Genetic Regulation of Adipose Gene Expression and Cardio-Metabolic Traits (original) (raw)
Abstract
Subcutaneous adipose tissue stores excess lipids and maintains energy balance. We performed expression quantitative trait locus (eQTL) analyses by using abdominal subcutaneous adipose tissue of 770 extensively phenotyped participants of the METSIM study. We identified _cis-_eQTLs for 12,400 genes at a 1% false-discovery rate. Among an approximately 680 known genome-wide association study (GWAS) loci for cardio-metabolic traits, we identified 140 coincident _cis_-eQTLs at 109 GWAS loci, including 93 eQTLs not previously described. At 49 of these 140 eQTLs, gene expression was nominally associated (p < 0.05) with levels of the GWAS trait. The size of our dataset enabled identification of five loci associated (p < 5 × 10−8) with at least five genes located >5 Mb away. These _trans_-eQTL signals confirmed and extended the previously reported _KLF14_-mediated network to 55 target genes, validated the CIITA regulation of class II MHC genes, and identified ZNF800 as a candidate master regulator. Finally, we observed similar expression-clinical trait correlations of genes associated with GWAS loci in both humans and a panel of genetically diverse mice. These results provide candidate genes for further investigation of their potential roles in adipose biology and in regulating cardio-metabolic traits.
Introduction
Genome-wide association studies (GWASs) have identified many loci for complex metabolic and cardiovascular traits, yet the underlying genes and mechanisms by which they affect disease remain poorly characterized.1, 2 The genetic analysis of gene expression by identification of expression quantitative trait loci (eQTLs) in relevant tissues has proven useful to predict candidate genes at GWAS loci and biological pathways that are perturbed in affected individuals.3, 4, 5, 6 Subcutaneous adipose tissue serves as a buffering system for lipid energy balance, particularly fatty acids,7, 8 and might play a protective role in metabolic and cardiovascular disease risk.9
Subcutaneous adipose eQTL studies have implicated genes involved in obesity and metabolic traits.10, 11, 12, 13 Recent GWASs for type 2 diabetes (T2D), cholesterol and triglyceride levels, body mass index, waist-hip ratio, and adiponectin have reported subcutaneous adipose eQTLs that are coincident with specific GWAS loci.14, 15, 16, 17 Similarly, a recent large GWAS for waist-hip ratio identified loci that were enriched for genes expressed in subcutaneous adipose tissue and for putative regulatory elements in adipocyte nuclei.16 Many GWAS loci for these traits do not yet have clear candidate genes, in part because of the limited statistical power of existing eQTL studies.
_trans_-eQTL associations between variants and transcripts located far from each other or on different chromosomes can identify downstream disease genes, including those not implicated by GWASs. Identifying these distant relationships is difficult because of the multiple testing burden in humans. Studies of natural variation in mice have identified a number of “hotspot” loci associated with trans regulation of genes and clinical traits.18, 19 One of the first reported human _trans_-acting eQTLs involved the KLF14 transcription factor in adipose tissue.20 The locus associated with T2D and HDL-cholesterol levels showed a _cis_-acting association with expression of KLF14 and ten distal genes.20 Studies of gene expression in circulating monocytes or whole-blood cells have also provided evidence of trans regulation of gene expression with linkage to traits relevant to lipid metabolism, type 1 diabetes, hypertension, celiac disease, and cancer.21, 22
In light of the widespread use of mice to help validate and gain mechanistic understanding of genes in GWAS loci, commonalities and differences in the regulatory networks of mice and humans are of clear relevance for studies of common diseases.6 A previous comparison between mouse and human adipose transcriptional networks detected a shared core-network module enriched for genes involved in the inflammatory and immune response causally associated with obesity-related traits.10 Recent analysis of DNase I hypersensitive sites and occupancy profiles of transcription-factor binding in humans and mice suggests the preservation of similar regulatory mechanisms for adipose gene expression in both species, and this preservation could be leveraged to inform disease pathways.23, 24
We describe here the analysis of gene expression in 770 subcutaneous adipose samples from Metabolic Syndrome in Men (METSIM), a study of 10,197 men, 45–73 years of age, living in the Kuopio area of Finland. Study data include dense genotypes and extensive metabolic and cardiovascular traits, such as plasma lipids, inflammatory markers, glycemic traits, and anthropometric traits.25 We identified _cis_-eQTLs at GWAS loci for cardio-metabolic traits, as well as _trans_-eQTL hotspots with at least five distant target genes. We also identified associations of gene expression with human clinical traits and compared our results with those of analogous studies in a set of 120 inbred strains of mice.
Subjects and Methods
METSIM Study Participants and Sample Characteristics
We analyzed samples from 770 males who are part of the METSIM study.25 The Ethics Committee of the Northern Savo Hospital District approved this study, and all participants gave written informed consent. The population-based METSIM study included 10,197 men, aged 45–73 years and randomly selected from the population register of Kuopio town in eastern Finland (population 95,000). Every participant had a 1 day outpatient visit to the Clinical Research Unit at the University of Kuopio, including an interview on the history of previous diseases and current drug treatment and an evaluation of glucose tolerance and cardiovascular risk factors. After 12 hr of fasting, a 2 hr oral 75 g glucose tolerance test was performed, and the blood samples were drawn at 0, 30, and 120 min. Plasma glucose was measured by enzymatic hexokinase photometric assay (Konelab Systems reagents; Thermo Fischer Scientific), and insulin and pro-insulin were determined by immunoassay (ADVIA Centaur Insulin IRI no. 02230141; Siemens Medical Solutions Diagnostics). Evaluation of insulin sensitivity (Matsuda index) and insulin secretion (calculated from area under the curve between 0 and 30 min of glucose tolerance test with the formula InsAUC0–30/GlucAUC0–30) have been previously described.25, 26 Plasma levels of lipids were determined via enzymatic colorimetric methods (Konelab System reagents, Thermo Fisher Scientific). Plasma adiponectin was measured with the Human Adiponectin Elisa Kit (Linco Research), C-reactive protein with high sensitive assay (Roche Diagnostics GmbH, Mannheim, Germany), and interleukin 1 receptor agonist with immunoassay (ELISA, Quantikine DRA00 Human IL-1RA, R&D Systems). Serum creatinine was measured by the Jaffe kinetic method (Konelab System reagents, Thermo Fisher Scientific) and was used for calculating the glomerular filtration rate. Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. Waist circumference (at the midpoint between the lateral iliac crest and lowest rib) and hip circumference (at the level of the trochanter major) were measured to the nearest 0.5 cm. Body composition was determined by bioelectrical impedance (RJL Systems) in participants in the supine position. The characteristics of the study participants are shown in Table S1. 770 participants were recruited for adipose-tissue needle biopsies. 61 participants were diagnosed with impaired glucose tolerance, and 27 participants had newly diagnosed type 2 diabetes at the time of the tissue collection.
Genotyping and Imputation
Genotyping of METSIM samples was performed with the Illumina HumanOmniExpress BeadChip array and the Illumina HumanCoreExome at the Center for Inherited Disease Research. Markers with poor mapping, no founder genotypes, call rate < 95%, deviation from Hardy-Weinberg equilibrium (p < 10−6), or more than two alleles were removed from subsequent imputation. We carried out genotype imputation of the 681,789 directly genotyped variants that passed quality control by applying the Markov Chain Haplotyping algorithm (MaCH) and the reference panel from the Haplotype Reference Consortium (see Web Resources). After imputation, variants were filtered on the basis of imputation quality (MaCH _r_ _2_ > 0.3) and minor-allele frequency (MAF ≥ 0.01). 7,677,146 variants were retained for subsequent analysis.
Gene Expression Profiling
Total RNA from METSIM participants was isolated from adipose tissue via the QIAGEN miRNeasy kit, according to the manufacturer’s instructions. RNA integrity numbers (RINs) were assessed with the Agilent Bioanalyzer 2100 instrument, and 770 samples with RIN > 7.0 were used for transcriptional profiling. Expression profiling with the Affymetrix U219 microarray was performed at the Department of Applied Genomics at Bristol-Myers Squibb according to the manufacturer’s protocols. The probe sequences were re-annotated so that probes that mapped to multiple locations, contained variants with MAF > 0.01 in the 1000 Genomes Project European samples, or did not map to known transcripts on the basis of the RefSeq (version 59) and Ensembl (version 72) databases were removed; 6,199 probe sets were removed in this filtering step. For subsequent analyses, we used 43,145 probe sets that represent 18,155 unique genes. The microarray image data were processed with the Affymetrix GCOS algorithm via the robust multiarray average (RMA) method for determination of the specific hybridizing signal for each gene.
PEER Factor Analysis
We applied the probabilistic estimation of expression residuals (PEER) method27 to infer and account for complex non-genetic factors affecting gene expression levels. This method is designed to detect the maximum number of _cis_-eQTLs. To optimize the discovery of _trans_-eQTLs within the same analysis, we performed PEER analysis by examining 10–50 inferred factors (Nk) at increments of five factors. We then used Matrix eQTL28 to assess the genetic association with inverse normal-transformed PEER-processed residuals from RMA-normalized expression data. The numbers of _cis_- and _trans_-eQTLs obtained at different Nk levels are shown in Table S2. We examined variants on chromosome 7, including rs4731702 at the known master regulator KLF14,20 to determine the number of _trans_-eQTL target genes by using various numbers of PEER factors. We selected Nk = 35 as a single analysis to maximize the number of target genes at this locus; this threshold captured the 94.8% of _cis-_eQTLs identified with 50 PEER factors. For downstream eQTL mapping, we used the inverse normal-transformed PEER-processed residuals after accounting for 35 factors.
eQTL Mapping
We performed eQTL mapping in 770 MESTIM individuals by using both FaST-LMM29 and EPACTS and implementing a linear mixed model to account for the population structure among the samples.30 Genotype dosages from all autosomal chromosomes and expression data that had passed the aforementioned quality-control measures were used. For FaST-LMM implementation, to improve power31 when testing all the variants on chromosome N for association, we constructed the kinship matrix by using the variants from all other chromosomes besides N. This procedure allowed us to include the variant being tested for association in the regression equation only once. Results obtained with FaST-LMM and EPACTS were similar. Results obtained from EPACTS analysis were used in the identification of coincident eQTL and GWAS signals. Results from the FaST-LMM analysis are available on our website.
eQTLs were defined as cis (local) if the peak association was within 1 Mb on either side of the exon boundaries of the gene or as trans (distal) if the peak association was at least 5 Mb outside of the exon boundaries. We used all association p values in the cis region to estimate the false-discovery rate (FDR-qvalue) by using the qvalue package in R. Variants with association p values < 2.46 × 10−4 corresponding to 1% FDR were considered significant. Considering the large number of analyses we performed to calculate _trans_-eQTL associations, we used the conservative Bonferroni-corrected p < 1.51 × 10−13 (0.05/[7.67 million variants × 43,145 probe sets]) to identify _trans_-eQTLs. To detect possible _trans_-eQTL hotspots, we report variants that are associated with at least five genes at a more liberal threshold of p < 5 × 10−8. These hotspots were visualized with Circos-0.66.32 LocusZoom was used for the regional visualization of eQTL results on the basis of linkage disequilibrium (LD) ascertained from the 770 METSIM samples.33
Heritability Calculations
Heritability was estimated via a linear mixed model with the GCTA software.34, 35 In this approach, gene expression phenotypes are assumed to be generated by genetic and environmental components. The assumption behind the linear-mixed-model approach is that the covariance of the genetic component of the phenotypic data is proportional to the kinship or genetic similarity matrix between the individuals. The analysis provides estimates of σ2u and σ2e, the variances corresponding to the genetic and environmental components, respectively. The heritability is then the fraction of the variance accounted for by the genetics
where var(u) and var(e) are, respectively, the genetic and residual variance components estimated by the restricted-maximum-likelihood approach using related individuals, and is computed for each probe set. A standardized kinship matrix, which has a mean of 1 along the diagonal, is used for these estimates so that they are consistent with the classical definition of heritability.36
Evaluation of eQTLs in Another Adipose eQTL Cohort
eQTLs identified in METSIM and MuTHER cohorts37 were compared in each study reciprocally. Of the ∼5 million significant variant-probe pairs identified in the METSIM study for cis associations (Table S3), 453,484 had an exact match for the variant in a corresponding variant-probe pair in the MuTHER study. A majority of the genes are represented by multiple probes on the Affymetrix microarray used in the METSIM study and the Illumina microarray used in the MuTHER study. Therefore, we required at least 50% of the variant-probe pairs for a given gene to have the same direction of effect in both studies at various p value thresholds (p < 5 × 10−2 to 5 × 10−6) in the MuTHER study. Of the 11,053 significant variant-probe pairs identified in the METSIM study for trans associations (Table S3), 2,312 had an exact match for the variant in a corresponding variant-probe pair in the MuTHER study. We report the METSIM-detected _trans_-eQTLs for which the exact variant-gene pair exists and shows the same direction of effect in MuTHER at several p value thresholds (p < 5 × 10−2 to 5 × 10−6) for which MuTHER data were available. _cis_- and _trans_-eQTLs detected in the MuTHER study were compared to those in the METSIM study according to the same criteria.
For the three _trans_-eQTL hotspots for which the peak variant or its LD proxy was available in the MuTHER dataset, we were able to find corresponding variant-probe pairs for 83 of the 106 genes. We report the variant-gene pairs with the same direction of effect size at p < 0.05 in the MuTHER study.
_cis_-eQTLs at GWAS Loci
We focused on the GWAS index variants reported to be associated at genome-wide significance (p < 5 × 10−8) with any of 91 cardio-metabolic diseases and related quantitative traits (Table S4). We downloaded these variants from the National Human Genome Research Institute–European Bioinformatics Institute GWAS Catalog (September 22, 2014) and included additional variants.16, 17 We estimated the number of loci for each trait group on the basis of the names of “reported gene(s)” and/or “mapped genes” shown in the catalog. Within the variants available in the METSIM eQTL data, we initially examined 115 variants at 68 loci for T2D, 114 variants at 68 loci for glycemic traits, 325 variants at 205 loci for obesity and related traits, 410 variants at 213 loci for lipid traits, 79 variants at 60 loci for metabolic syndrome, and 249 variants at 187 loci for other broadly defined cardiovascular risk traits. After removing duplicate variants present in at least one disease/trait group, we further examined the remaining 1,221 GWAS variants at approximately 680 loci for association with local gene expression levels.
For significant variant-transcript associations (FDR < 1%), if multiple probe sets mapped to the same transcript, we retained the probe sets for which the lead eSNP (the variant that exhibited the strongest evidence of association with the expression level of the corresponding transcript) had the largest pairwise LD _r_ _2_ estimate with the GWAS variant; LD was calculated from the 770 METSIM samples. If two or more highly correlated variants (LD _r_ _2_ > 0.8) were associated with the same transcript, we reported the variant with the largest LD r 2 with the lead eSNP. For two variants (pairwise LD r 2 = 0.79) associated with different GALNT2 probe sets, we kept the variant exhibiting higher LD r 2 with the GWAS index variant.
We further examined whether each eQTL was coincident with the GWAS signal. We defined GWAS-coincident eQTLs as loci with pairwise LD r 2 > 0.8 between the GWAS index variant and the lead eSNP. To evaluate association between the GWAS variant and the lead eQTL variant at each locus, we performed reciprocal conditional analyses; we tested association between the GWAS variant and transcript level when the lead eSNP was included in the model, and vice versa.
For 61 cardio-metabolic traits with publically available GWAS summary results14, 16, 17, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 (Table S5), we applied the summary-data-based Mendelian randomization (SMR) method to propose relevant genes at the GWAS loci.49 We performed a transcriptome-wide association for 61 cardio-metabolic traits and 24,383 probe sets with _cis_-eQTLs (<1 Mb) and focused on GWAS loci (p < 5 × 10−8). Probe sets with pSMR < 2.1 × 10−6 (0.05/24,383 probe sets) were then tested for pleiotropy via heterogeneity in dependent instruments (HEIDI). Probe sets with pHEIDI ≥ 0.05 were identified as potential gene targets of GWAS loci.
We also quantified the uncertainty in the inference that the _cis_-eQTL genes are causally mediating the association between the GWAS loci with cardio-metabolic traits by using the causal inference test (CIT).50 We used the GWAS index variants, expression level of the _cis_-eQTL genes, and the inverse normalized GWAS trait measured in METSIM to infer a causal relationship wherein for gene expression mediates the association between GWAS variant and trait when pCIT < 0.05.50
Association between Gene Expression Level and Phenotypic Traits
We conducted regression analyses to evaluate the association between gene expression and 23 cardio-metabolic-related traits in up to 770 METSIM individuals. The RMA-normalized expression levels were inverse normal transformed after age and BMI were accounted for. We used non-PEER-corrected expression levels for this analysis because correction for PEER factors can remove broadly acting phenotypic effects on gene expression. Eight of the 35 PEER factors had significant correlation with BMI (|r| = 0.10 to 0.50, p = 2.8 × 10−3 to 3.5 × 10−50). For genes coincident with GWAS loci, in addition to non-PEER-corrected associations, we calculated the association between PEER-corrected expression levels and traits. The 23 phenotypic traits examined included five obesity-related traits (BMI, waist-hip ratio, waist circumference, hip circumference, and fat-free mass), seven glycemic traits (glucose, insulin, proinsulin, HOMA-β, Hb1Ac, Matsuda index, and adiponectin), six lipid-related traits (total cholesterol, low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], triglycerides, total fatty acids, and free fatty acids), two inflammatory traits (C-reactive protein and interleukin 1 receptor antagonist), two blood-pressure traits (systolic and diastolic blood pressure, respectively), and one kidney-function trait (glomerular filtration rate). The phenotypic traits were adjusted for age and BMI before inverse normal transformation. When examining the association between gene expression and BMI, we applied no adjustment for BMI for either the phenotypic trait (BMI) or gene expression level. We used FDR < 1% (equivalent p < 6.5 × 10−4) to define a significant association between gene expression level and phenotypic-trait level. Genes were clustered via hierarchical clustering based on Euclidian distance as implemented in the heatmap2 function of the gplots package in R.
Mouse Expression and Phenotypic-Trait Data
The mouse studies using the Hybrid Mouse Diversity Panel (HMDP) have been described previously (GEO: GSE42890).51, 52 In brief, mice from 120 strains were obtained from The Jackson Laboratory and were bred at the University of California, Los Angeles. Mice were maintained on a chow diet (Ralston Purina Company) until 8 weeks of age, when they were either continued on a chow diet (n = 185 mice) or given a high-fat, high-sucrose diet (Research Diets-D12266B) for an additional 8 weeks (n = 227 mice). Body composition analysis was performed by nuclear magnetic resonance with a Bruker Minispec. Retro-orbital blood was collected under isoflurane anesthesia after mice fasted for 4 hr. Plasma lipids, insulin, glucose, and HOMA-IR were determined as previously described.52, 53 The animal protocol for the study was approved by the Institutional Care and Use Committee (IACUC) at the University of California, Los Angeles. Total RNA from flash-frozen epididymal adipose samples from 228 male mice was hybridized to Affymetrix HT_MG-430A arrays and scanned according to standard Affymetrix protocols. To reduce the chances of spurious association results, we performed RMA normalization after removing all individual probes with variants and all probe sets containing eight or more variant-containing probes, which resulted in 22,416 remaining probe sets. Correlations of non-PEER-corrected expression levels with phenotypic traits were calculated with the biweight midcorrelation, which is robust to outliers.54
Results
Genetic Variants Associated with Gene Expression in Subcutaneous Adipose Tissue
To identify genetic loci associated with transcript abundance in abdominal subcutaneous adipose tissue, we studied 770 extensively phenotyped men from the METSIM study. We analyzed ∼7.67 million variants and abundance of 43,145 probe sets corresponding to 18,155 unique genes. The mean narrow-sense heritability, _h_2, of the probe sets was 0.27, suggesting significant genetic effects on adipose gene expression (Figure S1).
eQTL mapping identified _cis-_eQTLs (<1 Mb) for 12,400 genes at a 1% FDR (p < 2.46 × 10−4) (Figure S2 and Table S3). The larger number of adipose eQTLs observed in comparison to that in previous adipose eQTL studies might be due to the larger sample size, denser imputation, and/or analysis of a larger number of transcripts; some differences might be due to different microarray platforms, statistical methodology, and p value thresholds (Table S6).10, 11, 37
30% of the _cis_-eQTLs discovered in METSIM at p < 5 × 10−6 showed consistent allelic direction of effect in the MuTHER study, and 79.1% of the _cis_-eQTLs reported in MuTHER at this threshold showed a consistent direction of effect in METSIM (Table S7).
Coincidence of _cis_-eQTLs and GWAS Loci
Hundreds of GWAS loci have been reported for cardio-metabolic diseases and related traits.55 Given the value of identifying candidate genes at GWAS loci and the observation that many eQTLs are shared across tissues,56 we investigated loci for a broad set of 91 cardio-metabolic diseases or traits (Table S4). Among the 1,221 GWAS signals (p < 5 × 10−8), we detected 944 initial unique GWAS variant and eQTL gene pairs (FDR < 1%, equivalent p < 2.4 × 10−4) (Table S8).
On the basis of pairwise LD r 2 > 0.8 between the GWAS variant and the variant that exhibited the strongest association with the gene expression level (lead eSNP), 140 (15%) of the 944 _cis-_eQTLs appear to be coincident with the GWAS signals. The coincidence is supported by reciprocal conditional analysis: after conditioning was performed on each lead eSNP, no GWAS variant remained significant (FDR < 1%), and after conditioning on each GWAS variant, 124 eSNP signals were no longer significant, and 16 were strongly attenuated (Δlog10(p) of 8.5 to 112 ) (Table S8). Of the 140 _cis-_eQTLs at GWAS loci, 93 (66.4%) were not previously reported by large-scale GWASs that interrogated available _cis_-eQTLs14, 15, 16, 17, 38, 45, 48, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 and 50 showed consistent direction of allelic effect at p < 0.05 in the MuTHER study. Table 1 shows 29 eQTLs for glycemic, obesity, and lipid traits at the LD threshold of _r_ _2_ > 0.9 between the GWAS variant and lead eSNP; three of these eQTLs were also identified with the SMR method. The full set of 944 adipose eQTLs at GWAS loci, evidence of their coincidence with the GWAS signals, and the corresponding p values in the MuTHER study are provided in Table S8.
Table 1.
Selected Adipose eQTLs Coincident with GWAS Signals for Cardiometabolic Risk
| GWAS Variant | GWAS Trait | GWAS Locus | eQTL Gene | GWAS Variant | Lead eSNP | LD r 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1/A2 | βinitial | pinitial | βcond | pcond | Lead eSNP | A1/A2 | βinitial | pinitial | βcond | pcond | |||||
| rs2013208 | HDL cholesterol | RBM5 | RBM6 | C/T | −0.987 | 4.4E−113 | 0.120 | 6.9E−01 | rs11130233 | G/T | −0.992 | 4.7E−116 | −1.099 | 2.2E−04 | 0.98 |
| rs12051272 | adiponectin | CDH13 | CDH13 | T/G | 1.370 | 4.3E−80 | 0.000 | 2.8E−01 | rs12051272 | T/G | 1.370 | 4.3E−80 | 0.000 | 2.8E−01 | 1.00 |
| rs6805251 | HDL cholesterol | GSK3B | GSK3B | C/T | −0.696 | 1.5E−43 | 0.067 | 7.4E−01 | rs334533 | C/T | −0.718 | 9.2E−47 | −0.786 | 1.3E−04 | 0.95 |
| rs8077889 | triglycerides | MPP3 | MPP3 | C/A | −0.696 | 9.2E−33 | −0.174 | 7.4E−01 | rs55768269 | T/C | −0.695 | 5.6E−33 | −0.569 | 2.8E−01 | 0.99 |
| rs4148008 | HDL cholesterol | ABCA8 | ABCA8 | G/C | −0.538 | 5.9E−26 | 0.415 | 2.1E−02 | rs1156340 | T/C | −0.598 | 1.8E−31 | −1.019 | 2.5E−08 | 0.92 |
| rs12489828 | waist-hip ratio | NT5DC2 | NT5DC2 | G/T | 0.503 | 4.0E−24 | 0.089 | 7.6E−01 | rs6778735 | T/C | 0.510 | 1.8E−24 | 0.398 | 1.9E−01 | 0.97 |
| rs138777 | total cholesterol | TOM1 | HMGXB4 | A/G | −0.495 | 1.7E−22 | 0.141 | 8.4E−01 | rs9306298 | T/C | −0.498 | 1.1E−22 | 0.636 | 3.7E−01 | 0.99 |
| rs2254287 | LDL cholesterol | B3GALT4 | HSD17B8 | G/C | −0.417 | 3.5E−17 | 0.000 | 2.1E−01 | rs2254287 | G/C | −0.417 | 3.5E−17 | 0.000 | 2.1E−01 | 1.00 |
| rs12679556 | waist-hip ratio | MSC | EYA1 | G/T | 0.463 | 1.1E−16 | −0.202 | 6.2E−01 | rs4738141 | G/A | 0.469 | 3.4E−17 | −0.677 | 9.7E−02 | 0.98 |
| rs12748152 | HDL cholesterol | NR0B2-PIGV | PIGV | T/C | −0.698 | 4.0E−14 | −0.054 | 8.5E−01 | rs6656815 | A/G | −0.732 | 2.7E−15 | −0.687 | 1.9E−02 | 0.90 |
| rs10919388 | waist-hip ratio | GORAB | PRRX1 | C/A | −0.448 | 4.5E−14 | −0.212 | 3.3E−01 | rs6427242 | G/C | −0.448 | 3.7E−14 | −0.245 | 2.5E−01 | 0.93 |
| rs8077889 | triglycerides | MPP3 | DUSP3 | C/A | 0.430 | 6.6E−13 | 0.000 | 5.6E−01 | rs2342310 | C/T | 0.430 | 6.6E−13 | 0.000 | 5.6E−01 | 1.00 |
| rs11136341 | LDL cholesterol | PLEC1 | PLEC | G/A | −0.358 | 9.5E−13 | 0.084 | 6.5E−01 | rs10107388 | C/T | −0.379 | 4.9E−14 | −0.455 | 1.5E−02 | 0.92 |
| rs2590838 | adiponectin | GNL3 | NEK4 | G/A | 0.346 | 8.4E−12 | −0.018 | 9.9E−01 | rs35212380 | C/G | 0.346 | 7.8E−12 | −0.415 | 6.7E−01 | 1.00 |
| rs439401 | triglycerides | APOE-TOMM40 | APOE | T/C | 0.384 | 2.1E−11 | 0.000 | 6.7E−01 | rs439401 | T/C | 0.384 | 2.1E−11 | 0.000 | 6.7E−01 | 1.00 |
| rs181362 | HDL cholesterol | UBE2L3 | YDJC | T/C | 0.327 | 7.6E−11 | −0.254 | 1.3E−01 | rs11089620 | G/C | 0.368 | 3.1E−13 | −0.621 | 2.3E−04 | 0.91 |
| rs7134375 | HDL cholesterol | PDE3A | PDE3A | C/A | 0.342 | 1.6E−10 | 0.000 | 3.5E−01 | rs7134375 | C/A | 0.342 | 1.6E−10 | 0.000 | 3.5E−01 | 1.00 |
| rs11869286 | HDL cholesterol | STARD3 | STARD3 | G/C | 0.333 | 1.1E−09 | 0.000 | 9.8E−01 | rs11869286 | G/C | 0.333 | 1.1E−09 | 0.000 | 9.8E−01 | 1.00 |
| rs9400239 | body mass index | FOXO3 | FOXO3 | C/T | 0.323 | 3.0E−09 | 0.008 | 9.7E−01 | rs3800228 | G/T | 0.337 | 5.9E−10 | 0.350 | 5.5E−02 | 0.92 |
| rs12748152 | HDL cholesterol | NR0B2-PIGV | ARID1A | T/C | −0.521 | 2.2E−08 | −0.091 | 7.8E−01 | rs34217609 | C/T | −0.515 | 1.1E−08 | −0.388 | 2.2E−01 | 0.92 |
| rs3812316 | triglycerides | MLXIPL | BCL7B | C/G | −0.411 | 6.5E−08 | −0.059 | 8.2E−01 | rs799166 | C/G | −0.431 | 2.1E−08 | −0.395 | 1.4E−01 | 0.92 |
| rs4846914 | HDL cholesterol | GALNT2 | GALNT2 | G/A | −0.267 | 4.1E−07 | 0.221 | 4.4E−01 | rs4631704 | C/T | −0.279 | 1.3E−07 | 0.503 | 8.4E−02 | 0.97 |
| rs12145743 | HDL cholesterol | PMVK-HDGF | RRNAD1 | T/G | −0.262 | 7.9E−07 | −0.115 | 8.1E−01 | rs3806415 | C/T | −0.263 | 7.8E−07 | −0.139 | 7.7E−01 | 0.99 |
| rs10501320 | proinsulin | MADD | ACP2 | G/C | 0.309 | 1.3E−06 | 0.024 | 9.7E−01 | rs11039149 | A/G | 0.310 | 1.2E−06 | 0.259 | 6.5E−01 | 0.99 |
| rs6784615 | waist-hip ratio | NISCH-STAB1 | NISCH | T/C | 0.649 | 1.6E−06 | 0.000 | 6.6E−01 | rs728408 | A/G | 0.649 | 1.5E−06 | 0.000 | 7.4E−01 | 1.00 |
| rs780094 | glucose, triglycerides | GCKR | EMILIN1 | T/C | 0.240 | 3.3E−06 | 0.000 | 7.2E−01 | rs780094 | T/C | 0.240 | 3.3E−06 | 0.000 | 7.2E−01 | 1.00 |
| rs10761731 | triglycerides | JMJD1C | NRBF2 | A/T | −0.231 | 5.8E−06 | 0.000 | 5.8E−02 | rs10761739 | G/C | −0.233 | 5.2E−06 | 0.000 | 4.3E−02 | 1.00 |
| rs1532624 | HDL cholesterol | CETP | CETP | C/A | 0.237 | 5.8E−06 | −0.549 | 2.1E−01 | rs4784741 | C/T | 0.246 | 2.4E−06 | −0.777 | 7.4E−02 | 0.99 |
| rs4311394 | adiponectin | ARL15 | FST | A/G | 0.287 | 6.2E−06 | −0.707 | 3.1E−01 | rs59061738 | A/G | 0.292 | 3.7E−06 | −1.011 | 1.5E−01 | 0.99 |
To consider potential pleiotropic effects of the GWAS variants on gene expression and cardio-metabolic traits, we performed two additional tests. We applied the SMR Mendelian randomization method to loci for 61 traits and identified 46 genes (pSMR < 2.1 × 10−6; pHEIDI ≥ 0.05_)_ (Table S9), 19 of which were identified via LD (_r_ _2_ > 0.8) and conditional analyses. The SMR analysis also detected nine genes (C18orf8, CABLES1, CADM1, CDK6, CENPW, LMOD1, MFAP2, MT1M, and STARD10) that were not identified via the conditional analysis approach.
We applied a causal-inference test50 and identified 15 variant-transcript pairs for seven traits that showed evidence of causal mediation (pCIT < 0.05) (Table S10 and Figure S3). None of the target genes identified by the causal-inference test overlap with the genes identified by the SMR analysis.
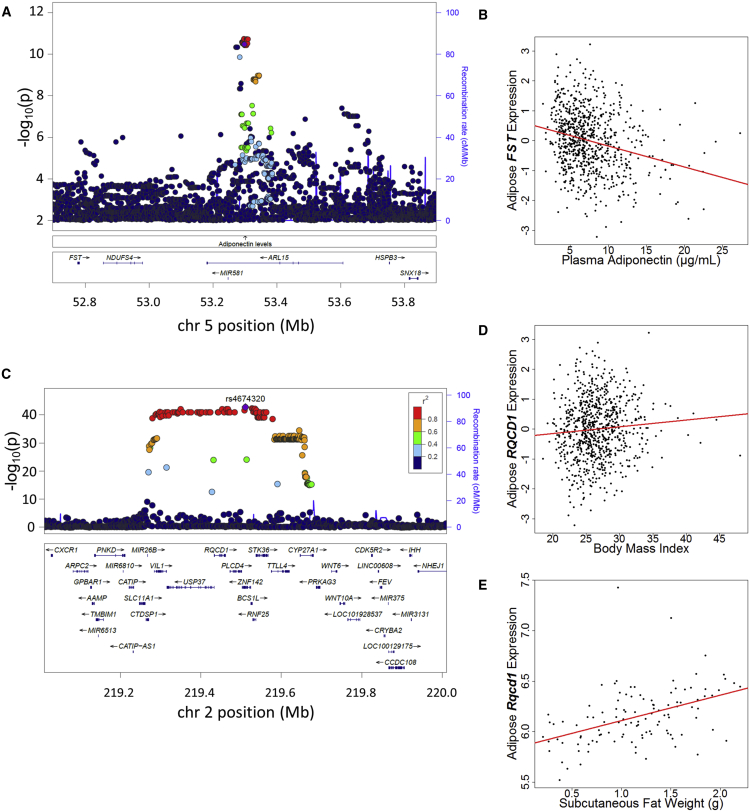
The coincident GWAS and eQTL signals suggest candidate genes that might influence cardio-metabolic risk. For example, at the ARL15 locus associated with adiponectin and HDL-C14, 38, the GWAS index variant rs6450176 was associated with expression of FST (p = 3.3 × 10−9), located ∼500 kb away, and exhibited strong LD (r 2 = 0.99) with the lead eSNP rs59061738, associated with FST expression (p = 1.8 × 10−9, Figure 1A and Table 1). Reciprocal conditional analyses provided additional evidence that this eQTL was coincident with the GWAS signal (pcond = 0.43 for rs6450176 and 0.17 for rs59061738). FST expression levels were negatively associated with HDL-C (p = 1.2 × 10−3) and adiponectin (p = 6.0 × 10−6, Figure 1B). Encoded by FST, the protein follistatin has been shown to promote adipocyte differentiation and reduce fat mass and insulin resistance.70, 71 The same GWAS variant showed no eQTL for the nearest gene, ARL15 (p = 0.43), and the expression level of ARL15 was not associated with HDL-C (p = 0.85) or adiponectin (p = 0.084). These data suggest that the associations with the levels of adiponectin and HDL-C at this GWAS locus might be mediated at least in part through the altered expression of FST.
Figure 1.
Example Subcutaneous Adipose eQTL Genes at GWAS Loci
(A) The adipose eQTL of FST is coincident with the adiponectin GWAS locus ARL15.69 Regional association of variants with expression level of FST is shown with the GWAS variant rs6450176 plotted as the index (purple diamond). LD is colored on the basis of the METSIM population.
(B) Association between adiponectin level and FST expression level in METSIM.
(C) The adipose eQTL of RQCD1 is coincident with the USP37 GWAS locus for BMI.17 Regional association of variants with expression level of RQCD1 is shown with the eSNP rs4674320 (_r_2 = 1.0 with BMI index SNP rs492400) plotted as the index.
(D and E) Association between (D) BMI and RQCD1 expression level in humans from the METSIM study and (E) body fat and Rqcd1 expression level in mice from the HMDP study.
Association between Gene Expression Level and Phenotypic Traits
We investigated the effects of gene expression levels on cardio-metabolic risk by evaluating the association between the expression levels of all 43,145 probe sets and 23 cardio-metabolic-related traits (Table S11). At FDR < 1% (equivalent p < 6.5 × 10−4), we observed 48,365 significant associations between probe set and trait, and these corresponded to 29,920 gene-trait associations and 7,643 genes that were associated with 1 to 16 cardio-metabolic traits (Table S12). 10,819 probe sets (6,064 genes) were associated with BMI, 6,640 probe sets (3,940 genes) with Matsuda index, and 4,933 probe sets (3,039 genes) with insulin levels.
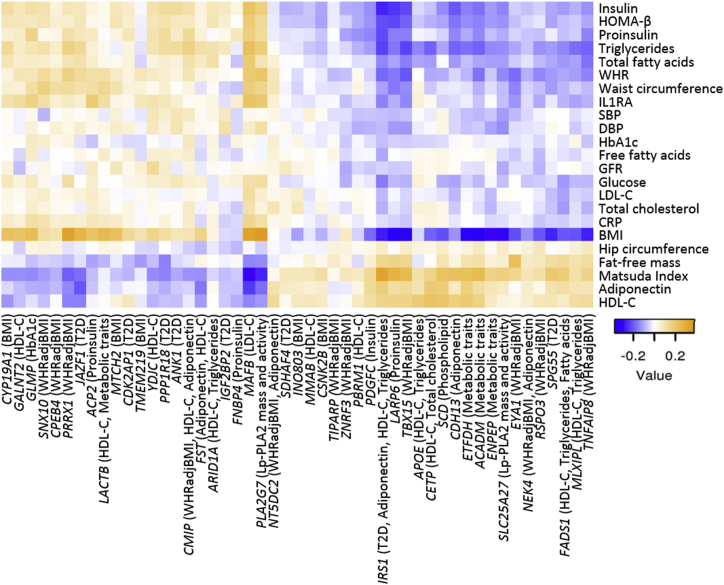
We next examined associations between the identified eQTL genes and cardio-metabolic traits measured in extensively phenotyped participants of the METSIM study. Among the genes for the 140 GWAS-relevant eQTLs, the expression levels of 49 were associated with the corresponding GWAS traits at p < 0.05 (Figure 2; also Figure S4 and Table S8). For example, TBX15 at the TBX15-WARS2 locus was associated with waist-hip ratio adjusted for BMI (p = 1.3 × 10−8) and 12 other traits (p = 4.3 × 10−21 to 2.6 × 10−4), and expression of GPR146 at the lipid locus GPR146 was associated with HDL-C (p = 3.3 × 10−13), triglycerides (p = 3.9 × 10−13), and ten other traits (p = 2.3 × 10−45 to 6.6 × 10−5, Figure 2). These gene expression and trait associations at GWAS loci further suggest plausible roles of these genes in mediating variant effects on cardio-metabolic disorders.
Figure 2.
Heatmap of Effect Sizes for Significant Associations between Gene Expression Level and Cardio-Metabolic-Trait Levels at GWAS Loci with Coincident eQTLs
Rows show 23 selected cardio-metabolic traits, and columns show the eQTL genes (and reported GWAS trait at the coincident locus). Negative values (blue) indicate that increased gene expression level was associated (p < 0.05) with decreased trait level, whereas positive values (orange) indicate that increased gene expression level was associated with increased trait level.
Human GWAS eQTL Genes in Mice
We next sought additional evidence for the involvement of GWAS-relevant eQTL genes in regulating cardio-metabolic traits by comparing results with those from a diverse panel of 120 inbred mouse strains known as the Hybrid Mouse Diversity Panel (HMDP).72 In the panel, we identified microarray probes for 107 of the 140 mouse orthologs and tested them for association between adipose expression level and metabolic traits, including plasma lipids, insulin, glucose, and body-fat composition. We observed a significant correlation between 70 genes and one of the metabolic traits (p < 5.2 × 10−5; 0.05/963 tests for nine traits × 107 genes) (Figure S5). Of these genes, 25 showed expression levels correlated with a similar metabolic trait in the same direction (Table S13). 13.1% of 10,771 orthologous genes had expression-trait correlations in the same direction in the adipose tissue of both species for waist-hip ratio, total cholesterol, insulin, and glucose. For example, GWAS variants associated with the homeostatic model of insulin resistance (HOMA-IR) are associated with decreased expression level of IRS1 (p = 2.0 × 10−50). IRS1 expression is negatively correlated with HOMA-IR (r = −0.40, p = 3.0 × 10−30) in METSIM and the HMDP (r = −0.50, p = 1 × 10−13) (Figure S6).
The HMDP data support a biological contribution of 25 eQTL genes at GWAS loci; 18 of these genes did not have significant associations in humans (p > 0.05) but had significant associations in mice, possibly because of the heterogeneous environmental effects in humans and controlled experimental environment in mice (Table S13). For example, the chromosome 2q35 variants reported by the GIANT consortium to be associated with BMI17 span ∼300 kb and nine genes. In METSIM, the BMI risk allele rs492400 was significantly associated with increased expression of RQCD1 (p = 8.9 × 10−43, Figure 1C), although RQCD1 expression was only nominally correlated with BMI (r = 0.09, p = 0.01, Figure 1D). In the HMDP, Rqcd1 expression was more strongly positively correlated with subcutaneous fat weight (r = 0.45, p = 1.2 × 10−12, Figure 1E), providing further support for the role of RQCD1 in BMI. Consistent correlation between gene expression and clinical traits in mice for _cis-_regulated genes at human GWAS loci supports the fruitful use of mouse models to further understand the roles of candidate genes for cardio-metabolic traits.
_trans_-eQTLs
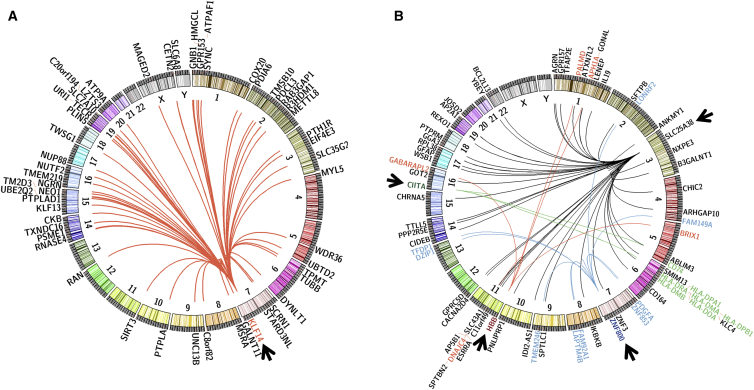
Associations of variants with expression of distant genes in trans allow the identification of regulatory networks in adipose tissue. We observed _trans_-eQTLs at a Bonferroni-corrected significance threshold (p < 1.51 × 10−13) for 90 genes (Table S3). To better identify regulatory networks in human samples, we searched for variants that were associated with at least five distal genes at a more liberal threshold of p < 5 × 10−8. These variants were located on chromosomes 3, 7, 11, and 16 in five independent loci (pairwise LD _r_2 = 0) that we termed _trans_-eQTL hotspots (Table S14, Figure 3). Four of the hotspot loci were associated with local (cis) gene expression at p < 1 × 10−3 (Figure S7).
Figure 3.
_trans_-eQTL Hotspots in Subcutaenous Adipose Tissue
(A) _KLF14 trans_-eQTL hotspot and target genes in subcutaneous adipose tissue. Representation of the location of 55 distal genes for which expression level is associated with rs12154627 at the KLF14 locus.
(B) Representation of the location of 65 distal genes for which expression level is associated with one of four _trans_-eQTL hotspots: SLC25A38 locus (black), ZNF800 locus (blue), CIITA locus (green), and HBB locus (red).
Arrowheads point to the _trans_-eQTL loci, and curves indicate the associations with the four sets of target genes.
40 of the 55 _trans_-mediated genes at the KLF14 locus were significantly associated with metabolic phenotypes at 1% FDR, representing a significant enrichment with respect to all the genes assayed on the microarray (Chi-square p value = 8.4 × 10−6) (Figure S8). The _trans_-regulated genes were co-expressed with pairwise correlations of −0.48 to 0.67 (p = 8.9 × 10−4 to 1.6 × 10−100); however, they were not enriched for known biological pathways according to the analysis involving the DAVID database.73 Some of the distal genes have been previously studied in relation to adipose biology, but their association with the KLF14 locus was not known. Among the _trans_-regulated genes, PLIN5 was associated with 13 of the metabolic phenotypes (Figure S8). PLIN5 encodes Perilipin 5, a lipid-droplet-associated protein that helps maintain a balance between lipolysis and lipogenesis and has been shown to play a role in fatty-acid oxidation in adipose tissue.74 The expanded _KLF14_-mediated trans network of genes suggests that these targets might also influence cardio-metabolic traits.
Variants located near CIITA (class II, major histocompatibility transactivator), which is known to activate the MHC class II genes at the HLA locus75 (Figure 3B), were associated with six MHC class II genes (HLA-DPA1, HLA-DMA, HLA-DPB1, HLA-DOA, HLA-DRA, and HLA-DMB) located on chromosome 6 and with CD74 on chromosome 5. CD74 encodes for a protein that associates with the class II MHC complex and regulates antigen presentation.76 The variants were also suggestively associated with CIITA expression (p = 1.0 × 10−3, Figure S7), suggesting that variation in its expression might be responsible for the _trans_-eQTL signals. Our eQTL results captured the known biology of CIITA regulation of MHC class II genes. Further, expression of CIITA and the seven _trans_-mediated genes was significantly associated with 14 of the metabolic traits (|β|= 0.12 to 0.37, p = 6.4 × 10−4 to 7.7 × 10−26), suggesting a role for this network of genes in adipose tissue (Figure S8).
The second largest _trans_-eQTL hotspot was located on chromosome 3 and was associated with the expression of 44 genes (Figure 3B). This locus has been reported in other tissues, including liver and omental fat,77 but the target genes identified in our study do not overlap with the previous ones, suggesting a unique _trans_-regulatory network in subcutaneous adipose tissue.11 Variants in this locus showed the strongest local association with the expression of SLC25A38, located ∼1 Mb away (p = 1.7 × 10−7, Figure S7). Missense mutations in SLC25A38, which encodes a mitochondrial solute carrier, lead to congenital sideroblastic anemia characterized by defective erythropoiesis and mitochondrial iron overload.78 The function of SLC25A38 in adipose tissue remains unclear. The signal observed at this locus might represent cell types other than adipocytes in light of the cellular heterogeneity of adipose tissue. The adipose expression level of SLC25A38 was nominally associated with 24 of the _trans_-mediated genes (|β| = 0.07 to 0.29, p = 6.8 × 10−16 to 4.7 × 10−2) and with 14 cardio-metabolic-related traits (|β| = 0.08 to 0.33, p = 6.7 × 10−21 to 3.1 × 10−2) (Figure S7). For example, SLC25A38 is negatively associated with BMI in METSIM participants (β = −0.33, p = 6.7 × 10−21). On the basis of structural similarity with other SLC25 proteins, SLC25A38 is predicted to transport glycine into the mitochondrial matrix for condensation with succinyl-coenzyme A to form 5-aminolevulinic acid, which is exported to the cytoplasm for haem synthesis.79, 80 Only a few of the _trans_-mediated genes have been shown to play important roles in adipocytes. For example, ESRRA encodes estrogen-related receptor alpha, which modulates the expression of adipogenesis genes during adipocyte differentiation.81 SPTLC1 encodes one of the subunits of serine palmitoyltransferase, the key enzyme in sphingolipid biosynthesis.82 These bioactive lipids are altered with obesity and can regulate inflammatory gene expression in adipocytes.83 Another target gene, GOT2, encodes glutamic-oxaloacetic transaminase, which functions in the inner mitochondrial membrane and has been shown to play a key role in amino acid metabolism.84 Although the function in adipose of the genes associated with SLC25A38 variants remains unclear, our results suggest a metabolic role.
We also observed a _trans_-eQTL hotspot, located on chromosome 7 but ∼3.3 Mb away from KLF14, associated with the expression of nine genes. The variants at this locus were independent from those at KLF14 and had distinct target genes (Figure 3B). The lead variant, rs62621812 in ZNF800, encodes a missense change (p.Pro103Ser) and is also associated with that gene’s expression level (p = 2.8 × 10−16), providing evidence suggesting that this gene regulates the target genes. ZNF800 encodes a C2H2 zinc finger protein and is a putative transcription factor.85 ZNF800 expression level is associated with the Matsuda Index (β = 0.12, p = 1.3 × 10−3), suggesting a role in insulin sensitivity.
A fifth _trans_-eQTL, located on chromosome 11, was associated with expression of five trans genes. The peak variant, rs10742583, is located ∼2 kb upstream of HBB, which encodes beta hemoglobin, and is in complete LD (_r_2 = 1.0) with a HBB synonymous variant, rs713040, although rs10742583 was not associated with the expression level of any local gene (p < 2.4 × 10−4). A functional role for HBB in adipose biology is not clear. Although HBB is distinctly and highly expressed in whole blood, the _trans_-mediated target genes are expressed in a range of tissues.56 This _trans_-eQTL signal might reflect cellular heterogeneity, especially blood cells present in adipose tissue.
To validate these _trans_-eQTL hotspots, we asked whether eQTLs were also observed in the MuTHER study. Variant information was available for three of the loci, near CIITA, SLC25A38, and KLF14. All seven genes that mapped to the CIITA locus, 35 of the 44 genes that mapped to the SLC25A38 locus, and 41 of the 55 genes that mapped to the KLF14 locus had corresponding probes available in the MuTHER study. Although only two of the 35 target genes for the SLC25A38 locus had evidence of association (FDR < 1%) in the MuTHER study, many KLF14 and CIITA target genes were replicated (Table S14). For the _KLF14 trans_-eQTL hotspot, originally described in MuTHER,20 25 of the 41 target genes showed consistent direction of effect and nominal associations (p < 0.05) in the MuTHER study, whereas none of the genes showed an opposite effect. For the CIITA locus, six of the seven target genes were validated in MuTHER with a consistent direction of effect (p < 0.05), indicating strong support for these two _trans_-eQTL hotspots.
Discussion
We report an analysis of genetic variants, adipose gene expression, and cardio-metabolic traits in a deeply phenotyped cross-sectional sample of 770 participants in the METSIM study and compare the results to those of studies performed in 120 diverse inbred strains of mice. These results help to prioritize potential target genes at GWAS loci, and they identify several _trans_-regulatory networks associated with metabolic syndrome and its component traits. In addition, the results reveal concordance between mice and humans in terms of correlations between gene expression and traits.
A main challenge with regard to gaining biological insights from genetic associations is to identify which genes and pathways explain the associations. Systems genetics aims to address this challenge by integrating genetic variants with molecular phenotypes to more comprehensively define the relationship between genotype and phenotype. Because a large fraction of the variation underlying common diseases appears to be regulatory,4, 86, 87 eQTLs that coincide with GWAS loci can link trait-associated loci with molecular perturbations. Overall, using reciprocal conditional analyses, we provided evidence for 140 genes at 105 loci that may be involved in metabolic traits (Table 1 and Table S8). This analysis of gene expression in a single tissue might not be relevant for the mechanisms of some of the cardio-metabolic traits. Although our analysis provides indirect evidence of an association, because the colocalization of an eQTL with a disease locus could be coincidental, the cardio-metabolic phenotypes available in the METSIM cohort provided additional evidence for 49 genes for which expression level was associated with the relevant trait. In addition, a subset of the 140 genes was also linked to GWAS traits via the summary-data-based Mendelian-randomization and causal-inference tests. Of note, we limited our analysis to lead eQTL variants and lead GWAS variants in high pairwise LD (_r_2 ≥ 0.8). This threshold might be conservative if the GWAS lead variant imperfectly tags a causal variant and might underestimate the number of GWAS loci that are coincident with eQTLs. Consistent with this possibility, we found evidence of association for 579 genes at 415 loci for which conditioning on the GWAS variant partially attenuated the eQTL effect (Table S8). Furthermore, secondary independent associations for both eQTLs and GWAS signals have been shown to play an important role in altering the expression of a gene in a locus;22 however, additional secondary and tertiary eQTL signals were not separately analyzed in this study. These genes provide a larger set of possible targets for GWAS loci.
Employing genotype imputation with a dense reference panel and analyzing 43,145 transcripts on a microarray to measure expression, we were able to identify more eQTLs than were identified in other subcutaneous adipose studies with similar sample sizes.10, 11, 37 Using these eQTLs, we implicated 50 genes that were distinct from the initial locus annotations used as signposts for the GWAS loci (Table S8). For example, rs11231693 is associated with waist-hip ratio adjusted for BMI16 and is located in the intron of MACROD1. Our results showed a strong association with the expression level of VEGFB (p = 2.2 × 10−67), located 140 kb from the variant. For ten loci, we identified multiple genes associated with the risk variants. For example, BMI-associated variants in the INO80E locus17 are also the strongest variants associated with the expression of five nearby genes: YPEL3, INO80E, TMEM219, TBX6, and HIRIP3 (p = 1.4 × 10−8 to 1.8 × 10−4), suggesting that variation in one or more of these genes might influence BMI. Finally, at the GCKR, LCAT, and LDLR genes that harbor common and rare coding variants that can affect plasma lipid levels, we observed eQTL effects on nearby genes, including EMILIN1 at the GCKR locus (p = 3.3 × 10−6), GFOD2 and NUTF2 at the LCAT locus (p = 1.9 × 10−6 and 1.6 × 10−4, respectively), and YIPF2 at the LDLR locus (p = 1.5 × 10−6). Some coincident associations between GWAS variants and eQTLs might not contribute strongly to trait variation.
Recent studies demonstrated the evolutionary conservation of regulatory networks that increase susceptibility to atherosclerosis and other cardio-metabolic traits in disease-relevant tissues in humans and mice.88 Genetically diverse mouse populations allow for the control of confounding factors, such as environmental exposure that are difficult to assess in humans. The HMDP has also helped researchers to understand contributions of genetic factors to cardio-metabolic traits.72 The results of our cross-species analysis showed consistent association between traits and the expression of 25 genes in humans and mice, providing support for further study of these genes in mouse models (Figure S5 and Table S13). Of course, the possibility exists that mechanisms might differ between species or that the wrong tissues are being compared. In addition to prioritizing candidate genes, studies of natural variation in gene expression in mice should be useful in elucidating mechanisms relating to the gene-by-gene, gene-by-environment, and gene-by-sex interactions.51, 52, 89 For example, although it is clear that genetic background contributes to weight gain and related traits in response to dietary challenge,90 the inability to accurately ascertain environmental factors over a lifetime in humans has made molecular dissection difficult. In contrast, studies of natural variation in mice have revealed that gene-by-environment interactions can contribute substantially to the overall variance in traits such as obesity and that the underlying genes and pathways can be identified.51, 52
Whereas numerous studies have successfully mapped cis associations, few studies have reported _trans_-eQTLs because of small effect sizes, multiple testing thresholds, and computational burden.22, 37, 91 These issues are consistent with the low reciprocal replication rates of _trans_-eQTLs identified in MuTHER and METSIM cohorts. However, in-depth characterization of the architecture of trans regulation of gene expression is useful in attempting to understand complex biological mechanisms, as shown by the expanded network of _trans_-regulated genes at the KLF14 locus, which has been associated with T2D and several metabolic traits. The 55 genes that map to this locus demonstrate the extensive downstream effects of an individual association signal and provide additional genes that might influence disease manifestation. The identification of _trans_-regulatory networks also offers opportunities for understanding fundamental biology. For example, ZNF800 is a putative transcription factor, and although little is known about the function of the encoded protein, our results implicate a novel role for ZNF800 in adipose biology. In addition, some _trans_-eQTL associations might be influenced by the cellular composition of the adipose tissue; for example, adipose tissue of obese individuals tends to have higher macrophage content.90, 91 Although we failed to observe any association between the _trans_-acting loci and expression of macrophage markers such as ABCG1 and CD68 (data not shown), we cannot rule out a role for cell-type heterogeneity. We also observed weaker cis associations compared to trans associations in the _trans_-eQTL hotspots. This may be an artifact of data pre-processing or inaccuracy in measuring low levels of expression. Future studies involving single-cell analyses might clarify the role of adipose tissue by identifying cell-type-specific eQTLs. Our study highlights the power of a systems-genetics approach in dissecting complex traits and identifying causal genes and pathways.
Conflicts of Interest
A.H., C.T., T.K., and P.S.G. are employees and shareholders of Bristol-Myers Squibb.
Acknowledgments
This study was supported by NIH grants R00HL121172 (M.C.), P01HL28481 (A.J.L. and P.P.), R01DK093757 (K.L.M.), U01DK105561 (K.L.M.), R01DK072193 (K.L.M.), U01DK062370 (M.B.), 1-ZIA-HG000024 (F.S.C.), R01HL095056 (P.P.), F31HL127921 (A.K.), and R00HL123021 (B.W.P.); Academy of Finland grants 77299 and 124243 (M.L.); the Finnish Heart Foundation (M.L.); the Finnish Diabetes Foundation (M.L.); Finnish Funding Agency for Technology and Innovation (TEKES) contract 1510/31/06 (M.L.); and the Commission of the European Community HEALTH-F2-2007-201681 (M.L.). C.K.R. was supported by NIH T32GM067553. Bristol-Myers Squibb supported the generation of METSIM and HMDP microarray data.
Published: March 2, 2017
Footnotes
Contributor Information
Karen L. Mohlke, Email: mohlke@med.unc.edu.
Aldons J. Lusis, Email: jlusis@mednet.ucla.edu.
Accession Numbers
Newly deposited human expression data can be obtained from the Gene Expression Omnibus with the accession number GEO: GSE70353.
Web Resources
- Efficient and Parallelizable Association Container Toolbox (EPACTS), http://genome.sph.umich.edu/wiki/EPACTS
- GWAS Catalog, https://www.ebi.ac.uk/gwas/
- The Haplotype Reference Consortium, http://www.haplotype-reference-consortium.org
- METSIM and HMDP, https://systems.genetics.ucla.edu/
Supplemental Data
Document S1. Figures S1–S8 and Tables S1–S14
Table S5. GWAS Summary Results Used in the Summary-Data-Based Mendelian-Randomization-Method Analysis
Table S8. Adipose eQTLs Identified at GWAS Loci for Cardiometabolic Dieseases and Traits
Table S9. Results of the Summary-Data-Based Mendelian-Randomization Analysis
The source of the GWAS summary results are given in Table S5.
Table S10. Results of the Causal-Inference Test
The causal-inference test was performed for the GWAS index SNP, expression of the cis-eQTL gene, and inverse-normalized traits measured in the METSIM population.
Table S11. Association of Gene Expression and Cardio-Metabolic Traits
Table S14. _Trans_-eQTL Hotspots Identified in METSIM Adipose Tissue and Their Look ups in the MuTHER Cohort
Red indicates a _cis_-regulated gene in the locus.
Document S2. Article plus Supplemental Data
References
- 1.Edwards S.L., Beesley J., French J.D., Dunning A.M. Beyond GWASs: Illuminating the dark road from association to function. Am. J. Hum. Genet. 2013;93:779–797. doi: 10.1016/j.ajhg.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visscher P.M., Brown M.A., McCarthy M.I., Yang J. Five years of GWAS discovery. Am. J. Hum. Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brænne I., Civelek M., Vilne B., Di Narzo A., Johnson A.D., Zhao Y., Reiz B., Codoni V., Webb T.R., Foroughi Asl H., Leducq Consortium CAD Genomics‡ Prediction of causal candidate genes in coronary artery disease loci. Arterioscler. Thromb. Vasc. Biol. 2015;35:2207–2217. doi: 10.1161/ATVBAHA.115.306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert F.W., Kruglyak L. The role of regulatory variation in complex traits and disease. Nat. Rev. Genet. 2015;16:197–212. doi: 10.1038/nrg3891. [DOI] [PubMed] [Google Scholar]
- 5.Conde L., Bracci P.M., Richardson R., Montgomery S.B., Skibola C.F. Integrating GWAS and expression data for functional characterization of disease-associated SNPs: an application to follicular lymphoma. Am. J. Hum. Genet. 2013;92:126–130. doi: 10.1016/j.ajhg.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Civelek M., Lusis A.J. Systems genetics approaches to understand complex traits. Nat. Rev. Genet. 2014;15:34–48. doi: 10.1038/nrg3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coelho M., Oliveira T., Fernandes R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013;9:191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustafson B., Hedjazifar S., Gogg S., Hammarstedt A., Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015;26:193–200. doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Porter S.A., Massaro J.M., Hoffmann U., Vasan R.S., O’Donnel C.J., Fox C.S. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emilsson V., Thorleifsson G., Zhang B., Leonardson A.S., Zink F., Zhu J., Carlson S., Helgason A., Walters G.B., Gunnarsdottir S. Genetics of gene expression and its effect on disease. Nature. 2008;452:423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- 11.Greenawalt D.M., Dobrin R., Chudin E., Hatoum I.J., Suver C., Beaulaurier J., Zhang B., Castro V., Zhu J., Sieberts S.K. A survey of the genetics of stomach, liver, and adipose gene expression from a morbidly obese cohort. Genome Res. 2011;21:1008–1016. doi: 10.1101/gr.112821.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nica A.C., Parts L., Glass D., Nisbet J., Barrett A., Sekowska M., Travers M., Potter S., Grundberg E., Small K., MuTHER Consortium The architecture of gene regulatory variation across multiple human tissues: the MuTHER study. PLoS Genet. 2011;7:e1002003. doi: 10.1371/journal.pgen.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong H., Beaulaurier J., Lum P.Y., Molony C., Yang X., Macneil D.J., Weingarth D.T., Zhang B., Greenawalt D., Dobrin R. Liver and adipose expression associated SNPs are enriched for association to type 2 diabetes. PLoS Genet. 2010;6:e1000932. doi: 10.1371/journal.pgen.1000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willer C.J., Schmidt E.M., Sengupta S., Peloso G.M., Gustafsson S., Kanoni S., Ganna A., Chen J., Buchkovich M.L., Mora S., Global Lipids Genetics Consortium Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahajan A., Go M.J., Zhang W., Below J.E., Gaulton K.J., Ferreira T., Horikoshi M., Johnson A.D., Ng M.C., Prokopenko I., DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium. South Asian Type 2 Diabetes (SAT2D) Consortium. Mexican American Type 2 Diabetes (MAT2D) Consortium. Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti-Ethnic Samples (T2D-GENES) Consortium Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat. Genet. 2014;46:234–244. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shungin D., Winkler T.W., Croteau-Chonka D.C., Ferreira T., Locke A.E., Mägi R., Strawbridge R.J., Pers T.H., Fischer K., Justice A.E., ADIPOGen Consortium. CARDIOGRAMplusC4D Consortium. CKDGen Consortium. GEFOS Consortium. GENIE Consortium. GLGC. ICBP. International Endogene Consortium. LifeLines Cohort Study. MAGIC Investigators. MuTHER Consortium. PAGE Consortium. ReproGen Consortium New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., Powell C., Vedantam S., Buchkovich M.L., Yang J., LifeLines Cohort Study. ADIPOGen Consortium. AGEN-BMI Working Group. CARDIOGRAMplusC4D Consortium. CKDGen Consortium. GLGC. ICBP. MAGIC Investigators. MuTHER Consortium. MIGen Consortium. PAGE Consortium. ReproGen Consortium. GENIE Consortium. International Endogene Consortium Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orozco L.D., Bennett B.J., Farber C.R., Ghazalpour A., Pan C., Che N., Wen P., Qi H.X., Mutukulu A., Siemers N. Unraveling inflammatory responses using systems genetics and gene-environment interactions in macrophages. Cell. 2012;151:658–670. doi: 10.1016/j.cell.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Nas A., Ingram-Drake L., Sinsheimer J.S., Wang S.S., Schadt E.E., Drake T., Lusis A.J. Expression quantitative trait loci: Replication, tissue- and sex-specificity in mice. Genetics. 2010;185:1059–1068. doi: 10.1534/genetics.110.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small K.S., Hedman A.K., Grundberg E., Nica A.C., Thorleifsson G., Kong A., Thorsteindottir U., Shin S.Y., Richards H.B., Soranzo N., GIANT Consortium. MAGIC Investigators. DIAGRAM Consortium. MuTHER Consortium Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat. Genet. 2011;43:561–564. doi: 10.1038/ng.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotival M., Zeller T., Wild P.S., Maouche S., Szymczak S., Schillert A., Castagné R., Deiseroth A., Proust C., Brocheton J., Cardiogenics Consortium Integrating genome-wide genetic variations and monocyte expression data reveals trans-regulated gene modules in humans. PLoS Genet. 2011;7:e1002367. doi: 10.1371/journal.pgen.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westra H.J., Peters M.J., Esko T., Yaghootkar H., Schurmann C., Kettunen J., Christiansen M.W., Fairfax B.P., Schramm K., Powell J.E. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y., Ma Z., Kim B.H., Wu W., Cayting P., Boyle A.P., Sundaram V., Xing X., Dogan N., Li J., Mouse ENCODE Consortium Principles of regulatory information conservation between mouse and human. Nature. 2014;515:371–375. doi: 10.1038/nature13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vierstra J., Rynes E., Sandstrom R., Zhang M., Canfield T., Hansen R.S., Stehling-Sun S., Sabo P.J., Byron R., Humbert R. Mouse regulatory DNA landscapes reveal global principles of cis-regulatory evolution. Science. 2014;346:1007–1012. doi: 10.1126/science.1246426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stancáková A., Javorský M., Kuulasmaa T., Haffner S.M., Kuusisto J., Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes. 2009;58:1212–1221. doi: 10.2337/db08-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 27.Stegle O., Parts L., Piipari M., Winn J., Durbin R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat. Protoc. 2012;7:500–507. doi: 10.1038/nprot.2011.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shabalin A.A. Matrix eQTL: Ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–1358. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippert C., Listgarten J., Liu Y., Kadie C.M., Davidson R.I., Heckerman D. FaST linear mixed models for genome-wide association studies. Nat. Methods. 2011;8:833–835. doi: 10.1038/nmeth.1681. [DOI] [PubMed] [Google Scholar]
- 30.Kang H.M., Sul J.H., Service S.K., Zaitlen N.A., Kong S.Y., Freimer N.B., Sabatti C., Eskin E. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 2010;42:348–354. doi: 10.1038/ng.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J., Zaitlen N.A., Goddard M.E., Visscher P.M., Price A.L. Advantages and pitfalls in the application of mixed-model association methods. Nat. Genet. 2014;46:100–106. doi: 10.1038/ng.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruim R.J., Welch R.P., Sanna S., Teslovich T.M., Chines P.S., Gliedt T.P., Boehnke M., Abecasis G.R., Willer C.J. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J., Benyamin B., McEvoy B.P., Gordon S., Henders A.K., Nyholt D.R., Madden P.A., Heath A.C., Martin N.G., Montgomery G.W. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Lee S.H., Goddard M.E., Visscher P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostem E., Eskin E. Improving the accuracy and efficiency of partitioning heritability into the contributions of genomic regions. Am. J. Hum. Genet. 2013;92:558–564. doi: 10.1016/j.ajhg.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grundberg E., Small K.S., Hedman A.K., Nica A.C., Buil A., Keildson S., Bell J.T., Yang T.P., Meduri E., Barrett A., Multiple Tissue Human Expression Resource (MuTHER) Consortium Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat. Genet. 2012;44:1084–1089. doi: 10.1038/ng.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dastani Z., Hivert M.F., Timpson N., Perry J.R., Yuan X., Scott R.A., Henneman P., Heid I.M., Kizer J.R., Lyytikäinen L.P., DIAGRAM+ Consortium. MAGIC Consortium. GLGC Investigators. MuTHER Consortium. DIAGRAM Consortium. GIANT Consortium. Global B Pgen Consortium. Procardis Consortium. MAGIC investigators. GLGC Consortium Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkler T.W., Justice A.E., Graff M., Barata L., Feitosa M.F., Chu S., Czajkowski J., Esko T., Fall T., Kilpeläinen T.O., CHARGE Consortium. DIAGRAM Consortium. GLGC Consortium. Global-BPGen Consortium. ICBP Consortium. MAGIC Consortium The influence of age and sex on genetic associations with adult body size and shape: A large-scale genome-wide interaction study. PLoS Genet. 2015;11:e1005378. doi: 10.1371/journal.pgen.1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berndt S.I., Gustafsson S., Mägi R., Ganna A., Wheeler E., Feitosa M.F., Justice A.E., Monda K.L., Croteau-Chonka D.C., Day F.R. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 2013;45:501–512. doi: 10.1038/ng.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikpay M., Goel A., Won H.H., Hall L.M., Willenborg C., Kanoni S., Saleheen D., Kyriakou T., Nelson C.P., Hopewell J.C., CARDIoGRAMplusC4D Consortium A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walford G.A., Gustafsson S., Rybin D., Stančáková A., Chen H., Liu C.T., Hong J., Jensen R.A., Rice K., Morris A.P. Genome-wide association study of the modified Stumvoll insulin sensitivity index identifies BCL2 and FAM19A2 as novel insulin sensitivity loci. Diabetes. 2016;65:3200–3211. doi: 10.2337/db16-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prokopenko I., Poon W., Mägi R., Prasad B R., Salehi S.A., Almgren P., Osmark P., Bouatia-Naji N., Wierup N., Fall T. A central role for GRB10 in regulation of islet function in man. PLoS Genet. 2014;10:e1004235. doi: 10.1371/journal.pgen.1004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manning A.K., Hivert M.F., Scott R.A., Grimsby J.L., Bouatia-Naji N., Chen H., Rybin D., Liu C.T., Bielak L.F., Prokopenko I., DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Multiple Tissue Human Expression Resource (MUTHER) Consortium A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 2012;44:659–669. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strawbridge R.J., Dupuis J., Prokopenko I., Barker A., Ahlqvist E., Rybin D., Petrie J.R., Travers M.E., Bouatia-Naji N., Dimas A.S., DIAGRAM Consortium. GIANT Consortium. MuTHER Consortium. CARDIoGRAM Consortium. C4D Consortium Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60:2624–2634. doi: 10.2337/db11-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soranzo N., Sanna S., Wheeler E., Gieger C., Radke D., Dupuis J., Bouatia-Naji N., Langenberg C., Prokopenko I., Stolerman E., WTCCC Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59:3229–3239. doi: 10.2337/db10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saxena R., Hivert M.F., Langenberg C., Tanaka T., Pankow J.S., Vollenweider P., Lyssenko V., Bouatia-Naji N., Dupuis J., Jackson A.U., GIANT consortium. MAGIC investigators Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat. Genet. 2010;42:142–148. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dupuis J., Langenberg C., Prokopenko I., Saxena R., Soranzo N., Jackson A.U., Wheeler E., Glazer N.L., Bouatia-Naji N., Gloyn A.L., DIAGRAM Consortium. GIANT Consortium. Global BPgen Consortium. Anders Hamsten on behalf of Procardis Consortium. MAGIC investigators New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Z., Zhang F., Hu H., Bakshi A., Robinson M.R., Powell J.E., Montgomery G.W., Goddard M.E., Wray N.R., Visscher P.M., Yang J. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 2016;48:481–487. doi: 10.1038/ng.3538. [DOI] [PubMed] [Google Scholar]
- 50.Millstein J., Zhang B., Zhu J., Schadt E.E. Disentangling molecular relationships with a causal inference test. BMC Genet. 2009;10:23. doi: 10.1186/1471-2156-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parks B.W., Nam E., Org E., Kostem E., Norheim F., Hui S.T., Pan C., Civelek M., Rau C.D., Bennett B.J. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17:141–152. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parks B.W., Sallam T., Mehrabian M., Psychogios N., Hui S.T., Norheim F., Castellani L.W., Rau C.D., Pan C., Phun J. Genetic architecture of insulin resistance in the mouse. Cell Metab. 2015;21:334–346. doi: 10.1016/j.cmet.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castellani L.W., Nguyen C.N., Charugundla S., Weinstein M.M., Doan C.X., Blaner W.S., Wongsiriroj N., Lusis A.J. Apolipoprotein AII is a regulator of very low density lipoprotein metabolism and insulin resistance. J. Biol. Chem. 2008;283:11633–11644. doi: 10.1074/jbc.M708995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langfelder P., Horvath S. Fast R functions for robust correlations and hierarchical clustering. J. Stat. Softw. 2012;46 [PMC free article] [PubMed] [Google Scholar]
- 55.Welter D., MacArthur J., Morales J., Burdett T., Hall P., Junkins H., Klemm A., Flicek P., Manolio T., Hindorff L., Parkinson H. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.GTEx Consortium Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorleifsson G., Walters G.B., Gudbjartsson D.F., Steinthorsdottir V., Sulem P., Helgadottir A., Styrkarsdottir U., Gretarsdottir S., Thorlacius S., Jonsdottir I. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 58.Kathiresan S., Willer C.J., Peloso G.M., Demissie S., Musunuru K., Schadt E.E., Kaplan L., Bennett D., Li Y., Tanaka T. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levy D., Ehret G.B., Rice K., Verwoert G.C., Launer L.J., Dehghan A., Glazer N.L., Morrison A.C., Johnson A.D., Aspelund T. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voight B.F., Scott L.J., Steinthorsdottir V., Morris A.P., Dina C., Welch R.P., Zeggini E., Huth C., Aulchenko Y.S., Thorleifsson G., MAGIC investigators. GIANT Consortium Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U., Lango Allen H., Lindgren C.M., Luan J., Mägi R., MAGIC. Procardis Consortium Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heid I.M., Jackson A.U., Randall J.C., Winkler T.W., Qi L., Steinthorsdottir V., Thorleifsson G., Zillikens M.C., Speliotes E.K., Mägi R., MAGIC Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat. Genet. 2010;42:949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teslovich T.M., Musunuru K., Smith A.V., Edmondson A.C., Stylianou I.M., Koseki M., Pirruccello J.P., Ripatti S., Chasman D.I., Willer C.J. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kooner J.S., Saleheen D., Sim X., Sehmi J., Zhang W., Frossard P., Been L.F., Chia K.S., Dimas A.S., Hassanali N., DIAGRAM. MuTHER Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat. Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.C4D Consortium A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat. Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 66.Morris A.P., Voight B.F., Teslovich T.M., Ferreira T., Segrè A.V., Steinthorsdottir V., Strawbridge R.J., Khan H., Grallert H., Mahajan A., Wellcome Trust Case Control Consortium. Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators. Genetic Investigation of ANthropometric Traits (GIANT) Consortium. Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium. South Asian Type 2 Diabetes (SAT2D) Consortium. DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kettunen J., Tukiainen T., Sarin A.P., Ortega-Alonso A., Tikkanen E., Lyytikäinen L.P., Kangas A.J., Soininen P., Würtz P., Silander K. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat. Genet. 2012;44:269–276. doi: 10.1038/ng.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabater-Lleal M., Huang J., Chasman D., Naitza S., Dehghan A., Johnson A.D., Teumer A., Reiner A.P., Folkersen L., Basu S., VTE Consortium. STROKE Consortium. Wellcome Trust Case Control Consortium 2 (WTCCC2) C4D Consortium. CARDIoGRAM Consortium Multiethnic meta-analysis of genome-wide association studies in >100 000 subjects identifies 23 fibrinogen-associated Loci but no strong evidence of a causal association between circulating fibrinogen and cardiovascular disease. Circulation. 2013;128:1310–1324. doi: 10.1161/CIRCULATIONAHA.113.002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richards J.B., Waterworth D., O’Rahilly S., Hivert M.F., Loos R.J., Perry J.R., Tanaka T., Timpson N.J., Semple R.K., Soranzo N., GIANT Consortium A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet. 2009;5:e1000768. doi: 10.1371/journal.pgen.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown M.L., Bonomi L., Ungerleider N., Zina J., Kimura F., Mukherjee A., Sidis Y., Schneyer A. Follistatin and follistatin like-3 differentially regulate adiposity and glucose homeostasis. Obesity (Silver Spring) 2011;19:1940–1949. doi: 10.1038/oby.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braga M., Reddy S.T., Vergnes L., Pervin S., Grijalva V., Stout D., David J., Li X., Tomasian V., Reid C.B. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J. Lipid Res. 2014;55:375–384. doi: 10.1194/jlr.M039719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lusis A.J., Seldin M.M., Allayee H., Bennett B.J., Civelek M., Davis R.C., Eskin E., Farber C.R., Hui S., Mehrabian M. The Hybrid Mouse Diversity Panel: a resource for systems genetics analyses of metabolic and cardiovascular traits. J. Lipid Res. 2016;57:925–942. doi: 10.1194/jlr.R066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 74.Mason R.R., Watt M.J. Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol. Metab. 2015;26:144–152. doi: 10.1016/j.tem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 75.LeibundGut-Landmann S., Waldburger J.M., Krawczyk M., Otten L.A., Suter T., Fontana A., Acha-Orbea H., Reith W. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 76.Wong D., Lee W., Humburg P., Makino S., Lau E., Naranbhai V., Fairfax B.P., Chan K., Plant K., Knight J.C. Genomic mapping of the MHC transactivator CIITA using an integrated ChIP-seq and genetical genomics approach. Genome Biol. 2014;15:494. doi: 10.1186/s13059-014-0494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X., Gierman H.J., Levy D., Plump A., Dobrin R., Goring H.H., Curran J.E., Johnson M.P., Blangero J., Kim S.K. Synthesis of 53 tissue and cell line expression QTL datasets reveals master eQTLs. BMC Genomics. 2014;15:532. doi: 10.1186/1471-2164-15-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guernsey D.L., Jiang H., Campagna D.R., Evans S.C., Ferguson M., Kellogg M.D., Lachance M., Matsuoka M., Nightingale M., Rideout A. Mutations in mitochondrial carrier family gene SLC25A38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat. Genet. 2009;41:651–653. doi: 10.1038/ng.359. [DOI] [PubMed] [Google Scholar]
- 79.Kannengiesser C., Sanchez M., Sweeney M., Hetet G., Kerr B., Moran E., Fuster Soler J.L., Maloum K., Matthes T., Oudot C. Missense SLC25A38 variations play an important role in autosomal recessive inherited sideroblastic anemia. Haematologica. 2011;96:808–813. doi: 10.3324/haematol.2010.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong W.S., Wong H.F., Cheng C.K., Chang K.O., Chan N.P., Ng M.H., Wong K.F. Congenital sideroblastic anaemia with a novel frameshift mutation in SLC25A38. J. Clin. Pathol. 2015;68:249–251. doi: 10.1136/jclinpath-2014-202211. [DOI] [PubMed] [Google Scholar]
- 81.Ijichi N., Ikeda K., Horie-Inoue K., Yagi K., Okazaki Y., Inoue S. Estrogen-related receptor alpha modulates the expression of adipogenesis-related genes during adipocyte differentiation. Biochem. Biophys. Res. Commun. 2007;358:813–818. doi: 10.1016/j.bbrc.2007.04.209. [DOI] [PubMed] [Google Scholar]
- 82.Weiss B., Stoffel W. Human and murine serine-palmitoyl-CoA transferase—Cloning, expression and characterization of the key enzyme in sphingolipid synthesis. Eur. J. Biochem. 1997;249:239–247. doi: 10.1111/j.1432-1033.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- 83.Samad F., Hester K.D., Yang G., Hannun Y.A., Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: A potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579–2587. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 84.Arrio-Dupont M., Coulet P.R., Gautheron D.C. Coupled reaction of immobilized aspartate aminotransferase and malate dehydrogenase. A plausible model for the cellular behaviour of these enzymes. Biochim. Biophys. Acta. 1985;829:58–68. doi: 10.1016/0167-4838(85)90068-8. [DOI] [PubMed] [Google Scholar]
- 85.Najafabadi H.S., Mnaimneh S., Schmitges F.W., Garton M., Lam K.N., Yang A., Albu M., Weirauch M.T., Radovani E., Kim P.M. C2H2 zinc finger proteins greatly expand the human regulatory lexicon. Nat. Biotechnol. 2015;33:555–562. doi: 10.1038/nbt.3128. [DOI] [PubMed] [Google Scholar]
- 86.Maurano M.T., Humbert R., Rynes E., Thurman R.E., Haugen E., Wang H., Reynolds A.P., Sandstrom R., Qu H., Brody J. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicolae D.L., Gamazon E., Zhang W., Duan S., Dolan M.E., Cox N.J. Trait-associated SNPs are more likely to be eQTLs: Annotation to enhance discovery from GWAS. PLoS Genet. 2010;6:e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Talukdar H.A., Foroughi Asl H., Jain R.K., Ermel R., Ruusalepp A., Franzén O., Kidd B.A., Readhead B., Giannarelli C., Kovacic J.C. Cross-tissue regulatory gene networks in coronary artery disease. Cell Syst. 2016;2:196–208. doi: 10.1016/j.cels.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tyler A.L., Donahue L.R., Churchill G.A., Carter G.W. Weak epistasis generally stabilizes phenotypes in a mouse intercross. PLoS Genet. 2016;12:e1005805. doi: 10.1371/journal.pgen.1005805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bouchard C., Tremblay A., Després J.P., Nadeau A., Lupien P.J., Thériault G., Dussault J., Moorjani S., Pinault S., Fournier G. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X., Johnson A.D., Hendricks A.E., Hwang S.J., Tanriverdi K., Ganesh S.K., Smith N.L., Peyser P.A., Freedman J.E., O’Donnell C.J. Genetic associations with expression for genes implicated in GWAS studies for atherosclerotic cardiovascular disease and blood phenotypes. Hum. Mol. Genet. 2014;23:782–795. doi: 10.1093/hmg/ddt461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1. Figures S1–S8 and Tables S1–S14
Table S5. GWAS Summary Results Used in the Summary-Data-Based Mendelian-Randomization-Method Analysis
Table S8. Adipose eQTLs Identified at GWAS Loci for Cardiometabolic Dieseases and Traits
Table S9. Results of the Summary-Data-Based Mendelian-Randomization Analysis
The source of the GWAS summary results are given in Table S5.
Table S10. Results of the Causal-Inference Test
The causal-inference test was performed for the GWAS index SNP, expression of the cis-eQTL gene, and inverse-normalized traits measured in the METSIM population.
Table S11. Association of Gene Expression and Cardio-Metabolic Traits
Table S14. _Trans_-eQTL Hotspots Identified in METSIM Adipose Tissue and Their Look ups in the MuTHER Cohort
Red indicates a _cis_-regulated gene in the locus.
Document S2. Article plus Supplemental Data