Interferon Regulatory Factor 1 Binding to p300 Stimulates DNA-Dependent Acetylation of p53 (original) (raw)
Abstract
Interferon regulatory factor 1 (IRF-1) and p53 control distinct sets of downstream genes; however, these two antioncogenic transcription factors converge to regulate p21 gene expression and to inhibit tumor formation. Here we investigate the mechanism by which IRF-1 and p53 synergize at the p21 promoter and show that stimulation of p21 transcription by IRF-1 does not require its DNA-binding activity but relies on the ability of IRF-1 to bind the coactivator p300 and to stimulate p53-dependent transcription by an allosteric mechanism. Deletion of the p300-binding sites in IRF-1 eliminates the ability of IRF-1 to stimulate p53 acetylation and associated p53 activity. Complementing this, small peptides derived from the IRF-1-p300 interface can bind to p300, stabilize the binding of p300 to DNA-bound p53, stimulate p53 acetylation in trans, and up-regulate p53-dependent activity from the p21 promoter. The nonacetylatable p53 mutant (p53-6KR) cannot be stimulated by IRF-1, further suggesting that p53 acetylation is the mechanism whereby IRF-1 modifies p53 activity. These data expand the core p300-p53 protein LXXLL and PXXP interface by including an IRF-1-p300 interface as an allosteric modifier of DNA-dependent acetylation of p53 at the p21 promoter.
The tumor suppressor protein p53 is a stress-responsive transcription factor that controls the expression of gene products implicated in cell cycle arrest and apoptosis. Well-characterized gene products which mediate the tumor suppressor function of p53 include the growth inhibitor p21WAF1 and the proapoptotic bcl-2 antagonist BAX (14, 23). Several functional domains of p53 are involved in promoting transactivation including (i) N-terminal LXXLL and PXXP activation motifs that anchor the transcriptional coactivator p300, (ii) a core sequence-specific DNA-binding domain, (iii) a tetramerization domain, and (iv) a C-terminal regulatory domain whose phosphorylation and acetylation stimulate p53-dependent transcription (1, 18).
The transactivation domain of p53 recruits the coactivator p300, while competition for binding by the oncogene MDM2 prevents p300 binding and blocks p53-dependent transcription. DNA damage-activated protein kinases phosphorylate three sites in the transactivation domain of p53, and these phosphorylation events have different effects on p300 and MDM2 binding. These kinases form part of the well-conserved ATM-CHK2 DNA damage signaling cascades that target p53 (38), and biochemical models of the effects of phosphorylation are consistent with an activating role on the p53 pathway. CHK2 can phosphorylate p53 at Thr18 or Ser20 (6, 30). Phosphorylation at the Thr18 site attenuates MDM2 binding (7, 28) and presumably relieves p53 from negative control by MDM2. In addition, phosphorylation at Thr18 or Ser20 by CHK2 stabilizes p300 binding to the LXXLL activation domain of p53 and promotes DNA-dependent acetylation of p53 by p300 (6, 8). Therefore, phosphorylation of the p53 activation domain may act as a switch to convert it from an MDM2-binding protein to a p300-binding protein, leading to enhanced DNA-dependent acetylation of the protein (18).
The stages in assembly of the p300-p53-DNA transactivation complex have been reconstituted in order to clarify the regulation and function of p53 acetylation. Such studies have identified four key stages in the assembly reaction. First, phosphorylation by CHK2 at Thr18 or Ser20 in the p53 activation domain stabilizes p300 docking to the LXXLL-p53 activation domain predominantly via the IBiD and IHD phosphopeptide-binding domains of p300 (9). Second, this docking of p300 is essential for sequence-specific DNA-dependent acetylation of p53, indicating that p53 tetramer acetylation has intrinsic conformational constraints in the absence of DNA (9). Third, dissecting the intrinsic conformational constraints on p53 acetylation has demonstrated that p300 docks not only to LXXLL motifs in p53 but also to the proline repeat domain (PXXP), and these dual docking interactions are required for DNA-dependent catalyzed acetylation (10). Fourth, the function of LXXLL- and PXXP-mediated acetylation of p53 as a post-DNA-binding event is to clamp the p300-p53AC complex into a stable state (9). This clamping of p300-p53 after acetylation is consistent with data demonstrating that acetylation recruits coactivator complexes to a promoter in vivo (2).
The tetrameric nature of p53 adds further combinatorial possibilities to the architecture of the substrate since the protein is octavalent with respect to the total number of LXXLL- and PXXP-binding sites for p300. The identification of this relatively complex multidomain LXXLL and PXXP docking interaction required for p300 to catalyze substrate acetylation is further complicated by the fact that this docking-dependent acetylation is also DNA dependent and therefore sensitive to the conformation of the p53 substrate (10). These data also suggest that the conformation of the many p300 subdomains plays an important role in mediating acetylation. In order to determine how p53 acetylation by p300 might be controlled in trans by other regulatory factors, we focused on dissecting the mechanism of interferon regulatory factor 1 (IRF-1) costimulation of p53 activity and whether novel insights can be obtained into mechanisms of p300-catalyzed acetylation.
IRFs were originally identified as transcriptional regulators of interferon (IFN) and IFN-stimulated genes (37). The founding member of the IRF family, IRF-1, regulates a diverse range of genetic programs, as it is implicated in the antiviral response (17), regulation of the cell cycle (32) and apoptosis (16, 34), development of T cells (19), susceptibility to transformation by oncogenes (35), and the response to genotoxic agents (25, 36). Furthermore, deletion or point mutation of the IRF-1 gene has been linked to the development of leukemia, myelodysplastic syndrome (3, 39), and solid-phase tumors of the gastrointestinal tract (22, 33), suggesting that IRF-1 has tumor suppressor properties.
Intriguingly, there appears to be a convergence of IRF-1 and p53 in the prevention of tumor formation that has led to the classification of IRF-1 as a tumor modifier (21). Thus, although loss of IRF-1 by itself has no statistically significant effect on the rate of tumor formation, loss of IRF-1 in a _p53_-null background gives a dramatic increase in both tumor incidence and spectrum over that with loss of p53 alone, providing genetic evidence of interplay between these two factors. Mouse embryonic fibroblasts deficient in IRF-1 are compromised in their ability to undergo growth arrest in response to ionizing radiation (IR) (36), and IRF-1-null hepatocytes are impaired in their ability to repair damaged DNA (26). In addition, maximal induction of p21 (WAF1/CIP1) in IR-treated cells requires both IRF-1 and p53 (36). Most recently the ATM kinase has been implicated as a coordinating factor for IRF-1 and p53 in damaged cells (25). However, the mechanism of IRF-1 cooperation with p53 is undefined.
Here we describe a novel form of cross talk between transcription factors involving the positive stimulation of p53 acetylation by IRF-1. This involves binding of IRF-1 to p300, which leads to the stabilization of p300 binding to the LXXLL transactivation domain of p53, enhancing docking-dependent and DNA-dependent acetylation of p53 and stimulating p53 activity. These molecular data indicate that acetylation of a substrate by p300 can be stimulated allosterically in trans, provide a model system to begin to dissect how the multidomain p300 protein is regulated as an acetyltransferase, and provide a molecular mechanism to account for genetic data demonstrating that IRF-1 is a tumor modifier.
MATERIALS AND METHODS
Plasmids.
EGFP-CT2 and -CT3 were constructed by ligating double-stranded oligonucleotides encoding amino acids (aa) 271 to 290 (DFSCKEEPEIDSPGGDIGLS) and 226 to 245 (DEDEEGKLPEDIMKLLEQSE) of human IRF-1, respectively, into XhoI-XbaI-digested EGFP-C3 plasmid (Clontech). EGFP-mCT2 and -mCT3 contained the following sequences, respectively: DFSCAEEAEIDSAGGDIGLS and DEDEEGALAEDIMALLEQSE. pcDNA-p53, p21-Luc, pCMVβ-p300, and pCMV-βGal have been previously described (8). pcDNA-p53-6KR (where K370, K372, K373, K381, K382, and K386 are mutated to R) was a gift from R. Hay (University of St. Andrews). p125-Luc and p55-Luc were gifts from T. Fujita (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). pcDNA3-IRF-1 was constructed from an EcoRI fragment encoding the full-length cDNA for human IRF-1. IRF-1YLP/A, in which Y109, L112, and P113 were mutated to A; IRF-1ΔCT2, which had a deletion of aa 276 to 285; IRF-1ΔCT3, which had a deletion of aa 231 to 240; and IRF-1ΔCT2/3, which had a deletion of aa 231 to 240 and aa 276 to 285, were generated for pcDNA3-IRF-1 by using a QuikChange kit (Stratagene). pVR-GAL4, GAL4-p300, GAL4-p300(1-504), GAL4-p300(1-703), GAL4-p300(192-504), GAL4-p300(192-600), GAL4-p300(192-703), GAL4-p300(192-1004), GAL4-p300(504-1238), GAL4-p300(852-1071), GAL4-p300(636-2414), GAL4-p300(1064-2414), and GAL4-p300(1757-2414) were a gift from N. Perkins (University of Dundee, Dundee, United Kingdom) under an agreement with Vical Inc. (San Diego, Calif.). pGAL4-N1, pGAL4-N2, pGAL4-N3, pGAL4-C1, pGAL4-C2, and pGAL4-C3 were a gift from Y. Shi (Harvard Medical School, Boston, Mass.).
Cell culture, transfections, gene reporter assays, ELISA, and immunoblotting.
HCT116 p53+/+ and p53−/− cells were a kind gift from B. Vogelstein (Johns Hopkins University School of Medicine) and were maintained in McCoy's 5A medium (Gibco BRL) supplemented with 10% (vol/vol) fetal bovine serum and incubated at 37°C with an atmosphere of 10% CO2. Transient transfections, gene reporter assays, and enzyme-linked immunosorbent assays (ELISAs) were carried out as previously described (8). Transfected lysates were analyzed by sodium dodecyl sulfate (SDS)-12% polyacrylamide gel electrophoresis, and immunoblotting was carried out as previously described (8). Anti-p53 monoclonal antibody (MAb) (DO-1) or anti-p53 MAb (19.1), anti-p21 MAb (Ab-1; Oncogene Research Products), anti-IRF-1 serum and anti-p300 serum (C20 and N15; Santa Cruz Biotechnology Inc.), anti-IRF-1 polyclonal antibody (1600034; Geneka), anti-IRF-1 polyclonal C20 antibody (Santa Cruz), MAb IRF-1 (BD Transduction Laboratories), anti-p300 (NM11; Pharmingen), anti-Ac-p53-K373/382 (Upstate Biotechnology), and anti-enhanced green fluorescent protein (anti-EGFP) MAb (Clontech) were used at 1 μg/ml and with the appropriate secondary antibody conjugated to horseradish peroxidase (HRP; DAKO). Antibody binding was detected by enhanced chemiluminescence and quantified using a Genegnome Bioimager plus Syngene analysis software.
EMSA.
IRF-1 and IRF-1YLP/A protein were expressed using a coupled reticulocyte lysate system (Promega) according to the manufacturer's instructions, and the protein was detected by immunoblot analysis. The lysates were assayed for DNA-binding activity with an IRF-1-specific oligonucleotide probe (IRFE) labeled with biotin and an electrophoretic mobility shift assay (EMSA) Gel-Shift kit (Panomics). Briefly, control lysate and lysate expressing either wild-type (wt) or mutant IRF-1 protein was incubated with binding buffer (Panomics) containing poly(I · C) (50 ng/μl) and 20 fmol of biotin end-labeled IRF-1 consensus site DNA (Panomics) and incubated at room temperature (RT) for 30 min. Binding specificity was assessed by the addition of 4 pmol of unlabeled IRF-1 consensus site DNA during the incubation. The reaction mixture was separated on a 6% polyacrylamide gel and transferred to a Biodyne B membrane (Pall). The DNA was cross-linked to the membrane by using a UV cross-linker (254 nm) at 120 mJ/cm2, and bound DNA was detected using a streptavidin-HRP conjugate and enhanced chemiluminescence.
Immunoprecipitation.
Cells were washed twice in phosphate-buffered saline and lysed in 50 mM HEPES, pH 7.8, containing 1% Triton X-100, 150 mM NaCl, 5 μM trichostatin A (TSA), 10 μg of leupeptin/ml, 4 μg of aprotinin/ml, 2 μg of pepstatin/ml, 1 mM benzamidine, 10 μg of soybean trypsin inhibitor/ml, 400 μg of Pefabloc/ml, and 10 mM EDTA for 15 min on ice and then passaged through a 21-gauge syringe needle 5 times and centrifuged at 11,000 × g for 15 min at 4°C. The supernatant (500 μg of total protein) was precleared using 30 μl of protein G-Sepharose beads prewashed in TX100WB (50 mM HEPES [pH 7.8], 1% [vol/vol] Triton X-100, 5 mM dithiothreitol [DTT], 10 μg of leupeptin/ml, 4 μg of aprotinin/ml, 2 μg of pepstatin/ml, 1 mM benzamidine, 10 μg of soybean trypsin inhibitor/ml, 400 μg of Pefabloc/ml, 1 mM EDTA, and 5 μM TSA). The supernatant was divided into three equal portions, and primary antibodies (1 μg) were added as follows: anti-Ac-p53-K373/382 (Upstate Biotechnology), anti-p53 (DO-1/ICA-9), or anti-p300 (NM11; BD Pharmingen) for 1 h at 4°C. Protein G-Sepharose beads, 20 μl of 80% slurry in TX100WB, was added to the antibody-lysate mixture and incubated overnight at 4°C. The beads were washed extensively in TX100WB, resuspended in SDS sample buffer, and heated to 90°C for 5 min. The samples were analyzed on 4 to 12% NuPAGE gels (Invitrogen) and transferred to nitrocellulose according to the manufacturer's instructions.
Protein purification.
Human p53 and p300 expressed in Sf9 cells were purified as previously described (8, 13, 31). Escherichia coli transformed with pET-IRF-1 (untagged human IRF-1) was grown at 37°C to an optical density at 600 nm of 0.6, arabinose was then added to a final concentration of 0.2%, and the bacteria were incubated at RT for 2 h. Following harvesting the bacteria were resuspended in an equal volume of 50 mM HEPES, pH 8.0, containing 10% sucrose and frozen in liquid nitrogen. The cells were thawed in an ice-water bath in the presence of 0.25 M KCl-2 mM DTT-0.5 mg of lysozyme/ml-400 μg of Pefabloc/ml (final concentrations) and incubated at 4°C for 30 min prior to centrifugation at 10,000 × g for 15 min. The supernatant was removed and diluted to 10 mg of total protein/ml with column buffer (50 mM HEPES [pH 7.6] containing 10% glycerol, 0.1 mM EDTA, 2 mM DTT, 0.1% Triton X-100, 400 μg of Pefabloc/ml, 1 mM benzamidine) and applied to a DEAE-Sepharose column equilibrated in column buffer plus 50 mM NaCl; the column was washed with column buffer plus 100 mM NaCl and eluted with a linear gradient of 0.1 to 1 M NaCl in column buffer. Fractions containing IRF-1 protein were detected by immunoblotting, pooled, and dialyzed against column buffer plus 100 mM NaCl before being applied to a heparin-Sepharose column equilibrated in column buffer plus 100 mM NaCl. The column was washed with column buffer plus 200 mM NaCl and eluted with a linear gradient of 0.2 to 1.0 M NaCl. Fractions containing IRF-1 protein were pooled and dialyzed against column buffer containing 50 mM NaCl and applied to a SP-Poros column equilibrated in column buffer plus 50 mM NaCl. The column was washed with column buffer plus 100 mM NaCl and eluted with a linear gradient of 100 to 600 mM NaCl. Fractions containing IRF-1 protein were pooled, concentrated to >1 mg/ml, and dialyzed as described above before being aliquoted and frozen in liquid nitrogen.
p53-p300 binding and acetylation assays.
Assays were carried out essentially as previously described (9). Briefly, heparin-Sepharose-purified p53 (200 ng) from Sf9 cells was incubated with 100 ng of double-stranded PolyGrip oligonucleotides and 400 ng of purified His-p300 in 100 μl of AT buffer (50 mM Tris-HCl [pH 8.0], 5% [vol/vol] glycerol, 0.1 mM EDTA, 1 mM DTT, 5 μM TSA) with or without 2 μM acetyl coenzyme A (CoA) for 10 min at 30°C; under these conditions the enzymatic reaction was linear; other additions are detailed in the figure legends. Reaction mixtures were preincubated on ice for 10 min prior to the start of the reaction by the addition of p300. p300 binding was determined by antibody-capture ELISA, where p53 was captured using anti-p53 MAb (ICA-9) and bound p300 was detected using anti-p300 (N15) at 1 μg/ml. Acetylation of p53 was detected by antibody-capture ELISA with the anti-p53 MAb ICA-9 (or by immunoblotting), and acetylation was determined using anti-Ac-p53-K373/382 (Upstate Biotechnology). Both p300 binding and acetylation were normalized to total p53 protein captured that was determined using polyclonal anti-p53 (CM5).
ChIP.
The chromatin immunoprecipitation (ChIP) assays were carried out essentially as described previously (2, 24) with some modifications. HCT116 (p53−/−) cells (107) were transfected with the indicated plasmids and after 48 h were cross-linked with 1% formaldehyde for 30 min at RT. The immunoprecipitation buffer was 16.7 mM Tris-HCl (pH 8.0)-0.01% (wt/vol) SDS-1% (vol/vol) Triton X-100-1.2 mM EDTA-167 mM NaCl-5 μM TSA-10 μg of leupeptin/ml-4 μg of aprotinin/ml-2 μg of pepstatin/ml-1 mM benzamidine-10 μg of soybean trypsin inhibitor/ml-400 μg of Pefabloc/ml. The precleared extract was split four ways for bead-alone control plus three primary antibodies (2 μg each of anti-Ac-p53-K373/382 [Upstate Biotechnology], anti-p53 [DO-1/ICA-9], or anti-p300 [NM11; BD Pharmingen]). The immune complexes were then captured with 40 μl of protein G beads for 1 h at 4°C and washed with 1 ml of buffer 1 (20 mM Tris-HCl [pH 8.1], 0.1% [4wt/vol] SDS, 1% [vol/vol] Triton X-100, 2 mM EDTA) plus 150 mM NaCl followed by 1 ml of buffer 1 plus 500 mM NaCl, followed by 1 ml of buffer 2 (10 mM Tris-HCl [pH 8.1], 0.25 M LiCl, 1% [vol/vol] NP-40, 1% [wt/vol] deoxycholate, 1 mM EDTA), and finally twice with 10 mM Tris-HCl (pH 8) containing 1 mM EDTA.
The PCRs were carried out according to the manufacturer's recommendations with HotStarTaq DNA polymerase. Into each 50-μl reaction mixture, 350 ng of each primer was incorporated, and titration of template (1, 2, and 5 μl) was carried out to verify linearity. An input DNA sample was also incorporated to act as a control for the PCRs. For p21 and glyceraldehyde phosphate dehydrogenase (GAPDH) PCRs, the cycle conditions were an initial 95°C, 10-min Taq activation step followed by 30 cycles of 1 min at 95°C, 1 min at 55°C, and 2 min at 72°C followed by a final 10-min extension at 72°C. The PCR products were analyzed on a 1% agarose gel and quantified using a Genegnome Bioimager and software. Primers used in the PCR were for the GAPDH promoter (5′-AAAAGCGGGGAGAAAGTAGG and 3′-CTAGCCTCCCGGGTTTCTCT) and for the p21 promoter (5′-CCAGCCCTTGGATGGTTT and 3′-GCCTCCTTTCTGTCCTGA).
RESULTS
The sequence-specific DNA-binding activity of IRF-1 is not required for cooperation with p53.
Genetic studies have suggested that p53 and IRF-1 can cooperate to prevent tumor development and that efficient expression of the growth-regulatory protein p21, following exposure to IR, requires the activity of both these factors (21, 36). The mechanism of this cooperation is undefined, and we have set up assay systems to study the IRF-1-mediated stimulation of p53 activity.
When p53 was expressed in HCT116 (p53−/−) cells, it induced expression from a p21 promoter construct (−2320 to +1; containing 2× p53 binding sites and 4× putative IRFEs) (Fig. 1A, upper panel) and led to an increase in the amount of endogenous p21 protein (Fig. 1A, lower panel). On the other hand, expression of IRF-1 alone gave a relatively weak activation of the p21 promoter construct (2.5-fold above background compared to 11.2-fold for p53) and no detectable change in the level of endogenous p21 protein (Fig. 1A). Activity from the p21 promoter was substantially increased (20.4-fold above background) when p53 and IRF-1 were coexpressed (Fig. 1A, upper panel), and this was mirrored by a substantial increase in the levels of endogenous p21 protein under conditions where p53 protein levels remained constant (Fig. 1A, lower panel). These data show that the levels of endogenous p21 protein expression are proportionate to the relative light units (RLUs) of a _p21_-Luc reporter construct and demonstrate a synergistic activation of p21 by p53 and IRF-1.
FIG. 1.
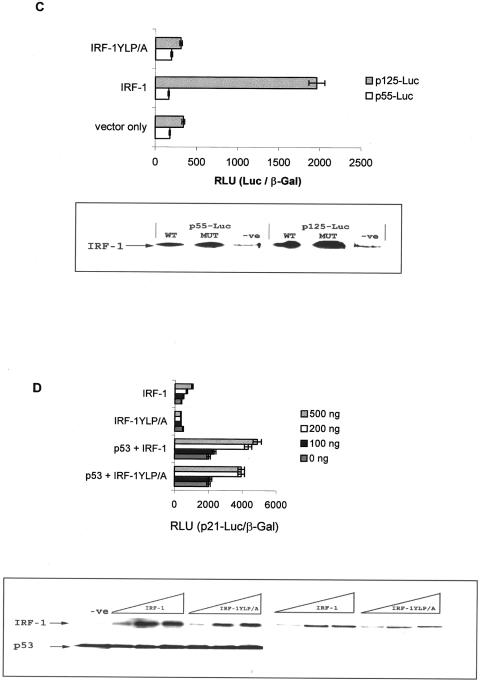
IRF-1 stimulates p53 activity. (A) HCT116 (p53−/−) cells were transfected with pcDNA3-p53 (500 ng) and/or pcDNA3-IRF-1 (500 ng) together with _p21_-Luc (1 μg) and pCMV-βGal (1 μg). Cells were harvested after 24 h; relative reporter activity is expressed as the ratio of luciferase to β-galactosidase activity (upper panel) and represents the mean ± standard deviation for three independent experiments. Immunoblot assays were performed to determine the levels of IRF-1, p53, and p21 by using C20, 19.1, and Ab-1, respectively (lower panel). (B) (Upper panel) Control reticulocyte lysate and lysate expressing IRF-1 or IRF-1YLP/A (1 and 3 μl) were assayed for their ability to bind to biotin-labeled IRF-1 consensus site DNA in the presence (+) or absence (−) of an excess of unlabeled probe. The assays were analyzed by EMSA, and the biotin-labeled DNA was detected following transfer and cross-linking to a Biodyne B membrane, by using streptavidin-HRP. NS represents a nonspecific DNA-binding activity contributed by the reticulocyte lysate. (Lower panel) An immunoblot showing the levels of IRF-1 protein added to the EMSA. IRF-1 protein was detected using the MAb IRF-1. The data shown are representative of two independent experiments. (C) HCT116 (p53−/−) cells were transfected with 500 ng each of pcDNA3-IRF-1, pcDNA-IRF-1YLP/A, or vector alone plus pCMV-βGal (1 μg) with either 1 μg of IFN-β promoter linked to luciferase (p125-Luc) or a control in which the IRF-1 binding sites were deleted (p55-Luc). Relative reporter activity was determined as described above (upper panel); an immunoblot shows the level of IRF-1 protein (lower panel). (D) HCT116 (p53−/−) cells were transfected with a titration of pcDNA3-IRF-1 or pcDNA3-IRF-1YLP/A in the presence and absence of pcDNA-p53 (500 ng) plus _p21_-Luc (1 μg) and pCMV-βGal (1 μg). Relative reporter activity was determined as described above (upper panel), and an immunoblot shows the level of IRF-1 and p53 protein detected using C20 and 19.1, respectively (lower panel).
The best-characterized activity of IRF-1 is as a sequence-specific DNA-binding protein and transcription factor; however, despite the fact that several putative IRFEs have been identified within the p21 promoter, deletion of these sites failed to prevent cooperative activation of the p21 promoter by IRF-1 and p53 (36). These data suggest that IRF-1 cooperation with p53 occurs independently of IRF-1 DNA binding to the p21 promoter. We therefore investigated whether the DNA-binding potential of IRF-1 was unnecessary for it to synergize with p53. An IRF-1 construct was generated that had been mutated within the DNA-binding domain (IRF-1YLP/A). IRF-1YLP/A mutant protein expressed in reticulocyte lysates was tested for its ability to bind DNA sequence specifically by an EMSA. wt IRF-1 bound in a titrative manner to a biotin-labeled DNA probe containing its consensus IRFE binding site (Fig. 1B, EMSA lanes 3 and 4), and binding was significantly reduced in the presence of an unlabeled competitor DNA probe (EMSA lane 5). Nonspecific DNA-binding activity (Fig. 1B, NS) was also detected; however, this was also present when control reticulocyte lysate was incubated with the labeled probe (EMSA lanes 1 and 2). Although the IRF-1YLP/A mutant was expressed at slightly higher levels in the reticulocyte lysate (Fig. 1B, immunoblot, compare lanes 4 and 7), no detectable specific DNA-binding activity was observed (EMSA lanes 6 and 7), confirming that this mutant is unable to form a specific isolatable complex with IRF-1 consensus site DNA. These conclusions are supported by the fact that the IRF-1YLP/A mutant displayed no activity against the beta IFN (IFN-β) promoter linked to luciferase (p125-Luc, Fig. 1C) or to the _p21_-Luc reporter (Fig. 1D). Strikingly, IRF-1YLP/A was able to synergize with p53 after cotransfection to stimulate _p21_-Luc expression with a potency similar to that of wt IRF-1 (Fig. 1D). These data suggest that, although IRF-1 DNA binding may play a minor role in activating low-level expression from the p21 promoter in the absence of p53 (Fig. 1D, IRF-1 compared to IRF-1YLP/A), the major activity of IRF-1 required for cooperation with p53 is independent of its sequence-specific DNA-binding activity. This observation is consistent with data indicating that deletion of IRF-1-binding sites in the p21 promoter failed to prevent cooperative activation of the p21 promoter by IRF-1 and p53 (36).
IRF-1 binding to p300 is required and is sufficient to stimulate p53 activity.
IRF-1 is known to play a role in coactivator recruitment when it is present within the IFN-β enhanceosome (20). We therefore investigated whether IRF-1 binding to transcriptional coactivators could play a role in the stimulation of p21 expression. Although there is evidence that IRF-1 and CBP/p300 can form a complex in vitro (20), less is known about the physiological relevance of the interaction and whether IRF-1/p300 complexes are generated in response to signals that promote p53 activity. The best-characterized pathway leading to p53-dependent induction of p21 is activated in response to DNA damage (5). We have recently demonstrated that common upstream elements are involved in the coordinated ATM-dependent up-regulation of IRF-1 and p53 in response to agents which generate DNA strand breaks (25) and DNA adducts (J. Pamment and K. L. Ball, unpublished data), supporting the idea that both these factors are required for the maximal expression of p21 in DNA-damaged cells (36). We therefore carried out immunoprecipitation assays in order to determine whether endogenous IRF-1 was found in a complex with p300 following the exposure of cells to DNA damage. When p300 was immunoprecipitated from A375 cells, no detectable IRF-1 was present in the immunocomplex (Fig. 2A, −ve). However, following treatment with IR (10 Gy), IRF-1 was present in the p300 immunocomplex (Fig. 2A, IR). Interestingly, the amount of IRF-1 protein in the p300 complex from IR-treated cells was high relative to that in cells treated with a viral mimetic [Fig. 2A, poly(I · C)] despite the fact that the absolute amount of IRF-1 protein in the poly(I · C)-treated cells was greater than that in cells exposed to IR [Fig. 2A, poly(I · C)]. As expected, the increase in p53 protein in IR-treated cells (Fig. 2A, IR versus −ve) was accompanied by an increase in p300-associated p53 (Fig. 2A, IR versus −ve). Thus, in response to signals that lead to p53-dependent activation of p21, endogenous p53 and IRF-1 are both found in complex with p300. We therefore mapped the IRF-1-p300 interface with the aim of determining whether p300-binding activity, rather than the DNA-binding activity of IRF-1, mediates the stimulation of p53 activity.
FIG. 2.
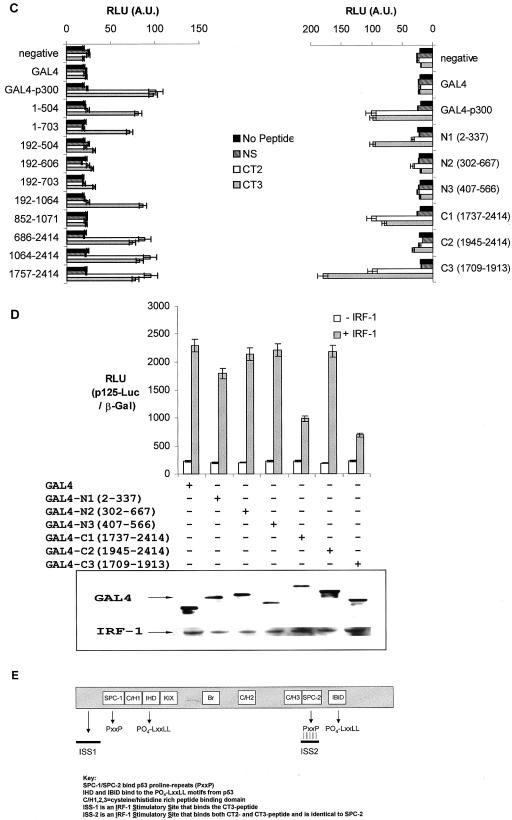
Characterizing the interaction between IRF-1 and p300. (A) A375 cells were untreated (−ve) or treated with 10 Gy of IR or 100 μg of poly(I · C)/ml for 5 and 4 h, respectively. p300-containing complexes were immunoprecipitated with NM11, and immunoblots were probed for p53 (DO-1), IRF-1 (C20), and p300 (NM11). (B) A peptide library spanning the entire sequence of IRF-1 (peptides 1 to 22) was screened by ELISA with full-length untagged p300 purified from Sf9 cells. Bound p300 protein was detected using N15 serum and quantified by chemiluminescence (RLU). Two peptides named CT2 (peptide 19) and CT3 (peptide 16) bound p300 with a high affinity. DBD, DNA-binding domain; A.U., arbitrary units. (C) GAL4-p300 fusion constructs (see Materials and Methods) which had been transiently expressed in HCT116 (p53−/−) cells were captured onto microtiter wells by using an anti-GAL4 antibody (RK5C1) and screened for binding to biotinylated CT2, CT3, or NS (20-aa nonspecific peptide). Peptide binding was detected using streptavidin-HRP and quantitated by chemiluminescence. (D) GAL4-p300 fusion constructs (as detailed) were transfected with or without IRF-1 (500 ng) into HCT116 (p53−/−) cells with p125-Luc (1 μg) and pCMV-βGal (1 μg). Cells were harvested after 24 h; relative reporter activity is expressed as the ratio of luciferase to β-galactosidase activity (upper panel) and represents the mean ± standard deviation for three independent experiments. Immunoblots show the levels of IRF-1 and GAL4 detected using C20 and anti-GAL4 (RK5C1), respectively (lower panel). (E) Schematic of p300 showing previously characterized p53-binding domains (9, 10) and the IRF-1-CT peptide-binding domains.
An overlapping peptide library, encompassing the entire sequence of IRF-1, was screened to identify potential p300 interaction sites (Fig. 2B). Consistent with results from Merika et al. (20), who found that the transactivation domain of IRF-1 interacted with CBP, there was a noticeable affinity of purified p300 for two peptides representing sequences from within the classic transactivation-enhancer domain of IRF-1 (Fig. 2B, peptides 16 and 19). The peptides displayed similar affinities for p300, and they were designated C-terminal p300-binding site 2 (CT2) (aa 271 to 290; DFSCKEEPEIDSPGGDIGLS) and CT3 (aa 226 to 245; DEDEEGKLPEDIMKLLEQSE). Domains in p300 that bind to the LXXLL and PXXP activation motifs on p53 have been mapped (9, 10), and we have used a similar approach to investigate whether the IRF-1 CT2 and CT3 motifs map to similar or distinct regions on p300. A series of GAL4-p300 fusion constructs captured from HCT116 cell lysates were immobilized on microtiter wells by using an anti-GAL4 immunoglobulin G (Fig. 2C). The corresponding biotinylated peptides (CT2, CT3, or no peptide) were added along with a nonspecific peptide (NS) derived from the nonphosphorylated LXXLL (BOX-I) domain of p53 that does not bind p300 (8), and p300-peptide complexes were detected using streptavidin-HRP and quantitated by chemiluminescence. The CT3 region of the IRF-1 transactivation domain displayed affinity for a number of N-terminal constructs, and we were able to define the interaction site as lying between aa 1 and 192 of p300 (Fig. 2C), which we have termed ISS-1 (IRF-1 stimulatory site 1; Fig. 2E). However, the N-terminal fragments of p300 that bind CT3 were unable to compete with endogenous coactivators, as no inhibition of IRF-1-dependent transcription from the IFN-β promoter was observed when they were expressed in cells (Fig. 2D).
Both the CT2 and CT3 activation domain fragments bound to a C-terminal domain of p300 outside the classic C/H3-binding domain and adjacent to the IBiD-binding domain (Fig. 2C and E). The minimal fragments of p300 that bind to both CT2 and CT3 encompassed aa 1737 to 1913 and could act as dominant-negative inhibitors of IRF-1 transcription from the IFN-β promoter (Fig. 2D). This suggests that the C terminus of p300 contains a high-affinity IRF-1-binding site that contacts the IRF-1 transactivation domain at more than one point and which is capable of competing with endogenous coactivators. aa 1737 to 1913 of p300 have recently been demonstrated to bind a proline repeat domain within p53 named SPC-2 (10), and they also lie within a region (aa 1623 to 2414) that has been shown to interact with the viral IRF family member vIRF-1 (4). These data together add complexity to p300-peptide-binding domains: the LXXLL and PXXP domains of p53 interact with the IBiD, IHD, SPC-1, and SPC-2 domains of p300, while the CT2/3 domains of IRF-1 bind to ISS-1 and SPC-2 (Fig. 2E).
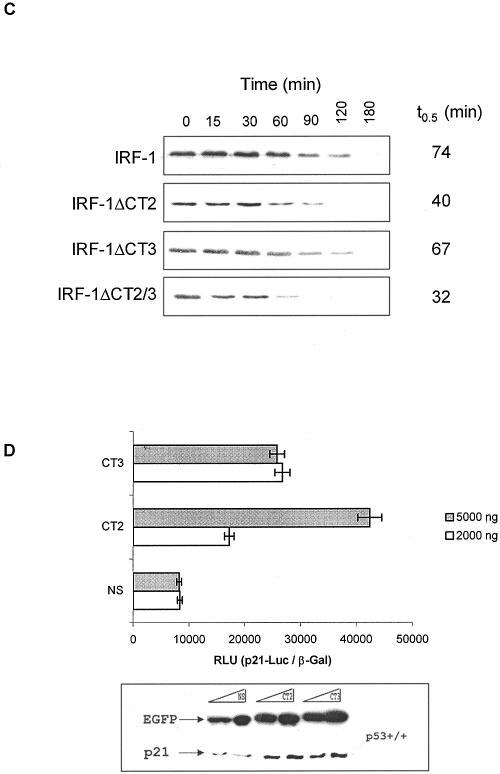
Having mapped the interaction between IRF-1 and p300, we then used this information to generate a series of IRF-1 constructs in which CT2 and CT3 were deleted individually (giving IRF-1ΔCT2 and IRF-1ΔCT3, respectively) or in combination (IRF-1ΔCT2/3). We first determined whether the CT2 and CT3 regions of IRF-1 were required for p300 to coactivate IRF-1-dependent transcription by using the IFN-β promoter (Fig. 3A). Coexpression of p300 with wt IRF-1 led to a 2.5-fold increase in IRF-1-dependent activity from the p125 reporter (Fig. 3A). However, the ability of p300 to stimulate IRF-1-dependent transcription from the _IFN_-β promoter was impaired by mutation of either the CT2 or CT3 region of IRF-1 (Fig. 3A). Consistent with data showing that constructs containing the SPC-2 domain of p300 inhibit IRF-1-dependent transcription (Fig. 2D), the basal transactivation potential of the ΔCT2/3 double mutant was partially compromised (Fig. 3A). In addition, the ΔCT2/3 mutant was even more insensitive to p300 stimulation than was either the ΔCT2 or ΔCT3 construct (Fig. 3A). Although the basal activity of IRF-1 on the IFN-β promoter was unaffected by mutations in CT2 or CT3 alone (Fig. 3A), the ability of the ΔCT2 and ΔCT3 mutants to stimulate p53-dependent transcription from the p21 promoter was attenuated (Fig. 3B). In this assay the ΔCT2/3 double mutant was essentially inactive as a p53 stimulator (Fig. 3B). In both the above assays it was noted that the expression level of the CT domain mutants differed from that of the wt IRF-1 protein; in particular the double mutant ΔCT2/3 displayed the lowest levels of protein expression. It has previously been noted that the ability of p53 to interact with p300 stabilizes p53 and is one key factor that attenuates its rate of degradation in DNA-damaged cells (15, 40). We therefore sought to investigate whether the differences in the expression of the IRF-1 constructs reflected changes in the rate of IRF-1 degradation. Consistent with a role for p300 in regulating IRF-1 degradation, mutations within the CT2 domain reduced the half-life of IRF-1 from 74 to 40 min (Fig. 3C). Although mutations in the CT3 domain did not have a significant effect on the _t_0.5, the double mutant (ΔCT2/3) turned over significantly faster than did the other IRF-1 constructs. The changes in the turnover of the IRF-1 constructs containing mutations within the p300-binding domain suggest that the interaction between these two proteins may regulate the availability of IRF-1 for degradation.
FIG. 3.
The CT domains of IRF-1 are required to promote p53-dependent expression of p21. (A) HCT116 (p53−/−) cells transfected with pcDNA3-IRF-1 (1 μg), pcDNA3-IRF-ΔCT2 (1 μg), pcDNA3-IRF-ΔCT3 (1 μg), pcDNA3-IRF-ΔCT2/3 (1 μg), and pCMVβ-p300 (1 μg) as detailed, plus p125-Luc (1 μg) and pCMV-βGal (1 μg). Cells were harvested after 24 h; relative reporter activity is expressed as the ratio of luciferase to β-galactosidase activity (upper panel) and represents the mean ± standard deviation for three independent experiments. Immunoblots show the levels of p300 and IRF-1 detected using NM11 and 1600034, respectively (lower panel). (B) HCT116 (p53−/−) cells transfected with pcDNA-p53 (500 ng), pcDNA3-IRF-1 (1 μg), pcDNA3-IRF-ΔCT2 (1 μg), pcDNA3-IRF-ΔCT3 (1 μg), and pcDNA3-IRF-ΔCT2/3 (1 μg) as detailed, plus _p21_-Luc (1 μg) and pCMVβ-Gal (1 μg). Relative reporter activity was determined as described above (upper panel), and immunoblots probed with DO-1 and 1600034 show the levels of p53 and IRF-1 (lower panel). (C) HCT116 (p53−/−) cells were transfected with 500 ng each of pcDNA3-IRF-1, pcDNA3-IRF-ΔCT2, pcDNA3-IRF-ΔCT3, or pcDNA3-IRF-ΔCT2/3; 24 h later they were treated with cycloheximide (30 μg/ml) and harvested at the times shown. An immunoblot shows the levels of IRF-1 detected using the IRF-1 MAb; the data were analyzed by densitometry, and the half-life was determined (_t_0.5). (D) HCT116 (p53+/+) cells were transfected with EGFP-CT2, EGFP-CT3, or EGFP-NS (0.2 or 0.5 μg), plus 1 μg each of _p21_-Luc and pCMV-βGal. Cells were harvested after 24 h; relative reporter activity is expressed as the ratio of luciferase to β-galactosidase activity (upper panel) and represents the mean ± standard deviation for three independent experiments. Immunoblots (lower panel) show the levels of EGFP-linked peptides and endogenous p21 protein detected with anti-EGFP MAb and Ab-1, respectively. (E) HCT116 (p53+/+) cells were transfected with EGFP-CT2, EGFP-mCT2, EGFP-CT3, EGFP-mCT3, or EGFP-NS (0.2 μg), plus 1 μg each of _p21_-Luc and pCMV-βGal. Cells were harvested after 24 h; relative reporter activity is expressed as the ratio of luciferase to β-galactosidase activity (upper panel) and represents the mean ± standard deviation for three sets of data. Immunoblots show the levels of endogenous p21 protein (middle panel) and EGFP-linked peptides (lower panel) detected with Ab-1 and anti-EGFP MAb, respectively. (F) Repeat of the experiment described for panel D but with HCT116 (p53−/−) cells.
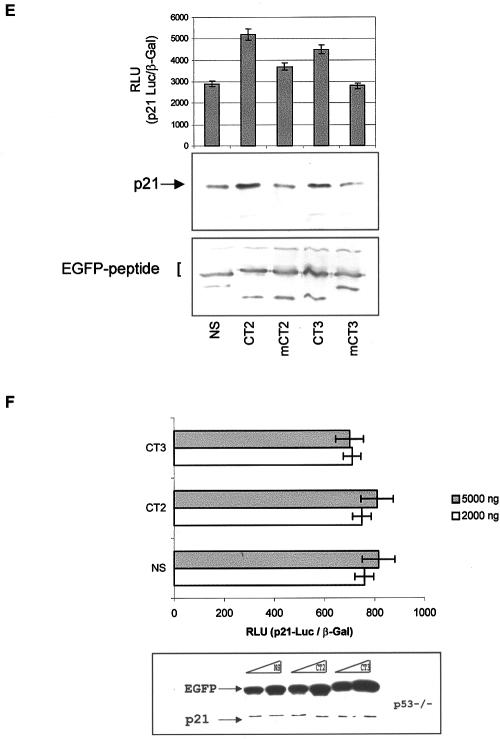
The above data suggest that the CT2 and CT3 p300-binding domains are required for IRF-1 to synergize with p53 to activate the expression of p21. Whether these domains by themselves were sufficient to stimulate p53-dependent regulation of p21 was addressed by generating EGFP fusion constructs for expression of CT2 and CT3 p300-binding peptides in cells. When EGFP-CT2 or EGFP-CT3 was expressed in HCT116 (p53+/+) cells together with the _p21_-Luc reporter, both of the constructs enhanced transactivation of the p21 promoter, compared to EGFP-NS (Fig. 3D, upper panel). Similarly, expression of both CT2 and CT3 in HCT116 (p53+/+) cells enhanced the expression of endogenous p21 protein (Fig. 3D, lower panel). Under the same conditions, EGFP-LXXLL and EGFP-PXXP peptide fusions from the p53-p300 interface attenuate p53 activity by blocking the p300-p53 interface (data not shown) as reported previously (10), thus highlighting a striking stimulatory effect of the IRF-1-derived p300-binding peptides. Both the CT2 and CT3 peptides are acidic and contain both Pro and Lys residues. However, mutation of groups of acidic residues did not prevent the peptides from activating p21 expression (data not shown), while mutation of the Lys and Pro residues to Ala significantly diminished the ability of the EGFP fusion peptides both to activate the p21 promoter and to elevate endogenous p21 protein levels (Fig. 3E). This suggests that the interaction between p300 and the CT domains may rely on the recognition of a structural motif that is disrupted upon mutation of the Pro residue(s).
If the CT2/3 peptides work through the p300/p53 axis to regulate p21 promoter activity, they should be inactive in the absence of p53. We therefore investigated the activity of the CT peptides in HCT116 (p53−/−) cells. The EGFP-CT peptides showed a total dependence on p53 for activity, as they had no effect on either p21 promoter activity (Fig. 3F, upper panel) or endogenous p21 protein expression (Fig. 3F, lower panel) in the HCT116 (p53−/−) cells.
Together the data presented here suggest that motifs from within the transactivation domain of IRF-1 (CT2 and CT3), which interact with the ISS-1 and/or SPC-2 domain on p300, are required and are sufficient for IRF-1-dependent stimulation of p21 expression. Thus, the major activity of IRF-1 required for synergistic activation of the p21 promoter with p53 is localized to two minidomains within IRF-1 that bind p300.
Transactivation domain fragments of IRF-1 stimulate acetylation of p53.
The data thus far suggest that the p300-IRF-1 interface plays a pivotal role in stimulating p53 activity. We therefore wished to establish whether the mechanism by which IRF-1 or the CT2/3 minidomains stimulated p53 activity on the p21 promoter involved enhanced p300-dependent acetylation of p53. It was first determined whether a nonacetylatable p53 mutant, in which six potential acetylation sites were mutated from lysine to arginine (p53-6KR), was responsive to IRF-1. If IRF-1 failed to stimulate basal transcription with this p53 mutant, it would suggest that acetylation was a likely mechanism by which IRF-1 was able to stimulate p53-dependent transcription from the p21 promoter. Consistent with previous findings (2), the p53-6KR mutant partially retained the ability to activate the p21 reporter (Fig. 4A and B). However, as expected, the activity of the p53-6KR mutant protein was not stimulated by exogenous p300 under conditions where wt p53 activity was stimulated 2.2-fold (Fig. 4A). Further, the p53-6KR mutant did not synergize with either wt IRF-1 or IRF-1YLP/A to regulate p21 promoter expression (Fig. 4B). In a separate experiment it was noted that, although p53-6KR was partially able to induce the expression of endogenous p21 protein compared to wt p53 (Fig. 4C), its activity was not further increased in the presence of IRF-1 under conditions where wt p53 and IRF-1 gave enhanced p21 protein levels. Together the above data suggest that the acetylation of p53 is required for IRF-1 to stimulate p53-dependent transcription from the p21 promoter.
FIG. 4.
A p53 acetylation mutant fails to cooperate with IRF-1. (A) HCT116 (p53−/−) cells were transfected with pcDNA-p53 (500 ng) and pcDNA-p53-6KR (500 ng) with or without pCMVβ-p300 (1 μg) as detailed, plus _p21_-Luc (1 μg) and pCMV-βGal (1 μg). Cells were harvested after 24 h; relative reporter activity is expressed as the ratio of luciferase to β-galactosidase activity (upper panel) and represents the mean ± standard deviation for three independent experiments. p53 and p300 protein levels were determined by immunoblotting with 19.1 and N15, respectively (lower panel). (B) HCT116 (p53−/−) cells were transfected with pcDNA-p53 (500 ng), pCMVp53-6KR (500 ng), pcDNA3-IRF-1 (1 μg), and pcDNA3-IRF-1YLP/A (1 μg) as detailed, plus _p21_-Luc (1 μg) and pCMV-βGal (1 μg). Relative reporter activity was determined as described above (upper panel), and immunoblots show the levels of p53 and IRF-1 determined using 19.1 and C20, respectively (lower panel). (C) HCT116 (p53−/−) cells were transfected with pcDNA-p53 (100 ng), pcDNA-p53-6KR (100 ng), pcDNA3-IRF-1 (150 ng), and pcDNA3-IRF-1YLP/A (150 ng) as detailed; all transfections were normalized for DNA with empty vector. Cells were harvested 24 h later, and immunoblots show the levels of endogenous p21 (upper panel), as well as IRF-1 (middle panel) and p53 (lower panel) expression levels; the proteins were detected using Ab-1, IRF-1 MAb, and DO-1, respectively. The results are representative of two independent experiments.
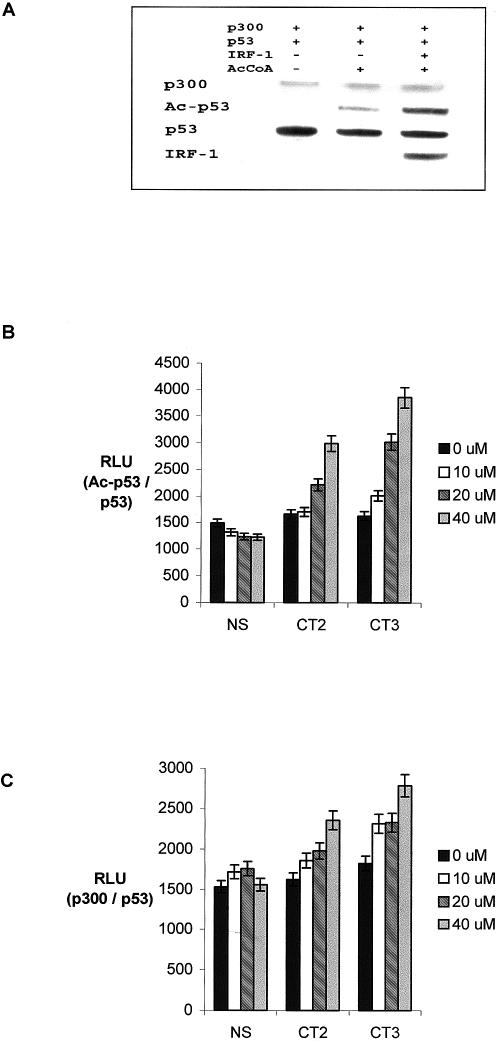
Whether IRF-1 binding to p300 actually promotes p53 acetylation was next investigated. We reason that, if IRF-1 was acting allosterically by enhancing p300-dependent acetylation of p53 through a direct interaction with the ISS-1 and/or SPC-2 domain, it should be possible to reconstitute IRF-1 and CT2/3 domain activity in vitro by using purified components (9). Acetylation reactions with reaction mixtures containing, as indicated, p53, p300, IRF-1, acetyl-CoA, and p53 consensus site DNA were carried out (Fig. 5A). The p53 consensus site was included since acetylation of native p53 tetramers is sequence specific and DNA dependent in vitro and in vivo (9). The relative acetylation of p53 was determined by immunoblotting with Ac-p53-K373/382 antibody, as described previously (9). The basal DNA-dependent acetylation (Fig. 5A, lane 2 from left) was stimulated 3.5-fold by the addition of purified IRF-1 (Fig. 5A, lane 3 from left), indicating that IRF-1 has the potential to stimulate p53 acetylation in vitro.
FIG. 5.
IRF-1 and the CT peptides stimulate p53 acetylation in vitro. (A) p53 protein (200 ng) was incubated with or without IRF-1 (200 ng) in the presence or absence of acetyl-CoA (2 μM); acetylation reactions were started by the addition of His-p300 (400 ng). p300, acetylated p53 (Ac-p53), total p53, and IRF-1 were detected by immunoblotting with N15, anti-Ac-p53-K373/382, DO-1/ICA-9, and C20, respectively. (B) Acetylation reactions were carried out in the presence of 0, 10, 20, or 40 μM CT2, CT3, or nonspecific peptide (NS) as described in Materials and Methods; acetylated p53 was detected by ELISA with anti-Ac-p53-K373/382 following capture of equal amounts of total p53 with MAb ICA-9. The results given as RLU are for acetylated p53 normalized to total p53 protein as the mean ± standard deviation for three independent experiments. (C) Acetylation reactions were carried out as described above but in the absence of acetyl-CoA; p300 binding was determined by ELISA with anti-p300 serum (N15) following capture of p53 by using ICA-9. The amount of bound p300 is given in RLU normalized to p53 protein.
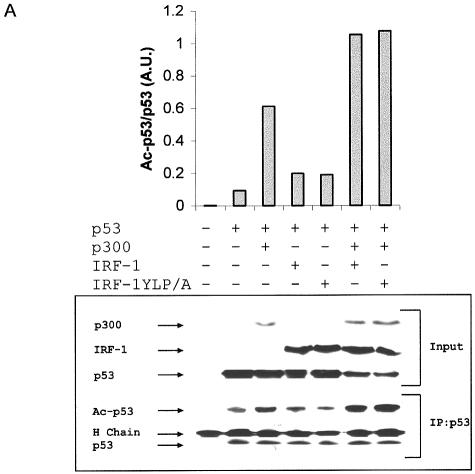
An ELISA-based immunochemical assay was used which measures the stability of the p300-p53 complex or the acetylation of p53 in vitro (9). Acetylation assays with reaction mixtures containing p53, p300, acetyl-CoA, and p53 consensus site DNA were carried out in the presence of increasing quantities of CT2, CT3, or a nonspecific peptide, NS. The relative acetylation of p53 was determined (Fig. 5B) using Ac-p53-K373/382 antibody following the capture of equal amounts of total p53 protein per assay. Under these conditions the acetylation of p53 was completely dependent on the presence of both acetyl-CoA and p300 (9). CT2 and CT3, but not a control peptide (NS), were able to significantly stimulate p300-dependent acetylation of p53 (Fig. 5B). Under these conditions, the phospho-LXXLL and proline repeat (PXXP) peptides derived from the two transactivation domains of p53 completely inhibit p53 acetylation (9, 10). These data indicate that the acetyltransferase activity of p300 can be inhibited by blocking the docking of p300 to the transactivation domain of p53 or that the acetyltransferase activity can be allosterically stimulated by IRF-1 towards p53 by an as-yet-undefined mechanism.
A p300-p53 binding assay was established to complement the above acetylation reactions except that it was carried out in the absence of acetyl-CoA (9). This allowed us to determine whether the increased acetylation seen in the presence of CT2 and CT3 correlated with an increase in p300 binding to p53. CT2 and CT3 stimulated acetylation-independent binding of p300 to p53 DNA complexes in a dose-dependent manner (Fig. 5C), suggesting that stimulation of p300-p53 binding by IRF-1 precedes enhanced acetylation of the p53 protein. Furthermore, there was good agreement between the ability of the peptides to stimulate acetylation of p53 and their ability to promote p300 binding to p53, suggesting that the CT peptides can replace full-length IRF-1 to promote, or stabilize, a more favorable p53 binding conformation in p300. Under these conditions, the phospho-LXXLL and PXXP peptides destabilized the p53-p300 complex (data not shown) (9, 10), indicating a specific effect of the CT2/3 peptides on the activity of p300. The fact that CT2/3 stabilize the binding of p300 to p53 suggests that the stimulation of p300 is not on the specific activity of the enzyme but on the stability of the docking-dependent acetylation reaction. Thus, binding of the CT domains of IRF-1 to p300 is sufficient to stabilize the complex among p300-p53-DNA, leading to enhanced p300-dependent acetylation of p53 protein.
IRF-1 stimulates acetylation of promoter-bound p53.
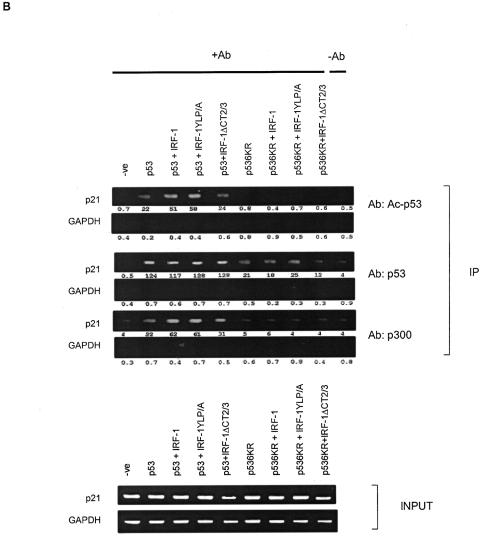
Finally, we determined whether full-length IRF-1 could stimulate p53 acetylation in cells and whether this could regulate the formation of p53-containing complexes at the p21 promoter sites within chromosomal DNA. When p53 protein was immunoprecipitated from HCT116 (p53−/−) cells expressing p53 and acetylation was quantified using an anti-Ac-p53-K373/382 antibody, basal acetylation was detected and this was increased in the presence of transfected p300 (Fig. 6A). The addition of wt IRF-1, or the IRF-1YLP/A mutant, in combination with p300 further enhanced the acetylation of p53 (Fig. 6A). Thus, IRF-1 stimulation of p53 activity against the p21 promoter correlated with increased acetylation of the p53 protein in cells.
FIG. 6.
IRF-1 stimulates the acetylation of p53 bound at the p21 promoter. (A) HCT116 (p53−/−) cells transfected with pcDNA-p53 (500 ng), pCMVβ-p300 (2 μg), pcDNA3-IRF-1 (1 μg), and pcDNA-IRF-1YLP/A (1 μg) as detailed. p53 was immunoprecipitated using MAb DO-1 and analyzed by immunoblotting; anti-Ac-p53-K373/382 was used to detect acetylated p53 protein (Ac-p53); p300, IRF-1, and total p53 were detected using N15, C20, and 19.1, respectively (lower panel). The immunoblot was quantitated, and relative acetylation of p53 is given in arbitrary units (A.U.) for acetylated p53 normalized to total immunoprecipitated p53 protein (upper panel). The antibody heavy chain is indicated by “H Chain.” (B) ChIP assay of HCT116 (p53−/−) cells transfected with 5 μg of pcDNA-p53 or pcDNA-p53-6KR and 10 μg each of pcDNA-IRF-1, pcDNA-IRF-1YLP/A, pcDNA-IRF-1ΔCT2/3, or empty vector (−ve) and irradiated with 20 J of UVC/m2. Protein DNA was cross-linked 5 h postirradiation with 1% formaldehyde. Protein-DNA complexes were immunoprecipitated with anti-p300 (N15), p53 (DO-1), and Ac-p53 (K373/382) antibodies. PCR analysis (with primers for p21 and GAPDH) is shown for input DNA or DNA after ChIP. The numbers below each agarose gel lane are given in arbitrary units and represent bands quantified by densitometry with a Genegnome Bioimager with Syngene software.
In order to ascertain whether there was a direct link between the ability of IRF-1 to enhance transcription from a p21 reporter and to stimulate p53 acetylation, we used ChIP assays (24). This allowed us to determine the effect of IRF-1 on promoter-bound p53 in DNA-damaged cells where the p21 promoter was present in the physiologically relevant context of chromatin. Interestingly, expression of IRF-1 had no effect on the total amount of p53 protein bound at the p21 promoter (Fig. 6B, Ab: p53). However, following immunoprecipitation with antibodies to acetylated p53, IRF-1 and IRF-1YLP/A gave a 2.3- and 2.6-fold increase in the acetylation of promoter-bound p53 protein, respectively (Fig. 6B, Ab: Ac-p53). The amount of endogenous p300 protein bound in transcription complexes at the p21 promoter following DNA damage also increased in the presence of IRF-1, as determined by analyzing DNA immunoprecipitated using p300 antiserum (Fig. 6B, Ab: p300). Thus, increased p53 acetylation was accompanied by a 2.8-fold increase in the amount of p300 protein associated with the p21 promoter in the presence of either IRF-1 or IRF-1YLP/A. In contrast, the p300-binding mutant IRF-1ΔCT2/3 did not have a significant effect on either p53 acetylation or p300 recruitment. As predicted the p53-6KR mutant showed low levels of p21 promoter binding compared to the wt protein, and no significant stimulation of binding was detected in the presence of the wt or mutant IRF-1 constructs. As a control for p53 acetylation, we were able to demonstrate that no p21 promoter DNA was detected in the presence of p53-6KR when an acetylation-specific p53 antibody was employed (Fig. 6B, Ab: Ac-p53). In addition the p300 protein was not associated with the p21 promoter in the presence of the p53-6KR mutant (Ab: p300).
The experiments presented above provide evidence that synergistic activation of the p21 promoter by IRF-1 and p53 involves the enhanced acetylation of promoter-bound p53 protein. The increase in acetylation of p53 observed in the presence of IRF-1 is accompanied by a similar increase in p21 promoter-associated p300 protein. Thus, IRF-1 appears to function by enhancing the recruitment of p300 to p53 prebound at the p21 promoter, leading to increased acetylation.
DISCUSSION
Gene disruption studies have shown that the two antioncogenic transcription factors IRF-1 and p53 are both required for maximal expression from the p21 promoter in damaged cells (36). We have built on these data to define in molecular detail how IRF-1 stimulates p53-dependent transcription through enhanced recruitment of the p300 coactivator protein to, and acetylation of, p53 that is bound to the p21 promoter.
p53 was the first nonhistone protein shown to be acetylated by p300 (12). Acetylation was originally shown to stimulate binding of p53 to DNA (12, 27). However, more recent studies have suggested that acetylation cannot activate p53 DNA binding when its consensus site is presented within the context of large DNA fragments or chromatin (11). Further, acetylation of the p53 tetramer is constrained by its conformation when in the native state and DNA binding can activate p300-dependent acetylation in vitro and in vivo (9). The mechanism of DNA-dependent acetylation involves the proline repeat domain of p53, which appears to be essential to maintain the conformational constraints imposed by acetylation when p53 is bound to DNA (10). Thus, the observation that acetylation of p53 enhances its transcriptional activity can be explained in part by the clamping of p300 to acetylated p53 when p53 is DNA bound (10). Presumably, the clamped p300 complex is then in a position to promote further coactivator binding and histone acetylation, producing an acetylation cascade (2). We found that there was a clear relationship between the ability of full-length IRF-1, or the small IRF-1-derived peptides (CT domain peptides), to promote p300 recruitment or p53 acetylation and their ability to promote p53-dependent transcription from the p21 promoter. In addition, while no increase in the total amount of p53 bound at the p21 promoter in damaged cells was detected following the expression of IRF-1 (Fig. 6B), increased acetylation of p53 and recruitment of p300 to promoter-bound complexes were readily observed. Thus, the data presented in the present study support the hypothesis that coactivator recruitment and acetylation promote p53-dependent transcription.
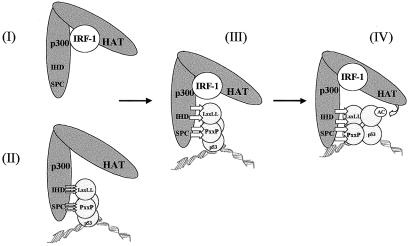
IR-treated p53−/− and IRF-1−/− MEFs are deficient in their ability to induce maximal expression of endogenous p21 protein and to undergo growth arrest (36). In addition, exposure of cells to agents that generate DNA strand breaks leads to the coordinated induction of IRF-1 and p53 proteins (25). In response to either IR or etoposide, IRF-1 protein levels are induced with kinetics strikingly similar to those of p53, and under these conditions induction of IRF-1 is ATM dependent. As the ATM kinase is a well-characterized member of the pathway leading to p53 activation in irradiated cells, this kinase appears to be a key component of the upstream pathway linking IRF-1 to p53 (25). Thus, synergy between p53 and IRF-1 to stimulate p21 expression during the response to DNA damage is likely to occur in an ATM-dependent manner. In the present study we have demonstrated that both IRF-1 and p53 are in complex with p300 in irradiated cells (Fig. 2A). We would therefore propose that, following the detection of DNA strand breaks and activation of ATM, induced IRF-1 protein binds to p300 promoting, or stabilizing, a favorable p53-binding conformation in p300, supporting the formation of a preinitiation complex at the p21 promoter. However, we cannot rule out the possibility that IRF-1 functions by additional mechanisms to cooperate with p53. Under certain cellular conditions, for example in the absence of functional p53, direct binding of IRF-1 to the p21 promoter may contribute to its activity (Fig. 1D) or IRF-1 may be involved in a direct interaction with p53 as has been proposed for the viral IRF homologue vIRF-1 (29). On the other hand the evidence presented here lends strong support for p300 as a mediator of IRF-1 effects on p53-dependent transcription. In this model the binding of IRF-1 to p300 facilitates recruitment of p300 to p53, leading to enhanced acetylation of p53 in a preinitiation complex; in turn this leads to an increase in p53-dependent transcription from the p21 promoter. These data expand on the protein-protein contacts that can operate on p53 at the p21 promoter and include (i) docking of p300 to the LXXLL and PXXP motifs on p53; (ii) the binding of IRF-1 CT2 and CT3 minidomains to p300, which further stabilizes the docking of p300 to p53; and (iii) DNA binding which changes the conformation of p53 to activate acetylation of the anchored p300 (Fig. 7). Future studies will be geared towards testing this model by identifying PXXP-containing modifiers of p300 docking and CT2/3 homology proteins that could modify IRF-1 effects on p300 binding to p53.
FIG. 7.
Stages in the assembly of the p300-p53-IRF-1 complex. Reconstitution of the p53-p300 complex has revealed several regulated stages that have been dissected biochemically. IRF-1 binding to p300 stabilizes the interaction of p300 with DNA-bound p53 (III) by either promoting a more favorable p53 binding conformation in p300 (I) or stabilizing a preformed p300-p53 complex (II). Stable binding of p300 to p53 then facilitates p53 acetylation, which clamps p53 to the DNA (IV).
The refinement of a minimal domain of IRF-1 into a stimulatory peptide identifies a novel lead for the development of low-molecular-weight stimulators of the p53 response. Prior to these studies, peptide leads that effect protein-protein contacts have been used to manipulate p53 protein-protein interactions and p53-dependent transcription. For example, modulation of the p53 inhibitor MDM2 by using a peptide that binds to the MDM2 active site can stimulate p53 activity (8). In contrast, peptides derived from the p53-p300 interface inhibit p53 and the combined use of LXXLL and PXXP peptides destabilizes the p53 protein (10). Both these approaches are important to the development of potential anticancer drugs, as both activation and inhibition of p53 can induce or sensitize cells to apoptosis, respectively. The IRF-1 peptide leads provide a novel type of agent that stimulates p53 activity in the absence of MDM2 modification and suggest that MDM2 control can be overridden by stabilizing p53 binding to a positive effector, in this case p300. Although it will prove relatively difficult to dissect the many p300 minidomains that contact p53 and IRF-1 in this three-component system (Fig. 7), the use of small peptides affords the possibility of fine-mapping and dissecting specific but dynamic protein-protein contacts in this transcription complex.
Acknowledgments
D.D. and M.E. were the recipients of BBSRC and CRUK Ph.D. studentships, respectively. T.R.H. is supported by a Cancer Research UK Programme grant and an MRC Career Establishment grant. K.L.B. is supported by a Cancer Research UK Programme grant.
REFERENCES
- 1.Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268**:**2764-2772. [DOI] [PubMed] [Google Scholar]
- 2.Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8**:**1243-1254. [DOI] [PubMed] [Google Scholar]
- 3.Boultwood, J., C. Fidler, S. Lewis, A. MacCarthy, H. Sheridan, S. Kelly, D. Oscier, V. J. Buckle, and J. S. Wainscoat. 1993. Allelic loss of IRF1 in myelodysplasia and acute myeloid leukemia: retention of IRF1 on the 5q- chromosome in some patients with the 5q- syndrome. Blood 82**:**2611-2616. [PubMed] [Google Scholar]
- 4.Burysek, L., W. S. Yeow, B. Lubyova, M. Kellum, S. L. Schafer, Y. Q. Huang, and P. M. Pitha. 1999. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J. Virol. 73**:**7334-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspari, T. 2000. How to activate p53. Curr. Biol. 10**:**R315-R317. [DOI] [PubMed] [Google Scholar]
- 6.Craig, A., M. Scott, L. Burch, G. Smith, K. Ball, and T. Hupp. 2003. Allosteric effects mediate CHK2 phosphorylation of the p53 transactivation domain. EMBO Rep. 4**:**787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig, A. L., L. Burch, B. Vojtesek, J. Mikutowska, A. Thompson, and T. R. Hupp. 1999. Novel phosphorylation sites of human tumour suppressor protein p53 at Ser20 and Thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers. Biochem. J. 342**:**133-141. [PMC free article] [PubMed] [Google Scholar]
- 8.Dornan, D., and T. R. Hupp. 2001. Inhibition of p53-dependent transcription by BOX-I phospho-peptide mimetics that bind to p300. EMBO Rep. 2**:**139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dornan, D., H. Shimizu, N. D. Perkins, and T. R. Hupp. 2003. DNA-dependent acetylation of p53 by the transcription coactivator p300. J. Biol. Chem. 278**:**13431-13441. [DOI] [PubMed] [Google Scholar]
- 10.Dornan, D., H. Shimizu, L. Burch, A. J. Smith, and T. R. Hupp. 2003. The proline repeat domain of p53 binds directly to the transcriptional coactivator p300 and allosterically controls DNA-dependent acetylation of p53. Mol. Cell Biol. 23**:**8846-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa, J. M., and B. M. Emerson. 2001. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol. Cell 8**:**57-69. [DOI] [PubMed] [Google Scholar]
- 12.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90**:**595-606. [DOI] [PubMed] [Google Scholar]
- 13.Hupp, T. R., and D. P. Lane. 1994. Allosteric activation of latent p53 tetramers. Curr. Biol. 4**:**865-875. [DOI] [PubMed] [Google Scholar]
- 14.Hupp, T. R., D. P. Lane, and K. L. Ball. 2000. Strategies for manipulating the p53 pathway in the treatment of human cancer. Biochem J. 352**:**1-17. [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer, N. G., S. F. Chin, H. Ozdag, Y. Daigo, D. E. Hu, M. Cariati, K. Brindle, S. Aparicio, C. Caldas, et al. 2004. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc. Natl. Acad. Sci. USA 101**:**7386-7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirchhoff, S., and H. Hauser. 1999. Cooperative activity between HER oncogenes and the tumor suppressor IRF-1 results in apoptosis. Oncogene 18**:**3725-3736. [DOI] [PubMed] [Google Scholar]
- 17.Kroger, A., M. Koster, K. Schroeder, H. Hauser, and P. P. Mueller. 2002. Activities of IRF-1. J. Interferon Cytokine Res. 22**:**5-14. [DOI] [PubMed] [Google Scholar]
- 18.Lane, D. P., and T. R. Hupp. 2003. Drug discovery and p53. Drug Discov. Today 8**:**347-355. [DOI] [PubMed] [Google Scholar]
- 19.Matsuyama, T., T. Kimura, M. Kitagawa, K. Pfeffer, T. Kawakami, N. Watanabe, T. M. Kundig, R. Amakawa, K. Kishihara, A. Wakeham, et al. 1993. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell 75**:**83-97. [PubMed] [Google Scholar]
- 20.Merika, M., A. J. Williams, G. Chen, T. Collins, and D. Thanos. 1998. Recruitment of CBP/p300 by the IFN beta enhanceosome is required for synergistic activation of transcription. Mol. Cell 1**:**277-287. [DOI] [PubMed] [Google Scholar]
- 21.Nozawa, H., E. Oda, K. Nakao, M. Ishihara, S. Ueda, T. Yokochi, K. Ogasawara, Y. Nakatsuru, S. Shimizu, Y. Ohira, K. Hioki, S. Aizawa, T. Ishikawa, M. Katsuki, T. Muto, T. Taniguchi, and N. Tanaka. 1999. Loss of transcription factor IRF-1 affects tumor susceptibility in mice carrying the Ha-ras transgene or nullizygosity for p53. Genes Dev. 13**:**1240-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogasawara, S., G. Tamura, C. Maesawa, Y. Suzuki, K. Ishida, N. Satoh, N. Uesugi, K. Saito, and R. Satodate. 1996. Common deleted region on the long arm of chromosome 5 in esophageal carcinoma. Gastroenterology 110**:**52-57. [DOI] [PubMed] [Google Scholar]
- 23.Oren, M. 2003. Decision making by p53: life, death and cancer. Cell Death Differ. 10**:**431-442. [DOI] [PubMed] [Google Scholar]
- 24.Orlando, V., H. Strutt, and R. Paro. 1997. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods 11**:**205-214. [DOI] [PubMed] [Google Scholar]
- 25.Pamment, J., E. Ramsay, M. Kelleher, D. Dornan, and K. L. Ball. 2002. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene 21**:**7776-7785. [DOI] [PubMed] [Google Scholar]
- 26.Prost, S., C. O. Bellamy, D. S. Cunningham, and D. J. Harrison. 1998. Altered DNA repair and dysregulation of p53 in IRF-1 null hepatocytes. FASEB J. 12**:**181-188. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi, K., J. E. Herrera, S. Saito, T. Miki, M. Bustin, A. Vassilev, C. W. Anderson, and E. Appella. 1998. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12**:**2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schon, O., A. Friedler, M. Bycroft, S. M. Freund, and A. R. Fersht. 2002. Molecular mechanism of the interaction between MDM2 and p53. J. Mol. Biol. 323**:**491-501. [DOI] [PubMed] [Google Scholar]
- 29.Seo, T., J. Park, D. Lee, S. G. Hwang, and J. Choe. 2001. Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J. Virol. 75**:**6193-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shieh, S. Y., J. Ahn, K. Tamai, Y. Taya, and C. Prives. 2000. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14**:**289-300. [PMC free article] [PubMed] [Google Scholar]
- 31.Shikama, N., C. W. Lee, S. France, L. Delavaine, J. Lyon, M. Krstic-Demonacos, and N. B. La Thangue. 1999. A novel cofactor for p300 that regulates the p53 response. Mol. Cell 4**:**365-376. [DOI] [PubMed] [Google Scholar]
- 32.Stevens, A. M., and L. Y. Yu-Lee. 1994. Multiple prolactin-responsive elements mediate G1 and S phase expression of the interferon regulatory factor-1 gene. Mol. Endocrinol. 8**:**345-355. [DOI] [PubMed] [Google Scholar]
- 33.Tamura, G., S. Ogasawara, S. Nishizuka, K. Sakata, C. Maesawa, Y. Suzuki, M. Terashima, K. Saito, and R. Satodate. 1996. Two distinct regions of deletion on the long arm of chromosome 5 in differentiated adenocarcinomas of the stomach. Cancer Res. 56**:**612-615. [PubMed] [Google Scholar]
- 34.Tamura, T., M. Ishihara, M. S. Lamphier, N. Tanaka, I. Oishi, S. Aizawa, T. Matsuyama, T. W. Mak, S. Taki, and T. Taniguchi. 1995. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature 376**:**596-599. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka, N., M. Ishihara, M. Kitagawa, H. Harada, T. Kimura, T. Matsuyama, M. S. Lamphier, S. Aizawa, T. W. Mak, and T. Taniguchi. 1994. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell 77**:**829-839. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, N., M. Ishihara, M. S. Lamphier, H. Nozawa, T. Matsuyama, T. W. Mak, S. Aizawa, T. Tokino, M. Oren, and T. Taniguchi. 1996. Cooperation of the tumour suppressors IRF-1 and p53 in response to DNA damage. Nature 382**:**816-818. [DOI] [PubMed] [Google Scholar]
- 37.Taniguchi, T., and A. Takaoka. 2002. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14**:**111-116. [DOI] [PubMed] [Google Scholar]
- 38.Wahl, G. M., and A. M. Carr. 2001. The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat. Cell Biol. 3**:**E277-E286. [DOI] [PubMed] [Google Scholar]
- 39.Willman, C. L., C. E. Sever, M. G. Pallavicini, H. Harada, N. Tanaka, M. L. Slovak, H. Yamamoto, K. Harada, T. C. Meeker, A. F. List, et al. 1993. Deletion of IRF-1, mapping to chromosome 5q31.1, in human leukemia and preleukemic myelodysplasia. Science 259**:**968-971. [DOI] [PubMed] [Google Scholar]
- 40.Yuan, Z. M., Y. Huang, T. Ishiko, S. Nakada, T. Utsugisawa, H. Shioya, Y. Utsugisawa, K. Yokoyama, R. Weichselbaum, Y. Shi, and D. Kufe. 1999. Role for p300 in stabilization of p53 in the response to DNA damage. J. Biol. Chem. 274**:**1883-1886. [DOI] [PubMed] [Google Scholar]