No increased risk of cardiovascular events in older adults initiating dipeptidyl peptidase 4 inhibitors versus therapeutic alternatives (original) (raw)
. Author manuscript; available in PMC: 2018 Jul 1.
Published in final edited form as: Diabetes Obes Metab. 2017 Mar 17;19(7):970–978. doi: 10.1111/dom.12906
Abstract
Objective
Randomized placebo-controlled trials have examined the cardiovascular (CV) effects of dipeptidyl peptidase-4 inhibitors (DPP-4i), but data on incidence relative to therapeutic alternatives are limited in the older US Medicare population. We compared the CV risk with DPP-4i relative to sulfonylureas (SU) and thiazolidinediones (TZD).
Methods
During 2007-2013, using Medicare beneficiaries >65 years we identified two new-user cohorts without the use of drugs being compared in the 6 months before initiation: DPP-4i versus SU and DPP-4i versus TZD. Using propensity score-adjusted Cox models accounting for competing risk by death, we estimated hazard ratios (HR), risk differences (RD) and 95% confidence intervals for myocardial infarction (MI), stroke, HF hospitalization, and a combined outcome (MI, stroke, all-cause mortality).
Results
In the DPP-4i vs SU comparison, there were 30,130 DPP-4i and 68,382 SU initiators with mean age 75 years, 41% males and 55% with a baseline CV condition. The HR for the composite outcome was 0.75 (95% CI: 0.72-0.79) over a median treatment duration of 1-year, but the 1-year MI risks were 1.00(0.89, 1.12) and 1.47(1.38, 1.56) per 100 patients for DPP-4i and SU, respectively and corresponding stroke risks were 0.98(0.87-1.10) and 1.09(1.01-1.17). For the DPP-4i vs TZD comparison, there were 20,596 DPP-4i and 13,526 TZD initiators without previous HF and mean age 74 years, 42% males and 30% with a baseline CV event. The composite outcome HR was 0.94 (0.86-1.02) over a median treatment duration of 1 year, with the 1-year risks ~0.90 for MI and ~0.80 per 100 patients for stroke in both DPP-4i and TZD.
Conclusion
Though limited by the short treatment duration, our study suggests no increased short-term risk of MI stroke or HF with DPP-4i versus SU/TZD.
Introduction
In the United States over 25% of the population 65 years or older has diabetes.1 Cardiovascular (CV) disease is a leading cause of morbidity and mortality in diabetes patients, with the risk increasing with age.2 While improved glycemic control by antihyperglycemic drugs reduces microvascular complications, uncertainty remains regarding risk reduction for CV events. International agencies now require a thorough assessment of CV risk in antihyperglycemic drug development programs.3,4
The dipeptidyl peptidase-4 inhibitors (DPP-4i) are relatively new antihyperglycemic drugs that were incorporated into diabetes treatment algorithms as second line therapy since 2011. These drugs have good tolerability, low risk of hypoglycemia and are weight neutral compared to other second line drugs.5 Three randomized placebo-controlled trials (RCT) have recently evaluated the CV safety of DPP-4i (saxagliptin, alogliptin and sitagliptin) in high-risk patients with type 2 diabetes.6-9 All RCTs found no increase in the risk of non-fatal myocardial infarction, stroke, CV death with adding a DPP-4i agent versus placebo to existing therapy. However, the saxagliptin trial found an increased risk of hospitalization for heart failure, whereas the other two trials did not find any association between DPP-4i treatment and heart failure.7
While randomized trials have had an important role in assessing the CV safety of DPP-4i, all the trials to date have compared the addition of a DPP-4i versus adding no drug to existing therapy which may not represent real world treatment patterns which involve a lot of switching or stopping treatments. Moreover, other than the ongoing trial comparing linagliptin to glimepiride (CAROLINA),10 all the completed trials were placebo-controlled making it difficult to assess the comparative incidence of cardiovascular events relative to therapeutic alternatives. Finally, the trials have recruited high risk populations, largely patients with a prior history of CV events.
Observational studies examining CV risk with DPP-4i report no increased relative risk of myocardial infarction and stroke with DPP-4i, but the evidence on heart failure is mixed.11-22 These studies mainly reported summary relative risk measures but not the absolute risk measures which may be important to put the issue in context. Further, some studies used a combined pool of non-DPP-4i drugs as the comparator making the results less useful for physicians for making treatment choices. To date there has not been any epidemiologic study comparing the incidence of CV events with DPP-4i versus clinically relevant comparators in a US population of older adults with a high prevalence of comorbidity and long duration of diabetes, both of which could affect the effects of DPP-4i on CV risk.
We therefore compared the relative and absolute risk of CV outcomes among initiators of DPP-4i versus relevant oral drug alternatives sulfonylureas (SU) and thiazolidinediones (TZD) using a 20% sample of the Medicare fee-for-service beneficiaries. Specifically, we examined the risk of non-fatal myocardial infarction (MI), stroke, hospitalization for heart failure (HF) and a composite outcome including MI, stroke, and all-cause mortality.
Methods
Study Population
We conducted an active-comparator new-user cohort study using a 20% random sample of Medicare beneficiaries >65 years with fee-for-service Part A, B and D enrollment in at least one month during January 1, 2007 to December 31, 2013. This dataset contains information about demographics, enrollment, diagnoses, procedures and prescription drugs for each enrollee and has been previously used to study antihyperglycemic drugs.23-25
From this population, we identified two new-user active-comparator cohort pairs mimicking a clinical treatment decision:26 1. DPP-4i versus SU (not exposed to either DPP-4i or SU in the previous 6 months) and 2. DPP-4i versus TZD (not exposed to either DPP-4i or TZD in the previous 6 months). Initiation was defined as the first prescription of the drug after a 6 month washout. Prevalent users of the drugs being compared during the washout period were excluded. To increase the probability of identifying second-line diabetes treatment initiators, all patients were required to have at least one metformin prescription in the 6 months before drug initiation. Patients were required to have at least 6 months of continuous Part D enrollment and at least 12 months parts A and B enrollment before initiation. To reduce the potential for bias toward the null due to secondary nonadherence, we restricted our cohorts to patients with a second prescription for the same drug class dispensed within 6 months after initiation and follow-up started from the second fill date. Since TZDs are contraindicated in patients with HF (which can lead to intractable confounding by contraindication), for DPP-4i versus TZD analyses we further excluded patients with diagnoses of HF and related conditions (cardiomyopathy, arrhythmias, chronic kidney disease, edema and loop diuretics use).
Outcomes
The outcomes assessed were non-fatal MI, stroke, HF hospitalization and all-cause mortality and a composite outcome of non-fatal MI, stroke and all-cause mortality based on the outcome definition in the RCTs. Medicare claims do not include information on causes of death and we could not identify cardiovascular death. However since cardiovascular deaths account for >50% deaths in diabetes patients, we used all-cause mortality as proxy.27,28 MI was defined using International Classification of Diseases, Ninth Revision (ICD-9) code 410 in the first or second position of the inpatient claims (definition with a positive predictive value of 94% in a Medicare population).29 Stroke was defined using ICD-9 codes 430, 431, 433.x1, 434.x1, and 436, located in the first position (specificity 95–97%, sensitivity 74–90%).30 HF hospitalization was defined using ICD-9 code 428.xx in the primary position which has a specificity >98% but a very low sensitivity of 21% in a Medicare population.31
Patients were followed from the second prescription until the earliest of: the outcome of interest, discontinuation, switching to or augmentation with the comparator drug, non-end point event (example, stroke is a non-end point event in the analysis of MI), end of enrollment, or December 31, 2013.
Confounding control and analysis
We used propensity scores (PS) to control for measured confounding. Using baseline variables (comorbidities, demographics, drug use and health care use) measured before initiation, we predicted the probability for initiating DPP-4i versus SU and DPP-4i versus TZD for each patient (PS) using two separate logistic regression models.32 We then assigned a weight of 1 to DPP-4i and a weight of (PS/(1-PS)) to SU and TZD. Such weighting creates pseudo-populations of SU and TZD initiators with similar covariate distribution as in DPP-4i.33, 34 Our weighted analysis thus answers the question “what would have happened to patients who initiated DPP-4i if they had initiated SU or TZD, instead”.35
Competing Risk
Competing risks arise when the occurrence of one event precludes the occurrence of other events. In our study of older adults, mortality is a competing event and standard Cox models censoring patients who die yield biased estimates because this type of censoring may be ‘informative’.36 We therefore used weighted cumulative incidence curves accounting for competing risk by death to estimate the risk, risk differences (RD) and risk ratios (RR) for non-fatal MI, stroke and HF hospitalizations among initiators of DPP-4i versus comparators.36 We obtained confidence intervals by bootstrapping 1000 replicates. We analyzed the composite outcome of non-fatal MI, stroke or all-cause mortality using traditional weighted Cox models.
Subgroup and Sensitivity analyses
Analyses were repeated in pre-specified subgroups based on CVD history. Several sensitivity analyses were performed. To increase the probability of CV death, we excluded the deaths of patients with codes for metastatic cancer anytime during follow-up. Finally, we repeated all analyses using an intent-to-treat approach where patients were not censored for treatment changes but followed from the second prescription to the earliest of the outcome, non-event end point, end of enrollment, or December 31, 2013.
Results
For the DPP-4i versus SU comparison (table 1), there were 30,130 DPP-4i and 68,382 SU initiators with mean age ~75 years. 39.8% of DPP-4i and 42.3% of the SU initiators were male while 55.4% DPP-4i and 54.8% of the SU initiators had a CV condition at baseline. Compared with DPP-4i, SU initiators were less likely to have hyperlipidemia, diabetes complications, less likely to be on statins, and less likely to have had influenza vaccinations and lipid panels at baseline. For the DPP-4i versus TZD comparison (table 2), there were 20,596 DPP-4i and 13,526 TZD initiators without previous HF/related diagnoses (excluded because TZD are contraindicated in those with pre-existing HF)37 The mean age in these cohorts was ~74 years, 40.4% of DPP-4i and 44.1% of the TZD initiators were male. 31.4% of the DPP-4i and 26.1% of the TZD initiators had a baseline CV condition. Compared with the DPP-4i initiators, the TZD initiators were less likely to have hyperlipidemia at baseline and less likely to get influenza vaccinations and lipid panels. After weighting all covariates (detailed in e-tables 1 and 2) in the weighted TZD and SU pseudo-populations were identical to the distribution of the DPP-4i initiators.
Table 1.
Characteristics of initiatorsa of dipeptidyl peptidase-4 inhibitors and sulfonylureas with a baseline metformin prescription : Medicare claims data 2006 – 2013
| Characteristic | DPP N=30,130 | SU N=68,382 | weighted SUb |
|---|---|---|---|
| Age 66-75 years | 18,312 (60.8%) | 41,310 (60.4%) | 18,344 (60.9%) |
| Age 76-85 years | 9,628 (32.0%) | 21,401 (31.3%) | 9,574 (31.8%) |
| Age 86+ years | 2,190 (7.3%) | 5,671 (8.3%) | 2,218 (7.4%) |
| Male | 11,984 (39.8%) | 28,937 (42.3%) | 11,945 (39.6%) |
| Diagnosis of acute myocardial infarction | 797 (2.6%) | 2,289 (3.3%) | 812 (2.7%) |
| Diagnosis of angina | 2,106 (7.0%) | 3,953 (5.8%) | 2,096 (7.0%) |
| Diagnosis of heart failure | 5,263 (17.5%) | 12,918 (18.9%) | 5,324 (17.7%) |
| Diagnosis of stroke or TIA | 2,126 (7.1%) | 5,320 (7.8%) | 2,140 (7.1%) |
| Diabetic nephropathy | 1,871 (6.2%) | 4,188 (6.1%) | 1,873 (6.2%) |
| Diabetic neuropathy | 6,023 (20.0%) | 11,637 (17.0%) | 6,028 (20.0%) |
| Diabetic retinopathy | 4,683 (15.5%) | 8,833 (12.9%) | 4,675 (15.5%) |
| Chronic kidney disease | 3,883 (12.9%) | 9,969 (14.6%) | 3,924 (13.0%) |
| Thiazolidinediones | 7,949 (26.4%) | 11,716 (17.1%) | 7,983 (26.5%) |
| Glucagon-like peptide-1 agonists | 574 (1.9%) | 1,045 (1.5%) | 578 (1.9%) |
| Long acting insulin | 4,723 (15.7%) | 8,084 (11.8%) | 4,814 (16.0%) |
| Short acting insulin | 2,079 (6.9%) | 4,113 (6.0%) | 2,103 (7.0%) |
| Statins | 22,286 (74.0%) | 46,842 (68.5%) | 22,325 (74.1%) |
Table 2.
Characteristics of initiatorsa of dipeptidyl peptidase-4 inhibitors and thiazolidinediones with a baseline metformin prescription : Medicare claims data 2006 – 2013
| Characteristic | DPP-4i N=20,596 | TZD N=13,526 | Weighted TZDb |
|---|---|---|---|
| Age 66-75 years | 14,017 (68.1%) | 9,385 (69.4%) | 14,023 (68.0%) |
| Age 76-85 years | 5,626 (27.3%) | 3,544 (26.2%) | 5,562 (27.0%) |
| Age 86+ years | 953 (4.6%) | 597 (4.4%) | 1,034 (5.0%) |
| Male | 8,313 (40.4%) | 5,963 (44.1%) | 8,302 (40.3%) |
| Diagnosis of acute myocardial infarction | 172 (0.8%) | 71 (0.5%) | 181 (0.9%) |
| Diagnosis of angina | 714 (3.5%) | 331 (2.4%) | 721 (3.5%) |
| Diagnosis of stroke or TIA | 913 (4.4%) | 515 (3.8%) | 918 (4.5%) |
| Diabetic nephropathy | 583 (2.8%) | 372 (2.8%) | 579 (2.8%) |
| Diabetic neuropathy | 3,179 (15.4%) | 1,802 (13.3%) | 3,270 (15.9%) |
| Diabetic retinopathy | 2,947 (14.3%) | 1,870 (13.8%) | 2,968 (14.4%) |
| Sulfonylureas | 11,372 (55.2%) | 8,007 (59.2%) | 11,362 (55.1%) |
| Glucagon-like peptide-1 agonists | 393 (1.9%) | 234 (1.7%) | 405 (2.0%) |
| Long acting insulin | 2,154 (10.5%) | 1,423 (10.5%) | 2,206 (10.7%) |
| Short acting insulin | 705 (3.4%) | 516 (3.8%) | 717 (3.5%) |
| Statins | 14,849 (72.1%) | 9,217 (68.1%) | 14,865 (72.1%) |
For the DPP-4i versus SU comparison, based on 2,424 composite events among DPP-4i initiators and 9,102 composite events among SU initiators, the adjusted hazard ratio was 0.75 (95% CI: 0.72-0.79) (table 3, figure 1a). This was mainly driven by death (E-figure 1) rather than MI and stroke for which risks were approximately 1% at median ~1 year of treatment (figure 2). The median time on treatment for the DPP-4i initiators was 1.00 years and for the SU initiators was 1.11 years. The adjusted 1-year RD per 100 patients for MI comparing DPP-4i versus SU was -0.47 (-0.61, -0.32) and the 1-year RD for stroke was -0.11 (-0.25, 0.02) (E-tables 3 and 4) indicating no meaningful difference in the risk of MI or stroke between DPP-4i and SU. In the subgroup without prior CVD, the adjusted risks of MI and stroke for both DPP-4i and SU groups were <1% at 1 year after initiation and the magnitudes of RDs were <1 per 100 patients (E-tables 5, 6). In the subgroup with prior CVD, the risks for MI and stroke were slightly higher (~1.3 – 2.0% at 1 year), but the magnitude of RD per 100 patients was <1 (E-tables 7, 8). No increased risk of HF hospitalization was observed with DPP-4i versus SU with a 5-year RD -1.12(-2.02, -0.21) (E-table 9).
Table 3.
Number of initiators, events (composite of nonfatal myocardial infarction, stroke and death), treatment duration, person–years, event rates and crude and adjusted hazard ratios for DPP-4i versus comparators in the entire population as well as subgroups based on prior cardiovascular disease (CVD)
| Comparison | Treatment | Number of new usersa | Events | Treatment Durationb IQR (median) | Total person-years | Incidence (per 100 person years) | Unadjusted HRc (95% CI) | Adjusted HRd (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Entire population | ||||||||
| DPP-4i vs SU | DPP-4ie | 30,130 | 2,424 | 0.56-1.90(1.00) | 41,388 | 5.9 | 0.67(0.64-0.70) | 0.75(0.72-0.79) |
| SU | 68,382 | 9,102 | 0.58-2.23(1.11) | 105,836 | 8.6 | 1.00 (reference) | 1.00 (reference) | |
| DPP-4i vs TZD | DPP-4ie | 20,596 | 1,220 | 0.59-2.04(1.08) | 30,580 | 4.0 | 0.94(0.86-1.02) | 0.95(0.86-1.03) |
| TZD | 13,526 | 920 | 0.67-2.24(1.16) | 21,603 | 4.3 | 1.00 (reference) | 1.00 (reference) | |
| Without CVD | ||||||||
| DPP-4i vs SU | DPP-4ie | 13,433 | 621 | 0.59-2.04(1.10) | 20,002 | 3.1 | 0.67(0.64-0.70) | 0.76(0.72-0.79) |
| SU | 30,877 | 2,539 | 0.66-2.53(1.30) | 53,630 | 4.7 | 1.00 (reference) | 1.00 (reference) | |
| DPP-4i vsTZD | DPP-4ie | 14,121 | 693 | 0.61-2.07(1.11) | 21,323 | 3.2 | 0.94(0.86-1.02) | 0.95(0.87-1.04) |
| TZD | 9,989 | 608 | 0.68-2.32(1.19) | 16,384 | 3.7 | 1.00 (reference) | 1.00 (reference) | |
| With CVD | ||||||||
| DPP-4i vs SU | DPP-4ie | 16,697 | 1,803 | 0.48-1.79(0.92) | 21,386 | 8.4 | 0.67(0.64-0.70) | 0.76(0.72-0.79) |
| SU | 37,505 | 6,563 | 0.52-1.98(0.98) | 52,206 | 12.6 | 1.00 (reference) | 1.00 (reference) | |
| DPP-4i vs TZD | DPP-4ie | 6,475 | 527 | 0.58-1.96(1.03) | 9,257 | 5.7 | 0.94(0.86-1.02) | 0.95(0.87-1.04) |
| TZD | 3,537 | 312 | 0.66-2.01(1.08) | 5,218 | 6.0 | 1.00 (reference) | 1.00 (reference) |
Figure 1. Weighted cumulative incidence for the composite outcome (non-fatal myocardial infarction, stroke and all-cause mortality).
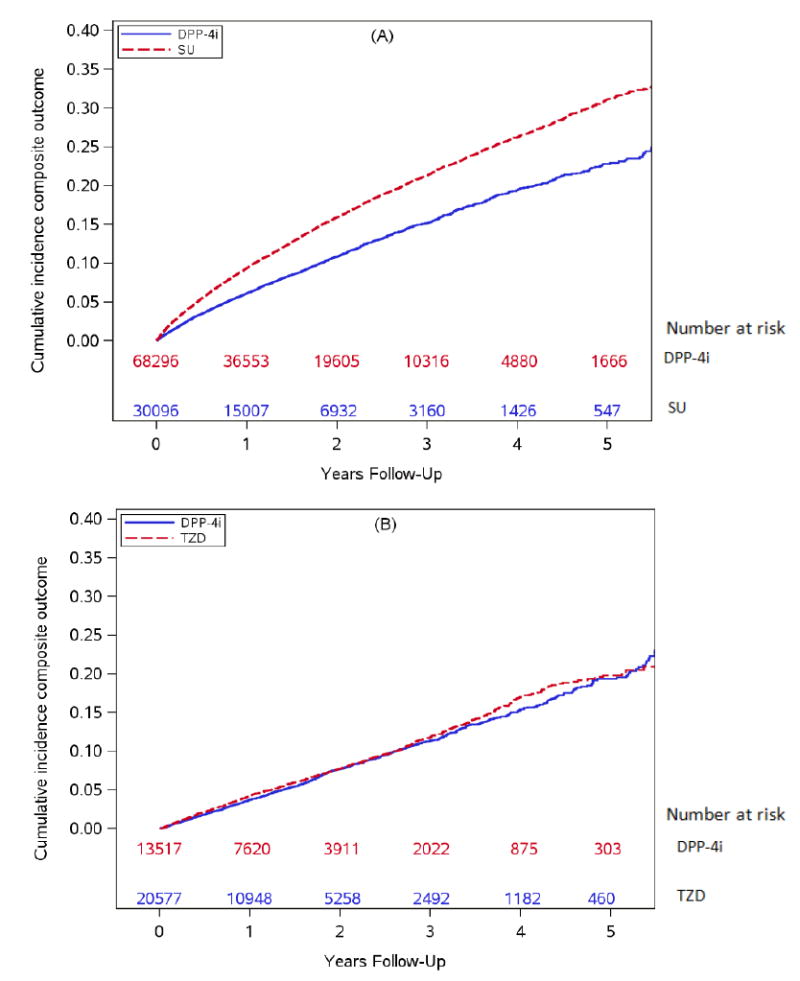
(A) Dipeptidyl peptidase-4 inhibitors (DPP-4i) versus sulfonylureas (SU)
(B) Dipeptidyl peptidase-4 inhibitors (DPP-4i) versus thiazolidinediones (TZD)
Figure 2. Weighted cumulative incidence for non-fatal myocardial infarction (MI) and stroke: DPP-4i versus SU and DPP-4i versus TZD.
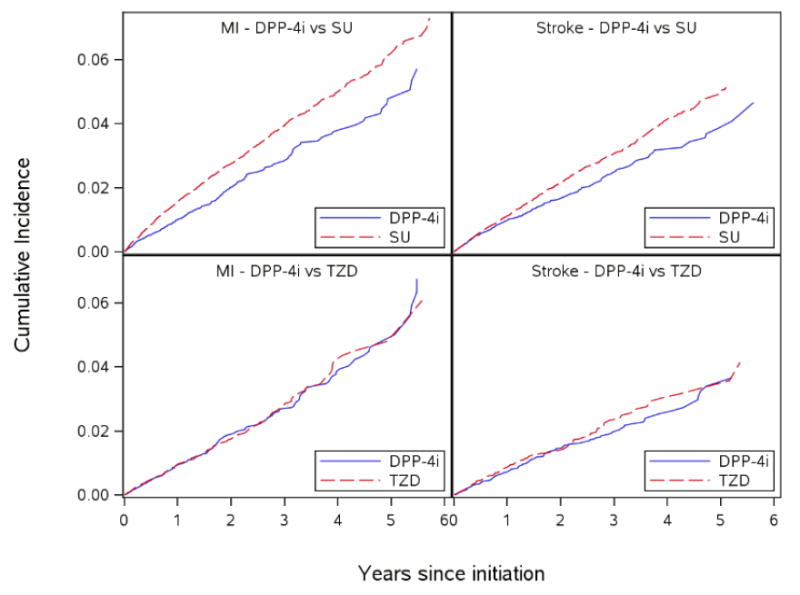
DPP-4i - dipeptidyl peptidase-4 inhibitors ; SU – sulfonylureas; TZD - thiazolidinediones
For DPP-4i versus TZD, based on 1,220 composite events among DPP-4i initiators and 920 composite events among TZD initiators the adjusted HR for the composite outcome was 0.95(0.86-1.03) (Figure 1b, table 3). The median time on treatment for the DPP-4i initiators was 1.08 years and for the TZD initiators was 1.16 years. The 1-year RD per 100 patients for MI was -0.04(-0.27, 0.18) and for stroke was -0.07 (-0.25, 0.11) (E-tables 10,11). In the subgroup without prior CVD, the 1-year risks of MI and stroke were <1% for DPP-4i and TZD (E-tables 12, 13). In the subgroup with prior CVD, the 1-year risks were slightly >1% for MI and stroke for both DPP-4i and TZD (E-tables 14, 15), but the RDs at 1 year were <1 per 100 patients. The RD per 100 patients for HF hospitalization comparing DPP-4i versus TZD were between 0 and -1 during the study period (E-table 16, E-figure 2).
Sensitivity analyses excluding metastatic cancer deaths from all-cause mortality (which accounted for 14-16% of deaths in all treatment groups) did not change the results (E-table 17). Additional analyses using an intent-to-treat approach did not change the results (E-table 18).
Discussion
We found no difference in the short-term risk of the composite CV outcome between second-line diabetes treatments DPP-4i versus TZD over a median treatment duration of 1 year in our study. The 1-year risk differences for MI, stroke and all-cause mortality were all between 0 and -1 per 100 patients again indicating no difference in risks of these outcomes among DPP-4i and TZD initiators.
The apparent decreased risk of the composite CV outcome with DPP-4i versus SU is mainly driven by all-cause mortality rather than by MI or stroke for which the 1-year risk differences were very small (<1 per 100 patients) indicating no difference in risk. Our results were consistent across subgroups based on prior CVD and several sensitivity analyses.While there is debate about increased risk of all-cause mortality with SU, the theoretical risk differs based on which SU agent is used. More information on the comparative risk with DPP-4i versus SU will be added once the CAROLINA trial comparing linagliptin with glimepiride is completed.10,38,39 Our results of no increased risk of MI and stroke with DPP-4i are consistent with the RCTs on saxagliptin (SAVOR-TIMI), alogliptin (EXAMINE) and sitagliptin (TECOS) which found no increased risk of MI, stroke with DPP-4i versus placebo in high risk populations.6,7,8
Several observational studies have compared the effect of DPP-4i on cardiovascular outcomes using different designs and populations and did not find an increased risk of CV events with DPP-4i.18-22 Some of the existing studies used a combined pool of non-DPP-4i drugs as the comparator making the results less useful for making treatment choices. For example, a study by Shih et al reported reduced risk of all-cause mortality and major adverse CV events with DPP-4i in an older population using Taiwanese insurance data. However, this study compared patients initiating DPP-4i versus non-users which potentially consisted of a mix of patients on other diabetes treatments and varying degrees of diabetes severity.13 Studies comparing metformin + DPP-4i versus metformin + SU found a reduced relative risk of CV mortality, all-cause mortality and disease with DPP-4i in UK, Danish, Swedish and Taiwanese populations, but the risks relative to TZD were not estimated.18-22 These studies mainly reported summary relative risk measures but not the absolute risks and risk differences which may be important to put the issue in context. Ours is the first study to examine this question in an older US population and reports both relative and absolute risks for CV events accounting for the competing risk by death. Taken together the evidence from our study and existing literature suggests that there is no concern of increased CV risk of with DPP-4i versus other second-line diabetes treatments. Further research is needed to investigate the relative effect of SU on all-cause mortality.
We did not observe an increased risk of HF hospitalization with DPP-4i versus SU. While the EXAMINE and TECOS trials did not find an increased HF risk with alogliptin and sitagliptin respectively, the SAVOR-TIMI 53 trial reported a 27% increased risk of HF hospitalization with saxagliptin versus placebo. Several factors could explain the discrepancy. First, the SAVOR-TIMI 53 examined saxagliptin alone while our DPP-4i cohort mainly consisted of sitagliptin initiators (~75%) and sample size was not sufficient to study saxagliptin alone. Second, the treatment duration in our study was shorter than in the trial. Observational studies examining HF risk with DPP-4i using different designs, populations and comparators report mixed results. Two studies reported a reduced rate of HF compared to other anti-hyperglycemic drugs11, 15, three studies suggest no difference in effect12,14,40, while two studies reported increased HF risk with DPP-4i compared to other antihyperglycemic drugs.16,17 Most of these studies used a heterogeneous comparator of ‘all other antihyperglycemic drugs’ makes interpretation of results hard particularly in cases where the risks differed greatly depending on the comparator.13,15-17,40 One study that reported increased HF risk compared sitagliptin initiators to matched controls who were prevalent users of antihyperglycemic therapy which could bias the results due to prevalent users possibly being tolerant to other antihyperglycemic therapy.17 Some clinical studies on the other hand, have suggested a protective role of DPP-4i in the pathogenesis of CHF.41,42
Since TZDs are known to be associated with an increased HF risk, we used HF hospitalization as a positive control outcome in the DPP-4i versus TZD analysis expecting no increased risk with DPP-4i relative to TZD and that is what we observed. Taken together the evidence from our study and existing literature suggests that there is no concern of increased risk of HF hospitalizations with DPP-4i.
A strength of our study is the use of a new-user active-comparator cohort design which is analogous to a head-to-head clinical trial and answers the more relevant question of ‘which second-line treatment to initiate’ rather than ‘treatment or not’.43 Specifically, from a pool of patients on metformin therapy, we identified initiators of DPP-4i or therapeutic alternatives (SU or TZD) after 6 months without use of DPP4i or comparators. Metformin is usually the first-line pharmacological treatment for diabetes and we required all patients to have a metformin prescription during the baseline period to be able to compare initiators of two second-line treatments. Further, since TZDs are contraindicated in patients with existing HF, for the DPP-4i versus TZD comparison we excluded patients with previous diagnoses of HF or related conditions in order to identify patients with treatment equipoise. Covariates were measured before initiation of second-line treatment thereby avoiding the problem of controlling for covariates potentially affected by treatment.43 The good balance of measured covariates achieved by the study design implies that unmeasured covariates could also be balanced, although this cannot be proven. The balance of measured covariates was further improved by PS weighting and reassures us about absence of confounding by these covariates. We also used two specific active comparator groups and reported results separately for each comparison unlike a few other observational studies where the comparator group consisted of ‘all other antihyperglycemic drugs’ making interpretation difficult. In our study, the TZD initiators appeared to be slightly healthier than the DPP-4i initiators while initiators of SU were slightly sicker relative to DPP-4i. Observation of similar results with both the analyses using two slightly different comparator populations further strengthens the finding of no increased CV risk with DPP-4i.
Following caveats should be considered. First, the time-on-treatment in our primary as-treated analysis was short (median ~1 year) but that is a function of the real-world treatment use among Medicare beneficiaries. Second, since Medicare claims do not contain information on causes of death, we could not identify cardiovascular death. Sensitivity analyses excluding deaths in patients with metastatic cancer to increase the contribution of cardiac death to all-cause mortality did not change results. Third, given the absence of clinical measures it is hard to identify HF using claims data and our definition of HF hospitalization had a near perfect specificity (which yields unbiased relative risks) but a low sensitivity which will lead to an underestimation of absolute risks. Fourth, since occurrence of one CV event during follow-up might affect the incidence of a subsequent CV event, (example, MI can affect the risk of stroke), we censored patients at non-end point CV events. This could theoretically lead to ‘competing risk’ from the non-end point event, but such censoring was extremely rare in our study. Fifth, because we required a baseline period to assess covariates in the period before initiation, patients did not become eligible for inclusion in our till they reached 66 years of age. We did not include the (very small) group of patients who were 65 at initiation because these were a ‘select’ group of sicker patients who were eligible for Medicare due to health reasons before they turned 65. Finally we were not able to measure and adjust for lifestyle variables like smoking and body mass index directly (BMI). However we adjusted for codes for tobacco use and chronic obstructive pulmonary disease as proxies for smoking. We also previously found that smoking and BMI do not meaningfully affect the choice of initiation of DPP-4i versus SU and TZD and therefore unlikely to be confounders in this setting.44
In summary, we did not observe an increased short-term risk of CV events with DPP-4i versus relevant oral second line diabetes drugs in an older population. Along with the RCT results, our results based on real-world drug use and effects are relevant to physicians for making antihyperglycemic treatment choices.
Supplementary Material
Supp data
Acknowledgments
The Medicare database infrastructure used for this project at UNC was supported by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL200811.0010), the Center for Pharmacoepidemiology, Department of Epidemiology, the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1TR001111), the Cecil G. Sheps Center for Health Services Research, and the School of Medicine. The development of the state-of-the art nonexperimental methodology was supported by an ongoing grant from the National Institute on Aging (R01 AG023178).
Footnotes
Author contributions:
All authors participated in study conception and design. M.G., M.J.F., J.L. and T.S. participated in the acquisition of the data. M.G.did the analysis and all authors contributed to the interpretation of the data. M.G. wrote the first draft of the manuscript. All authors reviewed and provided comments on the manuscript. M.G. is the guarantor of this work; M.G. and T.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. V.P. assisted with the re-analysis of data and helped with the interpretation of results. All authors contributed towards the revision of the manuscript.
Conflict of interest/disclosures:
M.G. was a doctoral student at University of North Carolina during the conduct of this study. She is now a full time employee of GlaxoSmithKline. Dr. Buse reports grants, non-financial support and other from Eli Lilly, grants, non-financial support and other from Bristol-Myers Squibb, grants, non-financial support and other from GI Dynamics, non-financial support and other from Elcylex, grants, non-financial support and other from Merck, non-financial support and other from Metavention, non-financial support and other from vTv Pharma, grants, personal fees, non-financial support and other from PhaseBio, grants, non-financial support and other from AstraZeneca, non-financial support and other from Dance Biopharm, non-financial support and other from Quest, grants from Medtronic Minimed, grants, non-financial support and other from Sanofi, grants from Tolerex, grants from Osiris, grants from Halozyme, grants from Johnson & Johnson, grants from Andromeda, grants from Boehringer-Ingelheim, grants from GlaxoSmithKline, grants from Astellas, grants from MacroGenics, grants from Intarcia Therapeutics, grants, non-financial support and other from Lexicon, grants from Scion NeuroStim, grants, non-financial support and other from Novo Nordisk, grants, non-financial support and other from Orexigen, grants, non-financial support and other from Takeda, non-financial support and other from Adocia, outside the submitted work; and I am or have been a member of a variety of non-profit boards: American Diabetes Association, DiabetesSisters, Taking Control of Your Diabetes, AstraZeneca Healthcare Foundation, Bristol-Myers Squib Together on Diabetes Foundation, the National Diabetes Education Program. T.S. receives investigator-initiated research funding and support as Principal Investigator (R01 AG023178) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453; R01 HL118255, R21-HD080214), National Institutes of Health (NIH). He also receives salary support as Director of the Comparative Effectiveness Research (CER) Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and as Director of the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, AstraZeneca) to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, Johnsen & Johnsen, and Novo Nordisk. Dr. Lund receives research support from the UNC Oncology Clinical Translational Research Training Program (K12 CA120780), as well as through a Research Starter Award from the PhRMA Foundation to the UNC Department of Epidemiology. M.J.F. receives investigator-initiated research funding and support as Principal Investigator from the National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI, R01 HL118255); as a Co-Investigator from the NIH National Institute on Aging (NIA, R01 AG023178), the NIH National Center for Advancing Translational Sciences (NCATS, 1UL1TR001111), AstraZeneca, and the Patient Centered Outcomes Research Institute (PCORI, 1IP2PI000075). Dr. Jonsson Funk does not accept personal compensation of any kind from any pharmaceutical company, though she receives salary support from the Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health (current members: GlaxoSmithKline, UCB BioSciences, Merck).
References
- 1.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halter JB, Musi N, McFarland Horne F, et al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes. 2014;63(8):2578–2589. doi: 10.2337/db14-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Medicines Agency. European Medicines Agency Recommends Suspension of Avandia, Avandamet and Avaglim. European Medicines Agency; 2010. [March 12, 2016]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2010/09/news_detail_001119.jsp=WC0b01ac058004d5c1. [Google Scholar]
- 4.FDA. Guidance for Industry Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. [February 3, 2016];2008 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf.
- 5.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 6.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889;10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 7.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684;10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 8.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 9.White WB, Baker WL. Cardiovascular Effects of Incretin-Based Therapies. Annu Rev Med. 2016;67:245–260. doi: 10.1146/annurev-med-050214-013431. [DOI] [PubMed] [Google Scholar]
- 10.Marx N, Rosenstock J, Kahn SE, et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA(R)) Diab Vasc Dis Res. 2015;12(3):164–174. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SC, Glynn RJ, Liu J, Everett BM, Goldfine AB. Dipeptidyl peptidase-4 inhibitors do not increase the risk of cardiovascular events in type 2 diabetes: a cohort study. Acta Diabetol. 2014;51(6):1015–1023. doi: 10.1007/s00592-014-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu AZ, Johnston SS, Ghannam A, et al. Association Between Hospitalization for Heart Failure and Dipeptidyl Peptidase-4 Inhibitors in Patients With Type 2 Diabetes: An Observational Study. Diabetes Care. 2016 doi: 10.2337/dc15-0764. [DOI] [PubMed] [Google Scholar]
- 13.Shih C, Chen H, Kuo S, Ou S, Chen Y. Cardiovascular outcomes of dipeptidyl peptidase-4 inhibitors in elderly patients with type 2 diabetes: A nationwide study. Journal of the American Medical Directors Association. 2016;17(1):59–64. doi: 10.1016/j.jamda.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Yu OH, Filion KB, Azoulay L, Patenaude V, Majdan A, Suissa S. Incretin-based drugs and the risk of congestive heart failure. Diabetes Care. 2015;38(2):277–284. doi: 10.2337/dc14-1459. [DOI] [PubMed] [Google Scholar]
- 15.Velez M, Peterson EL, Wells K, et al. Association of antidiabetic medications targeting the glucagon-like peptide 1 pathway and heart failure events in patients with diabetes. J Card Fail. 2015;21(1):2–8. doi: 10.1016/j.cardfail.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weir DL, McAlister FA, Senthilselvan A, Minhas-Sandhu JK, Eurich DT. Sitagliptin use in patients with diabetes and heart failure: a population-based retrospective cohort study. JACC: Heart Failure. 2014;2(6):573–582. doi: 10.1016/j.jchf.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Liu C, Chao T, et al. Sitagliptin and the risk of hospitalization for heart failure: a population-based study. Int J Cardiol. 2014;177(1):86–90. doi: 10.1016/j.ijcard.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Gamble JM, Thomas JM, Twells LK, et al. Comparative effectiveness of incretin-based therapies and the risk of death and cardiovascular events in 38,233 metformin monotherapy users. Medicine. 2016 Jun;95(26) doi: 10.1097/MD.0000000000003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson JW, Bodegard J, Nathanson D, et al. Sulphonylurea compared to DPP-4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all-cause mortality. Diabetes Research and Clinical Practice. 2016 Jul 31;117:39–47. doi: 10.1016/j.diabres.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 20.Morgan CL, Mukherjee J, Jenkins-Jones S, et al. Combination therapy with metformin plus sulphonylureas versus metformin plus DPP-4 inhibitors: association with major adverse cardiovascular events and all_cause mortality. Diabetes, Obesity and Metabolism. 2014 Oct 1;16(10):977–83. doi: 10.1111/dom.12306. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen UM, Andersson C, Fosbøl EL, et al. Cardiovascular safety of combination therapies with incretin-based drugs and metformin compared with a combination of metformin and sulphonylurea in type 2 diabetes mellitus–a retrospective nationwide study. Diabetes, Obesity and Metabolism. 2014 Oct 1;16(10):1001–8. doi: 10.1111/dom.12314. [DOI] [PubMed] [Google Scholar]
- 22.Ou SM, Shih CJ, Chao PW, et al. Effects on Clinical Outcomes of Adding Dipeptidyl Peptidase-4 Inhibitors Versus Sulfonylureas to Metformin Therapy in Patients With Type 2 Diabetes MellitusDPP-4 Inhibitors Versus Sulfonylureas as Add-ons to Metformin Therapy. Annals of internal medicine. 2015 Nov 3;163(9):663–72. doi: 10.7326/M15-0308. [DOI] [PubMed] [Google Scholar]
- [April, 2014];Medicare Program - General Information. http://www.cms.gov/Medicare/Medicare-General-Information/MedicareGenInfo/index.html.
- [April, 2014];Brief Summaries of Medicare & Medicaid. 2011 http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/downloads/MedicareMedicaidSummaries2011.pdf.
- 25.Gokhale M, Buse JB, Gray CL, Pate V, Marquis MA, Stürmer T. Dipeptidyl, peptidase-4 inhibitors and pancreatic cancer: a cohort study. Diabetes, Obesity and Metabolism. 2014 doi: 10.1111/dom.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brookhart MA. Counterpoint: the treatment decision design. Am J Epidemiol. 2015;182(10):840–845. doi: 10.1093/aje/kwv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrish N, Wang S, Stevens L, Fuller J, Keen H. WHO Multinational Study Group. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(2):S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 28.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304(4):411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 29.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33(10):2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 31.Kucharska-Newton AM, Heiss G, Ni H, et al. Identification of Heart Failure Events in Medicare Claims: The Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail. 2016;22(1):48–55. doi: 10.1016/j.cardfail.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 33.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 34.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 35.Stürmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiol Drug Saf. 2006;15(10):698–709. doi: 10.1002/pds.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole SR, Lau B, Eron JJ, et al. Estimation of the standardized risk difference and ratio in a competing risks framework: application to injection drug use and progression to AIDS after initiation of antiretroviral therapy. Am J Epidemiol. 2015;181(4):238–245. doi: 10.1093/aje/kwu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [February 3, 2016];Information for Healthcare Professionals: Pioglitazone HCl (marketed as Actos, Actoplus Met, and Duetact) http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124178.htm.
- 38.Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS, Featherstone TR. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. The Lancet Diabetes & Endocrinology. 2015;3(1):43–51. doi: 10.1016/S2213-8587(14)70213-X. [DOI] [PubMed] [Google Scholar]
- 39.Forst T, Hanefeld M, Jacob S, et al. Association of sulphonylurea treatment with all-cause and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Diab Vasc Dis Res. 2013;10(4):302–314. doi: 10.1177/1479164112465442. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Wang S, Mao C, et al. Sitagliptin and cardiovascular outcomes in diabetic patients with chronic kidney disease and acute myocardial infarction: a nationwide cohort study. Int J Cardiol. 2015;181:200–206. doi: 10.1016/j.ijcard.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 41.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109(8):962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 42.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12(9):694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 43.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 44.Gokhale M, Buse J, Sturmer T. Effect of Body Mass Index on Choice of Initiating Diabetes Therapies. Pharmacoepidemiology and Drug Safety; Special Issue: Abstracts of the 30th International Conference on Pharmacoepidemiology and Therapeutic Risk Management; October 24–27, 2014; Taipei, Taiwan. p. 23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp data