Coregulation of Fibronectin Signaling and Matrix Contraction by Tenascin-C and Syndecan-4 (original) (raw)
Abstract
Syndecan-4 is a ubiquitously expressed heparan sulfate proteoglycan that modulates cell interactions with the extracellular matrix. It is transiently up-regulated during tissue repair by cells that mediate wound healing. Here, we report that syndecan-4 is essential for optimal fibroblast response to the three-dimensional fibrin-fibronectin provisional matrix that is deposited upon tissue injury. Interference with syndecan-4 function inhibits matrix contraction by preventing cell spreading, actin stress fiber formation, and activation of focal adhesion kinase and RhoA mediated-intracellular signaling pathways. Tenascin-C is an extracellular matrix protein that regulates cell response to fibronectin within the provisional matrix. Syndecan-4 is also required for tenascin-C action. Inhibition of syndecan-4 function suppresses tenascin-C activity and overexpression of syndecan-4 circumvents the effects of tenascin-C. In this way, tenascin-C and syndecan-4 work together to control fibroblast morphology and signaling and regulate events such as matrix contraction that are essential for efficient tissue repair.
INTRODUCTION
Tissue architecture is defined by the three-dimensional (3D) organization of the proteins and proteoglycans that comprise the extracellular matrix (ECM). Individual ECM components influence cell arrangements and activities through binding to transmembrane receptors, thus initiating intracellular signaling and altering gene expression and other downstream events. Tissues undergo continual maintenance and remodeling through controlled deposition and removal of specific ECM components. These changes in ECM composition and organization modulate cell behaviors and serve important roles throughout development and in the adult during both physiological and pathological processes (Lukashev and Werb, 1998).
The provisional matrix that forms at sites of tissue injury undergoes extensive remodeling and turnover during wound repair. Composed predominantly of covalently cross-linked fibrin and plasma fibronectin (FN), this fibrin-FN provisional matrix coordinates the activities of cells involved in wound repair. It is remodeled over time to recapitulate normal tissue and this remodeling involves cellmediated contraction and deposition of new FN-rich matrix (Clark, 1996; Midwood et al., 2004). FN, a major component of the matrix, is a ubiquitously expressed, multifunctional extracellular glycoprotein that promotes cell adhesion. It controls many intracellular pathways and influences a wide range of cell functions (Hynes, 1990). For example, FN within a 3D fibrin-FN provisional matrix initiates signaling via focal adhesion kinase (FAK) and RhoA in order to promote fibroblast spreading, stress fiber formation, and focal adhesion assembly (Wenk et al., 2000; Midwood and Schwarzbauer, 2002).
The provisional matrix contacts many other ECM proteins that are induced at different stages of tissue repair, including thrombospondins, SPARC, and tenascin-C. These proteins are specifically up-regulated at wound sites where they modulate cell-ECM interactions (reviewed in Midwood et al., 2004). For example, both pro- and antiadhesive properties of thrombospondin-1 and -2 act to regulate angiogenesis during tissue repair (reviewed in Bornstein, 2001). Tenascin-C also has modulatory effects on cell-ECM interactions. In cell culture, it antagonizes the adhesive effects of FN (Chiquet-Ehrismann et al., 1988; Chiquet-Ehrismann and Chiquet, 2003). Within a 3D fibrin-FN matrix, tenascin-C impacts the ability of fibroblasts to deposit and contract the matrix by affecting the morphology and signaling pathways of adherent cells (Wenk et al., 2000; Midwood and Schwarzbauer, 2002).
Along with ECM proteins, proteoglycans also communicate extracellular information to the cell. These molecules, which consist of a protein core modified with glycosaminoglycan (GAG) side chains, are found at the cell surface, inside the cell, or incorporated into the ECM (Lander, 1998). Transmembrane heparan sulfate proteoglycans (HSPGs) of the syndecan family bind to ECM proteins and cooperate with heterodimeric integrin receptors to regulate cell adhesive activities (Bloom et al., 1999; Kusano et al., 2000; Woods and Couchman, 2000). One member of this family, syndecan-4, is up-regulated during tissue repair (Gallo et al., 1996) and syndecan-4–null mice show defects in wound healing (Echtermeyer et al., 2001). Syndecan-4 binds to FN and probably to tenascin-C via its GAG side chains (Salmivirta et al., 1991; Woods et al., 2000). In fact, fibroblasts use both integrin receptors and syndecan-4 to induce cell spreading, actin cytoskeleton organization, and signaling events on FN-coated substrates (Saoncella et al., 1999; Bass and Humphries, 2002). We know that fibroblasts require α5β1 integrins to bind FN within a provisional matrix and that this interaction is essential for cell adhesion, spreading, and matrix contraction (Corbett and Schwarzbauer, 1999). Here, we further determined that syndecan-4 is essential for signaling from the fibrin-FN provisional matrix and controls events such as matrix contraction. Syndecan-4 is also required for tenascin-C modulation of cell behavior. As such, syndecan-4 plays a vital role throughout tissue repair in communicating signals between the dynamic ECM and the cell.
MATERIALS AND METHODS
Protein Production
Rat plasma FN was purified by gelatin-Sepharose (Pharmacia Biotech, Arlington Heights, IL) affinity chromatography from freshly drawn plasma (Wilson and Schwarzbauer, 1992). The construction and production of the recombinant protein 70Ten has previously been described and this protein has been shown to have identical effects on cells as full-length tenascin-C (Wenk et al., 2000).
Cell Culture
NIH3T3 fibroblasts were maintained in DMEM and 10% calf serum (Hyclone Laboratories, Logan, UT). CHO K1 cells were maintained in DMEM, 2 mM glutamine, 1% nonessential amino acids, and 10% fetal clone serum (Hyclone Laboratories). CHO mutant cell lines were generously provided by Dr. Jeffrey Esko (University of California, San Diego; Esko, 1991), and grown in Ham's F-12 medium (Life Technologies, Rockville, MD) supplemented with 7.5% fetal bovine serum (Hyclone Laboratories). Fibroblasts derived from wild-type, heterozygous, or syndecan-4–deficient mouse dermis (Echtermeyer et al., 2001; gifts from Dr. Paul Goetinck, Massachusetts General Hospital) were cultured in DMEM plus 10% fetal bovine serum and used only up until 10 passages. Wild-type REF52 cells and REF52 cells stably overexpressing syndecan-4 (gifts from Dr. Getraud Orend, University of Basel; Orend et al., 2003) were maintained in DMEM plus 10% fetal calf serum and used between passage numbers 5 and 9. Cells were released from tissue culture dishes using 0.2 mg/ml EDTA in PBS or brief trypsinization, washed with PBS, resuspended in serum-free DMEM, and counted before use.
Transfection
Transfections were performed using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's guidelines. Syndecan-4–deficient mouse dermal fibroblasts were seeded at a density of 1 × 105 cells per 35-mm culture dish in antibiotic-free DMEM plus 10% fetal bovine serum. After 24 h at 37°C, cells were transfected with a pcDNA3.1 expression vector containing rat syndecan-4 cDNA (kindly provided by Dr. Paul Goetinck; Wilcox-Adelman et al., 2002) plus a pcDNA3-GFP expression vector (gift from Dr. Donald Winkelmann, Robert Wood Johnson Medical School) as a transfection reporter in LipofectAMINE 2000 reagent and OptiMem medium. In parallel, cells were transfected with the pcDNA3-GFP vector alone. Transfection medium was replaced with DMEM plus 10% fetal bovine serum after 5 h. Approximately 16% of cells were expressing GFP at 48 h posttransfection, at which time cells were replated on substrate-coated coverslips.
Immunofluorescence
Matrices were prepared as described previously (Midwood et al., 2002). Briefly, 600 μg/ml fibrinogen (America Diagnostica, Greenwich, CT), 30 μg/ml FN, and 15 μg/ml coagulation factor XIIIa (Calbiochem-Novabiochem, San Diego, CA) in the presence or absence of 120 μg/ml 70Ten were mixed with thrombin at 2 U/ml and the mixture was pipetted onto a glass coverslip (Fisher Scientific, Pittsburgh, PA). After overnight incubation at 4°C, the clots were aspirated from the coverslip and the substrate was blocked with 1% bovine serum albumin (BSA) in PBS.
Cells were plated at 4 × 104/well and allowed to spread on substrate-coated glass coverslips for 1 h, after which time cells were washed, fixed, and permeabilized (Midwood and Schwarzbauer, 2002). Cells were incubated with primary or secondary antibody or phalloidin in 2% ovalbumin (Sigma Chemical, St. Louis, MO) in PBS at 37°C for 1 h. Antibodies were used at the following dilutions: antivinculin monoclonal (Sigma Chemical) at 1:300 and fluorescein conjugated goat antimouse secondary antibody (Molecular Probes, Eugene, OR) at 1:500. For staining actin filaments, rhodamine-conjugated phalloidin (Molecular Probes) was used at 1:1000. Coverslips were mounted with SlowFade Light Antifade Kit (Molecular Probes). Cells were visualized with a Nikon Optiphot-2-microscope (Garden City, NY) and images were captured using a Photometrics Coolsnap camera (Tucson, AZ). Cell peripheries were outlined and areas were calculated using IPLab software (Madison, WI).
Immunoblotting
Matrices were prepared as for immunofluorescence studies. Immediately after the addition of thrombin, the mixture was pipetted onto 35-mm dishes. After overnight incubation at 4°C, the clots were aspirated and the substrate was blocked. Cells were plated at a density of 1.5 × 106 per 35-mm dish and allowed to spread on matrices for 60 min. At the end of the incubation period, cells were washed with PBS and then lysed in 200 μl RIPA lysis buffer (Wierzbicka-Patynowski and Schwarzbauer, 2002) and the protein concentration of the supernatant was determined using the BCA Protein Assay (Pierce, Rockford, IL). Samples were run on a 6% polyacrylamide-SDS gel and transferred to nitrocellulose (Sartorius, Long Island, NY; Midwood and Schwarzbauer, 2002). Proteins were detected using an anti-FAK mAb (Transduction Laboratories, Lexington, KY) at 1:1000 and an anti-FAK-397 polyclonal IgG (Biosource International, Camarillo, CA) at 1:5000. Primary antibodies were detected using horseradish peroxidase–conjugated goat anti-mouse or anti-rabbit secondary antibody (Pierce) diluted 1:50,000 and Supersignal chemiluminescent detection reagent (Pierce).
Contraction Assays
For culture in 3D matrices, cells were resuspended in 0.025 M HEPES, pH 7.4, 0.13 M NaCl at 1 × 106/ml as described previously (Midwood and Schwarzbauer, 2002). Matrices were prepared as for immunofluorescence except cells were added to the matrix components. Immediately after the addition of thrombin, the mixture was pipetted into 48-well plates, which had been coated with 1% BSA overnight at 4°C. The cell-matrix mixture was allowed to polymerize for 30 min at 37°C. To quantitate matrix contraction, cell-matrix mixtures were carefully detached from the dishes and matrix size was visualized as described previously (Midwood and Schwarzbauer, 2002). The area of the matrix was measured over time using a ruler, subtracted from the starting area and expressed as a percentage of the starting area.
Pretreatments
Cells were incubated with the indicated concentrations of heparin (Grade I-A) or chondroitin sulfate (Sigma Chemical), for 30 min at 37°C, and with chondroitinase ABC or heparinase I and III (Sigma Chemical) for 4 h at 37°C before addition to matrix proteins. Enzymes were stopped upon the addition of electrophoresis sample buffer. Cells were treated with 5 μM of lysophosphatidic acid (Sigma Chemical) for 30 min at 37°C as described previously (Midwood and Schwarzbauer, 2002). Cells were treated with antisyndecan-4 mAb D16 (Santa Cruz Biotechnology, Santa Cruz, CA) with or without pretreatment with blocking peptide, each at 50 μg/ml for 30 min at 37°C.
RESULTS
HSPGs Promote Cell Response to a Fibrin-FN Provisional Matrix
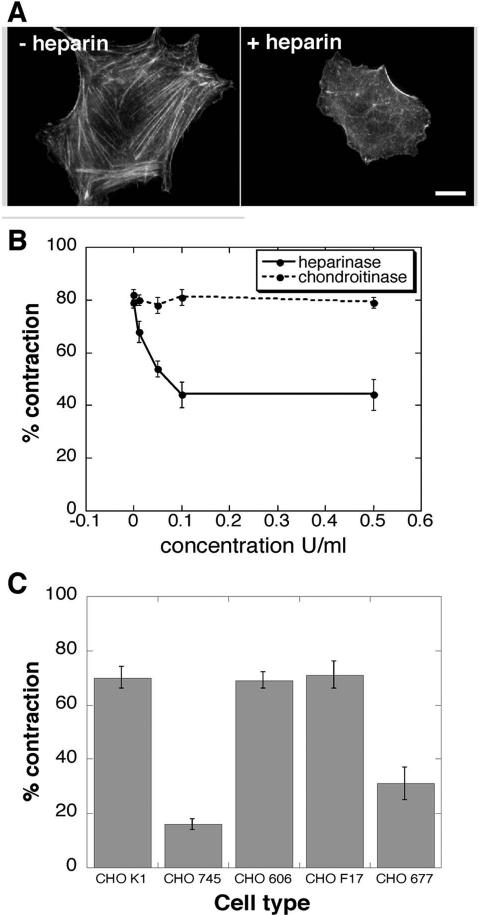
Within FN, binding sites for integrins lie adjacent to GAG binding domains (Hynes, 1990; Barkalow and Schwarzbauer, 1991; Moyano et al., 1999; Mostafavi-Pour et al., 2001). We have previously shown that α5β1 integrins are required for cell adhesion to fibrin-FN matrices (Corbett and Schwarzbauer, 1999). Here, we demonstrate the additional requirement for HSPGs for optimal cell response to fibrin-FN matrices. NIH3T3 fibroblasts plated on a fibrin-FN matrix became well spread and organized their actin into stress fibers (Figure 1A). HSPG function was compromised by treating cells with heparin, which competes with HSPGs for binding to FN. Heparin prevented cell spreading and organization of actin stress fibers (Figure 1A). The removal of cell surface HS GAGs by digestion with heparinase I and III had the same effect as soluble heparin (our unpublished results). Inhibition of chondroitin sulfate proteoglycans (CSPGs) function with chondroitin sulfate or chondroitinase ABC had no effect on cell morphology or cytoskeletal organization. These data indicate that HSPGs are essential for cell spreading and stress fiber formation in response to a fibrin-FN matrix.
Figure 1.
HSPGs impact actin organization and fibrin-FN matrix contraction. (A) NIH3T3 fibroblasts were incubated in suspension for 30 min, either with or without 100 μg/ml soluble heparin, before they were allowed to spread for 1 h on fibrin-FN matrix. Cells were washed, fixed, permeabilized, and stained with rhodamine-phalloidin to visualize filamentous actin. Representative cells are shown. Scale bar, 10 μm. (B) Fibroblasts were preincubated with the indicated concentrations of heparinase I/III or chondroitinase ABC for 4 h before polymerization together with fibrin-FN matrices. The matrix was detached from the dish and % contraction was measured after 4 h. The data are expressed as the mean ± SEM of triplicate experiments. (C) CHO wild-type and mutant cell lines were included during polymerization of fibrin-FN matrix and assayed for their ability to contract a fibrin-FN matrix. These data are expressed as the mean ± SEM of triplicate experiments.
Another measure of cell-FN interactions is the ability of cells to contract the surrounding matrix. Fibroblasts embedded within a fibrin-FN matrix rapidly contracted it (Figure 1B). Treatment of cells with heparinase, but not chondroitinase ABC, reduced their ability to contract this matrix (Figure 1B). Similarly, preincubation of cells with soluble heparin, but not chondroitin sulfate, inhibited matrix contraction in a dose-dependent manner (our unpublished results). These results indicate that HSPGs but not CSPGs are involved in mediating the contraction of a fibrin-FN matrix.
A role for HSPGs was clearly demonstrated using mutant Chinese hamster ovary (CHO) cell lines defective in different stages of GAG chain synthesis. Parental CHO K1 cells contracted fibrin-FN matrices to an extent comparable to fibroblasts (Figure 1C). CHO 745 cells can synthesize neither HS nor CS GAGs (Esko et al., 1985) and CHO 677 cells exhibit a reduced expression of HS GAGs and an increase in CS GAGs (Zhang and Esko, 1995). Both of these cells lines showed a significant reduction in fibrin-FN matrix contraction. In contrast, CHO 606 and CHO F17 cells with decreased sulfation (Bame and Esko, 1989; Bai and Esko, 1996) contracted fibrin-FN matrices equally as well as wild-type cells. These data show that CHO cell contractility specifically requires HS GAGs, but not CS GAGs or GAG sulfation. Furthermore, the absence of HS GAGs cannot be fully compensated for by increasing the level of expression of CSPGs. Therefore contraction of fibrin-FN matrices is a HSPG-dependent process.
Cell Contractility Requires Syndecan-4 Signaling
Syndecan-4 is a cell surface HSPG that functions cooperatively with α5β1 integrins in fibroblast binding to FN (Saoncella et al., 1999). Its expression by fibroblasts, among other cell types, is transiently up-regulated during tissue repair (Gallo et al., 1996). In addition, syndecan-4-null mice develop normally but show abnormal wound healing (Echtermeyer et al., 2001). For these reasons we analyzed the role of syndecan-4 in fibroblast response to the provisional matrix.
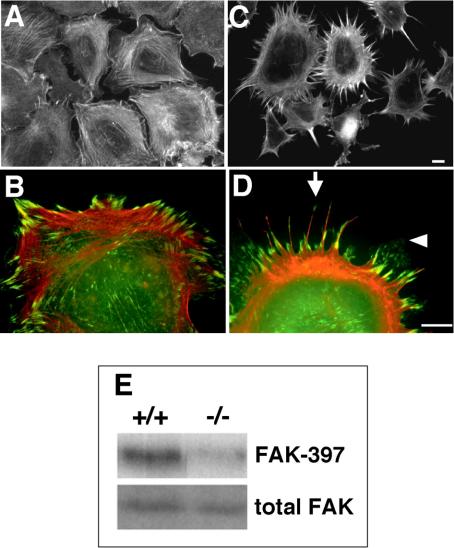
Dermal fibroblasts isolated from syndecan-4–deficient mice exhibited a distinctive morphology on a 3D fibrin-FN matrix (Figure 2C) that contrasted with the well-spread morphology and organized actin stress fibers of wild-type cells on this substrate (Figure 2A). Higher magnification of wild-type cells showed localization of vinculin to distinct focal adhesions at the ends of stress fibers on a fibrin-FN matrix (Figure 2B). However, syndecan-4–null cells, although spread, did not organize their actin into typical stress fibers. Instead these cells extended long actin-rich filopodia with vinculin localized at the base and tips (Figure 2, C and D, arrow). They also formed lamellae webbed between the filopodia that contained no detectable actin filaments but did contain punctate spots of vinculin (Figure 2D, arrowhead). Analysis of actin cytoskeleton organization over time shows that filopodia and lamellae are formed as early as 15 min and continue to extend up to 60 min (our unpublished results). On planar FN-coated substrates, both wild-type and syndecan-4–deficient fibroblasts became well spread and formed stress fibers and focal adhesions (our unpublished results; Ishiguro et al., 2000; Echtermeyer et al., 2001). In contrast, syndecan-4–null cells attached poorly and did not spread on 3D matrix composed of fibrin alone, similar to the behavior of NIH3T3 fibroblasts (Wenk et al., 2000), which indicates that the contribution of fibrin to the response is limited. Therefore, syndecan-4 plays an important role in the regulation of cell morphology, actin cytoskeleton organization, and focal adhesion formation in response to a 3D fibrin-FN matrix. Signaling was also compromised in cells lacking syndecan-4. Null cells showed a reduction in the phosphorylation of FAK compared with wild-type cells (Figure 2E), indicating that adhesive signals are reduced in the absence of syndecan-4.
Figure 2.
Syndecan-4–deficient fibroblasts have a distinct morphology on fibrin-FN matrix. Fibroblasts derived from wild-type mice (A and B) or syndecan-4–null mice (C and D) were allowed to spread on fibrin-FN matrices for 1 h before staining for actin (A and C) or for both actin (red) and vinculin (green) with an antivinculin mAb (B and D). Sites of colocalization are shown in yellow. Cells representative of the field are shown in B and D. The arrow indicates long actin-rich filopodia with vinculin localized at the base and tips and the arrowhead indicates vinculin-positive lamellae webbed between filopodia. Scale bars, 10 μm. (E) Wild-type (+/+) and syndecan-4–deficient (-/-) fibroblasts were allowed to adhere to fibrin-FN matrices for 1 h before lysis. Proteins were separated by SDS-PAGE, and immunoblots were probed with an anti-FAK mAb to detect total cellular FAK and a polyclonal antibody to detect FAK phosphorylated on tyrosine 397. Blots shown are representative of duplicate experiments.
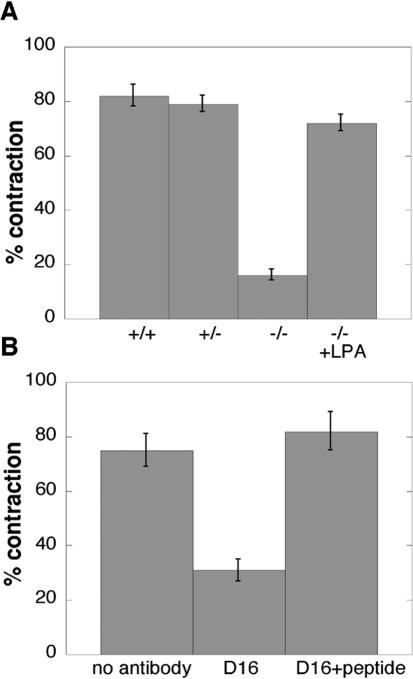
Syndecan-4–null cells were also defective in the contraction of a fibrin-FN matrix whereas wild-type and heterozygous cells contracted matrices to an extent comparable to NIH3T3 fibroblasts (Figure 3A). In further support of a syndecan-4 requirement, preincubation with D16 antisyndecan-4 monoclonal antibodies that have been shown to inhibit function (Kaneider et al., 2001, 2004) significantly reduced matrix contraction. However, antibodies treated with a blocking peptide had no effect on matrix contraction (Figure 3B). This function-blocking antibody also reduced the extent of cell spreading on fibrin-FN matrix (our unpublished results).
Figure 3.
Matrix contraction requires syndecan-4 signaling via RhoA. Fibrin-FN matrix contraction by fibroblasts isolated from wild-type (+/+), heterozygous (+/-), or syndecan-4–deficient mice (-/-) was measured with or without treatment with 5 μM lysophosphatidic acid (LPA) for 30 min before incorporation into the matrix (A). Fibrin-FN matrix contraction by NIH3T3 fibroblasts was measured with or without treatment with 50 μg/ml antisyndecan-4 antibody (D16) or antibody plus 50 μg/ml blocking peptide (D16+peptide) for 30 min before incorporation into the matrix (B). The data are expressed as the average of two experiments ± SEM.
Signaling through RhoA GTPase is required for maximal fibrin-FN matrix contraction (Midwood and Schwarzbauer, 2002) and expression of syndecan-4 contributes to the activation of RhoA (Wilcox-Adelman et al., 2002). We bypassed syndecan-4 and stimulated RhoA by treatment of syndecan-4–null cells with lysophosphatidic acid (LPA). This treatment rescued matrix contraction by these cells (Figure 3A). Therefore, fibrin-FN matrix contraction is mediated by syndecan-4 signaling through RhoA pathways.
Syndecan-4 Is Required for Tenascin-C Modulation of Cell Morphology and Contraction
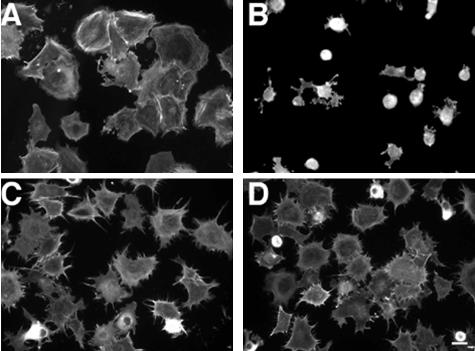
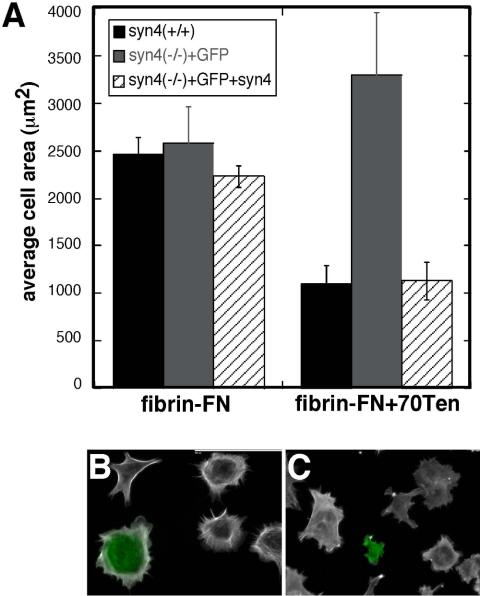
Our analyses of cell interactions with a fibrin-FN matrix have shown modulatory effects of both syndecan-4 and tenascin-C (Wenk et al., 2000; Midwood and Schwarzbauer, 2002). Indeed, the effect of compromising HSPG function mimics the effects of adding tenascin-C to the matrix, both morphologically and functionally. To determine whether tenascin-C is acting via a syndecan-4–dependent pathway, we analyzed the response of syndecan-4-null cells to fibrin-FN matrices containing 70Ten, a recombinant tenascin-C that we have previously shown to induce identical cell responses as native tenascin-C when incorporated into a fibrin-FN matrix (Wenk et al., 2000). Mouse dermal fibroblasts expressing syndecan-4 became well spread on fibrin-FN matrix (Figure 4A). With inclusion of 70Ten, these cells failed to spread (Figure 4B). In contrast, dermal fibroblasts isolated from syndecan-4–null mice were unaffected by the addition of tenascin-C to the matrix (Figure 4D) and showed a morphology that was comparable to these cells plated on a fibrin-FN matrix (Figure 4C). Syndecan-4–null cells transfected with a syndecan-4 expression construct showed a similar response to fibrin-FN + 70Ten matrix as wild-type cells. Measurements of cell areas showed that introduction of syndecan-4 caused a reduction in cell spreading (Figure 5A). Syndecan-4 transfectants, which also expressed a GFP cotransfection marker, were smaller in size and had fewer stress fibers than untransfected cells in the same population (Figure 5C). In contrast to the reduced size of wild-type fibroblasts in the presence of 70Ten, syndecan-4–null cells transfected with the GFP expression plasmid alone had cell areas that were not significantly different on fibrin-FN vs. fibrin-FN + 70Ten matrices (Figure 5, A and B). These results show that tenascin-C control of cell morphology is dependent on syndecan-4 expression.
Figure 4.
Syndecan-4–deficient cells are not susceptible to tenascin-C modulation of cell morphology. Fibroblasts derived from wild-type mice (A and B) or syndecan-4–null mice (C and D) were allowed to interact with fibrin-FN matrices with (B and D) or without (A and C) 70Ten for 1 h before staining for actin. Scale bar, 20 μm
Figure 5.
Transfection of syndecan-4 cDNA into syndecan-4–deficient cells restores tenascin-C modulation of cell morphology. (A) Wild-type mouse fibroblasts (black), syndecan-4–deficient fibroblasts transfected with a GFP plasmid (gray), or cotransfected with a GFP plasmid plus pcDNA3.1-syndecan-4 (hatched) were allowed to spread on fibrin-FN matrix with or without 70Ten for 1 h. Cells were stained with rhodamine-phalloidin and cell areas were measured using IPLab software. Eight random fields at 20× magnification were imaged per experiment and ∼20 green cells were measured per condition. Data are expressed as the average of two experiments. Fluorescence images are shown for syndecan-4–null cells transfected with pcDNA3-GFP alone (B) or with pcDNA3-GFP plus pcDNA3.1-syndecan-4 (C) plated on fibrin-FN + 70Ten matrix. Green cells are GFP-positive. Scale bar, 100 μm.
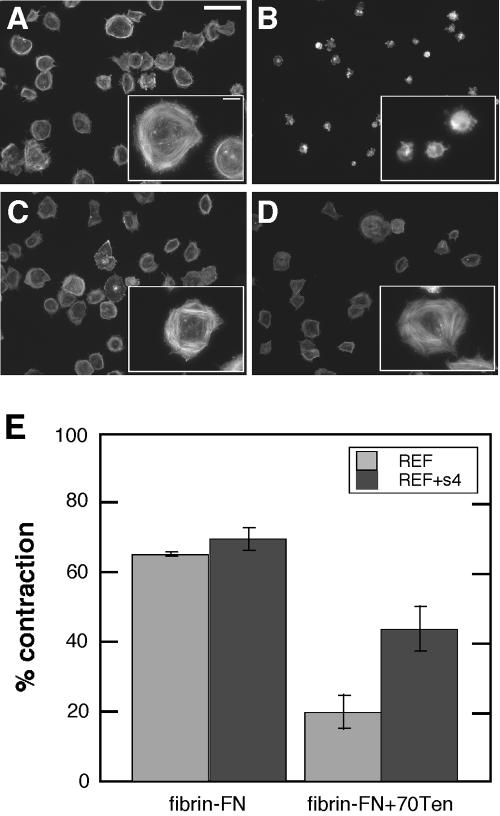
Overexpression of syndecan-4 also rescued changes in cell morphology and actin cytoskeleton organization caused by tenascin-C. Rat embryo fibroblasts (REFs) plated on fibrin-FN + 70Ten were poorly adherent and did not spread compared with REFs on fibrin-FN matrix (Figure 6, A and B). However, REFs overexpressing syndecan-4 did not respond to tenascin-C; they spread and formed stress fibers (Figure 6, C and D). Similarly, overexpression of syndecan-4 partially relieved the inhibition of contraction caused by tenascin-C. Both wild-type REFs and REFs overexpressing syndecan-4 contracted a fibrin-FN matrix equally well. Normal REF contraction was inhibited by the inclusion of 70Ten. However, cells overexpressing syndecan-4 showed a significant increase in contraction (Figure 6E) and had higher levels of active RhoA (our unpublished results). Thus overexpression of syndecan-4 can circumvent the inhibitory effects of tenascin-C.
Figure 6.
Overexpression of syndecan-4 bypasses the effects of tenascin-C. Wild-type REFs (A and B) or REFs overexpressing syndecan-4 (REF+s4) (C and D) were allowed to interact with fibrin-FN matrices with (B and D) or without (A and C) 70Ten for 1 h before staining for actin. Scale bar, 60 μm; inset, 10 μm. (E) Matrix contraction was measured for REF or REF+s4 cells in fibrin-FN or fibrin-FN + 70Ten matrix. Data are expressed as the average of two experiments.
Taken together these results show that tenascin-C acts by modulating syndecan-4 function and that syndecan-4 and tenascin-C act cooperatively to regulate cell morphology and matrix contraction during tissue repair.
DISCUSSION
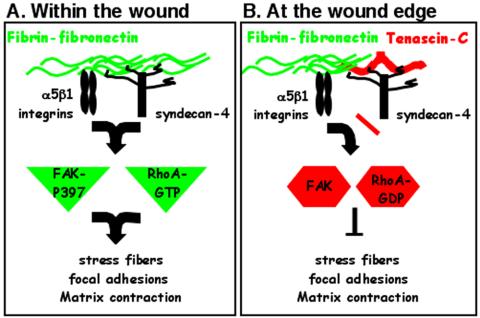
We have identified a novel mechanism for the coordinated regulation of cell behavior by multiple ECM components and their receptors during tissue repair. Syndecan-4, together with integrins, mediates cell response to matrix FN by initiating intracellular signaling pathways including the activation of RhoA and FAK (Figure 7A). ECM deposition of tenascin-C down-regulates these signals in a syndecan-4-dependent manner (Figure 7B). Thus, syndecan-4 acts as a regulatory receptor that monitors changes in ECM composition and organization, which it then communicates into the cell to coordinate appropriate responses including matrix contraction.
Figure 7.
Regulation of cell phenotype by tenascin-C and syndecan-4. Syndecan-4 and α5β1 integrins act in a concerted manner to initiate RhoA and FAK signaling pathways in response to a fibrin-FN provisional matrix (A). Tenascin-C modulates cell behavior by interfering with FN binding to the HS side chains of syndecan-4 and inhibiting downstream activation of RhoA and FAK (B). In this way distinct patterns of matrix proteins at different sites during tissue repair control the contribution of fibroblasts to wound healing. Cells in contact with fibrin-FN in the wound bed are able to induce matrix contraction (A), whereas cells at the edges of the injured tissue are not (B).
Studying cell responses to 3D matrices has highlighted differences in cell behavior depending on the organization of the ECM (Cukierman et al., 2001; Grinnell, 2003). On 3D fibrin-FN matrices, fibroblasts showed significantly reduced cell size and tenascin-C altered fibroblast morphology and signaling compared with cells on 2D FN-coated substrates (Corbett et al., 1996; Wenk et al., 2000). We show here that syndecan-4 activities are also affected by the dimensionality of the FN substrate. Syndecan-4-null cells formed stress fibers and focal adhesions on planar FN-coated surfaces but showed a distinctive cytoskeleton organization with actin-filled filopodia on a 3D fibrin-FN matrix. These results illustrate the effect of substrate presentation on cell response to ECM and suggest that there is some compensatory activity in these cells that takes over for syndecan-4 on 2D but not 3D substrates.
The response of syndecan-4–null cells to defined FN domains further supports a role for binding site presentation in regulating cell activities. On a surface coated with a mixture of cell- and heparin-binding (HepII) domains from FN, stress fibers terminating at focal adhesions were formed by these fibroblasts at levels comparable to intact FN (Ishiguro et al., 2000). However, addition of HepII fragment in soluble form to cells on the cell-binding domain induced very few focal adhesions. These cells clearly have a context-dependent requirement for the heparin-binding domain in order to assemble focal adhesions.
Whatever the context, fibroblast response to FN is initiated by coordinated action of several types of cell surface receptor. Integrin α5β1 binding to the FN cell-binding domain together with HS side chains of syndecan-4 interacting with heparin-binding sites on FN are essential for cells to spread and organize their cytoskeleton (Bloom et al., 1999; Saoncella et al., 1999; Woods et al., 2000; Echtermeyer et al., 2001). Of the multiple heparin domains in FN, syndecan-4 binds to the FNIII13 module in the HepII domain (Huang et al., 2001). This interaction contributes to the activation of RhoA and FAK (Wilcox-Adelman et al., 2002), two key signaling molecules in the formation of actin stress fibers and focal adhesions. Our results show that syndecan-4 is also a critical component of a matrix contraction pathway. Its primary role appears to be activation of RhoA because the over expression of syndecan-4 enhances RhoA activation and LPA-induced stimulation of RhoA circumvented the requirement for this receptor.
Similarities in the distribution and functional effects of tenascin-C and syndecan-4 at sites of injury suggest that these two proteins may coregulate cell activities during wound repair. Tenascin-C is highly expressed during tissue remodeling and repair (Jones and Jones, 2000; Chiquet-Ehrismann and Chiquet, 2003). Deposition of tenascin-C is markedly increased at the wound edges, particularly at the dermal-epidermal junction (Mackie et al., 1988). Its modulatory effects on cell adhesion suggest a role in promoting epidermal cell migration and proliferation. Syndecan-4 expression is also increased in response to tissue injury. In fact, as with tenascin-C, syndecan-4 expression is induced in the dermis adjacent to the injury where it has been shown to stimulate keratinocyte proliferation and migration during wound reepithelialization (Gallo et al., 1996; Echtermeyer et al., 2001). Also of significance to wound repair are the observations that syndecan-4–null mice and tenascin-C–null mice are both defective in resolving tissue damage in models of nephropathy (Nakao et al., 1998; Ishiguro et al., 2001).
Our results show that tenascin-C acts in a syndecan-4–dependent manner to suppress FN-mediated signaling via RhoA and FAK. In the absence of these signals, cells are unable to carry out matrix contraction. Tenascin-C binds to the heparin-binding FNIII13 module in the HepII domain of FN allowing it to compete with HSPGs such as syndecan-4 for binding to this site (Huang et al., 2001). In our experiments with fibroblasts, addition of soluble heparin or inclusion of tenascin-C in the fibrin-FN matrix blocked stress fiber formation indicating similar effects of these molecules on FN-syndecan-4 interactions. By masking FN-syndecan-4 interactions in this way, tenascin-C down-regulates syndecan-4–mediated signals required for matrix contraction (shown here) as well as for FN matrix assembly (K. Midwood, unpublished observations).
Like FN, tenascin-C can interact directly with the HS side chains of syndecans (Salmivirta et al., 1991) so, in addition to acting as a block to FN-HS interactions, tenascin-C can directly compete with FN for binding to HSPGs. In addition to its role in matrix contraction, tenascin-C has also been shown to act through syndecan-4 to affect cell cycle progression and proliferative capacities of normal fibroblasts and tumor cells (Huang et al., 2001; Orend et al., 2003). As such, tenascin-C may activate signaling pathways distinct from FN downstream of syndecan-4 while suppressing FN-mediated activation of RhoA and FAK.
How do these interactions regulate cell activities during the repair process? Differential patterns of expression of FN, tenascin-C, and syndecan-4 provide a mechanism for regulating cell phenotype throughout the injury (Midwood et al., 2004). At the center of the wound, the fibrin-FN provisional matrix replaces lost and damaged tissue. Fibroblasts move into this area and our previous results show that they use α5β1 integrin to attach and spread on a fibrin-FN matrix (Wenk et al., 2000). Fibroblasts also express syndecan-4, which facilitates intracellular communication from this matrix and promotes focal adhesion formation, cytoskeleton organization, and initiation of relevant signaling pathways (Figure 7A). These processes are required for deposition of new FN matrix to replace the provisional matrix and for matrix contraction to close the wound. Thus the combination of integrins and syndecan-4 binding to FN generates adhesive signaling required for replacement of damaged tissue. Furthermore, our results may help to explain one of the defects observed in the syndecan-4–null mouse, which exhibits delayed healing of dermal wounds due to insufficient matrix deposition and a failure of wound closure (Echtermeyer et al., 2001).
Tenascin-C expression is induced at the edges of the wound where it contacts the fibrin-FN provisional matrix (Mackie et al., 1988). The presence of tenascin-C in this area of the wound would serve to inhibit FN-initiated signaling events through blockade of syndecan-4 binding. The outcome would be to prevent inappropriate matrix deposition and premature contraction (Figure 7B). As such, syndecan-4 and tenascin-C coregulate the transmission of signals from a dynamic and complex extracellular environment to provide appropriate cell behaviors and efficient wound healing.
Acknowledgments
We thank Dr. Jeffrey Esko for providing CHO mutant cell lines, Dr. Paul Goetinck for syndecan-4–null cells and pcDNA3.1-syndecan-4, and Dr. Gertraud Orend for REFs over expressing syndecan-4. This work was supported by a grant from the National Institutes of Health (CA44627).
Abbreviations used: CHO, Chinese hamster ovary; CSPG, chondroitin sulfate proteoglycan; ECM, extracellular matrix; FAK, focal adhesion kinase; FN, fibronectin; GAG, glycosaminoglycans; HSPG, heparan sulfate proteoglycan; REF, rat embryo fibroblasts.
References
- Bai, X., and Esko, J.D. (1996). An animal cell mutant defective in heparan sulfate hexuronic acid 2-_O_-sulfation. J. Biol. Chem. 271, 17711-17717. [DOI] [PubMed] [Google Scholar]
- Bame, K.J., and Esko, J.D. (1989). Undersulfated heparan sulfate in a Chinese hamster ovary cell mutant defective in heparan sulfate _N_-sulfotransferase. J. Biol. Chem. 264, 8059-8065. [PubMed] [Google Scholar]
- Barkalow, F.J., and Schwarzbauer, J.E. (1991). Localization of the major heparin-binding site in fibronectin. J. Biol. Chem. 266, 7812-7818. [PubMed] [Google Scholar]
- Bass, M.D., and Humphries, M.J. (2002). Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling. Biochem. J. 368, 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, L., Ingham, K.C., and Hynes, R.O. (1999). Fibronectin regulates assembly of actin filaments and focal contacts in cultured cells via the heparin-binding site in repeat III13. Mol. Biol. Cell 10, 1521-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein, P. (2001). Thrombospondins as matricellular modulators of cell function. J. Clin. Invest. 107, 929-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann, R., and Chiquet, M. (2003). Tenascins: regulation and putative functions during pathological stress. J. Pathol. 200, 488-499. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann, R., Kalla, P., Pearson, C.A., Beck, K., and Chiquet, M. (1988). Tenascin interferes with fibronectin action. Cell 53, 383-390. [DOI] [PubMed] [Google Scholar]
- Clark, R.A.F., ed. (1996). The Molecular and Cellular Biology of Wound Repair, New York: Plenum Press.
- Corbett, S.A., and Schwarzbauer, J.E. (1999). Requirements for alpha(5)beta(1) integrin-mediated retraction of fibronectin-fibrin matrices. J. Biol. Chem. 274, 20943-20948. [DOI] [PubMed] [Google Scholar]
- Corbett, S.A., Wilson, C.L., and Schwarzbauer, J.E. (1996). Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood 88, 158-166. [PubMed] [Google Scholar]
- Cukierman, E., Pankov, R., Stevens, D.R., and Yamada, K.M. (2001). Taking cell-matrix adhesions to the third dimension. Science 294, 1708-1712. [DOI] [PubMed] [Google Scholar]
- Echtermeyer, F., Streit, M., Wilcox-Adelman, S., Saoncella, S., Denhez, F., Detmar, M., and Goetinck, P. (2001). Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Invest. 107, R9-R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko, J.D. (1991). Genetic analysis of proteoglycan structure, function and metabolism. Curr. Opin. Cell Biol. 3, 805-816. [DOI] [PubMed] [Google Scholar]
- Esko, J.D., Stewart, T.E., and Taylor, W.H. (1985). Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82, 3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, R., Kim, C., Kokenyesi, R., Adzick, N.S., and Bernfield, M. (1996). Syndecans-1 and -4 are induced during wound repair of neonatal but not fetal skin. J. Invest. Dermatol. 107, 676-683. [DOI] [PubMed] [Google Scholar]
- Grinnell, F. (2003). Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 13, 264-269. [DOI] [PubMed] [Google Scholar]
- Huang, W., Chiquet-Ehrismann, R., Moyano, J.V., Garcia-Pardo, A., and Orend, G. (2001). Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 61, 8586-8594. [PubMed] [Google Scholar]
- Hynes, R.O. (1990). Fibronectins, New York: Springer-Verlag.
- Ishiguro, K., Kadomatsu, K., Kojima, T., Muramatsu, H., Matsuo, S., Kusugami, K., Saito, H., and Muramatsu, T. (2001). Syndecan-4 deficiency increases susceptibility to kappa-carrageenan-induced renal damage. Lab. Invest. 81, 509-516. [DOI] [PubMed] [Google Scholar]
- Ishiguro, K., Kadomatsu, K., Kojima, T., Muramatsu, H., Tsuzuki, S., Nakamura, E., Kusugami, K., Saito, H., and Muramatsu, T. (2000). Syndecan-4 deficiency impairs focal adhesion formation only under restricted conditions. J. Biol. Chem. 275, 5249-5252. [DOI] [PubMed] [Google Scholar]
- Jones, P.L., and Jones, F.S. (2000). Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol. 19, 581-596. [DOI] [PubMed] [Google Scholar]
- Kaneider, N.C., Dunzendorfer, S., and Wiedermann, C.J. (2004). Heparan sulfate proteoglycans are involved in opiate receptor-mediated cell migration. Biochemistry 43, 237-244. [DOI] [PubMed] [Google Scholar]
- Kaneider, N.C., Egger, P., Dunzendorfer, S., and Wiedermann, C.J. (2001). Syndecan-4 as antithrombin receptor of human neutrophils. Biochem. Biophys. Res. Commun. 287, 42-46. [DOI] [PubMed] [Google Scholar]
- Kusano, Y., Oguri, K., Nagayasu, Y., Munesue, S., Ishihara, M., Saiki, I., Yonekura, H., Yamamoto, H., and Okayama, M. (2000). Participation of syndecan 2 in the induction of stress fiber formation in cooperation with integrin alpha5beta1, structural characteristics of heparan sulfate chains with avidity to COOH-terminal heparin-binding domain of fibronectin. Exp. Cell Res. 256, 434-444. [DOI] [PubMed] [Google Scholar]
- Lander, A.D. (1998). Proteoglycans: master regulators of molecular encounter? Matrix Biol. 17, 465-472. [DOI] [PubMed] [Google Scholar]
- Lukashev, M.E., and Werb, Z. (1998). ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 8, 437-441. [DOI] [PubMed] [Google Scholar]
- Mackie, E.J., Halfter, W., and Liverani, D. (1988). Induction of tenascin in healing wounds. J. Cell Biol. 107, 2757-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood, K.S., and Schwarzbauer, J.E. (2002). Tenascin-C modulates matrix contraction via focal adhesion kinase- and Rho-mediated signaling pathways. Mol. Biol. Cell 13, 3601-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood, K.S., Valenick Williams, L., and Schwarzbauer, J.E. (2004). Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 36, 1031-1037. [DOI] [PubMed] [Google Scholar]
- Midwood, K.S., Wierzbicka-Patynowski, I., and Schwarzbauer, J.E. (2002). Preparation and analysis of synthetic multicomponent extracellular matrix. Methods Cell Biol. 69, 145-161. [DOI] [PubMed] [Google Scholar]
- Mostafavi-Pour, Z., Askari, J.A., Whittard, J.D., and Humphries, M.J. (2001). Identification of a novel heparin-binding site in the alternatively spliced IIICS region of fibronectin: roles of integrins and proteoglycans in cell adhesion to fibronectin splice variants. Matrix Biol. 20, 63-73. [DOI] [PubMed] [Google Scholar]
- Moyano, J.V., Carnemolla, B., Albar, J.P., Leprini, A., Gaggero, B., Zardi, L., and Garcia-Pardo, A. (1999). Cooperative role for activated alpha4 beta1 integrin and chondroitin sulfate proteoglycans in cell adhesion to the heparin III domain of fibronectin. Identification of a novel heparin and cell binding sequence in repeat III5. J. Biol. Chem. 274, 135-142. [DOI] [PubMed] [Google Scholar]
- Nakao, N., Hiraiwa, N., Yoshiki, A., Ike, F., and Kusakabe, M. (1998). Tenascin-C promotes healing of Habu-snake venom-induced glomerulonephritis. Am. J. Pathol. 152, 1237-1245. [PMC free article] [PubMed] [Google Scholar]
- Orend, G., Huang, W., Olayioye, M.A., Hynes, N.E., and Chiquet-Ehrismann, R. (2003). Tenascin-C blocks cell-cycle progression of anchorage-dependent fibroblasts on fibronectin through inhibition of syndecan-4. Oncogene 22, 3917-3926. [DOI] [PubMed] [Google Scholar]
- Salmivirta, M., Elenius, K., Vainio, S., Hofer, U., Chiquet-Ehrismann, R., Thesleff, I., and Jalkanen, M. (1991). Syndecan from embryonic tooth mesenchyme binds tenascin. J. Biol. Chem. 266, 7733-7739. [PubMed] [Google Scholar]
- Saoncella, S., Echtermeyer, F., Denhez, F., Nowlen, J.K., Mosher, D.F., Robinson, S.D., Hynes, R.O., and Goetinck, P.F. (1999). Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl. Acad. Sci. USA 96, 2805-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk, M.B., Midwood, K.S., and Schwarzbauer, J.E. (2000). Tenascin-C suppresses Rho activation. J. Cell Biol. 150, 913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski, I., and Schwarzbauer, J.E. (2002). Regulatory role for SRC and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J. Biol. Chem. 277, 19703-19708. [DOI] [PubMed] [Google Scholar]
- Wilcox-Adelman, S.A., Denhez, F., and Goetinck, P.F. (2002). Syndecan-4 modulates focal adhesion kinase phosphorylation. J. Biol. Chem. 277, 32970-32977. [DOI] [PubMed] [Google Scholar]
- Wilson, C.L., and Schwarzbauer, J.E. (1992). The alternatively spliced V region contributes to the differential incorporation of plasma and cellular fibronectins into fibrin clots. J. Cell Biol. 119, 923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A., and Couchman, J.R. (2000). Integrin modulation by lateral association. J. Biol. Chem. 275, 24233-24236. [DOI] [PubMed] [Google Scholar]
- Woods, A., Longley, R.L., Tumova, S., and Couchman, J.R. (2000). Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch. Biochem. Biophys. 374, 66-72. [DOI] [PubMed] [Google Scholar]
- Zhang, L., and Esko, J.D. (1995). Accumulation of a pentasaccharide terminating in alpha-_N_-acetylglucosamine in an animal cell mutant defective in heparan sulfate biosynthesis. J. Biol. Chem. 270, 12557-12562. [DOI] [PubMed] [Google Scholar]