Intracellular Butyryl Phosphate and Acetyl Phosphate Concentrations in Clostridium acetobutylicum and Their Implications for Solvent Formation (original) (raw)
Abstract
It has been suggested (L. H. Harris, R. P. Desai, N. E. Welker, and E. T. Papoutsakis, Biotechnol. Bioeng. **67:**1-11, 2000) that butyryl phosphate (BuP) is a regulator of solventogenesis in Clostridium acetobutylicum. Here, we determined BuP and acetyl phosphate (AcP) levels in fermentations of C. acetobutylicum wild type (WT), degenerate strain M5, a butyrate kinase (buk) mutant, and a phosphotransacetylase (pta) mutant. A sensitive method was developed to measure BuP and AcP in the same sample. Compared to the WT, the buk mutant had higher levels of BuP and AcP; the BuP levels were high during the early exponential phase, and there was a peak corresponding to solvent production. Consistent with this, solvent formation was initiated significantly earlier and was much stronger in the buk mutant than in all other strains. For all strains, initiation of butanol formation corresponded to a BuP peak concentration that was more than 60 to 70 pmol/g (dry weight), and higher and sustained levels corresponded to higher butanol formation fluxes. The BuP levels never exceeded 40 to 50 pmol/g (dry weight) in strain M5, which produces no solvents. The BuP profiles were bimodal, and there was a second peak midway through solventogenesis that corresponded to carboxylic acid reutilization. AcP showed a delayed single peak during late solventogenesis corresponding to acetate reutilization. As expected, in the pta mutant the AcP levels were very low, yet this strain exhibited strong butanol production. These data suggest that BuP is a regulatory molecule that may act as a phosphodonor of transcriptional factors. DNA array-based transcriptional analysis of the buk and M5 mutants demonstrated that high BuP levels corresponded to downregulation of flagellar genes and upregulation of solvent formation and stress genes.
The anaerobic spore-forming bacterium Clostridium acetobutylicum is well known as a biological producer of the industrially important solvents acetone, ethanol, and butanol. In batch culture, the fermentation can be divided into two distinctive phases. In the initial acidogenic phase, the organism grows rapidly and produces acetate and butyrate, and there is a decrease in the medium pH resulting from acid accumulation. In the solventogenic phase, the organism grows slowly and produces acetone, butanol, and ethanol, and there is an increase in the medium pH due to acid reassimilation. The shift to solvent production is associated with the induction of solventogenic enzymes and a decrease in the activity of acidogenic enzymes (24, 32). Although many investigators have studied the biochemistry and molecular biology of the acetone-butanol fermentation, the detailed mechanisms of the shift to solvent formation are not well understood. Significant progress in the analysis of the enzyme activities and internal pH involved in the clostridial pathway has been reported. However, only recently have the in vivo levels of intermediate metabolites and cofactors, such as coenzyme A (CoA) and its derivatives, been studied (2).
Metabolic intermediates may directly affect the pattern of product formation in the fermentation. Two key compounds are acetyl phosphate (AcP) and butyryl phosphate (BuP). Until now, Escherichia coli was the only prokaryote in which AcP levels had been measured (19, 20, 33). AcP in E. coli not only is a key intermediate but also plays a global regulatory role (27).
BuP has a chemical structure similar to that of AcP and is likely to play a role similar to that of AcP. In solventogenic clostridia, minimal intracellular and/or extracellular levels of butyric and/or acetic acid have been deemed necessary for induction of solvent formation (21, 22, 29, 36). Based on correlative studies, it was suggested that a minimum intracellular concentration of butyric acid of 9 mM is necessary for induction of solvent formation and that intracellular acetic acid concentrations could not be related to the initiation of solvent formation (21, 22). Nevertheless, inclusion of low concentrations of acetate in fermentation media appears to enhance solvent formation (21). Recent studies with a butyrate kinase (BK) (buk) mutant of C. acetobutylicum led to the hypothesis that the presumed elevated levels of BuP are responsible for the early induction of solvent formation, in which butanol formation is initiated during the early exponential phase (15). Initiation of solvent formation typically commences during the transition from exponential growth to the stationary phase. Here, we tested the hypothesis that the buk mutant has elevated BuP levels. We also examined the possibility that BuP and possibly AcP levels correlate with initiation of solvent formation and other cellular programs that have been linked to AcP-mediated regulation in E. coli. In order to do this, we developed an assay to measure intracellular BuP levels simultaneously with and independent of AcP levels. We report here the intracellular concentrations of BuP and AcP over the course of fermentations for several C. acetobutylicum strains. We also examined if there is a correlation between BuP and AcP concentrations and the pattern of gene expression as measured by DNA microarrays.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or referenceb |
|---|---|---|
| C. acetobutylicum strains | ||
| ATCC 824 | Wild type | ATCC |
| PJC4BK | Emr, butyrate kinase mutant | 12 |
| PJC4PTA | Emr, phosphate acetyltransferase mutant | 12 |
| M5 | pSOL1− | 7 |
| E. coli TOP10 | Invitrogen | |
| Plasmids | ||
| pJC7 | Apr, BK gene | 4 |
| pTrcHis-TOPO | Apr, overexpression vector | Invitrogen |
| pTrcHis-BK | Apr, BK overexpression | This study |
Chemicals.
Butyryl phosphate was synthesized by the method of Stadtman (35). ADP was purchased from Sigma-Aldrich (St. Louis, Mo.). Luciferase and luciferin were purchased from Boehringer Mannheim (Indianapolis, Ind.). Acetate kinase (AcK) was purchased as a lyopholized powder from Sigma Chemical Company (St. Louis, Mo.), dissolved at a concentration of 1 U/μl in 25 mM Tris-HCl (pH 7.6) with 1 mM dithiothreitol (DTT), and stored at −80°C in small aliquots. All other chemicals, including acetyl phosphate, were purchased from Sigma Chemical Company.
Growth conditions and maintenance.
E. coli was grown aerobically at 37°C in Luria-Bertani medium. Media for E. coli recombinant strains were supplemented with ampicillin (100 μg/ml) and erythromycin (300 μg/ml) as needed. C. acetobutylicum was grown anaerobically at 37°C in Clostridium growth medium (17). Media for C. acetobutylicum recombinant strains were supplemented with erythromycin (40 μg/ml) as needed. For long-term storage of C. acetobutylicum, strains were cultivated, lyophilized, and stored in ampoules.
DNA isolation, manipulation, and transformation.
E. coli plasmid DNA was prepared by using Qiaprep Miniprep and Midiprep kits (QIAGEN, Valencia, Calif.) according to the manufacturer's instructions. Individual DNA fragments were isolated by agarose gel electrophoresis and extracted from the gel with a QIAquick gel extraction kit (QIAGEN). Restriction endonucleases, T4 ligase, and T4 polymerase were purchased from New England Biolabs (Beverly, Mass.) and were used according to the manufacturer's specifications. Transformation of E. coli was conducted by electroporation.
Fermentation experiments.
Static flask cultures containing 1 liter (AcP and BuP analysis) or 2 liters (AcP, BuP, and microarray analysis) of Clostridium growth medium were inoculated (1% inocula) with overnight cultures of C. acetobutylicum and grown in an anaerobic chamber. For AcP and BuP analysis, 100- to 200-ml samples were taken from each flask during the early exponential phase, and 30- to 50-ml samples were taken at later times. Cells were collected by centrifugation at 10,000 × g for 10 min at 4°C, and the pellet was immediately frozen on dry ice, stored at −85°C, and subsequently used in the AcP and BuP assays. Supernatant fluids were stored at −20°C for analysis of product (butanol, acetone, ethanol, butyrate, and acetate) concentrations by gas chromatography (42) or liquid chromatography (3). The variability of values for individual products was less than 8%.
RNA sampling, isolation, and purification.
RNA sampling, isolation, and purification were performed as previously described (39).
cDNA labeling and hybridization.
Labeled cDNA was synthesized by random hexamer-primed reverse transcription reactions (38). Labeled cDNA was hybridized on targeted cDNA arrays containing spots representing approximately one-fourth of the C. acetobutylicum genome (38). The genes in these targeted cDNA microarrays included, among others, 169 of 178 pSOL1 open reading frames (corrected from a previous publication [38]), 123 DNA replication and repair genes (90% of all such genes as identified by the genome annotation [30]), 97 cell division- and sporulation-related genes (92% of all such genes), 85 carbohydrate primary metabolism genes (31% of all such genes), including all known solventogenic genes, 67 energy production genes (52% of all such genes), 63 outer membrane and cell envelope genes (36% of all such genes), 48 lipid metabolism genes (80% of all such genes), 42 motility and chemotaxis genes (39% of all such genes), and all previously identified stress response genes. Arrays were hybridized as previously described (39), with the following modifications. Following array prehybridization, the arrays were washed twice in Millipore (Bedford, Mass.) water and twice in isopropanol. Each wash consisted of dipping the array five times completely and then dipping it five times to the top of the spotted area. The arrays were then immediately blown dry with filtered compressed air. If visible streaks were present, the washing process was immediately repeated. Before hybridization, labeled samples were mixed with an equal volume of 2× hybridization buffer (10× SSC, 50% formamide, 0.2% sodium dodecyl sulfate [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) containing 1 μl of sonicated salmon sperm DNA (10 mg/ml; Stratagene), and the mixture was denatured at 95°C for 3 min. All samples were analyzed in duplicate by using reverse-labeled samples (e.g., buk mutant-Cy3 versus strain M5-Cy5 and buk mutant-Cy5 versus strain M5-Cy3). The hybridized arrays were analyzed with an Agilent G2565BA scanner (Agilent, Palo Alto, Calif.). Spot intensities were quantitated with the GenePix Pro 5.0 analysis software (Axon Instruments, Union City, Calif.). The resulting array data were normalized, and genes exhibiting a high degree of differential expression (>95% confidence) were identified (39, 41). A complete list with expression profile data is available at http://www.chem-eng.northwestern.edu/Faculty/papou.html. The two sets of differentially expressed genes were analyzed by average-linkage hierarchical clustering (GeneCluster), and expression profiles were displayed (Eisen plots) by using TreeView (10).
Overexpression and purification of histidine-tagged butyrate kinase.
Plasmid pTrcHis-BK was constructed for overexpression of butyrate kinase. Primers BK-N (5′-ATG TAT AGA TTA CTA ATA ATC AAT-3′) and BK-C (5′-TTA TTT GTA TTC CTT AGC TTT-3′) were used to amplify the buk coding region with plasmid pJC7 (4) as the template DNA. The amplified region corresponded to positions 1074 to 2141 of the buk gene (GenBank accession no. L14744). The amplified 1,068-bp PCR product was gel band purified and cloned into pTrcHis-TOPO (Invitrogen) to form pTrcHis-BK.
His-tagged BK was purified from E. coli TOP10(pTrcHisBK) that was grown at 37°C in 50 ml of Luria-Bertani medium inoculated from an overnight culture. When the _A_600 of the culture reached 0.5, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.02 mM. After 4 to 6 h of induction, the cells were harvested and frozen at −20°C. The thawed cells were resuspended in 2 ml of lysis buffer (50 mM NaH2PO4, 5 mM Tris, 1 mM imidazole; pH 8.0) and sonicated. The sonicated lysate was centrifuged at 10,000 × g for 30 min, and the supernatant was loaded onto a QIAGEN Ni-nitrilotriacetic acid column. The column was washed three times with wash buffer (50 mM NaH2PO4, 5 mM Tris, 300 mM NaCl, 20 mM imidazole, 0.5% Triton X-100; pH 8.0) and was eluted with elution buffer (50 mM NaH2PO4, 5 mM Tris, 300 mM NaCl, 250 mM imidazole, 1 mM DTT; pH 8.0). The eluted enzyme was analyzed by electrophoresis on a 12.5% polyacrylamide gel. The purified BK was divided into aliquots and stored at −80°C.
Determination of intracellular concentrations of butyryl phosphate and acetate phosphate.
To determine the intracellular concentrations of BuP and AcP, the method of Prüss and Wolfe (33) was used, with a few modifications. Cell extracts were prepared by perchloric acid extraction. Stored cell pellets were resuspended in 3 ml of washing buffer (10 mM sodium phosphate [pH 7.5], 10 mM MgCl2, 1 mM EDTA), treated with ice-cold 3 M HClO4 (200 μl/ml of cell suspension), and incubated for 30 min on ice. The mixture was centrifuged for 5 min at 10,000 × g at 4°C. The supernatant was neutralized with saturated KHCO3 and centrifuged as described above. ATP and other small adenylated molecules were removed by incubating the neutralized extract with activated charcoal (50 mg/ml) for 15 min on ice. The charcoal was removed by filtration with a 0.22-μm-pore-size syringe filter unit (Millipore).
AcP or BuP was converted to ATP by adding 1 μl of 1 M MgCl2, 1 μl of 30 mM ADP, and an appropriate amount of AcK or BK to 1 ml of the extract, followed by incubation at 30°C for 30 min. Because of the decreased activity of AcK or BK when it was kept at −80°C, the appropriate amount of AcK or BK was determined independently for each set of analyses. The amounts of AcK and BK that could convert AcP and BuP, respectively, to ATP without converting the other acyl phosphate species were used. These amounts were 0.15 to 0.25 U of the kinase, and the results that demonstrated the specificity of each enzyme for each acyl phosphate under these assay conditions are shown in Fig. 1B. The concentration of ATP produced from AcP or BuP was determined by using the luciferase assay method (25) and a TD-20e luminometer (Turner Designs, Sunnyvale, Calif.). A 150-μl aliquot of the extract was assayed in a 1-ml reaction mixture containing 60 mM Tris acetate (pH 7.75), 1.7 mM EDTA, 60 μM DTT, 0.1% (wt/vol) bovine serum albumin, 17.5 mM magnesium acetate, 40 μM luciferin, and 600 U of luciferase (American firefly). The level of remaining ATP in each charcoal-treated extract and the ATP present as a contaminant in the ADP were determined by measurement of the reaction sample without addition of AcK or BK. The amount of ADP used (30 μM) in the conversion reaction was less than the amount used in the previously described assay (33), but a suitable signal was obtained. A series of AcP and BuP standards that had been subjected to the entire extraction and conversion procedure were used to obtain a standard curve for determining the AcP or BuP contents of the cell extracts. The variation in the assay was typically 10%. The intracellular concentrations of AcP and BuP are reported below in picomoles per gram (dry weight); i.e., the concentration was normalized to cell density. A mass extinction coefficient of 51 g of cells−1 cm−1 was used to convert optical densities (_A_600) to cell dry weights.
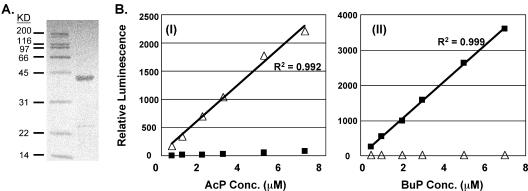
FIG. 1.
Development of AcP and BuP assay. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified His-tagged butyrate kinase. Left lane, protein marker; right lane, 3.6 μg of purified butyrate kinase. KD, kilodaltons. (B) Measurement of AcP (panel I) and BuP (panel II) standards by using acetate kinase and butyrate kinase, respectively. Symbols: ▵, AcP luminescence; ▪, BuP luminescence.
RESULTS
Development of an AcP and BuP assay.
Availability of a large amount of purified butyrate kinase is a precondition for measurement of BuP if a method similar to that for AcP is to be used. However, purified BK is not available commercially. The buk gene was PCR amplified and cloned into an E. coli expression vector. The molecular mass of BK is 39,027 Da, while the molecular mass of His-tagged BK is predicted to be 42,882 Da. The expressed His-tagged BK was purified, and one band of the predicted size (43 kDa) was observed (Fig. 1A). The eluted His-tagged BK exhibited a specific activity of 64 U/mg of protein when the method of Rose (34) was used.
AcK and BK exhibit a range of substrate specificities for the reversible reaction. Both enzymes can utilize AcP and BuP, but the relative activities are different. This made it particularly difficult to determine both AcP and BuP concentrations in the same clostridial extract when excess enzyme was used. However, a wide range of AcP or BuP concentrations could be measured with minimal background signals from the other acyl phosphate species by selecting an appropriate amount of AcK or BK (Fig. 1B). The amount of AcK or BK was determined independently for each assay as described in Material and Methods. The typical amount used ranged from to 0.15 to 0.25 U.
Time course of AcP and BuP levels in cultures of various strains.
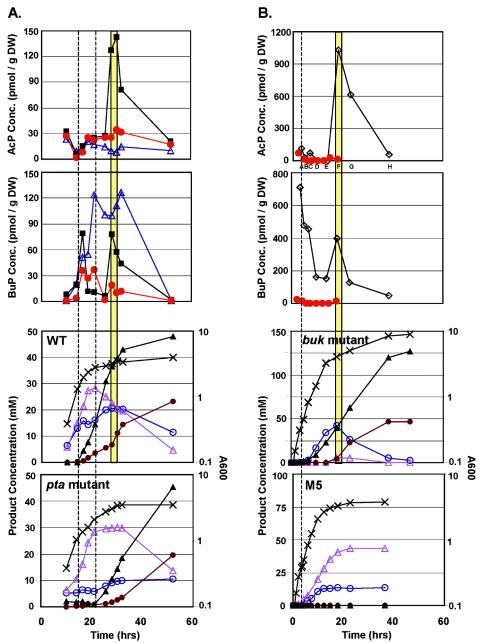
Time course profiles for AcP and BuP are shown in Fig. 2 for the wild type (WT), the pta and buk mutants, and strain M5. The pta and buk mutants are deficient in phosphotransacetylase (which converts acetyl-CoA to AcP, which is then converted to acetate by acetate kinase) and butyrate kinase, respectively. Strain M5 has lost the megaplasmid pSOL1 (8) and is asporogenous and deficient in solventogenesis. In all four strains, the AcP levels fell to nearly zero during the exponential phase. The levels of AcP in the M5 and pta mutant strains increased during the transition phase, but they only reached levels near those seen during the exponential phase. The AcP levels were significantly lower in the pta mutant throughout the study as a result of the inactivated phosphotransacetylase. In contrast to M5 and the pta mutant, the WT and the buk mutant were characterized by a peak AcP level (WT, 143 pmol/g [dry weight]; buk mutant, 1,032 pmol/g [dry weight]) midway through butanol and acetone formation during the early stationary phase, and there was a subsequent decrease during the late stationary phase. We also noted that the pta mutant produced high levels of butanol and at a higher flux than the WT (although initiation of butanol formation was delayed), yet the AcP levels remained low throughout the analysis. We concluded that AcP does not correlate with initiation of solventogenesis. However, the initial peak in the BuP levels coincided well with the onset of solvent production in all three solvent-producing strains (M5 does not produce solvent). The WT and the pta mutant had initial BuP peaks during the transition phase corresponding to initiation of solvent production. The initial BuP peak in the pta mutant was delayed compared to that in the WT, corresponding well with the delayed solvent production which has been noted previously (11). Both the WT and the pta mutant had a second BuP peak, before the levels finally fell to nearly zero at the end of the fermentations. The pta mutant, however, had a BuP profile that was sustained at higher levels, which may have been related to a stronger butanol formation flux and the stronger-than-usual acetone production during the late stationary phase. The BuP levels in the buk mutant were elevated during early exponential growth (711 pmol/g [dry weight]), which is consistent with early initiation of solvent (butanol) production. Initiation of solvent formation in the early exponential phase is atypical and well documented only in this strain (15). The second BuP peak (as well as the AcP peak) in the buk mutant also appeared to coincide with carboxylic acid reutilization and thus initiation of acetone production. The BuP concentrations in the buk mutant were five times higher than those in the WT, which is consistent with the data for an E. coli ack mutant which had higher levels of AcP than the WT (33).
FIG. 2.
BuP, AcP, growth, and product formation kinetics for C. acetobutylicum cultures. BuP and AcP kinetics are shown for the WT (▪), the pta mutant (▵), strain M5 (red circles), and the buk mutant (⋄). BuP and AcP levels are shown for two different M5 cultures (panels A and B). Transcriptional analysis was performed with samples taken from the fermentations depicted in panel B. The dashed vertical lines indicate the onset of solventogenesis in (from left to right) the WT and the pta mutant (panel A) and in the buk mutant (panel B). The vertical yellow bars indicate peaks in BuP and AcP concentrations corresponding to the onset of acetate reutilization in the WT and the buk mutant. Symbols: ○, acetate; brown circles, acetone; ▵, butyrate; ▴, butanol; ×, _A_600.
Transcriptional analysis and correlation with BuP and AcP levels.
A DNA array-based transcriptional analysis of the buk mutant and strain M5 was performed in order to examine the possible roles of BuP and AcP levels in transcriptional regulation. Samples for array analysis were taken during the exponential phase and into the transition phase to capture the switch from acid formation to solvent formation. All eight samples used for BuP and AcP analysis (Fig. 2B) were also used for array analysis. A time course transcriptional profile was obtained for the buk mutant by pairing the first labeled cDNA sample (sample A) with each of the following samples (samples B to H). Expression ratios were calculated relative to sample A. In Eisen plots, the expression ratios shown in red (see below) indicated higher relative expression compared to the expression in sample A, while the expression ratios shown in green indicated lower relative expression compared to the expression in sample A. A comparative transcriptional profile for the buk mutant versus strain M5 was obtained by pairing samples based on the stage of growth (data not shown). Relative expression profiles for BuP and AcP were determined from ratios of BuP or AcP levels by using the same sample pairs that were used in the microarray analysis. The profiles were included in the hierarchical clusters to identify genes with similar expression patterns.
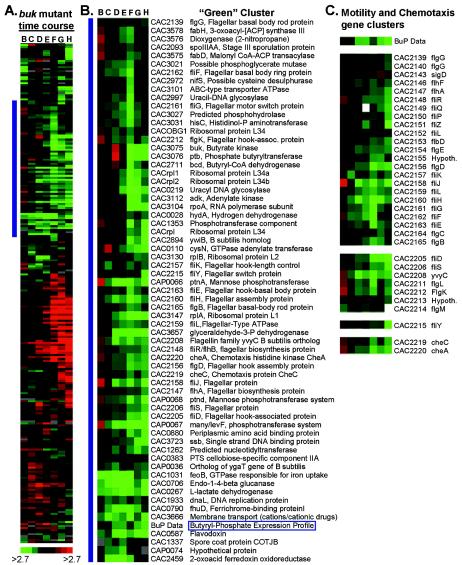
The hierarchical cluster from the buk mutant time course experiment is shown in Fig. 3A. The gene cluster containing the BuP expression data is shown in Fig. 3B. This green cluster contains 19 genes related to motility and chemotaxis, suggesting that BuP plays a role in regulating motility in C. acetobutylicum. AcP has been shown to regulate motility genes in E. coli (33). All known motility and chemotaxis genes present on the arrays (a total of 33 genes) showed a consistent pattern of decreased expression starting in the mid-exponential phase (Fig. 3C). Several phosphotransferase system (PTS) genes are also present. PTSs couple sugar uptake with phosphoenolpyruvate-dependent phosphorylation. Included in the BuP cluster are an operon (CAP0066 to CAP0068) with homology to the lev PTS operon in Bacillus subtilis (6), cellobiose-specific PTS component IIA (CAC0383), and an uncharacterized PTS component (CAC1353). Two genes involved in glycolysis are also included: glyceraldehyde-3-phosphate dehydrogenase and a possible phosphoglycerate mutase. Glyceraldehyde-3-phosphate dehydrogenase is involved in the formation of a high-energy sugar phosphate bond used in the net production of ATP during glycolysis. These findings suggest that BuP plays an important role in carbon metabolism. Other genes of interest include a predicted phosphohydrolase (CAC3027) and histidinol-phosphate aminotransferase (hisC; CAC3031).
FIG. 3.
Expression profiles from a buk mutant time course experiment. (A) Hierarchical cluster of differentially expressed genes. (B) Detailed view of the green cluster, next to the horizontal blue bar in panel A, which contains genes downregulated relative to the first time point (sample A). (C) Motility and chemotaxis gene clusters with BuP expression data. Red and green indicate higher and lower expression, respectively, relative to sample A. ABC, ATP binding cassette; ACP, acyl carrier protein; hook-assoc., hook-associated; Hypoth., hypothetical.
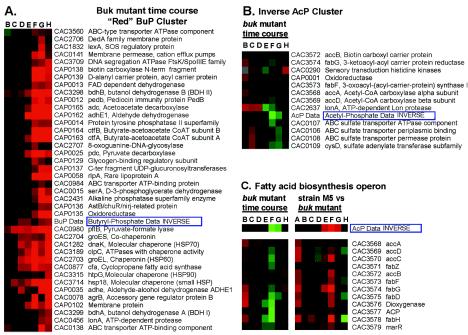
For temporarily upregulated genes (red cluster), the inverse of the BuP expression profile data was clustered with the buk mutant array data. Included in the red cluster (Fig. 4A) were the solvent formation genes, including adhE1 (CAP0162), ctfA (CAP0163), ctfB (CAP0164), adc (CAP0165), bdhA (CAC3298), bdhB (CAC3299), and a second aldehyde-alcohol dehydrogenase gene (adhE2; CAP0035). This cluster also contains a large number of stress response genes, including groESL (CAC2703 and CAC2704), dnaK (CAC1282), hsp18 (CAC3714), hsp90 (CAC3315), clpC (CAC3189), and lonA (CAC0456). The cfa gene coding for cyclopropane fatty acid synthase is also included in this cluster. Increased cfa expression has been shown to be associated with the solvent production phase (42). Other genes with very similar expression patterns include the genes encoding d-3-phosphoglycerate dehydrogenase (serA; CAC0015), alkaline phosphatase (CAC2431), pyruvate formate lyase (pflB; CAC0980), and pyruvate decarboxylase (pdc; CAP0025). The inverse of the AcP expression profile data was also clustered with the buk mutant array data, resulting in a smaller cluster (Fig. 4B) of genes inversely related to AcP levels. This cluster primarily included genes from one of two operons: the fatty acid biosynthesis (fab) operon and an ATP binding cassette sulfate transport operon. To better assess the relationship between AcP and the fab operon, the entire fab operon from both the buk mutant time course and the M5-buk mutant array data were compared to the inverse AcP data (Fig. 4C). This comparison suggested that there is a strong relationship between AcP and fab operon expression.
FIG. 4.
Hierarchical clusters of differentially expressed genes. (A) Genes with greater expression in the buk mutant relative to the first time point (sample A) (red cluster). (B) Genes in the buk mutant with expression patterns similar to the inverse AcP pattern. (C) Fatty acid biosynthesis operon, shown with inverse AcP data from both the buk mutant time course and the analyses of strain M5 versus the buk mutant. ABC, ATP binding protein; FAD, flavin adenine dinucleotide; HSP, heat shock protein; N term, N terminal; C ter, C terminal.
DISCUSSION
AcP and BuP are corresponding metabolic intermediates derived from acetyl-CoA and butyryl-CoA, respectively, during the acidogenic growth phase in C. acetobutylicum (24). At the end of acidogenic phase, in which the reactions which lead from butyryl-CoA via butyryl phosphate to butyrate formation are typically reversed (9, 14, 15), an elevated concentration of butyrate likely results in higher levels of BuP and butyryl-CoA as acids begin to be taken up. This does not happen frequently for the corresponding acetate-AcP system (9, 14, 15). Another main pathway for the uptake and reutilization of acetate and butyrate during the solvent-producing phase has been shown to be directly coupled to the production of acetone via acetoacetyl-CoA:acetate/butyrate:CoA transferase (CoAT) (9, 14, 15). The additional acetyl-CoA or butyryl-CoA formed from these processes can also be converted to AcP and BuP. The second BuP peak and the single AcP peak appear to be derived from these carboxylic acid reutilization reactions. The first BuP peak, however, is generated by another mechanism, such as an activity imbalance between the two enzymes (BK and phosphate butyryltransferase). This possibility is suggested by the early and large BuP peak in the buk mutant, by which the BK-phosphate butyryltransferase activity imbalance is genetically generated.
Levels of acetyl-CoA and butyryl-CoA in C. acetobutylicum have been measured (2, 13). During the shift from the acidogenic phase to the solventogenic or stationary phase, the concentration of butryl-CoA increased rapidly and paralleled that of BuP. In E. coli, the acetyl-CoA level also paralleled the AcP level (33). When incubated with AcP in vitro, several E. coli (and possibly other prokaryotic) response regulator proteins become phosphorylated; these proteins include CheY, NRI, PhoB, OmpR (26), RssB (1), and PhoP (5). Thus, AcP may act as an in vivo phosphodonor for these regulators. The intracellular concentration of AcP is strongly dependent on the metabolic state of the cell, as well as on the growth phase, carbon source, pH, and temperature (18, 27, 31, 33, 40). Therefore, the concentration of cytoplasmic AcP can be regarded as a physiologically relevant signal that feeds into the signal transduction system of E. coli and other prokaryotes. In E. coli, elevated levels of AcP have been shown to result in inhibition of flagellar synthesis and motility (33), and, in fact, AcP has been demonstrated to be the signal for control of flagellar gene expression (33, 40).
C. acetobutylicum exhibits morphological changes (differentiation) during fermentation (23). The initial acidogenic phase is normally characterized by the presence of highly motile elongated dividing rods. At entry into the stationary phase, which is typically coupled with the onset of solvent production, cell motility begins to decrease, and the swollen, cigar-shaped clostridial forms become abundant. Considering the correlation of the time of the sharp increase in BuP and the onset of solvent production, BuP in C. acetobutylicum might have a role in solventogenesis, chemotaxis, and motility. In C. acetobutylicum, Spo0A is the positive regulator of sporulation genes, as well as of the solvent genes adhE (aad), ctfA, ctfB, and adc (16, 37). BuP in C. acetobutylicum may act as the phosphodonor to phosphorylate Spo0A, thus triggering solvent production. The observation that the first BuP peak corresponds to initiation of solvent formation suggests that BuP (but not AcP) may regulate the Spo0A phosphorylation level, which then positively regulates solventogenic genes and may, similar to what happens in B. subtilis (28), negatively regulate flagellar, chemotaxis, and DNA replication genes (Fig. 2B and C). The finding that BuP but not AcP correlates with (and possibly regulates) initiation of solvent formation is consistent with the previous observation that threshold intracellular butyrate concentrations, but not threshold acetate concentrations, are necessary for initiation of solventogenesis (22).
Although some factors, such as carboxylic acid end products, nutrient (phosphate, nitrogen) limitation, and pH, have been considered as triggering mechanisms for solvent production (24, 32), the detailed molecular mechanism is still unclear. BuP may be a major molecule that mediates several of these solventogenic triggers. The connection between carboxylic acid, extracellular or intracellular pH, or phosphate limitation and BuP appears to be logical, but it requires further study.
Acknowledgments
This work was supported by National Science Foundation grants BES-9905669, BES-0001288, and BES-0418289 and by Robert A. Welch Foundation grants C-1268 and C-1372 to G.N.B. and F.B.R., respectively.
We thank the Microarray Core Facility of the Center for Genetic Medicine at Northwestern University.
REFERENCES
- 1.Bouché, S., E. Klauck, D. Fischer, M. Lucassen, K. Jung, and R. Hengge-Aronis. 1998. Regulation of RssB-dependent proteolysis in Escherichia coli: a role for acetyl phosphate in a response regulator-controlled process. Mol. Microbiol. 27**:**787-795. [DOI] [PubMed] [Google Scholar]
- 2.Boynton, Z. L., G. N. Bennett, and F. B. Rudolph. 1994. Intracellular concentrations of coenzyme A and its derivatives from Clostridium acetobutylicum ATCC 824 and their roles in enzyme regulation. Appl. Environ. Microbiol. 60**:**39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buday, Z., J. C. Linden, and M. N. Karim. 1990. Improved acetone butanol fermentation analysis using subambient HPLC column temperature. Enzyme Microbiol. Technol. 12**:**24-27. [Google Scholar]
- 4.Cary, J. W., D. J. Petersen, E. T. Papoutsakis, and G. N. Bennett. 1988. Cloning and expression of Clostridium acetobutylicum phosphotransbutyrylase and butyrate kinase genes in Escherichia coli. J. Bacteriol. 170**:**4613-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamnongpol, S., and E. A. Groisman. 2000. Acetyl phosphate-dependent activation of a mutant PhoP response regulator that functions independently of its cognate sensor kinase. J. Mol. Biol. 300**:**291-305. [DOI] [PubMed] [Google Scholar]
- 6.Charrier, V., J. Deutscher, A. Galinier, and I. Martin-Verstraete. 1997. Protein phosphorylation chain of a Bacillus subtilis fructose-specific phosphotransferase system and its participation in regulation of the expression of the lev operon. Biochemistry 36**:**1163-1172. [DOI] [PubMed] [Google Scholar]
- 7.Clark, S. W., G. N. Bennett, and F. B. Rudolph. 1989. Isolation and characterization of mutants of _Clostridium acetobutylicu_m ATCC 824 deficient in acetoacetyl-coenzyme A:acetate/butyrate:coenzyme A transferase (EC 2.8.3.9) and in other solvent pathway enzymes. App. Environ. Microbiol. 55**:**970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornillot, E., R. V. Nair, E. T. Papoutsakis, and P. Soucaille. 1997. The genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 reside on a large plasmid whose loss leads to degeneration of the strain. J. Bacteriol. 179**:**5442-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai, R. P., L. M. Harris, N. E. Welker, and E. T. Papoutsakis. 1999. Metabolic flux analysis elucidates the importance of the acid-formation pathways in regulating solvent production by Clostridium acetobutylicum. Metab. Eng. 1**:**206-213. [DOI] [PubMed] [Google Scholar]
- 10.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95**:**14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, E. M., and G. N. Bennett. 1998. Genetic manipulation of acid and solvent formation in Clostridium acetobutylicum ATCC 824. Biotechnol. Bioeng. 58**:**217-221. [DOI] [PubMed] [Google Scholar]
- 12.Green, E. M., Z. L. Boynton, L. M. Harris, F. B. Rudolph, E. T. Papoutsakis, and G. N. Bennett. 1996. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142**:**2079-2086. [DOI] [PubMed] [Google Scholar]
- 13.Grupe, H., and G. Gottschalk. 1992. Physiological events in Clostridium acetobutylicum during the shift from acidogenesis to solventogenesis in continuous culture and presentation of a model for shift induction. Appl. Environ. Microbiol. 58**:**3896-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris, L. M., L. Blank, R. P. Desai, N. E. Welker, and E. T. Papoutsakis. 2001. Fermentation characterization and flux analysis of recombinant strains of Clostridium acetobutylicum with an inactivated solR gene. J. Ind. Microbiol. Biotechnol. 27**:**322-328. [DOI] [PubMed] [Google Scholar]
- 15.Harris, L. M., R. P. Desai, N. E. Welker, and E. T. Papoutsakis. 2000. Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: need for new phenomenological models for solventogenesis and butanol inhibition? Biotechnol. Bioeng. 67**:**1-11. [PubMed] [Google Scholar]
- 16.Harris, L. M., N. E. Welker, and E. T. Papoutsakis. 2002. Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J. Bacteriol. 184**:**3586-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmanis, M. G. N., and S. Gatenbeck. 1984. Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl. Environ. Microbiol. 47**:**1277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyde, M., P. Laloi, and R. Portalier. 2000. Involvement of carbon source and acetyl phosphate in the external-pH-dependent expression of porin genes in Escherichia coli. J. Bacteriol. 182**:**198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt, A. G. 1986. Micromethod for the measurement of acetyl-phosphate and acetyl-CoA. Methods Enzymol. 122**:**43-50. [DOI] [PubMed] [Google Scholar]
- 20.Hunt, A. G., and J. Hong. 1980. A micromethod for the measurement of acetyl phosphate and acetyl coenzyme A. Anal. Biochem. 108**:**290-294. [DOI] [PubMed] [Google Scholar]
- 21.Hüsemann, M. H., and E. T. Papoutsakis. 1990. Effects of propionate and acetate additions on solvent production in batch cultures of Clostridium acetobutylicum. Appl. Environ. Microbiol. 56**:**1497-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hüsemann, M. H. W., and E. T. Papoutsakis. 1988. Solventogenesis in Clostridium acetobutylicum fermentations related to carboxylic-acid and proton concentrations. Biotechnol. Bioeng. 32**:**843-852. [DOI] [PubMed] [Google Scholar]
- 23.Jones, D. T., A. V. D. Westhuizen, S. Long, E. R. Allock, S. J. Reid, and D. R. Woods. 1982. Solvent production and morphological changes in Clostridium acetobutylicum. Appl. Environ. Microbiol. 43**:**1434-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, D. T., and D. R. Woods. 1986. Acetone-butanol fermentation revisited. Microbiol. Rev. 50**:**484-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundin, A., and A. Thore. 1975. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal. Biochem. 66**:**47-63. [DOI] [PubMed] [Google Scholar]
- 26.McCleary, W. R., and J. B. Stock. 1994. Acetyl phosphate and the activation of two-component response regulators. J. Biol. Chem. 269**:**31567-31572. [PubMed] [Google Scholar]
- 27.McCleary, W. R., J. B. Stock, and A. J. Ninfa. 1993. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 175**:**2793-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molle, V., M. Fujita, S. T. Jensen, P. Eichenberger, J. E. Gonzalez-Pastor, J. S. Liu, and R. Losick. 2003. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 50**:**1683-1701. [DOI] [PubMed] [Google Scholar]
- 29.Monot, F., J. M. Engasser, and H. Petitdemange. 1984. Influence of pH and undissociated butyric acid on the production of acetone and butanol in batch culture of Clostridium acetobutylicum. Appl. Microbiol. Biotechnol. 19**:**422-426. [Google Scholar]
- 30.Nölling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. D. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Y. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183**:**4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyström, T. 1994. The glucose-starvation stimulon of Escherichia coli: induced and repressed synthesis of enzymes of central metabolic pathways and role of acetyl phosphate in gene expression and starvation survival. Mol. Microbiol. 12**:**833-843. [DOI] [PubMed] [Google Scholar]
- 32.Papoutsakis, E. T., and G. N. Bennett. 1999. Molecular regulation and metabolic engineering of solvent production by Clostridium acetobutylicum, p. 253-279. In S. Y. Lee and E. T. Papoutsakis (ed.), Metabolic engineering. Marcel Dekker, Inc., New York, N.Y.
- 33.Prüss, B. M., and A. J. Wolfe. 1994. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol. Microbiol. 12**:**973-984. [DOI] [PubMed] [Google Scholar]
- 34.Rose, I. R. 1955. Acetate kinase of bacteria (acetokinase). Methods Enzymol. 1**:**591-595. [Google Scholar]
- 35.Stadtman, E. R. 1957. Preparation and assay of acetyl phosphate. Methods Enzymol. 3**:**229-231. [Google Scholar]
- 36.Terracciano, J. S., and E. R. Kashket. 1986. Intracellular conditions required for the initiation of solvent production by Clostridium acetobutylicum. Appl. Environ. Microbiol. 52**:**86-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thormann, K., L. Feustel, K. Lorenz, S. Nakotte, and P. Durre. 2002. Control of butanol formation in Clostridium acetobutylicum by transcriptional activation. J. Bacteriol. 184**:**1966-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomas, C. A., K. V. Alsaker, H. P. J. Bonarius, W. T. Hendriksen, H. Yang, J. A. Beamish, C. J. Parades, and E. T. Papoutsakis. 2003. DNA array-based transcriptional analysis of asporogenous, nonsolventogenic Clostridium acetobutylicum strains SKO1 and M5. J. Bacteriol. 185**:**4539-4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomas, C. A., J. A. Beamish, and E. T. Papoutsakis. 2004. Transcriptional analysis of butanol tolerance in Clostridium acetobutylicum. J. Bacteriol. 186**:**2006-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfe, A. J., D. E. Chang, J. D. Walker, J. E. Seitz-Partridge, M. D. Vidaurri, C. F. Lange, B. M. Prüss, M. C. Henk, J. C. Larkin, and T. Conway. 2003. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol. Microbiol. 48**:**977-988. [DOI] [PubMed] [Google Scholar]
- 41.Yang, H., H. Haddad, C. Tomas, K. Alsaker, and E. T. Papoutsakis. 2003. A segmental nearest neighbor normalization and gene identification method gives superior results for DNA-array analysis. Proc. Natl. Acad. Sci. USA 100**:**1122-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao, Y., L. A. Hindorff, A. Chuang, M. Monroe-Augustus, M. Lyristis, M. L. Harrison, F. B. Rudolph, and G. N. Bennett. 2003. Expression of a cloned cyclopropane fatty acid synthase gene reduces solvent formation in Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 69**:**2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]