Histone Deacetylases 5 and 9 Govern Responsiveness of the Heart to a Subset of Stress Signals and Play Redundant Roles in Heart Development (original) (raw)
Abstract
The adult heart responds to stress signals by hypertrophic growth, which is often accompanied by activation of a fetal cardiac gene program and eventual cardiac demise. We showed previously that histone deacetylase 9 (HDAC9) acts as a suppressor of cardiac hypertrophy and that mice lacking HDAC9 are sensitized to cardiac stress signals. Here we report that mice lacking HDAC5 display a similar cardiac phenotype and develop profoundly enlarged hearts in response to pressure overload resulting from aortic constriction or constitutive cardiac activation of calcineurin, a transducer of cardiac stress signals. In contrast, mice lacking either HDAC5 or HDAC9 show a hypertrophic response to chronic β-adrenergic stimulation identical to that of wild-type littermates, suggesting that these HDACs modulate a specific subset of cardiac stress response pathways. We also show that compound mutant mice lacking both HDAC5 and HDAC9 show a propensity for lethal ventricular septal defects and thin-walled myocardium. These findings reveal central roles for HDACs 5 and 9 in the suppression of a subset of cardiac stress signals as well as redundant functions in the control of cardiac development.
Postnatal growth of the heart occurs primarily through hypertrophy, in which cardiac myocytes increase in size but not in number (reviewed in reference 34). Hypertrophy can occur in response to physiological stimuli, such as exercise, or pathological stimuli, such as myocardial infarction, hypertension, aortic stenosis, or valve dysfunction. While stress-induced hypertrophy serves initially to normalize ventricular wall stress, this form of hypertrophy, when prolonged, can progress to dilated cardiomyopathy and sudden death. Pathological cardiac hypertrophy is a major predictor of human morbidity and mortality and a major cause of heart failure (17, 18, 25).
Numerous intracellular signaling pathways have been implicated in the transduction of hypertrophic signals from the cardiomyocyte cell surface to the nucleus (reviewed in references 2, 8, 22, and 34). Many hypertrophic agonists acting through cell surface receptors coupled to Gαq mobilize intracellular calcium, which activates downstream kinases and the calcium- and calmodulin-dependent phosphatase calcineurin. Activation of these effectors is sufficient and, in many cases, necessary for hypertrophic growth of the heart (14, 31, 35, 49). Elevation of cyclic AMP in response to β-adrenergic agonists also stimulates cardiac hypertrophy via protein kinase A and other downstream effectors (36). The identification of nodal points in hypertrophic signaling pathways and the mechanisms that link signaling in the cytoplasm with changes in gene expression that contribute to maladaptive growth of the heart represent major challenges in the field.
Pathological cardiac hypertrophy is coupled to the activation of a fetal cardiac gene program, which results in the expression of fetal proteins involved in contractility, metabolism, and calcium handling that are incompatible with sustained function of the adult myocardium (19). The myocyte enhancer factor 2 (MEF2) transcription factor activates many fetal cardiac genes and serves as a nuclear endpoint for stress signals in the adult myocardium (reviewed in reference 29). The transcriptional activity of MEF2 is tightly governed by its interaction with histone acetyltransferases (HATs) (39, 44) and histone deacetylases (HDACs) (9, 20, 21, 27, 28, 30, 40, 45-47, 50), which stimulate and suppress transcription, respectively, through their effects on histone acetylation and chromatin configurations. HATs acetylate the conserved amino-terminal tails of nucleosomal histones, resulting in relaxation of chromatin structure and consequent transcriptional activation (13, 37). The gene-activating functions of HATs are counteracted by HDACs, which remove acetate groups from histone tails, promoting chromatin condensation and transcriptional repression (41).
Mammalian HDACs can be classified into multiple classes based on their structure and homology to three Saccharomyces cerevisiae HDACs (41). Class I HDACs (HDACs 1, 2, and 3) are expressed ubiquitously and comprise simply a catalytic domain. The class II HDACs (HDACs 4, 5, 7, and 9) are expressed at the highest levels in the heart, brain, and skeletal muscle and contain a C-terminal catalytic domain and an N-terminal extension that mediates interactions with other transcriptional repressors and activators.
Interaction of MEF2 with class II HDACs silences the expression of MEF2 target genes (20, 21, 27, 28, 30, 40, 45-48, 50). Calcium- and calmodulin-dependent protein kinase and other kinases that are activated by stress signaling in the heart phosphorylate the amino-terminal extensions of class II HDACs, which results in their dissociation from MEF2 and export from the nucleus (27, 28, 45, 47). HATs are then enabled to interact with the HDAC binding region of MEF2 and promote transcription of MEF2 target genes and cardiac hypertrophy (reviewed in references 26 and 43).
Consistent with the notion that class II HDACs suppress pathological cardiac growth, at least in part by modulating MEF2 activity, mice lacking HDAC9 display enhanced hypertrophy and superactivation of MEF2 in response to cardiac stress signals (45). To further explore the functions of class II HDACs in vivo, we generated mice lacking HDAC5. Like HDAC9 mutant mice, these mice spontaneously develop cardiac hypertrophy with age and display cardiomegaly in response to constitutive calcineurin activation or pressure overload due to aortic constriction. In contrast, mice lacking either HDAC5 or HDAC9 display a normal hypertrophic response to chronic β-adrenergic signaling. We also show that compound mutant mice lacking both HDAC5 and HDAC9 are prone to embryonic and early postnatal death from a spectrum of cardiac abnormalities, including ventricular septal defects and thin-walled myocardium. These findings reveal redundant roles for HDACs 5 and 9 as counterregulators of a specific subset of hypertrophic signaling pathways in the adult heart as well as in normal cardiac growth and development during embryogenesis.
MATERIALS AND METHODS
Generation and genotyping of HDAC5 mutant mice.
A 129s6/SvEvTAC mouse genomic bacterial artificial chromosome library (BACPAC Resources) was screened for the HDAC5 gene with a PCR amplification product corresponding to the coding region for the MEF2 binding domain of mouse HDAC5. Two independent HDAC5 genomic clones were mapped by Southern blotting and partial sequencing. The HDAC5 targeting construct was generated with the pN-Z-TK2 vector, which contains a nuclear lacZ cassette and a neomycin resistance gene (kindly provided by R. Palmiter). The ≈1.5-kb 5′ arm was generated by PCR. The ≈6.5-kb 3′ arm was subcloned from a bacterial artificial chromosome clone. The lacZ cDNA and neomycin resistance cassette under control of the PGK promoter were fused in-frame with the 5′ region of exon 3, placing the β-galactosidase reporter gene under the control of the endogenous HDAC5 promoter. The targeting vector was linearized and electroporated into mouse D3 embryonic stem (ES) cells. Correctly targeted ES cell clones were identified by Southern blotting with both 5′ and 3′ probes. Of 361 clones tested, 17 were positive for correct recombination at the HDAC5 locus.
Two independent clones were injected into 3.5-day mouse C57BL/6 blastocytes, and the resulting chimeric males were bred to C57BL/6 females to achieve germ line transmission of the mutant allele. Subsequent genotyping was performed by PCR with the following primers: HD5gt 5′, 5′-CAAGGCCTTGTGCATGCTGGGCTGG-3′; HD5gt3′, 5′-CTGCTCCCGTAGCGCAGGGTCCATG-3′; and LacZ, 3′-GCCCGTTTGAGGGGACGACGACAGTATCG-3′.
Knockout and transgenic mice.
HDAC9 mutant mice (45) and mice bearing the α-myosin heavy chain (MHC)-calcineurin transgene were described previously (31).
Thoracic aorta banding and isoproterenol administration.
Six- to 8-week-old male mice either underwent a sham operation or were subjected to pressure overload induced by thoracic aorta banding as described (12).
For chronic isoproterenol administration, miniosmotic pumps (Alzet, Palo Alto, Calif.) containing isoproterenol or saline vehicle were inserted subcutaneously in the backs of 10- to 12-week-old male mice. The pumps delivered 28 μg of isoproterenol per h per 25 kg of body weight in a 0.9% saline solution or saline solution alone. Mice were sacrificed 7 days later for assessment of cardiac hypertrophy.
Histology.
Hearts were fixed in phosphate-buffered saline-buffered formalin, embedded in paraffin, and sectioned at 5 μm for histological examination. Sections were stained with hematoxylin and eosin and photographed under normal bright-field microscopic conditions. LacZ staining was performed as described (33).
RNA analysis.
Total RNA was isolated from intact hearts with Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA dot blot analysis was performed with 2 μg of total RNA, and transcripts were quantified with a Storm 820 phosphorimager (Molecular Dynamics). For reverse transcription-PCR, total RNA was used as a template for reverse transcriptase and random hexamer primers (Invitrogen). Reverse transcription-PCR was performed under conditions of linearity with respect to input mRNA.
RESULTS
Generation of HDAC5 mutant mice.
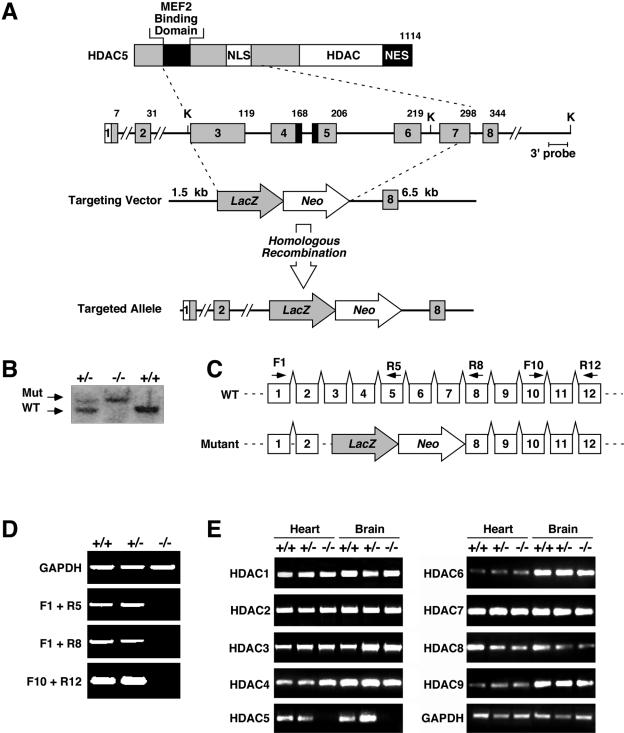
The mouse HDAC5 gene encodes a protein of 1,114 amino acids (Fig. 1A). We introduced a loss-of-function mutation in the HDAC5 gene by replacing coding exons 3 to 7 with a _lacZ_-neomycin resistance cassette through homologous recombination. These exons encode residues 32 to 298 of HDAC5, which include the region of the protein responsible for interaction with MEF2. Targeting of the HDAC5 allele in ES cells was confirmed by Southern blot and PCR of genomic DNA. ES cells heterozygous for the HDAC5 mutation were used to generate chimeric mice, which transmitted the mutation through the germ line (Fig. 1B). Breeding of HDAC5+/− mice in a mixed SvEv129/C57BL/6 background yielded HDAC5−/− mice at the predicted Mendelian ratios (data not shown). Homozygous mutants were viable and fertile and showed no abnormalities at early ages (data not shown).
FIG. 1.
Targeting the mouse HDAC5 gene. (A) A diagram of the HDAC5 protein is shown above a portion of the mouse HDAC5 locus encompassing coding exons 1 to 8. Amino acid numbers are shown above the exons. NES, nuclear export sequence; NLS, nuclear localization sequence. In the targeting vector, a nuclear lacZ reporter was inserted in-frame with exon 3. Homologous recombination resulted in deletion of exons 3 to 7, which encompass the MEF2 binding domain and nuclear localization sequence. The structure of the targeted allele is shown. K, KpnI sites. (B) Southern blot analysis of genomic DNA from mice of the indicated genotypes. A 500-bp DNA fragment downstream of 3′ arm sequences was used as to probe KpnI-digested tail DNA. Wild-type (WT) and mutant (Mut) bands of 8.5 and 15.5 kb, respectively, are shown. (C and D) RNA from hearts of mice of the indicated genotypes was analyzed by RT-PCR. The positions of the primers used for RT-PCR are shown above the corresponding exons of the wild-type and mutant HDAC5 alleles (panel C). No functional HDAC5 mRNA was detected in homozygous mutants. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts were measured as a control. (E) Transcripts for HDACs 1 to 9 were detected by RT-PCR with RNA isolated from the hearts and brains of mice of the indicated genotypes.
To confirm that the targeted mutation eliminated functional HDAC5, we sequenced the transcript from the mutant allele following RT-PCR. Sequencing showed that exon 2 was spliced to the 5′ end of exon 3, which was linked to the lacZ coding region (data not shown). Using primers representing exons within and surrounding the deleted region of the gene, we were unable to detect HDAC5 transcripts in RNA from the hearts of homozygous mutant mice (Fig. 1D). Thus, the mutant allele is expected to function as a null. We also examined the expression of HDACs 1, 2, 3, 4, 6, 7, 8, and 9 by RT-PCR in hearts and brains from wild-type and mutant mice. Transcripts encoding these HDACs were expressed at normal levels (Fig. 1E), indicating that other HDACs were not upregulated to compensate for the absence of HDAC5.
Expression of lacZ from the targeted HDAC5 allele.
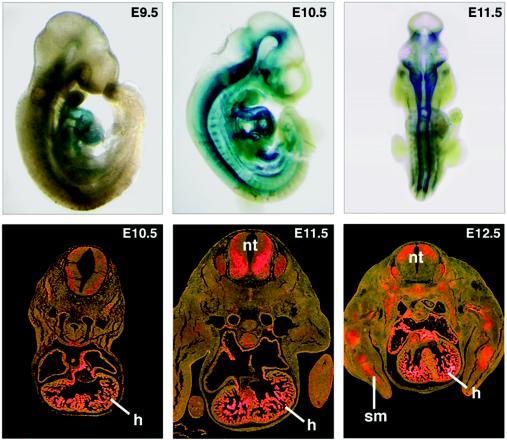
We stained embryos from HDAC5+/− intercrosses for lacZ expression in order to assess the tissue distribution of HDAC5 expression. As shown in Fig. 2, β-galactosidase staining was detected in the looping heart tube at embryonic day 9.5 (E9.5). At E10.5, expression in the heart increased further, and expression was also evident in the ventral region of the neural tube. β-Galactosidase staining was also apparent in a variety of adult tissues, including heart, brain, skeletal muscle, kidney, lung, and liver (data not shown). The expression pattern of β-galactosidase during pre- and postnatal development reflected that of the endogenous HDAC5 gene (data not shown).
FIG. 2.
Expression of lacZ from the targeted HDAC5 allele. Embryos heterozygous for the targeted HDAC5 allele were stained for lacZ expression on the indicated days of embryogenesis. Strong expression of LacZ was seen in the looping heart tube at E9.5 and in the heart and spinal cord at later stages. LacZ expression was also detected in the muscle forming regions of the limbs at E12.5. The lower panels show transverse sections through LacZ-stained embryos visualized in bright field, with LacZ staining indicated in pink. h, heart; nt, neural tube; sm, skeletal muscle.
Enhanced hypertrophy of HDAC5 mutant mice in response to calcineurin activation.
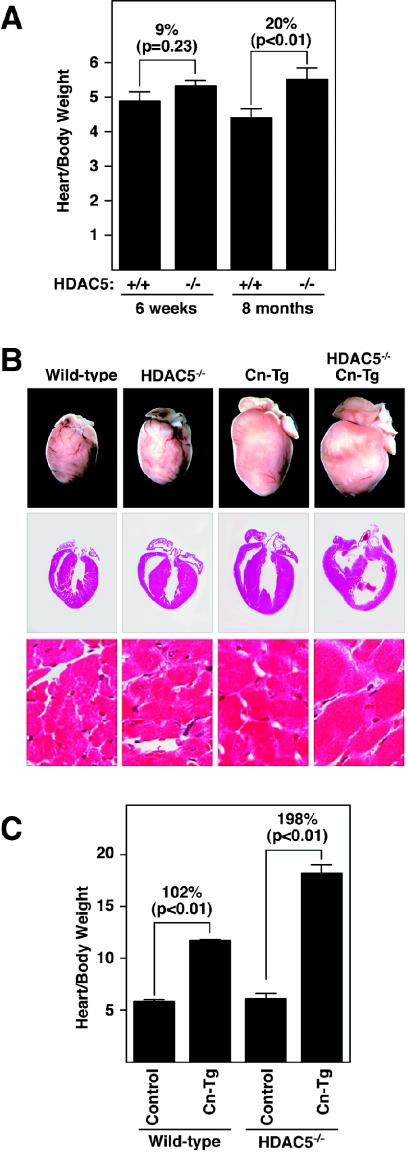
In light of the importance of HDAC9 in the control of cardiac growth (45), we focused on possible cardiac abnormalities in HDAC5 null mice. At 6 weeks of age, the hearts of HDAC5 mutant mice were similar in size to those of wild-type littermates (Fig. 3A). However, by 8 months of age, HDAC5 mutant hearts were 20% larger than normal (P < 0.01). Histological analysis indicated that age-dependent enlargement was due to hypertrophy (data not shown). Kaplan-Meier survival curves showed no difference in the survival of wild-type and HDAC5 mutant mice, indicating that the age-dependent hypertrophy in the homozygous mutants was not fatal.
FIG. 3.
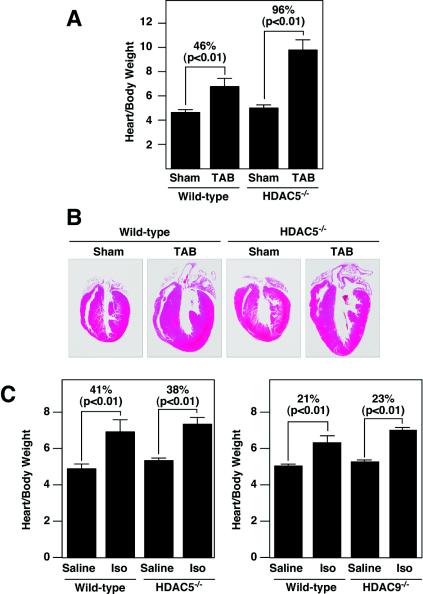
Enhanced hypertrophy in HDAC5 mutant mice. (A) Hearts were dissected from mice of the indicated genotypes at 6 weeks and 8 months of age, and heart weight-to-body weight ratios were determined. Values represent the mean ± standard deviation. The heart sizes of wild-type (n = 4) and HDAC5 mutant (n = 4) mice were similar at 6 weeks, but by 8 months, the hearts of the mutant mice (n = 5) were enlarged compared to those of the wild-type mice (n = 3). (B) HDAC5 mutant mice were bred with mice harboring theαMHC-calcineurin transgene (Cn-Tg). Hearts from 1-month-old mice of the indicated genotypes were isolated (top images), sectioned, and stained with hematoxylin and eosin (bottom images). (C) Heart weight/body weight ratios of mice of wild-type control (n = 6), αMHC-calcineurin transgene (n = 10), HDAC5−/− (n = 5), and HDAC5−/−/ αMHC-calcineurin transgene (n = 4) mice are shown.
To begin to investigate possible abnormalities in the cardiac stress responsiveness of HDAC5 mutant mice, we examined their response to constitutive calcineurin activation by intercrossing them with mice bearing an αMHC-calcineurin transgene that promotes cardiac hypertrophy (31). As shown in Fig. 3B and C, hearts from HDAC5 null mice showed an exaggerated hypertrophic response to activated calcineurin and had achieved a size approximately three times normal by 4 weeks of age. Analysis of histological sections indicated that the increase in cardiac mass in the mutant was due to hypertrophy of cardiac myocytes (Fig. 3B). We detected no evidence of cardiomyocyte hyperplasia in HDAC5 mutant hearts. HDAC5 mutant mice bearing the calcineurin transgene were highly prone to sudden death, and none of these mice survived beyond 8 weeks of age. Calcineurin transgenic mice in the wild-type background also die prematurely but not until at least 12 to 16 weeks of age. Thus, the lack of HDAC5 resulted in enhanced sensitivity to the pathological consequences of cardiac calcineurin signaling.
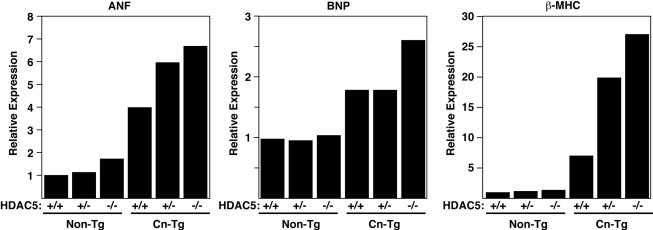
The remarkable hypertrophy in HDAC5 mutant mice was accompanied by a dramatic increase in expression of fetal cardiac genes. As shown in Fig. 4, expression of three representative fetal cardiac genes, ANF, BNP, and β-MHC, in response to calcineurin activation was enhanced in HDAC5 null mice. The ANF and β-MHC genes were particularly sensitive to the HDAC5 gene dose and showed a heightened response to calcineurin even in HDAC5+/− mice.
FIG. 4.
Fetal gene expression in HDAC5 knockout mice. RNA was isolated from the hearts of mice with the indicated genotypes, and expression of fetal cardiac genes was measured by dot blot analysis. Values are expressed as the level of expression of each transcript relative to that in hearts from wild-type mice.
Enhanced hypertrophy in HDAC5 mutant mice in response to thoracic aortic banding.
To further explore the role of HDAC5 in modulation of cardiac growth, we compared the responses of wild-type and HDAC5 mutant mice to thoracic aortic banding, which causes hypertrophy due to increased afterload. Constriction of the thoracic aorta for 21 days in wild-type mice resulted in a 46% increase in heart weight/body weight ratios (Fig. 5A and B). HDAC5 mutant mice showed an exaggerated hypertrophic response to thoracic aortic banding and an increase in heart size of approximately 96% over the same time period. Thus, the hearts of HDAC5 null mice were sensitized to pressure overload, as they were to calcineurin activation.
FIG. 5.
Cardiac responses to thoracic aortic banding and chronic isoproterenol administration. (A and B) Wild-type (non-Tg) and HDAC5 null mice at 6 weeks of age were subjected to thoracic aortic banding (TAB) for 21 days, at which time hearts were dissected and heart weight-to-body weight ratios were determined (A). Values represent the mean ± standard deviation. We tested sham-treated wild-type mice (n = 3), TAB-treated wild-type mice (n = 3), HDAC5−/− sham-treated mice (n = 4), and TAB-treated HDAC5−/− mice (n = 4). (B) Hearts were sectioned and stained with hematoxylin and eosin. (C) Wild-type or HDAC5 and HDAC9 mutant mice at 8 weeks of age were infused with isoproterenol (Iso) or saline alone for 7 days, at which time the hearts were dissected and heart weight-to-body weight ratios were determined. The hypertrophic responses of the HDAC5 and HDAC9 mutant mice were not statistically different from those of wild-type mice. The left panel shows saline-treated wild-type mice (n = 3), isoproterenol-treated wild-type mice (n = 4), sham-treated HDAC5−/− mice (n = 4), and isoproterenol-treated HDAC5−/− mice (n = 6). The right panel shows saline-treated wild-type mice (n = 4), wild-type isoproterenol-treated mice (n = 4), sham-treated HDAC9−/− mice (n = 4), and isoproterenol-treated HDAC9−/− mice (n = 4).
Normal responsiveness of HDAC5 and HDAC9 mutant mice to chronic isoproterenol stimulation.
We also investigated the potential role of HDAC5 in the hypertrophic growth response of the heart to β-adrenergic stimulation, using osmotic minipumps to deliver a chronic isoproterenol stimulus for 7 days. In contrast to the severe hypertrophy seen in the response to calcineurin activation or thoracic aortic banding, wild-type and HDAC5 mutant mice showed comparable responses to isoproterenol stimulation (Fig. 5C). HDAC9 mutant mice, which show an exaggerated response to calcineurin and pressure overload similar to that of HDAC5 mutants (45), also displayed a normal response to isoproterenol infusion (Fig. 5C). These findings suggest that HDAC5 and HDAC9 act selectively within the signaling pathways activated by calcineurin and pressure overload but not in the β-adrenergic signaling pathway.
Cardiac defects in HDAC5/HDAC9 double mutant mice.
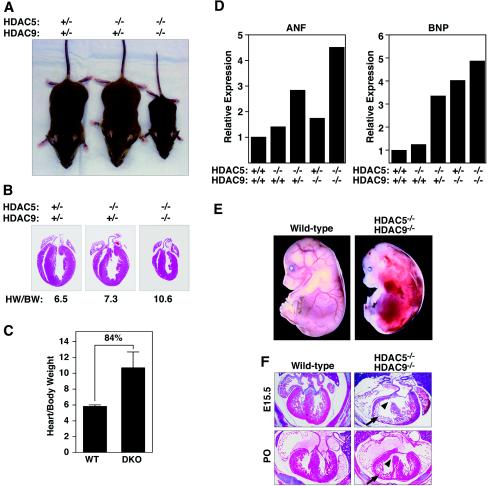
The similarity in the responses of HDAC5 and HDAC9 mutant mice to cardiac stresses suggested that these HDACs played similar roles in the control of cardiac growth. To explore the extent of their functional overlap, we generated compound HDAC5/HDAC9 mutant mice by interbreeding heterozygous mutants. The genotypes of offspring are shown in Table 1. Mice homozygous for either null allele and heterozygous for the other were viable and showed mild cardiac hypertrophy (Fig. 6A and data not shown). In contrast, HDAC5/HDAC9 double null offspring in the mixed C57BL6/129 genetic background were underrepresented at postnatal day 7 and showed severe growth retardation (Fig. 6A and Table 1). The few double mutants that survived to adulthood weighed about one-third as much as wild-type littermates.
TABLE 1.
Genotypes of offspring from intercrosses of HDAC5+/− and HDAC9+/− micea
| Mating no. | Genotype | Observed (no.) | Predicted (%) | Observed (%) | |
|---|---|---|---|---|---|
| HDAC5 | HDAC9 | ||||
| 1 | +/+ | +/+ | 15 | 6.25 | 6 |
| 2 | +/+ | +/− | 39 | 12.5 | 15 |
| 3 | +/+ | −/− | 15 | 6.25 | 6 |
| 4 | +/− | +/+ | 32 | 12.5 | 12 |
| 5 | +/− | +/− | 77 | 25.0 | 29 |
| 6 | +/− | −/− | 33 | 12.5 | 12 |
| 7 | −/− | +/+ | 18 | 6.25 | 7 |
| 8 | −/− | +/− | 33 | 12.5 | 12 |
| 9 | −/− | −/− | 3 | 6.25 | 1 |
FIG. 6.
Growth defects and cardiac abnormalities in HDAC5/HDAC9 double mutant mice. (A) One-month-old mice of the indicated genotypes are shown. The HDAC5/HDAC9 double mutant animals are severely growth retarded. (B) Hearts were dissected from the wild-type and HDAC5/HDAC9 double mutant mice shown in panel A, and heart weight-to-body weight ratios were determined. The double mutant mice had smaller hearts than the wild-type mice, but they were enlarged compared to body weight. Heart weight/body weight (HW/BW) ratios are shown. (C) Hearts were dissected from wild-type (n = 6) and HDAC5/HDAC9 double knockout (DKO) (n = 5) mice at 6 months of age, and heart/body weight ratios were determined. Values represent the mean ± standard deviation. (D) RNA was isolated from the hearts of adult mice with the indicated genotypes, and expression of fetal cardiac genes was measured by dot blot analysis. Values are expressed as the level of expression of each transcript relative to that in hearts from wild-type mice. (E) Wild-type and HDAC5/HDAC9 double mutant embryos at E15.5. The double mutant shows multifocal hemorrhages. (F) Hematoxylin and eosin staining of cardiac sections from wild-type and HDAC5/HDAC9 double mutant mice at E15.5 (top panels) and at birth (P0, bottom panels). Note the ventricular septal defect (arrowhead) and thin-walled myocardium (arrow) in the double mutant.
Analysis of the internal organs of double null mice that survived to 1 month of age did not reveal obvious abnormalities at either the gross organ or histological level. However, while the hearts were smaller than normal, reflecting the overall reduced size of the double mutant mice, as a fraction of overall body weight, the hearts from the double mutants were enlarged compared to those of wild-type littermates (Fig. 6B and C). Moreover, markers of cardiac hypertrophy were upregulated in the hearts of adult double mutant mice (Fig. 6D). One interpretation of these findings is that the lack of HDACs 5 and 9 imposes stress on the heart, which leads to augmentation of cardiac growth. Another interpretation is that the age-dependent hypertrophy seen in mice homozygous for either the HDAC5 or HDAC9 null alleles is accelerated in the absence of both genes. We also cannot rule out the possibility that the heart is less sensitive than the rest of the body to general growth retardation resulting from the lack of HDAC5 and HDAC9.
The underrepresentation of HDAC5/HDAC9 double mutant mice at postnatal day 7 suggested that this genotype caused embryonic or early perinatal lethality with variable penetrance. To establish the time of death of double mutants, we determined the genotypes of litters from timed matings. Double mutants were observed at predicted Mendelian frequencies until E15.5, at which time a subset of embryos displayed hemorrhages throughout the body (Fig. 6E). Thereafter, there was a gradual decline in the frequency of viable double mutants. Embryos that displayed multifocal hemorrhages at E15.5 also had ventricular septal defects and thin ventricular walls (Fig. 6F). Similar defects were seen in a subset of double mutant offspring that survived to birth (Fig. 6E). Overall, 77% of double mutants had ventricular septal defects and about 20% had thin-walled myocardium. However, the 12% of double mutants that survived to adulthood showed hypertrophy but no obvious cardiac malformations.
DISCUSSION
The results of this study demonstrate that HDAC5 acts as an antagonist of a specific set of pathological signaling pathways leading to cardiac hypertrophy. In the absence of HDAC5, the heart becomes profoundly enlarged in response to calcineurin signaling and pressure overload. The cardiac phenotype of HDAC5 mutant mice is remarkably similar to that of HDAC9 mutant mice (45), strongly suggesting that these two HDACs play comparable roles in the control of cardiac growth. HDACs 5 and 9 also appear to play overlapping roles in development of the heart, as evidenced by cardiac malformations that occur in mice lacking both genes but not in mice lacking only one gene or the other.
Suppression of stress-dependent cardiac growth by HDACs 5 and 9.
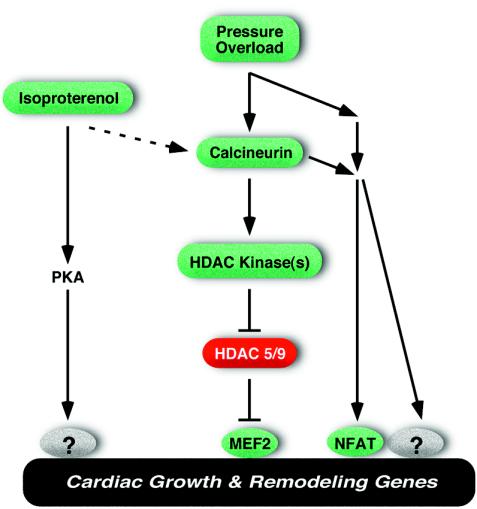
We showed previously that HDAC9 acts as a suppressor of cardiac hypertrophy and that hypertrophic signals inactivate HDAC9 and other class II HDACs through phosphorylation of their regulatory domains (45). Signal-dependent phosphorylation of class II HDACs creates binding sites for 14-3-3 chaperone proteins, which mediate their translocation from the nucleus to the cytoplasm and consequent derepression of the hypertrophic gene program (9, 27, 28). Calcineurin activation and pressure overload both activate a cardiac kinase(s) specific for the regulatory serine residues in class II HDACs (45), supporting a model in which these stress stimuli act, at least in part, by counteracting the growth-inhibitory functions of class II HDACs (Fig. 7). The notion that calcineurin and pressure overload promote hypertrophy through a common signaling pathway is compatible with studies showing that calcineurin is activated in response to pressure overload and that calcineurin inhibition prevents hypertrophy in response to thoracic aorta banding (reviewed in reference 7).
FIG. 7.
Schematic of the roles of HDACs 5 and 9 as antagonists of hypertrophic signaling. PKA, protein kinase A.
In contrast to their sensitized response to calcineurin and pressure overload, mice lacking HDAC5 or HDAC9 did not show a heightened hypertrophic response to chronic administration of isoproterenol. We suggest two possible explanations for this observation. Isoproterenol could induce hypertrophy through a signaling pathway independent of class II HDACs, or isoproterenol stimulation could superactivate an HDAC kinase(s) leading to the complete nuclear export and inactivation of class II HDACs, such that genetic deletion of HDACs 5 and 9 would not further enhance the hypertrophic response to this stimulus. On the contrary, the enhanced sensitivity to calcineurin and pressure overload of mice lacking either HDAC5 or HDAC9 suggests that these stimuli inactivate only a fraction of the entire pool of class II HDACs. Otherwise, if the entire pool of class II HDACs were inactivated in response to these stress stimuli, genetic deletion of HDACs would not be expected to increase the hypertrophic response.
Isoproterenol promotes hypertrophic growth via the β-adrenergic receptor, which stimulates the production of cyclic AMP by adenylyl cyclase with consequent activation of protein kinase A (36). Protein kinase A phosphorylates numerous proteins within the sarcomere and the sarcoplasmic reticulum that control cardiac contractility and calcium handling. The mechanisms by which protein kinase A signaling affects cardiac gene expression are less well understood, but the CREB transcription factor is one protein kinase A target that has been shown to promote cardiac growth (5). Neither cyclic AMP nor activated protein kinase A promotes the phosphorylation or nuclear export of class II HDACs (27). However, several studies have implicated calcineurin signaling in the pathway by which isoproterenol stimulates cardiac hypertrophy. Inhibition of calcineurin activity by elevation in the expression of modulatory calcineurin-interacting protein 1 or other inhibitory proteins, for example, can suppress hypertrophy in response to isoproterenol administration (4, 38). We have also found that isoproterenol efficiently stimulates protein kinase D, which acts as an HDAC nuclear export kinase (R. Vega, E. Olson, and T. McKinsey, unpublished results). Thus, signals emanating from the β-adrenergic receptor may modulate HDAC function through multiple mechanisms (Fig. 7). It should be noted in this regard that many forms of cardiac stress are accompanied by the activation of complex neurohumoral signals that act through interconnected signaling pathways (2, 8, 22, 34). Indeed, failing human hearts have been reported to demonstrate elevated activities of calcium- and calmodulin-dependent protein kinase (15), which phosphorylates HDACs (27, 28, 45), as well as protein kinase A, which does not act directly on HDACs.
While we favor the notion that MEF2 is a critical direct target for class II HDACs in the signaling pathway for cardiac hypertrophy, modulation of HDAC activity can also influence the activity of other transcription factors involved in hypertrophy, such as GATA4 and NFAT, which interact with MEF2 (32, 44). Stress signals that inactivate class II HDACs also activate p300, which serves as a coactivator for the above transcription factors (10, 43). Thus, there are multiple points of crossover among the signaling pathways that govern cardiac growth in response to stress signaling.
Potential roles of class II HDACs in heart development.
Whereas mice lacking either HDAC5 or HDAC9 display normal cardiac structure and function at birth, a high percentage of HDAC5/HDAC9 double mutants die during embryogenesis and the perinatal period from ventricular septal defects and thin ventricular walls, which typically arise from abnormalities in growth and maturation of cardiomyocytes. Both HDAC5 and HDAC9 are expressed in the developing myocardial chambers and interventricular septum during embryogenesis (48; this study).
Given the interaction between class II HDACs and MEF2 and the central role of MEF2 in the control of cardiomyocyte differentiation (29), the developmental cardiac defects in the double mutants may result from superactivation of MEF2 with consequent precocious differentiation and cell cycle withdrawal of cardiomyocytes, causing hypocellularity of the myocardium. In addition, class II HDACs participate in multiprotein repression complexes and modulate the activities of numerous transcription factors involved in myocardial growth, such as the retinoic acid receptor, serum response factor, and myocardin (3, 6, 16, 42; our unpublished results). The absence of HDACs 5 and 9 may therefore affect the activities of other cardiac transcriptional activators and repressors, thereby perturbing the precisely regulated programs of gene expression required for cardiac development.
It is interesting that genetic deletion of either HDAC5 or HDAC9 sensitizes the heart to pathological stress signals, yet developmental effects require the loss of both genes. These differential effects suggest that different types or strengths of signals regulate these HDACs during development and adult heart disease. Alternatively, or in addition, the responsiveness of HDAC5 and HDAC9 to developmental and stress signals could be influenced by other proteins that differ between embryonic and adult hearts.
Control of cell growth and homeostasis by class II HDACs.
Congenital and acquired cardiac disease phenotypes in humans and animal models frequently display variability in penetrance and expressivity, indicative of genetic modifiers. Whereas mice lacking as many as three of the four HDAC5/HDAC9 alleles are normal at an early age, deletion of even one HDAC alleles augments the growth response of the heart to hypertrophic signals. The sensitized phenotypes of HDAC5 and HDAC9 mutant mice demonstrate that heart size depends on class II HDAC gene dosage and point to HDACs 5 and 9 as modifiers of cardiac responses to pathological signals. It will be of interest to determine whether these HDACs modify cardiac disease phenotypes in humans.
The specific role of class II HDACs in a given cell type depends on the spectrum of available transcription factors with which they can interact. In addition to the influence of HDACs 5 and 9 on cardiac growth, we recently found that HDAC4 regulates bone growth and development by repressing chondrocyte hypertrophy (R. Vega and E. Olson, unpublished results). In mice lacking HDAC4, chondrocytes undergo inappropriate hypertrophy, which leads to premature and ectopic mineralization of the cartilaginous skeleton due to activation of the Runx2 transcription factor. Thus, the first three class II HDACs to be analyzed through genetic loss of function in the mouse (HDACs 4, 5, and 9) all result in abnormal hypertrophic phenotypes, suggesting that class II HDACs act as general regulators of cellular hypertrophy during development and disease.
Class II HDACs have also been implicated in cell proliferation and oncogenesis (23). The HDAC5 gene is located on human chromosome 17q21, which has frequently been associated with chromosomal alternations in human cancer (reviewed in reference 24). It will be interesting to determine whether mice lacking HDAC5 or other class II HDACs display a predisposition to tumorigenesis.
The signal responsiveness of class II HDACs provides a mechanism for linking extracellular signals with the genome during cellular transitions in development and disease and offers therapeutic opportunities for manipulating gene expression through the modulation of their regulatory kinases and phosphatases. Such approaches hold great promise for the modification of pathological cardiac growth and gene expression. Given the multiplicity of class I and II HDACs, there has also been interest in the identification of small-molecule inhibitors that can selectively modulate the functions of individual HDAC isoforms. In this regard, the recent finding that HDAC inhibitors block cardiac hypertrophy and thereby mimic the activity of class II HDACs (1, 11, 16) raises interesting questions about the possible antagonistic roles of class I and II HDACs in cardiac growth (1, 11, 16). Analysis of the responsiveness of cells derived from HDAC knockout mice should facilitate the characterization of such inhibitors and their specific enzymatic targets.
Acknowledgments
We thank A. Tizenor for graphics, J. Page for editorial assistance, Jianping Liang and Cheryl Nolan for technical assistance, and Hartmut Weiler (Blood Research Institute, Milwaukee, Wis.) for ES cell targeting.
This work was supported by grants from the NIH, the D. W. Reynolds Clinical Cardiovascular Research Center, the Robert A. Welch Foundation, and the Texas Advanced Technology Program to E.N.O.
REFERENCES
- 1.Antos, C. L., T. A. McKinsey, M. Dreitz, L. M. Hollingsworth, C. L. Zhang, K. Schreiber, H. Rindt, R. J. Gorczynski, and E. N. Olson. 2003. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. J. Biol. Chem. 278**:**28930-28937. [DOI] [PubMed] [Google Scholar]
- 2.Chien, K. R. 1999. Stress pathways and heart failure. Cell 98**:**555-558. [DOI] [PubMed] [Google Scholar]
- 3.Davis, F. J., M. Gupta, B. Camoretti-Mercado, R. J. Schwartz, and M. P. Gupta. 2003. Calcium/calmodulin-dependent protein kinase activates serum response factor transcription activity by its dissociation from histone deacetylase, HDAC4. Implications in cardiac muscle gene regulation during hypertrophy. J. Biol. Chem. 278**:**20047-20058. [DOI] [PubMed] [Google Scholar]
- 4.De Windt, L. J., H. W. Lim, O. F. Bueno, Q. Liang, U. Delling, J. C. Braz, B. J. Glascock, T. F. Kimball, F. del Monte, R. J. Hajjar, and J. D. Molkentin. 2001. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 98**:**3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fentzke, R. C., C. E. Korcarz, R. M. Lang, H. Lin, and J. M. Leiden. 1998. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative CREB transcription factor in the heart. J. Clin. Investig. 101**:**2415-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischle, W., F. Dequiedt, M. J. Hendzel, M. G. Guenther, M. A. Lazar, W. Voelter, and E. Verdin. 2002. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol. Cell 9**:**45-57. [DOI] [PubMed] [Google Scholar]
- 7.Frey, N., H. A. Katus, E. N. Olson, and J. A. Hill. 2004. Hypertrophy of the heart: a new therapeutic target? Circulation 109**:**1580-1589. [DOI] [PubMed] [Google Scholar]
- 8.Frey, N., and E. N. Olson. 2003. Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 65**:**45-79. [DOI] [PubMed] [Google Scholar]
- 9.Grozinger, C. M., and S. L. Schreiber. 2000. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl. Acad. Sci. USA 97**:**7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gusterson, R. J., E. Jazrawi, I. M. Adcock, and D. S. Latchman. 2003. The transcriptional co-activators CREB-binding protein (CBP) and p300 play a critical role in cardiac hypertrophy that is dependent on their histone acetyltransferase activity. J. Biol. Chem. 278**:**6838-6847. [DOI] [PubMed] [Google Scholar]
- 11.Hamamori, Y., and M. D. Schneider. 2003. HATs off to Hop: recruitment of a class I histone deacetylase incriminates a novel transcriptional pathway that opposes cardiac hypertrophy. J. Clin. Investig. 112**:**824-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill, J. A., M. Karimi, W. Kutschke, R. L. Davisson, K. Zimmerman, Z. Wang, R. E. Kerber, and R. M. Weiss. 2000. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation 101**:**2863-2869. [DOI] [PubMed] [Google Scholar]
- 13.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293**:**1074-1080. [DOI] [PubMed] [Google Scholar]
- 14.Kato, T., M. Sano, S. Miyoshi, T. Sato, D. Hakuno, H. Ishida, H. Kinoshita-Nakazawa, K. Fukuda, and S. Ogawa. 2000. Calmodulin kinases II and IV and calcineurin are involved in leukemia inhibitory factor-induced cardiac hypertrophy in rats. Circ. Res. 87**:**937-945. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhefer, U., W. Schmitz, H. Scholz, and J. Neumann. 1999. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc. Res. 42**:**254-261. [DOI] [PubMed] [Google Scholar]
- 16.Kook, H., J. J. Lepore, A. D. Gitler, M. M. Lu, W. Wing-Man Yung, J. Mackay, R. Zhou, V. Ferrari, P. Gruber, and J. A. Epstein. 2003. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J. Clin. Investig. 112**:**863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koren, M. J., R. B. Devereux, P. N. Casale, D. D. Savage, and J. H. Laragh. 1991. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann. Intern. Med. 114**:**345-352. [DOI] [PubMed] [Google Scholar]
- 18.Levy, D., R. J. Garrison, D. D. Savage, W. B. Kannel, and W. P. Castelli. 1990. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 322**:**1561-1566. [DOI] [PubMed] [Google Scholar]
- 19.Lowes, B. D., E. M. Gilbert, W. T. Abraham, W. A. Minobe, P. Larrabee, D. Ferguson, E. E. Wolfel, J. Lindenfeld, T. Tsvetkova, A. D. Robertson, R. A. Quaife, and M. R. Bristow. 2002. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N. Engl. J. Med. 346**:**1357-1365. [DOI] [PubMed] [Google Scholar]
- 20.Lu, J., T. A. McKinsey, R. L. Nicol, and E. N. Olson. 2000. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc. Natl. Acad. Sci. USA 97**:**4070-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, J., T. A. McKinsey, C. L. Zhang, and E. N. Olson. 2000. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol. Cell 6**:**233-244. [DOI] [PubMed] [Google Scholar]
- 22.MacLellan, W. R., and M. D. Schneider. 2000. Genetic dissection of cardiac growth control pathways. Annu. Rev. Physiol. 62**:**289-319. [DOI] [PubMed] [Google Scholar]
- 23.Mahlknecht, U., S. Schnittger, O. G. Ottmann, C. Schoch, M. Mosebach, W. Hiddemann, and D. Hoelzer. 2000. Chromosomal organization and localization of the human histone deacetylase 5 gene (HDAC5). Biochim. Biophys. Acta 1493**:**342-348. [DOI] [PubMed] [Google Scholar]
- 24.Marks, P., R. A. Rifkind, V. M. Richon, R. Breslow, T. Miller, and W. K. Kelly. 2001. Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer 1**:**194-202. [DOI] [PubMed] [Google Scholar]
- 25.Mathew, J., P. Sleight, E. Lonn, D. Johnstone, J. Pogue, Q. Yi, J. Bosch, B. Sussex, J. Probstfield, and S. Yusuf. 2001. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 104**:**1615-1621. [DOI] [PubMed] [Google Scholar]
- 26.McKinsey, T. A., and E. N. Olson. 2004. Cardiac histone acetylation-therapeutic opportunities abound. Trends Genet. 20**:**206-213. [DOI] [PubMed] [Google Scholar]
- 27.McKinsey, T. A., C. L. Zhang, J. Lu, and E. N. Olson. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408**:**106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2000. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA 97**:**14400-14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2002. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 27**:**40-47. [DOI] [PubMed] [Google Scholar]
- 30.Miska, E. A., C. Karlsson, E. Langley, S. J. Nielsen, J. Pines, and T. Kouzarides. 1999. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 18**:**5099-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93**:**215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morin, S., F. Charron, L. Robitaille, and M. Nemer. 2000. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 19**:**2046-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naya, F. J., C. Wu, J. A. Richardson, P. Overbeek, and E. N. Olson. 1999. Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development 126**:**2045-2052. [DOI] [PubMed] [Google Scholar]
- 34.Olson, E. N., and M. D. Schneider. 2003. Sizing up the heart: development redux in disease. Genes Dev. 17**:**1937-1956. [DOI] [PubMed] [Google Scholar]
- 35.Passier, R., H. Zeng, N. Frey, F. J. Naya, R. L. Nicol, T. A. McKinsey, P. Overbeek, J. A. Richardson, S. R. Grant, and E. N. Olson. 2000. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Investig. 105**:**1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rockman, H. A., W. J. Koch, and R. J. Lefkowitz. 2002. Seven-transmembrane-spanning receptors and heart function. Nature 415**:**206-212. [DOI] [PubMed] [Google Scholar]
- 37.Roth, S. Y., J. M. Denu, and C. D. Allis. 2001. Histone acetyltransferases. Annu. Rev. Biochem. 70**:**81-120. [DOI] [PubMed] [Google Scholar]
- 38.Rothermel, B. A., T. A. McKinsey, R. B. Vega, R. L. Nicol, P. Mammen, J. Yang, C. L. Antos, J. M. Shelton, R. Bassel-Duby, E. N. Olson, and R. S. Williams. 2001. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 98**:**3328-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartorelli, V., J. Huang, Y. Hamamori, and L. Kedes. 1997. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol. Cell. Biol. 17**:**1010-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sparrow, D. B., E. A. Miska, E. Langley, S. Reynaud-Deonauth, S. Kotecha, N. Towers, G. Spohr, T. Kouzarides, and T. J. Mohun. 1999. MEF-2 function is modified by a novel co-repressor, MITR. EMBO J. 18**:**5085-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verdin, E., F. Dequiedt, and H. G. Kasler. 2003. Class II histone deacetylases: versatile regulators. Trends Genet. 19**:**286-293. [DOI] [PubMed] [Google Scholar]
- 42.Wu, X. Y., H. Li, E. J. Park, and J. D. Chen. 2001. SMRTe inhibits MEF2C transcriptional activation by targeting HDAC4 and 5 to nuclear domains. J. Biol. Chem. 13**:**13. [DOI] [PubMed] [Google Scholar]
- 43.Yanazume, T., T. Morimoto, H. Wada, T. Kawamura, and K. Hasegawa. 2003. Biological role of p300 in cardiac myocytes. Mol. Cell. Biochem. 248**:**115-119. [DOI] [PubMed] [Google Scholar]
- 44.Youn, H. D., T. A. Chatila, and J. O. Liu. 2000. Integration of calcineurin and MEF2 signals by the coactivator p300 during T-cell apoptosis. EMBO J. 19**:**4323-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, C. L., T. A. McKinsey, S. Chang, C. L. Antos, J. A. Hill, and E. N. Olson. 2002. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110**:**479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, C. L., T. A. McKinsey, J. R. Lu, and E. N. Olson. 2001. Association of COOH-terminal-binding protein (CtBP) and MEF2-interacting transcription repressor (MITR) contributes to transcriptional repression of the MEF2 transcription factor. J. Biol. Chem. 276**:**35-39. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, C. L., T. A. McKinsey, and E. N. Olson. 2002. Association of class II histone deacetylases with heterochromatin protein 1: potential role for histone methylation in control of muscle differentiation. Mol. Cell. Biol. 22**:**7302-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, C. L., T. A. McKinsey, and E. N. Olson. 2001. The transcriptional corepressor MITR is a signal-responsive inhibitor of myogenesis. Proc. Natl. Acad. Sci. USA 98**:**7354-7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, T., E. N. Johnson, Y. Gu, M. R. Morissette, V. P. Sah, M. S. Gigena, D. D. Belke, W. H. Dillmann, T. B. Rogers, H. Schulman, J. Ross, Jr., and J. H. Brown. 2002. The cardiac-specific nuclear delta(B) isoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2A activity. J. Biol. Chem. 277**:**1261-1267. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, X., P. A. Marks, R. A. Rifkind, and V. M. Richon. 2001. Cloning and characterization of a histone deacetylase, HDAC9. Proc. Natl. Acad. Sci. USA 98**:**10572-10577. [DOI] [PMC free article] [PubMed] [Google Scholar]