Plk1 Regulates Activation of the Anaphase Promoting Complex by Phosphorylating and Triggering SCFβTrCP-dependent Destruction of the APC Inhibitor Emi1 (original) (raw)
Abstract
Progression through mitosis requires activation of cyclin B/Cdk1 and its downstream targets, including Polo-like kinase and the anaphase-promoting complex (APC), the ubiquitin ligase directing degradation of cyclins A and B. Recent evidence shows that APC activation requires destruction of the APC inhibitor Emi1. In prophase, phosphorylation of Emi1 generates a D-pS-G-X-X-pS degron to recruit the SCFβTrCP ubiquitin ligase, causing Emi1 destruction and allowing progression beyond prometaphase, but the kinases directing this phosphorylation remain undefined. We show here that the polo-like kinase Plk1 is strictly required for Emi1 destruction and that overexpression of Plk1 is sufficient to trigger Emi1 destruction. Plk1 stimulates Emi1 phosphorylation, βTrCP binding, and ubiquitination in vitro and cyclin B/Cdk1 enhances these effects. Plk1 binds to Emi1 in mitosis and the two proteins colocalize on the mitotic spindle poles, suggesting that Plk1 may spatially control Emi1 destruction. These data support the hypothesis that Plk1 activates the APC by directing the SCF-dependent destruction of Emi1 in prophase.
INTRODUCTION
To begin mitosis and the assembly of the bipolar spindle, cells undergo a series of timed, interdependent regulatory events. The activation of mitosis promoting factor (MPF), a complex of cyclin B and Cdk1, orchestrates a series of structural and regulatory events by phosphorylating key mitotic substrates (Murray, 2004). After MPF activation, the anaphase promoting complex (APC), an E3 ubiquitin ligase that controls the ubiquitin-dependent destruction of mitotic cyclins (Harper et al., 2002), is activated to direct the ordered destruction of several critical mitotic regulators. These include the early mitotic regulator cyclin A in late prophase, the chromosome cohesion regulator securin at metaphase, and a host of mitotic regulators, including cyclin B in late mitosis. This wave of late mitotic destruction allows the reversal of the mitotic state and mitotic exit. During the transition from G2 to prophase, mitotically activated kinases, including members of the Polo and Aurora kinase families, organize specific subprograms within mitosis (Nigg, 2001). How Cdk1 and these other mitotic regulators conspire to activate APC proteolytic events is only thinly outlined.
The characteristic timing of APC-dependent destruction of different substrates is achieved by mechanisms that are poorly understood, but several factors seem to be important. One is that APC activity is timed through binding of the APC complex with either of two activating subunits, Cdc20 or Cdh1 (Harper et al., 2002). Cdc20 activates the APC during early mitosis until anaphase, whereas Cdh1 activates the APC in late mitosis and G1 phase.
The activity of APCCdc20 is regulated through two inhibitory proteins or complexes that antagonize Cdc20 function: 1) the early mitotic inhibitor Emi1 (Reimann et al., 2001a,b; Hsu et al., 2002); or 2) components of the spindle assembly checkpoint, including the proteins Mad2 or BubR1 (Lew and Burke, 2003). Emi1 has recently emerged as a key regulator of both the G1-S transition and mitotic progression through its ability to inhibit the ubiquitin ligase activity of the APC (Reimann et al., 2001a; Hsu et al., 2002; Margottin-Goguet et al., 2003). During S and G2 phase, Emi1 stabilizes critical APC substrates, including the cyclins. The destruction of Emi1 in early mitosis requires the SCFβTrCP ubiquitin ligase (Guardavaccaro et al., 2003; Margottin-Goguet et al., 2003), and Emi1 destruction is required for the activation of the APC. Failure to destroy Emi1 during early mitosis results in a prometaphase block, and, ultimately, mitotic catastrophe (Margottin-Goguet et al., 2003). βTrCP, a substrate-adapting subunit of the SCF complex, specifically recognizes a canonical DSGXXS motif present within IκB, β-catenin, Emi1, and other substrates only when both of the motif's serine residues are phosphorylated (Yaron et al., 1998; Hart et al., 1999; Winston et al., 1999; Margottin-Goguet et al., 2003; Fuchs et al., 2004). The molecular basis for the phospho-specific interaction between βTrCP and β-catenin has been rationalized by a recently reported crystal structure (Wu et al., 2003). Because SCFβTrCP seems to be constitutively active, the timing of Emi1 destruction must be strongly governed by its phosphorylation on these sites. The kinase(s) responsible for mitotic phosphorylation of Emi1's DSGXXS motif is unknown, and its identification would greatly advance our understanding of mitotic progression.
Another mechanism regulating APCCdc20 activity is phosphorylation of the core APC during mitosis, which allows efficient binding to Cdc20 (Kramer et al., 2000). Treatment of fractionated or immunopurified interphase APC with active Cdk1 stimulates its ubiquitination activity, whereas the active, mitotic APC is inactivated by phosphatase treatment (Hershko et al., 1994; King et al., 1995; Lahav-Baratz et al., 1995; Sudakin et al., 1995). A number of the in vivo mitotic phosphorylation sites in APC subunits have been identified, and many are similar to those produced by phosphorylation of the APC by Cdk1 in vitro (Kraft et al., 2003). Mutation of Cdk sites in APC subunits Cdc16, Cdc23, and Cdc27 drastically reduced in vivo APC/Cdc20 binding, whereas treatment of interphase APC with Cdk1 in vitro stimulated APC/Cdc20 binding and activity (Shteinberg et al., 1999; Rudner and Murray, 2000; Kraft et al., 2003). The importance of Cdk1 phosphorylation of the APC seems to be conserved from yeast to humans
The Polo-like kinases also have been reported to be important for APC activation, but the precise means has remained controversial (Harper et al., 2002; Barr et al., 2004). Polo-like kinase 1 (Plk1) is a mitotically active kinase that enhances the G2-M transition and is required for bipolar spindle formation and cytokinesis (Barr et al., 2004). Polo mutants in Drosophila were originally identified because of failures in spindle pole assembly and mitosis (Sunkel and Glover, 1988; Llamazares et al., 1991). Two reports in 1998 suggested a requirement for Plk1 in APC activation in vertebrate systems. One report showed that treatment of meiotically arrested Xenopus egg extracts with immunodepletion of Xenopus Plk1 (Plx1) or saturation with catalytically inactive Plx1 prevented Ca2+-induced cyclin B destruction and meiotic exit (Descombes and Nigg, 1998). The other (Kotani et al., 1998) reported that Plk1 directly phosphorylated interphase APC in vitro, conferring ubiquitination activity, and that Plk1 activation required prior treatment with cyclin B/Cdk1, which fit with earlier suggestions that Cdk1's effects on APC activation might be indirect. However, subsequent work observed that direct Cdk1 phosphorylation did not activate Plk1 in vitro (Golan et al., 2002; Kelm et al., 2002), and that treatment of interphase APC in vitro with Plk1 alone had little or no activating effect (Golan et al., 2002; Kraft et al., 2003). It was reported that Plk1 could have a supplementary effect to Cdk1 for in vitro activation of the APC. However, Kraft et al. (2003) also demonstrated using RNA interference that mitotic APC immunopurified from HeLa cells was equally active in absence of Plk1, suggesting that the marginal effect seen in vitro may be a result of the in vitro promiscuity of Polo-like kinases, which has been reported elsewhere (Shou et al., 2002). So far, the in vivo or extract requirement of Plk1 for APC activation, most clearly seen in Xenopus eggs, does not seem to be recapitulated in an immunopurified, reconstituted reaction, suggesting that other factors present before Plk1 activation, such as APC inhibitors, might be the target of Plk1.
Here, we demonstrate in both human cells and Xenopus egg extracts that mitotic destruction of the APC inhibitor Emi1 requires Plk1, which provides a compelling explanation for the role of Plk1 in APC activation. We also show that the orderly recruitment of Plk1 and Emi1 on the poles of the mitotic spindle may provide a spatial element to the regulation of Emi1 destruction and the activation of the APC.
MATERIALS AND METHODS
Plasmids and Small-interfering RNA (siRNA) Oligonucleotides
Constructs for wild-type and K82R hPlk1 were obtained from G. Fang (Stanford Univ.) and E. Nigg (Max Planck Institute), respectively, and were polymerase chain reaction (PCR) subcloned into pCS2+HA, as were Plk1 N-terminal and C-terminal fragments. βTrCP and βTrCPΔF constructs were in pCS2+HA. All hEmi1 fragments and mutants used were in pCS2+myc. Emi1ΔN4 fragment consists of amino acids 135–244. Site-directed mutagenesis was performed by standard methods by using QuikChange protocol. For Plk1 knockdown by RNA interference we used a SMARTpool of four oligoduplexes targeted against Plk1 (MU-003290-00; Dharmacon Research, Lafayette, CO). Each oligoduplex in the pool was then tested for efficacy of Plk1 knockdown, and the most potent oligoduplex was used in subsequent experiments. The target sequence of this oligoduplex is AGAUUGUGCCUAAGUCUCU. All siRNA transfections were performed using Oligofectamine reagent (Invitrogen, Carlsbad, CA) as described previously (Hsu et al., 2002).
Antibodies and Purified Proteins
The following antibodies were used for immunoblotting: rabbit anti-myc (sc-789; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-hemagglutinin (HA) (PRB-101P; Covance, Cumberland, VA), mouse anti-Plk1 cocktail (33-1700; Zymed Laboratories, South San Francisco, CA), mouse anti-Cdc27 (610454; BD Transduction Laboratories, Lexington, KY), rabbit anti-cyclin A (sc-751; Santa Cruz Biotechnology), goat anti-actin (sc-1616; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-pTyr15-Cdc2 (9111; Cell Signaling Technology, Beverly, MA), and rabbit anti-hEmi1 (Hsu et al., 2002). Clone 3F10 rat anti-HA antibody (Roche Diagnostics, Indianapolis, IN) was used for coimmunoprecipitation from cell lysates. Rabbit anti-Plx1 was graciously provided by W. Dunphy (California Institute of Technology) for immunodepletion of Plx1 from Xenopus extracts. Anti-hEmi1, anti-Plk1 (Zymed Laboratories), and anti-cyclin A (Santa Cruz Biotechnology) antibodies also were used for immunofluorescence, as were mouse anti-γ-tubulin (T6557; Sigma-Aldrich, St. Louis, MO) and rat anti α-tubulin (MCAP77; Serotec, Oxford, United Kingdom).
Baculovirus encoding His6-Plx1 was obtained from W. Dunphy, and the protein was expressed in Sf9 cells and purified as described previously (Kumagai and Dunphy, 1996). MBP-xEmi1 and MBP-xEmi1-Cdkmut were described previously (Reimann et al., 2001a). The hEmi1ΔN4 fragment was PCR subcloned into pGEX-6P1, and these fusion proteins were expressed and purified by standard protocols.
Degradation Assays, Immunodepletion, and H1 Kinase Assays in Xenopus Extracts
Degradation assays were performed as described previously (Reimann et al., 2001a). For rescue of Plx1-depleted extract, 250 ng of His6-Plx1 was added to 10 μl of depleted mitotic extract. For Plx1 immunodepletion, 3 μg of anti-Plx1 or control IgG was used in each of two rounds of immunodepletion from 30 μl of Δ90-cyclin B-arrested mitotic extract. Antibodies were incubated with the extract on ice for 45 min per round of depletion and captured using protein A-coupled Dynabeads (Dynal Biotech, Lake Success, NY). H1 kinase assays were performed as described previously (Jackson et al., 1995).
Emi1 Binding and Stability Assays in Cultured Cells
HEK293T cells in 6-cm plates were transfected with pCS2+HA and pCS2+myc constructs (2 μg of total DNA) by using FuGENE6 (Roche Diagnostics), harvested after 48 h, frozen, resuspended, and lysed in TENIGAL buffer (50 mM Tris, pH 7.5, 1 mM EDTA, 150 mM NaCl, 0.5% Igepal [Sigma-Aldrich], 20 mM β-glycerophosphate, and aprotinin, leupeptin, pepstatin, and chymostatin protease inhibitors [Sigma-Aldrich]). Cell lysates were analyzed by immunoblotting or immunoprecipitation by using the antibodies described above. For immunoprecipitation, 0.5 μg of rat 3F10 anti-HA was combined with 0.5 mg of lysate protein for 1 h, captured for 30 min on protein G-Sepharose (Sigma-Aldrich), washed four times with TENIGAL, resuspended in sample buffer, and analyzed for coimmunoprecipitation by immunoblotting. MG132 (20 μM) or nocodazole (150 ng/ml) was applied during the last 12 h of transfection where indicated.
HeLa cells growing in six-well plates were synchronized at the G1-S transition by double thymidine block and released into medium containing 150 ng/ml nocodazole as described previously (Hsu et al., 2002). Control or Plk1 siRNA treatment was applied during the interval between the two thymidine blocks. Plk1 silencing was achieved using Dharmacon's SMARTpool oligos against Plk1, whereas control treatment used an oligoduplex targeted against green fluorescent protein (GFP). For each treatment, one well of cells was harvested at each time point and analyzed by immunoblotting.
In Vitro Phosphorylation and βTrCP Binding Assays
Kinase Assay Forty nanograms of His6-Plx1 was combined with 0.5 μg of glutathione _S_-transferase (GST)-Emi1ΔN4 in a 10-μl reaction volume containing 50 mM Tris, pH 7.5, 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, 200 μM ATP, and 0.25 μCi/μl [γ-32P]ATP (PerkinElmer Life and Analytical Sciences, Boston, MA). Reactions were incubated at 30°C for the indicated times and analyzed by SDS-PAGE, autoradiography, and densitometry using a PhosphorImager with ImageQuant software (Amersham Biosciences, Piscataway, NJ). βTrCP Binding Assays Indicated concentrations of His6-Plx1 were combined with GST-hEmi1ΔN4 (1 μM final concentration) in 50 μl of reaction buffer (50 mM Tris, pH 7.5, 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, and 300 μM ATP) and incubated at 30° for 1 h, after which reaction products were bound to 10 μl of glutathione resin for 45 min at 4°. Bead-bound Emi1ΔN4 was incubated with 1.5 μl of in vitro-translated (IVT)-radiolabeled βTrCP in 100 μl of TENIGAL buffer at 4° for 45 min, and the beads were then rinsed four times in TENIGAL. Bound proteins were eluted with sample buffer and analyzed by SDS-PAGE, autoradiography, and densitometry. Assays for Cdk1 costimulation were performed similarly but using MBP-xEmi1 (0.5 μM final concentration) in the presence or absence of cyclin B/Cdc2 (10 U, P6020S; New England Biolabs, Beverly, MA) in a volume of 50 μl of supplied Cdc2 reaction buffer. These phosphorylation reactions proceeded for 40 min, and reaction products were captured using amylose resin.
Ubiquitination Assays
Ubiquitination assays were performed based on a method used previously (Guardavaccaro et al., 2003). HeLa cell extracts were prepared as described previously (Montagnoli et al., 1999). IVT-radiolabeled Emi1ΔN4 (2 μl) was added to a 20-μl reaction mixture containing 40 mM Tris, pH 7.5, 5 mM MgCl2, 1 mM DTT, 10% glycerol, and 40 μg of HeLa cell extract, supplemented with 20 μg/ml E1, 100 μg/ml E2 (Ubc5c), 1 mg/ml ubiquitin, 1 μM ubiquitin aldehyde, 7.5 mM creatine phosphate, 1 mM ATP, and 0.5 μg of His6-Plx1 or buffer control. Reactions were incubated at 30°C. At the indicated times, aliquots of 5 μl were removed and quenched with sample buffer. Reaction products were analyzed by SDS-PAGE and autoradiography. To generate mitotic cell extracts with or without Plk1, cells were siRNA-treated with an oligo against Plk1 or a control oligo against GFP for 24 h, subjected to nocodazole treatment for another 24 h, and collected by mitotic shake-off.
Immunofluorescence and Microscopy
Immunofluorescence procedures were similar to those described previously (Hsu et al., 2002). U2OS cells were grown on coverslips and fixed for 10 min in phosphate-buffered saline (PBS) + 4% paraformaldehyde and permeabilized for 10 min in PBS + 0.5% Triton-X-100. Primary antibodies used were rabbit anti-Emi1 (1 μg/ml), mouse anti-Plk1 (5 μg/ml), rat anti-α-tubulin (1 μg/ml), and mouse anti-γ-tubulin (10 μg/ml). Secondary antibodies used were Alexa488-labeled donkey anti-rabbit (Molecular Probes, Eugene, OR) and Texas Red-labeled donkey anti-mouse or anti-rat (Jackson ImmunoResearch Laboratories, West Grove, PA). Images were obtained on a Zeiss Axiovert 200M, with a Plan-Apochromat 63×/1.4 numerical aperture lens, a Photometrics Coolsnap HQ digitial camera (Roper Scientific, Trenton, NJ), and Slidebook 4.0 software (Intelligent Imaging Innovations, Denver, CO) for image acquisition and deconvolution. Stacks were collected at 0.5-μm intervals and deconvolved using the nearest neighbor algorithm. Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA) was used for adjustment of image contrast and brightness.
RESULTS
Plk1 Is Essential for Emi1 Destruction and Efficient APC Activation during Mitosis
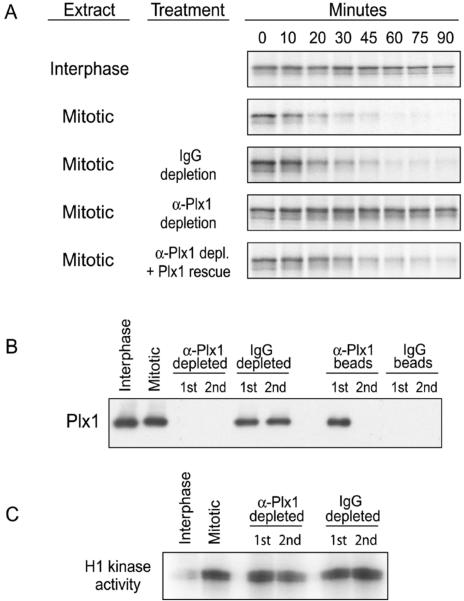
We previously validated an assay for Emi1 destruction in Xenopus egg extracts induced to enter mitosis by addition of nondestructible cyclin B (Margottin-Goguet et al., 2003). In this assay, Emi1 destruction requires the degron recognized by the SCFβTrCP ubiquitin ligase (DSGXXS), the activity of the 26S proteasome, and the mitotic state of the extracts. To identify kinases important for mitotic Emi1 destruction, we turned to candidates among the known mitotically activated kinases. Plk1 is a strong candidate because it is required for Ca2+-induced APC activation and meiotic exit in Xenopus egg extracts (Descombes and Nigg, 1998; Barr et al., 2004) and because of the similar timing of Plk1 activation and Emi1 destruction in somatic cells (Golsteyn et al., 1995; Lee et al., 1995; Hsu et al., 2002). We found that immunodepletion of Plx1 from mitotic extract completely stabilized Emi1, whereas mock-depleted extract still destroyed Emi1 efficiently (Figure 1, A and B). A potential concern was that the absence of Plx1 affected maintenance of MPF activity, but we observed that the Plx1-depleted extract maintained its mitotic kinase activity toward histone H1 (Figure 1C). Moreover, addition of recombinant Plx1 restored the Plx1-depleted extract's ability to destroy Emi1, demonstrating the reversibility of the immunodepletion and the specificity of the effect. Thus, Plx1 is required for Emi1 destruction in mitotic Xenopus extracts.
Figure 1.
Plk1 is essential for mitotic destruction of Emi1 in Xenopus extracts. (A) Immunodepletion of Plx1 from mitotic Xenopus egg extract prevents Emi1 destruction. Interphase egg extracts were induced to enter mitosis by addition of nondestructible cyclin B. These mitotic extracts were immunodepleted (2 rounds) by using anti-Plx1 or control IgG antibodies, supplemented with active Plx1 kinase or buffer, and assayed for destruction of IVT, radiolabeled Emi1. (B) Efficiency of depletion of Plx1. Immunodepleted extracts (after 1 or 2 rounds of depletion) and the equivalent amount of material bound to the immunodepleting beads were immunoblotted for Plx1. (C) Depletion of Plx1 does not affect MPF activity in mitotic extracts. Immunodepleted extracts were tested for H1 kinase activity as an assay of mitosis promoting factor (MPF or Cdk1) activity.
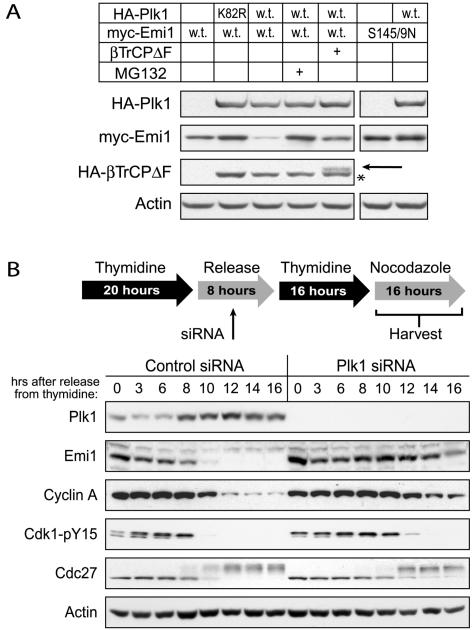
As a test of the sufficiency of Plk1 for Emi1 destruction, we examined the effects of Plk1 overexpression on Emi1 stability in cultured mammalian cells. When we cotransfected Plk1 and Emi1 into HEK293T cells, Plk1 overexpression substantially reduced Emi1 protein levels compared with vector control (Figure 2A). Moreover, this reduction was shown to be dependent on the SCFβTrCP ubiquitin ligase and on the proteasome, as either expression of a dominant negative βTrCP lacking the F-box domain (βTrCPΔF) or addition of the proteasome inhibitor MG132 prevented the reduction of Emi1 protein levels. Plk1 overexpression had no effect on a stable mutant of Emi1 lacking the serine residues of the βTrCP recognition motif, further demonstrating the specificity of Plk1's effects. Expression of a kinase-dead version of Plk1 (K82R) did not lower Emi1 levels, and in fact slightly increased Emi1 levels, suggesting a dominant negative inhibition of endogenous Plk1.
Figure 2.
Plk1 is essential for mitotic destruction of Emi1 in cultured human cells. A) Plk1 overexpression reduces Emi1 levels in a proteasome- and SCFβ-TrCP-dependent manner. HEK293T cells were cotransfected with either wild-type (w.t.) or nondestructible (S145/9N) myc-tagged hEmi1 and either wild-type (w.t.) or kinase-defective (K82R) HA-tagged Plk1. Additionally, cells cotransfected with wild-type Emi1 and Plk1 were also transfected with a dominant-negative HA-tagged βTrCP (βTrCPΔF) or treated with the proteasome inhibitor MG132. After 48 h, cells were harvested and processed for immunoblot analysis of transfected proteins. The identity of the faint band designated by the arrow is HA-βTrCPΔF. We have expressed this protein in previous studies (Margottin-Goguet et al., 2003) and are certain of its identity. The asterisk designates a breakdown product of HA-Plk1. Visualization of the bands designated by the arrow and the asterisk required much longer film exposure than the bands for full length HA-Plk1. (B) Inactivation of Plk1 in HeLa cells by siRNA treatment stabilizes Emi1 during mitosis. HeLa cells were synchronized by double thymidine block, released into nocodazole-containing medium, and harvested at the indicated times after release. The siRNA treatment was applied between the thymidine blocks as indicated in the protocol schematic. Cells were analyzed by immunoblot analysis for levels of Plk1, Emi1, and cyclin A. Markers of mitotic entry also were examined: tyrosine dephosphorylation of Cdk1 to indicate MPF activation, and mobility shift of the APC subunit Cdc27.
We next tested whether loss of Plk1 affected the mitotic destruction of Emi1 by using RNA interference. Because Plk1 is an important component of the positive feedback loop controlling the G2-M transition, cell populations in this experiment were synchronized and assayed for mitotic markers to control for any delay in Emi1 destruction linked to a delay in mitotic entry. HeLa cells were synchronized at the G1-S transition by using a double thymidine block and released into medium containing nocodazole, and the kinetics of mitotic entry and Emi1 stability were monitored in the presence or absence of Plk1 knockdown (Figure 2B). Mitotic entry was indicated by the disappearance of inhibitory tyrosine-15 phosphorylation on Cdk1, and by the mobility shift of the Cdc27 subunit of the APC. Cells treated with control siRNA entered mitosis ∼10 h after release from thymidine. In these cells, Emi1 destruction coincided with mitotic entry, with cyclin A destruction occurring shortly thereafter. Cells treated with siRNA targeting Plk1 were delayed in mitotic entry by ∼2 h. Inactivation of Plk1 was extremely effective in these cells. In contrast to control cells, Emi1 was still present at least 4 h after mitotic entry, and cyclin A also was markedly stabilized. Thus, inactivation of Plk1 caused a strong delay in the destruction of Emi1 and cyclin A, beyond the 2-h delay in mitotic entry. Thus, Plk1 is required for Emi1 destruction and for efficient APC activation, as monitored by cyclin A destruction. Experiments below support the interpretation that Plk1 directly phosphorylates Emi1's degron for βTrCP recognition, but we cannot exclude that Plk1 is an upstream regulator of other factors which control Emi1 degron phosphorylation. It is also possible that other cellular processes in which Plk1 participates (e.g., spindle pole organization) are involved in regulation of Emi1 stability.
The Phosphodegron in Emi1 Contains Consensus Motifs for Polo Binding and Phosphorylation
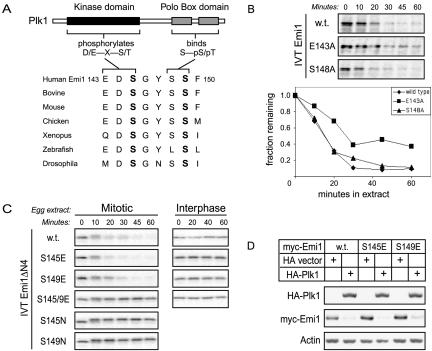
Because Plk1 was required for Emi1 destruction, we wanted to test whether Plk1 could directly phosphorylate the Emi1 degron. Inspection of the βTrCP binding site in Emi1 (Figure 3A) suggested that conserved elements in the Emi1 degron overlapped a Polo consensus phosphorylation site (E/D-X-S/T) containing glutamate-143 and serine-145 (Nakajima et al., 2003). Our earlier studies (Margottin-Goguet et al., 2003) showed serine-145 was required for Emi1 destruction, by using the assay for destruction in mitotic Xenopus egg extracts. Mutation of glutamate-143 (E143A) somewhat delayed the kinetics of Emi1 destruction (Figure 3B), consistent with other studies showing that the acidic residue in the –2 position is helpful but not essential for Plk1 phosphorylation (see Discussion).
Figure 3.
Both serine-145 and serine-149 in Emi1's degron are mitotically regulated. (A) The conserved Emi1 degron contains a Polo kinase consensus phosphorylation site and a Polo Box binding motif. (B) Emi1 destruction is partially blocked by mutation of the Polo consensus phosphorylation site, but not by mutation of the Polo Box consensus. The destruction of wild-type Emi1, the Polo consensus site mutant E143A, and the Polo Box consensus site mutant S148A was assayed in mitotic Xenopus egg extract. (C) Phosphomimic mutation of serine-145 (S145E) or serine-149 (S149E) to glutamate bypasses the dual phosphorylation requirement in the Emi1 degron. Wild-type, S145E, S149E, a S145E/S149E double mutant, S145N, and S149N versions of the Emi1ΔN4 fragment (residues 135–244) were assayed for destruction as in B. (D) Serine-145 and serine-149 are individually responsive to Plk1 overexpression. Human HEK293T cells were transfected with wild-type or the S145E or S149E mutants of myc-Emi1 and cotransfected with HA-Plk1 to trigger Emi1 destruction or HA vector control.
The βTrCP binding site in Emi1 also showed a perfect S-pS consensus motif at serines 148 and 149 for phosphorecognition by Plk1's Polo Box domain (PBD), defined by recent studies of Yaffe and coworkers (Elia et al., 2003a,b). Our previous studies showed that serine-149 is essential for Emi1 destruction (Margottin-Goguet et al., 2003), supporting the possibility that PBD binding is an important aspect of Emi1 destruction. We will show below that Emi1 interacts with the PBD of Plk1. We tested whether serine-148 was required for Emi1 destruction and did not find a significant effect (Figure 3B). One possibility is that the requirements for PBD binding may be more flexible than the consensus, originally defined in the context of a S-pT-P peptide. Another likely possibility, addressed below, is that there are additional sites on Emi1 for PBD recruitment (Figure 4E; see Discussion).
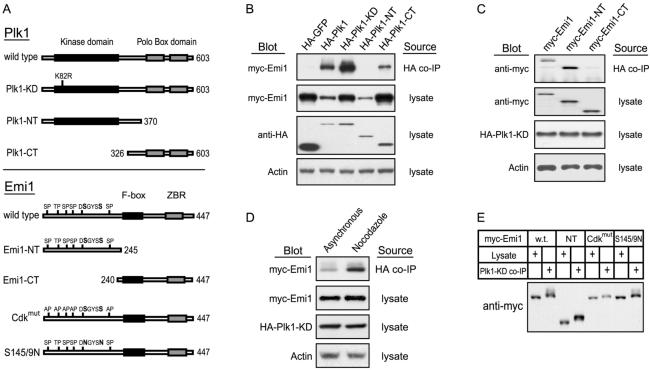
Figure 4.
Physical interaction of cotransfected Emi1 and Plk1. (A) Schematic of Plk1 and Emi1 mutants. (B) Plk1 association with Emi1 requires the C-terminal Polo Box domain, but not the kinase domain. HEK293T cells were cotransfected with the indicated variants of HA-Plk1 and myc-Emi1. After 48 h, cell lysates were prepared and analyzed by anti-HA immunoprecipitation and blotting for associated myc-Emi1. (C) The N terminus of Emi1, which confers mitotic instability, is sufficient for Plk1 association. HEK293T cells were cotransfected and analyzed as in part B. (D) Emi1-Plk1 association is enhanced in mitotic cells. HEK293T cells were cotransfected with myc-Emi1 and kinase dead HA-Plk1 and treated with nocodazole (Noc) to arrest cells in mitosis. HA-Plk1 immunoprecipitates from these cells were analyzed for myc-Emi1 association by immunoblot analysis. (E) Emi1 in Plk1 immunoprecipitates displays retarded gel mobility.
Both Phosphoserines of the Emi1 Degron Are Mitotically Regulated and Plk1 Responsive
We previously demonstrated that mutating either serine-145 or serine-149 in Emi1's βTrCP recognition motif to a nonphosphorylatable residue stabilizes Emi1 in mitotic extract, indicating that phosphorylation of both serines is required for efficient mitotic binding of βTrCP (Margottin-Goguet et al., 2003) (Figure 3C). It remained possible that either serine-145 or serine-149 is constitutively phosphorylated and that only one serine is mitotically regulated. To address this possibility, we replaced either serine-145 or serine-149 with a glutamate residue to mimic phosphorylation. The ability of glutamates to replace phosphoserines in binding to βTrCP is suggested by the noncanonical βTrCP degrons in Wee1 (Watanabe et al., 2004).
The S145E and S149E mutations allowed us to test the mitotic regulation of serine-149 and serine-145, respectively. The S145E mutant and the S149E mutant were each destroyed in mitotic extract with normal kinetics, indicating that both mutants retain the capacity for productive βTrCP association (Figure 3C). Moreover, each mutant remains stable in interphase extract, demonstrating that serine-145 and serine-149 phosphorylations are each mitosis-specific. Interestingly, the double S145/9E mutant is stable in both interphase and mitotic extract, suggesting that βTrCP recognition does require at least one actual phosphoserine residue on the substrate.
Whereas the presence in Emi1 of an acidic residue in the –2 position relative to serine-145 conforms to the minimal consensus motif for phosphorylation by Plk1, D/E-X-S/T (Kelm at al 2002, Nakajima et al., 2003), the sequence surrounding serine-149 does not conform to this consensus, leading us to question whether this residue is directly phosphorylated by Plk1. However, the individual S145E and S149E Emi1 mutants were each responsive to Plk1 overexpression in HEK293T cells (Figure 3D). This suggests either that serine-149 is phosphorylated directly by Plk1 or that Plk1 overexpression affects the activity of another kinase or phosphatase that governs serine-149 phosphorylation.
Physical Interaction of Plk1 and Emi1
To further investigate the directness of Plk1's involvement in triggering Emi1 destruction, we tested whether specific variants of Plk1 and Emi1 (depicted in Figure 4A) could associate in tissue culture cells. We cotransfected HEK293T cells with tagged versions of Plk1 and Emi1 and assayed them for coimmunoprecipitation of Emi1 with Plk1. We observed that Emi1 specifically coprecipitated with Plk1 or with catalytically inactive Plk1-KD (kinase dead K82R mutant), showing that binding does not require Plk1 kinase activity (Figure 4B). Indeed, deletion of the entire kinase domain showed that, although less efficient than full-length Plk1, the Plk1 C terminus (Plk1-CT) including the PBD was sufficient to associate with Emi1. Conversely, we detected no Emi1 coprecipitating with Plk1's N-terminal kinase domain (Plk1-NT), indicating that the PBD is required for stable complex formation with Emi1. Despite the absence of the PBD, Plk1-NT reduced Emi1 levels in cell lysates as effectively as did full-length Plk1, presumably because overexpression obviates the need for stable association and because of the higher specific activity of Plk1's kinase domain in the absence of its C terminus (Mundt et al., 1997; Jang et al., 2002). We also found that the N terminus of Emi1, which contains its degron, was sufficient to associate with Plk1, whereas the region of Emi1 containing the F-box and zinc-binding motif was dispensable for this interaction (Figure 4C).
Because the C-terminal PBD of Plk1 has recently been shown to bind phosphoproteins (Cheng et al., 2003; Elia et al., 2003a,b), we considered whether Plk1-bound Emi1 is phosphorylated and whether this interaction is enhanced during mitosis. For both full-length Emi1 and the Emi1 N terminus, the gel mobility of Plk1-bound Emi1 was slightly retarded compared with that of Emi1 from cell lysates, suggesting that Plk1 selectively binds a modified form of Emi1 (Figure 4E). We also observed that the amount of Emi1 present in Plk1 immunoprecipitates was significantly increased when cells were arrested in mitosis by using nocodazole, supporting that the Plk1–Emi1 interaction is mitosis specific (Figure 4D). In an attempt to define which phospho-residues of Emi1 mediate interaction with Plk1, we tried to see whether mutation either of the Cdk phosphorylation sites (Cdkmut) or the phosphorylation sites recognized by βTrCP (S145/9N) prevented association with Plk1. However, we observed that both mutants still bound Plk1, and that both Plk1-bound mutants still displayed retarded gel mobility (Figure 4E), demonstrating that neither the Cdk phosphorylation sites nor those recognized by βTrCP are exclusively required for Plk1 interaction.
Plk1 Stimulates βTrCP Binding and Emi1 Ubiquitination In Vitro
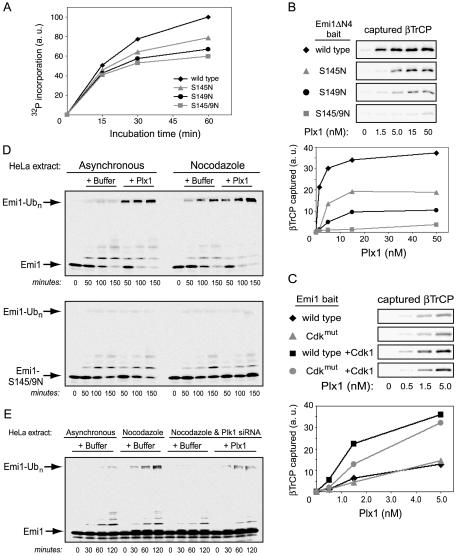
To further demonstrate that Plk1 directly participates in Emi1 phosphorylation and ubiquitination, we tested whether Plk1 can phosphorylate Emi1 in vitro and whether that phosphorylation results in βTrCP recognition and ubiquitination of Emi1. Using radiolabeled ATP, we found that recombinant Plx1 strongly phosphorylated a GST fusion of the Emi1ΔN4 fragment (residues 135–244), a fragment which mimics all known aspects of Emi1 stability and destruction (Margottin-Goguet et al., 2003). Mutation of both serines-145 and -149 to nonphosphorylatable residues substantially reduced the amount of 32P incorporation (Figure 5A). Because we detected no Plx1 activity toward GST alone (not depicted), the residual 32P incorporation in the S145/9N mutant means that additional Emi1 residues outside the βTrCP degron are phosphorylated by Plx1 in vitro. Single mutation of either serine-145 or serine-149 also noticeably reduced 32P incorporation, suggesting that Plk1 can phosphorylate both serines of the Emi1 degron critical for βTrCP binding, in agreement with the overexpression studies of Figure 3D. Interestingly, the S149N mutant reproducibly displays a more pronounced deficiency in 32P incorporation than the S145N mutant, implying that the S149N mutation somewhat compromises an additional phosphorylation event.
Figure 5.
Plk1 stimulates SCFβTrCP-dependent ubiquitination of Emi1 in vitro. (A) Phosphorylation of Emi1ΔN4 fragment by Plx1 in vitro. Purified Plx1 kinase was used to phosphorylate indicated versions of a GST-Emi1ΔN4 fusion protein over time in the presence of 32P-labeled ATP. Reaction products were resolved by SDS-PAGE, analyzed by autoradiography, and quantified by densitometry. (B) Plk1 stimulates specific βTrCP binding to the Emi1 degron. Purified Plx1 was used to phosphorylate wild-type or degron mutant versions of GST-Emi1ΔN4 fusion protein in vitro over a range of Plx1 concentrations. The products were then bound to glutathione resin and assayed for their ability to capture IVT-radiolabeled βTrCP, detected by SDS-PAGE and autoradiography. (C) βTrCP binding to Emi1 is stimulated cooperatively by Plx1 and Cdk1. Plx1 phosphorylation reactions were performed similar to as in B, but with MBP-xEmi1 fusion proteins, either wild-type or mutated in five Cdk sites, in the presence or absence of cyclin B/Cdk1 in Cdk1 reaction buffer. Reaction products were bound to amylose resin and assayed for ability to bind βTrCP. (D) Plx1 stimulates Emi1 ubiquitination in HeLa extracts from asynchronous cells. HeLa cell extracts supplemented with ATP, ubiquitin, E1, and E2 were incubated at 30° for the indicated times, with or without addition of purified Plx1 kinase, by using IVT-radiolabeled wild-type or nondestructible (S145/9N) Emi1ΔN4 as a ubiquitination substrate. Ubiquitinated forms of Emi1ΔN4 were resolved by SDS-PAGE and visualized by autoradiography. (E) Plk1 is essential for Emi1 ubiquitination in extract from nocodazole-arrested HeLa cells. HeLa cells were treated with siRNA for Plk1 or with control siRNA, arrested in mitosis with nocodazole, and extracts prepared and analyzed for Emi1ΔN4 ubiquitination activity as described in D with or without addition of purified Plx1.
Next, we conducted binding experiments between Emi1 and βTrCP to further demonstrate that Plk1 can directly phosphorylate the Emi1 degron. As expected, we found that Emi1ΔN4 phosphorylated in vitro by recombinant Plx1 became proficient in recruitment of βTrCP, whereas this response was abolished for the S145/9N double mutant (Figure 5B). Surprisingly, the individual S145N and S149N mutants, although substantially defective, each retained a measure of βTrCP binding capacity, despite the lack of negative charge at the consensus phosphoserine positions. This important result unambiguously shows that Plx1 can indeed phosphorylate both serine-145 and serine-149 in vitro. Again, the S149N mutant was more defective than the S145N mutant, possibly because 1) phosphoserine-149 contributes more strongly than phosphoserine-145 to the βTrCP interaction, or 2) the S149N mutation additionally causes a partial defect in phosphorylation of serine-145 (as hinted by Figure 5A). We should emphasize that despite their modest in vitro βTrCP binding capacity, the single S145N and S149N mutants are completely stable in mitotic Xenopus egg extracts (Figure 3C), meaning any interaction they may have with βTrCP in a more biological setting is unproductive and does not result in ubiquitination.
Because we have reported that Cdk phosphorylation of Emi1 plays a supportive but nonessential role in Emi1 destruction (Margottin-Goguet et al., 2003), we next tested whether cyclin B/Cdk1 could augment Plx1's stimulation of the Emi1/βTrCP interaction. Indeed, we did observe that, at lower concentrations of Plx1 (≤5 nM), Cdk1 significantly increased Plx1-dependent recruitment of βTrCP by Emi1 (Figure 5C). Moreover, Cdk1's costimulatory effect was somewhat dampened by mutation of the Cdk phosphorylation sites in Emi1's N terminus. At higher concentrations of Plx1 (≥50 nM), stimulation of Emi1/βTrCP binding was maximal and Cdk1 exhibited no costimulatory effect (our unpublished data).
Finally, we tested whether in vitro ubiquitination of Emi1 requires Plk1 by using a HeLa cell extract system that recapitulates the requirements for mitotic Emi1 ubiquitination, similar to the method used previously (Guardavaccaro et al., 2003). Whereas mitotic extract from nocodazole-arrested cells resulted in polyubiquitination of Emi1ΔN4, extract from asynchronously growing cells showed only background levels of activity (Figure 5D). However, supplementation of asynchronous extract with recombinant, active Plx1 stimulated Emi1 ubiquitination to a degree similar to that seen in mitotic extract. The stimulation of Emi1 polyubiquitination in mitotic extracts or in Plx1-supplemented extracts was not observed with the Emi1 S145/9N mutant, consistent with Emi1 ubiquitination activity requiring the SCFβTrCP ubiquitin ligase. (The spurious mono- and diubiquitination products seen are an artifactual result of supplementing the extract with E1 and E2, which is needed to obtain maximal polyubiquitination signal. They seem independent of cell cycle phase, Plk1 levels, and βTrCP binding and are unrelated to the polyubiquitination.) Emi1 ubiquitination activity in mitotic extract was also lost when the extract was generated from cells depleted of Plk1 by RNA interference (Figure 5E), and this deficiency was rescued by the addition of recombinant, active Plx1. Thus, the presence of Plk1 is necessary for Emi1 ubiquitination in mitotic extract, and its addition is sufficient to stimulate Emi1 ubiquitination in asynchronous extracts.
Colocalization of Emi1 and Plk1 on the Spindle Poles in Early Mitosis
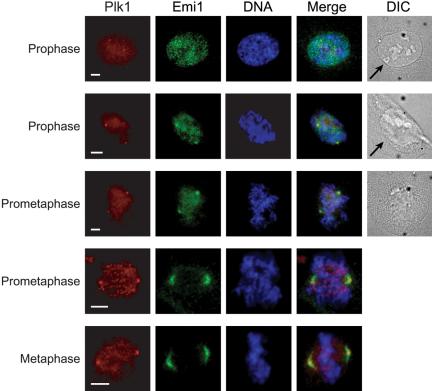
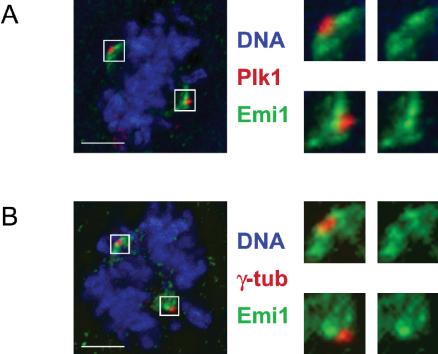
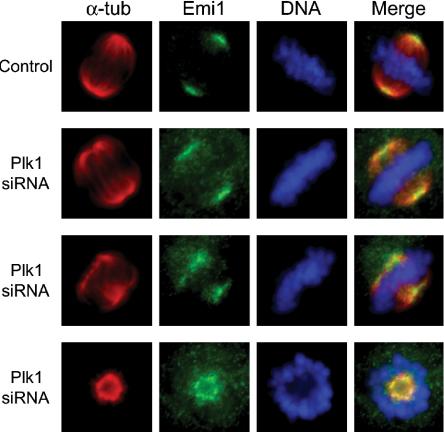
We used indirect immunofluorescence in U2OS cells to examine the extent of subcellular colocalization of Emi1 and Plk1, particularly during early mitosis. We observed that in early prophase before nuclear envelope breakdown (NEBD), Plk1 is localized both in the nucleus and at the centrosomes (Figure 6, row 1). At the same time, Emi1 localizes most noticeably to a series of punctate regions within the nucleus. These regions seem to be situated in interchromosomal regions. The nature of their localization is not understood. As NEBD takes place (as visualized by brightfield optics), Emi1 is dispersed from the nuclear spots and undergoes a redistribution to the spindle poles (Figure 6, rows 2 and 3). Throughout prometaphase and metaphase, Emi1 continues to colocalize at the spindle poles with Plk1, while Plk1 also can be seen at kinetochores in some cells (Figure 6, rows 4 and 5). We have previously reported the localization of Emi1 to the spindle poles (Reimann et al., 2001a; Hsu et al., 2002) but have not understood how spindle-associated Emi1 persists at a time when most Emi1 has been destroyed. Whereas Plk1 staining is tightly focused on the mitotic centrosome, Emi1 seems more broadly distributed on the spindle poles. We used deconvolution microscopy to examine this difference more closely and observed that Plk1 and Emi1 seem to occupy distinct subcompartments of the spindle pole. Although closely apposed to the staining of Plk1, Emi1 is consistently excluded from the region of the mitotic spindle pole occupied by Plk1 (Figure 7A). Costaining for γ-tubulin also verified that Emi1 seems excluded from the mitotic centrosome (Figure 7B). Whether this reflects a region on the spindle pole where Plk1 is triggering Emi1 destruction or whether there are distinct receptors for Plk1 and Emi1 in these adjacent regions is unclear, although both models are interesting. We noticed similar regions of Emi1–Plk1 juxta-position on the midzone and midbody later in mitosis in some cells (our unpublished data). Because we have shown that Plk1 can form a stable complex with Emi1 during mitosis (Figure 4), we tested whether knockdown of Plk1 altered localization of Emi1 to the spindle poles by inactivating Plk1 through RNA interference. Because of Plk1's well-documented role in spindle pole organization, poles in Plk1 knockdown cells were usually splayed and defocused or monoastral. We continued to observe Emi1 staining on these disrupted spindle pole structures (Figure 8), indicating that Emi1 does not exhibit a dependence on Plk1 for recruitment to the mitotic spindle. We are currently exploring whether Emi1 has an important function on the spindle and whether this function is compromised in the absence of Plk1.
Figure 6.
Emi1 and Plk1 colocalize on spindle poles in early mitosis. U2OS cells were fixed and visualized by indirect immunofluorescence by using α-Plk1 and α-Emi1 antibodies to monitor the appearance of Plk1 and Emi1 at the maturing spindle poles. Staining of DNA is shown to demonstrate chromosome condensation and brightfield images to visualize the integrity of the nuclear envelope and nuclear envelope breakdown. Arrows point to intact nuclear envelope. Bars, 5 μm.
Figure 7.
Emi1 and Plk1 localize to tightly juxtaposed compartments of the spindle pole. (A) Plk1 is on the mitotic centrosome immediately adjacent to, but excluding, spindle pole-associated Emi1. U2OS cells were processed as in Figure 6. Image stacks were collected and processed by deconvolution, and projected image presented. Spindle poles are magnified and shown with or without Plk1 to emphasize the absence of Emi1 from the mitotic centrosome. (B) Colocalization of Emi1 and γ-tubulin on mitotic spindle poles. U2OS cells were processed as in Figure 7A but with α-γ-tubulin instead of α-Plk1 antibodies. Bars, 5 μm.
Figure 8.
Emi1 localizes normally to spindle poles in the absence of Plk1. U2OS cells were synchronized by double thymidine block and siRNA treatment (as depicted in Figure 2B), released into normal medium and harvested 12 h after release. Cells were processed as in Figure 6 but with α-α-tubulin instead of α-Plk1 antibodies.
DISCUSSION
A recent review of the events orchestrating mitosis cast cyclin B/Cdk1 in the role of the conductor of mitosis, with the Polo kinases taking the role of first violin, leading specific episodes in the mitotic cycle (Barr et al., 2004). The question remains as to how conductor and first violin conspire to rouse the heavy drums signaling activation of the APC, the destruction of cyclins, and the final exit from mitosis. Here, we show that the Polo kinase Plk1 is required for triggering the destruction of the APC inhibitor Emi1, in both mitotic HeLa cells and in mitotic Xenopus egg extracts. Further, overexpression of Plk1 is sufficient to cause premature destruction of Emi1 in human cells, which can be rescued by proteasome inhibition or by a dominant negative form of βTrCP.
How Direct Is the Plk1 Effect on Emi1 Destruction?
Although it is formally possible that the in vivo effects of Plk1 overexpression or silencing on Emi1 destruction are indirect, we present several lines of evidence demonstrating that Plk1 directly phosphorylates the Emi1 degron to recruit the SCFβTrCP complex. First, in the Plx1 immunodepletion experiment, the Xenopus egg extract was fully mitotic before Plx1 removal. Thus, any Polo-dependent mitotic subprograms were functionally intact, at least at the time of immunodepletion, and yet the extract could not destroy Emi1. The addition of purified Plx1 to the depleted extract restored Emi1 destruction, arguing against the possibility that some other critical factor was codepleted with Plx1. Second, consistent with the model that Plk1 phosphorylates Emi1 directly, the Emi1 phosphodegron E-D-pS-G-Y-S-pS has consensus sites for both Polo kinase phosphorylation (E-X-S) and for binding of the PBD (S-pS). We show here that each site is mitotically regulated, Plk1-responsive, and critical for Emi1 destruction, although our mutational analysis somewhat questions whether the consensus residues surrounding the actual target serines are substantially important for Plk1 interaction (discussed below). Third, we demonstrate that Plk1 directly binds Emi1 in mitosis, likely through Plk1's PBD. Fourth, we also find that Plk1 can phosphorylate Emi1 in vitro, and that Plk1 can strongly stimulate Emi1 binding to βTrCP and in vitro ubiquitination by SCFβTrCP. Finally, immunofluorescence data show Emi1 and Plk1 are distinctively colocalized on mitotic spindle poles. Together, these tests support that Plk1 directly phosphorylates Emi1 to trigger its degradation but do not exclude roles for other mitotic kinases. Indeed, we show that Plk1 and cyclin B/Cdk1 can cooperate to stimulate Emi1 ubiquitination, supporting our earlier data that Cdk1 and a mitotic kinase stimulate Emi1 destruction (Margottin-Goguet et al., 2003). During the preparation of this manuscript, a report showing the ability of Plk1 and Cdk1 to phosphorylate and stimulate the in vitro ubiquitination of Emi1 was published (Moshe et al., 2004), in agreement with our in vitro findings.
The Nature of the Interactions between Plk1 and Its Targets
Emi1's degron (EDS145GYSS149) contains consensus motifs for both binding (S-pS149) and phosphorylation (E-X-S145) by Plk1. The consensus suggests a two-step, dual kinase model, similar to those proposed for β-catenin (Liu et al., 2002) and Cdc25A (Jin et al., 2003; Donzelli et al., 2004). In this model, phosphorylation of serine-149 by some unknown kinase would create a PBD binding site, which would then recruit Plk1 for phosphorylation of the consensus Plk1 target at serine-145. The dually phosphorylated degron would render Emi1 ripe for recognition and ubiquitination by the SCFβTrCP complex.
Although we have not yet excluded this model, our analysis does not favor such an orderly mode of interaction between Emi1 and Plk1. First, mutation of serine-149 to a nonphosphorylatable residue does not prevent Emi1's association with Plk1, indicating that other sites within Emi1 are capable of binding Plk1. We presume that this interaction depends on prior phosphorylation of Emi1 because Plk1's C-terminal domain that interacts with Emi1 has been shown to bind phosphopeptides (Elia et al., 2003a), and because all of the Emi1 in Plk1 immunoprecipitates displays retarded gel mobility, a characteristic feature of phosphorylated proteins. Second, we have shown using purified proteins that Plk1 can phosphorylate Emi1 on both serine-145 and serine-149 in vitro, by using βTrCP binding as a highly selective, contextual readout for these specific phosphoresidues. This dual phosphorylation by Plk1 occurs despite the absence of an acidic residue in the –2 position from serine-149. Because the sequence surrounding serine-149 does not match the consensus for Plk1 phosphorylation, it is possible that Plk1's activity toward serine-149 in our experiments is a result of in vitro promiscuity. However, we also demonstrated that serine-145 and 149 are each individually responsive to Plk1 overexpression in 293T cells, further supporting the notion that Plk1 is the kinase that generates both phosphoserines in Emi1's βTrCP degron.
Our studies illustrate some limitations of the consensus motifs for PBD binding or phosphorylation by Plk1. Although some Plk1 substrates do use the chemically defined, optimal D/E-X-S/T-Φ sequence (Nakajima et al., 2003), several mapped Plk1 phosphorylation sites do not (Yarm, 2002; Kraft et al., 2003; Erikson et al., 2004). Two of our observations—that Plk1 can phosphorylate Emi1 on serine-149, which lacks an acidic residue in the –2 position, and that mutation of glutamate-143 in front of serine-145 causes only a modest decrease in the rate of Emi1 destruction, suggest that the consensus is helpful, but not restrictive. Similarly, the sequence requirements for PBD binding may be more flexible than the S-pS/pT-P motif defined by the use of synthetic peptides (Elia et al., 2003a). Binding of Plk1 to MKLP2 represents a known example of Plk1's PBD binding to a phosphoserine lacking a preceding serine (Neef et al., 2003), consistent with the phosphoserine being the most critical determinant of PBD interaction, as suggested by the crystal structure (Cheng et al., 2003; Elia et al., 2003b). Accordingly, we currently favor a model for Plk1-Emi1 binding in which the extensive mitotic phosphorylation of Emi1 by Cdk1 and possibly other kinases produces a large number of phosphoresidues that serve as suboptimal sites for PBD association, perhaps similar to the allovalency mechanism of Sic1 recognition by Cdc4 (Orlicky et al., 2003). This model helps explain how the nonessential role of Cdk phosphorylation contributes to the efficiency of mitotic Emi1 destruction (Margottin-Goguet et al., 2003). We imagine that the cooperative activity of Cdk1, Plk1, and other mitotic factors in preparing Emi1 for ubiquitination by SCFβTrCP is more like a pack of hyenas unleashed upon prey rather than an orderly and processive assembly line.
Activating the Anaphase Promoting Complex
The requirement for the Polo kinase family in the mitotic program suggested that it might contribute to the destruction of mitotic cyclins (Sunkel and Glover, 1988; Llamazares et al., 1991). Early studies tried to directly link Polo phosphorylation to APC activation, but the emerging consensus is that direct phosphorylation of the APC by Plk1 contributes only marginally to APC activity, and most likely only in cooperation with cyclin B/Cdk1. The most convincing report of Plk1's importance in APC activation in vertebrate systems came from Descombes and Nigg (1998), who demonstrated in meiotically arrested Xenopus egg extracts that Plx1 is required for the Ca2+-induced destruction of mitotic cyclins. Here, we show that Polo kinases might trigger APC activation largely by causing Emi1 destruction. Fittingly, Emi1 is required to maintain the meiotic arrest in Xenopus egg extracts (Reimann and Jackson, 2002), suggesting a plausible explanation for the involvement of Plx1 in release from this arrest. Recent work by Liu et al. (2004) showed that inhibition of meiotic exit by dominant negative Plx1 can be almost entirely relieved by addition of excess Cdc20 —the same treatment that relieves the inhibition of meiotic exit by excess Emi1. This again suggests that Plk1 does not significantly regulate the intrinsic activity of the APC, but instead regulates some upstream aspect of Cdc20 function.
We have shown that silencing of Plk1 by RNA interference in HeLa cells delays the events of early mitosis. In addition to delaying mitotic entry by ∼2 h, Plk1 knockdown results in a marked stabilization of both Emi1 and cyclin A for at least 3–4 h after mitotic entry. This result seems at face value somewhat different than the analysis of Peters and coworkers (Kraft et al., 2003), who reported that Plk1 knockdown did not have an effect on the destruction of cyclin A as visualized by immunofluorescence assays of cyclin A staining or in situ destruction of a cyclin A-GFP fusion in HeLa cells. However, in their analysis the destruction of GFP-cyclin A does seem to take almost 2 h after NEBD, whereas the destruction of GFP-cyclin A in HeLa cells described previously (den Elzen and Pines, 2001) was completed within ∼20 min after NEBD. Additionally, in their Plk1 knockdown cells there seemed to be a plateau of ∼50% of the total GFP-cyclin A, which remained stable for several hours after NEBD. We think that these observations are potentially consistent with our data, which by immunoblot analysis indicate that, although cyclin A levels decline somewhat upon mitotic entry, about one-half of the cyclin A remains stable for at least 3–4 h. Alternatively, the observation by Kraft and coworkers that they do not see an effect of Plk1 silencing on APC activation might also result from an incomplete knockdown of Plk1 by RNA interference. The siRNAs used in their studies and in our studies are different and no direct comparison has been made, although the Plk1 siRNA we have used is very efficient (Figure 2). Another very recent article from Medema and coworkers suggests that Plk1 is not strictly required for APC activation in HeLa cells (van Vugt et al., 2004). Here, the authors cause Plk1 inactivation with a transfected pSUPER vector, a hairpin siRNA expression construct. In our experience, the pSUPER vectors take ∼24 h longer to have a maximal effect, compared with transfected siRNA duplexes (our unpublished data). Thus, whereas Plk1 activation of Emi1 destruction appears to be critical for the timing of APC activation, either the difficulty of achieving complete Plk1 silencing or the contribution of additional mitotic factors make it difficult to completely block APC activation.
Why do we observe any decline at all in cyclin A levels when Emi1 is not destroyed in the absence of Plk1? In these cells, we actually do see Emi1 levels begin to slowly decline ∼4 h after mitotic entry (Figure 2B), suggesting there may be redundant but inefficient modes of Emi1 destruction. We have found that the Plk1 homolog Plk3 was able to cause a strong reduction of Emi1 levels in an overexpression experiment, similar to that in Figure 2A (our unpublished data), whereas Plk2 failed to reduce Emi1 levels. Thus, it could be that the requirement for Polo-like kinases in triggering Emi1 destruction and APC activation includes both Plk1 and Plk3 or that in the absence of Plk1, Plk3 can substitute for Plk1. Some precedent exists for overlapping roles of Plk1 and Plk3 because both kinases can phosphorylate Cdc25C (Bahassi el et al., 2004). Furthermore, it is possible that mitotically phosphorylated APC is somewhat less susceptible to Emi1 inhibition. Finally, both Emi1 and the APC are strongly altered in their localization from interphase to mitosis, which might affect the efficiency with which Emi1 can inhibit the APC as both factors are redistributed.
Organization of Early Mitosis and the Mitotic Spindle Poles
The ability of Plk1 to trigger activation of Cdk1 by phosphorylation of the Cdk1-activating phosphatase Cdc25 forms a positive feedback loop that can strongly accelerate the G2-M transition (Kumagai and Dunphy, 1996; Qian et al., 1998; Abrieu et al., 1998). Phosphorylation of cyclin B1 by Plk1 also may provide a mechanism to allow localized activity of the cyclin B1/Cdk1 complex on prophase centrosomes (Jackman et al., 2003). The ability of Plk1 to accelerate and spatially organize the mitotic activation machinery is important in early mitosis and essential for the overall mitotic process. Similarly, the ability of Plk1 to cooperate with Cdk1 to trigger Emi1 destruction is critical for APC activation after NEBD. Emi1 is the strongest candidate for the factor that creates the lag between activation of MPF and the APC and is certainly essential to prevent premature destruction of mitotic cyclins.
The importance of Plk1 in organizing the mitotic spindle in early mitosis is well established (Barr et al., 2004). By promoting the destruction of Emi1, Plk1 takes a new role. Plk1 is first recruited to the mitotic centrosome before the recruitment of Emi1 to the adjacent spindle poles, suggesting that these two events may be linked. An interesting possibility is that assembly of the mitotic spindle poles is somehow connected to the destruction of Emi1 or that Plk1 organizes some other role for Emi1 on the mitotic spindle. Plk1 may organize a “destruction center” at the mitotic centrosome, with Emi1 on the adjacent spindle pole being actively transported along spindle microtubules to the centrosome, where it is phosphorylated by Plk1 and ubiquitinated. Components of the SCF ubiquitin ligase complex have been shown to localize to the centrosome (Freed et al., 1999). Alternatively, the Emi1 on the spindle poles could be a more static, structural or functional component of the mitotic spindle.
It may seem paradoxical that Emi1 persists on the spindle at a time when Plk1 is active and immunoblotting suggests that the majority of Emi1 is destroyed, but an analogous situation exists in vertebrate cells for cohesin. Whereas the large majority of cohesin is phosphorylated by Plk1 and removed from chromatin during early mitosis, a small amount of cohesin is somehow maintained at kinetochores to preserve sister chromatid cohesion until the metaphase–anaphase transition (Waizenegger et al., 2000). The localization of Plk1 and Emi1 to distinct subcompartments of the spindle pole suggests that spatial separation from Plk1 is important for the stability of spindle-associated Emi1. An exciting possibility is that the Emi1 retained on the spindle after destruction of the bulk pool is important for proper spindle function. It is very interesting to compare the spindle localization of Plk1 and Emi1 with that of the APC. Whereas the APC can be seen throughout the spindle, the phosphorylated, active form of the APC is only detected at the mitotic centrosome (Kraft et al., 2003), which correlates nicely with the presence of Cdk1 and Plk1 and the absence of Emi1. This suggests that one important function of Plk1 at the mitotic centrosome is organization of Emi1 destruction and APC activity. A role of the APC in cooperation with Polo for mitotic recruitment of centrosomal antigens has been reported in Drosophila (Deak et al., 2003). It is possible that the destruction of the bulk pool of Emi1 is only one aspect of APC activation and that spatially restricted pools of Emi1 provide critical timing mechanisms gating the APC-dependent destruction of regulators on the mitotic spindle. Here, the destruction of Emi1 may serve as an “egg timer,” providing a defined amount of APC inhibition, which thereby sets the persistence of assembled mitotic structures, until such time as the APC is released to trigger their maturation or disassembly.
Acknowledgments
We thank William Dunphy and Akiko Kumagai for the generous gift of anti-Plx1 antibody and baculovirus expressing Plx1. We also thank Michael Yaffe and Andrew Elia for noticing the Polo Box binding motif in Emi1. We thank and Guowei Fang for Plk1 constructs. This work was supported by grants from the Kirsch Foundation and the National Institutes of Health (to P.K.J.; R01 GM-53411 and GM-60439), a Stanford Presidential Fellowship (to D.V.H.), and a National Science Scholarship from the Agency for Science, Technology, and Research, Singapore (to K.H.B.). We also thank Palo Alto Fire Department for extending their expertise in extraction and preservation to D.V.H.
Abbreviations used: APC, anaphase promoting complex; MPF, mitosis promoting factor; Plk1, Polo-like kinase 1; Plx1, Xenopus polo-like kinase 1; PBD, Polo Box domain.
References
- Abrieu, A., Brassac, T., Galas, S., Fisher, D., Labbe, J.C., and Doree, M. (1998). The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 111, 1751-1757. [DOI] [PubMed] [Google Scholar]
- Bahassi el, M., Hennigan, R.F., Myer, D.L., and Stambrook, P.J. (2004). Cdc25C phosphorylation on serine 191 by Plk3 promotes its nuclear translocation. Oncogene 23, 2658-2663. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., Sillje, H.H., and Nigg, E.A. (2004). Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell. Biol. 5, 429-441. [DOI] [PubMed] [Google Scholar]
- Cheng, K.Y., Lowe, E.D., Sinclair, J., Nigg, E.A., and Johnson, L.N. (2003). The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J. 22, 5757-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak, P., Donaldson, M., and Glover, D.M. (2003). Mutations in makos, a Drosophila gene encoding the Cdc27 subunit of the anaphase promoting complex, enhance centrosomal defects in polo and are suppressed by mutations in twins/aar, which encodes a regulatory subunit of PP2A. J. Cell Sci. 116, 4147-4158. [DOI] [PubMed] [Google Scholar]
- den Elzen, N., and Pines, J. (2001). Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153, 121-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes, P., and Nigg, E.A. (1998). The polo-like kinase Plx1 is required for M phase exit and destruction of mititic regulators in Xenopus egg extracts. EMBO J. 17, 1328-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzelli, M., Busino, L., Chiesa, M., Ganoth, D., Hershko, A., and Draetta, G.F. (2004). Hierarchical order of phosphorylation events commits Cdc25A to betaTrCP-dependent degradation. Cell Cycle 3, 469-471. [PubMed] [Google Scholar]
- Elia, A.E., Cantley, L.C., and Yaffe, M.B. (2003a). Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 299, 1228-1231. [DOI] [PubMed] [Google Scholar]
- Elia, A.E., Rellos, P., Haire, L.F., Chao, J.W., Ivins, F.J., Hoepker, K., Mohammad, D., Cantley, L.C., Smerdon, S.J., and Yaffe, M.B. (2003b). The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115, 83-95. [DOI] [PubMed] [Google Scholar]
- Erikson, E., Haystead, T.A., Qian, Y.W., and Maller, J.L. (2004). A feedback loop in the polo-like kinase activation pathway. J. Biol. Chem. 279, 32219-32224. [DOI] [PubMed] [Google Scholar]
- Freed, E., Lacey, K.R., Huie, P., Lyapina, S.A., Deshaies, R.J., Stearns, T., and Jackson, P.K. (1999). Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 13, 2242-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, S.Y., Spiegelman, V.S., and Kumar, K.G. (2004). The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene 23, 2028-2036. [DOI] [PubMed] [Google Scholar]
- Golan, A., Yudkovsky, Y., and Hershko, A. (2002). The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J. Biol. Chem. 277, 15552-15557. [DOI] [PubMed] [Google Scholar]
- Golsteyn, R.M., Mundt, K.E., Fry, A.M., and Nigg, E.A. (1995). Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 129, 1617-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardavaccaro, D., Kudo, Y., Boulaire, J., Barchi, M., Busino, L., Donzelli, M., Margottin-Goguet, F., Jackson, P.K., Yamasaki, L., and Pagano, M. (2003). Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev. Cell 4, 799-812. [DOI] [PubMed] [Google Scholar]
- Harper, J.W., Burton, J.L., and Solomon, M.J. (2002). The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16, 2179-2206. [DOI] [PubMed] [Google Scholar]
- Hart, M. et al. (1999). The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 9, 207-210. [DOI] [PubMed] [Google Scholar]
- Hershko, A., Ganoth, D., Sudakin, V., Dahan, A., Cohen, L.H., Luca, F.C., Ruderman, J.V., and Eytan, E. (1994). Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J. Biol. Chem. 269, 4940-4946. [PubMed] [Google Scholar]
- Hsu, J.Y., Reimann, J.D., Sorensen, C.S., Lukas, J., and Jackson, P.K. (2002). E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat. Cell Biol. 4, 358-366. [DOI] [PubMed] [Google Scholar]
- Jackman, M., Lindon, C., Nigg, E.A., and Pines, J. (2003). Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5, 143-148. [DOI] [PubMed] [Google Scholar]
- Jackson, P.K., Chevalier, S., Philippe, M., and Kirschner, M.W. (1995). Early events in DNA replication require cyclin E and are blocked by p21CIP1. J. Cell Biol. 130, 755-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, Y.J., Lin, C.Y., Ma, S., and Erikson, R.L. (2002). Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc. Natl. Acad. Sci. USA 99, 1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, J., Shirogane, T., Xu, L., Nalepa, G., Qin, J., Elledge, S.J., and Harper, J.W. (2003). SCF(beta-TRCP) links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 17, 3062-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm, O., Wind, M., Lehmann, W.D., and Nigg, E.A. (2002). Cell cycle-regulated phosphorylation of the Xenopus polo-like kinase Plx1. J. Biol. Chem. 277, 25247-25256. [DOI] [PubMed] [Google Scholar]
- King, R.W., Peters, J.M., Tugendreich, S., Rolfe, M., Hieter, P., and Kirschner, M.W. (1995). A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279-288. [DOI] [PubMed] [Google Scholar]
- Kotani, S., Tugendreich, S., Fujii, M., Jorgensen, P.-M., Watanabe, N., Hoog, C., Hieter, P., and Todokoro, K. (1998). PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell 1, 371-380. [DOI] [PubMed] [Google Scholar]
- Kraft, C., Herzog, F., Gieffers, C., Mechtler, K., Hagting, A., Pines, J., and Peters, J.M. (2003). Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22, 6598-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, E.R., Scheuringer, N., Podtelejnikov, A.V., Mann, M., and Peters, J.M. (2000). Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11, 1555-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, A., and Dunphy, W.G. (1996). Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science 273, 1377-1380. [DOI] [PubMed] [Google Scholar]
- Lahav-Baratz, S., Sudakin, V., Ruderman, J.V., and Hershko, A. (1995). Reversible phosphorylation controls the activity of cyclosome-associated cyclin-ubiquitin ligase. Proc. Natl. Acad. Sci. USA 92, 9303-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.S., Yuan, Y.L., Kuriyama, R., and Erikson, R.L. (1995). Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol. Cell. Biol. 15, 7143-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, D.J., and Burke, D.J. (2003). The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 37, 251-282. [DOI] [PubMed] [Google Scholar]
- Liu, C., Li, Y., Semenov, M., Han, C., Baeg, G.H., Tan, Y., Zhang, Z., Lin, X., and He, X. (2002). Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837-847. [DOI] [PubMed] [Google Scholar]
- Liu, J., Lewellyn, A.L., Chen, L.G., and Maller, J.L. (2004). The polo box is required for multiple functions of Plx1 in mitosis. J. Biol. Chem. 279, 21367-21373. [DOI] [PubMed] [Google Scholar]
- Llamazares, S., Moreira, A., Tavares, A., Girdham, C., Spruce, B.A., Gonzalez, C., Karess, R.E., Glover, D.M., and Sunkel, C.E. (1991). polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 5, 2153-2165. [DOI] [PubMed] [Google Scholar]
- Margottin-Goguet, F., Hsu, J.Y., Loktev, A., Hsieh, H.M., Reimann, J.D., and Jackson, P.K. (2003). Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4, 813-826. [DOI] [PubMed] [Google Scholar]
- Montagnoli, A., Fiore, F., Eytan, E., Carrano, A.C., Draetta, G.F., Hershko, A., and Pagano, M. (1999). Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13, 1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshe, Y., Boulaire, J., Pagano, M., and Hershko, A. (2004). Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl. Acad. Sci. USA 101, 7937-7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt, K.E., Golsteyn, R.M., Lane, H.A., and Nigg, E.A. (1997). On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem. Biophys. Res. Commun. 239, 377-385. [DOI] [PubMed] [Google Scholar]
- Murray, A.W. (2004). Recycling the cell cycle: cyclins revisited. Cell 116, 221-234. [DOI] [PubMed] [Google Scholar]
- Nakajima, H., Toyoshima-Morimoto, F., Taniguchi, E., and Nishida, E. (2003). Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 278, 25277-25280. [DOI] [PubMed] [Google Scholar]
- Neef, R., Preisinger, C., Sutcliffe, J., Kopajtich, R., Nigg, E.A., Mayer, T.U., and Barr, F.A. (2003). Phosphorylation of mitotic kinesin-like protein 2 by pololike kinase 1 is required for cytokinesis. J. Cell Biol. 162, 863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg, E.A. (2001). Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell. Biol. 2, 21-32. [DOI] [PubMed] [Google Scholar]
- Orlicky, S., Tang, X., Willems, A., Tyers, M., and Sicheri, F. (2003). Structural basis for phosphodependent substrate selection and orientation by the SCF-Cdc4 ubiquitin ligase. Cell 112, 243-256. [DOI] [PubMed] [Google Scholar]
- Qian, Y.W., Erikson, E., Li, C., and Maller, J.L. (1998). Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol. 18, 4262-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann, J.D., Freed, E., Hsu, J.Y., Kramer, E.R., Peters, J.M., and Jackson, P.K. (2001a). Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105, 645-655. [DOI] [PubMed] [Google Scholar]
- Reimann, J.D., Gardner, B.E., Margottin-Goguet, F., and Jackson, P.K. (2001b). Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins. Genes Dev. 15, 3278-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann, J.D., and Jackson, P.K. (2002). Emi1 is required for cytostatic factor arrest in vertebrate eggs. Nature 416, 850-854. [DOI] [PubMed] [Google Scholar]
- Rudner, A.D., and Murray, A.W. (2000). Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 149, 1377-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou, W., Azzam, R., Chen, S.L., Huddleston, M.J., Baskerville, C., Charbonneau, H., Annan, R.S., Carr, S.A., and Deshaies, R.J. (2002). Cdc5 influences phosphorylation of Net1 and disassembly of the RENT complex. BMC Mol. Biol. 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteinberg, M., Protopopov, Y., Listovsky, T., Brandeis, M., and Hershko, A. (1999). Phosphorylation of the cyclosome is required for its stimulation by Fizzy/cdc20. Biochem. Biophys. Res. Commun. 260, 193-198. [DOI] [PubMed] [Google Scholar]
- Sudakin, V., Ganoth, D., Dahan, A., Heller, H., Hershko, J., Luca, F.C., Ruderman, J.V., and Herchko, A. (1995). The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6, 185-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkel, C.E., and Glover, D.M. (1988). polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J. Cell Sci. 89, 25-38. [DOI] [PubMed] [Google Scholar]
- van Vugt, M.A., van de Weerdt, B.C., Vader, G., Janssen, H., Calafat, J., Klompmaker, R., Wolthuis, R.M., and Medema, R.H. (2004). Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for APC/Cdc20 activation and initiation of cytokinesis. J. Biol. Chem. 279, 36841-36854. [DOI] [PubMed] [Google Scholar]
- Waizenegger, I.C., Hauf, S., Meinke, A., and Peters, J.M. (2000). Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103, 399-410. [DOI] [PubMed] [Google Scholar]
- Watanabe, N., Arai, H., Nishihara, Y., Taniguchi, M., Hunter, T., and Osada, H. (2004). M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. USA 101, 4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston, J.T., Strack, P., Beer-Romero, P., Chu, C.Y., Elledge, S.J., and Harper, J.W. (1999). The SCF(beta-TrCP)-ubiquitin ligase complex associates with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 13, 270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, G., Xu, G., Schulman, B.A., Jeffrey, P.D., Harper, J.W., and Pavletich, N.P. (2003). Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol. Cell 11, 1445-1456. [DOI] [PubMed] [Google Scholar]
- Yarm, F.R. (2002). Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol. Cell. Biol. 22, 6209-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron, A., Hatzubai, A., Davis, M., Lavon, I., Amit, S., Manning, A.M., Andersen, J.S., Mann, M., Mercurio, F., and Ben-Neriah, Y. (1998). Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature 396, 590-594. [DOI] [PubMed] [Google Scholar]