Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis (original) (raw)
Abstract
SJL mice are highly susceptible to experimental autoimmune encephalomyelitis (EAE) induced with myelin proteolipid protein (PLP) peptide 139-151, whereas H-2 congenic B10.S mice are resistant. Immunodominance and susceptibility to EAE are associated with a high precursor frequency of PLP 139-151-specific T cells in the naïve repertoire of SJL mice. To understand the mechanism of EAE resistance in B10.S mice, we determined the precursor frequency of PLP 139-151-reactive T cells in both strains by using IAs/PLP 139-151 tetramers. SJL and B10.S mice had similar frequencies of tetramer-reactive T cells in the naïve peripheral repertoire. However, in SJL mice, the majority of PLP 139-151 tetramer-positive cells were in the CD4+CD25- population, whereas there were more tetramer-positive cells in the CD4+CD25+ population of B10.S mice. Depletion of CD4+CD25+ cells in vivo facilitated the expansion of PLP 139-151-reactive cells with production of T helper 1 cytokines in EAE-resistant B10.S mice. Furthermore, anti-CD25 Ab treatment before immunization resulted in EAE induction in these otherwise resistant mice. These data indicate an important role for autoantigen-specific CD4+CD25+ cells in genetic resistance to autoimmunity.
Studies in various models of experimental autoimmune encephalomyelitis (EAE) have provided new insights into autoimmune disease mechanisms. In SJL mice, we and others have shown that proteolipid protein (PLP) 139-151 is the immunodominant encephalitogenic PLP epitope and that it induces severe EAE (1-3). In contrast, B10.S mice are highly resistant, even though both strains carry the H-2s MHC molecules (3), suggesting that differences in non-MHC genes contribute to susceptibility and resistance to EAE. By using crosses between SJL and B10.S mice, we and others have identified multiple loci that contribute to disease susceptibility (3-6), but the actual genes and cellular mechanisms determining susceptibility have not been elucidated.
There is now considerable evidence that CD4+CD25+ T cells play a critical role in the regulation of autoimmune diseases (7-9). They constitutively express Forkhead box P3 (10, 11). Emerging evidence suggests that CD4+CD25+ cells are generated in the thymus by a high-affinity interaction of the T cell receptor (TCR) with self-peptides bound to MHC II molecules rendering these self-reactive cells anergic (12-14). However, the antigen (Ag)-binding specificity of the TCR of regulatory CD4+CD25+ cells that suppress autoimmunity is not known. By using IAs/PLP 139-151 tetramers, we show here that EAE-resistant, B10.S mice possess significantly greater proportions of PLP 139-151-reactive CD4+CD25+ T cells in their periphery when compared with EAE-susceptible, SJL mice. CD4+CD25+ cells suppressed the expansion of PLP 139-151-responsive CD4+CD25- cells in naïve B10.S mice. Anti-CD25 Ab treatment of B10.S mice resulted in an increase in PLP 139-151-specific T cell proliferation with significant IFN-γ production. Furthermore, depletion of CD4+CD25+ cells in vivo with an anti-CD25 Ab resulted in a higher incidence of EAE in normally resistant B10.S mice. These results point to a pivotal role for Ag-specific CD4+CD25+ T cells in the regulation of susceptibility to EAE.
Materials and Methods
Mice. SJL mice were procured from The Jackson Laboratory (Bar Harbor, ME), and B10.S mice were obtained from the McLaughlin Research Institute (Great Falls, MT). The mice were maintained according to the animal protocol guidelines of Harvard Medical School.
Peptide Synthesis, Immunization Protocols, and Delayed Type Hypersensitivity (DTH). PLP 139-151 (HSLGKWLGHPDKF), Theiler's murine encephalomyelitis virus (TMEV) VP2 70-86 (W T TSQEAFSHIR IPLP), ovalbumin (OVA) 323-329 (ISQAVHAAHAEINEAGR), and neuraminidase (NASE) 101-120 (EALVRQGLAKVAYVYKPNNT) were synthesized on 9-fluorenylmethyloxycarbonyl chemistry (QCB, BioSource, Hopkinton, MA). For immunizations, 100-150 μg of each peptide emulsified in complete Freund's adjuvant was administered s.c. DTH responses were measured 7 days after immunization by injecting 100 μg of PLP 139-151 into the right footpad. Twenty-four hours later, DTH responses were assessed by measuring the thickness of the hind foot with a micrometer (Mitutoyo, Tokyo) and comparing it with the thickness of the uninjected left hind foot.
T Cell Proliferation. Single-cell suspensions were obtained from spleens and lymph nodes (LN) of naïve SJL and B10.S mice, and CD4+CD25+ and CD4+CD25- subsets were fractionated by magnetic separation using LS columns (Miltenyi Biotec, Auburn CA). For proliferation assays, 1.5 to 2.0 × 106 cells per ml CD4+CD25+ or CD4+CD25- cells or both were cultured with anti-CD3 Ab (0-1 μg/ml) in HL-1 medium (BioWhittaker) for 2 days or PLP 139-151 (0-150 μg/ml) for 3 days in the presence of antigen-presenting cells (APC). Sixteen hours after pulsing with 1 μCi of [3H]thymidine (1 Ci = 37 GBq), proliferation was measured as cpm by using a Wallac liquid scintillation counter (PerkinElmer). CD3+ T cells from draining LN were enriched by negative selection (R & D Systems). To determine recall responses to PLP 139-151 in B10.S mice depleted of CD25+ cells, 400 μg of anti-CD25 Ab (clone PC61, BioExpress, West Lebanon, NH) and its isotype control (rat IgG, ICN Biomedicals, Aurora, OH) were administered i.p. on days -5 and -3 into 4- to 8-week-old mice. The mice were immunized with 150 μg of PLP 139-151 in complete Freund's adjuvant on day 0. Ten days later, recall responses of CD3+ T cells to PLP 139-151 were tested, as described above.
IAs Tetramer Staining. IAs tetramers for PLP 139-151 and TMEV 70-86 were generated as described in ref. 15. TMEV tetramers were used as negative controls. T cells enriched from LN of naïve SJL and B10.S mice were treated with neuraminidase (0.7 units/ml) in serum-free medium (DMEM, BioWhittaker) at a density of 1 × 107 cells per ml for 1 h at 37°C. After washing, cells were incubated with the tetramers (30 μg/ml) for 3-4 h at 37°C in DMEM containing IL-2 (pH 8.0), followed by staining with anti-CD4-APC (clone RM4.5), anti-CD25-FITC (clone 7D4), and 7-amino-actinomycin D (7-AAD) (all three from Pharmingen). Cells were acquired by using the FACSort flow cytometer (Becton Dickinson), and tetramer-positive cells were determined in live CD4 or CD25+ populations after gating the dead (7-AAD+) cells by using the programs cellquest (Becton Dickinson) and flowjo (Tree Star, Ashland, OR).
Cytokine Measurement. To determine the frequency of cytokine-producing cells, LN cells (LNC) from SJL or B10.S mice immunized with PLP 139-151 with or without anti-CD25/control Ab treatment were restimulated twice with PLP 139-151 (20 μg/ml). Viable cells then were stimulated with anti-TCR Ab (clone H57597, 2.5 μg/ml) and anti-CD28 (clone 37.51, 4 μg/ml) and 2 mM monensin (GolgiStop, Pharmingen) for 4-6 h at 37°C. After staining with anti-CD4 (clone RM 4.5-APC) and 7-AAD, the cells were stained with cytokine Ab as recommended by the manufacturer (Pharmingen). Supernatants from the above cultures were tested for IL-2, IL-4, IL-10, and IFN-γ as described in ref. 16.
Induction of EAE. Eight- to twelve-week-old mice were immunized s.c. in the flanks with an emulsion containing PLP 139-151 and Mycobacterium tuberculosis, H37Ra extract (4 mg/ml) (Difco) in complete Freund's adjuvant. To determine the effect of anti-CD25 Ab treatment on the development of EAE, 4- to 8-week-old B10.S mice of either sex were treated with anti-CD25 Ab (clone PC61) or rat IgG isotype control twice on days -5 and -3 before inducing EAE. Pertussis toxin was administered (100 ng per mouse) i.p. on the day of immunization and day 2 after immunization. The mice were observed for signs of EAE for up to 4 weeks and scored as described in refs. 2 and 3.
Histopathology. Mice were killed when they reached a score of 4 or higher or when they began to recover from disease as indicated by a lack of further increase in clinical score. Brains and spinal cords from the mice were fixed in 10% phosphate-buffered formalin. Histological disease was evaluated by counting inflammatory foci in meninges and parenchyma as described in ref. 17.
Statistics. Comparisons of the differences in immune responses (proliferation, DTH, and IFN-γ production) and numbers of IAs tetramer-positive cells in SJL and B10.S mice were made by using Student's t test. The incidence of PLP 139-151-induced EAE in SJL and B10.S mice was analyzed by Fisher's exact test. P ≤ 0.05 was considered significant.
Results
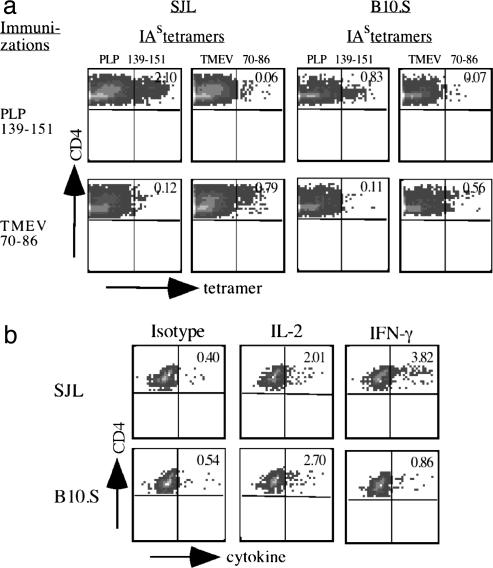
B10.S Mice Are Unresponsive to the Encephalitogenic PLP 139-151 Peptide. After immunization with PLP 139-151, the disease incidence and mean clinical scores in a cohort of B10.S mice were significantly lower than in SJL mice (P < 0.0001 for both) (see Table 2, which is published as supporting information on the PNAS web site). Thus, resistance to PLP 139-151-induced EAE in B10.S mice likely was influenced by non-MHC genes. To determine whether B10.S mice have a generalized defect in the expansion of T cells that recognize self or nonself Ags, B10.S mice were immunized with self (PLP 139-151) and foreign Ags (OVA), and recall responses to PLP 139-151, OVA, and purified protein derivative were tested. As shown in Table 2, proliferation and IFN-γ production in response to PLP 139-151 were significantly lower in B10.S mice compared with SJL mice (P = 0.0001 and P = 0.001, respectively). Correspondingly, the DTH response to PLP 139-151 was substantially lower in B10.S mice (P = 0.02). By contrast, LNC from B10.S and SJL mice responded comparably to foreign Ags (purified protein derivative and OVA) as indicated by the production of IFN-γ, suggesting that the defect in the T cell response in B10.S mice was restricted largely to the autoantigen. These results were verified further by using class II/IAs tetramers specific for PLP 139-151 or TMEV 70-86 peptides. In cultures derived from SJL mice immunized with PLP 139-151, there were nearly 3-fold more PLP 139-151 tetramer-reactive CD4 cells than there were in cells from B10.S mice (2.10% vs. 0.83%) (Fig. 1_a_). By contrast, in cultures from TMEV 70-86-immunized mice, the frequencies of TMEV 70-86 tetramer-reactive cells were similar in SJL and B10.S mice, although the frequency in B10.S mice was slightly lower (0.79% vs. 0.56%) (Fig. 1_a_). Recall responses to these peptides followed a similar trend (see Fig. 6, which is published as supporting information on the PNAS web site). The frequency of cytokine-secreting CD4+ cells in cultures stimulated with PLP 139-151 revealed comparable numbers of IL-2-producing cells in SJL and B10.S mice, but the frequency of IFN-γ-secreting cells was ≈4-fold higher in SJL mice (Fig. 1_b_).
Fig. 1.
B10.S mice have a diminished recall response to PLP 139-151. (a) Tetramer staining. LNC from SJL and B10.S mice immunized with PLP 139-151 or TMEV 70-86 peptide were restimulated with the corresponding peptides, and percentages of IAs tetramer-positive cells were determined in the viable (7-AAD-) CD4+ populations. (b) Intracellular cytokine staining. LNC from SJL and B10.S mice immunized with PLP 139-151 were restimulated with PLP 139-151. After 3 days, frequencies of IL-2- and IFN-γ-secreting cells were determined by flow cytometry. A representative experiment from three or four individual experiments involving two or three mice per group is shown in each plot.
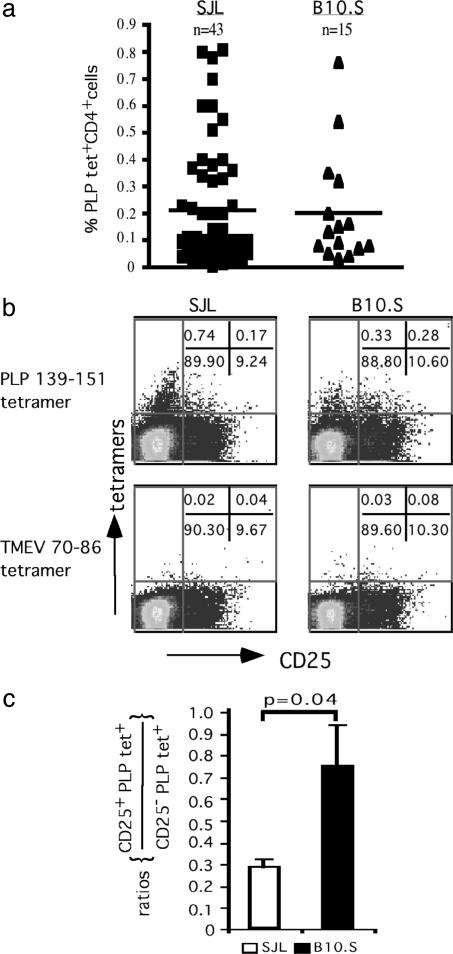
Naïve SJL and B10.S Mice Differ in the Frequency of PLP 139-151-Reactive T Cells in CD4+CD25+ and CD4+CD25- Subsets. The frequency of PLP 139-151-tetramer positive CD4+ cells in the naïve repertoire of SJL and B10.S mice varied greatly among individual mice (0.02-0.81%), but the average for large numbers of SJL and B10.S mice was ≈0.2% (Fig. 2_a_). This frequency (40 PLP-reactive cells in 20,000 CD4+ cells) is significantly higher than that determined by limiting dilution assay (1 in 20,000) (18) and may reflect enhanced sensitivity of detection with tetramers that does not depend on in vitro expansion of Ag-specific T cells. Reactivity to the TMEV 70-86 tetramer was present in both strains but at significantly lower frequencies (average = 0.07% and 0.09% in SJL and B10.S mice, respectively) than observed for PLP 139-151 tetramers. Therefore, these data indicate that differences in T cell expansion between SJL and B10.S mice to PLP 139-151 after immunization cannot be explained simply by a difference in precursor frequency in their naïve repertoires.
Fig. 2.
Naïve B10.S mice have greater proportions of PLP 139-151-reactive CD4+CD25+ T cells in their periphery than naïve SJL mice. (a) CD3+ T cells from naïve SJL and B10.S mice were stained with IAs tetramers (PLP 139-151 and TMEV 70-86) after treatment with neuraminidase, and the tetramer-positive cells were enumerated in the live CD4+ population. Horizontal bars indicate the average frequency of tetramer-positive CD4+ cells. (b) CD3+ T cells from naïve SJL and B10.S mice were incubated with IAs tetramers (PLP 139-151 or TMEV 70-86) after neuraminidase treatment, and frequencies of tetramer-positive cells were determined in the live CD4+ population in relation to the CD25 marker. (c) The relative precursor frequencies of PLP 139-151 tetramer-positive cells are expressed as the ratio of tetramer-positive cells in the CD4+CD25+ compartment to those in the CD4+CD25 negative compartment (n = 4 per group).
We next determined whether there are differences in IAs/PLP 139-151 tetramer-reactive T cells in the CD4+CD25+and CD4+CD25- compartments of these mice. We found that in SJL mice, the majority of PLP 139-151 tetramer-reactive cells were in the CD4+CD25- subset but that in the B10.S mice, the PLP 139-151 tetramer-reactive cells were present in nearly equal proportions in the CD4+CD25+ and CD4+CD25- populations (Fig. 2_b_). Furthermore, analysis of ratios of CD4+CD25+ to CD4+CD25- in the PLP 139-151 and TMEV 70-86 tetramer-reactive T cells demonstrated a significantly higher proportion of PLP 139-151 tetramer-reactive cells in the CD4+CD25+ cell subset in B10.S mice compared with SJL mice (Fig. 2_c_). There were no differences in the TMEV 70-86 tetramer-reactive populations. Because CD4+CD25+ cells include naturally occurring regulatory cell populations, this observation raised the possibility that PLP tetramer-reactive CD4+CD25+ T cells may influence susceptibility to EAE in B10.S mice.
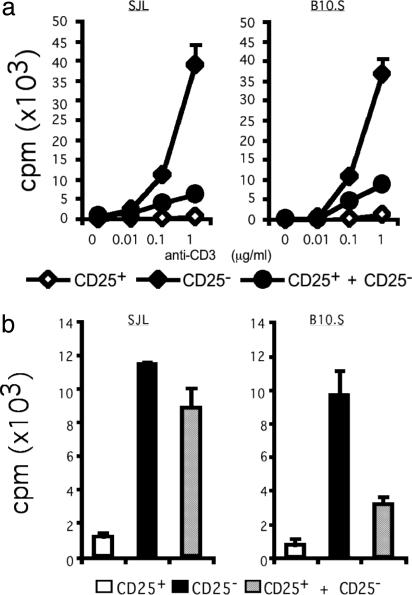
CD4+CD25+ Cells Are Functional and Their Suppressive Effect Is Ag-Specific. To determine whether CD4+CD25+ cells in SJL and B10.S mice are equally capable of regulating T cell responses, we first tested their ability to proliferate in the presence of the polyclonal activator, anti-CD3 Ab. As shown in Fig. 3_a_, CD4+CD25+ cells from both SJL and B10.S mice did not respond to anti-CD3 Ab stimulation, whereas CD4+CD25- cells showed significant proliferative responses to the anti-CD3 Ab. In a coculture of the two populations, CD4+CD25+ cells inhibited the proliferative responses of CD4+CD25- cells ≈5- to 8-fold. Therefore, there is no global defect in the functional activity of CD4+CD25+ cells to mediate their suppressive functions in either SJL or B10.S strains. Next, we compared the suppressive activity of CD4+CD25+ cells with the specific self-Ag PLP 139-151. As expected, CD4+CD25+ cells from either SJL or B10.S mice did not respond to PLP 139-151, and CD4+CD25- cells showed a strong proliferative response to PLP 139-151 (Fig. 3_b_). However, when CD4+CD25+ cells were cocultured with CD4+CD25- cells, the proliferative response of the CD4+CD25- cells to PLP 139-151 was inhibited (3- to 4-fold) in B10.S mice. In contrast, the CD4+CD25+ cells derived from the SJL mice did not inhibit responses to PLP 139-151 as effectively as in the B10.S mice (Fig. 3_b_). These data suggest that CD4+CD25+ cells may play a role in preventing expansion of PLP 139-151-specific T cells in B10.S mice. However, there was no inherent defect in the PLP-reactive effector cells in SJL mice, because CD4+CD25+ T cells from B10.S mice could inhibit the expansion of CD4+CD25- T cells from SJL mice as efficiently and effectively as they inhibited CD4+CD25- T cells from B10.S mice (data not shown). The efficient inhibition by B10.S derived CD4+CD25+ T cells may be attributed to a higher frequency of PLP 139-151-reactive, CD4+CD25+ T cells in B10.S than in SJL mice (Fig. 2 b and c).
Fig. 3.
CD4+CD25+ cells in SJL and B10.S mice are functional. (a) CD4+CD25+ and CD4+CD25- fractions were enriched from naïve SJL and B10.S mice. The different populations, either alone or together, were stimulated with anti-CD3 Ab in the presence of APC, and proliferative responses were measured as cpm. (b) CD4+CD25+ and CD4+CD25- populations as in a were stimulated with PLP 139-151 (150 μg/ml) in the presence of APC, and proliferative responses were measured. A representative experiment from three to five individual experiments is shown in each graph.
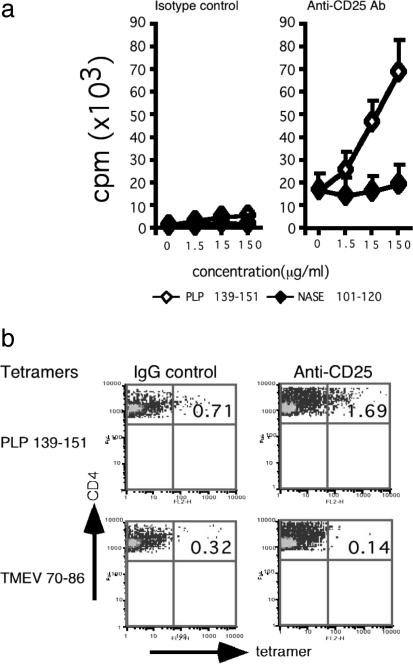
Depletion of CD25+ Cells in Vivo Enhances PLP 139-151-Specific T Cell Responses in B10.S Mice. We depleted CD25+ cells in B10.S mice before immunization with PLP 139-151 and tested recall responses to PLP 139-151 or NASE 101-120 (control) after 10 days. T cells from anti-CD25 Ab-treated mice responded more vigorously to PLP 139-151 than T cells from the control IgG-treated group (Fig. 4_a_). In the anti-CD25 Ab-treated group, there was also an increase in the basal proliferation of cells, i.e., without Ag, which may reflect a general lack of regulatory control mediated by CD4+CD25+ cells. The response to PLP 139-151 was specific because it followed a typical dose-response, and there was no response to the control peptide (Fig. 4_a_). By using IAs tetramers, we found that the PLP 139-151-reactive cells were present at a frequency ≈3-fold higher in the anti-CD25 treated group than in the IgG control Ab-treated group (Fig. 4_b_).
Fig. 4.
Depletion of CD25+ cells in vivo enhances PLP 139-151-specific T cell responses in B10.S mice. (a) Proliferation. CD3+ T cells from PLP 139-151-immunized B10.S mice that had been treated with anti-CD25 Ab or rat IgG control were restimulated with PLP 139-151, and proliferative responses were measured. (b) IAs tetramer staining. CD3+ T cells from PLP 139-151-immunized B10.S mice that were pretreated with anti-CD25 Ab or rat IgG were restimulated with PLP 139-151 for 4 days. The percentages of IAs tetramer (PLP 139-151, TMEV 70-86)-positive cells were determined in the viable (7-AAD-) CD4 subset. A representative experiment from five individual experiments involving two mice per group is shown.
T Cells from Anti-CD25-Treated B10.S Mice Produce Significant Amounts of IFN-γ. One factor involved in EAE resistance in B10.S mice is that immunization does not induce myelin basic protein (MBP)-specific IFN-γ production (19). Because B10.S mice possess significant numbers of PLP-reactive CD4+CD25+ cells, we hypothesized that they may keep the expansion of pathogenic effector cells that are capable of producing IFN-γ in check. In both anti-CD25 Ab- and isotype control-treated mice, there were cells capable of producing all of the cytokines tested (IL-2, IL-5, IL-10, and IFN-γ). However, in contrast to the isotype control-treated group, the frequency of IL-10-secreting cells was reduced and the frequency of IFN-γ-producing cells was increased in anti-CD25 Ab-treated mice (Fig. 5_a_). There was also an increase in the frequency of IL-2-producing cells in the anti-CD25-treated cultures. These profiles were verified by cytokine ELISA, which revealed that in the cultures from B10.S mice treated with anti-CD25 Ab, there was a significant increase in IFN-γ production with a concomitant decrease in IL-10 production (Fig. 5_b_). The data thus suggest that the anti-CD25 Ab treatment resulted in the expansion of PLP 139-151-reactive cells producing T helper 1 (Th1) cytokines after immunization with PLP 139-151.
Fig. 5.
Anti-CD25 Ab treatment alters cytokine production toward a Th1 phenotype. (a) Intracellular staining. LNC from PLP 139-151-immunized B10.S mice that were pretreated with anti-CD25 or rat IgG Ab were restimulated twice with PLP 139-151. Viable lymphoblasts were restimulated with anti-TCR and anti-CD28 Ab for 4-6 h. After staining with anti-CD4 Ab and 7-AAD, the frequency of IL-2-, IL-4-, IL-5-, IL-10-, and IFN-γ-secreting cells was determined in the live (7-AAD-) CD4+ subset by using flow cytometry. A representative sample from six individual experiments is shown. *, Isotype control for IL-2 and IL-10; **, Isotype control for IL-4 and IL-5 and IFN-γ. (b) Cytokine ELISA. Supernatants from the above cultures were analyzed by ELISA for production of IL-2, IL-4, IL-10, and IFN-γ. Each bar represents mean ± SEM values for a group of experiments (anti-CD25 Ab, n = 5; rat IgG, n = 6).
Depletion of CD25+ Cells in Vivo Induces EAE in B10.S Mice. Because depletion of CD25+ cells in vivo altered proliferative responses and IFN-γ production of PLP 139-151-reactive T cells, we determined whether anti-CD25 Ab treatment made B10.S mice susceptible to EAE. After depleting CD25+ cells with anti-CD25 Ab, the mice were immunized with PLP 139-151 in complete Freund's adjuvant. As shown in Table 1, B10.S mice treated with control Ab did not develop clinical EAE, whereas about one-third of the CD25+ Ab-treated mice developed clinical EAE (P = 0.0015). Inflammatory foci were detected in both meninges and parenchyma in anti-CD25 Ab-treated mice, whereas none of the control Ab-treated mice showed any histological disease (Table 1).
Table 1. Clinical and histological evaluation of EAE induced by PLP 139–151 in B10.S mice treated with anti-CD25 Ab or isotype control.
| Clinical disease* | Histopathology†, no. of inflammatory lesions | |||||
|---|---|---|---|---|---|---|
| Treatment | Incidence‡ (%) | Mean day of onset | Mean maximum score | Meninges | Parenchyma | Total |
| Anti-CD25 Ab | 9/32 (28.1) | 13.8 ± 1.4 | 2.2 ± 0.6 | 44.1 ± 14.0 | 42.6 ± 17.6 | 86.9 ± 31.1 |
| Rat IgG control | 0/34 (0) | 0 | 0 | 0 | 0 | 0 |
Discussion
In this study, we describe the cellular basis for resistance to EAE in B10.S mice after immunization with PLP 139-151. Both SJL and B10.S mice possess the same MHC haplotype H-2s, yet they differ in their susceptibility to EAE, suggesting that background genes play a crucial role in disease resistance in B10.S mice. A lack of expansion and production of IFN-γ after immunization with PLP 139-151 led us to investigate the cellular mechanisms that contribute to EAE resistance in this strain.
After immunization, B10.S mice showed a blunted T cell response to the self-Ag, PLP 139-151 but not to foreign Ags. We speculated that there might be differences in the frequency of PLP 139-151-reactive T cells in the periphery of naïve SJL and B10.S mice, but direct measurement with PLP 139-151-I-As tetramers showed that this was not the case. Expanded populations of autoreactive T cells have been demonstrated for two other autoantigens, i.e., pancreatic Ag-specific CD4+ cells in nonobese diabetic mice that are recognized by the BDC/IAg7 tetramer (20, 21) and the human CD8+ cells recognized by class I tetramers that react with the melanocyte-differentiating Ag, MELAN-A, in healthy HLA-A2+ individuals (22). How these autoreactive T cells are generated and maintained in the peripheral repertoire and whether they are critical for the development of autoimmune disease has not been determined.
When we analyzed IAs/PLP 139-151 tetramer-reactive cells in relation to CD25, the fraction of PLP 139-151 tetramer-reactive, CD4+CD25+ cells was strikingly higher in B10.S than in SJL mice (Fig. 2 b and c). This finding led us to question whether the difference in the fraction of regulatory PLP 139-151-specific, TCR-bearing CD4+ cells could explain partly the differences in the expansion of PLP 139-151-specific T cells, IFN-γ production, and disease susceptibility between the two strains.
It is now widely accepted that CD4+CD25+ cells can play a critical role in the development of autoimmune disease (7-9). CD4+CD25+ cells have been found to inhibit the expansion of CD4+CD25- cells in vitro in coculture systems by using polyclonal activators such as anti-CD3 and Con-A (9, 23, 24). However, it was not known whether the suppressive function of CD4+CD25+ cells is Ag-specific. Here, we demonstrate that in naïve animals, CD4+CD25+ cells act as natural regulatory cells in that they prevent expansion of PLP 139-151-reactive CD4+CD25- in an Ag-specific manner (Fig. 3_b_). This inhibitory function was more pronounced in EAE-resistant B10.S mice than in EAE-susceptible SJL mice, and these differences were reflected in the numbers of Ag-reactive T cells in CD4+CD25+ and CD4+CD25- compartments (Fig. 2 b and c). By depleting CD25+ cells in B10.S mice and immunizing with PLP 139-151, we confirmed that both Ag-specific proliferation (Fig. 4) and IFN-γ production (Fig. 5) were increased significantly. Consistent with these results, approximately one-third of the mice treated with anti-CD25 Ab developed clinical and histological evidence of EAE. Because not all of the mice developed EAE, the EAE resistance of B10.S mice may not lie entirely in the CD25+ compartment. Taken together, however, the data suggest that the CD4+CD25+ T cells specific to PLP 139-151 exist in the peripheral naïve repertoire and that they are functional.
Upon immunization with self-myelin Ags, B10.S mice preferentially produce more IL-4 and IL-10, prototypical Th2 cytokines, than SJL mice (25). The deviation of the immune response toward a Th1 phenotype as a consequence of CD25+ cell depletion that we describe here suggests that PLP 139-151-reactive CD4+CD25+ T cells are the source of Th2 cytokines, specifically IL-10, or promote the induction of IL-10 by other cells under normal conditions. By contrast, CD4+CD25- cells appear to secrete IFN-γ preferentially. Thus, CD4+CD25+ cells may control pathogenic T cells by direct inhibition or by inducing production of antiinflammatory cytokines such as IL-10 and control expansion of pathogenic effector T cells. Lack of such a control would allow expansion of pathogenic T cells, i.e., IFN-γ-producing CD4+CD25- T cells, to the threshold required to induce the disease. Nonetheless, because not all of the mice treated with anti-CD25 Ab developed EAE, other factors such as environmental effects, efficiency of CD25+ cell depletion, and effects of background genes may have influenced disease susceptibility. Indeed, IL-4 and IL-10 were still present in mice depleted of CD25+ cells. This observation implies that even in the mice made vulnerable to EAE by CD25+ cell depletion, there is a certain amount of regulatory control mediated by the antiinflammatory properties of IL-4 and IL-10 that prevented all of the mice treated with anti-CD25 Ab from developing the disease. It is known that resistance of B10.S mice to MBP-induced EAE is secondary to an Ag-specific defect in the generation of Th1 cells that produce IFN-γ and that this defect can be restored by supplementing the MBP-reactive T cells with IL-12 (19). In addition, B10.S mice have a defect in their capacity to up-regulate the IL-12R β2 subunit, which results from a failure of MBP-specific T cells to up-regulate CD40 ligand expression and to induce IL-12 production (26). Taken together, resistance to EAE in B10.S mice appears to be mediated by multiple factors. Here, we provide evidence that CD4+CD25+ cells may be one of the critical components that confers resistance to PLP 139-151-induced disease.
We have demonstrated that self-Ag reactive CD4+CD25+ cells exist in the periphery, but it is not known how they are generated. Generation of CD4+CD25+ cells requires high-avidity interactions of their TCR with self-peptide MHC complexes in the thymus, but the extent of their interactions must be low enough to avoid deletion (13). By real-time quantitative PCR analysis, we observed that B10.S mice express larger amounts of PLP mRNA in the thymus than SJL mice, although these strains have comparable levels of PLP expression in the brain (data not shown). Given that SJL mice have fewer PLP 139-151-reactive CD4+CD25+ cells in their naïve periphery than B10.S mice, higher thymic PLP expression in B10.S mice may favor the generation of PLP-specific CD4+CD25+ cells. This question can be investigated directly by testing the frequency of CD4+CD25+ T cells in PLP-deficient B10.S mice, once they become available.
In summary, we show here that the naïve repertoire of both B10.S and SJL mice contain T cells that recognize PLP139-151 at similar frequencies and that upon immunization, EAE-resistant B10.S mice fail to mount a sustained proliferative response. This blunted response in B10.S mice is at least in part due to a higher relative frequency of PLP 139-151 tetramer-reactive CD4+CD25+ cells in their peripheral repertoire. Depletion of CD25+ cells in vivo conferred EAE susceptibility to B10.S mice. Our data provide evidence that naturally occurring PLP 139-151-specific, tetramer-reactive CD4+CD25+ regulatory cells in part control genetic susceptibility and resistance to EAE.
Supplementary Material
Supporting Information
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 NS30843, PO1 NS38037, RO1 AI44880, NS 046414 (to R.A.S.), and PO1 A145757 (to K.W.W.) and National Multiple Sclerosis Society (NMSS) Grants 2571-D9 (to V.K.K.) and RG3257 (to L.N.). J.R. is a recipient of an advanced postdoctoral fellowship award from NMSS (New York).
Author contributions: J.R., Z.I., X.Z., J.E., and V.K.K. designed research; J.R., Z.I., J.E., and R.A.S. performed research; J.R., X.Z., J.P., and K.W.W. contributed new reagents/analytical tools; J.R., R.A.S., and V.K.K. analyzed data; and J.R., R.A.S., K.W.W., and V.K.K. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ag, antigen; APC, antigen-presenting cells; DTH, delayed type hypersensitivity; EAE, experimental autoimmune encephalomyelitis; LN, lymph node; LNC, LN cell; MBP, myelin basic protein; OVA, ovalbumin; PLP, proteolipid protein; TCR, T cell receptor; Th1, T helper 1; TMEV, Theiler's murine encephalomyelitis virus; 7-AAD, 7-amino-actinomycin D.
References
- 1.Greer, J. M., Sobel, R. A., Sette, A., Southwood, S., Lees, M. B. & Kuchroo, V. K. (1996) J. Immunol. 156**,** 371-379. [PubMed] [Google Scholar]
- 2.Tuohy, V. K., Lu, Z., Sobel, R. A., Laursen, R. A. & Lees, M. B. (1989) J. Immunol. 142**,** 1523-1527. [PubMed] [Google Scholar]
- 3.Encinas, J. A., Lees, M. B., Sobel, R. A., Symonowicz, C., Greer, J. M., Shovlin, C. L., Weiner, H. L., Seidman, C. E., Seidman, J. G. & Kuchroo, V. K. (1996) J. Immunol. 157**,** 2186-2192. [PubMed] [Google Scholar]
- 4.Linthicum, D. S. & Frelinger, J. A. (1982) J. Exp. Med. 156**,** 31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butterfield, R. J., Sudweeks, J. D., Blankenhorn, E. P., Korngold, R., Marini, J. C., Todd, J. A., Roper, R. J. & Teuscher, C. (1998) J. Immunol. 161**,** 1860-1867. [PubMed] [Google Scholar]
- 6.Encinas, J. A., Wicker, L. S., Peterson, L. B., Mukasa, A., Teuscher, C., Sobel, R., Weiner, H. L., Seidman, C. E., Seidman, J. G. & Kuchroo, V. K. (1999) Nat. Genet. 21**,** 158-160. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155**,** 1151-1164. [PubMed] [Google Scholar]
- 8.Sakaguchi, S. (2000) Cell 101**,** 455-458. [DOI] [PubMed] [Google Scholar]
- 9.Shevach, E. M. (2001) J. Exp. Med. 193**,** F41-F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. (2003) Nat. Immunol. 4**,** 337-342. [DOI] [PubMed] [Google Scholar]
- 11.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299**,** 1057-1061.12522256 [Google Scholar]
- 12.Jordan, M. S., Boesteanu, A., Reed, A. J., Petrone, A. L., Holenbeck, A. E., Lerman, M. A., Naji, A. & Caton, A. J. (2001) Nat. Immunol. 2**,** 301-306. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi, S. (2001) Nat. Immunol. 2**,** 283-284. [DOI] [PubMed] [Google Scholar]
- 14.Bensinger, S. J., Bandeira, A., Jordan, M. S., Caton, A. J. & Laufer, T. M. (2001) J. Exp. Med. 194**,** 427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy, J., Bettelli, E., Nicholson, L., Waldner, H., Jang, M. H., Wucherpfennig, K. W. & Kuchroo, V. K. (2003) J. Immunol. 170**,** 870-877. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson, L. B., Greer, J. M., Sobel, R. A., Lees, M. B. & Kuchroo, V. K. (1995) Immunity 3**,** 397-405. [DOI] [PubMed] [Google Scholar]
- 17.Sobel, R. A., Blanchette, B. W., Bhan, A. K. & Colvin, R. B. (1984) J. Immunol. 132**,** 2393-2401. [PubMed] [Google Scholar]
- 18.Anderson, A. C., Nicholson, L. B., Legge, K. L., Turchin, V., Zaghouani, H. & Kuchroo, V. K. (2000) J. Exp. Med. 191**,** 761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segal, B. M. & Shevach, E. M. (1996) J. Exp. Med. 184**,** 771-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang, M. H., Seth, N. P. & Wucherpfennig, K. W. (2003) J. Immunol. 171**,** 4175-4186. [DOI] [PubMed] [Google Scholar]
- 21.Stratmann, T., Martin-Orozco, N., Mallet-Designe, V., Poirot, L., McGavern, D., Losyev, G., Dobbs, C. M., Oldstone, M. B., Yoshida, K., Kikutani, H., et al. (2003) J. Clin. Invest. 112**,** 902-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zippelius, A., Pittet, M. J., Batard, P., Rufer, N., de Smedt, M., Guillaume, P., Ellefsen, K., Valmori, D., Lienard, D., Plum, J., et al. (2002) J. Exp. Med. 195**,** 485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornton, A. M. & Shevach, E. M. (1998) J. Exp. Med. 188**,** 287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton, A. M. & Shevach, E. M. (2000) J. Immunol. 164**,** 183-190. [DOI] [PubMed] [Google Scholar]
- 25.Maron, R., Hancock, W. W., Slavin, A., Hattori, M., Kuchroo, V. & Weiner, H. L. (1999) Int. Immunol. 11**,** 1573-1580. [DOI] [PubMed] [Google Scholar]
- 26.Chang, J. T., Shevach, E. M. & Segal, B. M. (1999) J. Exp. Med. 189**,** 969-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information