Lipid-based nanotherapeutics for siRNA delivery (original) (raw)
. Author manuscript; available in PMC: 2017 Feb 14.
Abstract
RNA interference (RNAi) is a specific gene-silencing mechanism triggered by small interfering RNA (siRNA). The application of RNAi in the clinic requires the development of safe and effective delivery systems. Inspired by progress with lipid-based systems in drug delivery, efforts have been dedicated to the development of liposomal siRNA delivery systems. Many of the lipid-based delivery vehicles self-assemble with siRNA through electrostatic interactions with charged amines, generating multi-lamellar lipoplexes with positively charged lipid bilayers separated from one another by sheets of negatively charged siRNA strands. Internalization of lipid-based siRNA delivery systems into cells typically occurs through endocytosis; accordingly, delivery requires materials that can facilitate endosomal escape. The size of the carrier is important as carriers <100 nm in diameter have been reported to have higher accumulation levels in tumours, hepatocytes and inflamed tissue, whereas larger particles tend to be taken up by Kupffer cells or other components of the reticuloendothelial system (RES). To reduce RES uptake and increase circulation time, carriers have been modified on the surface with hydrophilic materials, such as polyethyleneglycol. Herein, we review the molecular and structural parameters of lipid-based siRNA delivery systems.
Keywords: cationic/anionic lipid, cellular uptake, cholesterol, endocytosis, nanoparticle, siRNA
Introduction
The effects of RNA interference (RNAi) were first reported by Napoli et al. [1] in 1990, as a result of their attempt to overexpress chalcone synthetase (CHS), an enzyme largely responsible for plant colouration, in petunias. The authors were surprised to find that introducing the gene resulted in blocking pigment synthesis, and growth of white or partly white flowers instead of the purple ones [2, 3]. Although not fully understood at the time, an explanation for this result and other similar phenomena was revealed with the publication of Fire and Mello's seminal paper on RNAi in 1998. Fire, Mello and co-workers used double-stranded RNAs to manipulate gene expression in the nematode Caenorhabditis elegans and identified RNAi as a fundamental pathway in which sequence specific RNA strands are able to target and induce the silencing of complementary mRNA [4].
siRNA
Small interfering RNAs (siRNAs) are duplexes of 21–23 nucleotides, approximately 7.5 nm in length [5–7] and 2 nm in diameter [8]. siRNAs can be created intracellularly through cleavage of long double-stranded RNA by the enzyme Dicer [9, 10]. Once in the cytoplasm, the siRNA sense strand is cleaved and degraded, whereas the antisense strand is incorporated into the RNA-induced silencing complex (RISC) [11, 12]. RISC associates with and degrades complementary mRNA sequences; this prevents translation of the target mRNA into protein, ‘silencing’ the gene [12, 13]. As many diseases are caused by the overexpression of one or multiple genes, the therapeutic potential of RNA silencing has been investigated for a number of diseases, including cancer [14, 15], infection and inflammation [16], respiratory diseases [17], neurological diseases [18] and autoimmune diseases [19].
Small interfering RNA delivery and the delivery of large DNA sequences for gene therapy differ in several respects. Some of these differences include the site of action in the cell, molecular stability and molecular size [20]. The destination of an siRNA molecule is the cytoplasm, whereas the delivery of a gene requires that the genetic material pass the nuclear membrane. In either case, it is thought that the nucleic acids must ‘unpack’ from the lipid complex to interact with the appropriate cellular targets. Once the siRNA has been delivered to the cell, the duration of expression knockdown is often between 3 and 7 days (in dividing cells) or up to 3–4 weeks (in nondividing cells) [21]. Transgene expression as a result of DNA-based gene therapy is variable, and can range from short-term to permanent [20].
The molecular weight of a double-stranded siRNA molecule is in the order of 13 kDa, whereas the molecular weight of a double-stranded DNA molecule for gene therapy (not antisense therapy) is often several hundred times greater. Accordingly, those materials suited for DNA delivery may not be ideal for siRNA delivery. In part this is because the size of lipoplexes and polyplexes is affected by the size of the genetic material and the carrier [22]. The phosphodiester backbone of RNA is more sensitive to hydrolysis than in DNA; RNA can be degraded in vivo by RNAses. This has prompted development of chemical strategies to improve stability, including various modifications to the backbone that do not affect RISC complexation, and hydrophobic conjugates that improve serum stability [23–25].
The challenge – siRNA delivery
One of the primary challenges of siRNA-based therapeutics is delivery [15]. Therapeutic applications of siRNA require the development of carriers that will: (i) protect siRNA from degradation during circulation [26]; (ii) deliver siRNA at the target cells and avoid delivery to nontarget cell types; (iii) facilitate cellular uptake and endosomal escape; (iv) release siRNA intracellularly so that it will be accessible to the cellular machinery.
In general, siRNA delivery carriers are designed to accumulate at the target site, while avoiding non-specific uptake in nontarget tissue. Many carriers are designed to avoid nonspecific interactions with blood and extracellular elements [27]. This can be achieved by introducing a hydrated steric barrier to surround the carrier using materials such as polyethyleneglycol (PEG) [28, 29]. When a carrier is injected into a peripheral vein, it enters the right side of the heart and is pumped out to the lungs; the lungs contain the first capillary beds and act as an initial mechanical filtration barrier [27]. If small enough, the carriers leave the lungs and enter the left side of the heart and are pumped into the systemic circulation. Given that the liver blood vessels contain fenestrae that are, on average, 100 nm in diameter [30], particles smaller than 100 nm are considered necessary to target hepatocytes [27]. Inclusion of targeting ligands, such as galactose derivatives (recognized by the asioglycoprotein receptor) [31] or peptides from the T7 phage [27, 32], have been reported to improve hepatocellular uptake of some delivery systems. In certain tumour types, passive targeting has been reported via the enhanced permeability and retention (EPR) effect [33, 34], in which increased permeability of blood vessels surrounding tumours [35] and inflamed tissue [36, 37] is used to target these tissue.
Penetrating the cell
Small interfering RNA is negatively charged and typically cannot cross the cell membrane by free diffusion [38]. A number of approaches have been developed to facilitate siRNA uptake, including: (i) conjugating siRNA to a ligand, such as a cell-penetrating peptide or small molecule to facilitate siRNA uptake; (ii) endocytosis of siRNA encapsulated within nanoparticles; or (iii) fusion of the carrier with the cell membrane, thereby releasing the carriers’ content into the cytoplasm.
One study using siRNA lipoplexes generated from the commercially available cationic lipid DharmaFECT reported that ~95% of the lipoplexes enter cells through endocytosis [38]; ~50% of endocytosis was clathrin-mediated [38]. Typically, clathrin-mediated endocytosis is responsible for the uptake of many macromolecules from the extracellular medium. The vesicles generated by this pathway are about 100 nm in diameter and are decorated with a crystalline coat containing the protein clathrin [39]. In this same study about 20% of the remaining material delivered to the cytoplasm was internalized via lipid-raft/caveolin-mediated endocytosis [38]. Lipid-raft compartments are usually larger than 50 nm in diameter and consist of the cholesterol-binding protein caveolin and of liquid-ordered domains of cholesterol and glycosphingolipids [40, 41].
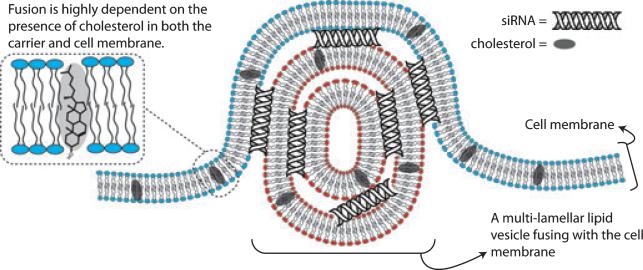
Xu and Szoka proposed that the release of nucleic acids from cationic lipid complexes may be facilitated by association of cellular anionic lipids with a carrier's cationic lipids, to form neutral ion pairs which ‘free’ the nucleic acid from the delivery system [27, 42]. Felgner et al. [43], discussing DNA delivery using liposomes composed of the cationic phospholipid DOTMA (1,2-di-O-octadecenyl-3-trimethylammonium propane), suggested that positively charged liposomes adhere to the negatively charged DNA, forming a complex in which the DNA is surrounded by charged liposomes. This complex adheres to, and then fuses with, the negative surface of cells, enabling internalization of DNA into the cell. As siRNA is smaller than DNA, it can be loaded into a single lipoplex which is capable of fusing with the cell membrane and subsequently freeing siRNA into the cytosol. A schematic representation of siRNA internalization by fusion of the carrier with the cell membrane is presented in Fig. 1.
Fig. 1.
A schematic representation of the fusion of a multilamellar small interfering RNA lipoplex with the cell membrane. The positively charged lipid bilayer adsorbs to the negatively charged surface of the cell, resulting in either an endocytosis process or by fusion of the lipoplex with the cell membrane, thereby releasing the nucleic payload into the cytosol [38]. During the process, the lipid membrane is stressed and lipids are freed to the intracellular and extracellular compartments.
Escaping the endosome
As siRNA carriers typically enter cells via endocytosis [38], a means of endosomal escape is necessary. Although the precise mechanisms of endosomal escape by siRNA delivery systems remains unclear, one hypothesis is that certain materials can facilitate endosomal escape via ‘the proton sponge effect’ [44, 45]. The mechanism is proposed to act as follows: the endosome acidifies after internalization, and amine groups on delivery materials that have a pKa in this range (typically between 7 and 5) are protonated. This is followed by influx of additional protons as well as chloride ions. The uptake of ions creates an osmotic imbalance; water enters the endosome to counter this effect, causing the endosome to inflate until it ruptures. Rupture of the endosome releases its contents to the cytoplasm [44, 45]. There are a number of intracellular delivery materials that have amines with pKa values in the endosomal pH range of 5–7 [46], such as polyethylenimine (PEI) and _β_-amino esters [44, 47–50].
Despite the fact that there is some controversy as to what extent endosomal escape affects transfection [38, 51, 52], we hypothesize that the influx of protons into the endosome may facilitate unpackaging of siRNA from some carriers. Internalization within acidified endosomes may facilitate siRNA release from the lipoplex prior to its release in the cytoplasm [27].
pH-sensitive bonds
Another strategy to improve nucleic acid delivery from lipid-based systems is the incorporation of pH-sensitive groups. These groups can induce phase or structural transformations that can promote unpackaging of siRNA from the complex [53, 54]. This approach has been used to also trigger drug release in tumours [55].
The pH level of the extracellular matrix and of blood is 7.4, whereas intracellularly the pH is 7.2 [56]. However, in a majority of tumours pH levels are lower both extracellularly and intracellularly [57, 58], reaching 5.7 in some cases [56]. This is primarily because of a higher rate of glycolysis in tumours [59]. An example of a pH-responsive phospholipid is citraconyl-DOPE (1,2-dioleoyl-3-phosphatidylethanotamine) modified by citraconic anhydride [60]. This lipid degrades under acidic conditions, destabilizing the siRNA lipid complex and promoting release of the siRNA. Degradation also releases a fusogenic entity, which can disrupt the endosome capsule, thereby releasing the free siRNA to the cytosol [46].
Cationic lipids as building blocks of siRNA delivery systems
The development of siRNA delivery systems has been influenced by the studies on intracellular DNA delivery [38]. However, there are significant differences between siRNA and DNA, including that: (i) the overall size and charge of siRNA is less than that of DNA, and (ii) siRNA needs to reach the cytosol for therapeutic effect, whereas DNA must enter the nucleus to be effective. As with DNA, siRNA carriers can be composed of polymers [61] (to form polyplexes), peptides, lipids (to form lipoplexes or liposomes) and their combinations [62]. This review will focus on lipid-based siRNA delivery systems.
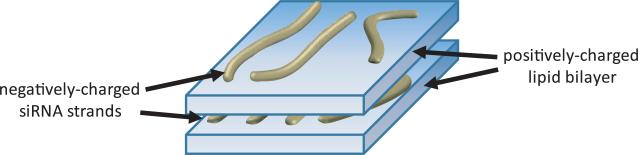
Cationic lipids were introduced as carriers for DNA and RNA over 20 years ago [63, 64]. Cationic lipids interact with negatively charged nucleic acids through electrostatic interactions forming complexes called lipoplexes [27]. The proposed [65] mechanism of formation of lipoplexes is that negatively charged nucleic acids bind to positively charged lipid vesicles. Additional positively charged vesicles adsorb to the solvent-exposed nucleic acids. This process causes formation of a multilamellar structure of positively charged lipid bilayers [66, 67] ~3.7 nm thick [65], spaced ~2 nm apart from each other by negatively charged nucleic acids [68]. A schematic representation of this structure appears in Fig. 2.
Fig. 2.
Multilamellar structure of cationic lipid and small interfering RNA (siRNA) lipoplexes. SiRNA double strands adsorb to the positively charged surfaces of lipid bilayers, to form a multilamellar structure in which, ~3.7-nm thick [65] lipid bilayers are separated ~2 nm apart from each other by siRNA strands [68].
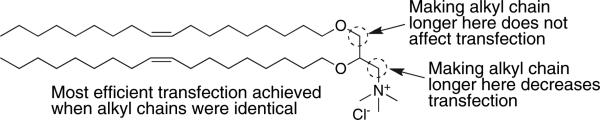
One of the first cationic lipids to be used for DNA delivery is DOTMA [63, 64], see Table 1. Upon hydration, DOTMA will form liposomes either alone, or in presence of other lipids. These liposomes can be down-sized into small unilamellar vesicles (SUVs) <100 nm in diameter. Liposomes differ from micelles; liposomes are spherical vesicles in which a single or several continuous lipid bilayers/s separate the external aqueous medium from the intraliposomal aqueous core, whereas micelles have an inner oil core. Based on the efficacy of DOTMA and other cationic lipids, Ren et al. [69] proposed structural features common to those lipids most effective for DNA delivery in vivo. These features, schematically represented in Fig. 3, include: (i) a cationic head group and its neighbouring aliphatic chain being in a 1,2-relationship on the backbone; (ii) an ether bond for bridging the aliphatic chains to the backbone; and (iii) paired oleyl chains as the hydrophobic anchor into the lipid assembly.
Table 1.
Some commonly used cationic lipids, their molecular structure and tail configuration. Molecules were drawn using ChemBioDraw Ultra 11.0.1 (CambridgeSoft, Cambridge, MA, USA)
Fig. 3.
Lessons learned from structural modifications of DOTMA acting as a transfection agent.
More recently [70], a combinatorial library of lipid-like molecules, termed lipidoids, was developed for siRNA delivery. The performance of the lipidoids was compared with different structural motifs including alkyl chain length and the degradability of the linker between amine and alkyl groups. Highest levels of knockdown were achieved using lipidoids with the following properties: (i) more than two amines per head unit; (ii) amide bonds between the amine ‘core’ and acyl tails; (iii) greater than two acyl chains; (iv) acyl chains between 8 and 12 carbon atoms; and (v) at least one secondary amine [70]. An example of a lipidoid, called 98N12, together with other commonly used cationic lipids, is presented in Table 1.
Cholesterol: common component in siRNA carriers
Cholesterol plays a role in many cellular membrane-related events such as membrane fusion, macropinocytosis and caveolin and lipid-raft-mediated endocytosis [38, 71, 72]. Introducing cholesterol as a component of certain DNA/RNA carriers has been reported to improve transfection in vivo in comparison with carriers not containing cholesterol [73–75]. When formulated in delivery vehicles at more than 25 mol%, cholesterol can decrease carrier permeability, increase carrier circulation time [76, 77] and increase structural rigidity and stability of the carrier [71]. Furthermore, cholesterol is reported to protect nucleic acids from extraliposomal degradative entities such as RNases [73, 74]. The importance of cholesterol for internalization of siRNA into cells has been exemplified by extracting cholesterol from cell membranes, and then exposing the cells to siRNA lipoplexes. In the cholesterol-depleted cells siRNA uptake and transfection were totally abolished [38].
Conjugating cholesterol to siRNA improves cellular uptake and transfection, and decreases siRNA degradation in serum [12, 15, 75]. Wolfrum et al. [78] showed that introducing cholesterol-conjugated siRNA into plasma resulted in association of these particles with either high-density lipoproteins (HDLs), which in vivo targets the liver, gut, kidney and steriodogenic organs, or with low-density lipoproteins (LDLs), which targeted the liver primarily [78]. siRNA conjugated to other hydrophobic molecules, with more than 22 carbons, also showed HDL and LDL association [78]. The association of the conjugated siRNA with HDL/LDL may protect siRNA from being degraded by plasma components.
Cholesterol may play a dual role in the delivery of siRNA. When incorporated in the carrier, cholesterol may help facilitate cell fusion or endosomal internalization of the carrier. When conjugated to siRNA, cholesterol seems to act as a targeting entity.
Derivatives of cholesterol have also been shown to improve the performance of cationic liposomes. Han et al. [79], showed that cationic liposomes enriched with an amine-based cholesterol derivative, cholesteryloxypropan-1-amine, increased delivery efficiency of siRNA in serum, in comparison with ordinary cholesterol.
Structural and physiological effects of carrier charge
A number of reports have addressed the relationship between lipid charge and lipid-to-RNA ratio in formulations on carrier shape, trafficking and efficacy. Pitard et al. [66] examined the morphology of siRNA lipoplexes prepared using lipids synthesized from aminoglyco-sides. In formulations with low lipid-to-siRNA ratios, the resulting lipoplex particles were small (<200 nm), stable and had overall negative charge. Increasing the concentration of lipids in the formulations neutralized the overall charge, but resulted in the formation of large (~700 nm) unstable aggregates [66]. Increasing the lipid/siRNA ratio further induced formation of small (<200 nm) stable particles with overall positive charge [66]. The ratio of lipid-to-siRNA strongly influences the shape, size and behaviour of a lipoplex. However, this effect depends on the lipid structure; different lipids at similar lipid/siRNA ratios will spontaneously form complexes of different sizes [80].
Safinya et al. [67] tested the effect of cationic lipid/siRNA charge ratio on particle uptake and gene knockdown in mammalian cells. They found that increasing the charge ratio had little effect on the total knockdown whereas it did however increase nonspecific knockdown. Interestingly, they found that multivalent (five-charge) lipids exhibited lower toxicity, higher total knockdown and lower nonspecific knockdown in comparison with a similar charge ratio carried by several univalent cationic lipids [67].
The overall charge of the carrier can affect its destination in vivo. Jain et al. [81] using 150-nm cationic liposomes composed of DOTAP (1,2-dioleoyl-3-trimethylammonium-propane), DOPC (1,2-dioleoyl-3-phosphatidylcholine), cholesterol and PEG-DSPE, showed that a majority (>55%) of liposomes accumulated in the liver. Increasing carrier charge (by introducing additional DOTAP to the carrier) reduced accumulation in the spleen and blood, and increased accumulation in the liver [81]. Although tumour uptake was not affected by particle charge, imaging analysis revealed that increasing positive charge on the particles did increase accumulation of the carriers in tumour vasculature. Litzinger et al. [82], using ~2 _μ_m diameter cationic liposomes, showed that biodistribution and cellular uptake were not affected by charge. In this case, a majority of liposomes accumulated in the Kupffer cells of the liver. Interestingly, they found that above a certain liposomal dose, the liver became saturated with liposomes, causing an ‘overflow’ of liposomes to accumulate in the spleen [82]. It should be noted that in this case accumulation in Kupffer cells (i.e. specialized macrophages located in the liver) may be owing to the large size of the carrier and not related to charge [83].
Anionic lipids
Although a majority of siRNA carriers are based on cationic lipids or polymers (e.g. DOTMA, DOTAP, poly-l-lysine, PEI and poly(2-(dimethylamino)ethyl methacrylate), several studies have tested the ability to deliver nucleic acids using combinations of cationic and anionic lipids. In one example, the negatively charged nucleic acid is complexed with an amine-based polypeptide (such as poly-l-lysine), generating a particle with a net positive charge. The particles are then treated with negatively charged lipids [84]. Mastrobattista et al. were able to improve transfection by preparing positively charged polyplexes coated with an anionic lipid [85]. The purpose of this strategy was to protect nucleic acids from deactivation by polyanions present in tumour ascitic fluid, such as hyaluronic acid (HA). Despite these reports, negatively charged complexes are uncommon in nucleic acid delivery as they can induce immunogenic responses, and are less likely to penetrate the negatively charged surface of the cells [27, 62, 86].
Effect of the carriers’ size on biodistribution
The size of the carrier seems to have a great effect on its biological fate and activity. Szoka et al. suggest that a size cutoff of 100 nm is highly important to overcome in vivo barriers for systemic gene delivery: blood components, RES uptake, tumour access, extracellular matrix components and intracellular barriers [83].
Kizelsztein et al. [87] showed that ~1% of the injected dose of neutral PEGylated liposomes ~ 80 nm in diameter will cross the blood–brain barrier and accumulate in brains of healthy mice. This number is tripled when the mice are suffering from a multiple sclerosis model disorder. Similarly, Avnir et al. [29], using ~80 nm PEGylated liposomes to treat an autoimmune arthritis model, showed that the levels of liposome accumulation in an arthritic joint were similar, or even higher, than those found in the liver, kidney or spleen. In these cases, increased accumulation is explained by the high permeability of vasculature surrounding the inflamed tissue.
Garbuzenko et al. [17, compared the pharmacokinetics of ~120 nm in diameter DOTAP-based liposomes containing siRNA that were administered either intravenously (IV) or intratrachealy. When administered IV, gradual accumulation of the liposomes in the liver, kidney and spleen were noticed over a period of 24 h; during this time, liposome concentration in the lungs increased for the first hour but declined thereafter [17]. Conversely, liposomes administered intratrachealy remained in the lungs for at least 72 h postadministration, with low levels in other organs [17]. In a different study by Ishiwata et al. [88] the majority of positively charged liposomes, ~150 nm in diameter, resided in the lung for the first hour post-IV administration, after which they mostly accumulated in the liver.
Chan et al. [89, 90] examined endocytosis of gold nanoparticles of varying sizes. They showed that uptake efficiency versus particle size followed a bell-shaped pattern with the most efficient uptake occurring in ~50-nm diameter particles. Although different from lipid-based carriers, studies with these particles provide insight into the optimal size of carriers that can enhance their efficiency and effectivity in vivo.
Carriers’ shape may affect delivery
The effect of the size of carriers on delivery has been studied for many years. However, limited in vitro and in vivo studies focused on the behaviour of carriers with regard to their shape and configuration. Discher et al. [91–94] formed worm-like micelles from degradable copolymers, with dimensions several nanometres wide and several microns long. These flexible filaments, named filomicelles, were shown to persist in rodent circulation for up to 1 week after IV injection, 10 times longer than spherical counter-particles, and were internalized by A549 human lung cancer cells. Sailor et al. [95] showed that dextran-coated magnetic iron oxide nanoparticles, elongated along one dimension, had longer circulation time, higher accumulation levels in murine MDA-MB-435 tumours and improved cellular uptake in comparison with the spherical ones. Similarly, it was shown [96] that single-walled carbon nanotubes coated with PEG-2000 accumulated at high levels in U87MG human glioblastoma tumours in mice.
Champion and Mitragotri [97], using alveolar macrophages as model phagocytes and polystyrene particles of various sizes and shapes as model targets, showed that target shape at the point of first contact by macrophages, and not size, decisively determines whether cells will proceed with phagocytosis or simply spread on the particle. While, prolate ellipsoids (major axis 2–6 _μ_m, aspect ratio 1.3–3) and elliptical discs (major axis 3–14 _μ_m, aspect ratio 2–4, thickness 400–1000 nm) were internalized by cells with great effectivity, spheres (radius 1.0–12.5 _μ_m) or oblate ellipsoids (major axis 4 _μ_m, aspect ratio 4) were covered by the cells and not internalized.
From the aforementioned studies it appears that carriers with a ‘pinhead’ format may be somewhat advantageous in penetrating cells, hence intracellular delivery. Recently, attempts to form microtubes and nanotubes from lipids have been reported [98–104], which are promising for use in nucleic acid delivery.
Current status and future prospective of clinical applications of siRNA nanotherapeutics
Since the first demonstrations of RNAi in C. elegans and mammalian cells about a decade ago, the development of RNAi therapeutics has progressed rapidly with a growing number of siRNA-based therapeutics currently in clinical trials (see Table 2). Early siRNA therapeutics for the treatment of age-related macular degeneration (AMD) and respiratory syncytial virus (RSV) [105] were administered locally using unmodified or chemically modified siRNA (in saline). More recently, formulations for systemic administration of siRNA packaged using polymers [106] or lipids have begun to be evaluated in the clinic. For example, a study conducted by Silence Therapeutics is testing a siRNA-liposomal formulation aimed at targeting protein kinase N3. This approach has proven to significantly inhibit tumour growth in prostate and pancreatic cancer models in mice [107], and is being tested in humans with advanced solid tumours. Alnylam Pharmaceuticals is investigating a lipid-based nanoformulation containing two different siRNA molecules aimed at targeting the kinesin spindle protein (KSP) and the vascular endothelial growth factor (VEGF) for their potential antiliver tumour activity. VEGF and KSP are upregulated in many tumour cells and play an important role in tumour proliferation and survival. Preclinical studies show that upon IV administration, the KSP/VEGF siRNAs in the lipid carrier target both KSP and VEGF messenger RNAs (mRNAs) [70, 108]. The results of these trials with lipid and formulated materials will provide important information regarding the translatability of delivery systems developed in rodents and primates.
Table 2.
Current clinical trials for small interfering RNA therapeutics
| Disease | Target | Formulation | Delivery mode | Company | Status | Preclinicalreference |
|---|---|---|---|---|---|---|
| Age-related macular degeneration; diabetic macular oedema | Vascular endothelial growth factor pathway | Saline | Topical; intravitreal injection | Opko Health; Allergan | Phases I–III | [109, 110] |
| Acute kidney injury | I5NP | IV | Quark | Phase I | ||
| Hypercholesterolemia | Liposome | IV | Tekmira | Phase I | [111, 112]a | |
| Cancer (solid tumour) | Ribonucleotide reductase subunit R2 | Cyclodextrin, PEG, transferrin- targeted | IV | Calando | Phase I | [106] |
| Cancer (solid tumour) | Protein kinase N3 | Liposome | IV | Silence Therapeutics | Phase I | [107] |
| Melanoma | Immunoproteasome | Transfected dendritic cells | Intradermal | Duke University | Phase I | [113] |
| Pachyonychia congenital | K6a keratin | – | Topical | Pachyonychia Congenita Project | Phase I | [114, 115] |
| Respiratory syncytial virus | RSV-P | Saline | Intranasal | Alnylam | Phase II | [105] |
| Cancer | Advanced solid tumours with liver involvement | Lipoplex | IV | Alnylam | Phase I | [70, 108]a |
Conclusions
Lipid-based carriers are promising candidates for therapeutic siRNA delivery. When designing carriers, consideration of both the molecular and meta-molecular scales must be taken into consideration. On the molecular scale, the building blocks, i.e. the lipids, must be able to assemble into stable delivery systems, which may or may not be affected by the nucleic acid payload. Complexation with siRNA often occurs via electrostatic interactions; therefore, the polar head of the lipid should contain a positive charge during siRNA complexation, carried in most cases by the amine groups. Electrostatic interactions must be stable enough to sustain the nucleic payload in the carrier en route, but must allow dissociation, to execute therapeutic activity, at the delivery site. Molecules containing several amines per head group, in which slight spacing exists between one amine to the other, are able to adhere to the negatively charged backbone of siRNA in a better manner than several lipids containing a single positive charge per headgroup. When assembling carriers from positively charged lipids, stability may be enhanced by addition of neutral lipids (sometimes referred to as helper lipids) to reduce repulsion between similar charges in the bilayer. Adding cholesterol, which resides in the hydrophobic region of the bilayer, improves carrier stability, and seems to play an important role in facilitating cellular uptake of siRNA. PEG lipids, which extend out of the lipid bilayer, presenting a highly hydrated corona surrounding the carrier, enhance circulation time and reduce carrier uptake by RES components. To enable carrier uptake and permeation across fenestrae, a size limit of less than 100 nm should be maintained.
Supplementary Material
ms + supp mats
Acknowledgements
The authors thank NIH grant RO1 EB00244, the David H. Koch Institute of Integrative Cancer Research and Alnylam Pharmaceuticals for financial support. A. S. thanks Dr. Michael Goldberg (Sharp Lab, MIT) for important discussions and insights.
Footnotes
Conflict of interest statement
R. Langer is a shareholder and member of the Scientific Advisory Board of Alnylam. D.G. Anderson is a consultant with Alnylam Pharmaceuticals. R. Langer and D.G. Anderson have sponsored research grants from Alnylam. Alnylam also has a license to certain intellectual property invented at Massachusetts Institute of Technology by Dr Anderson, Langer and colleagues.
References
- 1.Napoli C, Lemieux C, Jorgensen R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2:279–89. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Krol AR, Mur LA, Beld M, Mol JN, Stuitje AR. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990;2:291–9. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novina CD, Sharp PA. The RNAi revolution. Nature. 2004;430:161–4. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- 4.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 5.Hansen KM, Ji HF, Wu G, et al. Cantilever-based optical deflection assay for discrimination of DNA single-nucleotide mismatches. Anal Chem. 2001;73:1567–71. doi: 10.1021/ac0012748. [DOI] [PubMed] [Google Scholar]
- 6.Smith SB, Cui Y, Bustamante C. Overstretching B-DNA: the elastic response of individual double-stranded and single-stranded DNA molecules. Science. 1996;271:795–9. doi: 10.1126/science.271.5250.795. [DOI] [PubMed] [Google Scholar]
- 7.Baumann CG, Smith SB, Bloomfield VA, Bustamante C. Ionic effects on the elasticity of single DNA molecules. Proc Natl Acad Sci USA. 1997;94:6185–90. doi: 10.1073/pnas.94.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinden RR, Pearson CE, Potaman VN, Ussery DW. DNA: Structure and Function in Advances in Genome Biology. JAI Press; Greenwich, CT: 1998. [Google Scholar]
- 9.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 12.Rossi JJ. Medicine: a cholesterol connection in RNAi. Nature. 2004;432:155–6. doi: 10.1038/432155a. [DOI] [PubMed] [Google Scholar]
- 13.Check E. Hopes rise for RNA therapy as mouse study hits target. Nature. 2004;432:136. doi: 10.1038/432136b. [DOI] [PubMed] [Google Scholar]
- 14.Takeshita F, Ochiya T. Therapeutic potential of RNA interference against cancer. Cancer Sci. 2006;97:689–96. doi: 10.1111/j.1349-7006.2006.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponnappa BC. siRNA for inflammatory diseases. Curr Opin Invest Drugs. 2009;10:418–24. [PubMed] [Google Scholar]
- 17.Garbuzenko OB, Saad M, Betigeri S, et al. Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug. Pharm Res. 2009;26:382–94. doi: 10.1007/s11095-008-9755-4. [DOI] [PubMed] [Google Scholar]
- 18.Davidson BL, Paulson HL. Molecular medicine for the brain: silencing of disease genes with RNA interference. Lancet Neurol. 2004;3:145–9. doi: 10.1016/S1474-4422(04)00678-7. [DOI] [PubMed] [Google Scholar]
- 19.Prud'homme GJ. Gene Therapy of Autoimmune Diseases. Kluwer Academic/Plenum Publishers; New Delhi: 2005. [Google Scholar]
- 20.Gary DJ, Puri N, Won YY. Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J Control Release. 2007;121:64–73. doi: 10.1016/j.jconrel.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett DW, Davis ME. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006;34:322–33. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 23.Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–71. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8:173–84. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 25.De Paula D, Bentley MV, Mahato RI. Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting. RNA. 2007;13:431–56. doi: 10.1261/rna.459807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson SD, Jackson LN, Chen LA, Rychahou PG, Evers BM. Effectiveness of siRNA uptake in target tissues by various delivery methods. Surgery. 2007;142:262–9. doi: 10.1016/j.surg.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff JA, Rozema DB. Breaking the bonds: non-viral vectors become chemically dynamic. Mol Ther. 2008;16:8–15. doi: 10.1038/sj.mt.6300326. [DOI] [PubMed] [Google Scholar]
- 28.Torchilin VP, Papisov MI. Why do polyethylene glycolcoated liposomes circulate so long? J Liposome Res. 1994;4:725–39. [Google Scholar]
- 29.Avnir Y, Ulmansky R, Wasserman V, et al. Amphipathic weak acid glucocorticoid prodrugs remote-loaded into sterically stabilized nanoliposomes evaluated in arthritic rats and in a Beagle dog: a novel approach to treating autoimmune arthritis. Arthritis Rheum. 2008;58:119–29. doi: 10.1002/art.23230. [DOI] [PubMed] [Google Scholar]
- 30.Bacon BR, O'Grady JG. Comprehensive Clinical Hepatology. Elsevier Health Sciences; New York: 2006. [Google Scholar]
- 31.Wu J, Nantz MH, Zern MA. Targeting hepatocytes for drug and gene delivery: emerging novel approaches and applications. Front Biosci. 2002;7:d717–25. doi: 10.2741/A806. [DOI] [PubMed] [Google Scholar]
- 32.Wong SC, Wakefield D, Klein J, et al. Hepatocyte targeting of nucleic acid complexes and liposomes by a T7 phage p17 peptide. Mol Pharm. 2006;3:386–97. doi: 10.1021/mp050108r. [DOI] [PubMed] [Google Scholar]
- 33.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 34.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (Stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–6. [PubMed] [Google Scholar]
- 35.Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–92. [PubMed] [Google Scholar]
- 36.Oyen WJ, Boerman OC, Storm G, et al. Detecting infection and inflammation with technetium-99m-labeled Stealth liposomes. J Nucl Med. 1996;37:1392–7. [PubMed] [Google Scholar]
- 37.Schroeder A, Sigal A, Turjeman K, Barenholz Y. Using PEGylated nanoliposomes to target tissue invaded by a foreign body. J Drug Target. 2008;16:591–5. doi: 10.1080/10611860802228939. [DOI] [PubMed] [Google Scholar]
- 38.Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharm. 2009;6:763–71. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh M. Endocytosis. Oxford University Press; New York: 2001. [Google Scholar]
- 40.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–26. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 41.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–94. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Szoka FC., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–23. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 43.Felgner PL, Ringold GM. Cationic liposome-mediated transfection. Nature. 1989;337:387–8. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 44.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–63. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 45.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine–DNA polyplexes. J Biol Chem. 2003;278:44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 46.Reddy JA, Low PS. Enhanced folate receptor mediated gene therapy using a novel pH-sensitive lipid formulation. J Control Release. 2000;64:27–37. doi: 10.1016/s0168-3659(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 47.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001;123:8155–6. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 48.Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc. 2003;125:5316–23. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- 49.Klemm AR, Young D, Lloyd JB. Effects of polyethylenimine on endocytosis and lysosome stability. Biochem Pharmacol. 1998;56:41–6. doi: 10.1016/s0006-2952(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 50.Kichler A, Leborgne C, Coeytaux E, Danos O. Polyethylenimine-mediated gene delivery: a mechanistic study. J Gen Med. 2001;3:135–44. doi: 10.1002/jgm.173. [DOI] [PubMed] [Google Scholar]
- 51.Hoekstra D, Rejman J, Wasungu L, Shi F, Zuhorn I. Gene delivery by cationic lipids: in and out of an endosome. Biochem Soc Trans. 2007;35:68–71. doi: 10.1042/BST0350068. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J, Yockman JW, Kim SW, Kern SE. Intracellular kinetics of non-viral gene delivery using polyethylenimine carriers. Pharm Res. 2007;24:1079–87. doi: 10.1007/s11095-006-9229-5. [DOI] [PubMed] [Google Scholar]
- 53.Drummond DC, Zignani M, Leroux J. Current status of pH-sensitive liposomes in drug delivery. Prog Lipid Res. 2000;39:409–60. doi: 10.1016/s0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 54.Budker V, Gurevich V, Hagstrom JE, Bortzov F, Wolff JA. pH-sensitive, cationic liposomes: a new synthetic virus-like vector. Nat Biotechnol. 1996;14:760–4. doi: 10.1038/nbt0696-760. [DOI] [PubMed] [Google Scholar]
- 55.Simões S, Moreira JN, Fonseca C, Düzgüneş N, de Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv Drug Deliv Rev. 2004;56:947–65. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 56.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release. 2008;132:164–70. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volk T, Jahde E, Fortmeyer HP, Glusenkamp KH, Rajewsky MF. pH in human tumour xenografts: effect of intravenous administration of glucose. Br J Cancer. 1993;68:492–500. doi: 10.1038/bjc.1993.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–84. [PubMed] [Google Scholar]
- 60.Drummond DC, Daleke DL. Synthesis and characterization of N-acylated, pH-sensitive ‘caged’ aminophospholipids. Chem Phys Lipids. 1995;75:27–41. doi: 10.1016/0009-3084(94)02398-O. [DOI] [PubMed] [Google Scholar]
- 61.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41:749–59. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mastrobattista E, van der Aa MAEM, Hennink WE, Crommelin DJA. Artificial viruses: a nanotechnological approach to gene delivery. Nat Drug Discov. 2006;5:115–21. doi: 10.1038/nrd1960. [DOI] [PubMed] [Google Scholar]
- 63.Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123:1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 64.Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci USA. 1989;86:6077–81. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weisman S, Hirsch-Lerner D, Barenholz Y, Talmon Y. Nanostructure of cationic lipid–oligonucleotide complexes. Biophys J. 2004;87:609–14. doi: 10.1529/biophysj.103.033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desigaux L, Sainlos M, Lambert O, et al. Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference. Proc Natl Acad Sci USA. 2007;104:16534–9. doi: 10.1073/pnas.0707431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouxsein NF, McAllister CS, Ewert KK, Samuel CE, Safinya CR. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry. 2007;46:4785–92. doi: 10.1021/bi062138l. [DOI] [PubMed] [Google Scholar]
- 68.Radler JO, Koltover I, Salditt T, Safinya CR. Structure of DNA–cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997;275:810–14. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 69.Ren T, Song YK, Zhang G, Liu D. Structural basis of DOTMA for its high intravenous transfection activity in mouse. Gene Ther. 2000;7:764–8. doi: 10.1038/sj.gt.3301153. [DOI] [PubMed] [Google Scholar]
- 70.Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–9. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mouritsen OG. The Emerging Science of Lipidomics. Springer-Verlag; Berlin: 2005. Life as a Matter of Fat. [Google Scholar]
- 72.Umeda M, Nojima S, Inoue K. Effect of lipid composition on HVJ-mediated fusion of glycophorin liposomes to erythrocytes. J Biochem. 1985;97:1301–10. doi: 10.1093/oxfordjournals.jbchem.a135181. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Mounkes LC, Liggitt HD, et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol. 1997;15:167–73. doi: 10.1038/nbt0297-167. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Liggitt D, Zhong W, Tu G, Gaensler K, Debs R. Cationic liposome-mediated intravenous gene delivery. J Biol Chem. 1995;270:24864–70. doi: 10.1074/jbc.270.42.24864. [DOI] [PubMed] [Google Scholar]
- 75.Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–8. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 76.Senior J, Gregoriadis G. Stability of small unilamellar liposomes in serum and clearance from the circulation: the effect of the phospholipid and cholesterol components. Life Sci. 1982;30:2123–36. doi: 10.1016/0024-3205(82)90455-6. [DOI] [PubMed] [Google Scholar]
- 77.Mayhew E, Rustum YM, Szoka F, Papahadjopoulos D. Role of cholesterol in enhancing the antitumor activity of cytosine arabinoside entrapped in liposomes. Cancer Treat Rep. 1979;63:1923–8. [PubMed] [Google Scholar]
- 78.Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–57. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 79.Han SE, Kang H, Shim GY, et al. Novel cationic cholesterol derivative-based liposomes for serum-enhanced delivery of siRNA. Int J Pharm. 2008;353:260–9. doi: 10.1016/j.ijpharm.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 80.Schiffelers RM, Mixson AJ, Ansari AM, et al. Transporting silence: design of carriers for siRNA to angiogenic endothelium. J Control Release. 2005;109:5–14. doi: 10.1016/j.jconrel.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 81.Campbell RB, Fukumura D, Brown EB, et al. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62:6831–6. [PubMed] [Google Scholar]
- 82.Litzinger DC, Brown JM, Wala I, et al. Fate of cationic liposomes and their complex with oligonucleotide in vivo. Biochim Biophys Acta. 1996;1281:139–49. doi: 10.1016/0005-2736(95)00268-5. [DOI] [PubMed] [Google Scholar]
- 83.Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–49. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 84.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271:8481–7. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 85.Mastrobattista E, Kapel RH, Eggenhuisen MH, et al. Lipidcoated polyplexes for targeted gene delivery to ovarian carcinoma cells. Cancer Gene Ther. 2001;8:405–13. doi: 10.1038/sj.cgt.7700311. [DOI] [PubMed] [Google Scholar]
- 86.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–31. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 87.Kizelsztein P, Ovadia H, Garbuzenko O, Sigal A, Barenholz Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:20–5. doi: 10.1016/j.jneuroim.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 88.Ishiwata H, Suzuki N, Ando S, Kikuchi H, Kitagawa T. Characteristics and biodistribution of cationic liposomes and their DNA complexes. J Control Release. 2000;69:139–48. doi: 10.1016/s0168-3659(00)00293-5. [DOI] [PubMed] [Google Scholar]
- 89.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 90.Chithrani BD, Chan WCW. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–50. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 91.Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–55. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cai S, Vijayan K, Cheng D, Lima EM, Discher DE. Micelles of different morphologies – advantages of worm-like filomicelles of PEO-PCL in paclitaxel delivery. Pharm Res. 2007;24:2099–109. doi: 10.1007/s11095-007-9335-z. [DOI] [PubMed] [Google Scholar]
- 93.Geng Y, Ahmed F, Bhasin N, Discher DE. Visualizing worm micelle dynamics and phase transitions of a charged diblock copolymer in water. J Phys Chem B. 2005;109:3772–9. doi: 10.1021/jp0459559. [DOI] [PubMed] [Google Scholar]
- 94.Srinivas G, Discher DE, Klein ML. Key roles for chain flexibility in block copolymer membranes that contain pores or make tubes. Nano Lett. 2005;5:2343–9. doi: 10.1021/nl051515x. [DOI] [PubMed] [Google Scholar]
- 95.Park J-H, von Maltzahn G, Zhang L, et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv Mater. 2008;20:1630–5. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Z, Cai W, He L, et al. In-vivo biodistribution and highly efficient tumor targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 97.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. PNAS. 2006;103:4930–4. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martin CR, Kohli P. The emerging field of nanotube biotechnology. Nat Rev Drug Discov. 2003;2:29–37. doi: 10.1038/nrd988. [DOI] [PubMed] [Google Scholar]
- 99.Evans E, Bowman H, Leung A, Needham D, Tirrell D. Biomembrane templates for nanoscale conduits and networks. Science. 1996;273:933–5. doi: 10.1126/science.273.5277.933. [DOI] [PubMed] [Google Scholar]
- 100.Karlsson A, Karlsson R, Karlsson M, et al. Molecular engineering networks of nanotubes and containers. Nature. 2001;409:150–2. doi: 10.1038/35051656. [DOI] [PubMed] [Google Scholar]
- 101.Karlsson M, Sott K, Davidson M, et al. Formation of geometrically complex lipid nanotubevesicle networks of higher-order topologies. PNAS. 2002;99:11573–8. doi: 10.1073/pnas.172183699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karlsson M, Sott K, Cans A-S, Karlsson A, Karlsson R, Orwar O. Micropipette-assisted formation of microscopic networks of unilamellar lipid bilayer nanotubes and containers. Langmuir. 2001;17:6754–8. [Google Scholar]
- 103.Yager P, Schoen PE. Formation of tubules by a polymerizable surfactant. Mol Crystal Liquid Crystal. 1984;106:371–81. [Google Scholar]
- 104.Schnur JM. Lipid tubules: a paradigm for molecularly engineered structures. Science. 1993;262:1669–76. doi: 10.1126/science.262.5140.1669. [DOI] [PubMed] [Google Scholar]
- 105.Alvarez R, Elbashir S, Borland T, et al. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrob Agents Chemother. 2009;53:3952–62. doi: 10.1128/AAC.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6:659–68. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 107.Aleku M, Schulz P, Keil O, et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68:9788–98. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 108.Akinc A, Goldberg M, Qin J, et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol Ther. 2009;17:872–9. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen J, Samul R, Silva RL, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–34. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 110.Singerman L. Combination therapy using the small interfering RNA bevasiranib. Retina. 2009;29:S49–50. doi: 10.1097/IAE.0b013e3181ad2341. [DOI] [PubMed] [Google Scholar]
- 111.Judge AD, Robbins M, Tavakoli I, et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119:661–73. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chikh G, de Jong SD, Sekirov L, et al. Synthetic methylated CpG ODNs are potent in vivo adjuvants when delivered in liposomal nanoparticles. Int Immunol. 2009;21:757–67. doi: 10.1093/intimm/dxp044. [DOI] [PubMed] [Google Scholar]
- 113.Dannull J, Lesher DT, Holzknecht R, et al. Immunoproteasome down-modulation enhances the ability of dendritic cells to stimulate antitumor immunity. Blood. 2007;110:4341–50. doi: 10.1182/blood-2007-04-083188. [DOI] [PubMed] [Google Scholar]
- 114.Leachman SA, Hickerson RP, Hull PR, et al. Therapeutic siRNAs for dominant genetic skin disorders including pachyonychia congenita. J Dermatol Sci. 2008;51:151–7. doi: 10.1016/j.jdermsci.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hickerson RP, Smith FJ, Reeves RE, et al. Single-nucleotide-specific siRNA targeting in a dominant-negative skin model. J Invest Dermatol. 2008;128:594–605. doi: 10.1038/sj.jid.5701060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ms + supp mats