A Novel Environmental Azole Resistance Mutation in Aspergillus fumigatus and a Possible Role of Sexual Reproduction in Its Emergence (original) (raw)
ABSTRACT
This study investigated the dynamics of Aspergillus fumigatus azole-resistant phenotypes in two compost heaps with contrasting azole exposures: azole free and azole exposed. After heat shock, to which sexual but not asexual spores are highly resistant, the azole-free compost yielded 98% (49/50) wild-type and 2% (1/50) azole-resistant isolates, whereas the azole-containing compost yielded 9% (4/45) wild-type and 91% (41/45) resistant isolates. From the latter compost, 80% (36/45) of the isolates contained the TR46/Y121F/T289A genotype, 2% (1/45) harbored the TR46/Y121F/M172I/T289A/G448S genotype, and 9% (4/45) had a novel pan-triazole-resistant mutation (TR463/Y121F/M172I/T289A/G448S) with a triple 46-bp promoter repeat. Subsequent screening of a representative set of clinical A. fumigatus isolates showed that the novel TR463 mutant was already present in samples from three Dutch medical centers collected since 2012. Furthermore, a second new resistance mutation was found in this set that harbored four TR46 repeats. Importantly, in the laboratory, we recovered the TR463 mutation from a sexual cross between two TR46 isolates from the same azole-containing compost, possibly through unequal crossing over between the double tandem repeats (TRs) during meiosis. This possible role of sexual reproduction in the emergence of the mutation was further implicated by the high level of genetic diversity of STR genotypes in the azole-containing compost. Our study confirms that azole resistance mutations continue to emerge in the environment and indicates compost containing azole residues as a possible hot spot. Better insight into the biology of environmental resistance selection is needed to retain the azole class for use in food production and treatment of Aspergillus diseases.
KEYWORDS: Aspergillus fumigatus, ascospores, azole resistance, compost heap, conidiospores, hot spot for resistance development, novel mutation, sexual reproduction
IMPORTANCE
Composting of organic matter containing azole residues might be important for resistance development and subsequent spread of resistance mutations in Aspergillus fumigatus. In this article, we show the dominance of azole-resistant A. fumigatus in azole-exposed compost and the discovery of a new resistance mutation with clinical relevance. Furthermore, our study indicates that current fungicide application is not sustainable as new resistance mutations continue to emerge, thereby threatening the use of triazoles in medicine. We provide evidence that the sexual part of the fungal life cycle may play a role in the emergence of resistance mutations because under laboratory conditions, we reconstructed the resistance mutation through sexual crossing of two azole-resistant A. fumigatus isolates derived from the same compost heap. Understanding the mechanisms of resistance selection in the environment is needed to design strategies against the accumulation of resistance mutations in order to retain the azole class for crop protection and treatment of Aspergillus diseases.
INTRODUCTION
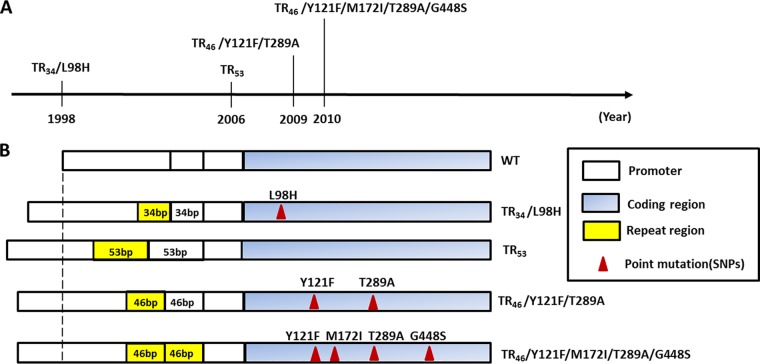
During the last decade, azole resistance has increasingly been reported in Aspergillus fumigatus, and it is now a global public health concern (1–11). Triazoles are the cornerstone of treatment of aspergillosis, and high mortality rates have been reported in patients with azole-resistant invasive aspergillosis (12–17). Azole resistance may emerge during azole therapy for patients; however, the extensive use of azole compounds in the environment is thought to be the major driver of resistance selection in A. fumigatus (18–20). The broad application of azoles for crop protection, material preservation, and clinical use has caused multiple resistance mutations to emerge. Although different azole compounds are used in the environment compared to those in medical applications, similarities in triazole molecule structure may explain the occurrence of strains that are cross-resistant to triazoles that are used for these different applications (19, 21). The majority of observed resistance mutations are caused by alterations in the cyp51A gene encoding the target protein sterol 14α-demethylase. Typically azole-resistant mutants have a combination of a tandem repeat (TR) in the promoter and single nucleotide polymorphisms (SNPs) in the coding sequence of this gene, such as TR34/L98H and TR46/Y121F/T289A (1, 10, 22–25). Figure 1 shows the history of discovery of promoter and coding region changes in the cyp51A gene of A. fumigatus in The Netherlands. These TR variants confer different azole resistance phenotypes, but most result in a pan-azole-resistant profile (26–32). The continued emergence of new resistance mutations can only be overcome if the critical steps in resistance development and selection are identified and understood.
FIG 1 .
(A) Year in which for the first time since 1998 each of the different azole-resistant A. fumigatus isolates with TR promoter mutations was observed in The Netherlands. (B) Genotype illustration of the azole-resistance mutations in the cyp51A gene (promoter and coding region).
Compost (decaying plant waste material) is believed to be an important biological niche for A. fumigatus, with high densities of conidiospores, where azole residues from agricultural waste may accumulate (33–35). Whether A. fumigatus is able to complete the whole life cycle in this harsh and competitive compost environment remains unclear. Use of variations in ways of reproduction exploiting various parts of the life cycle may be essential for resistance development, and such knowledge is thus critical for understanding potential hot spots of development, maintenance, and spread of azole resistance in A. fumigatus. We have previously shown that during azole exposure, resistance selection is enhanced when A. fumigatus reproduces asexually compared with nonsporulating controls (36). Beneficial spontaneous mutations produced during asexual reproduction were selected and accumulated over time. However, A. fumigatus can benefit from other reproduction modes, such as sexual or even parasexual reproduction, in order to create genetic variation (37). In the last decade, there has been accumulating evidence for cryptic sex in this fungus from population genetics studies, genome analysis, and the demonstration of a sexual cycle under laboratory conditions (38–44). However, direct observations or sampling of sexual structures in nature have not been reported, and the implication for resistance development is unclear.
In fungi, sexual reproduction can enhance adaptation to changing or new environments (45, 46) as sexual progeny have larger genetic variation compared with asexual populations. Greater genetic diversity is achieved through meiosis, by crossing over and chromosome reassortment. Genotyping studies of A. fumigatus show high diversity (39, 47–51), suggesting sexual reproduction in nature. In addition, both mating types are found in approximately equal proportions in such natural settings, as expected from balancing selection and Mendelian segregation in sexual crosses. Therefore, the sexual cycle, in addition to the asexual cycle, might play an important role in the ability of the fungus to adapt to the azole environment, and compost may provide the right conditions for sexual reproduction.
In this study, we investigated the azole phenotypes and genotypes of A. fumigatus in its natural habitat, the compost heap, but with contrasting azole exposures. One compost heap originated from organic waste with known azole exposure, while the other was azole free. Our hypothesis was that conditions that allow A. fumigatus to reproduce in the presence of azole pressure would select for azole-resistant A. fumigatus genotypes, whereas azole-sensitive A. fumigatus strains would be most frequent under azole-free conditions. In both settings, we searched for evidence of underlying reproduction modes, i.e., sexual versus asexual. In our opinion, insight into the dynamics of azole resistance selection of A. fumigatus in its natural habitat is essential to design strategies to prevent or reduce azole resistance development in the environment and ultimately help to manage the problem of azole-resistant Aspergillus diseases in patients.
RESULTS
Analysis of fungicide residues from the azole-free compost and azole-containing compost.
In the azole-free compost heap (Droevendaal, Wageningen, The Netherlands), no fungicide residues were detected (Table 1), while in the azole-containing compost from Hillegom, four fungicides were detected, three of which (prochloraz, prothioconazole, and tebuconazole) have been shown to cause cross-resistance to medical azoles (19) (Table 1). These fungicides are used in crop treatments—for instance for both spraying and immersion of bulbs prior to cultivation. In addition, in the azole-containing compost sample, the insecticide imidacloprid and another fungicide, boscalid, were detected. These pesticides are also used in conventional bulb cultivation in The Netherlands.
TABLE 1 .
Fungicides detected from the azole-free compost samples W5 and W6 and azole-containing compost samples H1 and H4
| Sample(s) | Detected fungicide | Fungicide concn (mg/kg) |
|---|---|---|
| W5, W6 | None | |
| H1 | Prothioconazole-desthio | 0.250 |
| Prochloraz | 0.020 | |
| Imidacloprid | 0.013 | |
| Boscalid | 0.026 | |
| H4 | Tebuconazole | 0.055 |
| Prothioconazole-desthio | 0.068 |
Isolation of A. fumigatus from azole-containing and azole-free compost before and after heat shock.
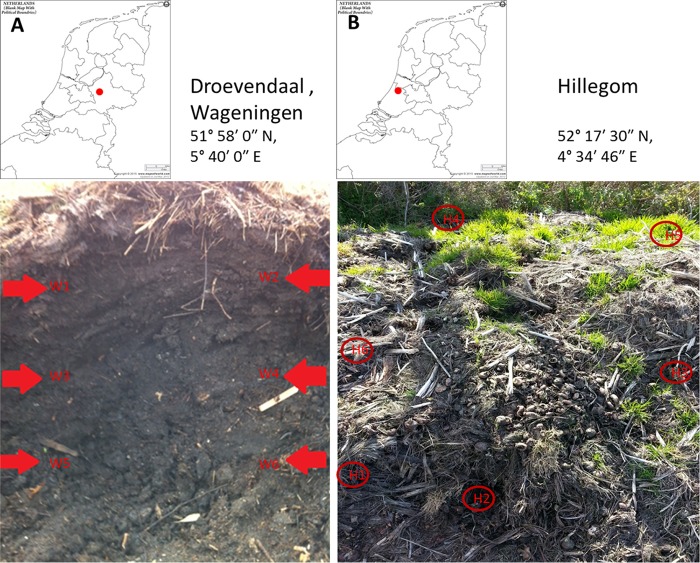
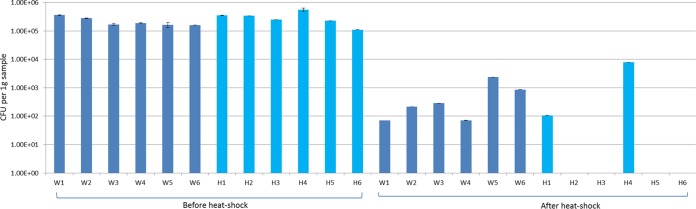
Before and after heat shock of each of the six samples from the two sample locations (Fig. 2), colonies with macroscopic and microscopic characteristics of A. fumigatus were recovered. From each sample, 20 randomly picked colonies were confirmed as A. fumigatus based on sequence analysis and their ability to grow at 48°C. Before heat shock, each sample contained more than 105 CFU. After heat shock, all six samples from the nonazole compost heap (W1 to W6) and two of the six samples from the azole compost heap (H1 and H4) yielded A. fumigatus isolates ranging from 71 to ~8,000 CFU per g of compost (Fig. 3).
FIG 2 .
Locations and positions of samples taken from azole-free compost in Wageningen (W1 to W6 [30 cm apart from top to bottom]) and azole-containing compost in Hillegom (H1 to H6).
FIG 3 .
A. fumigatus CFU density (CFU per gram of compost) before and after heat shock in samples taken at different positions in an azole-free compost heap (W1 to W6 [dark blue bars]) and an azole-containing compost heap (H1 to H6 [light blue bars]).
Azole resistance phenotypes and genotypes.
Azole-resistant A. fumigatus was found in both compost heaps, although the proportion of resistance was different, with 91% (41/45 colonies) resistance in the azole-containing compost compared to only 2% (1/50) in the azole-free compost. The genotypic variation in the cyp51A gene differed in the azole-free compost (98% wild type [WT], 2% TR34/L98H) and the azole-containing compost (9% WT, 80% TR46Y121F/T289A, and 2% TR46Y121F/M172I/T289A/G448S) (Tables 2 and 3). Moreover, in 9% of the phenotypic azole-resistant isolates, a new mutation was identified, consisting of a triple repeat of 46 bp of the promoter region that is present in duplicate in TR46 (TR463) combined with four mutations in the cyp51A gene, namely Y121F, M172I, T289A, and G448S.
TABLE 2 .
Genetic variation in azole-containing compost sample H4 from plant material waste in Hillegom, The Netherlandsa
| AZN_NMR no. | Sample | STR3 | STR4 | Cyp51A | Cyp51A SNP | Cyp51A SNP | Cyp51A SNP | Cyp51A | Mating type | Genotype × frequency | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |||||||||
| V181-07 | H1 | 33 | 10 | 23 | 6 | 13 | 8 | TR46 | Y121F | None | T289A | None | MAT1-2 | 1 × 3 |
| V181-08 | H2 | 44 | 8 | 61 | 6 | 12 | 8 | TR46 | Y121F | None | T289A | None | MAT1-2 | 2 × 1 |
| V181-09 | H3 | 44 | 8 | 7 | 10 | 9 | 7 | TR46 | Y121F | None | T289A | None | MAT1-2 | 3 × 11 |
| V181-11 | H5 | 42 | 8 | 61 | 7 | 10 | 8 | WT | None | None | None | None | MAT1-1 | 4 × 2 |
| V181-12 | H6 | 35 | 9 | 27 | 8 | 10 | 8 | WT | None | None | None | None | MAT1-1 | 5 × 1 |
| V181-13 | H7 | 44 | 8 | 12 | 10 | 8 | 18 | TR46 | Y121F | None | T289A | None | MAT1-2 | 6 × 1 |
| V181-14 | H8 | 35 | 11 | 16 | 8 | 7 | 6 | WT | None | None | NONE | None | MAT1-1 | 7 × 1 |
| V181-15 | H9 | 42 | 9 | 62 | 10 | 8 | 7 | TR46 | Y121F | None | T289A | None | MAT1-2 | 8 × 2 |
| V181-16 | H10 | 46 | 8 | 7 | 10 | 8 | 7 | TR46 | Y121F | None | T289A | None | MAT1-1 | 9 × 2 |
| V181-19 | H13 | 43 | 8 | 11 | 10 | 8 | 18 | TR46 | Y121F | None | T289A | None | MAT1-2 | 10 × 1 |
| V181-20 | H14 | 33 | 10 | 23 | 6 | 10 | 13 | TR46 | Y121F | None | T289A | None | MAT1-2 | 11 × 1 |
| V181-23 | H17 | 31 | 8 | 21 | 6 | 12 | 8 | TR46 | Y121F | None | T289A | None | MAT1-2 | 12 × 2 |
| V181-32 | H26 | 44 | 8 | 12 | 2 | 8 | 6 | TR46 | Y121F | None | T289A | None | MAT1-2 | 13 × 2 |
| V181-34 | H28 | 55 | 8 | 7 | 6 | 8 | 8 | TR46 | Y121F | None | T289A | None | MAT1-1 | 14 × 1 |
| V181-35 | H29 | 44 | 8 | 7 | 12 | 9 | 9 | TR46 | Y121F | M172I | T289A | G448S | MAT1-2 | 15 × 1 |
| V181-36 | H30 | 26 | 8 | 12 | 6 | 9 | 7 | TR463 | Y121F | M172I | T289A | G448S | MAT1-1 | 16 × 2 |
| V181-38 | H32 | 26 | 7 | 12 | 6 | 8 | 7 | TR46 | Y121F | None | T289A | None | MAT1-1 | 17 × 1 |
| V181-39 | H33 | 41 | 8 | 7 | 10 | 8 | 8 | TR463 | Y121F | M172I | T289A | G448S | MAT1-2 | 18 × 1 |
| V181-40 | H34 | 25 | 18 | 24 | 8 | 10 | 9 | TR46 | Y121F | None | T289A | None | MAT1-1 | 19 × 1 |
| V181-43 | H37 | 22 | 4 | 13 | 6 | 9 | 9 | TR463 | Y121F | M172I | T289A | G448S | MAT1-1 | 20 × 1 |
| V181-44 | H38 | 31 | 8 | 23 | 6 | 13 | 8 | TR46 | Y121F | None | T289A | None | MAT1-2 | 21 × 1 |
| V181-46 | H40 | 26 | 8 | 21 | 11 | 8 | 7 | TR46 | Y121F | None | T289A | None | MAT1-1 | 22 × 1 |
| V181-48 | H42 | 44 | 8 | 7 | 9 | 9 | 9 | TR46 | Y121F | None | T289A | None | MAT1-2 | 23 × 1 |
| V181-49 | H43 | 24 | 6 | 20 | 12 | 8 | 7 | TR46 | Y121F | None | T289A | None | MAT1-1 | 24 × 1 |
| V181-50 | H44 | 44 | 8 | 12 | 10 | 8 | 7 | TR46 | Y121F | None | T289A | None | MAT1-2 | 25 × 1 |
| V181-51 | H45 | 44 | 8 | 17 | 2 | 3 | 7 | TR46 | Y121F | None | T289A | None | MAT1-2 | 26 × 1 |
TABLE 3 .
Genetic variation in azole-free compost sample W5 from an organic plant waste compost heap in Wageningen, The Netherlandsa
| AZN_NMR no. | Sample | STR3 | STR4 | Cyp51A | Cyp51A | Mating type | Genotype × frequency | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | ||||||
| V182-18 | W1 | 34 | 9 | 8 | 7 | 8 | 31 | WT | WT | MAT1-2 | 1 × 1 |
| V182-19 | W2 | 38 | 8 | 12 | 8 | 10 | 20 | WT | WT | MAT1-1 | 2 × 1 |
| V182-20 | W3 | 30 | 8 | 11 | 8 | 9 | 20 | WT | WT | MAT1-2 | 3 × 4 |
| V182-22 | W5 | 38 | 10 | 46 | 10 | 8 | 8 | WT | WT | MAT1-1 | 4 × 1 |
| V182-23 | W6 | 29 | 7 | 11 | 9 | 9 | 18 | WT | WT | MAT1-1 | 5 × 1 |
| V182-24 | W7 | 26 | 10 | 21 | 8 | 7 | 5 | WT | WT | MAT1-2 | 6 × 1 |
| V182-25 | W8 | 30 | 8 | 11 | 8 | 9 | 20 | WT | WT | MAT1-2 | 7 × 1 |
| V182-27 | W10 | 25 | 8 | 8 | 6 | 9 | 8 | WT | WT | MAT1-2 | 8 × 1 |
| V182-28 | W11 | 13 | 18 | 8 | 7 | 26 | 5 | WT | WT | MAT1-2 | 9 × 1 |
| V182-29 | W12 | 30 | 8 | 11 | 5 | 9 | 20 | WT | WT | MAT1-2 | 10 × 1 |
| V182-30 | W13 | 9 | 8 | 8 | 8 | 8 | 6 | WT | WT | MAT1-2 | 11 × 1 |
| V182-31 | W14 | 30 | 8 | 8 | 7 | 9 | 19 | WT | WT | MAT1-1 | 12 × 2 |
| V182-32 | W15 | 13 | 8 | 10 | 8 | 8 | 3 | WT | WT | MAT1-1 | 13 × 1 |
| V182-33 | W16 | 30 | 8 | 8 | 5 | 9 | 19 | WT | WT | MAT1-1 | 14 × 1 |
| V182-34 | W17 | 27 | 8 | 30 | 7 | 6 | 5 | WT | WT | MAT1-2 | 15 × 1 |
| V182-35 | W18 | 27 | 8 | 26 | 10 | 8 | 5 | WT | WT | MAT1-2 | 16 × 4 |
| V182-36 | W19 | 24 | 8 | 8 | 8 | 11 | 33 | WT | WT | MAT1-1 | 17 × 2 |
| V182-37 | W20 | 50 | 8 | 8 | 8 | 9 | 25 | WT | WT | MAT1-2 | 18 × 1 |
| V182-38 | W21 | 10 | 8 | 8 | 5 | 7 | 18 | WT | WT | MAT1-1 | 19 × 1 |
| V182-39 | W22 | 30 | 8 | 11 | 5 | 7 | 18 | WT | WT | MAT1-2 | 20 × 1 |
| V182-40 | W23 | 36 | 10 | 30 | 22 | 9 | 8 | WT | WT | MAT1-1 | 21 × 1 |
| V182-42 | W25 | 50 | 8 | 8 | 10 | 8 | 5 | WT | WT | MAT1-1 | 22 × 1 |
| V182-43 | W26 | 8 | 7 | 11 | 7 | 8 | 10 | WT | WT | MAT1-1 | 23 × 1 |
| V182-45 | W28 | 30 | 8 | 11 | 8 | 9 | 21 | WT | WT | MAT1-2 | 24 × 1 |
| V182-46 | W29 | 25 | 8 | 21 | 14 | 8 | 5 | WT | WT | MAT1-2 | 25 × 1 |
| V182-51 | W34 | 28 | 10 | 8 | 8 | 8 | 8 | WT | WT | MAT1-1 | 26 × 1 |
| V182-52 | W35 | 29 | 16 | 8 | 10 | 26 | 5 | WT | WT | MAT1-2 | 27 × 1 |
| V182-54 | W37 | 38 | 8 | 12 | 5 | 10 | 21 | WT | WT | MAT1-1 | 28 × 1 |
| V182-55 | W38 | 32 | 8 | 8 | 7 | 9 | 19 | WT | WT | MAT1-2 | 29 × 3 |
| V182-57 | W40 | 28 | 9 | 12 | 8 | 6 | 11 | TR34 | L98H | MAT1-1 | 30 × 1 |
| V182-58 | W41 | 36 | 8 | 9 | 7 | 9 | 8 | WT | WT | MAT1-2 | 31 × 1 |
| V182-59 | W42 | 45 | 9 | 10 | 10 | 8 | 10 | WT | WT | MAT1-1 | 32 × 1 |
| V182-60 | W43 | 37 | 8 | 20 | 6 | 7 | 5 | WT | WT | MAT1-1 | 33 × 1 |
| V182-61 | W44 | 29 | 8 | 11 | 8 | 9 | 27 | WT | WT | MAT1-2 | 34 × 1 |
| V182-63 | W46 | 34 | 8 | 8 | 5 | 9 | 8 | WT | WT | MAT1-2 | 35 × 1 |
| V182-64 | W47 | 37 | 22 | 21 | 6 | 8 | 5 | WT | WT | MAT1-2 | 36 × 1 |
| V182-65 | W48 | 39 | 8 | 8 | 8 | 9 | 21 | WT | WT | MAT1-2 | 37 × 1 |
| V182-66 | W49 | 15 | 8 | 20 | 5 | 7 | 5 | WT | WT | MAT1-2 | 38 × 1 |
| V182-67 | W50 | 26 | 10 | 8 | 31 | 29 | 6 | WT | WT | MAT1-2 | 39 × 1 |
The ratio of MAT1-1 to MAT1-2 among the different genotypes was not significantly different from the expected 1:1 ratio (11:15 and 15:21 for the azole-containing and azole-free composts, respectively; chi-square values of P = 0.432 and P = 0.317). Ample genetic variation was present in both compost heaps based on six microsatellite markers; 26 unique genotypes from the azole-containing heap and 39 from the azole-free heap were identified (Tables 2 and 3).
Susceptibility testing and gene expression of TR34, TR46, and TR463.
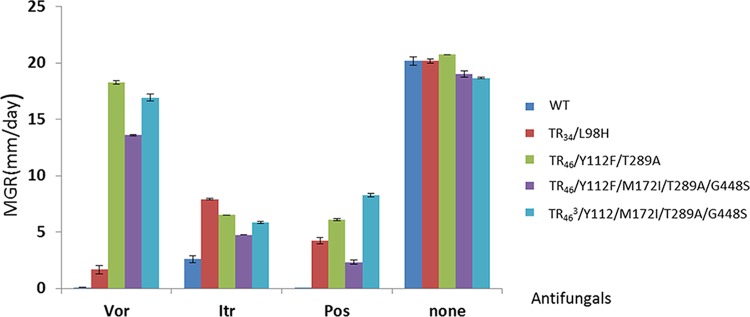
The mycelial growth rate (MGR) of the TR variants TR34, TR46, and TR463 isolated from the two compost heaps is shown in Fig. 4. TR463/Y121F/M172I/T289A/G448S exerted the fastest mycelial growth rate on medium supplemented with posaconazole, compared with TR34/L98H and TR46/Y121F/T289A (analysis of variance [ANOVA], _F_4, 10 = 369.674; P < 0.01) (Fig. 4). In addition, to compare the susceptibility of TR463 with those of TR34 and TR46, the high resistance of TR463/Y121F/M172I/T289A/G448S was confirmed by MIC testing, displaying a pan-triazole-resistant phenotype to posaconazole, itraconazole, and voriconazole (Table 4), indicating no _in vitro_ activity of itraconazole and voriconazole (MIC, >16 mg/liter) (26).
FIG 4 .
Azole-resistance assay based on MGR of the TR34, TR46, and TR463 variants against voriconazole (Vor), itraconazole (Itr), and posaconazole (Pos). MEA was supplemented with 2 mg/liter of Vor and Itr, respectively, with Pos at 0.5 mg/liter. None, without any azoles. Error bars indicate the standard error of the mean (SEM). The MGR was determined by averaging the colony diameters (in millimeters) as measured in two randomly chosen perpendicular directions.
TABLE 4 .
In vitro activities of itraconazole, voriconazole, and posaconazole against A. fumigatus isolates harboring TR463 compared with published resistance profiles of TR34, and TR46
| Isolate type | MIC range (mg/liter) fora: | Source or reference | ||
|---|---|---|---|---|
| Itr | Vor | Pos | ||
| TR34/L98H | >16 | 4–8 | 0.25–0.5 | 5 |
| TR46/Y121F/T289A | 4/16 | >16 | 0.25–2 | 21 |
| TR463/Y121F/M172I/T289A/G448S | >16 | >16 | 1 | This studyb |
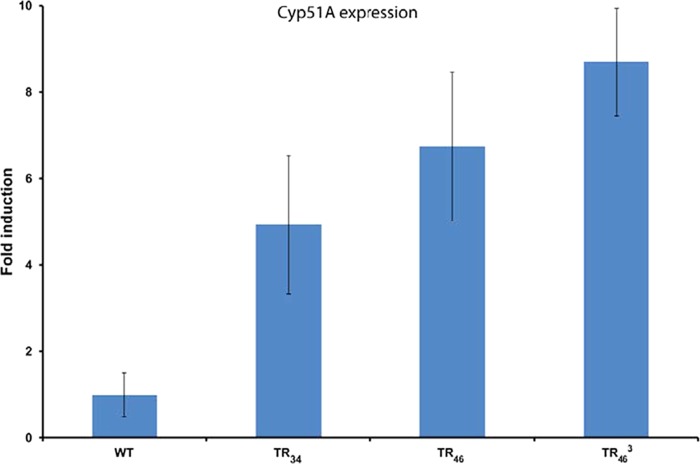
Expression levels assayed from Cyp51A/actin mRNA ratios showed that even in the absence of azoles, cyp51A gene expression levels in the TR463/Y121F/M172I/T289A/G448S strain were significantly higher than those in the WT by ANOVA (_F_3, 7 = 38.279, and P < 0.001) and post hoc least significant difference (LSD) test (P < 0.01 for TR463/Y121F/M172I/T289A/G448S versus TR34/L98H and P < 0.05 for TR463/Y121F/M172I/T289A/G448S versus TR46/Y121F/T289A), which suggests that the repeats in the promoter region are able to generate more cyp51A transcript and likely CYP51 protein to cope with azole stress and therefore contribute to a resistant phenotype (Fig. 5).
FIG 5 .
Gene expression levels of c_yp51A_ in TR variants (WT TR34, TR46, and TR463). Bars show averages from nine replicates, and error bars indicate the standard error of the mean (SEM).
Analysis of clinical isolates from the Dutch national surveillance program.
The A. fumigatus culture collection of the Dutch national Aspergillus resistance surveillance was used to determine whether TR463/Y121F/M172I/T289A/G448S was present in clinical isolates. All clinical A. fumigatus isolates collected between 2012 and 2015 that harbored a Y121F mutation were investigated for the presence of TR463 by PCR. Among 98 Y121F-harboring isolates, 3 were identified with TR463. In addition, one isolate was found with four TRs (TR464) in combination with the four mutations in the cyp51A gene: TR464/Y121F/M172I/T289A/G448S.
One TR463/Y121F/M172I/T289A/G448S isolate was recovered from a sputum sample from a 34-year-old female in 2013 in the western part of The Netherlands. The patient was hospitalized for treatment of synovial cell sarcoma with pleural metastasis. Computed tomography (CT) of the lungs showed no evidence for Aspergillus disease. A second patient was admitted to a hospital in the northwest part of The Netherlands in 2015. The patient was treated for asthma as an outpatient because of shortness of breath. Again, CT of the chest showed no evidence for Aspergillus disease.
A third patient was identified through an A. fumigatus isolate that was sent in for MIC testing. The isolate was cultured in 2012 from a 43-year-old patient with chronic obstructive pulmonary disease (COPD) admitted to a hospital in the eastern part of The Netherlands. The patient was admitted with a community-acquired pneumonia and received therapy with posaconazole in addition to antibacterial therapy. Because sputum cultures remained positive after 2 weeks of posaconazole therapy, the strain was sent for MIC testing. However, a definite diagnosis of invasive pulmonary aspergillosis could not be made.
The TR464 isolate was cultured in 2012 from the right ear of a 34-year-old male who was treated in the northern part of the country for cholesteatoma. The fungus may have contributed to the chronic infection, but there was no evidence for invasive disease.
The generation of TR463 via sexual reproduction of two TR46 strains from the azole-containing compost.
To obtain proof of principle that sexual reproduction could cause TR mutations, we performed a sexual cross between a pair of strains from the same azole-containing compost sample with the TR46/Y121F/T289A mutation but of opposite mating types (isolates H40 [MAT1-1/TR46/Y121F/T289A] and H35 [MAT1-2/TR46/Y121F/T289A]) (38, 44). After 4 months, cleistothecia were harvested, and 105 ascospores were heat shocked and plated on malt extract agar (MEA) with a high concentration of posaconazole (2 mg/liter), which showed the largest difference in MGR between TR46 (nearly no growth) and TR463 (with growth). Five hundred colonies that grew well in the presence of posaconazole were selected for sequence analysis of the cyp51A gene. Of these, 499 isolates were TR46/Y121F/T289A, but one colony contained the novel TR463/Y121F/T289A mutation. This shows that TR463 can indeed arise from a sexual cross between isolates with TR46/Y121F/T289A under laboratory conditions.
DISCUSSION
Discovery of novel azole resistance mutations and the hot spot for resistant A. fumigatus.
In this study, we observed that azole-containing compost harbored predominantly azole-resistant A. fumigatus, whereas in azole-free compost almost exclusively wild-type A. fumigatus was found. Our study suggests that azole exposure to A. fumigatus in its natural habitat at least sustains the presence of azole resistance. This, and similar habitats, could act as a “hot spot”—i.e., providing a source of azole-resistant A. fumigatus from which airborne conidia may migrate and cause disease in susceptible hosts, contribute to further azole resistance development, or both.
We identified a new resistance mutation (TR463/Y121F/M172I/T289A/G448S) in the azole-exposed compost. Analysis of clinical isolates that had been collected in the national surveillance program of The Netherlands showed that since 2012, each year at least one patient harbored an isolate with this new resistance mutation. Little is known about the generation mechanism and migration rate of resistant A. fumigatus, but the observation that the TR34/L98H variant is now the most frequently reported mutation globally (1–5, 7–10, 52, 53) suggests that spores can rapidly spread from a single source and over long distances. The TR46/Y121F/T289A mutation also showed rapid migration, as after its discovery in December 2009, the mutation was recovered from patients from six different hospitals in The Netherlands within 1 year (26). In line with these observations, the TR463/Y121F/M172I/T289A/G448S mutation was recovered from unrelated patients in three different hospitals in The Netherlands over a 3-year period, indicating that the resistance mutation might have migrated across the country, but at a slower pace compared to the other mutations. Although the mutation was found to only colonize patients, we believe that isolates harboring TR463/Y121F/M172I/T289A/G448S may ultimately cause invasive infections—potentially after accumulation of additional mutations allowing adaptation to the human host—as the resistance trait has probably survived in the environment for several years, in competition with other azole-resistant or wild-type isolates, indicating comparable fitness. This would imply that the cost of resistance of carrying TR463 is limited and/or that compensatory mutations easily emerge since otherwise selection would act against this mutation. In addition to TR463, a clinical azole-resistant isolate with four copies of TR46 was found. Although this genotype was not recovered from the environment, we believe that given the similarity of the resistance mutations to other TR46 variants, it originated via similar mechanisms to the other TR mutations, which included exposure to azole fungicides. These observations confirm our concern that new azole resistance mutations will continue to emerge in the environment if we persevere with our current practices of azole fungicide use. The azole-containing habitat may serve as an evolutionary incubator with increased selective pressure on recombination that benefits the fungus through increased fitness or increased resistance, thus facilitating its survival. With increasing azole resistance frequencies and greater diversity in resistance mutations, the consequence will be that the clinical role of medical triazoles for the management of Aspergillus diseases will be marginalized. As the arsenal of available drugs to treat Aspergillus diseases is already very limited, excess mortality due to azole resistance can be expected (54). Indeed, case studies show high mortality rates in patients with azole-resistant invasive aspergillosis (12, 16, 17, 55). Environmental resistance is especially difficult to manage as it is found in any Aspergillus disease and may occur in any patient, even those without previous azole therapy (54). Recently, mixed infections due to azole-susceptible and azole-resistant A. fumigatus strains were reported, which further complicates timely diagnosis and successful therapy (56, 57).
Our findings therefore underscore the urgent need to further investigate the evolution of A. fumigatus resistance in the environment as this will provide leads that enable us to prevent new mutations from emerging. In this way, investigation of the evolution of resistance may allow earlier detection and earlier intervention, especially in modification of patient therapy.
The role of sexual reproduction in the emergence of TR463/Y121F/M172I/T289A/G448S.
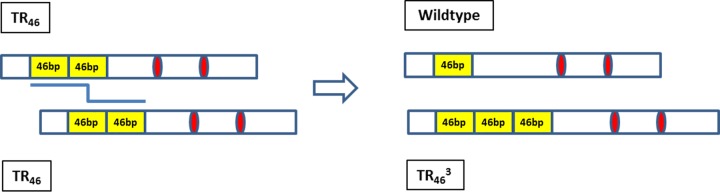
Theoretically, the isolates with TR463 mutations could have originated through both asexual and sexual reproduction. However, heat-shock treatment reduced the number of A. fumigatus CFU dramatically (Fig. 3), suggesting that most, if not all, conidiospores were killed and leaving only the heat-resistant ascospores in the compost sample. Furthermore, a high genetic diversity was found in the heat-shocked compost samples, as shown in Tables 2 and 3 for microsatellite loci and mating-type genes, which is indicative of sampling unique sexual spores rather than clonal conidiospores. Importantly, within the population the high microsatellite diversity and equal mating-type distribution are both consistent with sexual reproduction. Through sexual recombination, a multitude of combinations of microsatellite markers and mating-type loci can be generated, whereas balancing selection for the mating-type locus and Mendelian segregation accounts for equal distribution of the two mating types. Clonal expansion by asexual reproduction on the other hand would result in a population with low genetic variation and an unbalanced mating-type ratio. Here, we found TR463 isolates of either mating type and of different microsatellite types. Therefore, we expected that the novel TR463 genotype originated from a sexual cross; we tested progeny from a cross between two TR46 strains and were indeed able to recover this genotype. One mechanism that may underpin this new resistance genotype may have been unequal meiotic crossing over, as illustrated in Fig. 6 (58). The mispairing in the repeat region of 46 bp during the meiotic process may lead to even longer repeats in the promoter. As expected, the TR463 strain obtained from the cross contained only the two mutations Y121F and T289A present in the parental TR46 strains. The two additional mutations M172I and G448S, which are found in the natural TR463 isolates, must then have come from parental strains with TR46, of which one or both have all four mutations. Thus, this important observation suggests the potential occurrence of sex in the compost.
FIG 6 .
A possible scenario indicating how unequal crossing over in a sexual cross between two strains with a double repeat TR46 can result in a rare meiotic recombinant with the triple repeat TR463. Yellow boxes represent the tandem repeats in the promoter region of cyp51A gene, and red ovals represent point mutations in the coding region of the cyp51A gene. The bent blue line indicates unequal crossover.
In addition to these observations that support the notion of sexual reproduction, it is important to note that compost heaps provide favorable conditions for sex. Several key factors for sexual reproduction that have been identified under laboratory conditions seem to be present in compost (38). Compost contains multiorganic resources, with inside the heap a warm, dark, low-O2, and high-CO2 environment as a result of biological metabolic activity. A dynamic composting process with temperature gradients (20 to 70°C) and gas changes might therefore stimulate sexual reproduction of A. fumigatus (45, 59). The extent to which these favorable conditions for sex are present in the compost may differ for specific compost samples, which may explain the difference in number of ascospores between the two compost samples after heat shock (Fig. 3).
However, we are aware that the presence of sexual reproduction was not definitively proven in our study. The effectiveness of the heat shock treatment was proven with a suspension containing ascospores, but not asexual conidiospores, that were able to survive a heat shock of 70°C for 1 h (38, 60). However, whether the same effectiveness can be reached in a complex matrix such as compost has not yet been determined. We found that although indeed the ascospores were heat resistant in compost, not all of the 105 conidiospores were killed after 1 h at 70°C (J. Zhang, personal observation). A previous study has also shown that a population of conidiospores may not completely be killed by heat shock, depending on many factors, such as the freshness and concentration of conidiospores, the type of strain, and the pH (61). Therefore, we cannot definitively conclude that all colonies derived after heat shock of compost material have originated from ascospores. However, the significant reduction in the number of CFU of A. fumigatus after heat shock indicates that most if not all, conidiospores were killed. Furthermore, the observed genotype distribution among isolates obtained from heat-treated compost (Table 2) provides strong evidence that these isolates do originate from recombination through sexual reproduction. Direct light microscopic observation of ascospores in compost samples failed as they could not be clearly recognized, even after addition of ascospores (unpublished observations).
Conclusions and future outlook.
Our study shows that azole-exposed compost can serve as a hot spot as almost exclusively azole-resistant phenotypes were found there. In addition to known resistance mutations, we found two new mutations and evidence that these might evolve through sexual reproduction. Our data clearly indicate that the full life cycle of A. fumigatus needs to be taken into account to explain the emergence of azole resistance. The continued emergence of azole resistance mutations warrants research into the mechanisms of resistance selection in the environment. Insights into key factors involved in the selection or spread of resistance in A. fumigatus will help to develop strategies that prevent resistance selection. Only then can this important class be rescued for use for crop protection as well as treatment of Aspergillus diseases.
MATERIALS AND METHODS
Sampling and screening of A. fumigatus in azole-free and azole-containing compost.
Six samples were collected from each of two compost heaps: one originating from waste containing azoles (Hillegom, The Netherlands) and one free of azoles (Wageningen, The Netherlands) (Fig. 2). To document the presence of azole fungicides, two samples from each compost heap were analyzed for fungicide residues using gas chromatography-tandem mass spectrometry (GC-MS/MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) by Eurofins lab—Zeeuws Vlaanderen (http://www.labzvl.nl/en).
Samples of 1 g compost were added to 10 ml sterile saline with 0.05% Tween and screened for the presence of A. fumigatus by plating 50-µl samples on malt extract agar (MEA [36]) supplemented with two antibiotics (10 µg/ml streptomycin and 15 µg/ml tetracycline [Sigma-Aldrich, Germany]) and incubated at 37°C. Ten randomly selected colonies from each compost heap, which all showed Aspergillus morphology, were identified as A. fumigatus genetically by sequencing of the β-tubulin and carboxypeptidase-5 genes (38) and phenotypically by their capacity to grow at 48°C. The presence of A. fumigatus ascospores was investigated by subjecting the sample to heat shock (1 h at 70°C) before plating. This heat shock procedure has been shown to eliminate and greatly reduce A. fumigatus conidiospores (resulting from asexual reproduction), but not ascospores (resulting from sexual reproduction) (38, 41). As a consequence, any colony growing after heat shock is likely to have originated from a sexual spore. After 2 days, the colonies were counted and the survival rate was established.
Azole resistance phenotypes and genotypes of A. fumigatus cultures from compost.
As we were focused in this study on the sexual route of resistance development, we randomly picked 50 presumed ascospore-derived isolates after heat shock from a W5 (azole-free) compost sample and an H4 (azole-containing) compost sample with the highest A. fumigatus count. These isolates were genotyped for six microsatellite markers (STR3 A, B, and C and STR4 A, B, and C) and the cyp51A gene and its promoter region (62–64). The mating type was determined by sequencing the mating-type gene (40).
Susceptibility testing and cyp51A gene expression of TR variants (TR34, TR46, and TR463) found from compost.
Since we previously found that the level of resistance based on mycelial growth rate (MGR) is highly correlated with the results from the MIC test, here we used the more straightforward and reproducible MGR measurement as described before (36). The MGR of TR variants (TR34, TR46, and TR463) that were isolated from the compost heaps was assayed under the condition of exposure to three medical triazoles (itraconazole, voriconazole, and posaconazole). In addition, to compare the susceptibility of TR463 with those of TR34 and TR46, the high resistance of TR463 was confirmed by MIC testing, using a broth microdilution method according to EUCAST protocol E.DEF 60 9.2 (36, 65). The cyp51A expression was analyzed from duplicate cultures of three different strains per TR variant. Expression levels were calculated from Cyp51A/actin mRNA ratios and normalized for wild-type (WT) expression levels (25).
Clinical implications of environmental azole resistance mutations.
In five Dutch University Medical Centres, clinical A. fumigatus isolates are routinely screened for azole-resistant phenotype using agar supplemented with medical triazoles. Isolates that grow in the presence of azoles were sent to the National Mycology Reference Laboratory at Radboud University Medical Centre for MIC testing and genotypic characterization. Screening for triazole resistance mutations was performed using the Y121F mutation for the TR46/Y121F/T289A genotype and L98H for the TR34/L98H genotype, respectively (66). These two mutations have been shown to account for over 80% of clinical triazole resistance. If none of these mutations were found in the resistant isolates, the full cyp51A gene was sequenced. In this study, for all L98H- and Y121F-positive isolates collected between 2012 and 2015, the accompanying TR was determined by PCR analysis (67), and for selected isolates, relevant clinical information was retrieved.
Sexual cross between two TR46 strains of opposite mating types from the same azole-containing compost.
As our results indicated a possible involvement of sexual reproduction in the development of the new resistance mutation, we investigated the possibility that TR463 mutations arise through sexual reproduction, so a pair of strains from the same azole-containing compost sample with the TR46 resistance mutation and of the opposite mating type (isolates H40 [MAT1-1/TR46/Y121F/T289A] and H35 [MAT1-2/TR46/Y121F/T289A]) were used for a sexual cross following the protocol of O’Gorman et al. (38). After 4 months, cleistothecia were harvested, and 105 ascospores were heat shocked and plated on MEA with posaconazole (2 mg/liter) for selection of TR463.
Statistical analysis.
We tested the significance of differences in MGR and gene expression of the cyp51A gene of the different TR variants using a one-way ANOVA with number of repeats as a nominal factor. Pairwise differences between variants were tested post hoc using Fisher’s least significant difference (LSD). Whether the mating-type distribution in the two composts was a deviation from a 1:1 ratio was tested using a chi-square test.
ACKNOWLEDGMENTS
We gratefully acknowledge funding from the China Scholarship Council to J.Z.
We thank Bertha Koopmanschap and Marijke Slakhorst from the Genetics Laboratory of Wageningen University and Ton Rijs from the Department of Medical Microbiology, Radboud University Medical Centre, for technical assistance. We thank Peter Leendertse from CLM for the azole analyses in compost samples.
Footnotes
Citation Zhang J, Snelders E, Zwaan BJ, Schoustra SE, Meis JF, van Dijk K, Hagen F, van der Beek MT, Kampinga GA, Zoll J, Melchers WJG, Verweij PE, Debets AJM. 2017. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. mBio 8:e00791-17. https://doi.org/10.1128/mBio.00791-17.
REFERENCES
- 1.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendrup MC, Mavridou E, Mortensen KL, Snelders E, Frimodt-Møller N, Khan H, Melchers WJ, Verweij PE. 2010. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS One 5:e10080. doi: 10.1371/journal.pone.0010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother 55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortensen KL, Jensen RH, Johansen HK, Skov M, Pressler T, Howard SJ, Leatherbarrow H, Mellado E, Arendrup MC. 2011. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol 49:2243–2251. doi: 10.1128/JCM.00213-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller M, Boyken L, Hollis R, Kroeger J, Messer S, Tendolkar S, Diekema D. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Aspergillus species to the triazoles. J Clin Microbiol 49:586–590. doi: 10.1128/JCM.02136-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. 2012. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J Antimicrob Chemother 67:362–366. doi: 10.1093/jac/dkr443. [DOI] [PubMed] [Google Scholar]
- 7.Morio F, Aubin GG, Danner-Boucher I, Haloun A, Sacchetto E, Garcia-Hermoso D, Bretagne S, Miegeville M, Le Pape P. 2012. High prevalence of triazole resistance in Aspergillus fumigatus, especially mediated by TR/L98H, in a French cohort of patients with cystic fibrosis. J Antimicrob Chemother 67:1870–1873. doi: 10.1093/jac/dks160. [DOI] [PubMed] [Google Scholar]
- 8.Gisi U. 2014. Assessment of selection and resistance risk for demethylation inhibitor fungicides in Aspergillus fumigatus in agriculture and medicine: a critical review. Pest Manag Sci 70:352–364. doi: 10.1002/ps.3664. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhary A, Sharma C, Hagen F, Meis JF. 2014. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol 9:697–711. doi: 10.2217/fmb.14.27. [DOI] [PubMed] [Google Scholar]
- 10.Wiederhold NP, Gil VG, Gutierrez F, Lindner JR, Albataineh MT, McCarthy DI, Sanders C, Fan H, Fothergill AW, Sutton DA. 2016. First detection of TR34/L98H and TR46/Y121F/T289A Cyp51 mutations in Aspergillus fumigatus isolates in the United States. J Clin Microbiol 54:168–171. doi: 10.1128/JCM.02478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meis JF, Chowdhary A, Rhodes JL, Fisher MC, Verweij PE. 2016. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos Trans R Soc Lond B Biol Sci 371:20150460. doi: 10.1098/rstb.2015.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, Debets-Ossenkopp YJ, Kuijper EJ, Van Tiel FH, Melchers WJ, Verweij PE. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis 17:1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Linden JW, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJ, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2015. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front Microbiol 6:428. doi: 10.3389/fmicb.2015.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Paassen J, Russcher A, In’t Veld-van Wingerden AW, Verweij PE, Kuijper EJ. 2016. Emerging aspergillosis by azole-resistant Aspergillus fumigatus at an intensive care unit in the Netherlands, 2010 to 2013. Euro Surveill 21:30300. doi: 10.2807/1560-7917.ES.2016.21.30.30300. [DOI] [PubMed] [Google Scholar]
- 17.Steinmann J, Hamprecht A, Vehreschild M, Cornely O, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis J, Rath P-M. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 18.Faria-Ramos I, Farinha S, Neves-Maia J, Tavares PR, Miranda IM, Estevinho LM, Pina-Vaz C, Rodrigues AG. 2014. Development of cross-resistance by Aspergillus fumigatus to clinical azoles following exposure to prochloraz, an agricultural azole. BMC Microbiol 14:155. doi: 10.1186/1471-2180-14-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snelders E, Camps SM, Karawajczyk A, Schaftenaar G, Kema GH, van der Lee HA, Klaassen CH, Melchers WJ, Verweij PE. 2012. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 7:e31801. doi: 10.1371/journal.pone.0031801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowdhary A, Kathuria S, Xu J, Meis JF. 2013. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 9:e1003633. doi: 10.1371/journal.ppat.1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodriguez-Tudela JL. 2003. A point mutation in the 14α-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 47:1120–1124. doi: 10.1128/AAC.47.3.1120-1124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. 2004. Substitutions at methionine 220 in the 14α-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob Agents Chemother 48:2747–2750. doi: 10.1128/AAC.48.7.2747-2750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Li H, Li R, Bu D, Wan Z. 2005. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J Antimicrob Chemother 55:31–37. doi: 10.1093/jac/dkh507. [DOI] [PubMed] [Google Scholar]
- 25.Snelders E, Camps SMT, Karawajczyk A, Rijs AJMM, Zoll J, Verweij PE, Melchers WJG. 2015. Genotype-phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal Genet Biol 82:129–135. doi: 10.1016/j.fgb.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 26.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen E, Maertens J, Schoemans H, Lagrou K. 2012. Azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation emerging in Belgium, July 2012. Euro Surveill 17:20326http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20326. [PubMed] [Google Scholar]
- 28.Pelaez T, Monteiro MC, Garcia-Rubio R, Bouza E, Gomez-Lopez A, Mellado E. 2015. First detection of Aspergillus fumigatus azole-resistant strain due to Cyp51A TR46/Y121F/T289A in an azole-naive patient in Spain. New Microbes New Infect 6:33–34. doi: 10.1016/j.nmni.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavergne RA, Morio F, Favennec L, Dominique S, Meis JF, Gargala G, Verweij PE, Le Pape P. 2015. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother 59:4331–4335. doi: 10.1128/AAC.00127-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astvad KM, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, Christensen M, Hilberg O, Arendrup MC. 2014. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother 58:5096–5101. doi: 10.1128/AAC.02855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verweij PE, Kema GH, Zwaan B, Melchers WJ. 2013. Triazole fungicides and the selection of resistance to medical triazoles in the opportunistic mould Aspergillus fumigatus. Pest Manag Sci 69:165–170. doi: 10.1002/ps.3390. [DOI] [PubMed] [Google Scholar]
- 32.Verweij PE, van de Sande-Bruisma N, Kema GH, Melchers WJ. 2012. Azole resistance in Aspergillus fumigatus in the Netherlands—increase due to environmental fungicides? Ned Tijdschr Geneeskd 156:A4458 (In Dutch.). [PubMed] [Google Scholar]
- 33.Millner PD, Bassett DA, Marsh PB. 1980. Dispersal of Aspergillus fumigatus from sewage sludge compost piles subjected to mechanical agitation in open air. Appl Environ Microbiol 39:1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millner PD, Marsh PB, Snowden RB, Parr JF. 1977. Occurrence of Aspergillus fumigatus during composting of sewage sludge. Appl Environ Microbiol 34:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Moreno C, Lavergne R-A, Hagen F, Morio F, Meis JF, Le Pape P. 2017. Azole-resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci Rep 7:45631. doi: 10.1038/srep45631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Debets AJ, Verweij PE, Melchers WJ, Zwaan BJ, Schoustra SE. 2015. Asexual sporulation facilitates adaptation: the emergence of azole resistance in Aspergillus fumigatus. Evolution 69:2573–2586. doi: 10.1111/evo.12763. [DOI] [PubMed] [Google Scholar]
- 37.Verweij PE, Zhang J, Debets AJ, Meis JF, van de Veerdonk FL, Schoustra SE, Zwaan BJ, Melchers WJ. 2016. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: a dilemma for clinical management. Lancet Infect Dis 16:e251–e260. doi: 10.1016/S1473-3099(16)30138-4. [DOI] [PubMed] [Google Scholar]
- 38.O’Gorman CM, Fuller HT, Dyer PS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474. doi: 10.1038/nature07528. [DOI] [PubMed] [Google Scholar]
- 39.Varga J, Tóth B. 2003. Genetic variability and reproductive mode of Aspergillus fumigatus. Infect Genet Evol 3:3–17. doi: 10.1016/S1567-1348(02)00156-9. [DOI] [PubMed] [Google Scholar]
- 40.Paoletti M, Rydholm C, Schwier EU, Anderson MJ, Szakacs G, Lutzoni F, Debeaupuis JP, Latgé JP, Denning DW, Dyer PS. 2005. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr Biol 15:1242–1248. doi: 10.1016/j.cub.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 41.Kwon-Chung KJ, Sugui JA. 2009. Sexual reproduction in aspergillus species of medical or economical importance: why so fastidious? Trends Microbiol 17:481–487. doi: 10.1016/j.tim.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dyer PS, O’Gorman CM. 2012. Sexual development and cryptic sexuality in fungi: insights from Aspergillus species. FEMS Microbiol Rev 36:165–192. doi: 10.1111/j.1574-6976.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 43.Dyer PS, O’Gorman CM. 2011. A fungal sexual revolution: Aspergillus and Penicillium show the way. Curr Opin Microbiol 14:649–654. doi: 10.1016/j.mib.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Camps SM, Dutilh BE, Arendrup MC, Rijs AJ, Snelders E, Huynen MA, Verweij PE, Melchers WJ. 2012. Discovery of a hapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One 7:e50034. doi: 10.1371/journal.pone.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aanen DK, Hoekstra RF. 2007. Why sex is good: on fungi and beyond. Chapter 32. _In_Heitman J, Kronstad JW, Taylor JW, Casselton LA (ed), Sex in fungi: molecular determination and evolutionary implications. ASM Press, Washington, DC. [Google Scholar]
- 46.McDonald MJ, Rice DP, Desai MM. 2016. Sex speeds adaptation by altering the dynamics of molecular evolution. Nature 531:233–236. doi: 10.1038/nature17143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Losada L, Sugui JA, Eckhaus MA, Chang YC, Mounaud S, Figat A, Joardar V, Pakala SB, Pakala S, Venepally P, Fedorova N, Nierman WC, Kwon-Chung KJ. 2015. Genetic analysis using an isogenic mating pair of Aspergillus fumigatus identifies azole resistance genes and lack of MAT locus’s role in virulence. PLoS Pathog 11:e1004834. doi: 10.1371/journal.ppat.1004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pena GA, Coelho I, Reynoso MM, Soleiro C, Cavaglieri LR. 2015. Characterization and genetic variability of feed-borne and clinical animal/human Aspergillus fumigatus strains using molecular markers. Med Mycol 53:699–708. doi: 10.1093/mmy/myv040. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira J, Amorim A, Araujo R. 2015. Recombination detection in Aspergillus fumigatus through single nucleotide polymorphisms typing. Environ Microbiol Rep 7:881–886. doi: 10.1111/1758-2229.12321. [DOI] [PubMed] [Google Scholar]
- 50.Ashu EE, Hagen F, Chowdhary A, Meis JF, Xu J. 2017. Global population genetic analysis of Aspergillus fumigatus. mSphere 2:e00019-17. doi: 10.1128/mSphere.00019-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdolrasouli A, Rhodes J, Beale MA, Hagen F, Rogers TR, Chowdhary A, Meis JF, Armstrong-James D, Fisher MC. 2015. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. mBio 6:e00536-15. doi: 10.1128/mBio.00536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bignell E. 2014. Aspergillus fumigatus: saprotroph to pathogen, p 19–43. _In_Kurzai O. (ed), Human fungal pathogens. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 53.Chowdhary A, Kathuria S, Xu J, Sharma C, Sundar G, Singh PK, Gaur SN, Hagen F, Klaassen CH, Meis JF. 2012. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR 34/L98H mutations in the cyp 51A gene in India. PLoS One 7:e52871. doi: 10.1371/journal.pone.0052871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verweij PE, Chowdhary A, Melchers WJG, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuhren J, Voskuil W, Boel C, Haas P, Hagen F, Meis JF, Kusters J. 2015. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J Antimicrob Chemother 70:2894–2898. doi: 10.1093/jac/dkv177. [DOI] [PubMed] [Google Scholar]
- 56.Kolwijck E, van der Hoeven H, de Sévaux RG, ten Oever J, Rijstenberg LL, van der Lee HA, Zoll J, Melchers WJ, Verweij PE. 2016. Voriconazole-susceptible and voriconazole-resistant Aspergillus fumigatus coinfection. Am J Respir Crit Care Med 193:927–929. doi: 10.1164/rccm.201510-2104LE. [DOI] [PubMed] [Google Scholar]
- 57.Ahmad S, Joseph L, Hagen F, Meis JF, Khan Z. 2015. Concomitant occurrence of itraconazole-resistant and -susceptible strains of Aspergillus fumigatus in routine cultures. J Antimicrob Chemother 70:412–415. doi: 10.1093/jac/dku410. [DOI] [PubMed] [Google Scholar]
- 58.Jeffreys AJ, Wilson V, Thein SL. 1985. Hypervariable“minisatellite” regions in human DNA. Nature 314:67–73. [DOI] [PubMed] [Google Scholar]
- 59.Chamberlain M, Ingram DS. 1997. The balance and interplay between asexual and sexual reproduction in fungi. Adv Bot Res 24:71–73. [Google Scholar]
- 60.Sugui JA, Losada L, Wang W, Varga J, Ngamskulrungroj P, Abu-Asab M, Chang YC, O’Gorman CM, Wickes BL, Nierman WC, Dyer PS, Kwon-Chung KJ. 2011. Identification and characterization of an Aspergillus fumigatus “supermater” pair. mBio 2:e00234-11. doi: 10.1128/mBio.00234-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsukahara T. 1975. Studies on heat resistance of conidiospores of Aspergillus fumigatus. I. Qualitative observation of the resistance. Jpn J Med Mycol 16:27–34. doi: 10.3314/jjmm1960.16.27. [DOI] [Google Scholar]
- 62.de Valk HA, Meis JF, Curfs IM, Muehlethaler K, Mouton JW, Klaassen CH. 2005. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J Clin Microbiol 43:4112–4120. doi: 10.1128/JCM.43.8.4112-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balajee SA, Tay ST, Lasker BA, Hurst SF, Rooney AP. 2007. Characterization of a novel gene for strain typing reveals substructuring of Aspergillus fumigatus across North America. Eukaryot Cell 6:1392–1399. doi: 10.1128/EC.00164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snelders E, Huis In’t Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camps SM, Rijs AJ, Klaassen CH, Meis JF, O’Gorman CM, Dyer PS, Melchers WJ, Verweij PE. 2012. Molecular epidemiology of Aspergillus fumigatus isolates harboring the TR34/L98H azole resistance mechanism. J Clin Microbiol 50:2674–2680. doi: 10.1128/JCM.00335-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammadi F, Hashemi SJ, Zoll J, Melchers WJ, Rafati H, Dehghan P, Rezaie S, Tolooe A, Tamadon Y, van der Lee HA, Verweij PE, Seyedmousavi S. 2015. Quantitative analysis of single-nucleotide polymorphism for rapid detection of TR34/L98H- and TR46/Y121F/T289A-positive Aspergillus fumigatus isolates obtained from patients in Iran from 2010 to 2014. Antimicrob Agents Chemother 60:387–392. doi: 10.1128/AAC.02326-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, Melchers WJ. 2012. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob Agents Chemother 56:10–16. doi: 10.1128/AAC.05088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]