Identification of an hnRNP A1-Dependent Splicing Silencer in the Human Papillomavirus Type 16 L1 Coding Region That Prevents Premature Expression of the Late L1 Gene (original) (raw)
Abstract
We have previously identified _cis_-acting RNA sequences in the human papillomavirus type 16 (HPV-16) L1 coding region which inhibit expression of L1 from eukaryotic expression plasmids. Here we have determined the function of one of these RNA elements, and we provide evidence that this RNA element is a splicing silencer which suppresses the use of the 3′ splice site located immediately upstream of the L1 AUG. We also show that this splice site is inefficiently utilized as a result of a suboptimal polypyrimidine tract. Introduction of point mutations in the L1 coding region that altered the RNA sequence without affecting the L1 protein sequence resulted in the inactivation of the splicing silencer and induced splicing to the L1 3′ splice site. These mutations also prevented the interaction of the RNA silencer with a 35-kDa cellular protein identified here as hnRNP A1. The splicing silencer in L1 inhibits splicing in vitro, and splicing can be restored by the addition of RNAs containing an hnRNP A1 binding site to the reaction, demonstrating that hnRNP A1 inhibits splicing of the late HPV-16 mRNAs through the splicing silencer sequence. While we show that one role of the splicing silencer is to determine the ratio between partially spliced L2/L1 mRNAs and spliced L1 mRNAs, we also demonstrate that it inhibits splicing from the major 5′ splice site in the early region to the L1 3′ splice site, thereby playing an essential role in preventing late gene expression at an early stage of the viral life cycle. We speculate that the activity of the splicing silencer and possibly the concentration of hnRNP A1 in the HPV-16-infected cell determines the ability of the virus to establish a persistent infection which remains undetected by the host immune surveillance.
Human papillomaviruses (HPVs) are a group of nonenveloped, double-stranded DNA tumor viruses with tropism for epithelial cells (21, 62). HPV type 16 (HPV-16) is one of the most common sexually transmitted HPV types and is also the HPV type most frequently detected in cervical cancers (43). While many of the early HPV gene products are present in all layers of the squamous epithelium, expression of the late mRNAs encoding the L1 and L2 capsid proteins is restricted to the terminally differentiated cells in the upper layers of the epithelium (21). For this reason, it has been difficult to grow HPVs in vitro, and only organotypic raft cultures in which infected or transfected keratinocytes are grown in the air-liquid interface to induce terminal cell differentiation produce HPV virions (27). Expression of the late genes is regulated at the levels of transcription and RNA processing (1, 38, 39, 42, 55). Members of our group and others have previously shown that the late HPV-16 mRNAs contain _cis_-acting regulatory RNA elements (23, 24, 51), both in the 3′ untranslated region (UTR) and in the L1 and L2 coding regions (9, 33, 46, 51). It is likely that these elements are involved in the regulation of HPV late gene expression. Inhibitory RNA elements in the late 3′ UTR have been identified in multiple papillomavirus types, for example, bovine papillomavirus type 1 (BPV-1) (17), HPV-1 (52) and HPV-31 (11), in addition to HPV-16 (23, 24, 51). The BPV-1 and HPV-16 late UTR elements both appear to interact with U1 snRNP (10, 18), which inhibits polyadenylation, and possibly other factors (13, 25). The HPV-1 late UTR element is an AU-rich RNA instability element containing multiple UAUUUAU motifs, which reduce mRNA half-life and inhibit mRNA translation (47, 54). This element interacts with HuR, hnRNP C, and poly(A)-binding protein (44, 45, 54). The HPV-16 L1 and L2 coding regions were also found to contain _cis_-acting RNA sequences by virtue of their inhibitory properties when inserted downstream of a chloramphenicol acetyltransferase (CAT) reporter gene (8, 46, 51). Inhibitory RNA sequences were also present in the L1 coding region of multiple HPV types, including HPV-5, -6b, -16, -18, -31, -45, and -56 (9). To map the inhibitory RNA sequences in HPV-16 L1 and L2, hybrids between the L1 or L2 capsid genes and the highly expressed equine infectious anemia virus (EIAV) gag gene were constructed (9, 33). Inhibitory RNA elements were present in the first 514 nucleotides of L1 (9), and two inhibitory regions were found in L2 (33). We inactivated the inhibitory elements in L1 and L2 specifically by the introduction of nucleotide substitutions, which changed the RNA sequences without altering the protein sequences (9, 33). This resulted in mutant genes, which produced high levels of L1 or L2 mRNA and protein when expressed under control of the human cytomegalovirus immediate-early promoter (CMV), from mammalian expression plasmids transfected into HeLa cells (9, 33). Indeed, the mutant HPV-16 L1 gene induced potent immune responses in mice that were immunized with the L1 mutant expression plasmid, whereas the wild-type (wt) L1 gene did not (37). HIV-1 Rev in trans and the Rev-responsive element (RRE) in cis induced expression of the HPV-16 wt L1 gene from expression plasmids driven by the HIV-1 long terminal repeat promoter (33, 51), suggesting that the processing pathways of the HPV-16 late mRNAs are blocked by nuclear factors and that this effect can be overcome or bypassed by HIV-1 Rev and RRE. Different groups have shown that HIV-1 Rev and RRE can also overcome the effect of inhibitory sequences in the late 3′ UTR of BPV-1 (2), HPV-1 (52), and HPV-16 (51). Others have failed to see an effect of Rev and RRE on HPV gene expression (28). We reasoned that the RNA elements in the HPV-16 late coding region are likely to play an important regulatory role in the viral gene expression program (38, 39, 42). Mapping and functional inactivation of the inhibitory RNA elements in the L1 and L2 coding regions would therefore be helpful in studies of the regulated expression of late HPV-16 genes. Here we have used the L1 mutant gene to determine one function of the previously identified RNA elements in the HPV-16 L1 coding region.
MATERIALS AND METHODS
Plasmid constructions.
Plasmid pBEL was constructed in two steps. Firstly, HPV-16 sequences from genomic position 3395 to BamHI (numbers refer to nucleotide positions in the HPV-16R genomic sequence) were PCR amplified with primers E4S (the sequences of all oligonucleotides are shown in Tables 1, 2, and 3) and L1(AS)BamHI (9) and inserted into pC16L1, creating pC16L2L1splice. In the second step, early HPV-16 sequences were PCR amplified from nucleotide position 757 to 3395 using oligonucleotides 757SENSE and E4A followed by subcloning into the pCRII-TOPO cloning vector (Invitrogen) and transfer to pC16L2L1splice, thereby creating pBEL (see Fig. 1). To generate pBELM, PCR mutagenesis was performed using oligonucleotides L1MspliceFS and L1stopX or L2KPN1S and L1MspliceFA, followed by insertion of the KpnI- and BamHI-digested PCR fragment into pC16L2L1splice. An ApaI-BamHI fragment was then excised and subcloned into pBEL to produce pBELM. pBELMDC was generated by digestion of pBELM with HindIII and SalI, filling in, and religation. pBEL-pAE was generated by first PCR amplifying HPV-16 sequences from nucleotide 3402 to 4155 using oligonucleotides E4S (which introduces a BssHII site in the E4 reading frame) and K2, followed by cloning into pCRII-TOPO. Secondly, HPV-16 sequences from nucleotide 4221 to 5074 were PCR amplified with oligonucleotides K3 and L2 M, followed by cloning into pCRII-TOPO. The two PCR-amplified fragments were combined with MluI and SalI in one pCRII-TOPO plasmid, thereby deleting the pAE and 59 nucleotides of the early UTR. This fragment was subcloned into pBEL by using BssHII and StuI, generating pBEL-pAE. In order to generate pBELM-pAE, the mutant pAE-containing fragment was transferred into pBELM by BssHII and StuI. In order to generate pBEL-pAEPL, the SalI and MluI sites were first filled in, and a cryptic poly(A) signal in L1 was inactivated in pBEL-pAE by two point mutations as described previously (37). This plasmid was digested with ApaI and BamHI to insert a fragment amplified with oligonucleotides K3 and L1L2PK(AS). To generate pBEL-OPSA and pBEL-pAE-OPSA, the optimized splice sites were created by PCR mutagenesis using the following primers: L2B (46) and optimal 3′ss-ANTI, optimal 3′ss-sense, and L15′75AS (9). The PCR fragment was inserted into pBEL or pBEL-pAE with AvrII and BamHI. To generate pBEL-pAE-OPBP, PCR mutagenesis was performed with oligonucleotides L2B (46), Optimal3′ss(branch site)-antisense, Optimal 3′ss(branch site)-sense, and L15′75AS (9). The PCR fragment was inserted into pBEL-pAE by AvrII and BamHI. To generate pBEL-pAE-OPPy, PCR mutagenesis was performed with oligonucleotides L2B (46) and Optimal 3′ss(Y)n-antisense, Optimal 3′ss(Y)n-sense, and L15′75AS. The PCR fragment was inserted into pBEL-pAE by AvrII and BamHI.
TABLE 1.
Sequences of PCR oligonucleotides used to make L1 deletions
| Oligonucleotide | Sequence (5′→3′) |
|---|---|
| E4S | CGCGCGCCAACCACCCCGCCGCGACC |
| 757SENSE | GTCGACGGTATCGATCGGTTGTGCGTACAAAGCACACACG |
| E4A | CCGCGCGCTGCCTAATAATTTCAGGAGAGG |
| L1MspliceFS | GTTACGAAAACGACGTAAACGTTTACCATATTTTTTTTCAGATGAGCCTGTGG |
| L1stopX | CCTCGAGCTTACAGCTTACGTTTTTTG |
| L2KPN1S | CTACAACCCCGGTACCATCTGTACC |
| L1MspliceFA | CCACAGGCTCATCTGAAAAAAAATATGGTAAACGTTTACGTCGTTTTCGTAAC |
| K2 | ACGCGTGGTACCGTAAGTTATGGTATACAAC |
| K3 | ACGCGTGGTACCCTGTTATTACTTAACAATGCG |
| L2M | CGTCGACCTGGAGCTATATTAATAC |
| L1L2PK(AS) | GGATCCGATATCTCTAGACGCGTGTCGACCACTAGGCAGCCAAAGAGACA |
| Optimal 3′ss-ANTI | GGCCTCACTAGGCAGCCAAAGAGACATCTGAAAAAAAAAAAAAAAAAAAGAGTTAGTGGCTACGTCGTTT |
| Optimal 3′ss-sense | AAACGACGTAGCCACTAACTCTTTTTTTTTTTTTTTTTTTCAGATGTCTCTTTGGCTGCCTAGTGAGGCC |
| Optimal 3′ss(branch site)-antisense | GGCCTCACTAGGCAGCCAAAGAGACATCTGAAAAAAAATATGTTAGTGGCTACGTCGTTT |
| Optimal 3′ss(branch site)-sense | AAACGACGTAGCCACTAACATATTTTTTTTCAGATGTCTCTTTGGCTGCCTAGTGAGGCC |
| Optimal 3′ss(Y)n-antisense | GGCCTCACTAGGCAGCCAAAGAGACATCTGAAAAAAAAAAAAAAAAAAAGAGGGTAAACGTTTACGTCGTTT |
| Optimal 3′ss(Y)n-sense | AAACGACGTAAACGTTTACCCTCTTTTTTTTTTTTTTTTTTTCAGATGTCTCTTTGGCTGCCTAGTGAGGCC |
| F1-18wt | GGTACCGTCGACAAGATGTCTCTTTGGCTGCCT |
| 66-46R | TCTAGAACGCGTTACAACCTTAGATACTGGAC |
| 16L1(178)S | GCGCGCTCTAGAATATTAGTTCCTAAAGTATC |
| 16L1(318)AS | AAGCTTGGATCCCTCAACACCTACACAGGC |
| 16L1(270)AS | AAGCTTGGATCCAAATGAGGTGTCAGGAAAAC |
| 16L1(245)AS | AAGCTTGGATCCTTATTGGGGTCAGGTAAATG |
| 16L1(226)S | GCGCGCTCTAGACATTTACCTGACCCCAATAAG |
| 16L1(366)AS | AAGCTTGGATCCTAAAGGATGGCCACTAATGC |
| 16L1(270)S | GCGCGCTCTAGATTATAATCCAGATACACAGCG |
| 16L1(294)S | GCGCGCTCTAGAGGTTTGGGCCTGTGTAGGTG |
TABLE 2.
Sequences of annealing oligonucleotides used to make L1 deletions
| Oligonucleotide | Sequence (5′→3′) |
|---|---|
| 178-226(S) | CGCGTATATTAGTTCCTAAAGTATCAGGATTACAATACAGGGTATTTAGAATACG |
| 178-226(AS) | GATCCGTATTCTAAATACCCTGTATTGTAATCCTGATACTTTAGGAACTAATATA |
| 226-270(S) | CGCGTCATTTACCTGACCCCAATAAGTTTGGTTTTCCTGACACCTCATTTG |
| 226-270(AS) | GATCCAAATGAGGTGTCAGGAAAACCAAACTTATTGGGGTCAGGTAAATGA |
| 270-318(S) | CGCGTTTATAATCCAGATACACAGCGGCTGGTTTGGGCCTGTGTAGGTGTTGAGG |
| 270-318(AS) | GATCCCTCAACACCTACACAGGCCCAAACCAGCCGCTGTGTATCTGGATTATAAA |
| 318-366(S) | CGCGTGGTAGGTCGTGGTCAGCCATTAGGTGTGGGCATTAGTGGCCATCCTTTAG |
| 318-366(AS) | GATCCTAAAGGATGGCCACTAATGCCCACACCTAATGGCTGACCACGACCTACCA |
TABLE 3.
Sequences of PCR and annealing oligonuleotides used to make in vitro RNA synthesis plasmids
| Oligonucleotide | Sequence (5′→3′) |
|---|---|
| T7S | AATTCTAATACGACTCACTATAGGGGTAC |
| T7A | CCCTATAGTGAGTCGTATTAG |
| T7PKS | CGTCGACACGCGTGGATCCA |
| T7PKAS | AGCTTGGATCCACGCGTGTCGACGGTAC |
| AdenoS | GGTACCAACAAAAGCTTGCATGCCTG |
| AdenoAS | GTCGACCTGTGGAAAAAAAAGGGACAGGATA |
| 16L1-42wtS | CAGATGTCTCTTTGGCTGCCTAGTGAGGCCACTGTCTACTTGCCTA |
| 16L1-42wtAS | AGCTTAGGCAAGTAGACAGTGGCCTCACTAGGCAGCCAAAGAGACATCTGGTAC |
| 16L1-42mutS | CAGATGAGCCTGTGGCTGCCCAGCGAGGCCACCGTGTACCTGCCCA |
| 16L1-42mutAS | AGCTTGGGCAGGTACACGGTGGCCTCGCTGGGCAGCCACAGGCTCATCTGGTAC |
| 16L1(45)S | GTCGACGCGCGCGTCCCAGTATCTAAGGTTG |
| 16L1(177,BamHI,HindIII)AS | AAGCTTGGATCCTTTGTTATTGTTAGG |
| 16L1M(45)S | TCTAGAGCGCGCCGTGCCCGTGAGCAAGG |
| 16L1M(177,BamHI,HindIII)AS | AAGCTTGGATCCCTTGTTGTTGTTAGGCTTC |
FIG. 1.
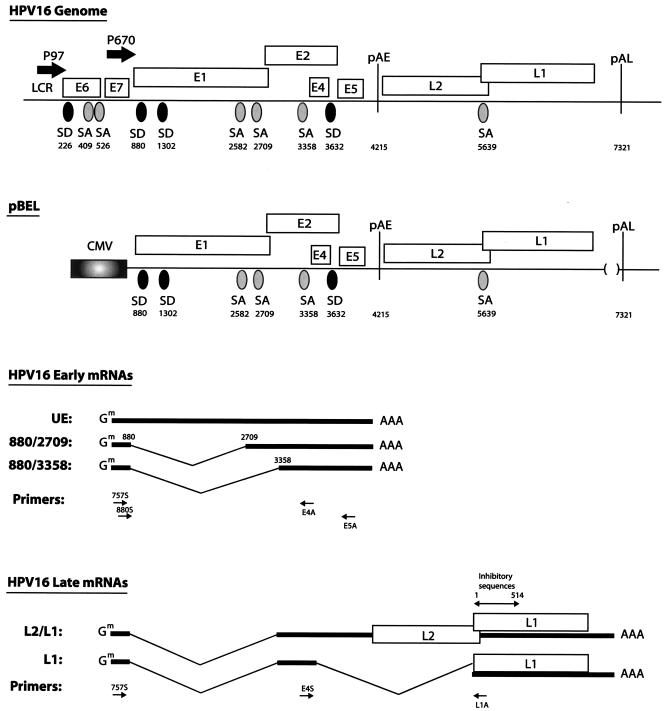
Schematic representation of the HPV-16 genome. Boxes indicate the protein coding regions. Numbers refer to nucleotide positions in the HPV-16R sequence. The major p97 promoter and the differentiation-dependent promoter p670 (19) are indicated. Splice sites and polyadenylation signals are shown. The late UTR which contains RNA instability elements was originally deleted to increases the chances of obtaining detectable levels of late mRNAs. The structure of the pBEL expression plasmid is shown, and the predicted major mRNAs are displayed. The RT-PCR primers used here are shown as arrows under the schematic structures of the mRNAs. The previously identified inhibitory element in the first 514 nucleotides of the L1 coding sequence is indicated above the L1 gene (9, 51). pAE, early poly(A) signal; pAL, late poly(A) signal, CMV, human cytomegalovirus immediate-early promoter; SD, 5′ ss; SA, 3′ ss.
To generate pPL1-520 and pPL1-520 M, pC16L1 (9) and pC16L1MUT#123 (9) were digested with MluI and BamHI and the L1 sequences were subcloned into pBEL-pAEPL. To generate pPL1-270, pPL1-129 and pPL1-129 M, L1 sequences were cut out from pCL1(+20)EIAV (9), pC16L1-129WT (9), and pC16L1-129MUT (9) with SalI and MluI and subcloned into pBEL-pAEPL. To generate pPL178-366 and pPL1-162, L1 sequences were either PCR amplified using primers L1(−15)start (9) and L15′25AS (9) or primers L1STARTSalI (9) and L1(+20)AS (9), followed by subcloning of the PCR fragments separately into pBEL-pAEPL by using SalI and MluI. pPL1-66 was generated by amplification of HPV-16 L1 nucleotides from position 1 to 66 of HPV-16 L1 by oligonucleotides F1-18wt and 66-46R, followed by insertion into pBEL-pAEPL at sites SalI and MluI.
To generate pPL178-318, pPL178-270, pPL178-245, pPL226-366, pPL270-366, pPL294-366, and pPL226-318, L1 sequences were PCR amplified with the following oligonucleotides: 16L1(178)S and 16L1(318)AS, 16L1(178)S and 16L1(270)AS, 16L1(178)S and 16L1(245)AS, 16L1(226)S and 16L1(366)AS, 16L1(270)S and 16L1(366)AS, and 16L1(294)S and 16L1(366)AS. The PCR fragments were inserted into pBEL-pAEPL by SalI and BamHI.
To generate pPL178-226, pPL178-226 M, pPL226-270, pPL270-318, and pPL318-366, the following oligonucleotides were annealed pair-wise: 178--226(S) and 178-226(AS), 226-270(S) and 226-270(AS), 270-318(S) and 270-318(AS) and 318-366(S) and 318-366(AS). The annealed fragments were cloned into MluI-BamHI-digested pBEL-pAEPL, respectively.
The bacteriophage T7 RNA polymerase promoter-containing plasmid pUCT7 was generated by insertion into EcoRI- and KpnI-digested pUC19 of annealed oligonucleotides T7S and T7A. To generate pT7PK, oligonucleotides T7PKS and T7PKAS were annealed and cloned into KpnI- and HindIII-digested pUCT7.
pTA was constructed by PCR amplifying adenovirus sequences from pAd1 (26) with primers AdenoS and AdenoAS, followed by subcloning of the PCR fragment into pT7PK using KpnI and SalI. pTA178-226 and pTA178-226 M were generated by transferring the MluI-BamHI fragments encompassing wt or mutant L1 sequences to pTA from plasmids pPL178-226 and pPL178-226 M, respectively.
To generate pT178-226 and pT178-226 M, the HPV sequences in pTA178-226 and pTA178-226 M were released with MluI and BamHI and transferred to pT7PK. To generate pT1-42 and pT1-42 M, oligonucleotides 16L1-42wtS and 16L1-42wtAS or 16L1-42mutS and 16L1-42mutAS were annealed pairwise and cloned into the KpnI and HindIII sites of pUCT7. To construct pT45-178 and pT45-178 M, wt and mutant HPV-16 L1 sequences were subjected to PCR with primers 16L1(45)S and 16L1(177,BamHI,HindIII)AS and 16L1 M(45)S and 16L1 M(177,BamHI,HindIII)AS, respectively. The PCR fragments were digested with BssHII and HindIII and inserted into MluI- and HindIII-digested pT7PK.
The constructed plasmids were all subjected to sequencing.
Transfections.
Transfections were performed in HeLa cells according to the Fugene 6 method (Roche Molecular Biochemicals). Briefly, 1 μg of DNA was mixed with 3 μl of Fugene 6 and subsequently added in 200-μl aliquots consisting of DNA, Fugene 6, and Dulbecco's modified Eagle's medium to 60-mm plates containing subconfluent HeLa cells. The transfected cells were harvested at 24 h posttransfection. The data variation in each transfection experiment was less than 20%.
RNA extraction and Northern blotting.
Nuclear and cytoplasmic RNA extraction were performed as previously described (51), and total RNA was prepared according to the RNeasy Mini protocol (QIAGEN). Northern blot analysis was performed by the separation of 10 μg of total, cytoplasmic, or nuclear RNA on 1% agarose gels containing 2.2 M formaldehyde, followed by transfer to a nitrocellulose filter and hybridization, as described previously (9, 54). Random priming of the DNA probe was performed using a Decaprime kit (Ambion) according to the manufacturer's instructions. The CMV probe has been described previously (9). The E1 probe was generated by PCR amplification of nucleotides 1268 to 2795 of the HPV16 genome with oligonucleotides 1302S (5′-GCAATACTGAAGTGGAAACTCAG-3′) and SA2582/2709Anti (5′-TCTAGATGTCCTGACACACATTTAAACG-3′), while the E4 probe was generated by PCR amplification of nucleotides 3401 to 3723 of the HPV16 genome, using oligonucleotides E4S and K1 (5′-ACGCGTGGTACCCCTGTCCAATGCCATGTAGACG-3′) and the L1 probe by digestion of pC16L1 with BamHI and XhoI. Restriction sites are underlined. All Northern blots were quantified in a Bio-Rad phosphorimager (GS-250).
Reverse transcription (RT)-PCR.
Total RNA was reverse transcribed at 42°C for 1 h in a total volume of 25 μl using random hexamers, as previously described (51). Reactions without reverse transcriptase were performed in parallel and served as a control for the presence of plasmid DNA contamination. A 5-μl aliquot of cDNA product was PCR amplified in a 100-μl reaction volume using the oligonucleotides indicated and shown in Fig. 1 and 2. The amplified products were cloned into a pCRII-TOPO vector (Invitrogen) and subjected to sequencing.
FIG.2.
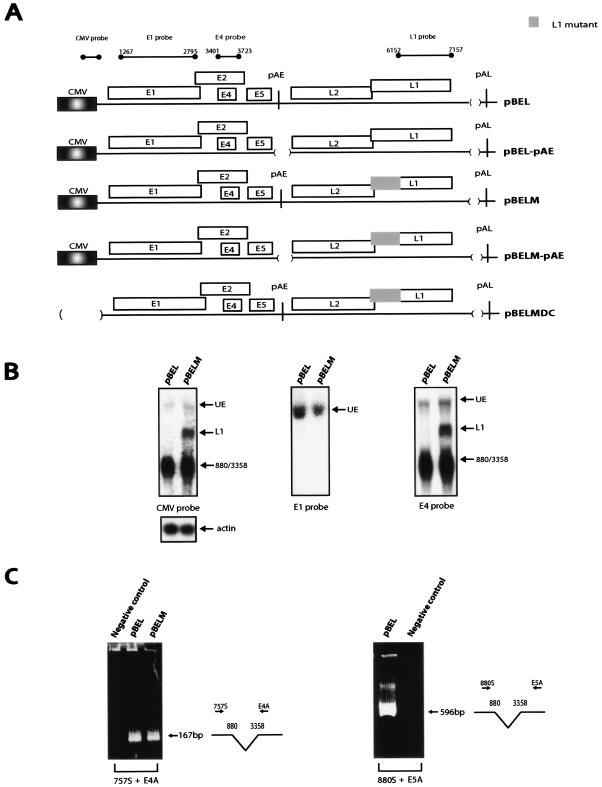
(A) Schematic representation of pBEL and the pBEL-derived plasmids. Boxes indicate the protein coding regions. The CMV promoter is indicated. The probes used in Northern blots are indicated above the diagram. The CMV probe detects all mRNAs produced from the CMV-driven plasmids and has been described previously (9). Numbers refer to nucleotide positions in the HPV-16R sequence. (B) Northern blots of total RNA extracted from HeLa cells transfected with pBEL and pBELM are shown hybridized to the indicated radiolabeled probes. UE, unspliced early mRNAs; L1, the spliced late mRNA; 880/3358, early mRNA spliced from the 5′ ss at position 880 to the 3′ss at position 3358 followed by polyadenylation at pAE. The same samples were hybridized to a human β-actin probe to control for loading. The data variation in each transfection experiment was less than 20%. (C) RT-PCR on the RNA samples shown in Fig. 2B using primers 757S or 880S in combination with either E4A or E5A (Fig. 1). Negative control, RNA sample from cells transfected with unrelated plasmid. (D) Northern blot of total RNA extracted from HeLa cells transfected with pBEL, pBELM, pBEL-pAE, pBELM-pAE, ors pBELMDC hybridized to the L1 probe. Both L2/L1 and L1 mRNAs are spliced from the 5′ ss at position 880 to the 3′ splice site at position 3358. The L2/L1 mRNA then remains unspliced until polyadenylation at pAL, whereas the L1 mRNA is spliced also between the 5′ss at position 3632 and the 3′ ss at position 5639. The truncated L1 mRNA is polyadenylated at a previously identified cryptic poly(A) signal at position 5170 in the HPV-16R genome (33). Spliced mRNA as a percentage of total late RNA in each lane is indicated at the bottom of the gel. The same samples were hybridized to a human β-actin probe to control for loading. The data variation in each transfection experiment was less than 20%. (E) RT-PCR of the RNA samples in Fig. 2D using primers 757S and L1A (left panel) or primers E4S and L1A (right panel). All RT-PCR products were cloned and sequenced. (F) Total, cytoplasmic, and nuclear RNAs were extracted from HeLa cells transfected with pBEL or pBELM. The blotted RNA was probed with the L1 probe.
UV cross-linking and preparation of cellular extracts and recombinant hnRNP A1.
In vitro synthesis of radiolabeled and unlabeled RNA was performed on linearized plasmid DNA. pT178-226, pT178-226 M, pT1-42, pT1-42 M, pT45-178, and pT45-178 M were linearized with HindIII. In vitro transcription was performed with T7-RNA polymerase in the presence of (32P)UTP, as previously described (46). The radiolabeled RNAs were purified by phenol-chloroform extraction and EtOH precipitation and resuspended in water. UV cross-linking and synthesis of radiolabeled RNA was performed as previously described (48). Radiolabeled RNA (105 cpm) was used in each UV cross-linking reaction.
HeLa cell nuclear and cytoplasmic extracts were prepared according to the method of Dignam (14). His-tagged hnRNP A1 was purified by using a HiTrap chelating column according to the manufacturer's instructions (Pharmacia Biotech). Twenty micrograms of nuclear extract or 50 ng of recombinant hnRNP A1 was used for UV cross-linking.
Antibodies and immunoprecipitation.
For immunoprecipitations, the UV cross-linking reactions were diluted up to 100 μl with radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl [pH 7.4], 75 mM NaCl, 0.5% Triton X-100, 0.5% sodium deoxycholate, 0.05% sodium dodecyl sulfate [SDS]) followed by the addition of monoclonal antibody. Monoclonal antibodies were CAMVIR-1 against HPV-16 L1 or 9H10 against hnRNP A1 (generously provided by G. Dreyfuss). The samples were incubated for 2 h at 4°C prior to the addition of protein A Sepharose, and incubation for 1 h at 4°C followed. The beads were washed three times in 1× RIPA buffer and resuspended in 20 μl of water mixed with 20 μl of 5× SDS loading buffer (625 mM Tris-HCl [pH 6.8], 6.25% SDS, 50% glycerol, 20% β-mercaptoethanol). The samples were boiled prior to the loading on SDS-12% polyacrylamide gel electrophoresis gels. Bands were visualized by autoradiography.
In vitro splicing.
RNAs for in vitro splicing were synthesized from 2 μg of linearized pTA, pTA178-226, or pTA178-226 M in a 25-μl reaction mixture containing 1× transcription buffer (40 mM Tris-HCl, pH 8, 15 mM MgCl2, 5 mM dithiothreitol [DTT], 0.5-mg/ml bovine serum albumin), 500 μM ATP and UTP, 50 μM CTP and GTP, 500 μM CAP, 0.5 U of RNAsin/μl, 50 μCi of [α-32P]CTP, and 4 U of T7 RNA polymerase/μl. The samples were incubated for 45 min at 37°C. The samples were DNase treated with 1 U of DNase RQ1 (Promega)/μl and incubated for 15 min at 37°C, followed by phenol-chloroform extraction and precipitation of synthesized RNA with ethanol. The samples were resuspended in loading buffer (90% [vol/vol] formamide, 50 mM Tris-HCl [pH 7.5], 1 mM EDTA), denatured at 65°C, and loaded onto 4% (acrylamide-bis, 29:1) denaturing polyacrylamide gels. The radiolabeled capped pre-mRNA was eluted with elution buffer (0.75 M NH4Ac, 10 mM MgCl2, 10 mM DTT) from gel slices, which contained the specific pre-RNA. In vitro splicing was performed in a 20-μl reaction mixture containing 70,000 cpm of radiolabeled capped pre-mRNA substrate, 1.5 mM MgCl2, 20 mM creatine phosphate, 5 mM DTT, 2 mM ATP, 0.5 U of RNasin/μl, and 40% (vol/vol) nuclear extract. After incubation for 1 h at 30°C, the reaction was stopped with stop solution (6% SDS, 0.2 M EDTA, 2.5 mg of proteinase K/ml, 2 mg of tRNA/ml), followed by incubation for 1 h at 37°C. The samples were precipitated and resuspended in loading buffer and loaded onto 8% (acrylamide-bis, 19:1) denaturing polyacrylamide gels. The assay was visualized by autoradiography.
RESULTS
Mutational inactivation of the early poly(A) signal induces expression of partially spliced L2 mRNAs and spliced L1 mRNAs.
In order to determine the function of previously identified RNA elements in the L1 coding region of the late HPV-16 mRNAs (9, 51) (Fig. 1), we first generated expression plasmid pBEL (Fig. 1), in which the HPV-16 sequences located between the late differentiation-dependent promoter p670 (19) and the late poly(A) signal (pAL) were inserted downstream of the constitutively active human cytomegalovirus immediate-early promoter (CMV) (Fig. 1). In essence, the pBEL plasmid contains the CMV promoter, the E1, E2, E4, and E5 early genes, and the early poly(A) signal (pAE), followed by the late region encoding L1 and L2 and the pAL (Fig. 1 and 2A). The late UTR, which contains RNA instability elements, was originally deleted to increase the chances of obtaining detectable levels of late mRNAs. The replacement of the differentiation-dependent promoter p670 (19), which is inactive in HeLa cells, with the constitutively active CMV promoter ensured efficient transcription in transfected cells. The pBEL plasmid also allowed a functional analysis of the RNA elements mentioned above in the context of genomic HPV-16 sequences and the authentic splice sites therein. The major early and late mRNAs predicted to be produced by pBEL are indicated in Fig. 1. These mRNAs were all identified, and their structures were confirmed, by a combination of Northern blotting and RT-PCR on RNA extracted from transfected cells (see below).
To investigate if late mRNAs could be produced from pBEL (Fig. 1 and 2A), this plasmid was transfected into HeLa cells, and total RNA was extracted and first analyzed by Northern blotting. The various probes used here are indicated at the top of Fig. 2A. The CMV probe spans the immediate 5′ end of the produced mRNAs and detects all mRNAs produced from pBEL. The E1 probe should detect unspliced early mRNAs, while the E4 probe is located in the central, early region, upstream of pAE, and would detect early mRNAs polyadenylated at the early poly(A) signal (Fig. 1 and 2A). Finally, the L1 probe would specifically detect late L1 and L2/L1 mRNAs polyadenylated at pAL (Fig. 1 and 2A). The results revealed that the CMV and E4 probes displayed the same pattern of bands (Fig. 2B). In contrast, the L1 probe failed to detect any of the mRNAs produced from pBEL (Fig. 2D). These results demonstrated that pBEL produced only early mRNAs. A combination of Northern blot and RT-PCR analysis revealed that the upper band seen with the CMV, E1, and E4 probes (Fig. 2B) represents unspliced early mRNAs (UE) (Fig. 1 and 2B), and the lower major band detected by the CMV and E4 probes represents splicing from the 880 5′ splice site (ss) to the 3358 3′ ss (Fig. 1 and 2B). The data variation in each transfection experiment was less than 20%. RT-PCR analysis with primers 757S or 880S and E4A or E5A (Fig. 1) confirmed that from the choice of 5′ splice sites downstream of the p670 promoter, the 880 5′ ss was used in the spliced mRNAs (Fig. 2C). Attempts to detect splicing from the 1302 5′ ss were unsuccessful (data not shown). Of the 3′ ss downstream of p670, the 3′ ss at position 3358 was clearly the predominant site (Fig. 2C). The 3′ ss at position 2709 was used at a very low frequency and was detected only in long exposures of the Northern blots or by RT-PCR (data not shown). The 3′ ss at position 2582 was not seen to be used (data not shown). All RT-PCR products were cloned and sequenced to confirm the splice junctions. Taken together, the results clearly demonstrated that replacement of the differentiation-dependent late promoter with the constitutively active CMV promoter did not activate HPV-16 late gene expression. We concluded that the major block in HPV-16 late gene expression in proliferating cells is at the level of RNA processing, as previously suggested (38, 39, 42). To induce expression of the late mRNAs from pBEL, the pAE was inactivated by a deletion that removed 59 nucleotides (nt) of the early UTR and the canonical poly(A) signal, resulting in pBEL-pAE (Fig. 2A). In contrast to pBEL, pBEL-pAE produced late mRNAs (Fig. 2D). The partially spliced L2/L1 and the fully spliced L1 mRNA species were detected with the L1 probe (Fig. 2D), whereas only the upper band was detected with an L2-specific probe (data not shown). To determine the exact splicing pattern of the late mRNAs, RT-PCR was performed on the same RNA samples, using either primer 757S or primer E4S in combination with primer L1A (Fig. 1). The results of both primer pairs are shown in the left and the right panels in Fig. 2E. Cloning and sequencing of the RT-PCR products revealed that the late mRNAs were spliced from the 5′ ss at position 880 to the 3′ ss at position 3358 to generate the L2/L1 mRNAs (Fig. 2E, left and right panels). Further splicing from the 5′ ss at position 3632 to the L1 3′ ss at 5639 produced the L1 mRNAs (Fig. 2E, left and right panels). We did not find any 3′ splice sites between the 3′ ss at position 3358 and the L2 AUG, despite multiple attempts. We concluded that the late mRNAs have the structures shown in Fig. 1 and that the pAE blocks late gene expression. In addition, plasmid pBEL-pAE could be used as a tool to study the regulation of HPV-16 late mRNA processing, since it produced detectable levels of the late L2/L1 and L1 mRNAs.
Mutational inactivation of negative RNA elements in the first 514 nucleotides of the HPV-16 L1 coding region results in enhanced splicing of the late mRNAs.
We have previously identified inhibitory RNA elements in the first 514 nt of the HPV-16 L1 coding region (46, 51), and we have inactivated these RNA elements by the introduction of point mutations that altered the RNA sequence but not the protein sequence of L1 (9). These point mutations were introduced in the first 514 nucleotides of L1 in pBEL-pAE (Fig. 2A) by PCR mutagenesis, resulting in plasmid pBELM-pAE (Fig. 2A). HeLa cells were transiently transfected with the generated plasmid, and the extracted RNA was analyzed by Northern blotting, using the L1 probe in the late region (Fig. 2D). The results revealed that pBELM-pAE, encoding the L1 mutant, produced primarily spliced L1 mRNA, whereas pBEL-pAE, which contained the wt L1 sequence, produced primarily the L2/L1 mRNA, which is unspliced in the late region (Fig. 2D). The additional truncated L1 mRNA seen in pBELM-pAE-transfected cells is polyadenylated at a previously described cryptic poly(A) signal at position 570 in the L1 coding sequence (33). These results suggested that the inhibitory sequences in the 5′ end of L1 encode RNA elements that inhibit splicing of the late mRNAs.
Mutational inactivation of the inhibitory sequences in L1 activates splicing directly from the major 5′ splice site at genomic position 880 to the L1 splice acceptor, thereby activating late gene expression through the bypassing of polyadenylation at the early poly(A) signal.
The mutant L1 sequence was also inserted into pBEL (Fig. 2A), which has a functional pAE, resulting in pBELM (Fig. 2A). Analysis of early mRNA levels produced from pBEL and pBELM revealed that similar levels of the early mRNAs were produced from the two plasmids (Fig. 2B). The E4 probe also detects the L1 and L2 mRNAs. In contrast, analysis of the late mRNAs showed that pBELM produced high levels of spliced L1 mRNA, whereas the L2/L1 mRNA was undetectable (Fig. 2D). These results verified that the presence of the mutations in L1 resulted in the production of primarily spliced late L1 mRNA at the expense of the L2/L1 mRNA. As expected, HPV mRNAs were not produced from pBELMDC (Fig. 2D). In addition, the presence of the mutations in L1 induced late gene expression (compare late L1 mRNA levels produced by pBEL and pBELM or by pBEL-pAE and pBELM-pAE [Fig. 2D]). This effect could be due to the fact that enhanced splicing accumulates all late mRNA species into one band, which reaches the levels of detection. Alternatively, a novel mRNA may be induced by the mutations in L1. To investigate this, RT-PCR was performed on the RNA above using primer 757S or E4S in combination with L1A (Fig. 1). Separation of the PCR products on acrylamide gels revealed low levels of the band corresponding to L1 mRNAs in pBEL-transfected cells, whereas high levels of the predicted band were seen with RNA from pBELM-, pBEL-pAE-, or pBELM-PAE-transfected cells (Fig. 2E, left and right panels). This comparison also revealed an additional band with primer pair 757S and L1A in cells transfected with plasmids containing the mutant L1 sequence (pBELM and pBELM-pAE) compared to cells transfected with pBEL and pBEL-pAE (Fig. 2E, left panel). Cloning and sequencing revealed that this band corresponded to an mRNA that is spliced from the major 5′ splice site at nucleotide position 880 in the early region directly to the L1 3′ss at position 5639. This novel 880-5639 spliced L1 mRNA is produced exclusively by the plasmids containing the mutant L1 RNA sequence (Fig. 2E, left panel). This splicing event is therefore induced by the mutations in L1. We also wished to investigate if the absence of late mRNA production from pBEL was caused by nuclear entrapment of late mRNAs produced from this plasmid. However, analysis of nuclear and cytoplasmic distribution of the late mRNAs produced from pBEL and pBELM did not detect late mRNAs trapped in the nuclei (Fig. 2F). In conclusion, the mutations in L1 activated the production of a novel late L1 mRNA which is expressed in the presence of a functional early poly(A) signal, suggesting that one important role of the splicing inhibitory RNA sequence in L1 is to prevent direct splicing from the 880 5′ ss in the early region to the 3′ ss at position 5639 immediately upstream of the L1 AUG. The mutations in L1 also enhanced splicing from the 3632 5′ ss to the L1 3′ ss. Splicing from the 880 and the 3633 5′ ss to the L1 3′ ss would lead to expression of HPV-16 L1 genes in proliferating cells, which would not be beneficial for continued, uninterrupted persistence of HPV-16 in the host.
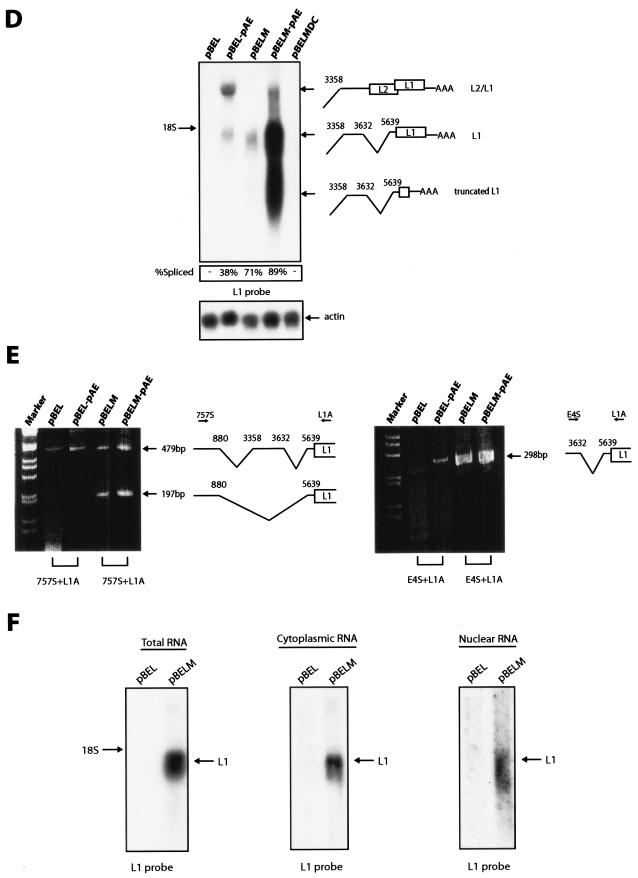
Extension of the polypyrimidine tract of the L1 3′ ss results in enhanced splicing of the late mRNAs.
Most 3′ splice sites under control of splicing silencer and/or enhancers are suboptimal (7). Inspection of the 3′ splice site at L1 revealed that the putative branch point sequence (BP) deviated from the consensus at one position and that the polypyrimidine tract (PPT) contained 9 consecutive pyrimidines compared to 10 to 18 in an optimal site (Fig. 3A). We optimized the putative BP or the PPT in pBEL-pAE or both in pBEL and pBEL-pAE and transfected the resultant plasmids into HeLa cells. Analysis of the extracted RNA with the L1 probe (Fig. 2A) revealed that the extension of the PPT from 9 consecutive pyrimidines to 23 or 25 resulted in a significant enhancement of splicing (Fig. 3B), whereas the optimization of the putative BP did not enhance splicing (Fig. 3B). The effect of the extended PPT mimicked the results obtained with the L1 mutants, in that both optimization of the PPT and mutational inactivation of the splicing silencer sequence resulted in enhanced splicing of the late mRNAs (Fig. 2D and 3B). These results demonstrated that the PPT was suboptimal. In addition, the inhibitory sequences in L1 were unable to inhibit splicing to the 3′ splice site with an extended PPT. Optimization alone was sufficient to activate splicing in the presence of the splicing suppressor.
FIG. 3.
(A) Schematic representation of the pBEL and pBEL-pAE plasmids. The wt and the various mutant branch-point and polypyrimidine sequences are shown. The optimal branch point is displayed above the wt sequence. The OPSA sequence contains optimized branch point and polypyrimidine tract, while OPBP contains only the optimized branch point, and OPPy contains the optimized polypyrimidine tract only. (B) Northern blots on total RNA extracted from HeLa cells transfected with the indicated plasmids. All blots were probed with the L1 probe (Fig. 2A). Spliced mRNA as a percentage of total late RNA in each lane is indicated at the bottom of each gel. The data variation in each transfection experiment was less than 20%.
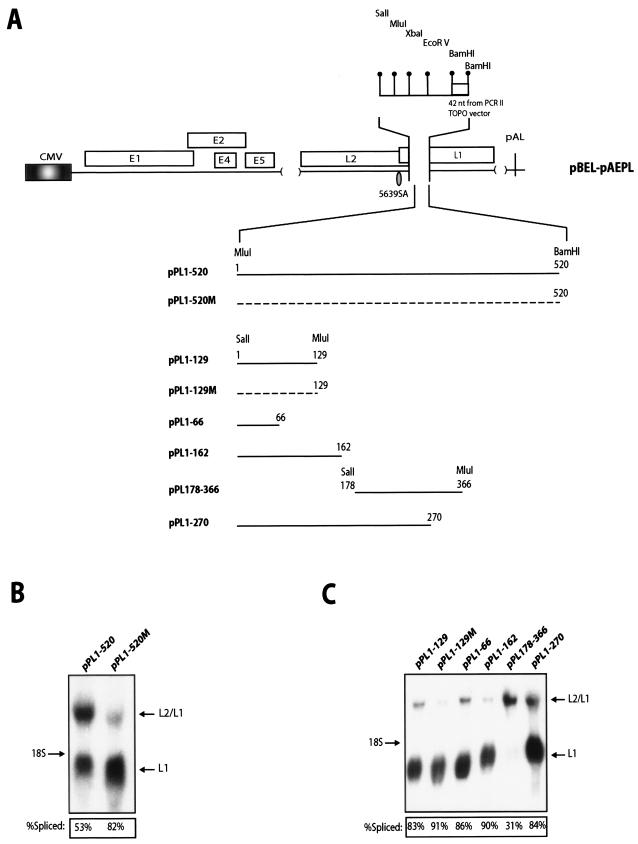
An RNA element located between nucleotide positions 178 and 366 in the L1 coding sequence inhibits splicing of the late mRNAs.
In order to map the RNA element which inhibited the use of the suboptimal 3′ splice site at position 5639, the L1 sequence between nucleotide positions 23 and 514 of L1 (numbering starts at the A in the L1 AUG) were deleted in plasmid pBEL-pAE and replaced with a polylinker sequence, creating pBEL-pAEPL (Fig. 4A). This was followed by insertion of various fragments of the first 514 nucleotides of L1 into the polylinker (Fig. 4A). Northern blot analysis of RNA extracted from HeLa cells transfected with plasmids containing the 1-520 wt or 1-520 mutant L1 sequences confirmed that the wt sequence inhibited splicing to a certain extent whereas the mutant did not (Fig. 4B). Analysis of the various deletion mutants showed that an L1 fragment encompassing nt 178 to 366 contained a strong inhibitor of splicing (Fig. 4C). We also observed that the plasmid with the polylinker sequence alone had a higher spliced-to-unspliced ratio than pBELM (data not shown), indicating that the short sequence from the pCRII-TOPO plasmid polylinker had an enhancing effect on splicing. However, this did not interfere with the mapping of the splicing silencer to a sequence between positions 178 and 366 of L1.
FIG. 4.
(A) Schematic diagram of the pBEL-pAEPL plasmid. A polylinker including a small sequence from the pCRII-TOPO cloning vector (Invitrogen) was inserted into L1, thereby replacing nucleotides 23 to 513 of L1 (numbering starts at A in the ATG of L1). The indicated wt and mutant L1 sequences of various lengths were inserted into the polylinker as MluI-BamHI or SalI-MluI fragments, as indicated. The numbering of the fragments starts at A in the L1 ATG. (B) Northern blot on total RNA extracted from HeLa cells transfected with pPL1-520 or pPL1-520 M. The blot is probed with the L1 probe (Fig. 2A). Spliced mRNA as a percentage of total late RNA in each lane is indicated at the bottom of each gel. (C) Northern blot on total RNA extracted from HeLa cells transfected with the indicated plasmids. The blot was probed with the L1 probe (Fig. 2A). Spliced mRNA as a percentage of total late RNA in each lane is indicated at the bottom of each gel. The data variation in each transfection experiment was less than 20%.
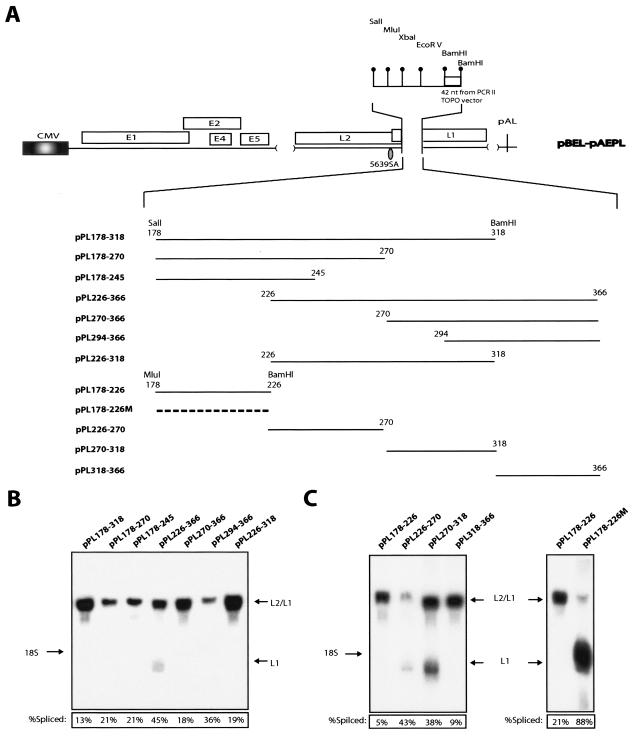
A strong splicing silencer located between positions 178 and 226 inhibits splicing in a sequence-specific manner in transfected cells and in vitro.
To map the splicing silencer further, 5′ or 3′ deletions of the 178-366 sequence were inserted into the pBEL-pAEPL plasmid with SalI and BamHI (Fig. 5A). All sequences inhibited splicing, but to various extents (Fig. 5B). The least efficient inhibitor of splicing was a fragment encompassing 226 to 366. Collectively, these results suggested that a strong silencer was present between 178 and 226 and indicated that multiple inhibitory sequences were present in the region located between 178 and 366. Although the results of the deletion analysis pointed to the existence of multiple RNA elements with positive and negative effects on splicing, we focused on the inhibitory element between 178 and 226. The sequence alterations in the mutant L1 in pBELM resulted in enhanced splicing, and it was therefore likely that the mutations affected a splicing silencer. Of the L1 sequences that negatively affected splicing, the sequence between 178 and 226 was one of the strongest.
FIG. 5.
(A) Schematic representation of the pBEL-pAEPL plasmid. A polylinker including a small sequence from the pCRII-TOPO cloning vector (Invitrogen) was inserted into L1, thereby replacing nucleotides 23 to 513 of L1 (numbering starts at A in the ATG of L1). The indicated L1 sequences of various length were inserted into the polylinker as SalI-BamHI or MluI-BamHI fragments. The numbering of the fragments start at the A in the L1 ATG. (B and C) Northern blot on total RNA extracted from HeLa cells transfected with the indicated plasmids. The blots were probed with the L1 probe (see Fig. 2A). Spliced mRNA as a percentage of total late RNA in each lane is indicated at the bottom of each gel. The data variation in each transfection experiment was less than 20%.
We divided the 178-366 region into four nonoverlapping short fragments and analyzed these in transfection experiments (Fig. 5A). The fragments spanning 178 to 226 and 318 to 366 displayed strong inhibitory effects on splicing, whereas the other two (226 to 270 and 270 to 318) did not (Fig. 5C, left panel). In contrast to the wt 178-226 sequence, the mutant 178-226 sequence did not inhibit splicing (Fig. 5C, right panel), demonstrating that the inhibitory effect on splicing was specific for the wt HPV-16 L1 sequence.
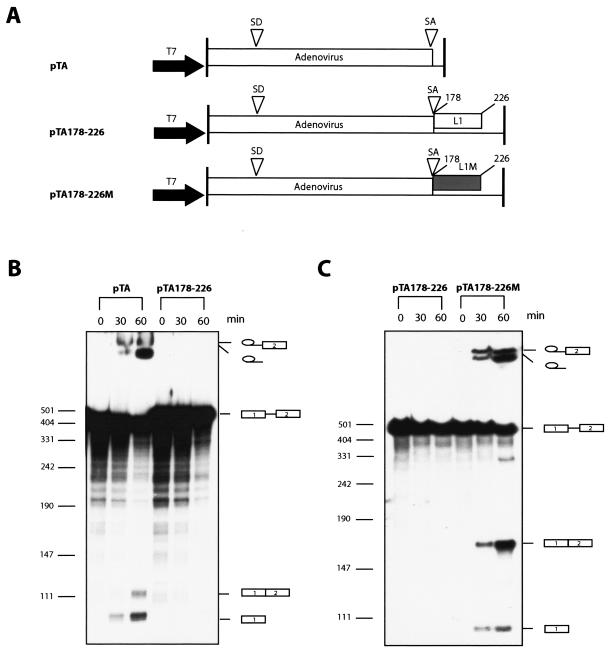
To confirm that the L1 sequence contained a splicing silencer, a fragment encompassing nucleotide 178 to 226 was inserted into pTA (Fig. 6A), which encodes an adenovirus mRNA which has been shown previously to splice efficiently in vitro (26), and in vitro splicing was performed (Fig. 6A). The results revealed that the RNA produced from the pTA vector was efficiently spliced as expected (Fig. 6B). In contrast, insertion of the wt 178-226 HPV-16 sequence into pTA efficiently inhibited splicing in vitro (Fig. 6B). As a control, the mutant 178-226 HPV-16 sequence was inserted in pTA (Fig. 6A). This sequence did not have a significant effect on the splicing of the mRNA produced from pTA (Fig. 6C). We concluded that the nucleotides between positions 178 and 226 in the HPV-16 L1 coding region contain a strong splicing silencer.
FIG. 6.
(A) Schematic representation of the pTA, pTA178-226, and pTA178-226 M plasmids used for in vitro synthesis of RNA substrates for the in vitro splicing reactions. The numbering of the inserted HPV-16 L1 fragments start at the A in the L1 ATG. L1, L1 wt sequence from position 178 to 226; L1 M, L1 mutant sequence from position 178 to 226; T7, T7 RNA polymerase promoter; SD, adenovirus 5′ ss; SA, adenovirus 3′ ss. (B) In vitro splicing using RNA derived from pTA or pTA178-226. The splicing products are indicated. (C) In vitro splicing using RNA derived from pTA178-226 or pTA178-226 M. The splicing products are indicated.
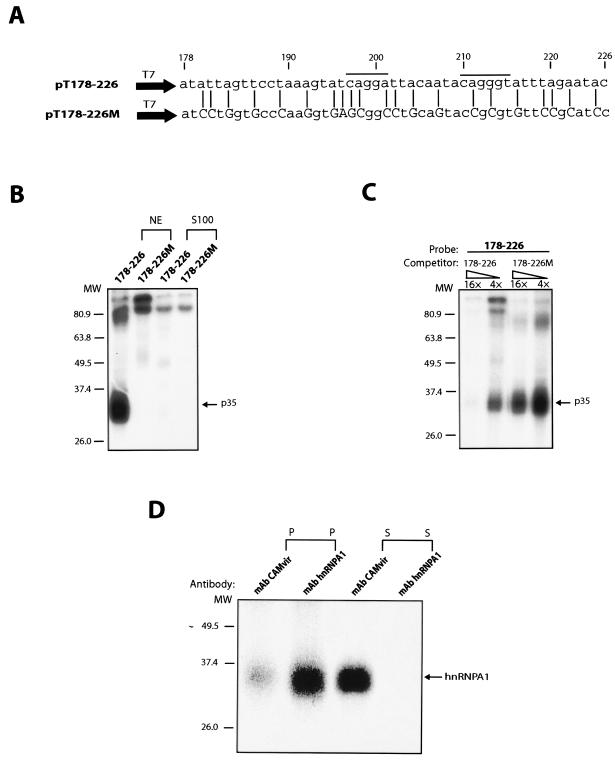
The splicing silencer interacts with hnRNP A1 in a sequence-specific manner.
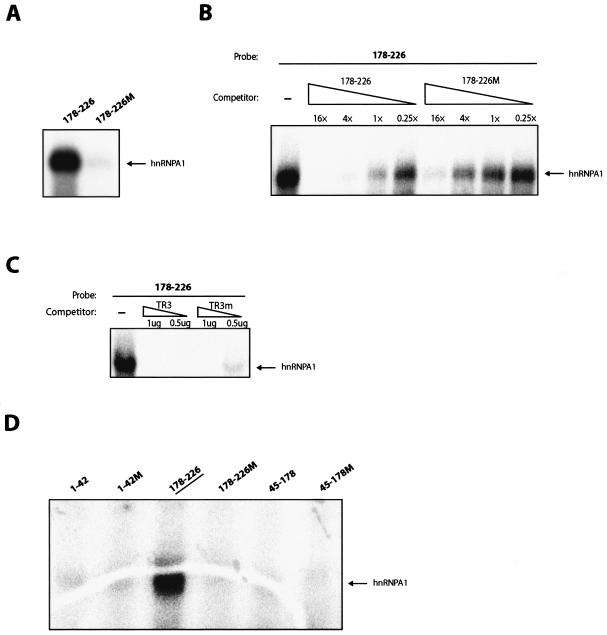
Having demonstrated that the 178-226 sequence inhibits splicing in a sequence-specific manner in living cells and in vitro, we tested if this sequence interacted with cellular factors. Radiolabeled wt 178-226 and mutant 178-226 (178-226 M) RNAs (Fig. 7A) were synthesized in vitro and subjected to UV cross-linking to nuclear and cytoplasmic S100 extracts. A 35-kDa nuclear factor cross-linked to the wt 178-226 sequence but not to the 178-226 M sequence (Fig. 7B). Excess unlabeled wt sequence competed with the probe for the 35-kDa protein (p35), whereas the mutant RNA sequence did not (Fig. 7C), demonstrating a sequence-specific interaction. Unrelated RNAs without hnRNP A1 binding sites did not compete for the 35-kDa protein (hnRNP A1) (data not shown). Immunoprecipitation of the cross-linked factor by using a monoclonal antibody against the HPV-16 L1 protein or against hnRNP A1 revealed that the anti-hnRNP A1 antibody specifically recognized p35 and depleted it from the cross-linked extract (Fig. 7D). In contrast, the HPV-16 L1 control antibody did not recognize p35, as expected (Fig. 7D). We concluded that p35 is hnRNP A1. Two potential hnRNP A1 binding sites are indicated above the wt 178-226 sequence (5).
FIG. 7.
(A) The sequences of the in vitro-transcribed inserts in plasmids pT178-228 and pT178-226 M are shown. The mutant positions are indicated with lines, and altered nucleotides are capitalized. These mutations were originally inserted in the L1 coding sequence in a way which did not affect the protein sequence of L1 while the L1 RNA sequence was altered (9). The numbering of the fragments start at the A in the L1 ATG. The two lines above the HPV-16 L1 sequence indicate two potential hnRNP A1 binding sites (5). (B) UV cross-linking of nuclear or cytoplasmic S100 extract to radiolabeled RNAs from the indicated plasmids. The p35 protein binding and cross-linking specifically to the wt HPV-16 L1 sequence is indicated. (C) UV cross-linking of nuclear extract to radiolabeled RNAs from plasmid pT178-226 in the presence of cold competitor RNA derived from the wt L1 sequence in pT178-226 or the mutant L1 sequence in pT178-226 M. Fold excess of the cold RNA is indicated. The p35 protein binding and cross-linking specifically to the wt HPV-16 L1 sequence is indicated. (D) Immunoprecipitation of the 35-kDa protein which UV cross-links specifically to the wt 178-226 sequence with a monoclonal antibody against hnRNP A1 (mAb hnRNP A1), but not with a monoclonal antibody against HPV-16 L1 capsid protein (mAb CAMvir).
Recombinant His-tagged hnRNP A1 cross-linked specifically to the 178-226 wt sequence and not to 178-226 M (Fig. 8A). Competition with unlabeled wt 178-226 sequence resulted in competition with the probe for hnRNP A1, whereas the mutant RNA sequence did not compete to the same extent (Fig. 8B). We also used single-stranded telomeric DNA repeats, named TR3, as competitor (60). hnRNP A1 has been shown previously to bind TR3 specifically (60). This sequence also competed efficiently with 178-226 RNA for hnRNP A1 (Fig. 8C), whereas a TR3-mutant single-stranded DNA (ssDNA) sequence competed less efficiently (Fig. 8C). In addition, recombinant hnRNP A1 did not cross-link to probes encompassing nucleotides 1 to 42 of the L1 sequence, wt or mutant, nor to RNA probes spanning 45 to 178, wt or mutant (Fig. 8D). Taken together, these results further demonstrated the specific interaction of hnRNP A1 with the splicing silencer sequence.
FIG. 8.
(A) UV cross-linking of recombinant His-tagged hnRNP A1 to radiolabeled RNAs from plasmid pT178-226 or pT178-226 M. (B) UV cross-linking of recombinant His-tagged hnRNP A1 to radiolabeled RNAs from plasmid pT178-226 in the presence of cold competitor RNA derived from the wt L1 sequence in pT178-226 or the mutant L1 sequence in pT178-226 M. _n_-fold excess of cold competitor is indicated. (C) UV cross-linking of recombinant His-tagged hnRNP A1 to radiolabeled RNA from plasmid pT178-226 in the absence or presence of wt or mutant single-stranded telomeric DNA repeats named TR3 and TR3m, respectively (60). hnRNP A1 has been shown previously to bind specifically to TR3 but less efficiently to the mutant named TR3m. (D) UV cross-linking of recombinant His-tagged hnRNP A1 to radiolabeled RNAs, which encode wt and mutant HPV-16 sequences derived from various parts of L1. The numbering of the fragments start at the A in the L1 ATG.
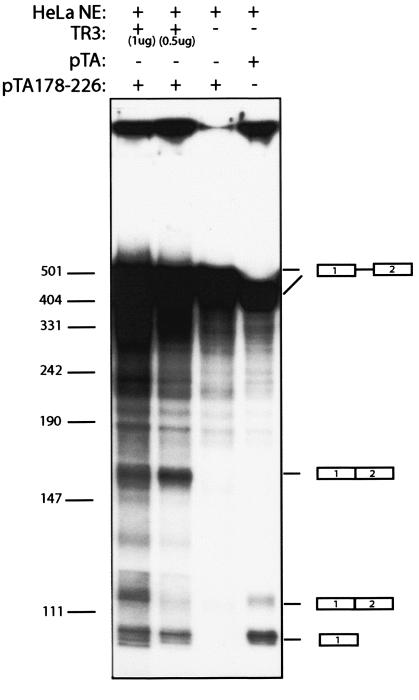
Titration of hnRNP A1 from nuclear extracts results in the induction of HPV-16 L1 splicing.
To obtain evidence for a functional interaction between hnRNP A1 and the HPV-16 splicing silencer, we included the single-stranded telomeric DNA repeats, TR3 (60), as a competitor for hnRNP A1 in an in vitro splicing reaction. pTA178-226, which contains the wt 178-226 HPV-16 L1 sequence with the splicing silencer and therefore does not splice, was subjected to in vitro splicing in the absence or presence of excess TR3 ssDNA. The results demonstrated that inclusion of TR3 oligonucleotide in the in vitro splicing reaction activated splicing of the RNA substrate that contained the HPV-16 L1 silencer element (Fig. 9). This effect was dose dependent (Fig. 9). In the absence of the TR3 oligonucleotide, splicing was suppressed by the silencer (Fig. 9; also shown in Fig. 6B and C). We concluded that the HPV-16 L1 silencer interacts with hnRNP A1 and that this interaction results in inhibition of splicing.
FIG. 9.
In vitro splicing using RNA derived from pTA or pTA178-226, in the absence or presence of the single-stranded telomeric DNA repeat competitor named TR3 (60). It has been shown previously that hnRNP A1 binds specifically to TR3 (60). The splicing products are indicated.
DISCUSSION
The splicing silencer acting on the L1 3′ splice site at position 5639 prevents direct splicing from one of the major 5′ splice sites at genomic nucleotide position 880 in the early region of the genome. This result is of particular interest, since it shows that the strong silencer at the L1 3′ ss is required to prevent expression of late mRNAs at an early stage of the infection. If this silencer at the L1 3′ ss at 5639 is not operational, one immediate consequence would be premature expression of at least the L1 late protein, an event that could lead to detection and elimination of the infected cell by the immune system before virions are produced. In addition, a persistent HPV-16 infection that would allow splicing to the late region and expression of late genes in a proliferating cell would most likely be detected and eliminated by the immune surveillance of the host and persistence would cease. The presence of a strong and functional splicing silencer at the L1 3′ splice site at 5639 therefore constitutes a prerequisite for the establishment of persistence. Since a persistent HPV-16 infection is a risk factor for development of cervical cancer (20) and high-risk HPV types are more likely to persist than low-risk types, it is reasonable to speculate that variations in the exact composition of the splicing silencer at the L1 3′ splice site in various HPV types may affect the outcome of the infection. Although we have previously shown that the L1 sequences of multiple HPV types (HPV-5, -6b, -16, -18, -31, -45, and -56) contain inhibitory RNA elements (9), it remains to be seen if these elements all correspond to functionally equivalent splicing silencers and if they inhibit splicing to the same extent. Interestingly, we have previously compared the inhibitory activity of the HPV-16 L1 and L2 sequences with those of the L1 and L2 sequenced from the benign, cutaneous low-risk HPV-1, and we found that the inhibitory activities of HPV-1 L1 and L2 were much lower than those for HPV-16 (46). Since there is a correlation between inhibitory activity of the HPV-16 L1 sequences and the presence of a splicing silencer, one may speculate that splicing into the late region occurs more frequently or earlier in the life cycle for the low-risk HPV-1 than for HPV-16. Indeed, it has been shown on in vivo material that HPV-1 late gene expression is activated at an earlier stage in the cellular differentiation program than HPV-16 late gene expression (35). It remains to be seen if further experiments will support a connection between the ability of the HPV type to persist and increase the risk of development of high-grade lesions and cervical cancer and the fine-tuned regulation of HPV mRNA processing.
The L2/L1 mRNA ratios produced by pBEL-pAE show that the proposed L2/L1 mRNAs are more abundant than the spliced wild-type L1 mRNA, suggesting that more L2 than L1 protein would be produced. This is unexpected, since there is more L1 than L2 in the virion and one would expect more L1 than L2 mRNA to be produced. Analysis by Northern blotting of late HPV-18 and HPV-31 mRNAs induced by cell differentiation in organotypic raft cultures showed that more L1 than L2/L1 mRNAs or similar levels of L1 and L2/L1 mRNAs were produced (15, 16, 22, 34). The ratio between L2/L1 and L1 mRNAs appeared to be lower than those observed here in the HeLa cells, indicating that a change in the splicing pattern of the late mRNAs towards higher levels of the spliced L1 mRNAs may occur as the infected cell differentiates. However, some variation in the ratio was seen in raft cultures, suggesting that splicing of the late mRNAs was sensitive to changes in the intracellular environment (15, 16, 22, 34). The comparison between the L2 and L1 ratio of the late mRNAs in HeLa cells and in terminally differentiated keratinocytes suggests that the intracellular concentration and/or activities of some RNA processing factors changes as the epithelial cell differentiates. In addition, intertype differences in the splicing pattern may be seen. It will be of interest to investigate the activity of the splice sites and their regulatory sequences in relation to cell differentiation and to determine if the intracellular concentration or subcellular localization of hnRNP A1 changes as the keratinocyte differentiates. In addition, the hnRNP A1 concentration may be different in HPV-infected cells from that in uninfected keratinocytes. For example, it has been shown that the mouse hepatitis virus alters the subcellular distribution of hnRNP A1 in murine cells, thereby gaining access to hnRNP A1 (29). In HPV-16-infected cells, alterations in the nuclear concentrations of hnRNP A1 are likely to affect late gene expression. Experiments designed to determine the concentration and subcellular localization of hnRNP A1 in normal stratified epithelium and in HPV-16-infected mucosa are currently under way.
The 3′ ss immediately upstream of L1 competes with all other 3′ ss at the HPV-16 genome, but due to regulation by a functional repressor at this site, the 3′ splice sites in the early region are favored. Since this must change during the course of the infection, the usage of the various splice sites in HPV-16 must be regulated and under the influence of enhancers and silencers which interact with different splicing factors. It will be interesting to investigate the regulation of splicing in the early region and to see if the utilization of the early 3′ ss affects splicing in the late region of the HPV-16 genome. Finally, we have previously identified the first 514 nucleotides of HPV-16 L1 as a region containing multiple RNA elements by the ability of multiple nonoverlapping sequences from this region to act in cis and inhibit production of L1 from an L1 cDNA (9) or by inhibiting CAT expression when inserted downstream of the CAT reporter gene (51). Therefore, there must be additional regulatory RNA elements within these 514 nucleotides. We have preliminary data that indicate the existence of one enhancer sequence and an additional silencer sequence in this region. Studies of regulatory RNA elements are in progress.
An exonic splicing suppressor named ESS1 has been identified in the early region of BPV-1 (57). This sequence can be divided into three regions that all interact with multiple cellular factors, for example, U2AF65, PTB, and serine/arginine-rich (SR) proteins, including ASF/SF2 (57). Only the C-rich region of the ESS is essential for suppression of splicing (56), and this region interacts with multiple SR proteins (57), suggesting that this silencer depends on SR proteins for its function. Similar to the silencer identified here, the ESS1 splicing suppressor in BPV-1 acts only on suboptimal splice sites (56, 58). A second silencer, named ESS2, acting on a differentiation-dependent 3′ splice site with suboptimal features, was identified in BPV-1 (59). The activity of this suppressor was 3′ splice site specific and enhancer specific. Taken together with the data presented here, one may speculate that the regulation of splicing in all papillomaviruses is very complex and plays an important role in the fine-tuned and differentiation-dependent expression of papillomavirus genes during the various stages of the viral life cycle.
We have previously shown that HIV-1 Rev and RRE can overcome the effect of the inhibitory RNA sequences in the HPV-16 L1 gene and induce expression of L1 from a transiently transfected L1 expression plasmid driven by the HIV-1 LTR promoter (51). The HIV-1 mRNAs are retained in the nucleus where they are degraded as a result of the presence of unutilized splice sites and intronic sequences on the mRNAs (36). These intronic sequences interact with nuclear factors, for example, hnRNP A1 (32, 61), and some encode splicing silencer, which interact with hnRNP A1 (3, 6, 30, 53). Therefore, HIV-1 Rev and RRE can overcome the inhibitory effect exerted by RNA sequence elements that interact with hnRNP A1, as well as the nuclear retention caused by unutilized splice sites. Both HPV-16 and HIV-1 mRNAs encode elements that interact with hnRNP A1, and as a result, expression of HPV-16 L1 from a cDNA expression plasmid can be enhanced by Rev and RRE as described previously (51).
In HIV-1, the pre-mRNA is spliced in a complex manner, which results in the production of a large number of differentially spliced mRNAs (40, 41). These mRNAs are inefficiently spliced as a result of suboptimal splice sites and the presence of splicing silencers on the mRNAs (49, 50). hnRNP A1 plays an important role in the inhibition of splicing of the HIV-1 mRNAs (3, 6, 30, 53). hnRNP A1 appears to bind at multiple sites on the HIV-1 pre-mRNA followed by protein-protein interactions between the hnRNP A1 molecules (4, 12). This bridging may prevent efficient recognition of a splice site. Alternatively, hnRNP A1 binds to a high-affinity site, which would allow hnRNP A1 molecules to fill up low-affinity sites in a cooperative manner to create a zone of RNA where spliceosome assembly is repressed (60). One may speculate that hnRNP A1 binding to the HPV-16 late 3′ splice site prevents the interaction of the splice site with the U2AF35/U2AF65 factors, thereby inhibiting splicing. The HPV-16 L1 3′ splice site at position 5639 is inefficiently utilized as a result of the suboptimal polypyrimidine tract. Suboptimal 3′ splice sites require splicing enhancers for their function (7). Alternatively, the sequence may act indirectly by interfering with the function of a proposed splicing enhancer. It is also well established that hnRNP A1 and ASF/SF2 are antagonistic factors (31). It remains to be investigated if the ratio between the concentrations of hnRNAP A1 and ASF/SF2 determines the levels of expression of the HPV-16 late genes and/or the ratios between the levels of HPV-16 L1 and L2/L1 mRNAs.
Acknowledgments
We thank Daniel Öberg for discussion, Brian Collier for materials and discussion, Elizabeth Doyle for RT-PCR analysis, Anette Carlsson for construction of pUCT7, Peter Kreivi and Anette Lindberg for materials, discussions, and help with in vitro splicing, Göran Akusjärvi for discussion, Tina Glisovic for His-tagged hnRNP A1, and G. Dreyfuss for monoclonal antibody against hnRNP A1.
Research was supported by grants from The Swedish Cancer Society and The Swedish Research Council.
REFERENCES
- 1.Baker, C. C. 1997. Posttranscriptional regulation of papillomavirus gene expression, p. 11-16. In S. R. Billakanti, C. E. Calef, A. D. Farmer, A. L. Halpern, and G. L. Myres (ed.), Human papillomaviruses: a compilation and analysis of nucleic acid and amino acid sequences. Theoretical Biology and Biophysics, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 2.Barksdale, S. K., and C. C. Baker. 1995. The human immunodeficiency virus type 1 Rev protein and the Rev-responsive element counteract the effect of an inhibitory 5′ splice site in a 3′ untranslated region. Mol. Cell. Biol. 15**:**2962-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilodeau, P. S., J. K. Domsic, A. Mayeda, A. R. Krainer, and C. M. Stoltzfus. 2001. RNA splicing at human immunodeficiency virus type 1 3′ splice site A2 is regulated by binding of hnRNP A/B proteins to an exonic splicing silencer element. J. Virol. 75**:**8487-8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchette, M., and B. Chabot. 1999. Modulation of exon skipping by high-affinity hnRNP A1-binding sites and by intron elements that repress splice site utilisation. EMBO J. 18**:**1939-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burd, C. G., and G. Dreyfuss. 1994. RNA binding specificity of hnRNP A1: significance of hnRNP A1 high-affinity binding sites in pre-mRNA splicing. EMBO J. 13**:**1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caputi, M., A. Mayeda, A. R. Krainer, and A. M. Zahler. 1999. hnRNP A/B proteins are required for inhibition of HIV-1 pre-mRNA splicing. EMBO J. 18**:**4060-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartegni, L., S. L. Chew, and A. R. Krainer. 2002. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat. Rev. Genet. 3**:**285-298. [DOI] [PubMed] [Google Scholar]
- 8.Collier, B., L. Goobar-Larsson, M. Sokolowski, and S. Schwartz. 1998. Translational inhibition in vitro of human papillomavirus type 16 L2 mRNA mediated through interaction with heterogenous ribonucleoprotein K and poly(rC)-binding proteins 1 and 2. J. Biol. Chem. 273**:**22648-22656. [DOI] [PubMed] [Google Scholar]
- 9.Collier, B., D. Öberg, X. Zhao, and S. Schwartz. 2002. Specific inactivation of inhibitory sequences in the 5′ end of the human papillomavirus type 16 L1 open reading frame results in production of high levels of L1 protein in human epithelial cells. J. Virol. 76**:**2739-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumming, S. A., M. G. McPhillips, T. Veerapraditsin, S. G. Milligan, and S. V. Graham. 2003. Activity of the human papillomavirus type 16 late negative regulatory element is partly due to four weak consensus 5′ splice sites that bind a U1 snRNP-like complex. J. Virol. 77**:**5167-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumming, S. A., C. E. Repellin, M. McPhilips, J. C. Redford, J. B. Clements, and S. V. Graham. 2002. The human papillomavirus type 31 untranslated region contains a complex bipartite negative regulatory element. J. Virol. 76**:**5993-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damgaard, C. K., T. O. Tange, and J. Kjems. 2002. hnRNP A1 controls HIV-1 mRNA splicing through cooperative binding to intron and exon splicing silencers in the context of a conserved secondary structure. RNA 8**:**1401-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich-Goetz, W., I. M. Kennedy, B. Levins, M. A. Stanley, and J. B. Clements. 1997. A cellular 65-kDa protein recognizes the negative regulatory element of human papillomavirus late mRNA. Proc. Natl. Acad. Sci. USA 94**:**163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11**:**1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frattini, M. G., H. B. Lim, J. Doorbar, and L. A. Laimins. 1997. Induction of human papillomavirus type 18 late gene expression and genomic amplification in organotypic cultures from transfected DNA templates. J. Virol. 71**:**7068-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frattini, M. G., H. B. Lim, and L. A. Laimins. 1996. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late expression. Proc. Natl. Acad. Sci. USA 93**:**3062-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furth, P. A., and C. C. Baker. 1991. An element in the bovine papillomavirus late 3′ untranslated region reduces polyadenylated cytoplasmic RNA levels. J. Virol. 65**:**5806-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furth, P. A., W. T. Choe, J. H. Rex, J. C. Byrne, and C. C. Baker. 1994. Sequences homologous to 5′ splice sites are required for the inhibitory activity of papillomavirus late 3′ untranslated regions. Mol. Cell. Biol. 14**:**5278-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassmann, K., B. Rapp, H. Maschek, K. U. Petry, and T. Iftner. 1996. Identification of a differentiation-inducible promoter in the E7 open reading frame of human papillomavirus type 16 (HPV-16) in raft cultures of a new cell line containing high copy numbers of episomal HPV-16 DNA. J. Virol. 70**:**2339-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho, G. Y., R. D. Burk, S. Klein, A. S. Kadish, C. J. Chang, P. Palan, J. Basu, R. Tachezy, R. Lewis, and S. Romney. 1995. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J. Natl. Cancer Inst. 87**:**1365-1371. [DOI] [PubMed] [Google Scholar]
- 21.Howley, P. M. 1996. Papillomavirinae: the viruses and their replication, p. 2045-2076. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 22.Hummel, M., H. B. Lim, and L. A. Laimins. 1995. Human papillomavirus type 31b late gene expression is regulated through protein kinase C-mediated changes in RNA processing. J. Virol. 69**:**3381-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy, I. M., J. K. Haddow, and J. B. Clements. 1990. Analysis of human papillomavirus type 16 late mRNA 3′ processing signals in vitro and in vivo. J. Virol. 64**:**1825-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy, I. M., J. K. Haddow, and J. B. Clements. 1991. A negative regulatory element in the human papillomavirus type 16 genome acts at the level of late mRNA stability. J. Virol. 65**:**2093-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koffa, M. D., S. V. Graham, Y. Takagaki, J. L. Manley, and J. B. Clements. 2000. The human papillomavirus type 16 negative regulatory RNA element interacts with three proteins that act at different posttranscriptional levels. Proc. Natl. Acad. Sci. USA 97**:**4677-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konarska, M. M., and P. A. Sharp. 1987. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell 49**:**763-774. [DOI] [PubMed] [Google Scholar]
- 27.Laimins, L. A. 1993. The biology of human papillomaviruses: from warts to cancer. Infect. Agents Dis. 2**:**74-86. [PubMed] [Google Scholar]
- 28.Leder, C., J. A. Kleinschmidt, C. Wiethe, and M. Müller. 2001. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J. Virol. 75**:**9201-9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, H. P., X. Zhang, R. Duncan, L. Comai, and M. M. Lai. 1997. Heterogeneous nuclear riboprotein A1 binds to the transcription-regulatory region of mouse hepatitis virus RNA. Proc. Natl. Acad. Sci. USA 94**:**9544-9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchand, V., A. Mereau, S. Jacquenet, D. Thomas, A. Mougin, R. Gattoni, J. Stevenin, and C. Branlant. 2002. A Janus splicing regulatory element modulates HIV-1 tat and rev mRNA production by coordination of hnRNP A1 cooperative binding. J. Mol. Biol. 323**:**629-652. [DOI] [PubMed] [Google Scholar]
- 31.Mayeda, A., and A. R. Krainer. 1992. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell 68**:**365-375. [DOI] [PubMed] [Google Scholar]
- 32.Najera, I., M. Krieg, and J. Karn. 1999. Synergistic stimulation of HIV-1 rev-dependent export of unspliced mRNA to the cytoplasm by hnRNP A1. J. Mol. Biol. 285**:**1951-1964. [DOI] [PubMed] [Google Scholar]
- 33.Öberg, D., B. Collier, X. Zhao, and S. Schwartz. 2003. Mutational inactivation of two distinct negative RNA elements in the human papillomavirus type 16 L2 coding region induces production of high levels of L2 in human cells. J. Virol. 77**:**11674-11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozbun, M. A., and C. Meyers. 1997. Characterization of late gene transcripts expressed during vegetative replication of human papillomavirus type 31b. J. Virol. 71**:**5161-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peh, W. L., K. Middleton, N. Christensen, P. Nicholls, K. Egawa, K. Sotlar, J. Brandsma, A. Percival, J. Lewis, W. J. Liu, and J. Doorbar. 2002. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J. Virol. 76**:**10401-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollard, V. W., and M. H. Malim. 1998. The HIV-1 Rev protein. Annu. Rev. Microbiol. 52**:**491-532. [DOI] [PubMed] [Google Scholar]
- 37.Rollman, E., L. Arnheim, B. Collier, D. Öberg, H. Hall, J. Klingström, J. Dillner, D. V. Pastrana, C. B. Buck, J. Hinkula, B. Wahren, and S. Schwartz. 2004. HPV-16 L1 genes with inactivated negative RNA elements induce potent immune responses. Virology 322**:**182-189. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz, S. 1998. Cis-acting negative RNA elements on papillomavirus late mRNAs. Semin. Virol. 8**:**291-300. [Google Scholar]
- 39.Schwartz, S. 2000. Regulation of human papillomavirus late gene expression. Upsala J. Med. Sci. 105**:**171-192. [PubMed] [Google Scholar]
- 40.Schwartz, S., B. K. Felber, D. M. Benko, E. M. Fenyö, and G. N. Pavlakis. 1990. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J. Virol. 64**:**2519-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz, S., B. K. Felber, E. M. Fenyö, and G. N. Pavlakis. 1990. Env and Vpu proteins of human immunodeficiency virus type 1 are produced from multiple bicistronic mRNAs. J. Virol. 64**:**5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz, S., M. Sokolowski, B. Collier, A. Carlsson, and L. Goobar-Larsson. 1999. Cis-acting regulatory sequences on papillomavirus late mRNAs. Recent Res. Dev. Virol. 1**:**53-74. [Google Scholar]
- 43.Shah, K. V., and P. M. Howley. 1996. Papillomaviruses, p. 2077-2109. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 44.Sokolowski, M., H. Furneaux, and S. Schwartz. 1999. The inhibitory activity of the AU-rich RNA element in the human papillomavirus type 1 late 3′ untranslated region correlates with its affinity for the elav-like HuR protein. J. Virol. 73**:**1080-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokolowski, M., and S. Schwartz. 2001. Heterogeneous nuclear ribonucleoprotein C binds exclusively to the functionally important UUUUU-motifs in the human papillomavirus type-1 AU-rich inhibitory element. Virus Res. 73**:**163-175. [DOI] [PubMed] [Google Scholar]
- 46.Sokolowski, M., W. Tan, M. Jellne, and S. Schwartz. 1998. mRNA instability elements in the human papillomavirus type 16 L2 coding region. J. Virol. 72**:**1504-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokolowski, M., C. Zhao, W. Tan, and S. Schwartz. 1997. AU-rich mRNA instability elements on human papillomavirus type 1 late mRNAs and c-fos mRNAs interact with the same cellular factors. Oncogene 15**:**2303-2319. [DOI] [PubMed] [Google Scholar]
- 48.Spångberg, K., L. Wiklund, and S. Schwartz. 2000. HuR, a protein implicated in oncogene and growth factor mRNA decay, binds to the 3′ ends of hepatitis C virus RNA of both polarities. Virology 274**:**378-390. [DOI] [PubMed] [Google Scholar]
- 49.Staffa, A., and A. Cochrane. 1995. Identification of positive and negative splicing regulatory elements within the terminal tat-rev exon of human immunodeficiency virus type 1. Mol. Cell. Biol. 15**:**4597-4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staffa, A., and A. Cochrane. 1994. The tat/rev intron of human immunodeficiency virus type 1 is inefficiently spliced because of suboptimal signals in the 3′ splice site. J. Virol. 68**:**3071-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan, W., B. K. Felber, A. S. Zolotukhin, G. N. Pavlakis, and S. Schwartz. 1995. Efficient expression of the human papillomavirus type 16 L1 protein in epithelial cells by using Rev and the Rev-responsive element of human immunodeficiency virus or the _cis_-acting transactivation element of simian retrovirus type 1. J. Virol. 69**:**5607-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan, W., and S. Schwartz. 1995. The Rev protein of human immunodeficiency virus type 1 counteracts the effect of an AU-rich negative element in the human papillomavirus type 1 late 3′ untranslated region. J. Virol. 69**:**2932-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tange, T. O., C. K. Damgaard, S. Guth, J. Valcarel, and J. Kjems. 2001. The hnRNP A1 protein regulates HIV-1 tat splicing via a novel intron silencer element. EMBO J. 20**:**5748-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiklund, L., M. Sokolowski, A. Carlsson, M. Rush, and S. Schwartz. 2002. Inhibition of translation by UAUUUAU and UAUUUUUAU motifs of the AU-rich RNA instability in the HPV-1 late 3′ untranslated region. J. Biol. Chem. 277**:**40462-40471. [DOI] [PubMed] [Google Scholar]
- 55.Zheng, Z. M. 2004. Regulation of alternative RNA splicing by exon definition and exon sequences in viral and mammalian gene expression. J. Biomed. Sci. 11**:**278-294.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng, Z. M., P. J. He, and C. C. Baker. 1999. Function of a bovine papillomavirus type 1 exonic splicing suppressor requires a suboptimal upstream 3′ splice site. J. Virol. 73**:**29-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng, Z. M., M. Huynen, and C. C. Baker. 1998. A pyrimidine-rich exonic splicing suppressor binds multiple RNA splicing factors and inhibits spliceosome assembly. Proc. Natl. Acad. Sci. USA 95**:**14088-14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng, Z. M., J. Quintero, E. S. Reid, C. Gocke, and C. C. Baker. 2000. Optimization of a weak 3′ splice site counteracts the function of a bovine papillomavirus type 1 exonic splicing suppressor in vitro and in vivo. J. Virol. 74**:**5902-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng, Z. M., E. S. Reid, and C. C. Baker. 2000. Utilization of the bovine papillomavirus type 1 late-stage-specific nucleotide 3605 3′ splice site is modulated by a novel exonic bipartite regulator but not by an intronic purine-rich element. J. Virol. 74**:**10612-10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu, J., A. Mayeda, and A. R. Krainer. 2001. Exon identity established through differential antagonism between exonic splicing silencer-bound hnRNP A1 and enhancer-bound SR proteins. Mol. Cell 8**:**1351-1361. [DOI] [PubMed] [Google Scholar]
- 61.Zolotukhin, A. S., D. Michalowski, J. Bear, S. V. Smulevitch, A. M. Traish, R. Peng, J. Patton, I. N. Shatsky, and B. K. Felber. 2003. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol. Cell. Biol. 23**:**6618-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.zur Hausen, H. 1999. Papillomaviruses in human cancers. Proc. Assoc. Am. Physicians 111**:**581-587. [DOI] [PubMed] [Google Scholar]