Human cytomegalovirus encodes a highly specific RANTES decoy receptor (original) (raw)
Abstract
The human cytomegalovirus pUL21.5 protein is a small, secreted glycoprotein whose mRNA is packaged into virions. We demonstrate that pUL21.5 protein is a soluble CC chemokine receptor that functions as a decoy to modulate the host immune response to infection. In contrast to other viral chemokine-binding proteins, which interact promiscuously with multiple chemokines, pUL21.5 selectively binds RANTES (regulated upon activation, normal T cell expressed and secreted) with high affinity. By binding RANTES, pUL21.5 blocks RANTES interaction with its cellular receptors. We propose that human cytomegalovirus directs the synthesis of a secreted, virus-coded protein that modulates the host antiviral response even before the newly infecting viral genome becomes transcriptionally active.
Human cytomegalovirus (HCMV) is the prototypical member of the β-herpes virus family. Seroepidemiologic studies have shown that the virus is widespread in the human population. HCMV causes life-threatening disease in immunologically immature or compromised individuals (1) including neonates, AIDS patients, and allogeneic transplant recipients. Most healthy adults with HCMV infection are asymptomatic, but the virus evades eradication by the immune system and persists in infected people for the rest of their lives. The ability of HCMV to establish life-long latency and to avoid extermination by the immune system depends on extensive immune modulation strategies including the ability to antagonize chemokine function.
Chemokines are small, chemoattractant cytokines divided into four subfamilies (CC, CXC, C, and CX3C) based on the arrangement of conserved cysteine residues (2). G protein-coupled receptors serve as chemokine receptors and propagate an intracellular signal in response to chemokine binding (3). Chemokines not only mediate inflammatory processes by recruiting leukocytes, but they also stimulate the effector mechanisms of lymphocytes (2). Consequently, chemokines play a pivotal role in the resolution of virus infections, and, not surprisingly, several viruses have developed strategies to antagonize the antiviral activities of chemokines (4). For example, the myxoma virus M-T1 protein (5), the vaccinia virus p35 (6, 7), and the murine gamma herpesvirus 68 M3 protein (8, 9) mimic the ligand-binding domains of chemokine receptors. Each of these proteins binds multiple chemokines with high affinity, blocking their ability to interact with cellular receptors.
Our laboratory has reported that the HCMV UL21.5 mRNA is packaged into virions (10). Viral and cellular mRNAs are packaged into virions (10, 11) in proportion to their abundance in infected cells (12). Inclusion of mRNAs in virions implies a role for at least a portion of the encoded protein before the viral genome reaches the nucleus and begins to be transcribed. The 103-aa pUL21.5 (13) has a relatively conserved sequence among clinical isolates (14, 15), and the presence of the UL21.5 mRNA appears to correlate directly with the occurrence of HCMV disease (16), but its role in disease had previously not been recognized. In this study, we demonstrate that pUL21.5 selectively binds the CC chemokine RANTES (regulated upon activation, normal T cell expressed and secreted) with high affinity and thereby blocks the ability of RANTES to bind to its receptor.
Materials and Methods
Cells, Viruses, and Biological Reagents. Primary human foreskin fibroblasts at passage 10–15 were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS. THP-1 cells were grown in RPMI medium 1640 with 10% FBS. Human umbilical vein endothelial cells were obtained by collagenase digestion of umbilical veins and were used at passage 3–6.
The AD169 laboratory strain (17), AD_wt_, and the VR1814 clinical isolate (18, 19), FIX_wt_, served as wild-type viruses in these studies. The pUL21.5-deficient mutant virus, AD-sub_UL21.5, was generated from AD_wt by homologous recombination within infected fibroblasts. The HCMV sequence from base pair 27105 to 27611 was substituted with a marker cassette containing the GFP under control of an SV40 promoter, followed by the internal ribosomal entry site and a puromycin resistance gene. A second UL21.5-null mutant was prepared from a VR1814-containing bacterial artificial chromosome (BAC), termed FIX BAC (19), which was modified by replacing the guanine phosphoribosyltransferase gene in the BAC sequence with a GFP sequence (S. Terhune, D. Yu, and T.S., unpublished data). The FIX substitution mutant, FIX-_sub_UL21.5, was constructed by linear recombination in Escherichia coli (20). The mutant FIX virus was reconstituted by electroporation into fibroblasts of BAC DNA, together with a plasmid expressing the viral pp71 protein, which has been shown to enhance the infectivity of viral DNA (21). Virus titers were determined by plaque assay on fibroblasts, and the growth of mutants was examined in both fibroblasts and endothelial cells.
An AD169 variant expressing a FLAG epitope-tagged pUL21.5, BAD_in_UL21.5F, was constructed by linear recombination in E. coli (20) by using an infectious AD169 BAC (22). The PCR-generated fragment used for recombination contained a kanamycin resistance gene flanked by Flp recognition target (FRT) sites. The 5′ end of the fragment also contained 50 base pairs derived from the 3′ end of the UL21.5 coding region followed by the FLAG tag sequence and a stop codon. After the initial recombination step, the kanamycin resistance marker was excised from the BAC by using Flp recombinase (23), and virus was recovered by electroporation of the BAC DNA into fibroblasts as described in the previous paragraph. The pUL21.5F fusion protein was purified from the medium of BAD_in_UL21.5F-infected cells by capturing the protein on anti-FLAG M2 affinity gel (Sigma) and eluting with buffer containing FLAG peptide. After two cycles of capture and elution, the protein was dialyzed overnight against PBS.
UL21.5-specific rabbit polyclonal antibody was produced by using a GST-pUL21.5 fusion protein as immunogen. The gamma herpesvirus 68 M3 protein was a gift from H. W. Virgin (Washington University School of Medicine, St. Louis). IE1 monoclonal antibody 1B12 was used to detect IE1 protein expression, and pp65 rabbit polyclonal antibody was obtained from M. Schrader (University of Marburg, Marburg, Germany).
Chemokine-Binding Assays. To prepare and study secreted pUL21.5, human fibroblasts were infected with wild-type (AD_wt_) or, as a control, pUL21.5-deficient virus (AD-_sub_UL21.5) at a multiplicity of 3 plaque-forming units (pfu) per cell and fed with medium containing 10% FCS. At 6–8 h after infection, cell monolayers were washed twice with serum-free medium, after which the infected cultures were maintained in serum-free medium. At 48 h after infection, the culture medium was collected, subjected to centrifugation to remove residual free virus, and concentrated 300-fold while exchanging the solvent to PBS by using a Centriprep-10 cartridge (Amicon).
To assay for chemokine-binding activity, 10 μl of concentrated medium from AD_wt_- or AD_sub_UL21.5-infected cells was incubated with 125I-labeled chemokines (0.4 nM, Amersham Pharmacia) for 2 h at room temperature. Protein complexes were then covalently crosslinked by the addition of EDC [1-ethyl-3-(3-dimethylaminopropyl) carbodimide hydrochloride] to a final concentration of 40 mM for 30 min at room temperature. The reaction was quenched by the addition of 0.1 vol of 1 M Tris·HCl (pH 7.4), and the resulting complexes were analyzed by electrophoresis in a polyacrylamide gel containing 10% SDS. Competition assays were performed similarly to the cross-linking experiment, except that a 10- to 500-fold excess of unlabeled chemokine was included in the assay.
Scatchard saturation analysis was used to determine the binding affinity of 125I-RANTES to pUL21.5. For each data point, pUL21.5F was captured on the M2 anti-FLAG affinity matrix, and the immobilized protein was then incubated for 2 h at room temperature with increasing concentrations of 125I-RANTES. The complex on the matrix was precipitated and washed twice, and radioactivity was quantified by using a scintillation counter.
To assay for effects of pUL21.5 on RANTES binding to cell receptors, 100 μl of concentrated protein derived from the medium from AD_wt_-, AD_sub_UL21.5-, or mock-infected fibroblasts was preincubated with 100 pM 125I-RANTES for 1 h at 4°C. Subsequently, 2 × 106 THP-1 cells were added in 50 μl and incubated for 2 h at 4°C. After incubation, the cells were centrifuged through 15% sucrose in RPMI medium 1640 containing 0.1% BSA and washed twice. Bound 125I-RANTES was quantified (24) by using a scintillation counter.
Results
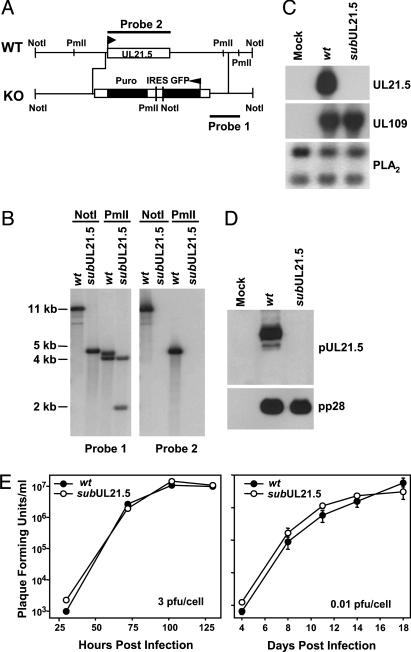
UL21.5 Is Dispensable for HCMV Replication in Cultured Cells. To explore the function of pUL21.5, we initially constructed a UL21.5-deficient virus (AD_sub_UL21.5) in the AD169 laboratory strain of HCMV in which the UL21.5 ORF is replaced with a marker gene cassette (Fig. 1_A_). We verified the junctions of the substitution mutation by sequence analysis. Southern blot analysis of AD_sub_UL21.5 virion DNA demonstrated the loss of the UL21.5 gene (Fig. 1_B_), and the loss of UL21.5 mRNA and protein was confirmed by Northern (Fig. 1_C_) and Western (Fig. 1_D_) blot assay, respectively. To evaluate the growth characteristics of the mutant virus, human fibroblasts were infected and the production of infectious progeny was monitored. No growth defect was observed (Fig. 1_E_) after infection at relatively high (3 pfu per cell) or low (0.01 pfu per cell) input multiplicities.
Fig. 1.
Construction and analysis of a UL21.5-deficient virus, AD_sub_UL21.5, in the HCMV AD169 laboratory strain. (A) Schematic representation of the UL21.5 region of the HCMV genome. A marker cassette containing the GFP coding region, an internal ribosomal entry site (IRES), and a puromycin resistance coding region (Puro) controlled by the SV40 promoter and poly(A) site was substituted for the UL21.5 ORF. The probes (1 and 2) and restriction enzyme cleavage sites (_Not_I and Pml_I) used to characterize the mutant virus are shown. (B) Analysis of AD_wt (wt) and AD_sub_UL21.5 (_sub_UL21.5) viral DNA. DNA was prepared from virions, digested with Not_Ior Pml_I, and analyzed by Southern blot by using 32P-labeled DNA probe 1 or probe 2. The sizes (in kb) of radioactive bands relative to marker DNAs are indicated. (C) Analysis of UL21.5 mRNA accumulation after infection of fibroblasts with AD_wt or AD-sub_UL21.5. Total RNA was prepared at 72 h after infection and analyzed by Northern blot with 32P-labeled DNA probe 2. Northern blots also were performed on the RNA samples by using 32P-labeled probes for the HCMV UL109 or cellular PLA2 RNA, to demonstrate that cells receiving AD_sub_UL21.5 were infected and to serve as a loading control. (D) Analysis of pUL21.5 accumulation at 72 h after infection of fibroblasts with AD_wt or AD_sub_UL21.5. Western blot assays were performed by using antibodies specific for pUL21.5 or, as a control, the HCMV pp28 protein. (E) Growth kinetics of AD_wt and AD-_sub_UL21.5 in human fibroblasts. Cells were infected at a multiplicity of 3 or 0.01 pfu per cell. Cultures were harvested at the indicated times after infection, and infectious virus was quantified by plaque assay on fibroblasts.
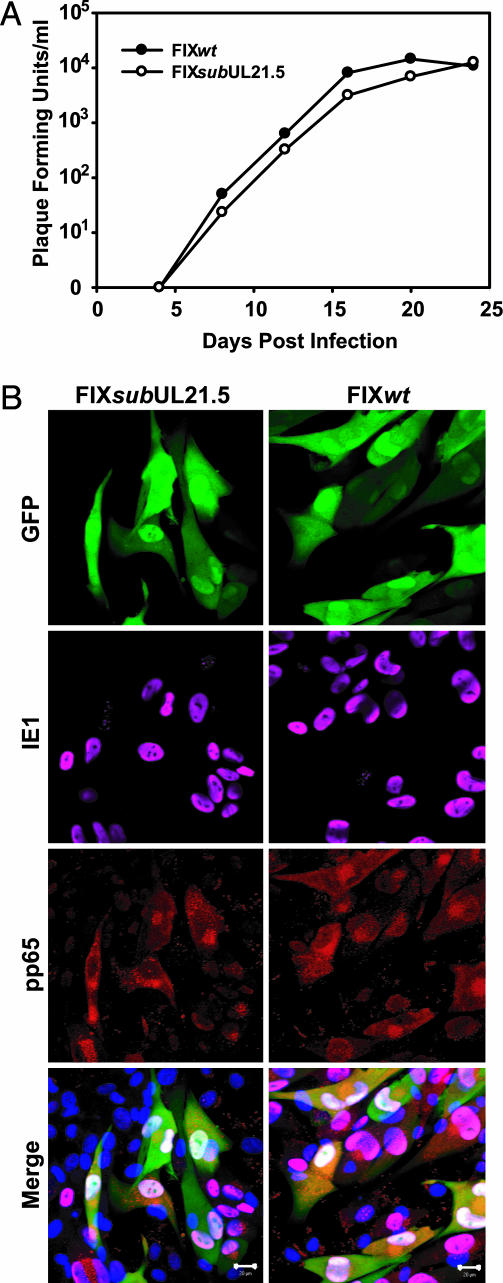
The AD169 laboratory strain is genetically and biologically different from clinical isolates of HCMV (reviewed in ref. 15). AD169 replicates effectively only in fibroblasts, whereas clinical isolates grow in a variety of additional cell types including endothelial cells. To test whether pUL21.5 is required for replication of a clinical virus in fibroblasts and for infection of endothelial cells by a clinical virus, we generated a derivative of the clinical virus VR1814 (18, 19), termed FIX_sub_UL21.5, which lacked the entire UL21.5 ORF. As expected, the mutant failed to express pUL21.5 (data not shown), and, as observed for the AD169 mutant, FIX_sub_UL21.5 grew with similar kinetics and reached similar yields as its wild-type parent after infection of fibroblasts (Fig. 2_A_). Immunofluorescent analysis demonstrated that the mutant virus also successfully infected endothelial cells, expressing a virus-coded immediate-early protein (UL123-coded IE1) and a late protein (UL83-coded pp65) that were properly localized (Fig. 2_B_). We conclude that pUL21.5 mediates a function that is dispensable for efficient virus replication in fibroblasts and for infection of endothelial cells.
Fig. 2.
Analysis of a UL21.5-deficient virus, FIX_sub_UL21.5, produced in the HCMV VR1814 clinical isolate. (A) Growth kinetics of FIX_wt_ and FIX_sub_UL21.5 in human fibroblasts. Cells were infected at a multiplicity of 0.01 pfu per cell, cultures were harvested at the indicated times after infection, and infectious virus was quantified by plaque assay on fibroblasts. (B) FIX_wt_ and FIX-sub_UL21.5 infection of endothelial cells. Cells were infected at a multiplicity of 0.1 pfu per cell with FIX_wt or FIX_sub_UL21.5. Cells were fixed 72 h after infection, and infected cells were identified by their GFP expression from a marker cassette on the FIX viruses. UL123-coded IE1 protein was visualized by using a specific monoclonal antibody plus an Alexa 647-conjugated secondary antibody (pink). UL83-coded pp65 protein was stained with a rabbit polyclonal antibody plus an Alexa 546-conjugated secondary antibody (red). The endothelial cell nuclei were stained with 4′,6-diamidino-2-phenylindole (blue).
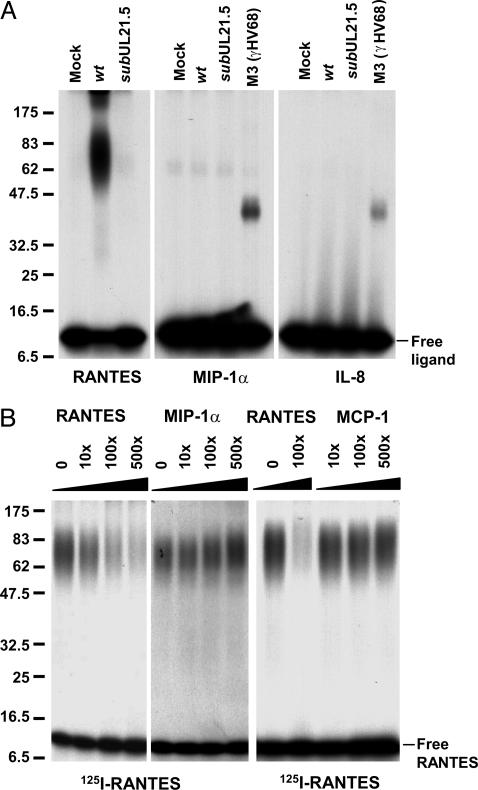
pUL21.5 Selectively Binds RANTES. The potential to express pUL21.5 from mRNAs delivered to the cell by infecting virions (10), coupled with the observation that it is not required for growth in cultured cells, led us to speculate that it might act to modulate the host immune response to infection. Accordingly, we used an in vitro binding assay to test the hypothesis that pUL21.5 may act as a soluble chemokine receptor. Aliquots of concentrated protein from the medium of mock-infected and virus-infected cells were incubated with 125I-labeled RANTES, MIP-1α, or IL-8, followed by chemical crosslinking and electrophoretic analysis in an SDS-containing polyacrylamide gel. The addition of the CC-chemokine, RANTES, to the medium of cultured cells infected with wild-type virus generated a complex migrating as a broad band with a median molecular mass of ≈70 kDa relative to marker proteins (Fig. 3_A_ Left). The complex was not detected when the radiolabeled ligand was incubated in medium from cells infected with the pUL21.5-deficient virus, arguing for pUL21.5 as a RANTES-binding protein. In contrast to the gamma herpesvirus 68 M3 protein, which binds to a variety of chemokines (9), the activity that accumulated in the medium of HCMV-infected cells did not bind at a detectable level to a second radiolabeled CC-chemokine, MIP-1α (Fig. 3_A_ Center), or to a CXC-chemokine, IL-8 (Fig. 3_A_ Right). To confirm the specificity of the binding activity, we performed a competition assay in which pUL21.5 binding to 125I-RANTES was competed with excess, unlabeled RANTES, MIP-1α, or MCP-1. The binding was efficiently competed by RANTES, but no inhibition was observed for the other CC chemokines (Fig. 3_B_).
Fig. 3.
Medium from HCMV-infected cultures contains RANTES-binding activity. (A) Crosslinking assay demonstrating pUL21.5 chemokine-binding activity. Concentrated protein preparations from the medium of mock-, AD_wt_ (wt)-, and AD_sub_UL21.5 (_sub_UL21.5)-infected cultures were incubated with 125I-RANTES, MIP-1α, and IL-8. The mixtures were treated at room temperature for 30 min with the crosslinking agent EDC (40 mM), and the resulting crosslinked complexes were analyzed by electrophoresis in SDS-containing polyacrylamide gels. Size markers are identified by their molecular masses (kDa). (B) Chemokine binding specificity of pUL21.5. Concentrated protein from the medium of AD_wt_-infected infected cultures was incubated with 125I-RANTES in the presence of unlabeled RANTES, MIP-1α, or MCP-1, the mixtures were treated with EDC, and the resulting crosslinked complexes were analyzed by electrophoresis. The fold-excess of unlabeled ligand is indicated above each lane. Size markers are identified by their molecular masses (kDa).
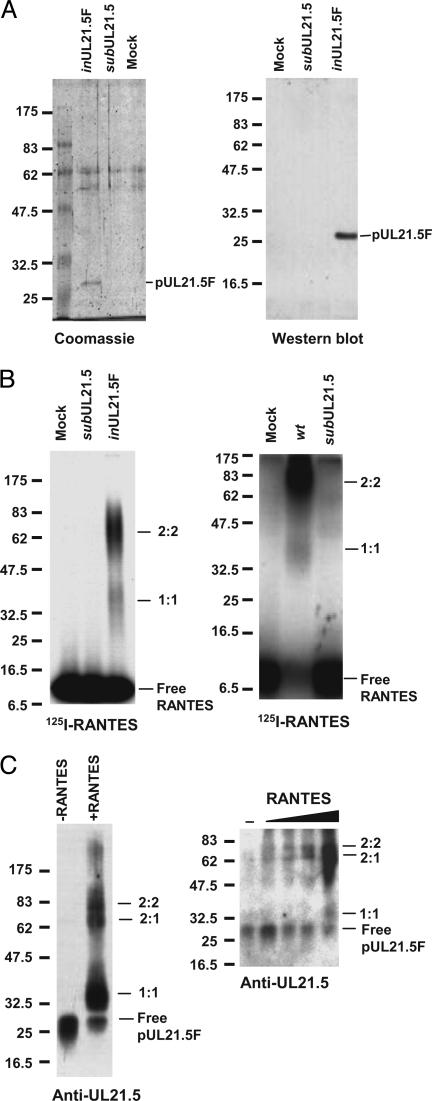
For a direct demonstration that pUL21.5 binds RANTES, we constructed a recombinant HCMV (BAD_in_UL21.5F) in which the wild-type UL21.5 locus was replaced with a modified sequence encoding pUL21.5 with a C-terminal FLAG epitope tag (pUL21.5F), and then we partially purified the fusion protein from the medium of infected cells by using an affinity matrix containing antibody specific to the FLAG epitope. This material was analyzed by PAGE and Coomassie blue staining, and three major polypeptides were evident (Fig. 4_A_ Left). One of the bands was identified as pUL21.5F by using a Western blot assay (Fig. 4_A_ Right). When the partially purified protein was mixed with 125I-labeled RANTES, crosslinked, and examined by electrophoresis, the ≈70-kDa complex was readily detected (Fig. 4_B_ Left), indicating that the complex did indeed result from the binding of pUL21.5 to RANTES. A relatively minor (≈35-kDa) complex was also observed in this assay. An ≈35-kDa complex was also seen in crosslinking reactions using medium from wild-type-virus-infected cells after a long exposure of the auto-radiogram (Fig. 4_B_ Right). To test for the presence of pUL21.5 in the two complexes detected by using 125I-labeled RANTES, we repeated the experiment with unlabeled RANTES, and complex formation was detected by Western blot with a pUL21.5 polyclonal antibody. The antibody reacted with the two complexes (Fig. 4_C_ Left), demonstrating that both contain pUL21.5. We conclude that the complexes each contain both RANTES and pUL21.5, and their size (≈35 and ≈70 kDa, respectively) supports the argument that they are most likely comprised of one molecule each (1:1) and two molecules each (2:2) of RANTES (8 kDa) and pUL21.5F (27 kDa), respectively. When pUL21.5 was incubated with increasing amounts of RANTES, the 2:2 complex could be detected at a lower concentration of RANTES than could the 1:1 complex (Fig. 4_C_ Right), indicating that the 2:2 complex was formed preferentially. A 2:2 stoichiometry is consistent with earlier demonstrations that the gamma herpesvirus 68 M3 protein and vaccinia virus p35 chemokine-binding proteins form dimers (25, 26).
Fig. 4.
Direct demonstration of a pUL21.5–RANTES interaction. (A) Purification and characterization of pUL21.5F. Epitope-tagged protein (pUL21.5F) purified by two sequential rounds of capture and elution from a matrix containing antibody to the FLAG epitope was subjected to electrophoresis and analyzed by Coomassie blue staining (Left) or Western blot assay (Right) by using anti-UL21.5 antibody. Size markers are identified by their molecular masses (kDa). (B) Purified pUL21.5F binds RANTES. Purified pUL21.5F (Left) or concentrated protein preparations from the medium of mock-, _wt_-, and _sub_UL21.5-infected cultures (Right) were incubated with 125I-RANTES and crosslinked by treatment with EDC, and the resulting complexes were analyzed by electrophoresis. Complexes were detected by antoradiography. Size markers are identified by their molecular masses (kDa). (C) RANTES binding stoichiometry. Purified pUL21.5F was incubated with unlabeled RANTES and crosslinked, and the resulting complexes were analyzed by Western blot with anti-UL21.5 antibody. Size markers are identified by their molecular masses (kDa).
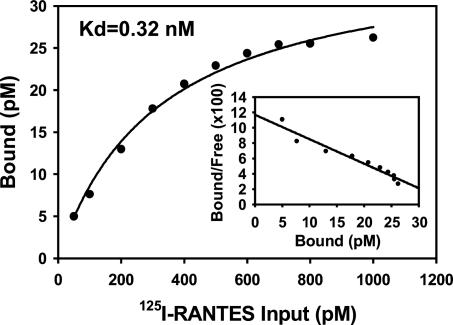
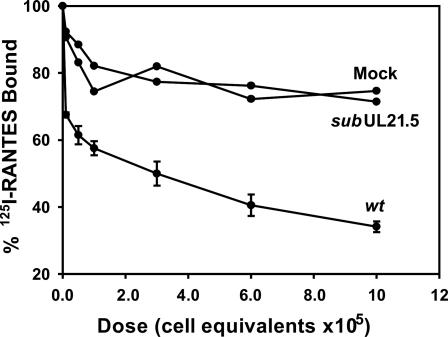
We quantified the binding affinity of pUL21.5 and RANTES by Scatchard analysis (Fig. 5). The interaction was saturable with an apparent affinity of 0.32 nM, comparable to the affinity of the interaction of RANTES with its cellular receptor (0.4 nM) (24). pUL21.5 Blocks the Interaction of RANTES with Cellular Receptors. To investigate the biological consequence of the interaction between pUL21.5 and RANTES, we tested whether the viral protein could block RANTES binding to its cell receptors. As shown in Fig. 6, the binding of RANTES to its native receptors on THP-1 cells was inhibited in a dose-dependent manner by concentrated protein from the medium of cells infected with wild-type HCMV. In contrast, the modest inhibitory effect of medium from cells infected by the pUL21.5-deficient virus was that same as that observed for medium from mock-infected cells. This experiment demonstrates that secreted pUL21.5 protein can block the biological function of RANTES by competing for binding to the CC-chemokine with the normal RANTES cell receptors.
Fig. 5.
Saturation binding of 125I-RANTES to pUL21.5F. Increasing amounts of 125I-RANTES were incubated for 2 h at room temperature with partially purified pUL21.5F that was bound to an anti-FLAG tag affinity matrix. The amount of bound radioactivity was then determined.
Fig. 6.
Inhibition by pUL21.5 of RANTES binding to cellular receptors. Concentrated protein from the medium of mock-, AD_wt_ (wt)-, or AD-_sub_UL21.5 (_sub_UL21.5)-infected fibroblast cultures was preincubated with 125I-RANTES for 1 h at 4°C, followed by the addition of THP-1 cells for an additional 2 h at 4°C. The radioactivity specifically bound to THP-1 cells was determined. The dose of protein from the medium is reported as cell equivalents, i.e., the number of cells in the culture that produced the secreted protein in the assay.
Discussion
HCMV infection activates the production of multiple chemokines, such as RANTES and MCP-1, in endothelial cells (27, 28) and fibroblasts (28, 29). To counteract chemokine-mediated immune responses, HCMV has evolved multiple strategies including the direct suppression of chemokine production (27, 30) and sequestration of chemokine by the virus-encoded US28 G protein-coupled receptor, which acts as a chemokine sink (28, 31). Here we demonstrate that HCMV encodes a secreted protein, pUL21.5, which binds to RANTES (Figs. 3, 4, 5) and antagonizes its function (Fig. 6). Two other CC chemokines, MCP-1 and MIP-1α, as well as the CXC chemokine, IL-8, did not bind pUL21.5 at a detectable level in cross-linking or competition assays (Fig. 3). The RANTES specificity of pUL21.5 is in contrast to the other known viral decoys, which bind multiple chemokines (5–9).
Most chemokines possess two major binding surfaces: a high-affinity site responsible for specific G protein-coupled receptor interactions and a lower-affinity site, also called the glycosaminoglycan-binding domain, believed to be responsible for the establishment of chemokine gradients on the surface of endothelial cells and within the extracellular matrix (32). The specificity and high affinity of the pUL21.5–RANTES interaction argues that it is not likely to be mediated by the RANTES glycosaminoglycan-binding site. MCP-1, MIP-1α, and IL-8 did not interact at a detectable level with pUL21.5 (Fig. 3), although all these chemokines are able to bind glycosaminoglycans. Further, the high affinity of the pUL21.5–RANTES interaction (_K_d ≈ 0.35 nM; Fig. 5) prevents RANTES from binding to its cellular receptors (Fig. 6).
The reason that HCMV produces a protein that specifically antagonizes RANTES and not other CC chemokines is not clear. The inability of pUL21.5 to bind to additional chemokines is not restricted to the AD169 laboratory strain that we have studied, because the sequence of pUL21.5 encoded by the FIX clinical isolate is identical to that produced by AD169. RANTES is a proinflammatory chemokine with a broad range of activities. It is generated by a variety of cell types, including CD8+ T cells, epithelial cells, fibroblasts, and platelets, and its receptors reside in monocytes, dentritic cells, T cells, NK cells, neutrophils, and eosinophils. In addition to its chemotactic activity, RANTES promotes Th1 cell differentiation and activates T cells at high concentrations (33). The unique specificity of pUL21.5 suggests that, during acute or chronic infection, RANTES may play a critical role in mediating an anti-HCMV immune response.
The HCMV pUS28 G protein-coupled receptor homolog has been shown to bind and internalize a variety of CC chemokines including RANTES, removing them from the environment as they contact the receptor in the membranes of infected cells (28, 31). Given the scavenger function of pUS28, why does the virus also produce pUL21.5? It seems likely that one utility of pUL21.5 to the virus is that the receptor decoy is a secreted protein with the potential to act at a distance from the infected cell. Consequently, it can neutralize RANTES-binding activity in the vicinity of uninfected cells that neighbor the infected cells. This neutralization could be particularly useful in the case of local T cells. Oligomerized RANTES is believed to be responsible for T cell activation (34). Thus, by binding to oligomerized RANTES, pUL21.5 would be expected to inhibit RANTES-mediated T cell activation.
Secreted pUL21.5 might serve a second function during the late stage of infection, when the protein accumulates to high levels. Because RANTES can bind to glycosaminoglycans (32), oligomers of RANTES could potentially facilitate the binding of enveloped viruses, such as HCMV, to the surface of cells by bridging between glycosaminoglycans on the viral envelope and cell membrane. Perhaps it is important for secreted pUL21.5 to bind RANTES and antagonize its ability to bridge between newly produced viruses and the surface of the cell in which it was produced as well as other nearby cells. Such an activity could prevent RANTES from trapping the virus in the microenvironment in which it was synthesized and, consequently, facilitate its spread.
In conclusion, pUL21.5 represents a previously uncharacterized class of viral chemokine decoy protein, both in terms of the mechanism by which it is initially expressed in infected cells and in terms of its highly selective targeting of RANTES.
Acknowledgments
We thank H. W. Virgin for generously providing purified recombinant gamma herpesvirus 68 M3 protein, M. Schrader for providing the pp65 polyclonal antibody, G. Hahn (Ludwig-Maximillian University, Munich) for providing the FIX-BAC clone, C. Kulesza (Princeton University) for endothelial cells, J. Schroer (Princeton University) for help with microscopy, and J. Alwine (University of Pennsylvania, Philadelphia) for critical reading of the manuscript. This work was supported by National Institutes of Health Grant CA85786 (to T.S.). D.W. was a Postdoctoral Fellow of the New Jersey Commission on Cancer Research.
Author contributions: T.S. designed research; D.W. and W.B. performed research; D.W. analyzed data; and D.W. and T.S. wrote the paper.
Abbreviations: HCMV, human cytomegalovirus; RANTES, regulated upon activation, normal T cell expressed and secreted.
References
- 1.Britt, W. J. & Alford, C. A. (1996) in Fields Virology, eds. Fields, B. N., Knipe, D. M. & Howley, P. M. (Lippincott-Raven, Philadelphia), Vol. 2, pp. 2493–2523. [Google Scholar]
- 2.Zlotnik, A. & Yoshie, O. (2000) Immunity 12**,** 121–127. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto, F., Mackay, C. R. & Lanzavecchia, A. (2000) Annu. Rev. Immunol. 18**,** 593–620. [DOI] [PubMed] [Google Scholar]
- 4.Murphy, P. M. (2001) Nat. Immunol. 2**,** 116–122. [DOI] [PubMed] [Google Scholar]
- 5.Graham, K. A., Lalani, A. S., Macen, J. L., Ness, T. L., Barry, M., Liu, L. Y., Lucas, A., Clark-Lewis, I., Moyer, R. W. & McFadden, G. (1997) Virology 229**,** 12–24. [DOI] [PubMed] [Google Scholar]
- 6.Smith, C. A., Smith, T. D., Smolak, P. J., Friend, D., Hagen, H., Gerhart, M., Park, L., Pickup, D. J., Torrance, D., Mohler, K., et al. (1997) Virology 236**,** 316–327. [DOI] [PubMed] [Google Scholar]
- 7.Alcami, A., Symons, J. A., Collins, P. D., Williams, T. J. & Smith, G. L. (1998) J. Immunol. 160**,** 624–633. [PubMed] [Google Scholar]
- 8.Parry, C. M., Simas, J. P., Smith, V. P., Stewart, C. A., Minson, A. C., Efstathiou, S. & Alcami, A. (2000) J. Exp. Med. 191**,** 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Berkel, V., Barrett, J., Tiffany, H. L., Fremont, D. H., Murphy, P. M., McFadden, G., Speck, S. H. & Virgin, H. I. (2000) J. Virol. 74**,** 6741–6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bresnahan, W. A. & Shenk, T. (2000) Science 288**,** 2373–2376. [DOI] [PubMed] [Google Scholar]
- 11.Greijer, A. E., Dekkers, C. A. J. & Middeldorp, J. M. (2000) J. Virol. 74**,** 9078–9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terhune, S. S., Schroer, J. & Shenk, T. (2004) J. Virol. 78**,** 10390–10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullberg, J., Hsu, M. L., Rauch, C. T., Gerhart, M. J., Kaykas, A. & Cosman, D. (1999) J. Gen. Virol. 80**,** 437–440. [DOI] [PubMed] [Google Scholar]
- 14.Scott, G. M., Barrell, B. G., Oram, J. & Rawlinson, W. D. (2002) Virus Genes 24**,** 39–48. [DOI] [PubMed] [Google Scholar]
- 15.Murphy, E., Yu, D., Grimwood, J., Schmutz, J., Dickson, M., Jarvis, M. A., Hahn, G., Nelson, J., Myers, R. M. & Shenk, T. (2003) Proc. Natl. Acad. Sci. USA 100**,** 14976–14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andre, E., Imbert-Marcille, B. M., Cantarovich, D., Besse, B., Ferre-Aubineau, V. & Billaudel, S. (1999) Diagn. Microbiol. Infect. Dis. 34**,** 287–291. [DOI] [PubMed] [Google Scholar]
- 17.Elek, S. D. & Stern, H. (1974) Lancet 1**,** 1–5. [DOI] [PubMed] [Google Scholar]
- 18.Revello, M., Baldanti, F., Percivalle, E., Sarasini, A., De-Giuli, L., Genini, E., Lilleri, D., Labo, N. & Gerna, G. (2001) J. Gen. Virol. 83**,** 1993–2000. [Google Scholar]
- 19.Hahn, G., Khan, H., Baldanti, F., Koszinowski, U. H., Revello, M. G. & Gerna, G. (2002) J. Virol. 76**,** 9551–9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borst, E. M., Mathys, S., Wagner, M., Muranyi, W. & Messerle, M. (2001) J. Virol. 75**,** 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldick, C. J., Jr., Marchini, A., Patterson, C. E. & Shenk, T. (1997) J. Virol. 71**,** 4400–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu, D., Smith, G. A., Enquist, L. W. & Shenk, T. (2002) J. Virol. 76**,** 2316–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brune, W., Nevels, M. & Shenk, T. (2003) J. Virol. 77**,** 11633–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, J. M., McVicar, D. W., Oppenheim, J. J. & Kelvin, D. J. (1993) J. Exp. Med. 177**,** 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carfi, A., Smith, C. A., Smolak, P. J., McGrew, J. & Wiley, D. C. (1999) Proc. Natl. Acad. Sci. USA 96**,** 12379–12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexander, J. M., Nelson, C. A., van Berkel, V., Lau, E. K., Studts, J. M., Brett, T. J., Speck, S. H., Handel, T. M., Virgin, H. W. & Fremont, D. H. (2002) Cell 111**,** 343–356. [DOI] [PubMed] [Google Scholar]
- 27.Billstrom Schroeder, M. & Worthen, G. S. (2001) J. Virol. 75**,** 3383–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph-Habecker, J. R., Rahill, B., Torok-Storb, B., Vieira, J., Kolattukudy, P. E., Rovin, B. H. & Sedmak, D. D. (2002) Cytokine 19**,** 37–46. [DOI] [PubMed] [Google Scholar]
- 29.Michelson, S., Dal Monte, P., Zipeto, D., Bodaghi, B., Laurent, L., Oberlin, E., Arenzana-Seisdedos, F., Virelizier, J. L. & Landini, M. P. (1997) J. Virol. 71**,** 6495–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch, A. J. & Shenk, T. (1999) J. Virol. 73**,** 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodaghi, B., Jones, T. R., Zipeto, D., Vita, C., Sun, L., Laurent, L., Arenzana-Seisdedos, F., Virelizier, J. L. & Michelson, S. (1998) J. Exp. Med. 188**,** 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez, E. J. & Lolis, E. (2002) Annu. Rev. Pharmacol. Toxicol. 42**,** 469–499. [DOI] [PubMed] [Google Scholar]
- 33.Appay, V. & Rowland-Jones, S. L. (2001) Trends Immunol. 22**,** 83–87. [DOI] [PubMed] [Google Scholar]
- 34.Appay, V., Brown, A., Cribbes, S., Randle, E. & Czaplewski, L. G. (1999) J. Biol. Chem. 274**,** 27505–27512. [DOI] [PubMed] [Google Scholar]