Bacterial subversion of the host secretory pathway (original) (raw)
The endoplasmic reticulum (ER) can be thought of as a large, membrane-bound factory in which many of the proteins to be delivered to other cellular organelles are produced. Residing in the ER are chaperones and modifying enzymes that ensure newly manufactured proteins are packaged properly and addressed for correct delivery to other organelles. Cargo to be shipped from the ER is loaded into vesicles at specialized subdomains of the ER, sometimes called ER exit sites. Central to the processes of packaging and export of cargo from the ER is the protein known as Sar-1 and a complex of cytosolic coat proteins called CopII (1). Sar-1 is a regulatory GTPase, which means that in the GTP-bound state it is active and recruits the CopII coat to membranes, and in the GDP-bound state it is inactive. After exit, the retrieval of material back to the ER is controlled by another GTPase, called Arf, and a complex of cytosolic proteins called CopI (2). Thus, ER exit sites act as loading docks through which vesicular traffic is occurring, and this traffic is being carefully controlled by the Cops (Fig. 1 A and B). In this issue of PNAS, Celli et al. (3) make the exciting discovery that the microbial pathogen Brucella abortus is able to fool the host cell Cops and gain entry to the ER, which allows this bacterium to rob the cell of nutrients stored within this privileged site and to replicate intracellularly.
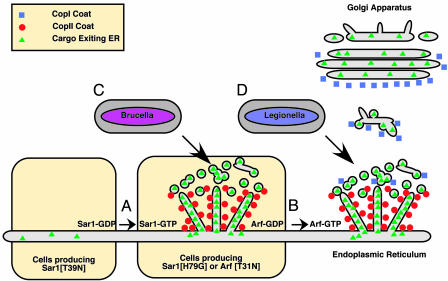
Fig. 1.
Vacuoles containing Brucella and Legionella converge on the secretory pathway at different stages. (A) Sar-1 activation leads to the recruitment of the CopII coat complex to ER membranes. CopII recruits cargo that is to be delivered to other organelles and packages the cargo into membrane tubules and vesicles. In cells producing Sar-1[T39N], CopII recruitment to membranes is inhibited, and ER exit sites do not function. In cells producing Sar-1[H79G] protein or Arf[T31N] protein, ER exit sites are functional, but cargo is not transported away from the ER. (B) Arf activation leads to the recruitment of the CopI coat complex to early secretory vesicles. The exchange of the CopII coat for the CopI coat on these membranes allows further protein sorting to occur on these vesicles and transport of cargo away from the ER. Cells are able to assemble a functional Golgi apparatus, and the secretory pathway is fully operational. (C) Vacuoles containing Brucella require the activities of the Sar-1/CopII system for productive interactions with the ER. Cells producing the Sar-1[T39N] protein are nonpermissive for Brucella replication. (D) Vacuoles containing Legionella require the sequential activities of the Sar-1/CopII system and the Arf/CopI system for productive interactions with the ER. Cells producing either Sar-1[H79G] or Arf[T31N] are nonpermissive for Legionella replication.
The ability to subvert host cellular processes to create a niche that permits intracellular replication is a common trait among intracellular pathogens (4). Whereas most organisms that enter an animal cell will remain in a membrane-bound compartment and be transported to lysosomes for destruction, many intracellular pathogens have evolved ways to avoid this fate. Some disrupt the membrane of the vacuole in which they are contained to gain direct access to the cytosol. Although there are many phospholipases and pore-forming toxins that can lyse a vacuole membrane, recent studies have suggested that nutrients essential for the growth of most bacteria are tightly sequestered in the host cytosol, making this a poor environment for the replication of microbes (5). An inhospitable cytosol might be one of the reasons many successful intracellular pathogens remain within a vacuole. However, replication within a vacuole is not easily achieved. To import nutrients and expand this vacuole, it is becoming increasingly clear that vacuoles must retain the ability to receive membrane and cargo from vesicles traveling through the host cell, which sometimes involves intercepting host secretory vesicles. For example, maintenance of vacuoles containing Chlamydia trachomatis has been shown to involve transport of lipids from the trans-Golgi network (6). Recent data show that Salmonella enterica has the ability to interact with the Golgi apparatus and that this interaction is important for intracellular replication (7). Understanding how these pathogens are able to promote interactions between the vacuole in which they reside and host secretory vesicles has become an area of important investigation.
Similar to what has been shown for Brucella, vacuoles containing the bacterium Legionella pneumophila are remodeled into ER-derived compartments (8). Despite the morphological similarities between the ER-derived vacuoles in which Legionella and Brucella replicate, the article by Celli et al. (3) indicates that the point at which these vacuoles intercept the secretory pathway of the host is different (Fig. 1 C and D). Previous studies have shown that conversion of the _Legionella_-containing vacuole into a replicative niche requires the sequential activities of both the Sar-1/CopII and Arf/CopI systems of the host cell (9). Fusion of secretory vesicles with the _Legionella_-containing vacuole also involves the proteins Sec22b and Rab1 (10, 11), host components that facilitate the docking and fusion of ER-derived vesicles with Golgi membranes. These data indicate that the _Legionella_-containing vacuole interacts with host vesicles after they have exited the ER and are in transit to other locations in the cell.
The findings of Celli et al. (3) suggest that Brucella takes a more direct route to the ER and show that interfering with the function of the Arf/CopI system has no effect on the ability of Brucella to gain access to the ER and replicate. It is known that in the absence of Arf/CopI function, secretory vesicles generated by the Sar-1/CopII system fail to mature, and there is evidence that in mammalian cells, cargo within these vesicles is incorporated back into the ER (12-14). A similar defect in transport of cargo out of the ER is observed in cells producing the Sar-1[H79G] variant, which is sometimes called a constitutively active mutant because it is defective in hydrolysis of bound GTP. Production of the Sar-1[H79G] protein prevents the removal of the CopII coat from ER-derived membranes and the subsequent exchange for the CopI coat. Thus, vesicles generated from the ER exit sites are not transported away from the ER in cells producing Sar-1[H79G] (13, 14). Celli et al. find that Brucella can still access the ER in cells producing the Sar-1[H79G] protein, providing further evidence that secretory transport from the ER is not essential for Brucella entry into the ER.
Unlike the H79G variant, the Sar-1[T39N] protein remains locked in an inactive GDP-bound state and interferes with the recruitment of CopII to ER membranes (14). Because CopII plays an important role in the recruitment of cargo proteins to ER exit sites and is directly involved in the creation of the membrane tubules and vesicles that are enriched for these cargo proteins (1), disruption of CopII function in cells producing Sar-1[T39N] interferes with ER exit site function. Surprisingly, Brucella could not access the ER in cells producing Sar-1[T39N]. These data suggest a role for the ER exit site machinery in creating specialized domains that are essential for the docking and delivery of Brucella to the ER.
Secretory transport is not essential for Brucella entry into the endoplasmic reticulum.
What might the Sar-1/CopII system be doing to facilitate access of Brucella into the ER? Most likely, the CopII coat is organizing host proteins on the ER membrane that are important for fusion of the _Brucella_-containing vacuole with the ER. The fusion of vesicles with target membranes usually involves protein-protein interactions between different soluble _N_-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins residing on the surface of the opposing membranes. Recent studies indicate that in addition to playing a role in the selection of cargo that will exit the ER, the CopII coat can also interact with SNARE proteins and direct the packaging of SNARE complexes into ER-derived vesicles (15, 16). One possibility is that in the absence of CopII function, a host SNARE protein that is important for fusion of ER with the _Brucella_-containing vacuole is not organized or presented correctly on the surface of the ER membrane. Future studies aimed at determining which, if any, of the host SNARE proteins are important for Brucella entry into the ER should help determine whether concentration of that SNARE into ER exit sites by CopII is important for fusion of the _Brucella_-containing vacuole with the ER.
How Brucella is able to modulate host membrane transport to promote interactions between the vacuole in which it resides and the ER is the other important question that remains unanswered. The data produced by Celli et al. (3) clearly indicate that the _Brucella virB_-encoded type IV secretion system is involved in this process, because mutants defective in the VirB system are unable to acquire ER membranes and replicate intracellularly. Based on similarities to other type IV secretion systems, it is assumed that the Brucella VirB system translocates bacterial proteins into the cytosol of the host cell; however, substrates translocated into host cells by the Brucella VirB system have not been identified. Transport of Legionella to the ER also requires a type IV secretion system, and a number of proteins translocated into host cells by the Legionella type IV system have been identified (17-19). Several of these factors interact specifically with host factors that facilitate fusion of ER-derived vesicles with target membranes, such as Arf and Rab1 (10, 11, 19), validating studies that revealed an important role for these host proteins in biogenesis of the organelle that supports Legionella replication. Thus, it is expected that the identification and characterization of the Brucella proteins that are translocated into host cells by the VirB system will provide important clues to the mechanism by which these bacteria are able to subvert ER exit sites and gain access to the ER.
The relative ease with which bacteria can exchange genetic material in conjunction with their longstanding associations with eukaryotic hosts has fueled the evolution of some very sophisticated mechanisms for subversion of host cellular processes. Given that we have much to learn about how vesicular transport in and out of the ER is controlled, understanding the molecular mechanisms used by pathogens such as Brucella and Legionella to co-opt host secretory organelles will provide new and important insight into how the cell controls secretory traffic. As the work by Celli et al. (3) reveals, this bacterial cops-and-robbers story is an exciting, action-packed adventure. So stay tuned. I'm sure future chapters will provide more unexpected twists and turns!
See companion article on page 1673.
References
- 1.Barlowe, C. (2000) Traffic 1**,** 371-377. [DOI] [PubMed] [Google Scholar]
- 2.Serafini, T., Orci, L., Amherdt, M., Brunner, M., Kahn, R. A. & Rothman, J. E. (1991) Cell 67**,** 239-253. [DOI] [PubMed] [Google Scholar]
- 3.Celli, J., Salcedo, S. P. & Gorvel, J.-P. (2005) Proc. Natl. Acad. Sci. USA 102**,** 1673-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finlay, B. B. & Falkow, S. (1997) Microbiol. Mol. Biol. Rev. 61**,** 136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Riordan, M. & Portnoy, D. A. (2002) Trends Microbiol. 10**,** 361-364. [DOI] [PubMed] [Google Scholar]
- 6.Hackstadt, T., Rockey, D. D., Heinzen, R. A. & Scidmore, M. A. (1996) EMBO J. 15**,** 964-977. [PMC free article] [PubMed] [Google Scholar]
- 7.Salcedo, S. P. & Holden, D. W. (2003) EMBO J. 22**,** 5003-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz, M. A. (1983) J. Exp. Med. 158**,** 1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagan, J. C. & Roy, C. R. (2002) Nat. Cell. Biol. 4**,** 945-954. [DOI] [PubMed] [Google Scholar]
- 10.Kagan, J. C., Stein, M. P., Pypaert, M. & Roy, C. R. (2004) J. Exp. Med. 199**,** 1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derre, I. & Isberg, R. R. (2004) Infect. Immun. 72**,** 3048-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scales, S. J., Pepperkok, R. & Kreis, T. E. (1997) Cell 90**,** 1137-1148. [DOI] [PubMed] [Google Scholar]
- 13.Aridor, M., Bannykh, S. I., Rowe, T. & Balch, W. E. (1995) J. Cell Biol. 131**,** 875-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward, T. H., Polishchuk, R. S., Caplan, S., Hirschberg, K. & Lippincott-Schwartz, J. (2001) J. Cell Biol. 155**,** 557-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossessova, E., Bickford, L. C. & Goldberg, J. (2003) Cell 114**,** 483-495. [DOI] [PubMed] [Google Scholar]
- 16.Miller, E. A., Beilharz, T. H., Malkus, P. N., Lee, M. C., Hamamoto, S., Orci, L. & Schekman, R. (2003) Cell 114**,** 497-509. [DOI] [PubMed] [Google Scholar]
- 17.Luo, Z. Q. & Isberg, R. R. (2004) Proc. Natl. Acad. Sci. USA 101**,** 841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, J., de Felipe, K. S., Clarke, M., Lu, H., Anderson, O. R., Segal, G. & Shuman, H. A. (2004) Science 303**,** 1358-1361. [DOI] [PubMed] [Google Scholar]
- 19.Nagai, H., Kagan, J. C., Zhu, X., Kahn, R. A. & Roy, C. R. (2002) Science 295**,** 679-682. [DOI] [PubMed] [Google Scholar]