The combined effects of 3,4-methylenedioxymethamphetamine (MDMA) and selected substituted methcathinones on measures of neurotoxicity (original) (raw)
. Author manuscript; available in PMC: 2018 May 1.
Published in final edited form as: Neurotoxicol Teratol. 2017 Feb 16;61:74–81. doi: 10.1016/j.ntt.2017.02.003
Abstract
The rise in popularity of substituted methcathinones (aka “bath salts”) has increased the focus on their neurotoxic effects. Two commonly abused methcathinones, 3,4-methylenedioxymethcathinone (methylone, MDMC) and 3,4-methylenedioxypyrovalerone (MDPV), are often concomitantly ingested with the illicit drug 3,4-methylenedioxymethamphetamine (MDMA). To examine potential neurotoxic effects of these drug combinations, C57BL/6J mice were administered 4 i.p. injection of the drugs, at 2 h intervals, either singularly: MDMA 15 or 30 mg/kg, methylone 20 mg/kg, MDPV 1 mg/kg; or in combination: methylone/MDMA 20/15 mg/kg, MDPV/MDMA 1/15 mg/kg. Drug effects on thermoregulation were characterized and striatal tissue analyzed after 2 or 7 days for dopamine (DA) and tyrosine hydroxylase (TH) levels, as well as glial fibrillary acidic protein (GFAP) expression. Two days following drug administration, DA and TH were decreased only in the MDMA 30 mg/kg group, whereas GFAP expression was dose-dependently increased by MDMA alone. While the combination of the methcathinones with the lower MDMA dose did not affect DA or TH levels, both blocked the MDMA-induced increase in GFAP expression. Seven days following drug administration, there were no significant differences in DA, TH, or GFAP for any treatment group, indicating that changes in DA, TH, and GFAP were transient. Five of the six drug groups exhibited acute hypothermia followed by gradually increasing temperatures. Animals treated with MDPV did not exhibit these biphasic temperature changes, and resembled the saline group. These results indicate that specific effects of both methylone and MDPV on DA depletion or astrocyte activation in the striatum are not additive with effects of MDMA, but block astrogliosis caused by MDMA alone. Additionally, MDPV modulates thermoregulation through a different mechanism than methylone or MDMA.
Keywords: MDMA, methylone, MDPV, neurotoxicity, dopamine, GFAP
1. Introduction
Illicit use of 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) continues unabated (UNODC, 2014;NFLIS, 2015). Additionally, new psychoactive substances in the form of synthetic cathinones (referred to collectively as “bath salts”) have emerged. Originally sold as legal alternatives to MDMA, the U.S. Drug Enforcement Administration (DEA) emergency scheduled three substituted methcathinones in 2011: methylone, MDPV, and 4-methylmethcathinone (mephedrone, 4-MMC) making them illegal to possess, use or manufacture (Drug Enforcement Administration, 2013). The latest report of drugs seized and analyzed in the U.S. (2014) reported methylone to be just as prevalent as MDMA (NFLIS, 2015). Although MDPV is not as common as MDMA or methylone, it is responsible for more severe adverse effects, resulting in psychosis and increased fatalities among users (White, 2016;Vallersnes et al., 2016). The methcathinones’ danger to health is reflected in the large number of emergency department visits. In the U.S.: during 2011, there were 22,904 methcathinone-related visits, 67% involving combinations with other drugs, similar to the number of visits involving MDMA (22,498) (DAWN, 2013). Further complicating matters, the majority of MDMA users are poly-drug users (Mohamed et al., 2011), and the content of “ecstasy” tablets varies in purity and adulterants (Vogels et al., 2009) including methcathinones (Brunt et al., 2011;Gonzalez et al., 2013). Although there is considerable co-ingestion of MDMA and substituted methcathinones, both intentionally and unknowingly (Palamar et al., 2016;Caudevilla-Galligo et al., 2013), the neurotoxic effects of these drug combinations remain unclear.
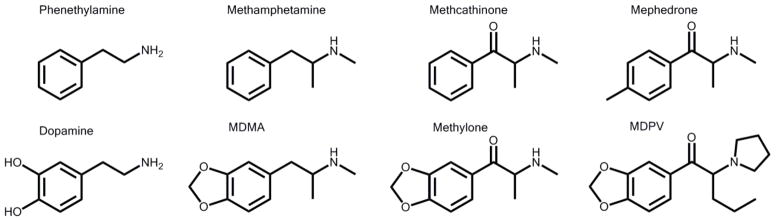
MDMA, methylone, and MDPV are all phenethylamines possessing a methylenedioxy bridge (Fig. 1). Methylone is the methcathinone analogue of MDMA, differing only in a ketone group attached to the β-carbon. MDPV has two additional moieties: a propane group and a pyrrolidine ring attached at the α-carbon. Both methylone and MDPV act primarily on the monoamine transporters, but through different mechanisms and with different potencies. Methylone, like MDMA, is a transporter substrate with dual function; both act as potent inhibitors of neurotransmitter uptake at the dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET), while also inducing release of neurotransmitters via these transporters (Baumann et al., 2013). MDPV is a selective inhibitor of DAT and NET (with little effect on SERT) and is more than 10 times more potent at inhibition of DAT than MDMA or methylone (Eshleman et al., 2013). A primary difference between both methcathinones and MDMA is their affinity for and potency at the vesicular monoamine transporter 2 (VMAT2). MDMA is a substrate of VMAT2, but the methcathinones are not: they have low affinity for VMAT2 and low potency at either inhibiting VMAT2-mediated uptake or inducing VMAT2-mediated release of monoamines (Cozzi et al., 1999;Eshleman et al., 2013;Lopez-Arnau et al., 2012).
Fig. 1.
Molecular structures for phenethylamine and related compounds: DA; methamphetamine and substituted derivative MDMA; methcathinone and substituted derivatives mephedrone, methylone, and MDPV.
Although MDMA is predominantly a serotonergic neurotoxin in humans, primates, and rats, it selectively affects the dopaminergic system in mice (Logan et al., 1988;Mueller et al., 2013). Following MDMA administration, striatal DA overflow is acutely increased in mice (Camarero et al., 2002;Hagino et al., 2011). Subsequently, decreased striatal DA and TH levels are observed as early as 1 day later and as late as 21 days, indicative of DA terminal degeneration (O’Callaghan & Miller, 1994;Reveron et al., 2005;O’Callaghan et al., 2014). The neurotoxic effects of MDMA on DA nerve terminals is restricted to the nigrostriatal pathway, as there is no decrease in these markers in the nucleus accumbens following MDMA administration (Granado et al., 2008). Reactive gliosis (astrogliosis) is also a robust indicator of neurotoxicity, and is the dominant response of astrocytes to all types of injury of the central nervous system (CNS) (O’Callaghan & Sriram, 2005). Astrogliosis can be quantified by assaying CNS tissue for expression of glial fibrillary acidic protein (GFAP), the major intermediate filament protein of astrocytes. Striatal GFAP expression peaks 2–3 days after MDMA administration (O’Callaghan et al., 2014;Granado et al., 2008;O’Callaghan & Miller, 1994). Striatal DA markers decrease prior to an increase in GFAP expression (O’Callaghan & Miller, 1994;O’Callaghan et al., 2014) and pharmacological blockade of dopaminergic neurotoxicity decreases GFAP expression as well (O’Callaghan et al., 2014), findings consistent with dopaminergic damage serving as the inducer of the observed striatal astrogliosis. By themselves mephedrone, methylone and MDPV do not alter striatal DA, TH levels or GFAP expression in rodent models (den Hollander et al., 2013;Anneken et al., 2015;Baumann et al., 2012).
Although co-ingestion of MDMA and methylone is common due in part to the similarity in subjective effects reported (Palamar et al., 2016;Karila et al., 2016), the neurochemical effects of combining methylone and MDMA previously have not been investigated. Mephedrone (a transporter substrate like methylone) potentiates MDMA neurotoxicity when co-administered in mice, decreasing striatal DA levels 2 days after drug administration (Angoa-Perez et al., 2013). A more recent study found that methylone also increases METH neurotoxicity under similar conditions, decreasing striatal DA and TH levels as well as increasing GFAP expression beyond the effects of METH alone (Anneken et al., 2015). In the same study, MDPV was co-administered with MDMA and found to be neuroprotective. MDPV alone does not affect DA levels or GFAP expression (similar to methylone), but in combination with MDMA mitigates the DA and TH level decrease and GFAP expression increase elicited by MDMA alone. This is supported by previous research on combinations of MDMA and DAT inhibitors, such as the co-administration of MDMA with cocaine or GBR 12909(a selective DAT uptake inhibitor), both which attenuate MDMA-induced striatal DA level decrease (O’Shea et al., 2001;Peraile et al., 2013). As such, we hypothesized that a combination of the two transporter substrates (MDMA and methylone) would have an additive neurotoxic effect, resulting in lower DA and TH levels and greater GFAP expression, whereas a combination of a transporter substrate (MDMA) and a reuptake inhibitor (MDPV) would have a neuroprotective effect, mitigating decreased DA and TH levels and increased GFAP expression. Because of their prevalence in the illicit drug market, we investigated the neurotoxic effects of methylone and MDPV alone and in combination with MDMA.
Hyperthermia in response to substituted amphetamines significantly contributes to neurotoxicity, but can serve as a confound in determining the pharmacological effects of these drugs. A hyperthermic increase in core body temperature (above 40 °C) following drug administration is strongly correlated with striatal terminal degeneration and monaminergic fluctuations (Docherty & Green, 2010;Bowyer & Hanig, 2014). This neurotoxicity is exacerbated when drugs are administered at elevated environmental temperatures (27 °C) causing increased hyperthermia (Miller & O’Callaghan, 2003). However, drug-induced temperature-independent neurotoxicity occurs when hyperthermia is not present, though to a lesser degree. MDMA administered under normothermic conditions (21 °C) decreases mouse striatal DA and TH levels 7 days later (O’Shea et al., 2001), as does METH at a lowered environmental temperature of 13 °C (Bowyer et al., 2001). Both effects involve conditions under which hyperthermia was not induced. By administering MDMA, methylone, and MDPV at a normothermic environmental temperature (21 °C) that does not elicit hyperthermia, this study aimed to investigate the pharmacological effects of these drugs, independent of temperature effects.
2. Materials and Methods
2.1 Drugs and chemicals
Racemic MDMA, methylone, and MDPV hydrochloride were generously provided by the National Institute on Drug Abuse (NIDA) Research Resources Drug Supply program. All chemicals and reagents used to assay DA levels, as included in the Dopamine Research ELISA kit, were purchased from Rocky Mountain Diagnostics, Inc. (Colorado Springs, CO). The materials used in the TH immunoassay have been described previously (Sriram et al., 2006;Sriram et al., 2004). All chemicals and reagents used to assay GFAP expression levels, as included in the GFAP ELISA kit, were purchased from EMD Millipore (Billerica, MA). All other reagents were obtained from standard commercial sources, unless otherwise noted. 2.2 Animals
Female C57BL/6J mice (N = 112) were purchased from The Jackson Laboratory (Bar Harbor, ME) and tested at 11–15 weeks of age. Animals were group-housed, four to a poly-carbonate cage (19.6 × 30.9 × 13.3 cm) that was lined with BioFresh Performance Bedding (Absorption Corp, Ferndale, WA), and fitted with a wire top. The colony room was maintained at an environmental temperature of 21 ± 1 °C, on a 12 h light/dark schedule (lights on at 0600 h). Temperature measurements were made between 0700 and 1700 h. Food (Purina Laboratory Rodent Chow formulation 5LOD; Purina Mills, St. Louis, MO) and water were provided ad libitum. All procedures were approved by the VA Portland Health Care System’s Institutional Animal Care and Use Committee and followed the requirements of the Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to use alternatives to in vivo techniques when available. All animals acclimated to the vivarium at least one week prior to testing.
2.3 Temperature recording and drug treatment
Two days prior to drug administration, mice were implanted with IPTT-300 temperature transponders from BioMedic Data Systems (Seaford, DE) to assess body temperature via telemetry. Animals were anesthetized with isoflurane (5% induction, 2.5% maintenance) and transponders were subcutaneously injected dorsally between the shoulders.
On the day of drug administration, animals were weighed (Mean = 20.3 g, SEM = 1.2 g) and transferred from group to individual cages to avoid temperature changes associated with interaction (e.g., huddling). After a 1 h acclimation period, temperature recording began and was measured every 15 min for 8 h. Temperatures were non-invasively recorded using the DAS-8001 reader console and smart probe from BioMedic Data Systems. Animals were removed from the cage and the smart probe placed within 5 cm of the embedded transponder to acquire temperature readings. The environmental temperature of the testing environment was 21 ± 1 °C. Although increased ambient temperature correlates with increased neurotoxicity, MDMA-induced neurotoxicity occurs at the temperature used (21 °C) (O’Shea et al., 2001;Peraile et al., 2013). This temperature was selected in order to differentiate neurotoxic effects of the drugs from those exacerbated by elevated environmental temperatures.
Following the first recording (baseline temperature), each animal received four intra-peritoneal injections (same drug and dose for a particular animal) with a 2 h interval between injections. The seven treatment groups were: saline 0.9%, MDMA 15 mg/kg, methylone 20 mg/kg, MDPV 1 mg/kg, MDMA 30 mg/kg, methylone/MDMA 20/15 mg/kg combined, and MDPV/MDMA 1/15 mg/kg combined. An MDMA dose of 15 mg/kg was chosen for combination treatment groups as it decreases striatal DA levels without inducing fatalities (O’Shea et al., 2001;Herndon et al., 2014;Sanchez et al., 2003). MDMA is modestly more potent than methylone as a transporter substrate, whereas MDPV is 15 times more potent than MDMA as an uptake inhibitor at the DAT (Eshleman et al., 2013). This rank order of potencies (MDPV≫MDMA> methylone) has been demonstrated behaviorally in rodents (Dal Cason et al., 1997;Gatch et al., 2013) and is reflected in estimates of human recreational doses: MDPV = 8–15 mg; MDMA = 75–125 mg; methylone = 100–250 mg (Erowid, 2016). Single drug doses of methylone (20 mg/kg) and MDPV (1 mg/kg) were selected to approximate an equivalent dose to MDMA (15 mg/kg). All drugs were dissolved in 0.9% saline and injected in a final volume of 10 ml/kg.
2.4 Brain dissection and tissue preparation
Mice were euthanized 2 or 7 days after drug treatment and temperature recording, by cervical dislocation, followed by decapitation. The striatum was removed using blunt dissection, flash-frozen, and weighed prior to being stored at −70 °C until time of assay. Striatal tissue from each animal was dissected and each half used for either the DA or GFAP assays (counterbalanced by side of brain).
2.5 Quantification of dopamine levels
Tissue was homogenized in a 1 mM EDTA, 4 mM metabisulfite, 0.01 N HCl solution**,** using a 5 mg/ml dilution, and sonicated for 30 sec using a 2-mm ultra-sonification microprobe. Protein density was determined using the bicinchoninic acid (BCA) assay protocol. Striatal dopamine levels were quantified using a competitive enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions. 5 μl of tissue homogenate was used for DA analysis in duplicate. Two samples were excluded after meeting the outlier criterion.
2.6 Quantification of tyrosine hydroxylase levels
Tissue was prepared as previously outlined (O’Callaghan, 2002). Striatal tissue was homogenized in 10 volumes of 1% hot (85–95 °C) sodium dodecyl sulfate (SDS) by sonification and the total protein concentration was determined by BCA assay. TH holoenzyme protein was assessed using a fluorescence-based ELISA developed in the laboratory (Sriram et al., 2004). In brief, a mouse anti-rat tyrosine hydroxylase monoclonal antibody (1:500; Sigma-Aldrich, St. Louis, MO) was coated on the wells of Immulon-2 microliter plates (Thermo Labsystems, Franklin, MA). The SDS homogenates and standards (prepared from control mouse striatum) were diluted in phosphate-buffered saline (pH 7.4) containing 0.5% Triton X-100. After blocking non-specific binding with 5% non-fat dry milk, aliquots of the homogenate and standards were added to the wells in duplicate and incubated. Following washes, a rabbit anti-rat TH polyclonal antibody (1:500; Calbiochem, San Diego, CA) was added to ‘sandwich’ the TH protein between the two antibodies. The amount of sandwich antibody bound to TH was then detected using a peroxidase-labeled antibody directed against rabbit IgG (1:3000; Artisan Technology Group, Champagne, IL. Peroxidase activity was detected using the fluorogenic substrate Quantablu (Pierce), which has excitation and emission maxima of 325 and 420 nm, respectively (read at 320/405 nm). The amount of TH in the samples was calculated and expressed as micrograms TH per milligram total protein.
2.7 Quantification of GFAP expression
Tissue homogenate for GFAP analysis was prepared in the same manner as for TH analysis. Striatal GFAP levels were quantified using a sandwich ELISA kit (EMD Millipore, Billerica, MA) according to the manufacturer’s instructions. 100 μl of tissue homogenates, in duplicate, were used for GFAP analysis against a standard curve using a GFAP standard provided by the manufacturer.
2.8 Data analysis
One-way analysis of variance (ANOVA) was used to test the significance of drug treatment on striatal DA, TH levels, and GFAP expression at each time point, independently. Dunnett’s multiple comparison test was used for follow-up mean comparisons with the saline group. Mouse temperature data at each time point, grouped by drug treatment, were analyzed using a two-way repeated measures ANOVA. To follow up significant drug group × time interactions and examine the specific effects of each drug, data for each drug group were compared to the saline group data, using separate repeated measures ANOVAs, followed by Bonferroni’s multiple comparison test for mean comparisons at individual time points. Overall temperature change for comparison of drug effects was assessed by subtracting the mean temperature 15 min after the first injection (selected because maximal hypothermic effects were elicited at this time point) from the final mean temperature at the 8 h time point for each treatment group and analyzed by one-way ANOVA, using Dunnett’s multiple comparison test for follow-up mean comparisons with the saline group. Data were analyzed for outliers using Dixon’s Q test at 90% confidence. All statistical analyses were performed using Prism v.6.04 (GraphPad Software, La Jolla, CA). Differences were considered significant at p < 0.05.
3. Results
3.1 Dopamine levels
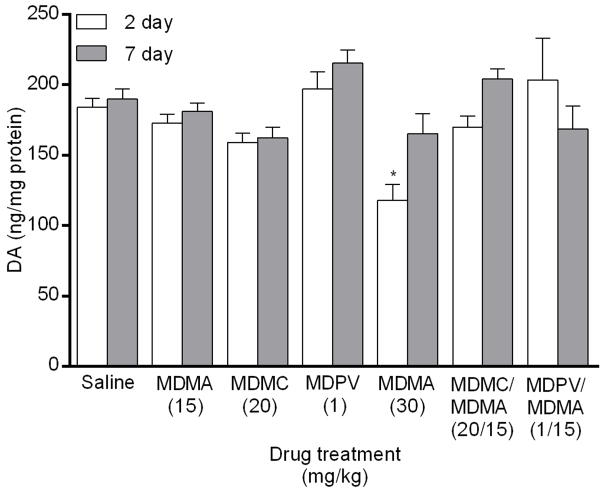
A main effect of drug group on DA levels (Fig. 2) was found in the striatum 2 days after drug treatment (_F_6,48 = 4.09, p < 0.01). Post hoc analysis revealed that only the MDMA 30 mg/kg group significantly decreased DA levels in comparison to the saline group. There was also a main effect of drug 7 days after drug treatment (_F_6,48 = 3.81, p < 0.01), but none of the drug groups differed significantly from the saline group for DA level.
Fig. 2.
Striatal DA levels measured 2 and 7 days following drug treatment via competitive ELISA. Groups of mice received 4 i.p. injections of the same drug/dose (saline; MDMA 15 mg/kg; methylone (MDMC) 20 mg/kg; MDPV 1 mg/kg; MDMA 30 mg/kg; methylone (MDMC)/MDMA 20/15 mg/kg combined; or MDPV/MDMA 1/15 mg/kg combined), spaced 2 h apart, and were euthanized either 2 or 7 days later for striatal tissue collection. DA values were normalized to the amount of protein in each tissue sample. Data represent mean ± SEM of 7–8 mice for each of the 14 treatment groups. *: p < 0.05 compared to the saline group (Dunnett’s post hoc test).
3.2 Tyrosine hydroxylase levels
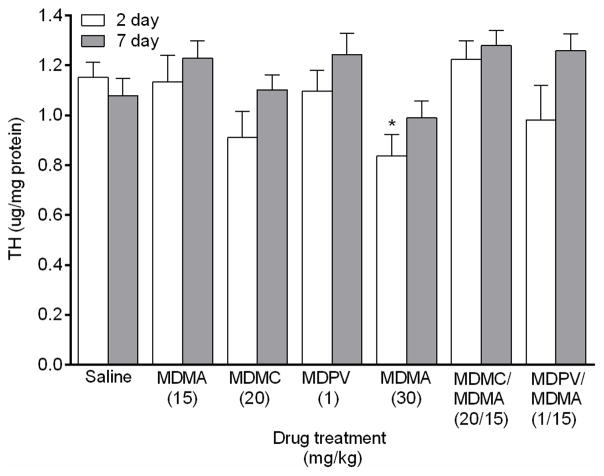
A main effect of drug group on TH levels (Fig. 3) was found in the striatum 2 days after drug treatment (_F_6,39 = 2.40, p < 0.05). Post hoc analysis revealed that only the MDMA 30 mg/kg group significantly decreased TH levels in comparison to the saline group. There was also a main effect of drug 7 days after drug treatment (_F_6,42 = 2.56, p < 0.01), but none of the drug groups differed significantly from the saline group for TH level.
Fig. 3.
Striatal TH levels measured 2 and 7 days following drug treatment via sandwich ELISA. Groups of mice received 4 i.p. injections of the same drug/dose (saline; MDMA 15 mg/kg; methylone (MDMC) 20 mg/kg; MDPV 1 mg/kg; MDMA 30 mg/kg; methylone (MDMC)/MDMA 20/15 mg/kg combined; or MDPV/MDMA 1/15 mg/kg combined), spaced 2 h apart, and were euthanized either 2 or 7 days later for striatal tissue collection. TH values were normalized to the amount of protein in each tissue sample. Data represent mean ± SEM of 7–8 mice for each of the 14 treatment groups. *: p < 0.05 compared to the saline group (Dunnett’s post hoc test).
3.3 GFAP expression
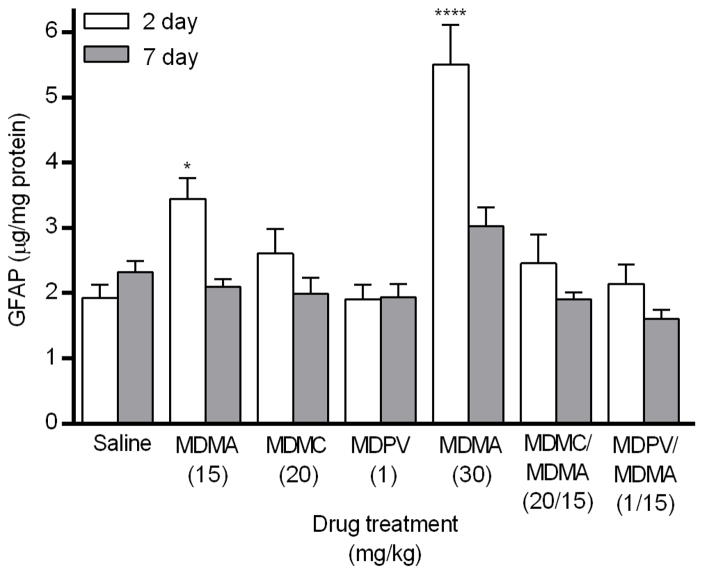
A main effect of drug group on GFAP expression (Fig. 4) was found in the striatum 2 days after drug treatment (_F_6,49 = 11.78, p < 0.0001). Post hoc analysis revealed that GFAP expression was significantly increased only in the two drug groups that received MDMA alone, compared to the saline group. There was also a main effect of drug 7 days after drug treatment (_F_6,49 = 5.47, p < 0.001), but again, none of the drug groups differed significantly from the saline group.
Fig. 4.
Striatal GFAP expression measured 2 and 7 days following drug treatment via sandwich ELISA. Groups of mice received 4 i.p. injections of the same drug/dose (saline; MDMA 15 mg/kg; methylone (MDMC) 20 mg/kg; MDPV 1 mg/kg; MDMA 30 mg/kg; methylone (MDMC)/MDMA 20/15 mg/kg combined; or MDPV/MDMA 1/15 mg/kg combined), spaced 2 h apart, and were euthanized either 2 or 7 days later for striatal tissue collection. GFAP values were normalized to the amount of protein present in each tissue sample. Data represent mean ± SEM of 8 mice for each of the 14 treatment groups. *: p < 0.05, ****: p < 0.0001 compared to the saline group (Dunnett’s post hoc test).
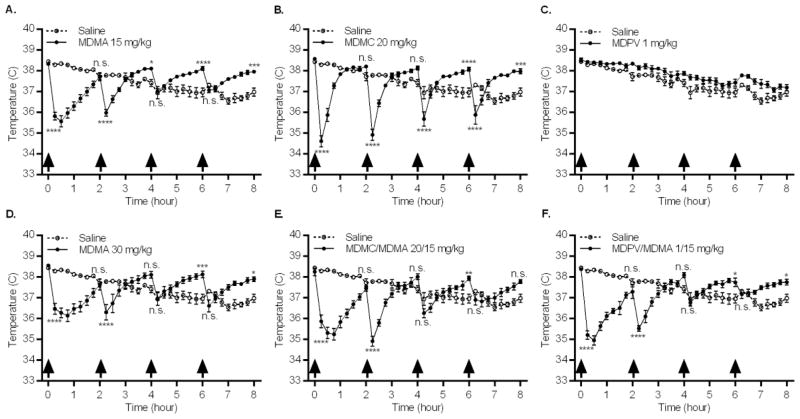
3.4 Thermoregulation
Following acclimation and immediately prior to the first injection, the mean baseline temperature of all animals was 38.4 °C (SEM = 0.04 °C**),** and there was no significant between-group difference. Temperature data (Fig. 5) analyzed using a two-way repeated measures ANOVA, revealed a significant drug group × time interaction (_F_192,1568 = 19.31, p < 0.0001). Examination of each drug group, in comparison to the saline group, revealed a significant drug group × time interaction for 5 of the 6 drug groups: MDMA 15 mg/kg (_F_32,448 = 42.98, p < 0.0001), MDMA 30 mg/kg (_F_32,448 = 24.51, p < 0.0001), methylone 20 mg/kg (_F_32,448 = 32.01, p < 0.0001), methylone/MDMA 20/15 mg/kg (_F_32,448 = 32.32, p < 0.0001), and MDPV/MDMA 1/15 mg/kg (_F_32,448 = 39.38, p < 0.0001). These five groups displayed a biphasic temperature pattern: an acute temperature decrease immediately after drug injection followed by an increase in temperature until the next dose was administered. To statistically investigate the data, post hoc comparisons were performed between each drug group and the saline group, but only at the 15 min and 2 h time-points following each of the 4 injections. Analyses were limited to these 8 time-points based on maximal temperature effects (Fig. 5) and to decrease error inflation associated with multiple comparisons. The MDPV 1 mg/kg group did not display the temperature pattern elicited by other drug treatments (Fig. 5C), as it paralleled the saline group. No further analysis was conducted for this drug group, as there was no significant drug × time interaction.
Fig. 5.
Effects of repeated drug injections on core body temperature. Groups of mice received 4 i.p. injections (arrows indicate time of drug injection) of the same drug/dose (A. MDMA 15 mg/kg; B. methylone (MDMC) 20 mg/kg; C. MDPV 1 mg/kg; D. MDMA 30 mg/kg; E. Methylone (MDMC)/MDMA 20/15 mg/kg combined; or F. MDPV/MDMA 1/15 mg/kg combined), and temperature was measured every 15 min via telemetry over 8 h. Data represent temperature for each saline and drug group (mean ± SEM) at that given time point, n = 8 mice for each group. *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001, compared to the saline group. Time points selected for comparison were 15 min and 2 h after each injection, with the 2 h measurement occurring just prior to the subsequent injection (Bonferroni post hoc test).
Analysis of overall temperature change data (from the initial hypothermic decrease in temperature seen following first injection to the final temperature measurement) identified a significant main effect of drug group (_F_6,49 = 55.10, p < 0.0001). In comparison to the saline group, which exhibited an overall decrease in temperature, the same 5 drug groups, for which significant drug group × time interactions were detected when compared to the saline group (Fig. 5), expressed highly significant temperature increases from an initial hypothermic drop in temperature: MDMA 15 mg/kg, MDMA 30 mg/kg, methylone 20 mg/kg, methylone/MDMA 20/15 mg/kg, and MDPV/MDMA 1/15 mg/kg. The temperature change of the MDPV 1 mg/kg group was not significantly different from that of the saline group and exhibited a decrease in overall temperature over time, similar to the saline group.
4. Discussion
The aim of this study was to investigate the combined effects of MDMA with selected and frequently abused substituted methcathinones. Acute thermoregulatory effects were recorded during drug administration and measures of neurotoxicity were assessed 2 and 7 days (transient and sustained) following drug treatment. Methylone and MDPV were chosen for comparison based on their different mechanisms of action at monoamine transporters and their reported concomitant use with MDMA.
Methylone and MDPV failed to affect striatal DA or TH levels (Fig. 2 and 3). These results support previous findings that by themselves, these methcathinones do not affect striatal dopaminergic markers of neurotoxicity (DA, DAT or TH expression) in mice 2–3 days following drug administration (Anneken et al., 2015;Lopez-Arnau et al., 2014). Another study in mice reported no change in striatal DA levels two weeks after drug administration (2 daily injections of methylone 30 mg/kg for 4 consecutive days), although there was a decrease in striatal DA levels in rats using the same dosing regimen, reinforcing inter-species response variability (den Hollander et al., 2013). However, we were surprised by the lack of methcathinone-induced changes in striatal DA and TH levels when combined with MDMA. This finding is discordant with similar amphetamine-methcathinone combination studies from a research group that found the administration of mephedrone and MDMA (4 injections of 20 mg/kg each) decreases striatal DA, DAT, and TH measured 2 days later as compared to effects of MDMA alone (Angoa-Perez et al., 2013). The same group found the co-administration of methylone and METH (4 injections of 20 and 2.5 mg/kg, respectively) also decreases striatal DA, DAT, and TH measured 2 days later (Anneken et al., 2015). Alternatively, they found in the same study the combination of the transporter inhibitor MDPV and MDMA (4 injections of 30 and 20 mg/kg) mitigated MDMA-induced decreases in these markers.
It is possible that our findings differed from reported effects due to our use of a lower dose of MDMA (15 mg/kg), which did not decrease striatal DA or TH levels by itself, either 2 or 7 days following drug administration. These markers were decreased at the higher dose of MDMA (30 mg/kg), though only at 2 days following drug administration, indicating the effects were transient. The lower dose of MDMA was chosen as it was sufficient to elicit DA terminal degeneration in previous studies (Sanchez et al., 2003; Johnson et al., 2004) and in order to avoid a potential floor effect when combined with the methcathinones. As there was no change in these markers when this dose of MDMA was combined with methylone and MDPV, it is possible these methcathinones only have modulatory effects on striatal DA and TH levels following an MDMA-induced decrease of these markers. Additionally, although the same dose of methylone was used in both studies, Anneken et al. (2015) administered a dose of MDPV 30× larger than that used in our experiment. We chose a dose of 1 mg/kg based on its increased potency at DAT in relation to MDMA, reflected in recreational doses reported by humans, where MDMA doses are estimated to be 20× greater than MDPV (Simmler et al., 2013;Erowid, 2016). This dose (1 mg/kg) is also sufficient to induce conditioned place preference and significantly increase locomotor activity in mice (Karlsson et al., 2014;Fantegrossi et al., 2013).
MDMA increased GFAP expression 2 days following drug administration in a dose-dependent fashion, but not 7 days later (Fig. 4). This is in agreement with previous research demonstrating that GFAP expression in the striatum peaks 2–3 days following MDMA administration, correlating with localized decreased DA, DAT, and TH levels (Granado et al., 2008;Frau et al., 2013;O’Callaghan et al., 2014). Neither methcathinone increased GFAP expression 2 or 7 days following drug administration, also in accordance with previous research where methcathinones by themselves (methylone, MDPV, and mephedrone) do not alter striatal GFAP expression (Angoa-Perez et al., 2013;Lopez-Arnau et al., 2014;Anneken et al., 2015). The increase in GFAP seen in response to the lower dose of MDMA (Fig. 4) was abolished when either methylone or MDPV were co-administered. Although Anneken et al. (2015) found the combination of MDMA and MDPV similarly decreases GFAP expression, they reported the combination of METH and methylone potentiates GFAP expression. This discrepancy in methylone-induced GFAP expression is potentially attributable to differences in drug combinations (METH vs. MDMA) or dose of MDMA (20 mg/kg vs. 15 mg/kg), though the same dose of methylone (20 mg/kg) was used in both studies. To further explore the effects of these drug combinations on reactive gliosis, other markers for astrogliosis such as activation of the janus kinase 2 – signal transducer activator of transcription (JAK2-STAT3) pathway, which precedes increased GFAP expression in models of neurotoxicity (O’Callaghan et al., 2014) could be characterized. Additionally, experiments could investigate microglial activation through quantification of microglial markers, such as complement type 3 receptor (CD11b) or translocator protein (TSPO) binding.
As DA terminal damage induces astrogliosis (O’Callaghan et al., 2014), the absence of changes in DA and TH levels (Figs. 2 and 3), following the MDMA 15 mg/kg dose, in contrast to an increase in GFAP expression (Fig. 4) suggests the methods employed to measure these markers may not be sensitive enough to detect changes at this dose. Our finding that both methylone and MDPV mitigated MDMA-induced astrogliosis indicates the methcathinones may be competing with MDMA (at this dose) at the DAT, thereby preventing uptake of MDMA into the cell, subsequent dopaminergic damage, and the eventual induction of astrogliosis, whether they are transporter substrates or inhibitors. Both methcathinones have a higher affinity for DAT than MDMA (methylone, Ki = 5.02 μM; MDPV, Ki = 0.02 μM; MDMA, Ki = 22.00 μM (Eshleman et al., 2013)). At higher doses of MDMA, it is possible that MDMA is more readily taken up into the cell by DAT than is the transporter substrate methylone. Due to MDPV’s substantially higher affinity for DAT and potency at inhibiting DA uptake, it is likely that MDPV inhibits uptake of even high doses of MDMA by the DAT.
Another potential mechanism underlying our results is the difference between substituted amphetamine and substituted methcathinone effects at VMAT2. The vesicular transporter is recognized as a key modulator of neurotoxicity as interruption of its function leads to increased cytosolic DA levels, oxidative stress, and terminal degeneration (Fleckenstein et al., 2007). Substituted amphetamines, including MDMA, act as substrates at VMAT2 as well as DAT, depleting vesicular stores of monoamines and increasing cytosolic DA levels. Conversely, methylone and MDPV lack affinity for VMAT2 as well as potency at inhibiting monoamine uptake (Cozzi et al., 1999;Eshleman et al., 2013;Lopez-Arnau et al., 2012). As a DAT uptake inhibitor, MDPV’s action is extracellular, inhibiting the uptake of DA and MDMA into the cell. As DAT substrates, both methylone and MDMA are taken into the cell and contact with VMAT2. The difference in affinity for VMAT2 between substituted amphetamines (MDMA and METH) and methcathinones (methylone and mephedrone) that are transporter substrates may underlie the different neurotoxic profiles of the two categories of drugs, providing an explanation for why methcathinones do not elicit DA terminal degeneration (Pifl et al., 2015;Angoa-Perez et al., 2013). This may also contribute to the discrepancy between our findings and those of others that used differing doses of MDMA. As mentioned above, at the lower dose of MDMA, methylone may decrease the amount of MDMA that enters the cell, preventing MDMA-induced vesicular DA release and leading to less neurotoxicity as reflected here by mitigation of astrogliosis. At a higher dose, more MDMA may enter the cell, increasing cytosolic DA levels, as well as extracellular DA levels potentiated by the DAT substrate methylone. Thus, these drugs are excellent tools to investigate effects of drug combinations on VMAT2 function and expression to better understand the potential role of VMAT2 in the underlying mechanism of neurotoxicity.
None of the drugs, by themselves or in combination, elicited hyperthermia (> 40° C) under our experimental conditions. Additionally, thermic response was not correlated with a drug’s effects on markers of neurotoxicity. With the exception of MDPV, all drug treatments elicited a biphasic temperature response (Fig. 5). This pattern was characterized by an initial strong hypothermic reaction to drug administration followed by a steady increase in temperature. However, the increased temperature never exceeded 1.2 °C above the temperature of the saline group nor rose above the initial baseline temperature of 38.4 °C, and therefore cannot be considered hyperthermia. As hypothermic conditions provide neuroprotection from MDMA (Mueller et al., 2013;Miller & O’Callaghan, 1995;Fantegrossi et al., 2003), it is possible that the hypothermic response to these drugs blunted their neurotoxic effects or that the normothermic environmental temperature (21° C) and single-housing of animals during drug administration diminished hyperthermic effects (Carvalho et al., 2002;Fantegrossi et al., 2003;Gannon et al., 2016). The transporter substrate, methylone, both alone and in combination with MDMA, elicited the same biphasic pattern as MDMA alone, at either dose. As such, the differences in measures of neurotoxicity (DA, TH, and GFAP), both dose-dependently between MDMA doses and in comparison to methylone, must be considered independent of their elicited thermic responses. Although this agrees with findings that substituted methcathinones do not alter thermic response to METH or MDMA when combined (Angoa-Perez et al., 2013;Anneken et al., 2015), it should be noted that under the conditions of those studies, METH and MDMA administered alone elicited hyperthermia. Thus, a difference in findings may be driven by environmental factors, such as ambient temperatures, as even our dose of MDMA 30 mg/kg did not elicit hyperthermia in contrast to their lower dose of MDMA 20 mg/kg. Further investigation of these drugs’ combinatory effects under elevated environmental temperatures is needed to resolve this discrepancy.
The transporter inhibitor MDPV was the only drug that did not elicit the biphasic thermic pattern or produce a significant overall temperature change from the saline group (Fig. 5c). Previous reports indicate a similar lack of core temperature change following MDPV administration, despite doses up to 30 times greater than the dose used in our experiments (Anneken et al., 2015;Aarde et al., 2013;Gannon et al., 2016). MDPV is also a selective uptake inhibitor of DAT without effect on the serotonergic system, as opposed to methylone and MDMA which are non-selective transporter substrates. This finding indicates substituted methcathinones and amphetamines that act as transporter substrates thermoregulate through different mechanisms than transporter uptake inhibitors, potentially involving SERT. Though difficult to untangle the underlying mechanisms, the thermoregulatory effects of MDMA appear to override those of MDPV, as the biphasic pattern re-emerged when the two drugs were co-administered.
5. Conclusions
Our results provide new and additional insight into the neurotoxic profiles of methylone and MDPV. In comparison to MDMA, neither drug alone elicited neurotoxic effects in the striatum, as indicated by their lack of effect on DA, TH levels, or GFAP expression. They also did not have regulatory effects on DA or TH levels when combined with MDMA, although both methcathinones mitigated astrogliosis induced by MDMA, an effect previously only observed with MDPV. In agreement with previous research, thermoregulation did not correlate with changes in striatal DA. While all transporter substrate treatment groups (alone or in combination) elicited a biphasic temperature response, only the transporter uptake inhibitor MDPV did not evoke this response. Though these results do not indicate a strong neurotoxic profile for either methylone or MDPV, caution must be exercised before drawing such conclusions. More research is needed on these substances both alone and in combination with MDMA at a higher dose in order to better determine their modulatory neurotoxic effects. Stronger effects may be seen at elevated environmental temperatures, which more accurately reflect the environment in which these substances are often ingested.
Highlights.
- Neurotoxic injection regimen of MDMA or methcathinones, alone or in combination.
- MDMA decreased striatal DA and TH, and increased striatal GFAP levels 2 days later.
- Combination of MDMA and methcathinones (methylone or MDPV) blocked astrogliosis.
- Transporter substrates (MDMA and methylone) elicited biphasic temperature response.
- Transporter inhibitor (MDPV) did not affect thermoregulation.
Acknowledgments
This research was generously funded by the Methamphetamine Abuse Research Center (P50 DA018165), T32 NS007466, and the VA Merit Review and Research Career Scientist Programs. The authors gratefully acknowledge the excellent technical assistance of Christopher M. Felton.
Abbreviations
DA
dopamine
DAT
dopamine transporter
ELISA
enzyme-linked immunosorbent assay
GFAP
glial fibrillary acidic protein
JAK2-STAT3
janus kinase 2 – signal transducer activator of transcription
MDMA
3,4-methylenedioxymethamphetamine
MDMC
3,4-methylenedioxymethcathinone, methylone
MDPV
3,4-methylenedioxypyrovalerone
METH
methamphetamine
4-MMC
mephedrone, 4-methylmethcathinone
NET
norepinephrine transporter
SERT
serotonin transporter
TH
tyrosine hydroxylase
Footnotes
Disclosure
The authors do not have any conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. S0028-3908(13)00140-8 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angoa-Perez M, Kane MJ, Herrera-Mundo N, Francescutti DM, Kuhn DM. Effects of combined treatment with mephedrone and methamphetamine or 3,4-methylenedioxymethamphetamine on serotonin nerve endings of the hippocampus. Life Sci. 2013 doi: 10.1016/j.lfs.2013.07.015. S0024-3205(13)00402-5 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anneken JH, Angoa-Perez M, Kuhn DM. 3,4-Methylenedioxypyrovalerone (MDPV) prevents while methylone enhances methamphetamine-induced damage to dopamine nerve endings: beta-ketoamphetamine modulation of neurotoxicity by the dopamine transporter. J Neurochem. 2015 doi: 10.1111/jnc.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. npp2011304 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol. 2013;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. S0014-2999(12)00951-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowyer JF, Hanig JP. Amphetamine- and methamphetamine-induced hyperthermia: Implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity. Temperature (Austin ) 2014;1:172–182. doi: 10.4161/23328940.2014.982049. [doi];982049 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowyer JF, Holson RR, Miller DB, O’Callaghan JP. Phenobarbital and dizocilpine can block methamphetamine-induced neurotoxicity in mice by mechanisms that are independent of thermoregulation. Brain Res. 2001;919:179–183. doi: 10.1016/s0006-8993(01)03051-7. S0006-8993(01)03051-7 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol. 2011;25:1543–1547. doi: 10.1177/0269881110378370. 0269881110378370 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Camarero J, Sanchez V, O’Shea E, Green AR, Colado MI. Studies, using in vivo microdialysis, on the effect of the dopamine uptake inhibitor GBR 12909 on 3,4-methylenedioxymethamphetamine (‘ecstasy’)-induced dopamine release and free radical formation in the mouse striatum. J Neurochem. 2002;81:961–972. doi: 10.1046/j.1471-4159.2002.00879.x. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho M, Carvalho F, Remiao F, de Lourdes PM, Pires-das-Neves R, de Lourdes BM. Effect of 3,4-methylenedioxymethamphetamine (“ecstasy”) on body temperature and liver antioxidant status in mice: influence of ambient temperature. Arch Toxicol. 2002;76:166–172. doi: 10.1007/s00204-002-0324-z. [DOI] [PubMed] [Google Scholar]
- 11.Caudevilla-Galligo F, Ventura M, Indave Ruiz BI, Fornis I. Presence and composition of cathinone derivatives in drug samples taken from a drug test service in Spain 2010–2012. Hum Psychopharmacol. 2013;28:341–344. doi: 10.1002/hup.2296. [DOI] [PubMed] [Google Scholar]
- 12.Cozzi NV, Sievert MK, Shulgin AT, Jacob P, III, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. S0014-2999(99)00538-5 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Dal Cason TA, Young R, Glennon RA. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. S0091-3057(97)00323-7 [pii] [DOI] [PubMed] [Google Scholar]
- 14.DAWN. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. 2013. [Google Scholar]
- 15.den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanpera I, Korpi ER. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol Biochem Behav. 2013;103:501–509. doi: 10.1016/j.pbb.2012.10.006. S0091-3057(12)00289-4 [pii]; [DOI] [PubMed] [Google Scholar]
- 16.Docherty JR, Green AR. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and its derivatives. Br J Pharmacol. 2010;160:1029–1044. doi: 10.1111/j.1476-5381.2010.00722.x. BPH722 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drug Enforcement Administration. Establishment of drug codes for 26 substances. Final rule. Federal Register. 2013;78:664–666. [PubMed] [Google Scholar]
- 18.Erowid . Psychoactive chemicals. 2016. [Google Scholar]
- 19.Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. S0006-2952(13)00228-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. npp2012233 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl) 2003;166:202–211. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- 22.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 23.Frau L, Simola N, Plumitallo A, Morelli M. Microglial and astroglial activation by 3,4-methylenedioxymethamphetamine (MDMA) in mice depends on S(+) enantiomer and is associated with an increase in body temperature and motility. J Neurochem. 2013;124:69–78. doi: 10.1111/jnc.12060. [DOI] [PubMed] [Google Scholar]
- 24.Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective Effects of Abused “Bath Salt” Constituent 3,4-Methylenedioxypyrovalerone in Mice: Drug Discrimination, Locomotor Activity, and Thermoregulation. J Pharmacol Exp Ther. 2016;356:615–623. doi: 10.1124/jpet.115.229500. jpet.115.229500 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez D, Ventura M, Caudevilla F, Torrens M, Farre M. Consumption of new psychoactive substances in a Spanish sample of research chemical users. Hum Psychopharmacol. 2013;28:332–340. doi: 10.1002/hup.2323. [DOI] [PubMed] [Google Scholar]
- 27.Granado N, O’Shea E, Bove J, Vila M, Colado MI, Moratalla R. Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J Neurochem. 2008;107:1102–1112. doi: 10.1111/j.1471-4159.2008.05705.x. JNC5705 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Hagino Y, Takamatsu Y, Yamamoto H, Iwamura T, Murphy DL, Uhl GR, Sora I, Ikeda K. Effects of MDMA on Extracellular Dopamine and Serotonin Levels in Mice Lacking Dopamine and/or Serotonin Transporters. Curr Neuropharmacol. 2011;9:91–95. doi: 10.2174/157015911795017254. CN-9-91 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herndon JM, Cholanians AB, Lizarraga LE, Lau SS, Monks TJ. Catechol-o-methyltransferase and 3,4-({+/−})-methylenedioxymethamphetamine toxicity. Toxicol Sci. 2014;139:162–173. doi: 10.1093/toxsci/kfu035. kfu035 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson EA, O’Callaghan JP, Miller DB. Brain concentrations of d-MDMA are increased after stress. Psychopharmacology (Berl) 2004;173:278–286. doi: 10.1007/s00213-003-1740-3. [DOI] [PubMed] [Google Scholar]
- 31.Karila L, Billieux J, Benyamina A, Lancon C, Cottencin O. The effects and risks associated to mephedrone and methylone in humans: A review of the preliminary evidences. Brain Res Bull. 2016;126:61–67. doi: 10.1016/j.brainresbull.2016.03.005. S0361-9230(16)30048-X [pii] [DOI] [PubMed] [Google Scholar]
- 32.Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, Methylone and 3,4-Methylenedioxypyrovalerone (MDPV) Induce Conditioned Place Preference in Mice. Basic Clin Pharmacol Toxicol. 2014 doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- 33.Logan BJ, Laverty R, Sanderson WD, Yee YB. Differences between rats and mice in MDMA (methylenedioxymethylamphetamine) neurotoxicity. Eur J Pharmacol. 1988;152:227–234. doi: 10.1016/0014-2999(88)90717-0. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Arnau R, Martinez-Clemente J, Abad S, Pubill D, Camarasa J, Escubedo E. Repeated doses of methylone, a new drug of abuse, induce changes in serotonin and dopamine systems in the mouse. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3493-6. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller DB, O’Callaghan JP. The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol. 1995;11:177–192. doi: 10.1007/BF02740694. [DOI] [PubMed] [Google Scholar]
- 37.Miller DB, O’Callaghan JP. Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res. 2003;92:48–53. doi: 10.1016/s0013-9351(02)00051-8. S0013935102000518 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Mohamed WM, Ben HS, Cassel JC, de Vasconcelos AP, Jones BC. MDMA: interactions with other psychoactive drugs. Pharmacol Biochem Behav. 2011;99:759–774. doi: 10.1016/j.pbb.2011.06.032. S0091-3057(11)00236-X [pii] [DOI] [PubMed] [Google Scholar]
- 39.Mueller M, Maldonado-Adrian C, Yuan J, McCann UD, Ricaurte GA. Studies of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) metabolism and disposition in rats and mice: relationship to neuroprotection and neurotoxicity profile. J Pharmacol Exp Ther. 2013;344:479–488. doi: 10.1124/jpet.112.201699. jpet.112.201699 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NFLIS. National Forensic Laboratory Information System 2015 Annual Report. 2015. [Google Scholar]
- 41.O’Callaghan JP. Measurement of glial fibrillary acidic protein. Curr Protoc Toxicol. 2002;Chapter 12(Unit 12) doi: 10.1002/0471140856.tx1208s11. [DOI] [PubMed] [Google Scholar]
- 42.O’Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, Miller DB. Early Activation of STAT3 Regulates Reactive Astrogliosis Induced by Diverse Forms of Neurotoxicity. PLoS One. 2014;9:e102003. doi: 10.1371/journal.pone.0102003. PONE-D-14-12852 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- 44.O’Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- 45.O’Shea E, Esteban B, Camarero J, Green AR, Colado MI. Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (‘ecstasy’) on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology. 2001;40:65–74. doi: 10.1016/s0028-3908(00)00106-4. S0028390800001064 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Palamar JJ, Salomone A, Vincenti M, Cleland CM. Detection of “bath salts” and other novel psychoactive substances in hair samples of ecstasy/MDMA/”Molly” users. Drug Alcohol Depend. 2016;161:200–205. doi: 10.1016/j.drugalcdep.2016.02.001. S0376-8716(16)00057-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peraile I, Granado N, Torres E, Gutierrez-Lopez MD, Moratalla R, Colado MI, O’Shea E. Cocaine potentiates MDMA-induced oxidative stress but not dopaminergic neurotoxicity in mice: implications for the pathogenesis of free radical-induced neurodegenerative disorders. Psychopharmacology (Berl) 2013;230:125–135. doi: 10.1007/s00213-013-3142-5. [DOI] [PubMed] [Google Scholar]
- 48.Pifl C, Reither H, Hornykiewicz O. The profile of mephedrone on human monoamine transporters differs from 3,4-methylenedioxymethamphetamine primarily by lower potency at the vesicular monoamine transporter. Eur J Pharmacol. 2015;755:119–126. doi: 10.1016/j.ejphar.2015.03.004. S0014-2999(15)00171-5 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Reveron ME, Monks TJ, Duvauchelle CL. Age-dependent (+)MDMA-mediated neurotoxicity in mice. Neurotoxicology. 2005;26:1031–1040. doi: 10.1016/j.neuro.2005.05.006. S0161-813X(05)00090-2 [pii] [DOI] [PubMed] [Google Scholar]
- 50.Sanchez V, Camarero J, O’Shea E, Green AR, Colado MI. Differential effect of dietary selenium on the long-term neurotoxicity induced by MDMA in mice and rats. Neuropharmacology. 2003;44:449–461. doi: 10.1016/s0028-3908(02)00411-2. S0028390802004112 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem. 2004;279:19936–19947. doi: 10.1074/jbc.M309304200. M309304200 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Sriram K, Miller DB, O’Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. JNC3566 [pii] [DOI] [PubMed] [Google Scholar]
- 54.UNODC. United Nations Office on Drugs and Crime: United Nations World Drug Report. 2014. [Google Scholar]
- 55.Vallersnes OM, Dines AM, Wood DM, Yates C, Heyerdahl F, Hovda KE, Giraudon I, Dargan PI. Psychosis associated with acute recreational drug toxicity: a European case series. BMC Psychiatry. 2016;16:293. doi: 10.1186/s12888-016-1002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogels N, Brunt TM, Rigter S, van DP, Vervaeke H, Niesink RJ. Content of ecstasy in the Netherlands: 1993–2008. Addiction. 2009;104:2057–2066. doi: 10.1111/j.1360-0443.2009.02707.x. ADD2707 [pii] [DOI] [PubMed] [Google Scholar]
- 57.White CM. Mephedrone and 3,4-Methylenedioxypyrovalerone (MDPV): Synthetic Cathinones With Serious Health Implications. J Clin Pharmacol. 2016;56:1319–1325. doi: 10.1002/jcph.742. [DOI] [PubMed] [Google Scholar]