Broad Sensitivity of Saccharomyces cerevisiae Lacking Ribosome-Associated Chaperone Ssb or Zuo1 to Cations, Including Aminoglycosides (original) (raw)
Abstract
The Hsp70 Ssb and J protein Zuo1 of Saccharomyces cerevisiae are ribosome-associated molecular chaperones, proposed to be involved in the folding of newly synthesized polypeptide chains. Cells lacking Ssb and/or Zuo1 have been reported to be hypersensitive to cationic aminoglycoside protein synthesis inhibitors that affect translational fidelity and to NaCl. Since we found that Δ_ssb1_ Δ_ssb2_ (Δ_ssb1_,2), Δ_zuo1_, and wild-type cells have very similar levels of translational misreading in the absence of aminoglycosides, we asked whether the sensitivities to aminoglycosides and NaCl represent a general increase in sensitivity to cations. We found that Δ_ssb1_,2 and Δ_zuo1_ cells are hypersensitive to a wide range of cations. This broad sensitivity is similar to that of cells having lowered activity of major plasma membrane transporters, such as the major K+ transporters Trk1 and Trk2 or their regulators Hal4 and Hal5. Like Δ_hal4_,5 cells, Δ_ssb1_,2 and Δ_zuo1_ cells have increased intracellular levels of Na+ and Li+ upon challenge with higher-than-normal levels of these cations, due to an increased rate of influx. In the presence of aminoglycosides, Δ_ssb1_,2, Δ_zuo1_, and Δ_hal 4_,5 cells have similarly increased levels of translational misreading. We conclude that, in vivo, the major cause of the aminoglycoside sensitivity of cells lacking ribosome-associated molecular chaperones is a general increase in cation influx, perhaps due to altered maturation of membrane proteins.
Molecular chaperones such as Hsp70s, characterized by their ability to bind to short hydrophobic stretches of polypeptides, facilitate protein folding in living cells (18). The highly conserved Hsp70 genes have evolved into complex multigene families in many organisms. For example, the yeast Saccharomyces cerevisiae has 14 Hsp70 genes. Two of these, SSB1 and SSB2, encode the 99% identical proteins Ssb1 and Ssb2 (hereafter referred to as Ssbs) (25). Ssbs, abundant proteins stochiometrically associated with ribosomes, can be cross-linked to nascent polypeptide chains that extend only 10 to 20 amino acids beyond the exit tunnel of the ribosome (15, 19, 30). The evolution of Hsp70s that are ribosome associated and their association with newly synthesized proteins have led to the hypothesis that Ssbs are involved in the folding of proteins as they emerge from the ribosome.
Hsp70s do not function alone, but rather with cochaperones called J proteins, which serve to stimulate Hsp70's ATPase activity, thus facilitating their interaction with polypeptide substrates (5). Zuo1, a ribosome-associated J protein, is the proposed partner of the Ssb Hsp70s. Δ_ssb1_ Δ_ssb2_ (Δ_ssb1_,2) and Δ_zuo1_ cells, as well as Δ_ssb1_,2 Δ_zuo1_ cells, have the same phenotypes: slow growth, particularly at low temperatures, and sensitivity to the aminoglycoside class of protein synthesis inhibitors and NaCl (14, 19, 42). This similarity in phenotypes among strains lacking Ssb and Zuo1 individually, or together, is consistent with a required partnership between the two proteins.
Aminoglycosides, antibiotics that bind to the small ribosomal subunit, affect translational fidelity, as well as the rate of translational elongation (4, 27). Particular alterations in rRNA or certain ribosomal proteins that render cells more sensitive to aminoglycosides also increase the amount of misreading, causing nonsense suppression, that is, insertion of amino acids rather than chain termination at stop codons, and missense suppression, the substitution of an inappropriate amino acid (7, 28, 37). In addition, because aminoglycosides are cations, mutations in genes encoding certain transporters in the plasma membrane (21, 24) or components of the secretory machinery (9) affect sensitivity to aminoglycosides.
Ion homeostasis is maintained within cells by a complex network of transporters and their regulators (33). Critical to ion transport is the highly negative membrane potential, which is determined primarily by the relative activities of the proton-pumping ATPase Pma1 (11) and the Trk1 and Trk2 K+ transporters (13), which pump large amounts of K+, thus maintaining the high potassium levels required within the cell. Low sodium levels are maintained within the cell in good part by the action of the Na+ exporter Ena1 (16, 41). Other cation transporters of the plasma membrane have been genetically identified in yeast (40). Additional, yet to be identified, transporters are thought to be present in the plasma membrane as well. Their existence is only surmised, based on the observed transport of some cations in the absence of the known K+ transporters. However, this prediction is supported by the presence of unstudied open reading frames in the yeast genome that encode proteins having sequence similarity with known transporters (2). The activity and expression of transporters are regulated by a complex network of transcriptional and posttranslational regulators. The Hal4 and Hal5 kinases, which activate the Trk1 and -2 transporters (23), are one such example.
The physiological basis of the ssb1,2 and zuo1 phenotypes is not known. Based on the belief that a better understanding of the cellular defects caused by the absence of these chaperones will aid in understanding their in vivo function(s), we set out to establish the basis of the sensitivity to aminoglycosides. We found ssb1,2 and zuo1 mutants to be sensitive to all cations tested and to have increased intracellular Li+ and Na+ concentrations compared to wild-type cells after exposure to these cations. We conclude that a defect in ion homeostasis is responsible for many pleiotropic effects of the absence of the ribosome-associated chaperones Ssb and Zuo1, including sensitivity to aminoglycosides.
MATERIALS AND METHODS
Strains and growth media.
Yeast strains used are isogenic with either a derivative of S288C, DS10 (_his3_-11,_15 leu2_-3,112 lys1 lys2 Δ_trp1 ura3_-52), or W303, W303-A1 (_his3_-11,_15 leu2_-3,112, _trp1_-_1 ura3_-_1 ade2_-_1 can1_-100). In both strain backgrounds the same deletions, ssb1::TRP1 and ssb2::LEU2 (HE1 or NL226a) or a zuo1::HIS3 deletion (HE13 or HE5) were used (10, 29, 38). These strains are [PSI _+_]. Very similar results were obtained in the two strain backgrounds. In addition, the following strains derived from W303-1A were used: SKY637 (hal4::LEU2 hal5::HIS3), SKY697 (_ena1_-4::HIS3), and WΔ3 (trk1::LEU2 trk2::HIS3) (17, 23).
Strains were generally grown in rich medium containing 1% yeast extract-2% peptone-2% dextrose (YPD) or minimal medium (0.67% yeast nitrogen base without amino acids-2% dextrose, supplemented with all amino acids and bases except those needed for selection). pH 3.5 YPD plates were prepared by adjusting a twofold-concentrated YPD stock solution containing 50 mM succinic acid to the desired pH with Tris prior to autoclaving and then mixing with a concentrated agar solution before pouring. pH 5.5 and 7.5 YPD plates were prepared by adjusting the pH with potassium phosphate buffer. For testing potassium-dependent phenotypes, strains were grown in synthetic dextrose arginine phosphate medium, pH 5.8 (1, 32), which is deficient in K+.
For comparison of growth log-phase liquid cultures grown in YPD were diluted and spotted onto agar plates and incubated at 30°C. Plates were monitored for growth after 1, 2, and 3 days. Growth was scored on a scale of 4 to 0, with 4 indicating growth of the particular strain at 30°C on normal YPD (pH 5.5) and 0 indicating absence of growth. Plus and minus signs next to the numbers were used to indicate slightly faster or slower growth, respectively.
Assays for translational fidelity.
Cells were grown in selective medium to mid-exponential phase at 30°C. The amount of aminoglycoside added to the medium was that which had been previously determined to slow the growth of the mutant cells by approximately 50%. Because of the decreased sensitivity of strains growing in minimal medium compared to that of strains growing in rich medium, those concentrations of hygromycin in rich and minimal media were 3 and 100 μg/ml, respectively. The drug was added approximately 8 h prior to harvest of the cells. For experiments in which hal4,5 cells were tested, cells were grown overnight in selective minimal medium, harvested by centrifugation, and resuspended in rich medium prior to the addition of the drug due to the poor growth of hal4,5 cells in minimal medium. Plasmid retention was determined to be comparably efficient in all strains over the 8-h period of the experiment.
β-Galactosidase.
Yeast strains were transformed with one of the pUKC815, -817, -819 vector series, having either a wild-type lacZ gene or a stop codon inserted after the translational initiation codon (36). β-Galactosidase activity was determined as previously described (10, 12, 35) and was calculated as nanomoles of _ortho_-nitrophenol formed per minute per unit of cell culture optical density at 600 nm per milliliter of culture volume and expressed as a percentage of control β-galactosidase activity measured in a transformant having the wild-type lacZ gene. Variability in measured β-galactosidase levels among different transformants of the same strain was found to be <10%. Assays from cultures of individual transformants were performed in duplicate.
CAT.
Chloramphenicol acetyltransferase (CAT) activity was measured with the fluorescent FAST CAT Green (deoxy) CAT assay kit (Molecular Probes), according to the manufacturer's instructions, with minor modifications. Yeast strains were transformed with one of the pUKC618, -619 vector series (37) carrying the wild-type or mutant CAT genes. Cells were harvested, washed, and resuspended in 50 μl of 40 mM Tris-HCl (pH 7.4)-1 mM EDTA-180 mM NaCl solution, glass beads were added, and the mixture was subjected to vortexing for 5 min. Sixty microliters of cell lysates was mixed with 10 μl of FAST CAT substrate reagent and 10 μl of 9 mM acetyl-coenzyme A. The assay reactions were performed at 37°C for 3 h, and the reaction was stopped by addition of 1 ml of ice-cold ethyl acetate. The reaction substrate and product were resolved by thin-layer chromatography on silica gel 60 plates (Merck) in chloroform-methanol (85:15, vol/vol) solvent. The unconverted CAT substrate and the acetylated product derivative were scraped from the plate after visualization by UV illumination and dissolved in 200 μl of methanol, and fluorescence was measured. The percentage of conversion of substrate to acetylated product was calculated by dividing the product fluorescence intensity by the sum of the product and the substrate fluorescence intensities.
Analysis of intracellular levels of salts.
Cells were grown in YPD to an absorbance of 0.6 to 0.7 at 600 nm, centrifuged for 5 min at 1,900 × g, resuspended at the same concentration in YPD containing salt, and incubated at 30°C. Aliquots of 10 ml were removed, centrifuged for 5 min at 2,000 × g at 4°C, and washed twice with 10 ml of ice-cold washing solution (20 mM MgCl2 and 1.5 M sorbitol) by resuspension and subsequent centrifugation. The cell pellets were resuspended in 1 ml of cold washing solutions, centrifuged again, and resuspended in 0.5 ml of 20 mM MgCl2. Ions were extracted by heating the cells for 15 min to 95°C. After centrifugation to remove cellular debris, aliquots of the supernatant were analyzed with a flame atomic absorption spectrophotometer (Solaar Unicam 969).
For lithium efflux experiments, cells were incubated for 1 h with LiCl as described above, centrifuged, washed once, and resuspended at the same concentration in YPD. Aliquots of 10 ml were processed as described above.
RESULTS
Increased misreading in ssb and zuo1 cells in the presence, but not absence, of hygromycin B, relative to wild-type cells.
Our goal was to understand the basis of the aminoglycoside sensitivity of strains lacking the Ssb and Zuo1 molecular chaperones. Since Ssb and Zuo1 are ribosome-associated chaperones and since aminoglycosides bind to ribosomes and affect translational fidelity, we first tested if cells lacking these chaperones had increased levels of misreading. We used two assays to assess misreading. First, we tested suppression of nonsense codons using genes encoding β-galactosidase having either a TAA or TGA stop codon near the translation initiation codon. In wild-type cells, the β-galactosidase activity in cells harboring the gene with a TAA stop codon was 0.013% of the activity in cells having the control gene lacking the stop codon (Table 1). For the TGA codon, 0.017% readthrough was observed. Very similar values were obtained for both the ssb1,2 and zuo1 mutants, with the greatest difference being a 1.4-fold-higher value for TAA codon readthrough in ssb1,2 cells than in wild-type cells, that is, 0.018 compared to 0.013%.
TABLE 1.
Translational misreading in ssb1,2 and zuo1 strains
| Strain | Codona | % Misreading (10−2)b | Fold increase in misreading | |
|---|---|---|---|---|
| −hygB | +hygB | |||
| wtc | TAA | 1.30 ± 0.24 | 2.30 ± 0.03 | 1.8 |
| ssb1,2 | TAA | 1.80 ± 0.14 | 7.00 ± 0.66 | 4.0 |
| zuo1 | TAA | 1.30 ± 0.04 | 8.20 ± 0.15 | 6.1 |
| wt | TGA | 1.70 ± 0.16 | 3.90 ± 0.62 | 2.3 |
| ssb1,2 | TGA | 2.00 ± 0.18 | 10.0 ± 0.63 | 5.1 |
| zuo1 | TGA | 1.70 ± 0.09 | 12.3 ± 0.30 | 4.6 |
| wt | CAC | 0.37 ± 0.02 | 0.67 ± 0.17 | 1.8 |
| ssb1,2 | CAC | 0.39 ± 0.04 | 2.40 ± 0.80 | 6.2 |
| zuo1 | CAC | 0.35 ± 0.04 | 1.80 ± 0.28 | 5.2 |
To assess suppression of missense codons, we utilized a previously described system that is based on a mutation that encodes a single amino acid change, His195(CAC) to Tyr195(UAC), in the active site of type III CAT (CATIII) (37). CATIII having tyrosine at position 195 is completely inactive but is as stable as the wild-type protein (37). Activity is restored if histidine is incorporated by a misreading of the UAC codon. In our wild-type strain that carried the CATIII gene having the mutation, enzyme activity was 0.0037% of that found in a strain harboring the wild-type gene. Very similar levels of CAT activity were found in the ssb1,2 and zuo1 strains, 0.0039 and 0.0035%, respectively. Thus we conclude that no significant increase in misreading of either nonsense or missense mutations occurred in the ssb1,2 and zuo1 strains under the conditions we tested.
To compare the levels of misreading in the presence of the aminoglycoside hygromycin B in wild-type and mutant strains, we added the drug to cultures growing in minimal media at a concentration that slowed the growth rate of the mutant strains by 50%. Under these conditions, ssb and zuo1 cells displayed significantly more misreading of both nonsense and missense codons than the wild-type strain. While the wild-type strain had approximately twofold-higher misreading in the presence of the drug, the mutant strains had between four- and sixfold higher rates (Table 1). Similar results were found in the presence of another aminoglycoside known to affect translational fidelity, paromomycin (data not shown).
ssb1,2 and zuo1 cells are hypersensitive to a variety of cations.
Since we found significant enhancement of misreading in mutant cells compared to wild-type cells only in the presence of the drug, other possible reasons for the extreme sensitivity of the ssb1,2 and zuo1 strains to aminoglycosides were investigated. We previously reported that ssb1,2 and zuo mutants were sensitive to NaCl (42). Since both NaCl and aminoglycosides are cations, we tested whether ssb1,2 and zuo1 mutants were hypersensitive to other cations added to the growth medium. ssb1,2 and zuo1 cells were found to be sensitive to the addition of a variety of cations other than Na+, particularly Mn2+ and Li+ (Table 2). However, growth was not inhibited in the presence of 1 M sorbitol, indicating that the observed sensitivity to cations was not due to an increase in osmotic strength of the medium.
TABLE 2.
Growth of ssb1,2 and zuo1 cells in the presence of cations
| Addition | Relative growtha of: | ||
|---|---|---|---|
| wt | ssb1,2 | zuo1 | |
| —b | 4 | 4 | 4 |
| 0.5 M NaCl | 3 | 1 | 1 |
| 0.1 M CaCl2 | 4 | 2 | 2 |
| 0.3 M MgCl2 | 4 | 3 | 3 |
| 2.0 mM MnCl2 | 2 | 0 | 0 |
| 0.1 M LiCl | 4 | 1 | 1 |
| 1.0 M KCl | 4 | 2 | 2 |
| 1.0 M sorbitol | 4 | 4 | 4 |
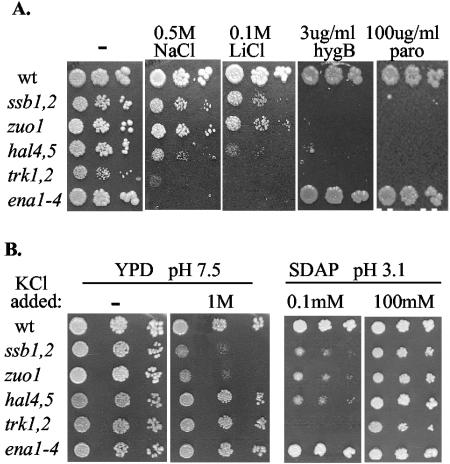
The general sensitivity of ssb1,2 and zuo1 mutants to cations is reminiscent of the sensitivity of mutants having altered activity of transporters located in the plasma membrane (21-23). Therefore, we compared the growth rates of strains containing deletions of the genes encoding the major K+ transporters Trk1 and -2 and the Na+ exporter Ena1. We also tested a hal4,5 mutant, which lacks kinases that activate Trk1 and -2. All mutant strains showed an enhanced sensitivity under a subset of conditions compared to the wild type (Fig. 1A; Table 2). As reported previously, _ena1_-4 mutants were extremely sensitive to Na+ and Li+, but not to aminoglycosides (16, 41). This phenotype is expected for a mutant having a defective transporter that is known to export Na+ and Li+, but not aminoglycosides. On the other hand, trk1,2 and hal4,5 mutants were sensitive to Na+ and Li+, as well as aminoglycosides, as were ssb1,2 and zuo1 mutants. ssb1,2 and zuo1 strains showed reduced growth in YPD medium containing 1 M NaCl at any pH tested (pH 3.5, 5.5, and 7.5), similar to _ena1_-4 and trk1,2 mutants (Table 3). In addition, ssb1,2 and zuo1 cells, as well as trk1,2, hal4,5, and _ena1_-4 cells, grew slowly in minimal medium having very low levels of potassium, while wild-type cells did not (Fig. 1B). This growth inhibition was reversed by the addition of K+ to the medium. However, the phenotypes of ssb and zuo1 cells were not identical to those of trk1,2 or hal4,5 cells. Under one condition ssb and zuo1 cells displayed growth defects when trk 1,2, hal 4,5, or _ena 1_-4 cells did not: the presence of high levels of potassium in medium having a pH of 7.5, rather than 5.5, the pH at which yeast is usually grown (Fig. 1B).
FIG. 1.
Sensitivity of chaperone and transporter mutants to cations. Tenfold serial dilutions of the indicated strains were spotted onto rich media containing the indicated additions. Plates were incubated at 30°C for 3 days. Shown are results for rich medium (YPD) at the normal pH of 5.5 (A) and at pH 7.5 (B) and for minimal medium lacking K+ (synthetic dextrose arginine phosphate medium [SDAP]) at pH 3.1 (B). wt, wild type; hygB, hygromycin B; paro, paromomycin.
TABLE 3.
Effect of pH, Na+, and K+ on growth of chaperone and transporter mutants
| pH | Addition | Relative growtha of: | |||||
|---|---|---|---|---|---|---|---|
| wt | ssb1,2 | zuo1 | hal4,5 | trk1,2 | ena1,4 | ||
| 5.5b | —c | 4 | 4 | 4 | 4 | 4 | 4 |
| 1.0 M KCl | 4 | 3+ | 3+ | 4 | 4+ | 4 | |
| 0.5 M NaCl | 4 | 2 | 2 | 2 | 1 | 1 | |
| 3.5 | — | 4 | 4 | 4 | 4 | 0 | 4 |
| 1.0 M KCl | 4 | 4− | 4− | 4 | 4 | 4 | |
| 0.5 M NaCl | 4 | 2 | 2 | 2 | 1 | 2 | |
| 7.5 | — | 4 | 4 | 4 | 4 | 4+ | 4 |
| 1.0 M KCl | 4 | 1 | 1 | 4 | 4+ | 4 | |
| 0.5 M NaCl | 4 | 2− | 2− | 3 | 2 | 0 |
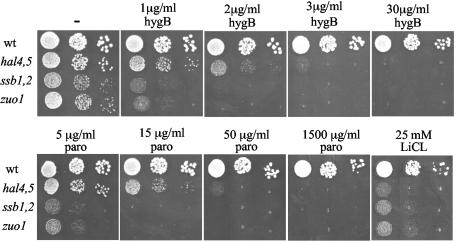
Experiments described in the ensuing sections of this report were performed in order to compare phenotypes of chaperone mutants with those of mutants having lower activity of plasma membrane transporters. Most were done with the hal4,5 strain, rather than the trk1,2 strain. The trk1,2 strain grew very slowly, particularly in liquid medium, and rapidly accumulated suppressors. While ssb1,2, zuo1, and hal4,5 cells displayed very similar sensitivities to LiCl, ssb1,2 and zuo1 strains were slightly more sensitive than hal4,5 cells to the aminoglycosides hygromycin B and paromomycin. Growth of ssb1,2 and z_uo1_ cells was partially inhibited when 0.5-μg/ml hygromycin B was added to plates and was completely inhibited at 2 μg/ml (Fig. 2; data not shown). On the other hand, hal4,5 cell growth was partially inhibited at 1.5 μg/ml and was completely inhibited at 4 μg/ml. On paromomycin-containing plates, ssb1,2 and zuo1 cells were partially inhibited at 5 μg/ml and completely inhibited at 15 μg/ml and hal4,5 cells were partially inhibited at 15 μg/ml and completely inhibited at 50 μg/ml. Therefore, hal4,5 cells required three- to fourfold more aminoglycoside for growth to be inhibited to the same extent as it was for ssb1,2 or zuo1 cells. However, this difference is minimal when considering that no inhibition of growth of wild-type cells was observed at the highest concentrations tested, 30 μg/ml for hygromycin or 1,500 μg/ml for paromomycin.
FIG. 2.
Comparison of the sensitivities of ssb1,2, zuo1, and hal 4,5 mutants to hygromycin B (hygB), paromomycin (paro), and LiCl. Tenfold serial dilutions of the indicated strains were spotted onto rich media containing the indicated additions. wt, wild type. Plates were incubated at 30°C for 2 days.
The rate of cation influx is greater in ssb and zuo1 cells than in wild-type cells.
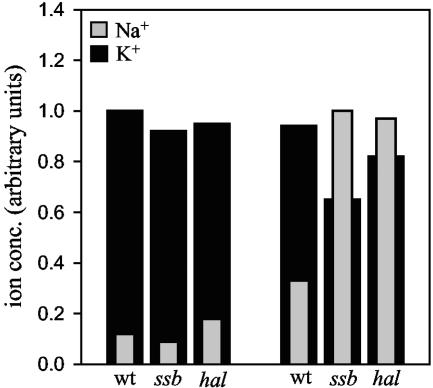
Previous work demonstrated that trk1,2, hal4,5, and _ena1_-4 mutants accumulate higher levels of toxic cations than wild-type cells. The sensitivity of ssb1,2 and zuo1 mutant cells to toxic cations suggested to us that the intracellular ion level may also be higher in the chaperone mutants. To test this idea directly, we first measured the intracellular Na+ concentrations in wild-type, ssb1,2, and hal4,5 cells by atomic absorption spectrometry, both before and 60 min after challenge with 0.1 M NaCl (Fig. 3). Similar to previously reported results (23), wild-type cells had approximately a threefold increase in intracellular Na+ concentration. Both hal4,5 and ssb1,2 cells had intracellular concentrations that were threefold higher than that found in wild-type cells. Li+ treatment had a similar effect on intracellular Li+ concentrations. While the Li+ concentration in all strains was unmeasurable in the absence of additional LiCl, a measurable level of Li+ was present after challenge in all strains tested. The chaperone and transporter mutants all showed larger increases in intracellular Li+ concentrations than the wild-type control (Table 4).
FIG. 3.
Intracellular cation concentration in ssb1,2 and hal4,5 cells after NaCl addition. NaCl was added to a final concentration of 0.1 M to log-phase cultures of the indicated strains. Sixty minutes after addition of salt, the cells were washed and lysed as described in Materials and Methods. The concentrations of K+ and Na+ in the cell lysate were measured by atomic absorption spectroscopy. The highest concentration of K+ or Na+ was arbitrarily set at 1. wt, wild type.
TABLE 4.
Increase in Li+ in chaperone and transporter mutants
| Strain | Li+ accumulationa |
|---|---|
| wtb | 1.0 |
| ssb1,2 | 3.8 |
| zuo1 | 3.6 |
| hal4,5 | 2.4 |
| trk1,2 | 3.4 |
| ena1-4 | 2.0 |
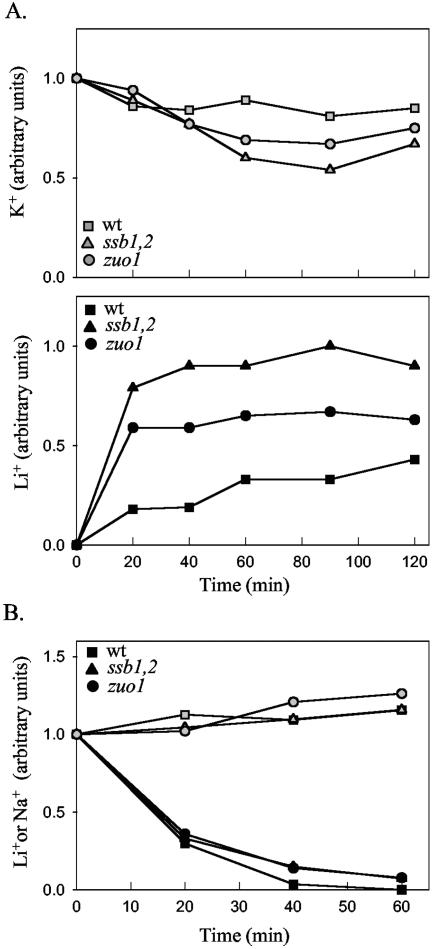
It was demonstrated previously that the increase in intracellular cations in hal4,5 and trk1,2 cells is due to an increased rate of influx (23). To assess whether the increase in cation concentration in the chaperone mutants was due to an increased influx or decreased efflux, we measured the rate of decrease in cations after challenge. First, we carried out a time course of intracellular Li+ concentration after the addition of LiCl to wild-type, ssb1,2, and zuo1 cells. Within 20 min of LiCl addition, the intracellular Li+ levels in ssb1,2 and zuo1 cells had plateaued. At 20 min the concentration in wild-type cells reached a level four to five times lower than that found in the mutants; however, this level gradually increased, reaching 50 to 60% of the level found in the mutants by 120 min after addition (Fig. 4A). In summary, the chaperone mutants showed increased accumulation of the cations Na+ and Li+.
FIG. 4.
Time course of the change in intracellular cation concentration after addition and removal of LiCl. (A) At time zero LiCl was added to a concentration of 0.1 M to cultures of the indicated strains growing at 30°C. Samples were removed at the indicated times, and the intracellular K+ (top) and Li+ (bottom) concentrations were determined as described in the legend to Fig. 3. (Top) The concentration of K+ at time zero was arbitrarily set at 1; (bottom) the highest concentration of Li+ (90-min time point for ssb1,2 cells) was set at 1. (B) Cultures of the indicated strains were treated as for panel A except that, 60 min after addition of LiCl, the cells were harvested, washed, and resuspended in rich media without added salt. Samples were removed at the indicated times after resuspension, and the intracellular K+ and Li+ contents were determined. The concentrations of K+ (open symbols) and Li+ (solid symbols) at the time of Li removal were set at 1. wt, wild type.
To assess the relative rates of efflux of Li+, cells were harvested by centrifugation 60 min after addition of 0.1 M LiCl, washed, and resuspended in media containing no additions. At various times, aliquots were removed and the intracellular Li+ levels were determined (Fig. 4B). The rates of decrease in intracellular Li+ in all three strains were very similar. Therefore, we conclude that, in the ssb1,2 and zuo1 mutant cells, the increase in intracellular Li+ concentration is primarily due to an increased influx of the cation.
hal4,5 mutants show an increase in misreading in the presence of aminoglycosides, as do ssb mutants.
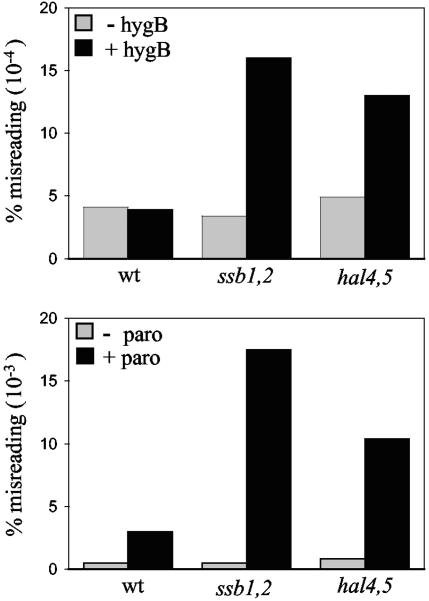
The results presented above demonstrate that ssb1,2 cells accumulate higher intracellular concentrations of the cations Li+ and Na+ than do wild-type cells. These results suggested to us that the cause of increased misreading in these mutants might be that they had a higher internal concentration of the cationic aminoglycosides than wild-type cells, even when the concentration in the surrounding medium is the same. If so, then mutants having defects in transporter function ought to also have increased levels of misreading in the presence of the drug. Therefore we compared levels of misreading using the CATIII missense assay described above for wild-type, ssb1,2, and hal4,5 cells in the presence of paromomycin or hygromycin B (Fig. 5). While in the absence of the drug there was less than a twofold difference in misreading among the strains, both ssb1,2 and hal4,5 mutants displayed significantly greater misreading in the presence of the drug than did the wild-type strain. For example, under conditions where the wild type displayed a 6-fold increase in misreading in the presence of paromomycin (0.003%) compared to that in the absence of the drug (0.0005%), ssb1,2 and hal4,5 mutants displayed 36- and 12.5-fold increases (0.018 and 0.010%), respectively. Similarly, in the presence of a concentration of hygromycin B in which the wild type showed the same percentage of misreading as it showed in the absence of the drug (0.0004%), misreading in ssb1,2 and hal4,5 cells increased 4- and 3.2-fold, respectively, to 0.0016 and 0.0013%. This increased misreading relative to the wild type in a hal4,5 strain in the presence of aminoglycosides supports the idea that alteration in cation homeostasis substantially affects the translational misreading caused by aminoglycosides.
FIG. 5.
Comparison of translational misreading in wild-type, ssb1,2, and hal4,5 cells. The indicated strains harboring a CATIII gene, either the wild-type (wt) gene or a gene with a missense codon, were grown in rich media either containing or lacking hygromycin B (hygB; 3 μg/ml) or paromomycin (paro; 30 μg/ml). Cells were harvested, and CAT activity was determined. The percentage of misreading was calculated based on the activities in lysates from cells having mutant and wild-type genes.
DISCUSSION
The results reported here demonstrate that ssb1,2 and zuo1 cells are sensitive to a wide range of cations. That these chaperone mutants have increased internal concentrations of Na+ and Li+ after challenge with NaCl and LiCl relative to the wild type indicates that these mutant cells have defects in cation homeostasis. Consistent with this idea, Jones et al. (20) reported a six- to sevenfold-higher intracellular level of guanidine in ssb1,2 mutant cells than in wild-type cells after addition of radiolabeled guanidine. Although not discussed in that report, guanidine is a cation. This increase is quite comparable, considering the differences in experimental conditions, to the three- to fourfold increase relative to wild-type cells that we report here for Li+ and Na+. We propose that the transport of cations across the plasma membrane is altered in mutants lacking Ssb and Zuo1 and that this results in wide-ranging pleiotropic effects, including the hypersensitivity of ssb1,2 strains to the curing effects of guanidine on the prion [PSI _+_] (8, 20).
The phenotypes of ssb1,2 and zuo1 mutants described were not identical to those of mutants having defects in a single type of transporter. However, the chaperone mutants had sensitivities very similar to those of strains having decreased function of Trk1 and Trk2, the major transporters of K+ into the cell. At first glance, an increased influx in cations in a transporter mutant is counterintuitive. However, it is well established that the highly negative potential across the plasma membrane is the driving force of cation transport. This potential is “set” by the relative activities of Pma1 H+-pumping ATPase, the main generator of the membrane potential, and Trk transporters, the main consumers. Thus, trk mutants, or mutants whose mutations affect the expression of Trk transporters, such as the hal4,5 mutant, are sensitive to a wide variety of cations due to an increased influx, primarily because of the increased membrane potential in such strains. Presumably this increased transport occurs through the yet to be molecularly defined nonselective channels (1-3). Unfortunately, the direct analysis of Trk1 and -2 and the measurement of the membrane potential in yeast are problematic. Nevertheless, our data are very consistent with the idea that the general sensitivity of ssb1,2 and zuo1 mutants to toxic cations is caused by an increased influx due to an increased membrane potential. This idea is supported by the fact that hal4,5 cells show similarly increased levels of misreading in the presence of aminoglycosides as ssb1,2 and zuo1 cells, as well as lowered accumulation of Li+.
We initiated the experiments reported here to test our hypothesis that the aminoglycoside sensitivity of the ssb1,2 and zuo1 strains was due to an innate difference in the ribosomes or the translation process in cells having mutations in genes encoding ribosome-associated molecular chaperones. Even though our results strongly indicate that cation influx defects are the primary cause of the hypersensitivity to aminoglycosides, they do not necessarily mean that there are no differences between the translational apparatuses of mutant and wild-type cells that could contribute to the aminoglycoside sensitivity. In light of the similar sensitivities of the chaperone and hal4,5 mutants to LiCl, it is possible that the slight difference in drug sensitivity between strains is due to an additional effect of the lack of Ssb or Zuo1 directly on the structure of ribosomes, or the fidelity of translation in the presence of aminoglycosides. A paper published when this report was under review (31) supports this possibility. A twofold increase in nonsense suppression, but not missense suppression, in ssb1,2 and zuo1 strains in vivo in the absence of aminoglycosides was reported. The difference between their observation and ours may be due to the constructs used to monitor translational fidelity. We were measuring normal, low levels of misreading, between 0.003 and 0.02%, while Rakwalska and Rospert (31) used a construct having a nonsense codon in a nucleotide context that promotes high levels of misreading, ∼10%.
However, it is also possible that differences in other physiological parameters, such as intracellular pH, caused by the alteration in ion homeostasis may be the cause of the slightly different sensitivities to aminoglycosides. Intracellular pH is intimately tied to ion transport and is normally tightly regulated because of its effects on proteins and cellular biochemical reactions (26), which may well include translation. Effects of aminoglycosides could be magnified by changes in the translational apparatus caused by differences in intracellular pH. Regardless of the cause of these differences in aminoglycoside sensitivity between chaperone mutants and mutants having decreased transporter activity, the minimal differences compared to the exquisite sensitivities of both types of mutants relative to those of wild-type strains make a strong argument for the major cause of aminoglycoside sensitivity of ssb1,2 and zuo1 cells being altered cation transport.
Although our results support increased influx of cations in the chaperone mutants, they do not explain the basis of this effect. We favor the idea that there is a flaw in some aspect of the biogenesis of the plasma membrane. Such a general defect would be consistent with our observation that the sensitivities of ssb1,2 and zuo1 mutants were not identical with those caused by disruption of a single transporter and the well-established fact that mutations in genes encoding components of the secretory pathway are sensitive to aminoglycosides (9). Also, supporting this idea, our preliminary results suggest decreased levels of a number of plasma membrane proteins (data not shown).
Based on reported analyses of ssb1,2 mutants, we can envision two possible reasons for a general defect in plasma membrane biogenesis. First, since interaction between WD40 proteins and Ssb have been observed in vivo, it has been proposed that Ssbs are particularly important in the folding of proteins containing this fold (34). Yeast contains 89 proteins having the WD40 motif (6, 39). Of those 16 have been shown to be involved in the secretory pathway. Thus it is plausible that ssb1,2 mutants have a general defect in the secretory pathway, which is responsible for the maturation of integral membrane proteins, including those of the plasma membrane. Alternatively, Ssb and Zuo1, as ribosome-associated chaperones, could play a more direct role in the cotranslational insertion of proteins into the endoplasmic reticulum membrane, also a process critical to maturation of membrane proteins. Further analysis is required to distinguish among such possibilities.
Acknowledgments
We thank Ian Stansfield, Rosaro Haro, and Serge Potier for their generous gifts of yeast strains and plasmids. We are also very grateful to Robert McClain (Department of Chemistry, University of Wisconsin-Madison) for his help with the atomic absorption spectroscopy, Mick Tuite and Ian Stansfield for their interest and help in the initial stages of this work, William Walter for help with some of the experiments, Thomas Rauch for thoughtful discussions, and Heather Hundley for critical reading of the manuscript.
This work was supported by a Public Health Services grant (R37GM31107) to E.A.C.
REFERENCES
- 1.Bertl, A., J. Ramos, J. Ludwig, H. Lichtenberg-Frate, J. Reid, H. Bihler, F. Calero, P. Martinez, and P. Ljungdahl. 2003. Characterization of potassium transport in wild-type and isogenic yeast strains carrying all combinations of trk1, trk2 and tok1 null mutations. Mol. Microbiol. 47**:**767-780. [DOI] [PubMed] [Google Scholar]
- 2.Bihler, H., C. Slayman, and A. Bertl. 2002. Low-affinity potassium uptake by Saccharomyces cerevisiae is mediated by NSC1, a calcium-blocked non-specific cation channel. Biochim. Biophys. Acta 1558**:**109-118. [DOI] [PubMed] [Google Scholar]
- 3.Bihler, H., C. Slayman, and A. Bertl. 1998. NSC1: a novel high-current inward rectifier for cations in the plasma membrane of Saccharomyces cerevisiae. FEBS Lett. 432**:**59-64. [DOI] [PubMed] [Google Scholar]
- 4.Brodersen, D., W. J. Clemons, A. Carter, R. Morgan-Warren, B. Wimberly, and V. Ramakrishnan. 2000. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 103**:**1143-1154. [DOI] [PubMed] [Google Scholar]
- 5.Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92**:**351-366. [DOI] [PubMed] [Google Scholar]
- 6.Camasses, A., A. Bogdanova, A. Shevchenko, and W. Zachariae. 2003. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol. Cell 12**:**87-100. [DOI] [PubMed] [Google Scholar]
- 7.Chernoff, Y., A. Vincent, and S. Liebman. 1994. Mutations in eukaryotic 18S ribosomal RNA affect translational fidelity and resistance to aminoglycoside antibiotics. EMBO J. 13**:**906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernoff, Y. O., G. P. Newnam, J. Kumar, K. Allen, and A. D. Zink. 1999. Evidence for a protein mutator in yeast: role of the Hsp70-related chaperone ssb in formation, stability, and toxicity of the [PSI] prion. Mol. Cell. Biol. 19**:**8103-8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean, N. 1995. Yeast glycosylation mutants are sensitive to aminoglycosides. Proc. Natl. Acad. Sci. USA 92**:**1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenman, H., and E. Craig. 2004. Activation of pleiotropic drug resistance by the J.-protein and Hsp70-related proteins, Zuo1 and Ssz1. Mol. Microbiol. 53**:**335-344. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira, T., A. Mason, and C. Slayman. 2001. The yeast Pma1 proton pump: a model for understanding the biogenesis of plasma membrane proteins. J. Biol. Chem. 276**:**29613-29616. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein, D., and S. Strausberg. 1983. Heat shock-regulated production of Escherichia coli beta-galactosidase in Saccharomyces cerevisiae. Mol. Cell. Biol. 3**:**1625-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaber, R. 1992. Molecular genetics of yeast ion transport. Int. Rev. Cytol. 137**:**299-353. [DOI] [PubMed] [Google Scholar]
- 14.Gautschi, M., H. Lilie, U. Funfschilling, A. Mun, S. Ross, T. Lithgow, P. Rucknagel, and S. Rospert. 2001. RAC, a stable ribosome-associated complex in yeast formed by the DnaK-DnaJ homologs Ssz1p and zuotin. Proc. Natl. Acad. Sci. USA 98**:**3762-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautschi, M., A. Mun, S. Ross, and S. Rospert. 2002. A functional chaperone triad on the yeast ribosome. Proc. Natl. Acad. Sci. USA 99**:**4209-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haro, R., M. Banuelos, F. Quintero, F. Rubio, and A. Rodriguez-Navarro. 1993. Genetic basis of sodium exclusion and sodium tolerance in yeast. A model system for plants. Physiol. Plant. 89**:**868-874. [Google Scholar]
- 17.Haro, R., L. Sainz, F. Rubio, and A. Rodríguez-Navarro. 1999. Cloning of two genes encoding potassium transporters in Neurospora crassa and expression of the corresponding cDNAs in Saccharomyces cerevisiae. Mol. Microbiol. 31**:**511-520. [DOI] [PubMed] [Google Scholar]
- 18.Hartl, F., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295**:**1852-1858. [DOI] [PubMed] [Google Scholar]
- 19.Hundley, H., H. Eisenman, W. Walter, T. Evans, Y. Hotokezaka, M. Wiedmann, and E. Craig. 2002. The in vivo function of the ribosome-associated Hsp70, Ssz1, does not require its putative peptide-binding domain. Proc. Natl. Acad. Sci. USA 99**:**4203-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, G., Y. Song, and D. Masison. 2003. Deletion of the Hsp70 chaperone gene SSB causes hypersensitivity to guanidine toxicity and curing of the [PSI+] prion by increasing guanidine uptake in yeast. Mol. Genet. Genomics 269**:**304-311. [DOI] [PubMed] [Google Scholar]
- 21.Madrid, R., M. J. Gomez, J. Ramos, and A. Rodriguez-Navarro. 1998. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly polarized membrane potential. J. Biol. Chem. 273**:**14838-14844. [DOI] [PubMed] [Google Scholar]
- 22.Mercier, R., N. Rabinowitz, R. Ali, R. Gaxiola, and G. Berkowitz. 2004. Yeast hygromycin sensitivity as a functional assay of cyclic nucleotide gated cation channels. Plant Physiol. Biochem. 42**:**529-536. [DOI] [PubMed] [Google Scholar]
- 23.Mulet, J., M. Leube, S. Kron, G. Rios, G. Fink, and R. Serrano. 1999. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 19**:**3328-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarre, C., and A. Goffeau. 2000. Membrane hyperpolarization and salt sensitivity induced by deletion of PMP3, a highly conserved small protein of yeast plasma membrane. EMBO J. 19**:**2515-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson, R. J., T. Ziegelhoffer, C. Nicolet, M. Werner-Washburne, and E. A. Craig. 1992. The translation machinery and seventy kilodalton heat shock protein cooperate in protein synthesis. Cell 71**:**97-105. [DOI] [PubMed] [Google Scholar]
- 26.Nuccitelli, R., and J. Heiple. 1982. Summary of the evidence and discussion concerning the evidence and discussion concerning the involvement of pH in the control of cellular function, p. 567-586. In R. Nuccitelli and D. Deamer (ed.), Intracellular pH: its measurement, regulation, and utilization in cellular functions. Alan R. Liss, New York, N.Y.
- 27.Ogle, J., A. Carter, and V. Ramakrishnan. 2003. Insights into the decoding mechanism from recent ribosome structures. Trends Biochem. Sci. 28**:**259-266. [DOI] [PubMed] [Google Scholar]
- 28.Parker, J. 1989. Errors and alternatives in reading the universal genetic code. Microbiol. Rev. 53**:**273-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfund, C., P. Huang, N. Lopez-Hoyo, and E. Craig. 2001. Divergent functional properties of the ribosome-associated molecular chaperone Ssb compared to other Hsp70s. Mol. Biol. Cell 12**:**3773-3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfund, C., N. Lopez-Hoyo, T. Ziegelhoffer, B. A. Schilke, P. Lopez-Buesa, W. A. Walter, M. Wiedmann, and E. A. Craig. 1998. The molecular chaperone SSB from S. cerevisiae is a component of the ribosome-nascent chain complex. EMBO J. 17**:**3981-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakwalska, M., and S. Rospert. 2004. The ribosome-bound chaperones RAC and Ssb1/2p are required for accurate translation in Saccharomyces cerevisiae. Mol. Cell. Biol. 24**:**9186-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Navarro, A., and J. Ramos. 1984. Dual system for potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 159**:**940-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrano, R., and A. Rodriguez-Navarro. 2001. Ion homeostasis during salt stress in plants. Curr. Opin. Cell Biol. 13**:**399-404. [DOI] [PubMed] [Google Scholar]
- 34.Siegers, K., B. Bölter, J. P. Schwarz, U. Böttcher, S. Guha, and F.-U. Hartl. 2003. TRiC/CCT cooperates with different upstream chaperones in the folding of distinct protein classes. EMBO J. 22**:**5230-5240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Slater, M., and E. A. Craig. 1987. Transcriptional regulation of an hsp70 heat shock gene in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 7**:**1906-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stansfield, I., Akhmaloka, and M. Tuite. 1995. A mutant allele of the SUP45 (SAL4) gene of Saccharomyces cerevisiae shows temperature-dependent allosuppressor and omnipotent suppressor phenotypes. Curr. Genet. 27**:**417-426. [DOI] [PubMed] [Google Scholar]
- 37.Stansfield, I., K. Jones, P. Herbert, A. Lewendon, W. Shaw, and M. Tuite. 1998. Missense translation errors in Saccharomyces cerevisiae. J. Mol. Biol. 282**:**13-24. [DOI] [PubMed] [Google Scholar]
- 38.Stone, D. E., and E. A. Craig. 1990. Self-regulation of 70-kilodalton heat shock proteins in Saccharomyces cerevisiae. Mol. Cell. Biol. 10**:**1622-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valpuesta, J. M., J. Martin-Benito, P. Gomez-Puertas, J. L. Carrascosa, and K. R. Willison. 2002. Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett. 529**:**11-16. [DOI] [PubMed] [Google Scholar]
- 40.Van Belle, D., and B. Andre. 2001. A genomic view of yeast membrane transporters. Curr. Opin. Cell Biol. 13**:**389-398. [DOI] [PubMed] [Google Scholar]
- 41.Wieland, J., A. Nitsche, J. Strayle, H. Steiner, and H. Rudolph. 1995. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 14**:**3870-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan, W., B. Schilke, C. Pfund, W. Walter, S. Kim, and E. A. Craig. 1998. Zuotin, a ribosome-associated DnaJ molecular chaperone. EMBO J. 17**:**4809-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]