High- and Low-Threshold Genes in the Spo0A Regulon of Bacillus subtilis (original) (raw)
Abstract
The master regulator for entry into sporulation in Bacillus subtilis is the response regulator Spo0A, which directly governs the expression of about 121 genes. Using cells in which the synthesis of Spo0A was under the control of an inducible promoter or in which production of the regulatory protein was impaired by a promoter mutation, we found that sporulation required a high (threshold) level of Spo0A and that many genes in the regulon differentially responded to high and low doses of the regulator. We distinguished four categories of genes, as follows: (i) those that required a high level of Spo0A to be activated, (ii) those that required a high level of Spo0A to be repressed, (iii) those that were activated at a low level of the regulator, and (iv) those that were repressed at a low dose of the regulator. Genes that required a high dose of Spo0A to be activated were found to have low binding constants for the DNA-binding protein. Some genes that were turned on at a low dose of Spo0A either had a high binding constant for the regulatory protein or were activated by an indirect mechanism involving Spo0A-mediated relief of repression by the repressor protein AbrB. We propose that progressive increases in the level of Spo0A leads to an early phase of transcription in which genes that play auxiliary roles in development, such as cannibalism and biofilm formation, are turned on and a later phase in which genes that play a direct role in sporulation are activated.
Entry into the developmental process of spore formation in Bacillus subtilis is governed by a master regulatory protein known as Spo0A (1, 21). Spo0A, which is a member of the response regulator family of DNA-binding proteins, is activated at the start of sporulation by a multicomponent phosphorelay consisting of at least three histidine autokinases, KinA, KinB, and KinC, and the phosphorelay proteins Spo0F and Spo0B (6). The kinases phosphorylate Spo0F. Spo0F∼P, in turn, transfers the phosphoryl group to Spo0B. Finally, Spo0B∼P transfers the phosphoryl group to, and thereby activates, Spo0A. Spo0A is additionally subject to control at the level of its synthesis by a positive feedback loop in which the regulatory protein indirectly stimulates the synthesis of the RNA polymerase sigma factor σH, which, in turn, stimulates transcription of the gene for Spo0A as well as the genes for the phosphorelay components KinA and Spo0F (21). The level of phosphorylation of Spo0A∼P is also influenced by dedicated phosphatases that remove phosphoryl groups from Spo0F∼P (e.g., RapA) and from Spo0A∼P itself (Spo0E) (17, 24, 25). These regulatory mechanisms act in effect as a bistable switch in that under conditions that induce sporulation, only a portion of the cells in the population activate Spo0A, whereas the remainder of the cells do not (9, 16).
Once activated by phosphorylation, the master regulator binds to a DNA sequence element known as the 0A box (21, 23, 33). In certain cases, such as the well-studied example of abrB (14, 15, 26, 27, 35, 36, 39), binding of Spo0A∼P to the 0A box results in repression of an otherwise vegetatively expressed gene. In other cases, such as those of the classic sporulation operons spoIIA, spoIIE, and spoIIG, Spo0A∼P acts in conjunction with RNA polymerase containing the housekeeping sigma factor σA (as exemplified by spoIIE and spoIIG) or with the alternative sigma factor σH (as exemplified by spoIIA) to turn on transcription (28). Thus, Spo0A∼P is both a repressor and an activator and is responsible for effecting a switch in the global pattern of gene transcription at the start of sporulation (10). Although Spo0A is known principally for its role in governing the initiation of sporulation, recent work indicates that Spo0A continues to function at intermediate stages of sporulation, when it accumulates to high levels and directs transcription in the mother cell compartment of the developing sporangium (13).
In recent work, we identified 121 genes that are under the direct control of Spo0A∼P (10, 23). These genes are organized as 30 single-gene units and 24 operons (or multigene clusters) and were assigned to the regulon based on a variety of criteria, including transcriptional profiling, chromatin-immunoprecipitation experiments, gel electrophoretic mobility shift assays, and computational approaches. There was, however, reason to suspect that not all members of the regulon are equally responsive to Spo0A. Earlier work based on flow cytometry and the use of a mutant (kinA) that was partially impaired in Spo0A activation indicated that at least one gene (spoVG, which is indirectly under the control of Spo0A via Spo0A-mediated repression of abrB, which encodes a repressor of spoVG [15, 36, 39] and other genes [27, 34]) is activated at a low dose of Spo0A, whereas other genes (e.g., the spoIIG operon) require a high threshold level of Spo0A (9). Motivated by this earlier work, we were interested in revisiting the issue of whether some genes under Spo0A control respond to a low dose of the regulatory protein and others respond to a high dose and to extend this analysis to the entire regulon. Here, we report that many genes in the regulon are differentially responsive to high and low doses of Spo0A, with some genes being activated or repressed at a low dose of the regulatory protein and others requiring a threshold level of Spo0A in order to be turned on or off. Our analysis leads us to propose that different genes are turned on or off at different times as Spo0A levels progressively increase, with genes that do not contribute to sporulation directly, such as genes involved in cannibalism and biofilm formation, being activated earlier and at lower doses of Spo0A than genes that play a direct role in spore formation.
MATERIALS AND METHODS
Plasmid construction.
All plasmid constructions were performed with Escherichia coli DH5α by using standard methods. The plasmid used to generate amyE::P_spac_ -gfp spc (pMF302) was created by ligating the HindIII_-SphI PCR fragment containing gfp (oligonucleotide primers omf316 and omf317 and template DNA pMF35 [12]), and pDG1728 (18) cut with HindIII_-SphI. The plasmid used to generate amyE::P_spoIIA -lacZ spc (pMF223) was created by ligating the MfeI-HindIII PCR fragment containing PspoIIA (oligonucleotide primers omf187 and omf188 and template DNA PY79) into pDG1728 between EcoRI and HindIII. The plasmid used to generate amyE::P_racA -lacZ spc (pMF225) was created by ligating the EcoRI_-HindIII PCR fragment containing PracA (oligonucleotide primers omf191 and omf192 and template DNA PY79) into pDG1728 between EcoRI and HindIII. The plasmid used to generate amyE::P_abrB -lacZ spc (pMF172) was created by ligating the EcoRI_-HindIII PCR fragment containing PabrB (oligonucleotide primers omf114 and omf115 and template DNA PY79) into pDG1728 between EcoRI and HindIII. The plasmid used to generate amyE::PΔV_-Spo0A cm (pMF171) was created by ligating the HindIII-BamHI PCR fragment containing P_ΔV-Spo0A_ (oligonucleotide primers omf28 and omf111 and template DNA PY79) into pDG1662 (18) between EcoRI and HindIII. The plasmid used to generate amyE::P_skf_ -lacZ spc (pMF289) was created by ligating the EcoRI_-_BamHI fragment from plasmid pEG101 (16) containing Pskf into pDG1728 between EcoRI and BamHI. To construct pEG112 (sdp_Ω_sdpABC::lacZ cm), a 609-bp DNA fragment containing part of sdpC was amplified with the primers YVAYLACECO and YVAYLACBAM. The PCR fragment was digested with EcoRI and BamHI and was cloned into the plasmid pEX44 (16), which was digested with the same restriction enzymes. All plasmids and oligonucleotide primers used for this study are listed in Tables 1 and 2, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype | Reference, source, or construction |
|---|---|---|
| Strain | ||
| PY79 | Prototroph | 38 |
| JDB322 | zej-82::Tn_917_::pTV21_Δ_2::pD177.1::pd179.1 kan cm | 13 |
| RL620 | _abrB_Δ::erm | Lab stock |
| RL1024 | amyE::Pspac-spo0A cm, trpC2, phe-1 | Lab stock |
| RL1105 | amyE::Pspac-spo0A cm, _spo0A_Δ::erm trpC2, phe-1 | Lab stock |
| RL2242 | _spo0A_Δ::spc | Lab stock |
| EG276 | amyE::P_skf-lacZ cm_ | 16 |
| EG297 | P_skfA-skf_′′-gfp spc | 16 |
| EG316 | amyE::P_skf-lacZ spc spo0A_Δ::spc | 16 |
| EG329 | sdp_Ω_sdpABC::lacZ cm | pEG112→PY79 |
| EG455 | amyE::P_sdp-lacZ cm_ | 16 |
| EG517 | amyE::P_sdp-lacZ cm spo0A_Δ::spc | 16 |
| MF290 | amyE::P_spoIIG-lacZ spc_ | 12 |
| MF327 | zej-82::amyE::P_spoIIG-lacZ spc cm_ | pMF223→JDB322 |
| MF476 | _spo0A_Δ::erm | RL1105→PY79 |
| MF813 | zej82::Tn_917_::pTV21_Δ_2::pD177.1::pD179.1::pCm::Tc kan tet | pCm::Tc (32)→JDB322 |
| MF815 | zej-82::amyE::P_spoIIG-lacZ spc tet_ | pCm::Tc→MF327 |
| MF1127 | _spo0A_Δ::erm, P_skfA-skf_′′-gfp spc | MF476→EG297 |
| MF1138 | zej-82::amyE::PΔ_v_ -spo0A cm tet | pMF171→MF813 |
| MF1206 | zej-82::amyE::PΔ_v_ _-spo0A cm tet spo0A_Δ::erm | MF576→MF1138 |
| MF1207 | amyE::P_abrB-lacZ spc_ | pMF172→PY79 |
| MF1248 | amyE::P_skf-lacZ spc_ | pDAG32→EG276 |
| MF1249 | sdp_Ω_sdpABC::lacZ spc | pCm::Tc→EG329 |
| MF1250 | amyE::P_spac-spo0A cm_ | RL1024→PY79 |
| MF1256 | amyE::P_spac-spo0A cm spo0A_Δ::spc | RL2242→MF1250 |
| MF1278 | zej-82::amyE::PΔ_V-spo0A cm tet spo0A_Δ::erm amyE::P_spoIIG-lacZ spc_ | pCm::Tc→EG276 |
| MF1282 | zej-82::amyE::PΔ_V-spo0A cm tet spo0A_Δ::erm amyE::P_skf-lacZ cm_ | MF1248→MF1206 |
| MF1283 | zej-82::amyE::PΔ_V-spo0A cm tet spo0A_Δ::erm sdp_Ω_sdpABC::lacZ cm | MF1249→MF1206 |
| MF1317 | zej-82::amyE::PΔ_V-spo0A cm tet spo0A_Δ::erm P_skfA_-_skf_′′-gfp spc | EG297→MF1206 |
| MF1521 | zej-82::amyE::P_spoIIG-lacZ spc tet amyE_::P_spac-spo0A cm_ | RL1024→MF815 |
| MF1532 | zej-82::amyE::P_spoIIG-lacZ spc tet amyE_::P_spac-spo0A cm spo0A_Δ::erm | MF476→MF1521 |
| MF1565 | amyE::P_spac-spo0A cm sdp_<!48>sdpABC::lacZ cm | MF1250→MF1249 |
| MF1566 | amyE::P_spac-spo0A cm sdp_Ω_sdpABC_::_lacZ cm spo0A_Δ::erm | MF476→MF1565 |
| MF1579 | zej-82::amyE::P_spoIIA-lacZ spc tet_ | pCm::Tc→MF327 |
| MF1580 | zej-82::amyE::P_abrB-lacZ spc tet_ | pMF172→MF813 |
| MF1582 | zej-82::amyE::P_spoIIA-lacZ spc tet amyE_::P_spac-spo0A cm_ | MF1250→MF1579 |
| MF1588 | zej-82::amyE::P_spoIIA-lacZ spc tet amyE_::P_spac-spo0A cm spo0A_Δ::erm | MF476→MF1582 |
| MF1589 | zej-82::amyE::P_abrB-lacZ spc tet amyE_::P_spac-spo0A cm_ | MF1250→MF1580 |
| MF1594 | zej-82::amyE::P_abrB-lacZ spc tet amyE_::P_spac-spo0A cm spo0A_Δ::erm | MF476→MF1589 |
| MF1621 | amyE::P_spoIIA-lacZ spc_ | pMF223→PY79 |
| MF1623 | amyE::P_racA-lacZ spc_ | pMF225→PY79 |
| MF1631 | zej-82::amyE::P_racA-lacZ spc tet_ | pMF225→MF813 |
| MF1637 | zej-82::amyE::P_racA-lacZ spc tet amyE_::P_spac-spo0A cm_ | MF1250→MF1631 |
| MF1649 | zej-82::amyE::P_racA-lacZ spc tet amyE_::P_spac-spo0A cm spo0A_Δ::erm | MF476→MF1637 |
| MF1685 | zej-82::amyE::PΔ_v-spo0A cm tet spo0A_Δ::erm amyE::P_spaIIA-lacZ spc_ | MF1621→MF1206 |
| MF1686 | zej-82::amyE::PΔ_v-spo0A cm tet spo0A_Δ::erm amyE::P_racA-lacZ spc_ | MF1623→MF1206 |
| MF1756 | zej-82::amyE::PΔ_v-spo0A cm tet spo0A_Δ::erm amyE::P_abrB-lacZ spc_ | MF1207→MF1206 |
| MF1822 | amyE::P_sdp-lacZ cm abrB_Δ::erm | RL620→EG455 |
| MF1830 | amyE::P_sdp-lacZ cm spo0A_Δ::_spc abrB_Δ::erm | RL2242→MF1822 |
| MF1895 | amyE::P_skf-lacZ cm abrB_Δ::erm | RL620→EG276 |
| MF1898 | amyE::P_skf-lacZ cm spo0A_Δ::_spc abrB_Δ::erm | RL2242→MF1895 |
| MF2039 | zej-82::amyE::P_skf-lacZ spc tet_ | pMF289→MF813 |
| MF2049 | zej-82::amyE::P_skf-lacZ spc tet amyE_::P_spac-spo0A cm_ | MF1250→MF2039 |
| MF2060 | zej-82::amyE::P_skf-lacZ spc tet amyE_::P_spac-spo0A cm spo0A_Δ::erm | MF476→MF2049 |
| MF2233 | amyE::P_spac-gfp spc_ | pMF302→PY79 |
| Plasmid | ||
| pCm::Tc | Cmr→Tcr | Lab stock |
| pDAG32 | Cmr→Spcr | Lab stock |
| pEG112 | sdp_Ω_sdpABC::lacZ cm | This study |
| pMF171 | amyE::PΔ_v_-Spo0A cm | This study |
| pMF172 | amyE::P_abrB-lacZ spc_ | This study |
| pMF223 | amyE::P_spoIIA-lacZ spc_ | This study |
| pMF225 | amyE::P_racA-lacZ spc_ | This study |
| pMF289 | amyE::P_skf-lacZ spc_ | This study |
| pMF302 | amyE::P_spac-gfp spc_ | This study |
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequencea |
|---|---|
| For plasmid construction: | |
| omf28 | 5′-gccGGATCCttaagaagccttatgctctaa-3′ |
| omf111 | 5′-gcgAAGCTTggtaaaatatacaaaagaagatttttcgaca-3′ |
| omf114 | 5′-gccGAATTCatcgatatttatggaaaaga-3′ |
| omf115 | 5′-gccAAGCTTgagatacttatttgtttaaat-3′ |
| omf187 | 5′-gccCAATTGatcaaacagtagcaaaagtaaaggtc-3′ |
| omf188 | 5′-gccAAGCTTgatatgatcggataatgagtg-3′ |
| omf191 | 5′-gccGAATTCgatttgtcgcttaacggctcgtatg-3′ |
| omf192 | 5′-gccAAGCTTaagctaggtattcgaccatag-3′ |
| omf316 | 5′-gccAAGCTTacataaggaggaactactatgagtaaaggagaagaactt-3′ |
| omf317 | 5′-gccGCATGCttatttgtatagttcatccatgcc-3′ |
| YVALYACECO | 5′-ctacGAATTCtacttaggctattgattgtttcca-3′ |
| YVAYLACBAM | 5′-actatcGGATCCttataatggattattgatgaatca-3′ |
| For gel mobility shift assay: | |
| abrB-U | 5′-taaatatttataaaatgctgttat-3′ |
| abrB-L | 5′-aataactacacgtcctaattcatc-3′ |
| spoVG-U | 5′-gctttatgacctaattgtgt-3′ |
| spoVG-L | 5′-cgtaatcttacgtcagtaac-3′ |
| skf-U | 5′-tcagaattctaagatgtttaacccctctgga-3′ |
| skf-L | 5′-tgaggatcccctctcaatttttgcatagagt-3′ |
| sdp-U | 5′-agtctcgaattcgaagaaaaagtgaatgagctg-3′ |
| sdp-L | 5′-actacaaagcttattacagtaataattccctttttt-3′ |
| spoIIG-U | 5′-cagcaggaattcagtgatcgtccgagatgatt-3′ |
| spoIIG-L | 5′-cagcagaagctttgcctcacgctgttcccctt-3′ |
| spoIIE-U | 5′-gccgaattcgatcccctccgccgctacca-3′ |
| spoIIE-L | 5′-gccaagcttttatattcgttgcctgtcat-3′ |
| spoIIA-U | 5′-gcccaattgatcaaacagtagcaaaagtaaaggtc-3′ |
| spoIIA-L | 5′-gccaagcttgatatgatcggataatgagtg-3′ |
Strain construction.
All strains used in this study are listed in Table 1. The parent strain for all experiments was B. subtilis strain PY79 (38).
Transcriptional profiling.
In one kind of experiment, we compared the relative levels of transcript accumulation in cells harboring the P_Δv-spo0A_ (MF1206) versus cells with a deletion of spo0A (MF476). In the other kind of experiment, we compared transcript levels between cells harboring the P_Δv-spo0A_ (MF1206) construct and cells that were wild type for spo0A (PY79). Cells were induced to sporulate in Sterlini-Mandelstam (SM) medium at 37°C. Each experiment was carried out twice with cells collected from two independently prepared cultures at 2 h after the start of sporulation. Sample preparation, RNA isolation, labeling, hybridization, and data analyses were performed as previously described (5).
General methods.
Media, culture conditions, preparation of competent cells, preparation of chromosomal and plasmid DNA, assays of sporulation efficiency, and assays of β-galactosidase activity were as previously described (12).
Gel mobility shift assays.
Gel mobility shift assays were carried out as described previously (14, 23). DNA fragments of interest were amplified by PCR from chromosomal DNA from strain PY79 with the primer sets shown in Table 2.
Fluorescence microscopy.
Fluorescence microscopy was performed as described previously (12).
Immunoblot analysis.
Immunoblot analysis was performed as described previously (12). Polyclonal anti-Spo0A (11) and σA antibodies (11) were used for the detection of Spo0A and σA, respectively.
RESULTS
Efficient sporulation requires a threshold level of Spo0A.
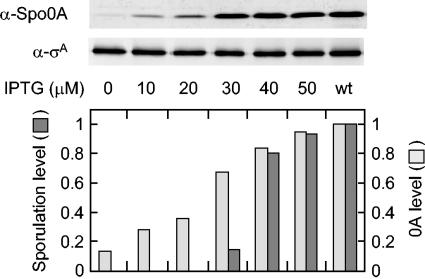
We wished to determine whether sporulation is triggered by a threshold level of Spo0A or whether the efficiency of sporulation is simply proportional to the amount of the regulatory protein. To address this issue, we used a strain that had been engineered to produce Spo0A in response to IPTG (isopropyl-β-d-thiogalactopyranoside) by using the IPTG-inducible promoter P_spac_. Cells were induced to sporulate by suspension in SM medium in the presence of various concentrations of IPTG. The results in Fig. 1 show that Spo0A levels, as measured by immunoblot analysis, were related linearly to the concentration of IPTG but that sporulation efficiency, as measured by the production of heat-resistant CFU at 24 h, was not. Whereas the level of Spo0A increased approximately in proportion to the concentration of IPTG over the range of 0 to 50 μM, the efficiency of sporulation abruptly increased over a narrow range, rising from <0.4% of the wild-type level at 20 μM to 15% of the wild-type level at 30 μM and 80% of the wild-type level at 40 μM. In a direct comparison of sporulation to the level of Spo0A, the efficiency of sporulation was 80% of that of the wild type at a Spo0A level that was 80% of that of the wild type but was markedly lower at Spo0A levels that were 65% of the wild-type level or below.
FIG. 1.
Sporulation is triggered by a threshold level of Spo0A. The upper panels show immunoblots of extracts from cells in which spo0A was under the control of the IPTG-inducible promoter P_spac_ (MF1256) and from cells of the wild type (PY79). Synthesis of Spo0A was induced in MF1256 by the addition of the indicated concentrations of IPTG just after the cells were suspended in SM medium. Extracts were prepared from cells collected at 2 h after suspension, and proteins were fractionated in a sodium dodecyl sulfate gel containing 16% polyacrylamide. Immunoblot analysis was carried out with polyclonal anti-Spo0A antibodies and polyclonal anti-σA antibodies. The anti-σA immunoblot served as a control for loading. The lower panel shows the corresponding levels of sporulation and of Spo0A at the indicated concentrations of IPTG. The number of spores per milliliter of culture at 24 h after suspension was measured by the number of heat-resistant (80°C for 15 min) CFU on agar plates containing Luria broth. Sporulation levels were normalized to the number of spores produced by the wild type (∼3 × 108). Spo0A levels from the immunoblot analysis were quantified and normalized to both the levels of σA and the levels of Spo0A produced by the wild type.
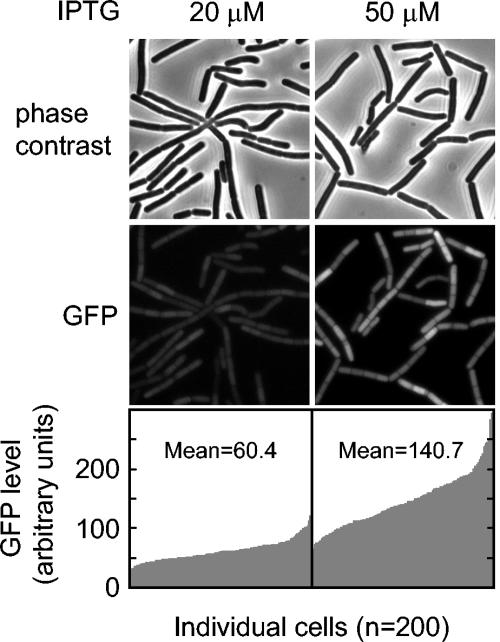
An assumption in the above experiment was that the level of Spo0A was increasing in a similar manner in all cells in the population with the increasing concentrations of IPTG. An alternative possibility was that the P_spac_ promoter responds to IPTG in an all-or-nothing manner, with some cells in the population producing a high level of Spo0A and others producing little or none for any given concentration of the inducer. To distinguish between these possibilities, we created a construct in which the P_spac_ was joined to the gene for the green fluorescent protein (GFP) instead of the gene for Spo0A. We then examined GFP levels by fluorescence microscopy in fields of individual cells at various concentrations of inducer (Fig. 2). The results showed that the fluorescence intensity was approximately proportional to the IPTG concentration (the mean value of GFP intensities at a 20 μM concentration of IPTG was approximately half of that with 50 μM IPTG) and that, at a particular concentration of IPTG, the level of fluorescence varied from cell to cell by a factor of only about two for the majority (80%) of the cells. We conclude that most cells responded in a similar manner to the dose of IPTG in the medium and, hence, that the level of Spo0A was likely to be largely similar from cell to cell in the population for any given concentration of IPTG.
FIG. 2.
The response of the P_spac_ promoter to IPTG is moderately uniform from cell to cell. Cells of strain MF2233, which contains a fusion of the gene for GFP to P_spac_, were treated with 20 and 50 μM IPTG after suspension in SM medium. The production of GFP was monitored by fluorescence microscopy at hour 2 after suspension. The cells were also visualized by phase contrast microscopy. The lower panels show the relative levels of fluorescence from GFP in individual cells (n = 200) at 20 and 50 μM concentrations of IPTG. The mean values of relative fluorescence at the two concentrations of inducer are indicated with the graphs.
Influence of the level of Spo0A on the level of expression of various Spo0A-controlled genes.
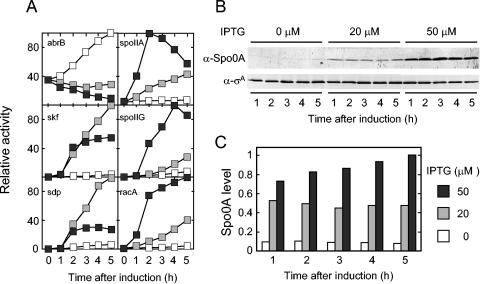
Next, we examined the influence of the level of Spo0A on the level of transcription from the promoters for five genes or operons known to be under the positive control of the sporulation regulatory protein (Fig. 3). These were the promoters for the classic sporulation operons spoIIA and spoIIG and for three recently characterized genes and operons, skf, sdp, and racA (2, 16, 28). Transcription was measured by using fusions to lacZ for the genes under study and the P_spac_-spo0A construct to control the levels of Spo0A synthesis. As a control and for comparison, we also examined abrB, a gene that is repressed by Spo0A but that does not require Spo0A for its transcription (27, 34). As expected, β-galactosidase from an abrB-lacZ fusion accumulated rapidly in the absence of IPTG (i.e., when little or no Spo0A was being synthesized) and at low rates when inducer was present (Fig. 3A).
FIG. 3.
Transcription from Spo0A-controlled promoters at various levels of Spo0A. (A) β-Galactosidase levels were measured in strains harboring the P_spac_-spo0A construct and lacZ fused to the following promoters: abrB (MF1594), skf (MF2060), sdp (MF1566), spoIIA (MF1588), spoIIG (MF1532), and racA (MF1649). Cells were treated with 0 (open boxes), 20 (gray boxes), or 50 (filled boxes) μM concentrations of IPTG after suspension in SM medium. Samples were collected at the indicated times after suspension and assayed for β-galactosidase activity. For each experiment, the specific activities (Miller units) were normalized to the maximum specific activity observed for each lacZ fusion (typically, 200 U for abrB, spoIIA, skf, and racA; 500 U for sdp, and 80 U for spoIIG). (B) Shown are immunoblots of extracts prepared from the same cells used for panel A for β-galactosidase measurements for spoIIG. Immunoblot analysis was carried as described in the legend to Fig. 2. (C) Shown is the quantification of the time course of Spo0A accumulation from the immunoblot analysis shown in panel B, normalized to σA levels and then to the maximum level of Spo0A.
In keeping with the idea that spoIIA, spoIIG, skf, sdp, and racA require Spo0A for their expression, it can be seen that in each case little or no synthesis of β-galactosidase was observed in the absence of inducer (Fig. 3A). However, the five genes fell into two categories with respect to how they responded to Spo0A: those that required a high dose of Spo0A for maximal expression and those that were maximally expressed at a low dose of the regulatory protein. Thus, β-galactosidase from lacZ fusions to spoIIA, spoIIG, and racA accumulated at low rates when the concentration of IPTG was at 20 μM and at severalfold-higher rates when the inducer was at 50 μM (Fig. 3A). It can be seen from the results shown in Fig. 3B and C that the steady-state level of Spo0A at 20 μM IPTG was approximately 50% of the level seen with the wild type and that at a 50 μM concentration of inducer the level of Spo0A was equivalent to that of the wild type.
In contrast, _skf_-lacZ and _sdp_-lacZ were expressed at maximum levels at IPTG concentrations of either 20 or 50 μM (Fig. 3A). Interestingly, however, by 2 h after the addition of a high concentration (50 μM) of inducer, the accumulation of β-galactosidase from the fusions was curtailed. This observation suggests that transcription from skf and sdp was repressed as Spo0A accumulated to high levels.
Use of a construct that produces a reduced level of Spo0A to distinguish between high- and low-threshold genes.
As an alternative to P_spac_, we modified the regulatory region for spo0A so as to achieve a reduced level of synthesis of the sporulation regulatory protein. The spo0A gene is transcribed under the direction of σA and σH from distinct promoters known as P_v_ (for vegetative) and P_s_ (for sporulation), respectively (8). We built a modified spo0A gene that lacked P_v_ but retained P_s_. When suspended in SM medium, cells harboring the P_v_ deletion-mutated gene (P_Δv-spo0A_) sporulated at an efficiency of 1 to 3% compared to the wild type. Quantitative immunoblot analysis showed that the level of accumulation of Spo0A was about two- to threefold lower in sporulating cells harboring P_Δv-spo0A_ than in sporulating cells containing the wild-type gene (Fig. 4B and C). Thus, cells harboring the P_Δv-spo0A_ construct were locked into producing Spo0A at a modestly reduced level and did so in a manner that was not dependent on the level of inducer in the medium. The observation that cells harboring the P_Δv-spo0A_ construct were strongly impaired in sporulation is in keeping with the conclusion reached above, that efficient sporulation requires a threshold level of Spo0A.
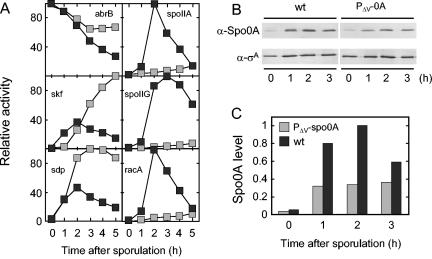
FIG. 4.
Use of a promoter-mutated spo0A gene to distinguish between high- and low-threshold genes. (A) β-Galactosidase activity was measured after suspension in SM medium of cells containing either wild-type spo0A (filled boxes) or the promoter-mutated gene P_Δv-spo0A_ (gray boxes) and lacZ fusions to the following promoters: abrB (spo0A+ [MF1207], P_Δv-spo0A_ [MF1756]), skf (spo0A+ [MF1248], P_Δv-spo0A_ [MF1282]), sdp (spo0A+ [MF1249], P_Δv-spo0A_ [MF1283]), spoIIA (spo0A+ [MF1621], P_Δv-spo0A_ [MF1685]), spoIIG (spo0A+ [MF290], P_Δv-spo0A_ [MF1278]), and racA (spo0A+ [MF1623], P_Δv-spo0A_ [MF1686]). Specific activities (Miller units) were normalized as described for Fig. 3. (B) Extracts were prepared and subjected to immunoblot analysis as described for Fig. 3. (C) Spo0A levels shown in panel B were quantified, normalized to σA levels, and then normalized to the maximum level of Spo0A.
Next, we examined the expression of the lacZ fusions in cells harboring P_Δv-spo0A_ (Fig. 4A). The results show that the pattern of β-galactosidase accumulation from abrB-lacZ, skf-lacZ, and sdp-lacZ was similar to that seen with the wild type during the early stages of sporulation (1 to 2 h of sporulation), but reached substantially higher levels than in the wild type later in sporulation. These results are consistent with the idea that skf and sdp are fully activated at a low concentration of Spo0A but also show that the expression of skf and sdp is inhibited by Spo0A as the regulatory protein attains progressively higher levels during the course of sporulation.
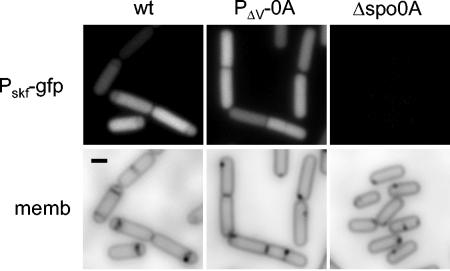
That skf was efficiently expressed at a reduced level of Spo0A was also demonstrated by fluorescence microscopy using a fusion of the gene to gfp (Fig. 5). The results show that the skf-gfp fusion was expressed as strongly in cells harboring P_Δv-spo0A_ as in wild-type cells (although as expected, no expression was seen in cells with a deletion of spo0A). We also noticed that the frequency of polar septa (as observed by the use of the red membrane stain FM4-64) was noticeably lower in cells containing P_Δv-spo0A_ than in wild-type cells. This observation is in keeping with the conclusion reached above that the efficiency of sporulation is markedly impaired when the level of Spo0A is reduced below a threshold level and suggests that the defect in sporulation occurs, at least in part, at the stage of asymmetric division.
FIG. 5.
Visualization of transcription from the skf promoter in cells producing a reduced level of Spo0A. Shown are fluorescence micrographs of cells harboring gfp fused to the skf promoter fusion and wild-type spo0A (EG297), P_Δv-spo0A_ (MF1317), or a spo0A null mutation spo0AΔ::erm (MF1127) at hour 2 after suspension and treated with the membrane stain FM4-64. The upper panels show fluorescence from GFP, and the lower panels show fluorescence from the same cells from FM4-64. Scale bar, 1 μm.
The most striking results were obtained with spoIIA-lacZ, spoIIG-lacZ, and racA-lacZ, which were expressed at only very low levels in cells with the deletion-mutated gene. Evidently, transcription of all three genes requires a threshold level of Spo0A, and its concentration is brought below this threshold by only a modest (two- to threefold) reduction in the level of Spo0A. That such a threshold exists was even more apparent from these results with PΔv-spo0A, for which the cells are locked into producing a level of Spo0A that was modestly reduced from that observed with the P_spac_ construct.
Binding affinity of Spo0A to the regulatory region for high- and low-threshold genes.
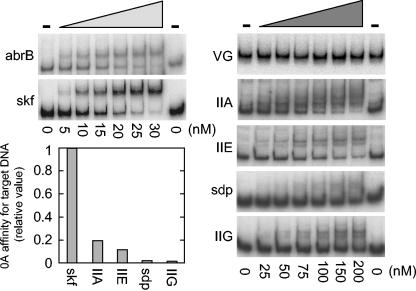
We wondered whether high- and low-threshold genes differed in the binding affinities of their regulatory regions for Spo0A. To investigate this possibility, we carried out electrophoretic mobility shift assays using radiolabeled DNAs that contained the binding site for Spo0A and a truncated form of Spo0A that corresponded to its DNA-binding domain, which is located in the C-terminal portion of the protein (23). This truncated protein lacks the region in which phosphorylation takes place and is known to bind DNA in a manner that is not dependent on phosphorylation (30). We therefore assume for the purposes of this analysis that the DNA-binding properties of the truncated protein mimicked those of Spo0A∼P. The results indicated apparent Kd values of 26 nM for skf, 140 nM for spoIIA, 230 nM for spoIIE, 1,300 nM for sdp, and 1,700 nM for spoIIG (Fig. 6). For comparison, the Kd for abrB was 64 nM. Also, no binding to spoVG, which does not contain a Spo0A-binding site, was detected; spoVG served as the negative control.
FIG. 6.
Gel electrophoretic mobility shift analysis of the binding of Spo0A to the regulatory regions of various genes. The regulatory regions of target genes were amplified by PCR with radioactive primers, incubated with the purified DNA-binding domain of Spo0A at the indicated concentrations, and subjected to nondenaturing polyacrylamide gel electrophoresis. abrB is the positive control, and spoVG is the negative control. The data were plotted as fractions of free DNA versus protein concentration to determine Kd, which was approximately equal to the protein concentration at which half of the free DNA was bound (7). The graph shows the relative binding affinities of Spo0A for the indicated DNAs.
In plots of relative binding affinities (Fig. 6, lower left), it can be seen that the affinity of skf for Spo0A was much higher than that for spoIIA, spoIIE, spoIIG, and sdp (with the affinities of the last two being especially low). This finding is in keeping with our observation that transcription of skf is switched on at a low cellular level of Spo0A and that spoIIA, spoIIE, and spoIIG require a high concentration of the sporulation regulatory protein. But how are we to explain the anomalous results with sdp, which was turned on at a low concentration of Spo0A in vivo, but whose binding affinity for Spo0A was almost as low as that for spoIIG?
Transcription of sdp is under the negative control of AbrB.
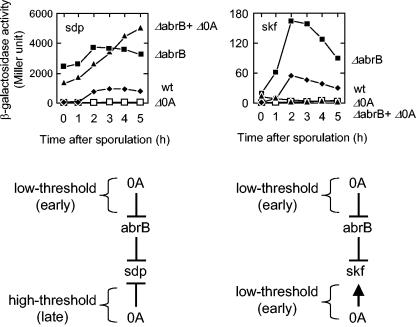
A possible explanation for the apparent anomaly is that sdp is indirectly activated by Spo0A through relief of repression by the DNA-binding protein AbrB. AbrB is known to repress a variety of genes that are activated at an early stage of sporulation, including spoVG, spo0E, and spo0H (15, 27, 34, 39). AbrB is an unstable protein and its gene is under the negative regulation of Spo0A, which, as we have seen (Fig. 6), binds to abrB with relatively high affinity. Thus, as Spo0A levels rise early in sporulation, abrB is repressed, leading to a drop in cellular levels of AbrB and activation of genes under its negative control. To investigate whether sdp is similarly activated by Spo0A-mediated repression of abrB, we examined the effect of an abrB mutation on the expression of sdp-lacZ. The results show that the deletion of abrB resulted in high levels of sdp-lacZ expression and bypassed the dependence of sdp-lacZ expression on spo0A (Fig. 7). We note that although sdp-lacZ was activated at a low level of Spo0A, its expression was curtailed at a high level of the regulatory protein (Fig. 4A). We interpret this result to indicate that Spo0A is a repressor of sdp and that when Spo0A accumulates to high levels it is able to adhere to its (relatively weak) binding site, shutting off further transcription of the operon. Thus, as summarized in the model at the bottom of Fig. 7, our results are consistent with the view that sdp is both indirectly under the control of Spo0A via repression by AbrB (representing a low-threshold mode of activation) and under its direct negative control through the presence of a weak binding site for Spo0A (representing a high-threshold mode of repression).
FIG. 7.
Direct and indirect modes of low-threshold activation by Spo0A. (Top) The graphs are the time course of the accumulation of β-galactosidase from P_sdp_ -lacZ (left) and P_skf_ -lacZ (right) in wild-type (filled diamonds) cells (EG455 for sdp; EG276 for skf), spo0A mutant cells (open boxes; EG517 for sdp and EG316 for skf), abrB mutant cells (filled boxes; MF1822 for sdp and MF1895 for skf), and cells with both spo0A and abrB mutations (filled triangles; MF1830 for sdp and MF1898 for skf). Samples were collected at the indicated times after the initiation of sporulation and assayed for β-galactosidase activity. (Bottom) The figure shows models for the regulation of sdp and skf by Spo0A and AbrB (see the text for details).
Interestingly, the results shown in Fig. 7 indicate that skf was both subject to repression by AbrB and under the direct positive control of Spo0A. Thus, the expression of the skf-lacZ fusion was enhanced in otherwise wild-type cells by a deletion of abrB, but expression was almost entirely prevented by a deletion of spo0A whether or not abrB had been deleted. Thus, as summarized in Fig. 7, low levels of Spo0A activate skf by two routes: relieving AbrB-mediated repression and direct activation of the skf promoter.
We conclude that there are two categories of low-threshold-activated genes, those like skf, which have a high affinity for Spo0A (and hence require only a low concentration of Spo0A to be activated), and those like sdp, which are under the negative control of AbrB and are indirectly activated at a low concentration of Spo0A through repression of the AbrB gene. In some cases, as exemplified by skf, the same gene is subject to both mechanisms of low-threshold activation.
Identifying other high- and low-threshold genes in the Spo0A regulon.
In a previous work, we assigned 121 genes, which are organized as 30 single-gene units and 24 multigene units or operons, to the Spo0A regulon (23). In the present investigation, we sought to carry this analysis further by taking advantage of the P_Δv-spo0A_ construct to identify genes whose expression was differentially affected at low or high doses of Spo0A. To do this, we carried out two kinds of gene microarray experiments. In one, we compared the relative levels of transcript accumulation in cells harboring the P_Δv-spo0A_ versus cells with a deletion of spo0A. In the other, we compared transcript levels between cells harboring the P_Δv-spo0A_ construct and cells that were wild type for spo0A. Our experiments were performed with cells harvested at a time (2 h after suspension in sporulation medium) when Spo0A had reached peak levels in wild-type cells. Because Spo0A is known to influence the expression of many genes that are not under its direct control (e.g., genes under the control of downstream regulatory proteins in the sporulation pathway), we restricted our analysis to genes that had previously been assigned as direct targets of Spo0A (that is, members of the Spo0A regulon).
Our analysis distinguished among four categories of genes. Genes whose expression was at least twofold higher in the wild type than in cells containing the P_Δv-spo0A_ construct are referred to as high-threshold-activated genes. Conversely, genes whose expression was at least twofold higher in cells harboring P_Δv-spo0A_ than in the wild type are referred to as high-threshold-repressed genes. Similarly, in the low-threshold category, genes whose expression was at least twofold higher in P_Δv_-_spo0A_-containing cells than in a spo0A null mutant were referred to as low-threshold-activated genes whereas genes whose expression was at least twofold higher in spo0A null than in P_Δv-spo0A_-containing bacteria were referred to as low-threshold-repressed genes. Note that some genes fell into two categories. For example, sdp was activated at a low level of Spo0A (it is a low-threshold-activated gene), but its expression was repressed at high levels of the DNA-binding protein (hence, it is also a high-threshold-repressed gene).
Table 3 summarizes the results of our analysis for the 53 targets of Spo0A regulation (29 single-gene units plus 24 multigene clusters or operons, leaving aside the unusual case of the late-sporulation-activated gene cotD [23]), which are listed according to the gene that is most proximal to the 0A box in each case. As expected, spoIIA, spoIIG, spoIIE, and racA fell into the high-threshold-activated category. Three additional genes, yneE, yttP, and sinI, also were in this category, the results being most robust in the case of yneE, a gene of unknown function. Ten genes fell into the category of high-threshold-repressed genes, whose expression was inhibited at a high dose of Spo0A. As expected, and as indicated above, sdp fell into this category. Two other noteworthy members of the high-threshold-repressed category were rapA and divIVA. The rapA gene encodes a phosphatase that dephosphorylates an intermediate (Spo0F∼P) in the phosphorelay (24); hence, its repression by high levels of Spo0A could contribute to maintaining Spo0A activity at a high level. divIVA is a vegetative gene whose product (together with the product of the high-threshold-activated gene racA) is involved in anchoring chromosome replication origin regions to the cell poles shortly after the start of sporulation (2, 37), among other functions. Because the origin region is released from the pole prior to engulfment, DivIVA is required only early in sporulation, and thus its repression when Spo0A reaches high levels is not inconsistent with the idea that DivIVA acts in only a small window of time.
TABLE 3.
High- and low-threshold genes in the Spo0A regulona
| Category and gene | Transcriptional ratio | Function | |
|---|---|---|---|
| PΔv/Δ0A | wt/PΔv | ||
| High-threshold activated | |||
| spoIIG | 1.2 | 15.9 | pro-σE processing protease/σE factor |
| spoIIE | 1.0 | 14.3 | Ser phosphatase (σF activation)/asymmetric septum formation |
| racA | 0.8 | 9.2 | Remodeling and anchoring of the chromosome |
| spoIIA | 2.0 | 6.8 | Anti-anti-σF/anti/σF/σF |
| yneE | 1.8 | 6.2 | Unknown |
| yttP | 1.1 | 3.0 | Similar to transcriptional regulator (TetR/AcrR family) |
| sinI | 1.6 | 2.6 | Antagonist of SinR (transcriptional regulator) |
| High-threshold repressed | |||
| rapA | 4.9 | 0.2 | Asp phosphatase (dephosphorylation of Spo0F-P) |
| flgB | 0.7 | 0.3 | fla/che operon: motility and chemotaxis |
| ftsE | 0.9 | 0.3 | Cell division protein (ATP binding) |
| lytE | 0.6 | 0.3 | Cell wall hydrolase |
| divIVA | 1.0 | 0.3 | Cell division protein |
| yfmI | 19.2 | 0.3 | Similar to macrolide efflux transporter |
| yxbC | 51.3 | 0.4 | Unknown |
| sdp | 50.1 | 0.4 | Sporulation delaying protein |
| dltA | 1.1 | 0.4 | d-Alanyl-d-alanine carrier protein ligase |
| rocD | 0.5 | 0.4 | Omithine aminotransferase |
| Low-threshold activated | |||
| yxbC | 51.3 | 0.4 | Unknown |
| sdp | 50.1 | 0.4 | Sporulation delaying protein |
| yfmI | 19.2 | 0.3 | Similar to macrolide efflux transporter |
| yqcG | 9.0 | 0.5 | Unknown |
| yybN | 9.0 | 0.5 | Unknown |
| skf | 8.2 | 1.0 | Sporulation killing factor |
| yjcM | 5.7 | 0.5 | Unknown |
| rapA | 4.9 | 0.2 | Asp phosphatase (dephosphorylation of Spo0F-P) |
| spo0A | 3.5 | 1.1 | Transcriptional master regulator for sporulation |
| yqxI | 3.5 | 0.6 | Unknown |
| kinA | 2.5 | 0.6 | Sensor histidine kinase (initiation of sporulation) |
| ykzF | 2.5 | 0.7 | Unknown |
| spo0F | 2.5 | 0.5 | Response regulator (multicomponent P-relay) |
| Low-threshold repressed | |||
| abrB | 0.2 | 0.6 | Transcriptional pleiotropic regulator of transition stage genes |
| fruR | 0.3 | ND | Transcriptional repressor of the fructose operon |
| yqzD | 0.4 | 0.6 | Unknown |
| ykaA | 0.4 | 0.8 | Unknown |
| purT | 0.4 | 1.0 | Phosphoribosylglycinamide formyltransferase 2 |
| med | 0.4 | 1.1 | Positive regulator of comK |
| Not differentially expressed | |||
| accD | 0.8 | 1.2 | Acetyl coenzyme carboxylase (beta subunit) |
| dnaA | 0.8 | 0.7 | Initiation of chromosome replication |
| dnaG | 1.3 | 0.6 | DNA primase |
| holB | 0.9 | 0.5 | DNA polymerase III (delta′ subunit) |
| kinC | 1.8 | 0.8 | Sensor histidine kinase |
| metS | 0.9 | 1.3 | Methionyl-tRNA synthetase |
| nfrA | 1.1 | 1.8 | FMN-containing NADPH-linked nitro/flavin reductase |
| relA | 1.1 | 0.6 | GTP pyrophosphokinase (stringent response) |
| rok | 1.3 | 0.6 | Repressor of comK |
| soj | 1.1 | 0.5 | Negative regulation of sporulation initiation |
| tkt | 0.5 | 0.7 | Transketolase |
| yaaD | 0.9 | 0.5 | Unknown |
| yerB | 0.8 | 1.0 | Unknown |
| ycgM | 0.6 | 2.0 | Similar to proline oxidase |
| ylmD | 1.6 | 0.7 | Unknown |
| yocH | 1.4 | 1.6 | Similar to cell wall-binding protein |
| yppD | 1.6 | 1.6 | Unknown |
| yrrL | 0.7 | 0.9 | Similar to folate metabolism |
| yusE | 1.0 | 0.8 | Unknown |
| yvyE | 0.6 | 0.5 | Unknown |
| σK regulated | |||
| cotD (σK) | 0.5 | ND | Spore coat protein (inner) |
| Read-through from yuzC (σE) | |||
| yuxH | 0.2 | 13.3 | Unknown |
Thirteen genes fell into the category of low-threshold-activated genes, that is, genes whose expression was higher in the P_Δv-spo0A_-containing cells than in spo0A mutant cells. As expected, skf fell into this category, as did the phosphorelay genes, kinA and spo0F, and spo0A itself. Four members of the low-threshold-activated genes were also members of the high-threshold-repressed category. One of the four, as expected and as indicated above, was sdp, with the other three being rapA, yfmI, and yxbC. Expanding on our discussion of rapA (above), we suppose that low doses of Spo0A stimulate synthesis of the Spo0F∼P phosphatase and thereby help to prolong the period at which Spo0A activity is at a low level, whereas when Spo0A finally accumulates to a high level, further synthesis of the phosphatase is curtailed to help maintain the regulatory protein at a high-threshold level.
Finally, six genes fell into the low-threshold-repressed category, in which expression was higher in the spo0A mutant than in cells containing the P_Δv-spo0A_ construct. Notable in this category was abrB, whose expression is repressed at a relatively low level of Spo0A. This observation reinforces the view that some of the effects of low doses of Spo0A are exerted indirectly by blocking further synthesis of the unstable AbrB repressor, which we have proposed is the basis for the activation of sdp.
In toto, 32 genes and operons out of the total list of 53 fell into one of the four categories of high- and low-threshold response, with some genes being assigned to two categories. This left 20 of the Spo0A-regulated targets that did not fall into any of the four categories (leaving aside the case of yuxH, which we discuss below). An inspection of the transcriptional profiling data of Molle et al. (23) indicates that many of these 20 genes are cases in which the microarray data revealed a relatively small influence of Spo0A-Sad67 (the activated form of Spo0A used in the experiments of Molle et al.) on transcript levels and, hence, represent weak targets of Spo0A regulation. In other words, the majority of genes in the regulon for which the influence of Spo0A on transcript levels was relatively strong responded to the transcriptional regulator in a manner that was dependent upon its dose.
Finally, we consider the case of yuxH, which we had previously determined was under the negative control of Spo0A (23) but which in our present investigation appeared to behave as a high-threshold-activated gene (the transcriptional ratio of transcript accumulation in the wild type versus P_Δv-spo0A_ being 13.3.) A possible explanation for this discrepancy was that the cells used in the present study were wild type for the sporulation regulatory protein σE, whereas the experiments of Molle et al. (23) were conducted under conditions in which σE was absent. Further, adjacent to and in convergent orientation with yuxH is a gene (yucZ) which is under the control of σE. Therefore, the strong spo0A+-dependent signal detected in our microarray analysis might have been an indirect consequence of σE-dependent, read-through transcription from yucZ (the microarray was constructed with PCR-amplified open reading frames and hence contained both strands for each gene). We therefore fused lacZ to the promoter for yuxH so that its expression could be studied independently of read-through from yucZ. The results showed that yuxH-lacZ was expressed more strongly in spo0A mutant cells than in the wild type (data not shown) and, hence, that yuxH is indeed under the negative control of Spo0A as reported previously.
Incidental to our analysis, we observed indirect effects of high and low doses of Spo0A on the expression of many additional genes that are not members of the regulon. These data are available as supplemental material (Table S1).
DISCUSSION
The principal contribution of this investigation is the finding that many of the genes in the Spo0A regulon respond to the transcription factor in a dose-dependent manner. Indeed, this was true for the large majority of the genes whose transcription was strongly influenced by Spo0A. We assume in our analysis that the amount of Spo0A was proportional to the amount of Spo0A∼P, the active form of the response regulator. We were unable to test this assumption experimentally (because of the instability of Spo0A∼P), but there is likely to be at least a partial correlation between the level of Spo0A and the level of its phosphorylation as Spo0A is part of a positive feedback loop in which the response regulator directly and indirectly stimulates the expression of genes involved its phosphorylation (21).
Our analysis distinguished four categories of responses to Spo0A: genes that were activated at a low dose of Spo0A, genes that required a high-threshold level of Spo0A to be activated, genes that were repressed at a low level of Spo0A and, finally, genes that were repressed in a manner that required a high-threshold level of the regulatory protein. We distinguish between direct and indirect mechanisms by which genes respond to low and high doses of Spo0A. One mechanism involves the affinity of the gene in question for Spo0A. Thus, the regulatory region for the skf operon has a relatively high-affinity binding site for Spo0A, explaining its activation at a low level of the regulatory protein. In contrast, the regulatory regions for the sporulation operons spoIIA, spoIIE, and spoIIG have relatively weak affinities for Spo0A, which is consistent with the observation that these transcription units require a high level of Spo0A to be switched on. The second mechanism is indirect activation via repression of the gene for the unstable repressor protein AbrB, a well-known global regulator of numerous genes that commence expression at the end of the exponential phase of growth (15, 27, 34-36, 39). Thus, and as we have shown here, the sdp operon is subject to repression by AbrB. At low doses, Spo0A represses abrB, which leads to depletion of the unstable regulatory protein and, hence, relief from AbrB-mediated repression. Indeed, from the point of view of its activation, sdp would not be considered a member of the Spo0A regulon. However, sdp is additionally and directly subject to repression by Spo0A through the presence of a weak binding site for the regulatory protein, which is responsible for shutting off continued transcription of the operon at high doses of Spo0A.
Interestingly, several genes in the regulon are subject to both direct and indirect modes of regulation by Spo0A. Thus, skf was subject to repression by AbrB, but the expression of the operon was also almost entirely dependent on Spo0A in a manner that was independent of AbrB. That sdp and skf are subject to negative control by AbrB was also observed by M. Strauch (personal communication), who has also demonstrated the presence of binding sites for the repressor protein in the regulatory regions of both operons.
Two other examples of genes that are directly and indirectly regulated by Spo0A are the phosphorelay genes kinA and spo0A itself. Both genes have binding sites for Spo0A (21), and biochemical evidence indicates that both are repressed by the response regulator at high concentrations (14). Yet, as shown here, kinA and spo0A fell into the category of low-threshold-activated genes. It is known that both genes are transcribed by σH-containing RNA polymerase (14, 29). Thus, low-threshold activation of kinA and spo0A is likely to be mediated by the positive feedback loop discussed above, in which Spo0A stimulates σH synthesis by relieving AbrB-mediated repression of spo0H (the structural gene for σH).
The case of the rapA gene is of special interest because it is both activated at a low dose of Spo0A and repressed at a high dose. The rapA gene encodes a phosphatase that drains phosphoryl groups from the phosphorelay (specifically from the phosphorelay intermediate Spo0F∼P) that is responsible for activating Spo0A (24). As discussed above, the activation of rapA at a low dose of Spo0A would be expected to retard Spo0A levels from rising rapidly and would thus facilitate the persistence of Spo0A at a low concentration. As noted above, Spo0A indirectly stimulates its own transcription by inhibiting the synthesis of AbrB, which is a repressor of the gene for σH (21, 27, 34). The σH factor, in turn, is responsible for directing transcription from one of the two promoters that govern the expression of the spo0A gene. Thus, diminished phosphorylation of Spo0A via the action of RapA would limit the rate of Spo0A synthesis. Conversely, repression of rapA at a high dose of Spo0A would curtail further synthesis of the phosphatase. Thus, once Spo0A does eventually reach a high-threshold level, the diminished synthesis of RapA would help ensure that phosphorylation and synthesis of Spo0A continue at a high rate.
What is the biological significance of having differential responses to high and low doses of Spo0A? A possible clue comes from the nature of the genes that are turned on at different levels of Spo0A, some of which are directly involved in sporulation and some of which have other functions. Thus, racA, spoIIA, spoIIE, and spoIIG require high levels of Spo0A to be activated and all are directly involved in sporulation. Moreover, and as we have seen, sporulation is itself a high-threshold process, requiring a high level of Spo0A in order to proceed efficiently. In contrast, skf and sdp are switched on at a low level of Spo0A, in keeping with the idea that the products of these operons are involved in a process that delays cells that have activated Spo0A from becoming committed to sporulation. Conditions of nutrient limitation, which lead to entry into sporulation, typically cause the appearance of two kinds of cells: those that have activated Spo0A and those that have not (9, 16). The skf and sdp operons are responsible for the production of a killing factor and a sporulation-delaying protein that block sibling cells that have not activated Spo0A from entering sporulation. Instead, the sibling cells undergo lysis, thereby providing a source of nutrients for the cells that have activated Spo0A and hence blocking them from progressing further into sporulation. Seen in the light of this cannibalistic process, it makes sense that genes that delay progression into sporulation are activated at a low level of Spo0A and that genes that are directly involved in sporulation require high levels of the regulatory protein to be switched on.
Yet another biological process that helps to explain the meaning of high- and low-threshold responses to Spo0A is biofilm formation. Biofilms are multicellular communities of cells, and recent work has shown that cells of B. subtilis are capable of assembling into such communities (3, 4, 20). Importantly, in the present context, biofilm formation is dependent upon Spo0A (3, 19). One of the targets of Spo0A that is important in biofilm formation is abrB, which is evidently responsible for repressing one or more genes involved in multicellularity (20) and which, as we have seen, is repressed at a low dose of Spo0A. Another potential target of Spo0A in biofilm formation is sinI, whose product is an antagonist of the repressor protein SinR (1) and whose expression is stimulated by Spo0A (31). Recent work has shown that SinR plays a central role in biofilm formation by controlling the expression of the eps operon, which is responsible for the production of the exopolysaccharide that is believed to be responsible for binding chains of cells together in the biofilm (22). Thus, the Spo0A-directed expression of sinI would be expected to (indirectly) turn on expression of the eps operon and thereby promote biofilm formation. Our analysis classified sinI as a high-threshold-activated gene, but inspection of the results in Table 3 shows that its differential response to high levels of Spo0A was the least robust of that of all of the genes in the category. Conceivably, therefore, transcription of sinI is stimulated at a somewhat lower level of Spo0A than is required to activate sporulation-specific genes. If so, then biofilm formation is a low-threshold response to Spo0A or, at least, a lower dose response than is sporulation. Wild strains of B. subtilis produce particularly robust biofilms with complex architectural features, including fruiting-body-like aerial structures in which spore formation preferentially takes places at the tips (3). Biofilm formation in wild strains can therefore be seen as a prelude to spore formation, with a low level of Spo0A promoting the formation of fruiting bodies and the eventual attainment of high levels of Spo0A-activating genes that mediate the process of spore formation itself.
In sum, and in an extension of earlier work, our findings lead to the view that cells exist in at least four states with respect to Spo0A, each with its own biological significance. First, activation of Spo0A in response to conditions of nutrient limitation is governed by a bistable switch in which some cells in the population activate the regulatory protein and others do not. Second, cells that have activated Spo0A initially produce the regulatory protein at a low level that is sufficient to activate genes involved in specialized processes, such as cannibalism and biofilm formation, but not spore formation. Indeed, the cannibalism phenomenon depends on the existence of a population of cells that have not activated Spo0A (16). Third, cells that have activated Spo0A enter a state in which the transcription factor accumulates to high levels, thereby unleashing the expression of genes critical for the initial stages of spore formation. Finally, at intermediate stages of sporulation, Spo0A accumulates to very high levels in a cell-specific manner when it promotes gene expression in the mother cell compartment of the sporangium (13).
Supplementary Material
[Supplemental material]
Acknowledgments
We thank M. Strauch for communicating unpublished results and M. Strauch, S. Branda, and D. Kearns for helpful comments.
This work was supported by NIH grant GM18568.
Footnotes
REFERENCES
- 1.Bai, U., I. Mandic-Mulec, and I. Smith. 1993. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 7**:**139-148. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Yehuda, S., D. Z. Rudner, and R. Losick. 2003. RacA, a bacterial protein that anchors chromosomes to the cell poles. Science 299**:**532-536. [DOI] [PubMed] [Google Scholar]
- 3.Branda, S. S., J. E. Gonzalez-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98**:**11621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branda, S. S., J. E. Gonzalez-Pastor, E. Dervyn, S. D. Ehrlich, R. Losick, and R. Kolter. 2004. Genes involved in formation of structured multicellular communities by Bacillus subtilis. J. Bacteriol. 186**:**3970-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton, R. A., P. Eichenberger, J. E. Gonzalez-Pastor, P. Fawcett, R. Monson, R. Losick, and A. D. Grossman. 2002. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J. Bacteriol. 184**:**4881-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burbulys, D., K. A. Trach, and J. A. Hoch. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64**:**545-552. [DOI] [PubMed] [Google Scholar]
- 7.Carey, J. 1991. Gel retardation. Methods Enzymol. 208**:**103-117. [DOI] [PubMed] [Google Scholar]
- 8.Chibazakura, T., F. Kawamura, and H. Takahashi. 1991. Differential regulation of spo0A transcription in Bacillus subtilis: glucose represses promoter switching at the initiation of sporulation. J. Bacteriol. 173**:**2625-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, J. D., G. Stephanopoulos, K. Ireton, and A. D. Grossman. 1994. Gene expression in single cells of Bacillus subtilis: evidence that a threshold mechanism controls the initiation of sporulation. J. Bacteriol. 176**:**1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fawcett, P., P. Eichenberger, R. Losick, and P. Youngman. 2000. The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 97**:**8063-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita, M. 2000. Temporal and selective association of multiple sigma factors with RNA polymerase during sporulation in Bacillus subtilis. Genes Cells 5**:**79-88. [DOI] [PubMed] [Google Scholar]
- 12.Fujita, M., and R. Losick. 2002. An investigation into the compartmentalization of the sporulation transcription factor σE in Bacillus subtilis. Mol. Microbiol. 43**:**27-38. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, M., and R. Losick. 2003. The master regulator for entry into sporulation in Bacillus subtilis becomes a cell-specific transcription factor after asymmetric division. Genes Dev. 17**:**1166-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita, M., and Y. Sadaie. 1998. Feedback loops involving Spo0A and AbrB in in vitro transcription of the genes involved in the initiation of sporulation in Bacillus subtilis. J. Biochem. (Tokyo) 124**:**98-104. [DOI] [PubMed] [Google Scholar]
- 15.Furbass, R., M. Gocht, P. Zuber, and M. A. Marahiel. 1991. Interaction of AbrB, a transcriptional regulator from Bacillus subtilis with the promoters of the transition state-activated genes tycA and spoVG. Mol. Gen. Genet. 225**:**347-354. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Pastor, J. E., E. C. Hobbs, and R. Losick. 2003. Cannibalism by sporulating bacteria. Science 301**:**510-513. [DOI] [PubMed] [Google Scholar]
- 17.Grossman, A. D. 1995. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu. Rev. Genet. 29**:**477-508. [DOI] [PubMed] [Google Scholar]
- 18.Guerout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180**:**57-61. [DOI] [PubMed] [Google Scholar]
- 19.Hamon, M. A., and B. A. Lazazzera. 2001. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 42**:**1199-1209. [DOI] [PubMed] [Google Scholar]
- 20.Hamon, M. A., N. R. Stanley, R. A. Britton, A. D. Grossman, and B. A. Lazazzera. 2004. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol. Microbiol. 52**:**847-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoch, J. A. 1991. spo0 genes, the phosphorelay, and the initiation of sporulation, p. 747-755. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 22.Kearns, D. B., F. Chu, S. S. Branda, R. Kolter, and R. Losick. 2004. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 52**:**357-369. [DOI] [PubMed] [Google Scholar]
- 23.Molle, V., M. Fujita, S. T. Jensen, P. Eichenberger, J. E. Gonzalez-Pastor, J. S. Liu, and R. Losick. 2003. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 50**:**1683-1701. [DOI] [PubMed] [Google Scholar]
- 24.Perego, M., C. Hanstein, K. M. Welsh, T. Djavakhishvili, P. Glaser, and J. A. Hoch. 1994. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell 79**:**1047-1055. [DOI] [PubMed] [Google Scholar]
- 25.Perego, M., and J. A. Hoch. 2002. Two-component systems, phosphorelays, and regulation of their activities by phosphatases, p. 473-481. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 26.Perego, M., G. B. Spiegelman, and J. A. Hoch. 1988. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol. Microbiol. 2**:**689-699. [DOI] [PubMed] [Google Scholar]
- 27.Phillips, Z. E., and M. A. Strauch. 2002. Bacillus subtilis sporulation and stationary phase gene expression. Cell. Mol. Life Sci. 59**:**392-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piggot, P. J., and R. Losick. 2002. Sporulation genes and intercompartmental regulation, p. 483-518. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 29.Predich, M., G. Nair, and I. Smith. 1992. Bacillus subtilis early sporulation genes kinA, spo0F, and spo0A are transcribed by the RNA polymerase containing σH. J. Bacteriol. 174**:**2771-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe-Magnus, D. A., and G. B. Spiegelman. 1998. Contributions of the domains of the Bacillus subtilis response regulator Spo0A to transcription stimulation of the spoIIG operon. J. Biol. Chem. 273**:**25818-25824. [DOI] [PubMed] [Google Scholar]
- 31.Shafikhani, S. H., I. Mandic-Mulec, M. A. Strauch, I. Smith, and T. Leighton. 2002. Postexponential regulation of sin operon expression in Bacillus subtilis. J. Bacteriol. 184**:**564-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142**:**79-83. [DOI] [PubMed] [Google Scholar]
- 33.Strauch, M., V. Webb, G. Spiegelman, and J. A. Hoch. 1990. The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc. Natl. Acad. Sci. USA 87**:**1801-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauch, M. A. 1993. AbrB, a transition state regulator, p. 757-764. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 35.Strauch, M. A., and J. A. Hoch. 1993. Signal transduction in Bacillus subtilis sporulation. Curr. Opin. Genet. Dev. 3**:**203-212. [DOI] [PubMed] [Google Scholar]
- 36.Strauch, M. A., G. B. Spiegelman, M. Perego, W. C. Johnson, D. Burbulys, and J. A. Hoch. 1989. The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein. EMBO J. 8**:**1615-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, L. J., and J. Errington. 2003. RacA and the Soj-Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol. Microbiol. 49**:**1463-1475. [DOI] [PubMed] [Google Scholar]
- 38.Youngman, P. J., J. B. Perkins, and R. Losick. 1983. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn_917_. Proc. Natl. Acad. Sci. USA 80**:**2305-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuber, P., and R. Losick. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169**:**2223-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]