Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification (original) (raw)
Abstract
Collagenase-3 (MMP13), a member of the matrix metalloproteinase (MMP) family of neutral endopeptidases, is expressed in the skeleton during embryonic development and is highly overexpressed in human carcinomas and in chondrocytes and synovial cells in rheumatoid arthritis and osteoarthritis. To determine the functional roles of Mmp13, we generated _Mmp13_-null mice that showed profound defects in growth plate cartilage with markedly increased hypertrophic domains as well as delay in endochondral ossification and formation and vascularization of primary ossification centers. Absence of Mmp13 resulted in significant interstitial collagen accumulation due, in part, to the lack of appropriate collagenase-mediated cleavage that normally occurs in growth plates and primary ossification centers. Cartilaginous growth plate abnormalities persisted in adult mice and phenocopied defects observed in human hereditary chondrodysplasias. Our findings demonstrate a unique role of Mmp13 in skeletal development.
Keywords: collagen, extracellular matrix, vascularization
Collagenases, a group of matrix metalloproteinases (MMPs) that act at neutral pH (1–4), have been postulated to have a role in skeletal development and bone remodeling (5–8). The MMPs are members of a large family of proteinases that have several structural features in common including the presence of a conserved zinc-binding catalytic domain (1–4). Only the products of specific MMP genes, MMP1, -2, -8, -13, and -14, however, have the capacity to cleave native, undenatured, interstitial collagens at a specific helical locus (9–13). Of the collagenases, MMP13 (collagenase-3) has been considered to have an important role in skeletal biology in view of its exclusive presence in the skeleton during embryonic development in cartilaginous growth plates and primary centers of ossification (5–8). MMP13 is also a downstream target of parathyroid hormone (PTH)-related protein (PTHrP) (14) and the transcription factor Osf2/Cbfa1/Runx2 in growth plate chondrocytes (15, 16). In contrast to humans, where MMP1 may be strongly expressed, e.g., in inflammation, the orthologue of MMP1, McolA (12), is expressed in mice only at low levels.
To examine possible functional roles of collagenases during skeletal development in vivo, we targeted a null mutation to the Mmp13 gene in mice. Our targeting strategy resulted in splicing out exon 5 that encodes the zinc-binding residues in the catalytic domain. As described here, deletion of functional Mmp13 had profound effects on skeletal development. In _Mmp13_–/– embryos compared with WT embryos, the growth plates were strikingly lengthened, a defect ascribable predominantly to a delay in terminal events in the growth plates, with failure to resorb collagens, as well as a delay in ossification at the primary centers.
Materials and Methods
Generation of _Mmp13_–/– Mice. We isolated two Mmp13 genomic clones from a 129/J1 library to construct the knockout vector. The first was a _Bam_HI/Sal_I fragment that spanned from ≈3.4 kb of promoter sequence through the first ≈1.4 kb of intron 4, cloned into pT7Blue-3. A PGK_neo cassette was inserted between _Sal_I and _Xba_I sites that resulted from the cloning. The second genomic clone served as template for PCR with primers in intron 4 and exon 9 that included new _Xba_I sites to serve as the 3′ arm (≈4.5 kb) of the final construct. The forward primer also contained sequences of the intron 4/exon 5 junction followed by codons for the first two amino acids (YN), a TAA stop codon with the last 2 bp of the F262 codon deleted, altering the coding for the critical zinc-binding sequences in the catalytic domain. This targeting construct thus encodes a truncated protein, but during transcription, exon 5 might be spliced out.
For gene targeting, the knockout vector was purified and two clones were linearized in the 5′ or 3′ polylinker and electroporated into J1 embryonic stem (ES) cells. With the vector linearized at the 5′ end, of 200 G418-resistant clones screened by PCR, seven had correct integration, confirmed in four by Southern blotting. With the vector linearized at the 3′ end, four clones had correct integration. ES cells with correct integration were injected into C57BL/6 blastocysts, and overtly chimeric males were obtained and mated with WT C57BL/6 females. DNA from tail snips of agouti offspring was analyzed by PCR and Southern blotting. Germline transmission was successful when using each of the two independently derived ES cell clones and resulted in similar phenotypes.
Tissue Processing and Analysis. Mice were killed by CO2 narcosis to obtain tissue. Bones were removed, cleaned, fixed in 10% phosphate-buffered formalin, decalcified in 14% EDTA, and dehydrated in graded alcohol for paraffin embedding. Longitudinal 5-μm sections were prepared from embryos and newborns through the epiphyses and shafts of long bones. Femurs from adult mice were fixed in 70% ethanol and embedded in glycol methacrylate, and undecalcified 3-μm sections were stained with toluidene blue.
Immunohistochemistry and Immunoblotting. Antibodies used for immunohistochemistry and Western blotting were as follows. A rabbit polyclonal antibody to the N-terminal neoepitope QRGIV (amino acids 779–783) in the collagenase cleavage C-terminal 1/4 fragment (Bα1[II]) of mouse and chick type II collagen and QRGVV in Bα1[I] of type I collagen was generously provided by Eunice Lee (Shriners Hospital for Children, Montreal). Epitope specificity of the antibody to QRGIV was demonstrated in Western blots of collagenase-digested collagens. Type I collagen was extracted and purified from mouse skin (17), and chick type II collagen was from Sigma. Human MMP13 cDNA in the pcDNA 3.1 vector was transfected into human embryonic kidney (HEK) 293 cells, and conditioned medium was harvested. Collagenase digestion and analysis was performed as described in ref. 17; proMMP13 was activated by using 1 mM _p_-aminophenylmercuric acetate. The mouse mAb 9A4 (18), which detects a C-terminal neoepitope (amino acids 769–775) in collagenase-cleaved collagens [in the α 1(II) chains of type II collagen and in the α 1(I) chains of type I collagen], was generously provided by Ivan Otterness (Central Research Division, Pfizer, Groton, CT) and was used as described in ref. 19. The rabbit polyclonal antibody against the C-terminal domain of mouse type X collagen was generously provided by Bjorn Olsen (Harvard Medical School). The rabbit polyclonal antibody to CD31 was from Santa Cruz Biotechnology.
Aliquots of conditioned medium of calvarial cultures or Mmp13 digests of mouse type I and chick type II collagens were resolved by SDS/PAGE with 10% polyacrylamide gels (17), and the proteins were transferred to polyvinylidene difluoride membranes (Hybond-P, Amersham Biosciences). After blocking for 1 h with 5% nonfat dry milk in PBS/0.1% Tween 20 at 4°C, membranes were incubated for 2 h with an anti-Mmp13 mAb (Chemicon). After incubation with horseradish peroxidase-conjugated rabbit anti-mouse IgG for 1 h, immunoreactive bands were detected by using an ECL system (Amersham Biosciences).
For tissue immunohistochemistry, paraffin sections were rehydrated with graded alcohol and blocked with 5% nonfat dry milk for 30 min, and primary antibodies were applied for 2 h. After incubation with biotin-conjugated secondary antibody for 1 h, sections were reacted either with the AEC substrate kit for peroxidase (Vector Laboratories) for light microscopy or with streptavidin-conjugated Alexa Fluor 568 (Molecular Probes) for fluorescence microscopy. Images were obtained by using a Nikon metallurgical microscope (Nikon, Melville, NY) and recorded by using the SPOT RT Slider digital camera system (Diagnostic Instruments, Sterling Heights, MI).
In Situ Hybridization and RNA Analysis. Paraffin sections were used for in situ hybridization with uridine 5′-[α-35S]thiotriphosphate-labeled antisense riboprobes (Amersham Biosciences) (20). Two different Mmp13 cRNA probes were used: 5′probe, bp 1–695; exon 5 probe, bp 627–816 (GenBank accession no. NM_008607). RNA was isolated from distal femurs and proximal tibias, including knee joints, and analyzed by Northern blotting (21, 22). Quantification of mRNA levels was achieved by real-time PCR with either TaqMan probes or SYBR Green and the ABI Prism 7700 sequence detector (Applied Biosystems). Standard curves for each gene were established by using cDNA templates. Primer sequences are available on request.
Calvarial Organ Cultures. Calvariae from 5-day-old mice were isolated aseptically, cleaned, and cultured for 16 h at 37°C under 5% CO2 in air in 0.5 ml of BGJb medium (Life Technologies) containing 1 mg/ml BSA (fraction V, Sigma) (21, 22). Half calvariae were transferred to fresh medium with or without 0.1 μM PTH or 1 nM IL-1α and cultured for an additional 5 days. Conditioned medium was collected for analysis by Western blotting, and RNA was extracted from tissue for Northern blotting and quantification by real-time PCR.
Statistical Analysis. Some data are expressed as means ± SEM. Significance of differences was analyzed by using Student's t test.
Results
_Mmp13_-Null Mutation. The proportion of WT (+/+), Mmp13+/–, and _Mmp13_–/– embryos, based on Southern blot (Fig. 1 A and B) and PCR analysis, was consistent with Mendelian predictions. Newborn _Mmp13_–/– mice appeared healthy. To assess transcription of mutant Mmp13, total RNA extracted from calvariae of 5-day-old mice was analyzed by RT-PCR with primers that spanned from exon 4 to exon 7. We found a single band of ≈430 bp, as predicted, in WT calvariae but a band of only ≈268 bp in _Mmp13_–/– calvariae, consistent with a transcript lacking exon 5 (confirmed by DNA sequencing). No band of 333 bp corresponding to a TAA stop codon-truncated exon 5 was detected. We expressed in E. coli a Mmp13 cDNA with exon 5 sequences deleted and found it to be devoid of collagenase activity (data not shown).
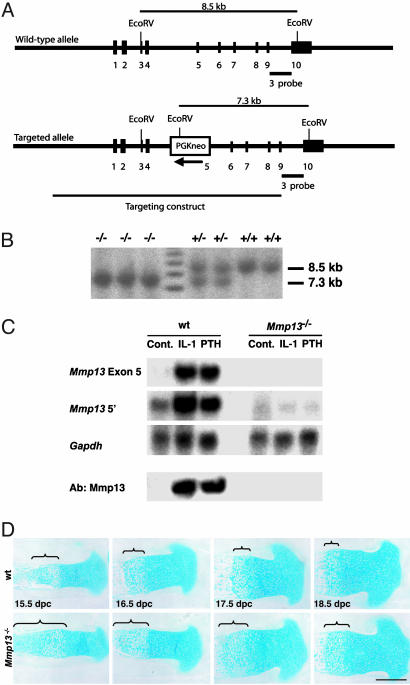
Fig. 1.
Generation of _Mmp13_–/– mice. (A) Schematic representation of the WT Mmp13 locus, the targeting vector, the targeted allele, the position of _Eco_RV sites, and the probe used for Southern blot analysis. (B) Southern blot analysis of genomic DNA from littermate progeny of Mmp13+/– breeding. Probes of _Eco_RV-digested DNA identified the fragments of 8.5 and 7.3 kb for WT and disrupted alleles, respectively. (C) Northern blot analysis of calvarial RNA after calvarial organ cultures for 5 days with or without IL-1α or PTH, and Western blot analysis of conditioned medium from these organ cultures. Levels of Mmp13 mRNA increased in WT calvariae exposed to IL-1α or PTH in contrast with _Mmp13_–/– calvariae. A marked increase in Mmp13 (Ab:Mmp13) is seen in Western blots from WT calvarial conditioned medium, but no increase in Mmp13 is seen in _Mmp13_–/– calvarial conditioned medium. (D) Skeletal development in WT and _Mmp13_–/– 15.5- to 18.5-dpc embryos. Sections of proximal tibias were stained with Alcian blue. Increases in length of the hypertrophic zones of the growth plates, indicated by the brackets, are seen in the _Mmp13_–/– tibias. (Scale bar: 400 μm.)
Levels of Mmp13 mRNA increased markedly in cultured WT calvariae incubated either with IL-1α or PTH (Fig. 1_C_). In contrast, there was no detectable Mmp13 mRNA in _Mmp13_–/– calvariae when using the exon 5 probe and barely detectable mRNA when using the 5′ probe. Western blots of conditioned medium showed a marked increase in Mmp13 levels induced by PTH or IL-1α in cultures of WT calvariae; no Mmp13 was detected in medium from cultures of _Mmp13_–/– calvariae (Fig. 1_C_). The antibody used in these assays recognizes _Escherichia coli_-expressed mutant (exon 5 deleted) Mmp13.
Abnormalities of the Limb Growth Plates and Delay in Ossification in _Mmp13_–/– Mice. In sections of long bones from hind limbs (Fig. 1_D_), the length of the growth plates in _Mmp13_–/– embryos was increased compared with _Mmp1_3+/– or WT embryos and was maintained throughout development. The length of the total growth plate in proximal tibias (measured from articular surface to primary spongiosa) was increased in _Mmp13_–/– embryos vs. WT embryos by ≈20% and that of the hypertrophic zone by ≈70%. In addition, the chondrocyte columns in _Mmp13_–/– mice were less well aligned and were disordered compared with those in WT mice. These abnormalities persisted in newborns and adults and were still evident in 4-month-old mice.
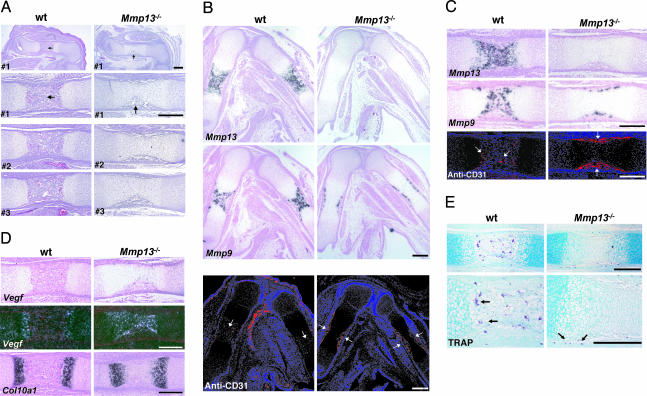
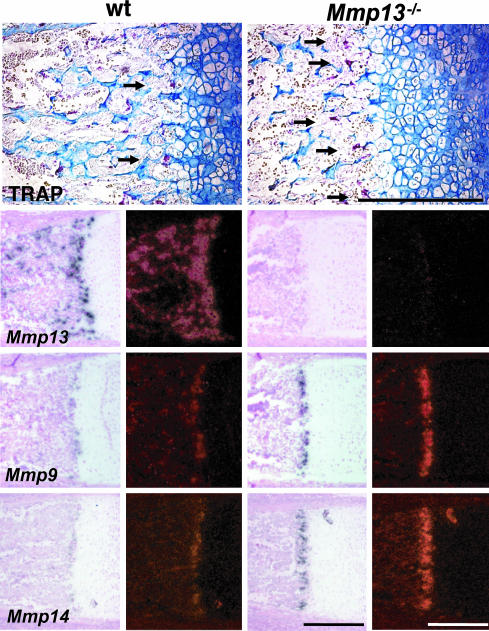
There was a profound delay in development of the primary ossification centers in _Mmp13_–/– mice. In 15.5-dpc WT embryos (dpc, days postconception), primary ossification centers were fully formed in femurs and tibias (Fig. 2_A_), in contrast with _Mmp13_–/– embryos, where primary centers were not formed and only a diaphyseal periosteal collar was visible. In adjacent sections (Fig. 2 B and C), Mmp13 expression was prominent in the primary ossification centers in WT embryos but was not detected in _Mmp13_–/– embryos. The pattern of immunostaining for CD31, an endothelial cell marker, in WT embryos correlated with that of Mmp13 expression, i.e., prominent within the well formed primary ossification centers. Staining was limited to the periosteal collar, however, in the _Mmp13_–/– embryos (Fig. 2 B and C). Osteoclasts that express tartrate resistant acid phosphatase (TRAP) as well as Mmp9 are apparently important in initial phases of development of primary ossification centers (23). In 15.5-dpc embryos, the pattern of Mmp9 (Fig. 2 B and C) expression in WT and _Mmp13_–/– embryos mirrored that of CD31. Abundant TRAP+ osteoclasts (Fig. 2_E_) were present in WT ossification centers, but TRAP+ cells were limited to the diaphyseal collar in _Mmp13_–/– mice. In _Mmp13_–/– mice, impaired vascularization (shown by CD31 staining) correlated with the defect in development of primary ossification centers and the absence of Mmp13; expression of Mmp9 (24) limited to the periosteal cuff did not compensate for the absence of Mmp13. Nevertheless, by 17.5 dpc, when growth plates were still lengthened, primary ossification centers had formed and abundant TRAP+ cells were found there in _Mmp13_–/– and WT mice (arrows in Fig. 3) and were particularly prominent in the primary spongiosa near the edge of the cartilaginous growth plates where Mmp9 was also highly expressed. Osteoclasts express Mmp14 (MT1-Mmp) as well, and _Mmp14_–/– mice have defects in endochondral ossification (25, 26). In our studies, Mmp14 was expressed in 17.5-dpc embryos in a pattern similar to that of TRAP and Mmp9 with higher levels in the _Mmp13_–/– bones compared with WT bones (Fig. 3). Collagenase-2 (Mmp8) was not detected in 15.5- or 17.5-dpc WT embryos but was expressed at low levels in distal growth plates and primary centers of ossification of 15.5- and 17.5-dpc _Mmp13_–/– embryos (data not shown). In newborn WT mice, expression of Mmp8 was noted in bone marrow cavities; levels higher than in WT were observed in newborn _Mmp13_–/– mice (data not shown). Using real-time PCR with RNA extracted from the distal femurs and proximal tibias of newborn WT and _Mmp13_–/– mice, Mmp8 mRNA levels were higher in _Mmp13_–/– mice than in WT mice (mean 12.6 vs. 6.8 × 103 molecules per ng of RNA). Mmp9 mRNA levels were also higher in _Mmp13_–/– than in WT mice (mean 3.6 vs. 2.2 × 104 molecules per ng of RNA), consistent with the results obtained by in situ hybridization.
Fig. 2.
Analysis of long bones from WT and _Mmp13_–/– mice. (A) Sections of femurs from three different WT and _Mmp13_–/– 15.5-dpc embryos stained with hematoxylin/eosin. The top panels are low-power views, and the other panels are higher-power views. Primary ossification centers are fully formed in WT samples, but only periosteal cuffs (arrows) are fully formed in _Mmp13_–/– samples. (Scale bar: 300 μm.) (B) Adjacent sections of femurs and tibias from 15.5-dpc embryos. In situ hybridization was used for analysis of Mmp13 and -9 mRNAs, and immunohistochemistry was used for analysis of CD31 (seen as red on blue-black background). Note CD31 (arrows) in primary centers of WT bones but restricted to periosteal cuffs in _Mmp13_–/– bones. (Scale bar: 300 μm.) (C) Higher-power images of mid-femurs shown in B. (Scale bar: 300 μm.) (D) Mid-femurs as in C. In situ hybridization was used for analysis of Vegf and Col10a1 mRNAs. (Scale bar: 300 μm.) (E) Mid-femurs as in A stained for TRAP. Note the TRAP+ cells (arrows). (Scale bar: 300 μm.)
Fig. 3.
Proximal tibias from 17.5-dpc embryos showing TRAP+ cells (arrows) and analysis by in situ hybridization for Mmp13,-9, and -14 mRNAs. (Scale bar: 300 μm.)
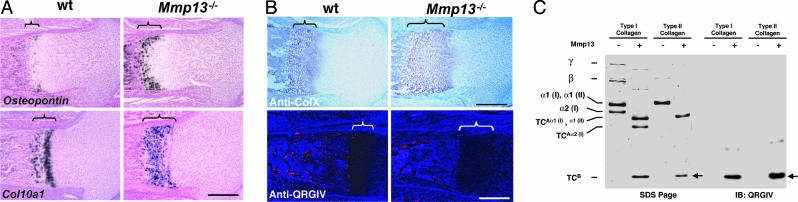
Extracellular Matrix (ECM) Proteins in Endochondral Ossification. Type X collagen is normally produced only by hypertrophic chondrocytes in the distal growth plate (27, 28), and mutations in Col10a1 cause chondrodysplasias (28). As shown in Fig. 2_D_, in 15.5-dpc embryos and in Fig. 4_A_, in newborns, there was comparable intensity of expression of Col10a1 in growth plates from WT and _Mmp13_–/– mice. The anatomic domain where Col10a1 was expressed, however, was increased in _Mmp13_–/– mice vs. WT mice, corresponding to the increase in length of the zone of hypertrophy and consistent with increased numbers of hypertrophic chondrocytes in _Mmp13_–/– mice vs. WT mice. There was also a striking increase in the domain where type X collagen was deposited (immunohistochemistry) in the growth plates in _Mmp13_–/– mice vs. WT mice (Fig. 4_B_). The increase in deposition of type X collagen in the _Mmp13_–/– embryos could thus be due to decreased resorption (lack of collagenase) or increased synthesis. The domain of expression of osteopontin, another marker of chondrocyte hypertrophy (29, 30), was also increased in the distal growth plates from the _Mmp13_–/– mice vs. WT mice (Fig. 4_A_), consistent with the observed increase in hypertrophic chondrocytes and delay in ossification.
Fig. 4.
Analysis of tibias and femurs. (A) In situ hybridization for Osteopontin and Col10a1 mRNAs in proximal tibias from 18.5-dpc embryos. Brackets indicate the lengths of hypertrophic zones. (Scale bar: 300 μm.) (B) Proximal femurs from newborn (P0) mice. Sections were stained for type X collagen and for QRGIV epitope in collagenolytic TCB fragments. Brackets indicate the lengths of hypertrophic zones. Note the absence of anti-QRGIV staining [shown as red on blue-black background] in distal growth plates and primary centers of _Mmp13_–/– bones. (Scale bar: 300 μm.) (C) SDS/PAGE stained with Coomassie blue (left) and Western blots (right) of collagenase (MMP13) digests of types I and II collagens. The TCBα 1 fragments are indicated by arrows. The faster-moving TCBα2(I) fragment of type I collagen moved off the gel. The QRGIV antibody, which detects the neoepitope in the TCBα 1(I) and TCBα 1(II) collagenase-cleavage fragments of types I and II collagens, respectively, was used in the immunoblots (IB:QRGIV).
We provide evidence that Mmp13 expressed in distal growth plates and primary ossification centers normally functions in collagen degradation. Staining with the antibody that detects the QRGIV sequence in the C-terminal (TCB) type II collagen fragment was observed in distal growth plates in WT mice (Fig. 4_B_). This antibody also detects the QRGVV epitope in the TCBα1(I) type I collagen fragment (Fig. 4_B_), which can account for staining also observed in the WT primary ossification centers. In contrast, no staining was detected in distal growth plates from _Mmp13_–/– mice and only faint staining was detected in the bone marrow cavities where other _Mmp_s, e.g., Mmp8, are also expressed, particularly after birth when these cavities are populated by _Mmp8_-expressing hematopoietic cells. Staining with mAb 9A4 (18) that detects sequences in TCA type II collagenolytic fragments was seen in the intercellular septae of distal growth plates and primary centers from newborn WT mice but not from _Mmp13_–/– mice (data not shown). Lack of staining with antibody to QRGIV and mAb 9A4 in growth plates from _Mmp13_–/– mice suggests that Mmp13 is normally the collagenase responsible for collagen degradation in growth plate cartilage during endochondral ossification.
Expression of Regulatory Genes in Growth Plates and Primary Ossification Centers. Intensity of expression by in situ hybridization of PTH/PTHrP receptor, Indian hedgehog, and its receptor, patched, all regulatory factors in developing growth plates (31, 32), was similar in WT and _Mmp13_–/– mice (data not shown). Because vascularization and influx of osteogenic cells from the primary center of ossification are determinants of the length of the hypertrophic zone in developing growth plates, we assessed expression of vascular endothelial growth factor (Vegf) (24, 33, 34). As shown in long bones from 15.5-dpc embryos (Fig. 2_D_), expression of Vegf, using in situ hybridization, was limited to cells in the distal growth plates in WT mice but included cells in the entire primary center of ossification in _Mmp13_–/– mice. The observed pattern of CD31 staining in 15.5-dpc embryos (Fig. 2 B and C) is consistent with decreased vascular ingrowth and might be explained by sequestration of Vegf in the cartilage ECM as proposed by Vu et al. (24), to explain the phenotype of _Mmp9_–/– mice.
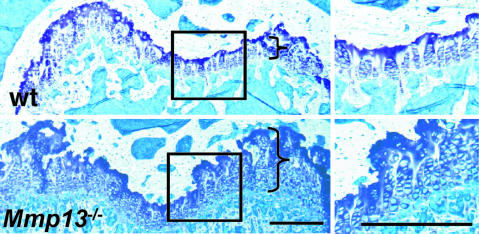
Persistent Abnormal Skeletal Phenotype in Adult _Mmp13_–/– Mice. We emphasize that the formation of primary ossification centers in the _Mmp13_–/–mice, although delayed during embryonic development, began to normalize after birth. We found no gross abnormality in skull or spine of newborn mice. Abnormal growth plates were still present, however, in 4-wk-old mice and were associated with a tendency toward metaphyseal flaring and increased trabecular bone mass (data not shown). The _Mmp13_–/– femurs were also shorter than the WT femurs by ≈8%, measured in mice at ages 4 wk (P < 0.01) and 12 wk (P < 0.001). As shown in sections of distal femurs from 12-wk-old _Mmp13_–/– mice compared with WT mice (Fig. 5), there was persistent lengthening of growth plates, increased numbers of chondrocytes, and irregularly aligned chondrocyte columns as shown previously in embryos (Figs. 2_E_ and 3).
Fig. 5.
Sections of distal femoral growth plates from 12-wk-old WT and _Mmp13_–/– mice. Brackets indicate the approximate lengths of the growth plates. Higher-magnification views of the boxed areas in Left are shown in Right. Note the increased lengths of the growth plates and the irregular arrangement of the chondrocyte columns in _Mmp13_–/– samples vs. WT samples. (Scale bar: 300 μm.)
Discussion
MMP13 (collagenase-3), a highly expressed collagenolytic MMP in developing bone and cartilage, has been assigned a role in the joint tissue destruction that is a major feature of various forms of human arthritis (3, 35, 36). We demonstrate here that a targeted null mutation in mouse Mmp13 resulted in a profound embryonic and adult skeletal phenotype characterized by abnormal growth plates and delayed ossification. During embryonic development at the earliest stage examined, _Mmp13_–/– mice had growth plates in long bones almost double in length, accounted for by increases in the zone of hypertrophy. Among the processes that could account for increase in the hypertrophic zone are decreased proteolysis of the ECM, increased proliferation of chondrocytes in more proximal portions of growth plates, decreased apoptosis of the terminal hypertrophic chondrocytes and decreased resorption of calcified cartilage by cells entering with vascular ingrowth from the primary centers of ossification. Several of these potential mechanisms are operative in the Mmp13-deficient mice.
With regard to decreased proteolysis, we focused on collagens in the ECM in distal growth plates. Mmp13, produced by chondrocytes but not by osteoclasts/chondroclasts, is particularly effective in proteolysis of type II compared to type I collagen with a high _V_max and low _K_M (35). Using antibodies that detect epitopes in the specific proteolytic fragments, we obtained evidence for Mmp13 cleavage of type II collagen in vivo in WT mice but not in _Mmp13_–/– mice. It is thus unlikely that other Mmps compensate for the loss of Mmp13 function in cartilage. The delay in ossification so prominent in 15.5-dpc _Mmp13_–/– embryos is largely transient. Mmp8 is clearly expressed in newborn _Mmp13_–/– and WT skeletons (37), and the faint signal in the primary centers of ossification that we detected in newborn _Mmp13_–/– mice by using the QRGIV TCB cleavage fragment antibody could be ascribed to action of Mmp8, produced by hematopoietic cells, on type I collagen. Type X collagen is also a substrate for MMP1 and Mmp13 (38, 39). We found that the area of type X collagen deposition was significantly increased in growth plates from _Mmp13_–/– mice, consistent with decreased proteolysis. Thus, decreased proteolysis of cartilage ECM is the most likely explanation for our findings in _Mmp13_–/– mice. Nevertheless, because the area where Col10a1 was expressed was also greater in _Mmp13_–/– mice, increased synthesis of type X collagen could also contribute. The expression of osteopontin, another molecular marker of chondrocyte hypertrophy (29, 30), was also increased in _Mmp13_–/– growth plates. Expanded domains of type X collagen and osteopontin have also been found in mice with targeted misexpression of Dlx5, a positive regulator of chondrocyte maturation (40).
Increased accumulation of type II collagen in the absence of functional Mmp13 and decreased collagen degradation could secondarily regulate development of the hypertrophic chondrocyte phenotype. An α2-integrin-mediated interaction of osteoblasts with type I collagen is required for activation of Osf2/Cbfa1/Runx2 and induction of osteoblast-specific gene expression in bone (41, 42). Osf2/Cbfa1/Runx2 also acts as a chondrocyte differentiation factor (42–44). Type II and/or type X collagens might thus have roles in the regulation of cartilage-specific gene expression similar to those of type I collagen in the regulation of bone-specific gene expression. Further support for a regulatory role for Mmp13 comes from observations on _c-maf_-null mice (45). The transcription factor c-Maf is normally expressed in late hypertrophic chondrocytes and in 15.5-dpc _c-maf_-null embryos; intensity of Mmp13 expression in these cells was markedly reduced. Thus, Mmp13 could be a direct target of c-Maf (45). Later in development, however, when the hypertrophic domain was expanded, Mmp13 expression normalized in the _c-maf_-null mice. Because we found increased numbers of hypertrophic chondrocytes in growth plates from _Mmp13_–/– mice, increased entry of cells into the hypertrophic chondrocyte pool is a possibility. The cyclin-dependent kinase inhibitor p57Kip2, which normally participates in the coordination and differentiation of growth plate chondrocytes (46), might have a role. The metatarsal growth plates from 2-wk-old _Mmp9_–/– mice were markedly lengthened (24). Mmp9 is not a collagenase, however, and in our _Mmp13_–/– mice, growth plates were abnormal despite increased Mmp9 expression. We tried terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling staining (19) to assess apoptosis of hypertrophic chondrocytes but noted so few apoptotic cells in the distal growth plate in any of the mice (data not shown) that apoptosis could not explain the differences in _Mmp13_–/– and WT mice.
Our observations are also consistent with altered vascular penetration into the primary centers of ossification in _Mmp13_–/– mice. Binding of Vegf by infusion of dimerized soluble receptor Flt-1 reversibly delayed formation of the primary spongiosa and increased the length of the hypertrophic zone of distal growth plates (33). Evidence was presented suggesting that Vegf was sequestered in the cartilage ECM of _Mmp9_–/– mice and unavailable to bind to its receptor on vascular endothelial cells (24). Decreased availability of Vegf in the absence of the proteolytic function of Mmp13 might also contribute to the widened growth plates. Defects in general postnatal angiogenesis as well as more specific vascularization of cartilage have been described in _Mmp14_–/– mice where hypertrophic zones in growth plates are also lengthened (25, 26). An aspect of the phenotype in the _Mmp14_–/– mice that was not observed in the _Mmp13_–/– mice was abnormal vascularization of the epiphyses and delayed formation of secondary centers of ossification. Mmp14 deficiency might result in failure to activate proMmp13 through a proteolytic cascade also involving Mmp2 (26). Defects in vascularization might therefore contribute to the delayed osteoclast migration observed in the _Mmp13_–/– mice because, despite normal or increased Mmp9 and Mmp14 expression, osteoclast penetration is clearly retarded.
Based on our results, Mmp13 has a critical role in regulating events in the growth plate beginning in embryonic development. Deficiency of Mmp13 with failure to normally resorb collagens in the cartilage ECM profoundly affects cellular activities that underlie differentiation of hypertrophic chondrocytes that persist in newborn and adult mice. The phenotype of adult _Mmp13_–/– mice with increased length of growth plates, increased numbers of chondrocytes, and distortion of alignment of the rows of chondrocytes thus has features of a chondrodysplasia. It is pertinent that a form of human chondrodysplasia, the Missouri variant of spondyloepimetaphyseal dysplasia (47), is caused by a mutation in MMP13.∥
Acknowledgments
We thank Dr. En Li and the Massachusetts General Hospital Transgenic Core Service for help with the Mmp13 knockout procedures, Dr. Ernestina Schipani (Endocrine Unit, Massachusetts General Hospital) for help with in situ hybridization, and Dr. Eunice Lee for the generous gift of the antibody to the QRGIV sequence in collagens. This work was supported by a Schering Fellowship from the Japanese Menopause Society (to M.I.), European Union Grant FP6-CancerDegradome (to C.L.-O.), and National Institutes of Health Grants RO1AR44815 and P50AR44855 (to S.M.K.).
Abbreviations: dpc, days postconception; ECM, extracellular matrix; PTH, parathyroid hormone; TRAP, tartrate resistant acid phosphatase.
Footnotes
∥
Kennedy, A. M., Christie, P. T., Harding, B., Pannett, A. A. J., Dearlove, A., Whyte, M. P. & Thakker, R. V. (2002) J. Bone Miner. Res. 17, Suppl 1., S175 (abstr.).
References
- 1.Nagase, H. & Woessner, J. F., Jr. (1999) J. Biol. Chem. 274**,** 21491–21494. [DOI] [PubMed] [Google Scholar]
- 2.Sternlicht, M. D. & Werb, Z. (2001) Annu. Rev. Cell Dev. Biol. 17**,** 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy, G., Knäuper, V., Atkinson, S., Butler, G., English, W., Hutton, M., Stracke, J. & Clark, I. (2002) Arthritis Res. 4**,** Suppl. 3, S39–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamenkovic, I. (2003) J. Pathol. 200**,** 448–464. [DOI] [PubMed] [Google Scholar]
- 5.Mattot, V., Raes, M. B., Henriet, P., Eeckhout, Y., Stehelin, D., Vandenbunder, B. & Desbiens, X. (1995) J. Cell Sci. 108**,** 529–535. [DOI] [PubMed] [Google Scholar]
- 6.Fuller, K. & Chambers, T. J. (1995) J. Cell Sci. 108**,** 2221–2230. [DOI] [PubMed] [Google Scholar]
- 7.Gack, S., Vallon, R., Schmidt, J., Grigoriadis, A., Tuckermann, J., Schenkel, J., Weiher, H., Wagner, E. F. & Angel, P. (1995) Cell Growth Differ. 6**,** 759–767. [PubMed] [Google Scholar]
- 8.Stähle-Backdahl, M., Sandstedt, B., Bruce, K., Lindahl, A., Jimenez, M. G., Vega, J. A. & López-Otín, C. (1997) Lab. Invest. 76**,** 717–728. [PubMed] [Google Scholar]
- 9.Aimes, R. T. & Quigley, J. P. (1995) J. Biol. Chem. 270**,** 5872–5876. [DOI] [PubMed] [Google Scholar]
- 10.Seandel, M., Noack-Kunnmann, K., Zhu, D., Aimes, R. T. & Quigley, J. P. (2001) Blood 97**,** 2323–2332. [DOI] [PubMed] [Google Scholar]
- 11.Ohuchi, E., Imai, K., Fujii, Y., Sato, H., Seiki, M. & Okada, Y. (1997) J. Biol. Chem. 272**,** 2446–2451. [DOI] [PubMed] [Google Scholar]
- 12.Balbín, M., Fueyo, A., Knäuper, V., López, J. M., Álvarez, J., Sánchez, L. M., Quesada, V., Bordallo, J., Murphy, G. & López-Otín, C. (2001) J. Biol. Chem. 276**,** 10253–10262. [DOI] [PubMed] [Google Scholar]
- 13.Gross, J. (1981) in Cell Biology of the Extracellular Matrix, ed. Hay, E. D. (Plenum, New York), pp. 217–258.
- 14.Selvamurugan, N., Pulumati, M. R., Tyson, D. R. & Partridge, N. C. (2000) J. Biol. Chem. 275**,** 5037–5042. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez, M. J., Balbín, M., López, J. M., Alvarez, J., Komori, T. & López-Otín, C. (1999) Mol. Cell. Biol. 19**,** 4431–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porte, D., Tuckermann, J., Becke, M., Baumann, B., Teurich, S., Higgins, T., Owen, M. J., Schorpp-Kistner, M. & Angel, P. (1999) Oncogene 18**,** 667–678. [DOI] [PubMed] [Google Scholar]
- 17.Krane, S. M., Byrne, M. H., Lemaitre, V., Henriet, P., Jeffrey, J. J., Witter, J. P., Liu, X., Wu, H., Jaenisch, R. & Eeckhout, Y. (1996) J. Biol. Chem. 271**,** 28509–28515. [DOI] [PubMed] [Google Scholar]
- 18.Otterness, I. G., Downs, J. T., Lane, C., Bliven, M. L., Stukenbrok, H., Scampoli, D. N., Milici, A. J. & Mezes, P. S. (1999) Matrix Biol. 18**,** 331–341. [DOI] [PubMed] [Google Scholar]
- 19.Zhao, W., Byrne, M. H., Wang, Y. & Krane, S. M. (2000) J. Clin. Invest. 106**,** 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, W., Byrne, M. H., Boyce, B. F. & Krane, S. M. (1999) J. Clin. Invest. 103**,** 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusano, K., Miyaura, C., Inada, M., Tamura, T., Ito, A., Nagase, H., Kamoi, K. & Suda, T. (1998) Endocrinology 139**,** 1338–1345. [DOI] [PubMed] [Google Scholar]
- 22.Miyaura, C., Inada, M., Suzawa, T., Sugimoto, Y., Ushikubi, F., Ichikawa, A., Narumiya, S. & Suda, T. (2000) J. Biol. Chem. 275**,** 19819–19823. [DOI] [PubMed] [Google Scholar]
- 23.Engsig, M. T., Chen, Q. J., Vu, T. H., Pedersen, A. C., Therkidsen, B., Lund, L. R., Henriksen, K., Lenhard, T., Foged, N. T., Werb Z, et al. (2000) J. Cell Biol. 151**,** 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vu, T. H., Shipley, J. M., Bergers, G., Berger, J. E., Helms, J. A., Hanahan, D., Shapiro, S. D., Senior, R. M. & Werb, Z. (1998) Cell 93**,** 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmbeck, K., Bianco, P., Caterina, J., Yamada, S., Kromer, M., Kuznetsov, S. A., Mankani, M., Robey, P. G., Poole, A. R., Pidoux, I., et al. (1999) Cell 99**,** 81–92. [DOI] [PubMed] [Google Scholar]
- 26.Zhou, Z., Apte, S. S., Soininen, R., Cao, R., Baaklini, G. Y., Rauser, R. W., Wang, J., Cao, Y. & Tryggvason, K. (2000) Proc. Natl. Acad. Sci. USA 97**,** 4052–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linsenmayer, T. F., Chen, Q. A., Gibney, E., Gordon, M. K., Marchant, J. K., Mayne, R. & Schmid, T. M. (1991) Development (Cambridge, U.K.) 111**,** 191–196. [DOI] [PubMed] [Google Scholar]
- 28.Warman, M. L., Abbott, M., Apte, S. S., Hefferon, T., McIntosh, I., Cohn, D. H., Hecht, J. T., Olsen, B. R. & Francomano, C. A. (1993) Nat. Genet. 5**,** 79–82. [DOI] [PubMed] [Google Scholar]
- 29.Gerstenfeld, L. C. & Shapiro F. D. (1996) J. Cell. Biochem. 62**,** 1–9. [DOI] [PubMed] [Google Scholar]
- 30.Koyama, E., Golden, E. B., Kirsch, T., Adams, S. L., Chandraratna, R. A., Michaille, J. J. & Pacifici, M. (1999) Dev. Biol. 208**,** 375–391. [DOI] [PubMed] [Google Scholar]
- 31.Kronenberg, H. M. (2003) Nature 423**,** 332–336. [DOI] [PubMed] [Google Scholar]
- 32.Ingham, P. W. & McMahon, A. P. (2001) Genes Dev. 15**,** 3059–3087. [DOI] [PubMed] [Google Scholar]
- 33.Gerber, H. P., Vu, T. H., Ryan, A. M., Kowalski, J., Werb, Z. & Ferrara, N. (1999) Nat. Med. 5**,** 623–628. [DOI] [PubMed] [Google Scholar]
- 34.Zelzer, E., McLean, W., Ng, Y. S., Fukai, N., Reginato, A. M., Lovejoy, S., D'Amore, P. A. & Olsen, B. R. (2002) Development (Cambridge, U.K.) 129**,** 1893–1904. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell, P. G., Magna, H. A., Reeves, L. M., Lopresti-Morrow, L. L., Yocum, S. A., Rosner, P. J., Geoghegan, K. F. & Hambor, J. E. (1996) J. Clin. Invest. 97**,** 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuhold, L. A., Killar, L., Zhao, W., Sung, M. L., Warner, L., Kulik, J., Turner, J., Wu, W., Billinghurst, C., Meijers, T., et al. (2001) J. Clin. Invest. 107**,** 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasano, Y., Zhu, J. X., Tsubota, M., Takahashi, I., Onodera, K., Mizoguchi, I. & Kagayama, M. (2002) J. Histochem. Cytochem. 50**,** 325–332. [DOI] [PubMed] [Google Scholar]
- 38.Schmid, T. M., Mayne, R., Jeffrey, J. J. & Linsenmayer, T. F. (1986) J. Biol. Chem. 261**,** 4184–4189. [PubMed] [Google Scholar]
- 39.Welgus, H. G., Fliszar, C. J., Seltzer, J. L., Schmid, T. M. & Jeffrey, J. J. (1990) J. Biol. Chem. 265**,** 13521–13527. [PubMed] [Google Scholar]
- 40.Ferrari, D. & Kosher, R. A. (2002) Dev. Biol. 252**,** 257–270. [DOI] [PubMed] [Google Scholar]
- 41.Xiao, G., Wang, D., Benson, M. D., Karsenty, G. & Franceschi, R. T. (1998) J. Biol. Chem. 273**,** 32988–32994. [DOI] [PubMed] [Google Scholar]
- 42.Enomoto, H., Enomoto-Iwamoto, M., Iwamoto, M., Nomura, S., Himeno, M., Kitamura, Y., Kishimoto, T. & Komori, T. (2000) J. Biol. Chem. 275**,** 8695–8702. [DOI] [PubMed] [Google Scholar]
- 43.Takeda, S., Bonnamy, J. P., Owen, M. J., Ducy, P. & Karsenty, G. (2001) Genes Dev. 15**,** 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueta, C., Iwamoto, M., Kanatani, N., Yoshida, C., Liu, Y., Enomoto-Iwamoto, M., Ohmori, T., Enomoto, H., Nakata, K., Takada, K., et al. (2001) J. Cell Biol. 153**,** 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLean, H. E., Kim, J. I., Glimcher, M. J., Wang, J., Kronenberg, H. M. & Glimcher, L. H. (2003) Dev. Biol. 262**,** 51–63. [DOI] [PubMed] [Google Scholar]
- 46.Yan, Y., Frisén, J., Lee, M. H., Massagué, J. & Barbacid, M. (1997) Genes Dev. 11**,** 973–983. [DOI] [PubMed] [Google Scholar]
- 47.Patel, A. C., McAlister, W. H. & Whyte, M. P. (1993) Medicine 72**,** 326–342. [PubMed] [Google Scholar]