleaved NS2-3 is required for production of infectious bovine viral diarrhea virus. J. Virol. 78**:**2414-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, J. C. 1987. Bovine viral diarrhea virus: a review. J. Am. Vet. Med. Assoc. 190**:**1449-1458. [PubMed] [Google Scholar]
- 4.Baroth, M., M. Orlich, H.-J. Thiel, and P. Becher. 2000. Insertion of cellular NEDD8 coding sequences in a pestivirus. Virology 278**:**456-466. [DOI] [PubMed] [Google Scholar]
- 5.Becher, P., M. Orlich, and H.-J. Thiel. 2000. Mutations in the 5′ nontranslated region of bovine viral diarrhea virus result in altered growth characteristics. J. Virol. 74**:**7884-7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becher, P., M. Orlich, and H.-J. Thiel. 2001. RNA recombination between persisting pestivirus and a vaccine strain: generation of cytopathogenic virus and induction of lethal disease. J. Virol. 75**:**6256-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens, S.-E., C. W. Grassmann, H.-J. Thiel, G. Meyers, and N. Tautz. 1998. Characterization of an autonomous subgenomic pestivirus RNA replicon. J. Virol. 72**:**2364-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behrens, S.-E., L. Tomei, and R. de Francesco. 1996. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 15**:**12-22. [PMC free article] [PubMed] [Google Scholar]
- 9.Birghan, C., E. Mundt, and A. E. Gorbalenya. 2000. A non-canonical lon proteinase lacking the ATPase domain employs the Ser-Lys catalytic dyad to exercise broad control over the life cycle of a double-stranded RNA virus. EMBO J. 19**:**114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corapi, W. V., R. O. Donis, and E. J. Dubovi. 1990. Characterization of a panel of monoclonal antibodies and their use in the study of the antigenic diversity of bovine viral diarrhea virus. Am. J. Vet. Res. 51**:**1388-1394. [PubMed] [Google Scholar]
- 11.Corapi, W. V., R. O. Donis, and E. J. Dubovi. 1988. Monoclonal antibody analyses of cytopathic and noncytopathic viruses from fatal bovine viral diarrhea infections. J. Virol. 62**:**2823-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Moerlooze, L., M. Desport, A. Renard, C. Lecomte, J. Brownlie, and J. A. Martial. 1990. The coding region for the 54-kDa protein of several pestiviruses lacks host insertions but reveals a “zinc finger-like” domain. Virology 177**:**812-815. [DOI] [PubMed] [Google Scholar]
- 13.Deng, R., and K. V. Brock. 1992. Molecular cloning and nucleotide sequence of a pestivirus genome, noncytopathogenic bovine viral diarrhea virus strain SD-1. Virology 191**:**867-879. [DOI] [PubMed] [Google Scholar]
- 14.Dryga, S. A., O. A. Dryga, and S. Schlesinger. 1997. Identification of mutations in a Sindbis virus variant able to establish persistent infection in BHK cells: the importance of a mutation in the nsP2 gene. Virology 228**:**74-83. [DOI] [PubMed] [Google Scholar]
- 15.Frolov, I., E. Agapov, T. A. Hoffman, Jr., B. M. Pragai, M. Lippa, S. Schlesinger, and C. M. Rice. 1999. Selection of RNA replicons capable of persistent noncytopathic replication in mammalian cells. J. Virol. 73**:**3854-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gmyl, A. P., E. V. Pilipenko, S. V. Maslova, G. A. Belov, and V. I. Agol. 1993. Functional and genetic plasticities of the poliovirus genome: quasi-infectious RNAs modified in the 5′-untranslated region yield a variety of pseudorevertants. J. Virol. 67**:**6309-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorbalenya, A. E., and E. J. Snijder. 1996. Viral cysteine proteinases. Perspect. Drug Discov. Des. 6**:**64-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosert, R., D. Egger, and K. Bienz. 2000. A cytopathic and a cell culture adapted hepatitis A virus strain differ in cell killing but not in intracellular membrane rearrangements. Virology 266**:**157-169. [DOI] [PubMed] [Google Scholar]
- 19.Grakoui, A., D. W. McCourt, C. Wychowski, S. M. Feinstone, and C. Rice. 1993. A second hepatitis C virus-encoded proteinase. Proc. Natl. Acad. Sci. USA 90**:**10583-10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grassmann, C. W., O. Isken, N. Tautz, and S. E. Behrens. 2001. Genetic analysis of the pestivirus nonstructural coding region: defects in the NS5A unit can be complemented in trans. J. Virol. 75**:**7791-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin, D. E. 2001. Alphaviruses, p. 917-962. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 22.Hahn, C. S., and J. H. Strauss. 1990. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J. Virol. 64**:**3069-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellen, C. U. T., M. Fäcke, H.-G. Kräusslich, C.-K. Lee, and E. Wimmer. 1991. Characterization of poliovirus 2A protease by mutational analysis: residues required for autocatalytic activity are essential for induction of cleavage of eucaryotic initiation factor 4F polypeptide p220. J. Virol. 65**:**4226-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henikoff, S., and J. G. Henikoff. 1994. Position-based sequence weights. J. Mol. Biol. 243**:**574-578. [DOI] [PubMed] [Google Scholar]
- 25.Hijikata, M., H. Mizushima, T. Akagi, S. Mori, N. Kakiuchi, N. Kato, T. Tanaka, K. Kimura, and K. Shimotohno. 1993. Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J. Virol. 67**:**4665-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, J. L., K. A. Morgenstern, C. Lin, T. Fox, M. D. Dwyer, J. A. Landro, S. P. Chambers, W. Markland, C. A. Lepre, E. T. O'Malley, S. L. Harbeson, C. M. Rice, M. A. Murcko, P. R. Caron, and J. A. Thomson. 1996. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell 87**:**343-355. [DOI] [PubMed] [Google Scholar]
- 27.Kolykhalov, A. A., K. Mihalik, S. M. Feinstone, and C. M. Rice. 2000. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 74**:**2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kümmerer, B., D. Stoll, and G. Meyers. 1998. Bovine viral diarrhea virus strain Oregon: a novel mechanism for processing of NS2-3 based on point mutations. J. Virol. 72**:**4127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence, C. E., S. F. Altschul, M. S. Boguski, J. S. Liu, A. F. Neuwald, and J. C. Wootton. 1993. Detecting subtle sequence signals: a Gibbs sampling strategy for multiple alignment. Science 262**:**208-214. [DOI] [PubMed] [Google Scholar]
- 30.Lawson, M. A., and B. L. Semler. 1991. Poliovirus thiol proteinase 3C can utilize a serine nucleophile within the putative catalytic triad. Proc. Natl. Acad. Sci. USA 63**:**5013-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemm, J. A., T. Rümenapf, E. G. Strauss, J. H. Strauss, and C. M. Rice. 1994. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 13**:**2925-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1042. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 33.Love, R. A., H. E. Parge, J. A. Wickersham, Z. Hostomsky, N. Habuka, E. W. Moomaw, T. Adachi, and Z. Hostomska. 1996. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell 87**:**331-342. [DOI] [PubMed] [Google Scholar]
- 34.Mendez, E., N. Ruggli, M. S. Collett, and C. M. Rice. 1998. Infectious bovine viral diarrhea virus (strain NADL) RNA from stable cDNA clones: a cellular insert determines NS3 production and viral cytopathogenicity. J. Virol. 72**:**4737-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyers, G., D. Stoll, and M. Gunn. 1998. Insertion of a sequence encoding light chain 3 of microtubule-associated proteins 1A and 1B in a pestivirus genome: connection with virus cytopathogenicity and induction of lethal disease in cattle. J. Virol. 72**:**4139-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers, G., N. Tautz, P. Becher, H.-J. Thiel, and B. Kümmerer. 1996. Recovery of cytopathogenic and noncytopathogenic bovine viral diarrhea viruses from cDNA constructs. J. Virol. 70**:**8606-8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyers, G., and H.-J. Thiel. 1996. Molecular characterization of pestiviruses. Adv. Virus Res. 47**:**53-117. [DOI] [PubMed] [Google Scholar]
- 38.Nicholas, K. B., N. H. B. J. Nicholas, and D. W. Deerfield. 1997. GeneDoc: analysis and visualization of genetic variation. EMBNET News 4**:**1-4. [Google Scholar]
- 39.Pallaoro, M., A. Lahm, G. Biasiol, M. Brunetti, C. Nardella, L. Orsatti, F. Bonelli, S. Orru, F. Narjes, and C. Steinkuhler. 2001. Characterization of the hepatitis C virus NS2/3 processing reaction by using a purified precursor protein. J. Virol. 75**:**9939-9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen, J. F., M. M. Cherney, H. D. Liebig, T. Skern, E. Kuechler, and M. N. James. 1999. The structure of the 2A proteinase from a common cold virus: a proteinase responsible for the shut-off of host-cell protein synthesis. EMBO J. 18**:**5463-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pocock, D. H., C. J. Howard, M. C. Clarke, and J. Brownlie. 1987. Variation in the intracellular polypeptide profiles from different isolates of bovine viral diarrhea virus. Arch. Virol. 94**:**43-53. [DOI] [PubMed] [Google Scholar]
- 42.Qu, L., L. K. McMullan, and C. M. Rice. 2001. Isolation and characterization of noncytopathic pestivirus mutants reveals a role for nonstructural protein NS4B in viral cytopathogenicity. J. Virol. 75**:**10651-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Racaniello, V. R. 2001. Picornaviridae: the viruses and their replication, p. 685-722. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 44.Reed, K. E., A. Grakoui, and C. M. Rice. 1995. Hepatitis C virus-encoded NS2-3 protease: cleavage-site mutagenesis and requirements for bimolecular cleavage. J. Virol. 69**:**4127-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinck, G., C. Birghan, T. Harada, G. Meyers, H.-J. Thiel, and N. Tautz. 2001. A cellular J-domain protein modulates polyprotein processing and cytopathogenicity of a pestivirus. J. Virol. 75**:**9470-9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santolini, E., L. Pacini, C. Fipaldini, G. Migliaccio, and N. La Monica. 1995. The NS2 protein of hepatitis C virus is a transmembrane polypeptide. J. Virol. 69**:**7461-7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166**:**368-379. [DOI] [PubMed] [Google Scholar]
- 48.Schuler, G. D., S. F. Altschul, and D. J. Lipman. 1991. A workbench for multiple alignment construction and analysis. Proteins 9**:**180-190. [DOI] [PubMed] [Google Scholar]
- 49.Sutter, G., M. Ohlmann, and V. Erfle. 1995. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 371**:**9-12. [DOI] [PubMed] [Google Scholar]
- 50.Tautz, N., T. Harada, A. Kaiser, G. Rinck, S. E. Behrens, and H.-J. Thiel. 1999. Establishment and characterization of cytopathogenic and noncytopathogenic pestivirus replicons. J. Virol. 73**:**9422-9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tautz, N., G. Meyers, R. Stark, E. J. Dubovi, and H.-J. Thiel. 1996. Cytopathogenicity of a pestivirus correlated with a 27-nucleotide insertion. J. Virol. 70**:**7851-7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25**:**4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vassilev, V. B., and R. O. Donis. 2000. Bovine viral diarrhea virus induced apoptosis correlates with increased intracellular viral RNA accumulation. Virus Res. 69**:**95-107. [DOI] [PubMed] [Google Scholar]
- 54.Yu, S. F., and R. E. Lloyd. 1992. Characterization of the roles of conserved cysteine and histidine residues in poliovirus 2A protease. Virology 186**:**725-735. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, H., S. F. Chao, L. H. Ping, K. Grace, B. Clarke, and S. M. Lemon. 1995. An infectious cDNA clone of a cytopathic hepatitis A virus: genomic regions associated with rapid replication and cytopathic effect. Virology 212**:**686-697. [DOI] [PubMed] [Google Scholar]