Prader-Willi Syndrome: Current Understanding of Cause and Diagnosis (original) (raw)
. Author manuscript; available in PMC: 2017 Jun 30.
Published in final edited form as: Am J Med Genet. 1990 Mar;35(3):319–332. doi: 10.1002/ajmg.1320350306
Abstract
Prader-Willi syndrome (PWS) is characterized by hypotonia, obesity, hypogonadism, short stature, small hands and feet, mental deficiency, a characteristic face, and an interstitial deletion of the proximal long arm of chromosome 15 in about one-half of the patients. The incidence is estimated to be about 1 in 25,000, and PWS is the most common syndromal cause of human obesity. DNA abnormalities, usually deletions or duplications of chromosome 15, have been identified in individuals with PWS with or without recognizable chromosome 15 deletions. Paternal origin of the chromosome 15 deletion by cytogenetic and DNA studies has been found in nearly all PWS individuals studied. No cytogenetic evidence for chromosome breakage has been identified, although an environmental cause (e.g., paternal hydrocarbon-exposed occupations) of the chromosome 15 abnormality has been proposed. PWS patients with the chromosome 15 deletion are more prone to hypopigmentation compared with PWS individuals with normal chromosomes, but no other clinical differences are consistently identified between those with and without the chromosome deletion. Anthropometric, dermatoglyphic, and other clinical findings indicate homogeneity of PWS patients with the chromosome deletion and heterogeneity of the nondeletion patients. A review of our current understanding of the major clinical, cytogenetic, and DNA findings is presented, and clinical manifestations and cytogenetic abnormalities are summarized from the literature.
Keywords: clinical manifestations, cytogenetic findings, DNA probes, review
BACKGROUND
Introduction
The Prader-Willi syndrome (PWS) was first described by Prader et al. [1956]; subsequently, over 700 cases have been reported. PWS, generally sporadic in occurrence, is characterized by infantile hypotonia (94%), early onset of childhood obesity (94%), mental deficiency (average IQ of 65, range from 20 to 90; 97%), short stature (76%), small hands and feet (83%), hypogenitalism/hypogonadism (95%), a characteristic face (e.g., narrow bifrontal diameter, almond-shaped eyes, and triangular mouth) and an interstitial deletion of chromosome 15 (q11–13) in about one-half of the patients [Laurance, 1967; Dunn, 1968; Zellweger and Schneider, 1968; Hall and Smith, 1972; Dunn et al., 1981; Ledbetter et al., 1982; Mattei et al., 1983; Bray et al., 1983; Cassidy et al., 1984; Fukushima et al., 1984; Niikawa and Ishikiriyama, 1985; Fear et al., 1985; Takano et al., 1986; Butler et al., 1986; Wenger et al., 1987; Greenswag, 1987]. Figure 1 shows the chromosome 15 deletion recognized in about one-half of PWS patients. Figure 2 shows typical clinical manifestations of two individuals with PWS (one with and one without the chromosome 15 deletion). The incidence of PWS is estimated to be about 1 in 25,000 live births [Zellweger and Soper, 1979], and it is the most common syndromal cause of marked human obesity. A review of our current understanding of the major clinical, cytogenetic, and molecular manifestations of this unique syndrome will be presented.
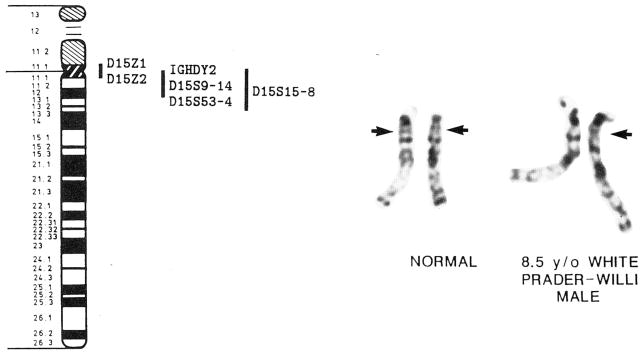
Fig. 1.
A prometaphase chromosome 15 idiogram (850 band level) and representative prometaphase chromosomes of a normal control individual and a Prader-Willi syndrome individual with an interstitial deletion of chromosome 15 are shown. The 15q12 band is indicated by the arrow in each of the normal chromosomes. Known DNA probes and loci on proximal 15q(11–13) [Cox and Gedde-Dahl, 1988; Donlon, 1988; Nicholls et al., 1989]. D15S15–18 lie outside of 15q11.1–12 but within 15q11–13. IGHDY2, immunoglobulin heavy chain diversity region 2.
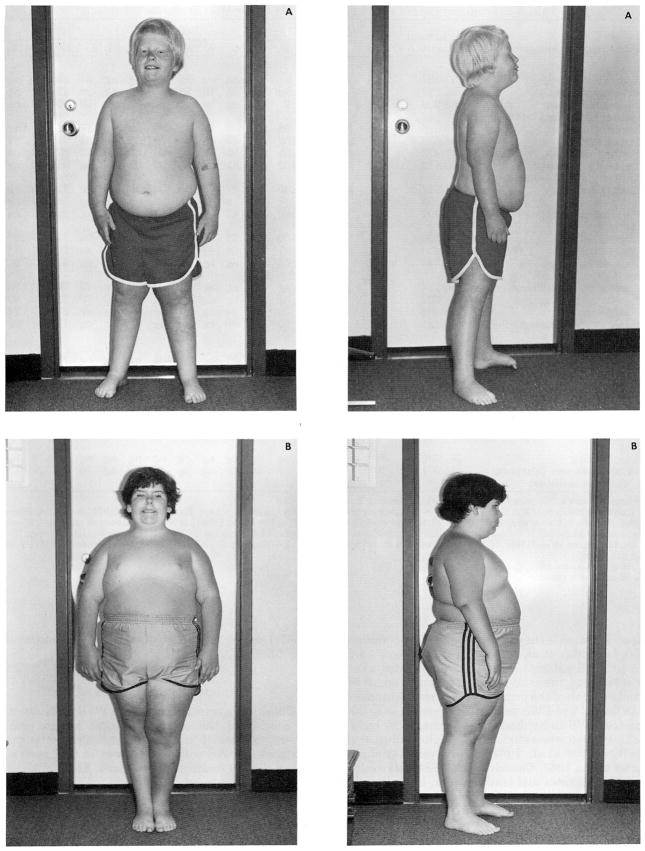
Fig. 2.
Frontal and profile views of two males (patient A is 8.5 years of age, with the chromosome 15[q11–13] deletion; patient B is 11 years of age, with apparently normal chromosomes) with Prader-Willi syndrome. Note the typical facial appearance (e.g., narrow bifrontal diameter, almond-shaped eyes, triangular mouth), small hands and feet, characteristic obesity, and hypopigmentation (in patient A).
Clinical Stages and Cytogenetic Findings
The course and natural history of PWS can be divided into two distinct clinical stages. The first stage is characterized by varying degrees of hypotonia during the neonatal period and early infancy, a weak cry, hypothermia, hypogenitalism and a poor suck reflex [Hall and Smith, 1972; Zellweger, 1981; Butler et al., 1986; Greenberg et al., 1987]. The hypotonia is central in origin, nonprogressive, and, on the average, begins to improve between 8 and 11 months of age [Hall and Smith, 1972; Butler et al., 1986]. Electromyograms, motor nerve conduction velocity, serum creatinine phosphokinase, and results of light microscopy of muscles are usually normal [Holm, 1981], while specialized histochemistry studies of muscles demonstrate type II fiber atrophy consistent with disuse [Afifi and Zellweger, 1969]. Sucking and feeding difficulties in PWS infants usually require gavage feeding. As muscle tone improves and the child becomes more alert, an increased appetite and weight gain develop, which characterizes the beginning of the second stage of PWS.
The second stage, which usually occurs between ages 1 and 2 years, is characterized by psychomotor retardation, with an average onset for crawling, walking, and talking (>10 words) at 16, 28, and 39 months, respectively, and early onset of childhood obesity [Hall and Smith, 1972; Holm, 1981; Butler et al., 1986; Wenger et al., 1987]. Other recognized findings seen in PWS individuals during the second stage include speech articulation problems, foraging for food, rumination, unmotivated sleepiness, physical inactivity, decreased pain sensitivity, picking at sores and insect bites, prolonged periods of hyperthermia, hypopigmentation, and skeletal and dental problems. Clinical manifestations in 538 individuals who were diagnosed with PWS and summarized from the literature are shown in Table I.
TABLE I.
Frequency (%) of Clinical findings in 538 Prader-Willi Syndrome Individuals*
| Clinical manifestations | Affected/total patients | Overall | Referencesa | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |||
| Gestation | |||||||||||||||||
| Reduced fetal activity | 137/181 | 76 | ngb | 56 | ng | 74 | 56 | ng | 85 | 84 | ng | ng | 61 | 83 | 86 | ng | ng |
| Breech presentation | 56/212 | 26 | 33 | 22 | ng | 40 | 25 | 12 | 22 | 38 | ng | ng | 28 | 22 | 30 | ng | ng |
| Nonterm delivery | 83/203 | 41 | 22 | 33 | 21 | 43 | ng | 24 | 43 | 33 | ng | ng | 39 | 50 | 59 | ng | ng |
| Neonatal period and infancy | |||||||||||||||||
| Low birth weight (<2.27 kg) | 68/226 | 30 | 11 | 78c | 36c | 21 | 70 | 12 | 41 | 20 | ng | ng | 17 | 21 | 17 | ng | ng |
| Hypotonia | 504/538 | 94 | 100 | 100 | 100 | 100 | 82 | 65 | 100 | 100 | 100 | 100 | 83 | 91 | 100 | 92 | 94 |
| Feeding problems | 445/479 | 93 | 100 | 100 | 100 | 100 | 91 | 41 | 100 | 90 | 100 | 100 | 72 | 87 | 100 | ng | 94 |
| Delayed milestones | 405/412 | 98 | 100 | 100 | 100 | 97 | ng | ng | 100 | 90 | 92 | ng | ng | 95 | 97 | ng | 100 |
| CNS function and behavior | |||||||||||||||||
| Mental deficiency | 504/517 | 97 | 100 | 100d | 100 | 97 | 87 | 100 | 100 | 100 | 100 | 100 | 100 | 91 | 100 | ng | 97 |
| Seizures | 40/199 | 20 | ng | 11 | 0 | 16 | 9 | 29 | 20 | 20 | ng | ng | ng | 38 | 24 | ng | ng |
| Personality problems | 161/397 | 41 | 11 | 44 | ng | 71 | 47 | ng | 60 | 71 | 78 | ng | ng | ng | 72 | ng | >25 |
| Growth | |||||||||||||||||
| Obesity | 287/306 | 94 | 100 | 100 | 100 | 100 | 78 | 76 | 100 | 100 | 100 | 100 | 94 | 79 | 90 | 100 | ng |
| Short stature (< −1 SD) | 232/306 | 76 | 78 | 56 | 57 | 94 | 96 | 47 | 90 | 90 | 83 | 100 | 44 | 70 | 71 | 74 | ng |
| Delayed bone age | 74/148 | 50 | 33 | 67 | 86 | 50 | ng | ng | ng | 90 | ng | ng | ng | 17 | 35 | ng | ng |
| Face | |||||||||||||||||
| Narrow bifrontal diameter | 138/184 | 75 | ng | ng | ng | 40 | ng | ng | ng | ng | 100 | ng | 72 | 79 | 69 | 93 | ng |
| Almond-shaped eyes | 151/202 | 75 | ng | 67 | ng | 19 | ng | ng | ng | ng | 92 | 100 | 72 | 91 | 74 | 93 | ng |
| Strabismus | 259/494 | 52 | 33 | 78 | 29 | 40 | 74 | ng | 67 | 95 | 67 | ng | ng | 30 | 67 | 41 | <50 |
| Early dental caries/enamel hypoplasia | 56/141 | 40 | ng | 86 | 50 | 12 | 80 | ng | ng | ng | ng | ng | ng | 13 | 46 | ng | ng |
| Sexual development | |||||||||||||||||
| Cryptorchidism | 240/273 | 88 | 83 | 100 | 100 | 84 | 87 | ng | 87 | 100 | ng | ng | ng | 79 | 100 | 81 | 86 |
| Hypogenitalism/hypogonadism | 270/285 | 95 | 100 | 100 | 100 | 100 | ng | 88 | 87 | 100 | 83 | 100 | 89 | 95 | 100 | ng | 92 |
| Menstruation | 38/98 | 39 | ng | ng | ng | ng | ng | ng | ng | 33 | ng | ng | ng | ng | 36 | ng | 40 |
| Skeletal | |||||||||||||||||
| Small hands and feet | 237/286 | 83 | 76 | 100 | 86 | 79 | 91 | ng | 100 | 100 | 67 | 100 | 72 | 81 | 71 | 76 | ng |
| Scoliosis | 159/360 | 44 | 33 | 22 | ng | ng | ng | ng | ng | ng | 50 | ng | ng | ng | 38 | 29 | <50 |
| Other | |||||||||||||||||
| Skin picking | 261/330 | 79 | ng | ng | ng | ng | ng | ng | ng | ng | ng | ng | ng | ng | 79 | 83 | 78 |
| Reduced glucose tolerance/diabetes mellitus | 74/371 | 20 | 22 | 25 | 7 | 30 | ng | ng | ng | 12 | ng | ng | 39 | ng | 15 | ng | 19 |
Early in the second stage, infants and toddlers are usually easy going and affectionate, but, in about one-half of PWS individuals, personality problems develop between ages 3 and 5 years. These problems include temper tantrums, depression, stubbornness, and sudden acts of violence [Hall and Smith, 1972; Bray et al., 1983; Cassidy, 1984]. These behavioral changes may be initiated by withholding of food, but may also occur with little provocation, particularly in the adolescents and young adults. Poor peer interactions, immaturity and inappropriate social behavior may also occur during this time.
Although 60% of PWS individuals have IQs in the normal or borderline range, cognitive dysfunction is nearly always present. Specific academic weaknesses in arithmetic and writing are noted in PWS individuals, but reading and art skills are considered strengths. Individual educational and remedial programs should be advised after careful intellectual assessment of PWS children.
The vast majority of PWS cases are sporadic occurrences, but at least 10 putative families with two or more relatives with characteristics of PWS have been reported, with the most recent documentation by Burke et al. [1987] and Lubinsky et al. [1987]. In a recent review of PWS individuals and their families, an empiric risk for recurrence of PWS was estimated at less than 1/1,000 [Cassidy, 1987]. Normal chromosomes have been reported in suspected familial cases, suggesting either an autosomal recessive mode of inheritance or a new autosomal dominant mutation.
Although normal chromosomes have been found in affected patients from families with more than one probable PWS individual, Hawkey and Smithies [1976] reviewed the literature and noted that 10 of 61 patients with PWS had a chromosome abnormality identified with standard banded or unbanded chromosome preparations. Several PWS patients were reported to have Robertsonian 15q translocations with deletions of the short arm of chromosome 15, which was thought to be causally related to the phenotype of PWS individuals. In 1980, Ledbetter et al. reported a chromosome 15 deletion in PWS patients. Using high-resolution chromosome banding, these investigators found a small interstitial deletion of the proximal long arm of chromosome 15 in four of five PWS patients. Currently, over 50% of reported PWS individuals are discovered to have the 15q12 deletion when studied with high-resolution analysis [Ledbetter et al., 1981, 1982; Wyandt et al., 1981; Mattei et al., 1983; Cassidy et al., 1984; Fukushima et al., 1984; Niikawa and Ishikiriyama, 1985; Fear et al., 1985; Takano et al., 1986; Butler et al., 1986; Labidi and Cassidy, 1986; Wenger et al., 1987; Wiesner et al., 1987]. DNA studies are also in progress to gain an insight into the molecular genetics of PWS and to identify sub-microscopic deletions or rearrangements of the proximal long arm of chromosome 15 [Donlon et al., 1986; Nicholls et al., 1987; Tasset et al., 1988; Gregory and Hamerton, 1987; Donlon, 1988; Nicholls et al., 1989; Tantravahi et al., 1989].
DISCUSSION
Clinical Manifestations of PWS
Obesity is a cardinal feature in PWS individuals and is considered the most significant health problem. Therefore, obesity and related anthropometric characteristics in PWS will be discussed in this review and other clinical findings summarized. About one-third of PWS patients weigh more than 200% of ideal body weight [Schoeller et al., 1988; Meaney and Butler, 1989a], and, without intervention, significant morbidity and mortality may occur from the complications of obesity (e.g., cardiopulmonary compromise, hypertension, diabetes mellitus). Obesity is thought to result from hyperphagia, persistent hunger, decreased perception of satiety, and an uncontrollable appetite with impaired emesis [Holm, 1981; Cassidy, 1984]. The role of energy expenditure in the causation of obesity is not clear, although a combination of excessive caloric intake, decreased energy expenditure, and/or decreased physical activity leads to the morbid obesity [Holm, 1981; Nardella et al., 1983; Cassidy, 1984; Schoeller et al., 1988].
Medications to suppress appetite have met with little success in PWS individuals, but more research is needed. Naloxone, an opiate antagonist, has been used in PWS individuals to determine if their hyperphagia was related to an abnormality of endorphin activity, but no consistent weight loss was achieved [Kyriakides et al., 1980]. Also, recent evidence indicates that endorphin levels are normal in PWS individuals [Hawkins et al., 1989]. Fenfluramine, an appetite-suppressing drug, was used with success in one PWS patient [Bray et al., 1983]. Tryptophan, a precursor of brain serotonin and associated with a reduction of food intake in animal studies, was used in the treatment of four PWS individuals, but no effect on food intake was observed [Bray et al., 1983].
The onset of obesity usually occurs between ages 1 and 6 years, with an average age of onset of 2 years. This follows the period of failure to thrive and hypotonia seen during the first stage of PWS. Recent evidence with skinfold measurements of PWS infants indicates that excessive fat may be present as early as 6 months and before the infant is judged to be obese by weight/height parameters [Butler et al., 1988]. The fat pattern is characteristic in PWS individuals and most prominently distributed over the buttocks, trunk, and thighs but spares the distal extremities [Cassidy, 1984; Schoeller et al., 1988; Meaney and Butler, 1989a,b]. An estimate of 42% body fat, or a two- to threefold increase, compared with the general population was recently determined on 29 PWS individuals in whom skinfold standards (triceps, subscapular, and medial calf) were available for determination of percent body fat [Meaney and Butler, 1989b].
Interestingly, children with PWS who have lost weight must be kept on caloric intakes averaging about 60% of normal for age to maintain their reduced weight [Holm, 1981; Cassidy, 1984]. Therefore, 8 to 9 kcal/cm of height usually allows for slow weight loss in PWS children, whereas 10 to 11 kcal/cm of height is usually sufficient for maintenance of weight [Pipes and Holm, 1973; Cassidy, 1984]. Energy expenditure is apparently abnormal in PWS children, although energy expenditure studies in PWS individuals are few and inconclusive.
Basal metabolic rates are considered normal in PWS individuals when expressed per unit of body surface area in some reports, but reduced by 20 to 50% in other reports [Bray et al., 1983; Schoeller et al, 1988]. Butler et al. [1989b] recently measured resting metabolic rates using indirect calorimetry, and by comparing body composition values determined by bioelectrical impedance and skinfold measurements in 15 PWS, 23 nonobese, and 11 obese (>130% ideal body weight) individuals, they found that PWS patients had lower resting metabolic rates per given body weight or fat-free mass than the obese or nonobese control individuals. These data suggest that low resting metabolic rates in PWS individuals are not due simply to a reduced fat-free mass; additional research is needed.
Surgical procedures such as gastroplasty and intestinal bypass to correct or prevent the obesity have met with only limited success and should be considered in older adolescents and adults who are unable to lose weight by dietary and exercise programs [Soper et al., 1981; Cassidy, 1984]. Calorie-controlled ketogenic diets individualized for each patient have been effective, but patient compliance is a problem [Nelson et al., 1981]. With early diagnosis, diet intervention, behavior modification, and increased physical activity, excessive weight may be eliminated and the life-threatening obesity controlled in PWS individuals.
Metabolic studies to explain the obesity in PWS are few, and sparse information is available on adipose tissue metabolism. Thyroid hormone, lipid profiles, insulin, glucocorticoid, and amino acid levels are comparable to those in obese individuals, although reduced glucose tolerance is reported in one-fifth of PWS patients [Hall and Smith, 1972; Parra et al., 1973; Holm, 1981; Cassidy, 1984; Butler et al., 1989c, d]. In six PWS patients, no abnormalities in fat metabolism or transport were identified in response to norepinephrine, insulin, and glucose [Bier et al., 1977]. While fat cells were found to be larger than in control individuals, uptake of fat and fatty acid composition in adipose tissue was normal and the fat cell number not increased [Bier et al., 1977; Ginsberg-Fellner, 1981; Gurr et al., 1982]. Levels of adipose tissue lipoprotein lipase, an enzyme that regulates uptake and storage of triglycerides, were increased 10-fold in fat biopsy specimens from seven PWS patients compared with those of control individuals when adjusted for percent ideal body weight and fat cell size [Schwartz et al., 1981]. Thus this enzyme is apparently elevated in PWS, but additional research is needed to determine whether the elevation is a primary defect or a response to other metabolic derangements. Recently, derangements of steroid metabolism were reported by Chasalow et al. [1987], but these abnormalities and their role, if any, in the manifestations (e.g., obesity, mental deficiency, hypogonadism) seen in PWS are not understood.
Serum cholesterol and triglyceride levels are apparently normal in patients with PWS [Butler et al., 1989c], but fat biopsy specimens from nine PWS patients showed that triglycerides make up more than 98% of the fat [Nelson et al., 1981]. There was also a demonstrated threefold increase in long chain polyunsaturated fatty acids, which may suggest a resistance to lipolysis. Body composition and substrate utilization studies in 11 PWS patients showed a high percentage of adipose tissue but an apparent normal substrate utilization with a normal percentage of fat used for basal metabolism when compared with other obese control individuals [Nelson et al., 1981]. The roles of the elevated long chain fatty acids, increased levels of adipose tissue lipase suggested from fat biopsy specimens, and the genetic alterations seen in chromosome 15(q11–13) in the development of obesity in PWS patients are not understood.
Short stature is also a recognized finding in PWS individuals and occurs because of linear growth retardation and lack of a pubertal growth spurt in both sexes. Of those PWS patients reported in the literature, 76% have short stature while birth length is usually normal; birth weight is low in 30% of patients (Table I). The average adult height is 150 cm in women and 155 cm in men [Bray et al., 1983]. The bone age may be equivalent to the chronological age but is delayed in 50% of PWS patients (Table I). Although no specific lesion of the hypothalamus has been identified in PWS necropsy reports [Holm, 1981; Cassidy, 1984], thermolability, hyperphagia, hypogonadism, and growth hormone abnormalities producing a “blunted” response to provocative stimuli suggest a possible hypothalamic dysfunction as an important factor in the short stature seen in PWS individuals [Bray et al., 1983; Lee et al., 1987]. The use of oxandrolone, an anabolic steroid, and growth hormone therapy has had limited success in achieving growth in PWS individuals, but more research is needed [Lee et al., 1987].
Hypogonadism and hypogenitalism are major findings in PWS and can be identified early in the male but with more difficulty in the female [Zellweger and Schneider, 1968; Hall and Smith, 1972; Bray et al., 1983; Butler et al., 1986]. Sertoli cells and variable numbers of Leydig and germinal cells are usually present in the testicles, although PWS males are infertile. The tubules are usually small and atrophic. The hypogonadism is thought to be due to hypothalamic hypogonadotropism since it is often associated with low gonadotropin and gonadal steroid levels [Hamilton et al., 1972; Morgner et al., 1974].
Cryptorchidism, micropenis, and scrotal hypoplasia are recognized in the neonatal period in 80 to 100% of males, whereas a hypoplastic labia minora and clitoris are seen in most female patients [Hall and Smith, 1972; Holm, 1981; Bray et al., 1983; Cassidy, 1984; Butler et al., 1986; Wenger et al., 1987]. Treatment of the small penis with topical or parenteral testosterone has been effective in achieving penile growth in PWS males [Cassidy, 1984]. Gonadotropin treatment may also be helpful in treating PWS males with cryptorchidism. Delayed, precocious, or incomplete sexual maturation are also recognized but probably in less than 5% of PWS patients.
Menarche is often late or does not occur in PWS females. In 98 appropriately aged females reported in the literature, 38 developed menstruation (Table I). There is one report of fertility in two females who were thought to have PWS [Laxova et al., 1973].
Because few anthropometric studies in PWS individuals were previously reported, weight, height, sitting height, and 24 other anthropometric variables were obtained on 38 PWS individuals, who ranged in age from 2 weeks to 38.5 years [Butler and Meaney, 1987]. Significant differences were not found in the measurements between those PWS individuals with and without the chromosome 15 deletion. The average percent ideal body weight was 180% for the PWS individuals, and skinfold thicknesses at six sites were found to be higher in PWS males than in PWS females. This represented an abnormal fatness pattern with sex-reversal compared with control individuals [Meaney and Butler, 1989b].
Anthropometric measurements confirmed the presence of short stature, small hands and feet with the feet smaller in relationship to the hands, obesity, and narrow bifrontal diameter in PWS. Inverse correlations were produced with linear measurements (e.g., height, hand and foot lengths) and age, which indicated a deceleration of linear growth with increasing age relative to normal individuals [Butler and Meaney, 1987]. A relative deceleration in growth of certain craniofacial dimensions (e.g., head circumference, head length) was also suggested [Meaney and Butler, 1987]. Therefore, dolichocephaly may be considered a early helpful diagnostic sign of PWS.
To determine the effects of familial background on physical characteristics in PWS individuals, anthropometric measurements, including weight; height; sitting height; longitudinal and breadth measurements of the head, hands, and feet; head, arm, and calf circumferences; and triceps and subscapular skinfolds were obtained on 28 PWS individuals and their natural parents [Butler et al., 1989a], Intrafamilial and midparental PWS correlations and heritability estimates were calculated from the anthropometric data. Midparental-PWS child correlations were significant, and higher heritability estimates were calculated for height and foot length but not for the other measurements. These data suggest that taller parents with longer foot length would have a taller than the average PWS individual with longer feet. Therefore, these physical characteristics in PWS were apparently influenced by the genetic background while soft tissue variables such as arm and calf circumferences and triceps skinfold have low heritability estimates.
Clinical Differences Between Deletion and Nondeletion PWS Individuals
Butler et al. [1986] reported on the clinical differences between PWS patients with or without the chromosome 15 deletion, the former having lighter hair, eye, and skin colors and higher intelligence scores than PWS patients with apparently normal chromosomes and compared 39 PWS patients with 124 PWS individuals from the literature. Correlation studies of metacarpophalangeal pattern profile variables from hand radiographs of PWS patients standardized for age and sex and palmar and plantar dermatoglyphic findings indicated apparent homogeneity of the deletion PWS group and apparent heterogeneity of individuals with PWS and normal chromosomes [Butler et al., 1982; Reed and Butler, 1984; Butler and Meaney, 1985, 1987].
Wiesner et al. [1987] and Butler [1989] reported that 48% of their PWS patients, respectively, were hypopigmented and that the decreased pigmentation correlated with the chromosome 15 deletion. It is postulated that a DNA segment on proximal 15q, which is deleted in about 50% of PWS patients, may play a role in melanin production and decreased pigment in PWS patients with the deletion. Wiesner et al. [1987] reported decreased hairbulb tyrosinase activity, glutathione, and urine cysteinyldopa levels (all components of melanin synthesis), although (β-melanocyte stimulating hormone and biochemical (phenylalanine, tyrosine, and catecholamine) levels were found to be normal [Butler, et al., 1987; Butler, 1989]. Wenger et al. [1987] did not identify a difference in hair or eye colors in their PWS patients with or without the chromosome deletion. Wiesner et al. [1987] also reported abnormal melanosomes in one PWS patient. Misrouting of optic fibers, a finding consistent with oculocutaneous or ocular albinism, was also found in several PWS patients reported by Creel et al. [1986].
Cytogenetic and DNA Findings
In a literature review by Mattei et al. [1984], 94 anomalies of chromosome 15 from PWS individuals were found. The 94 anomalies were divided into 19 reciprocal translocations involving chromosome 15 (2 balanced and 17 unbalanced); 9 small bisatellited additional chromosomes derived from chromosome 15 (e.g., idic [15][q11]); 12 Robertsonian translocations with chromosome 15; 52 interstitial deletions of chromosome 15 identified with high resolution analysis; and 2 pericentric inversions of chromosome 15. In the current literature, a total of 243 individuals with PWS have been reported with chromosome 15 abnormalities. These include 168 with interstitial deletions of chromosome 15, or 69% of the total, 6 (2 %) with apparently balanced reciprocal translocations involving chromosome 15, 32 (13%) with unbalanced translocations involving chromosome 15, 14 (6%) with Robertsonian translocations involving chromosome 15, 17 (7%) with small additional chromosomes, 2(1%) with pericentric inversions of chromosome 15, and 4 (2%) with duplications of chromosome 15 (Table II). Thus various chromosome anomalies have been found in PWS individuals, but it is the proximal region of the long arm of chromosome 15 that is usually involved.
TABLE II.
Anomalies of Chromosome 15 Associated With Prader-Willi Syndrome Individuals
| Karyotype | No. of Patients | References |
|---|---|---|
| Interstitial deletion | ||
| 46,XX,del(15)(q11q13) | 4 | Ledbetter et al. [1981] |
| 46,XX or XY,del(15)(q12) | 7 | Wyandt et al. [1981] |
| 46,XY,del(15)(q11q13) | 1 | Bonuccelli et al. [1982] |
| 46,XX or XY,del(15)(q11q13) | 17 | Ledbetter et al. [1982] |
| 46,XX or XY,del(15)(q11q11) | 2 | Ledbetter et al. [1982] |
| 46,XY,?del(15)(q11q12) | 1 | Fesseler and Bierich [1983] |
| 46,XX,del(15)(q11q13) | 1 | Fraccaro et al. [1983] |
| 46,XX or XY,del(15)(q11q12) | 8 | Mattei et al. [1983] |
| 46,XX or XY,del15)(q11q13) | 11 | Cassidy et al. [1984] |
| 46,XX,del(15)(q11q13) | 1 | Golden et al. [1984] |
| 46,XX or XY,del(15)(q11q12) | 9 | Fukushima et al. [1984] |
| 46,XX or XY,del(15)(q12) | 9 | Fear et al. [1985] |
| 46,X,der(X),t(X;14)(q22;q24.3)mat,del(15)(q11.1q11.3)mat | 1 | Markovic et al. [1985] |
| 46,XX or XY,del(15)(q11q12) | 11 | Niikawa and Ishikiriyama [1985] |
| 46,XX or XY,del(15)(q11q13) | 21 | Butler et al. [1986] |
| 46,XY,del(15)(q11q13) | 1 | Kadotani et al. [1986] |
| 46,XX or XY,del(15)(q11q13) | 9 | Labidi and Cassidy [1986] |
| 46,XX or XY,del(15)(q11.2q13) | 11 | Takano et al. [1986] |
| 46,XX,del(15)(q11.2q12) | 1 | Takano et al. [1986] |
| 46,XY,del(15)(q11q13) | 1 | Jaskulsky and Stone [1987] |
| 46,XY,del(15)(q11q13) | 1 | Reynolds et al. [1987] |
| 46,XX or XY,del(15)(q11q13) | 13 | Wiesner et al. [1987] |
| 46,XX or XY,del(15)(q12) | 25 | Wenger et al. [1987] |
| 46,XY,del(15)(q11.2q13) | 1 | Miike et al. [1988] |
| 46,XX,del(15)(q11.2q13) | 1 | Phelan et al. [1988] |
| Balanced reciprocal translocations | ||
| 46,XY,t(3;15)(q29;q11) | 1 | Aurias et al. [1978] |
| 46,XY,t(5;15)(pter;q12)/46,XY,t(8;15)(qter;q12)/46,XY,t(12;15)(qter;q12) | 1 | Lejeune et al. [1979] |
| 46,XY,t(15;18)(q11;q22) | 1 | Fraccaro et al. [1983] |
| 46,XX,t(8;15)(p11;p13) | 1 | Fraccaro et al. [1983] |
| 46,X,t(X;15)(q12.2;p12) | 1 | Gustavson et al. [1984] |
| 46,XY,t(7;15) | 1 | Garau et al. [1986] |
| Unbalanced reciprocal translocations | ||
| 45,XY,t(6;15)(q25;q12) | 1 | Mikkelsen and Dyggve [1973] |
| 46,XY,t(9;15)(?;q11) | 1 | Zuffardi et al. [1978] |
| 45,XX or XY,t(15;20)(q15;p13) | 1 | H. Kawashima (personal communication as cited by Mattei et al. [1984]) |
| 45,XY,−3,−15, + t(3;15)(p25;q15) | 1 | Kucerova et al. [1979] |
| 46,XY,t(9;15)(p24;q11) | 1 | Guanti [1980] |
| 45,XY,t(14;15)(q32;q12–13) | 1 | Kozma et al. [1980] |
| 45,X,−15,−Y,t(Y;15)(p11;q11) | 1 | Berry et al. [1981] |
| 45,XY,t(7;15)(q36;q13) | 1 | Cassidy et al. [1981] |
| 45,XX,−15,−19, + der(19),t(15;19)(q12;q13) | 1 | Moric-Petrovic et al. [1981] |
| 45,XY,t(1;15)(q36;q13) | 1 | Styles and Popkin [1981] |
| 45,X,−15,t(X;15;16)(p22.2;q12;q11.1) | 1 | Temperani and Forabosco [1981] |
| 45,XY,t(9;15) with del(15pter→ 15q11) | 1 | Wajntal et al. [1981] |
| 45,XY,t(15;17)(q13;p13) | 1 | Cavalli et al. [1982] |
| t(7q;15q) | 1 | D. Ledbetter (personal communication as cited in Kousseff [1982]) |
| 45,XY,t(15;19)(q12;pter) | 1 | D. Ledbetter (personal communication as cited in Charrow et al. [1983]) |
| 45,XY,t(15;20)(q12;qter) | 1 | D. Ledbetter (personal communication as cited in Charrow et al. [1983]) |
| 45.XX,−1,−15, + der(1),t(1;15)(pter;q11)/46,XX−1, −15, + der(1), + idic(15)(q11) | 1a | Wulfsberg et al. [1982] |
| 46,XX,t(11;15)(q25;q11–12) | 1 | Charrow et al. [1983] |
| 45,XY,−9,−15, + der(9),t(9;15)(q34;q11) | 1 | Fraccaro et al. [1983] |
| 45,XX,− 15,− 18, + der(18),t(15;18)(q12;q23) | 1 | Fraccaro et al. [1983] |
| 45,XX,−15,−19, + der(19),t(15;19)(q11 or q13;p13) | 1 | Fraccaro et al. [1983] |
| 45,XY,−11,− 15,t(11;15)(q25;q15) | 1 | Pauli et al. [1983] |
| 45,XY,−15,der(7),t(7;15)(q36;q13) | 1 | Cassidy et al. [1984] |
| 45,XX,−15,−17, + der(17),t(15,17)(q13;p13.1) | 1 | Elder et al. [1985] |
| 46,XX or XY,− 15, + der(14),t(14;15)(q11.1;q13) | 1 | Hasegawa et al. [1984] |
| 45,XX,− 15,− 17, + der(17),t(15;17)(q13;q25) | 1 | Duckett et al. [1984] |
| 46,XY,t(15;22)(q13;q11.2) | 1 | Fear et al. [1985] |
| 46,XY,−5,−15,+der(5),t(5;15)(5pter → 5q35::15q13 → 15qter), + idic(15)(pter → q1?3:q1?3 → pter) | 1 | Murdock and Wurster-Hill [1986] |
| 46,XY,− 15,+der(22) | 1a | Fernandez et al. [1987] |
| 46,XY,t(15;22)(q13;q12)pat | 1 | Fernandez et al. [1987] |
| 45,XX, − 7, − 15, + der,t(7;15)(q36;q15) | 1 | Pfeiffer et al. [1987] |
| 45.XY, −15, − 20, + der,t(15;20)(q14;p13) | 1 | Pfeiffer et al. [1987] |
| Robertsonian translocations | ||
| 45,XY,t(15q;15q) | 1 | Hawkey and Smithies [1976] |
| 45,XX,t(15;15)(p11;q11) | 1 | Emberger et al. [1977] |
| 45,XY,t(15;15) dicentric | 1 | Fraccaro et al. [1977] |
| 45,XY,t(15,15) monocentric | 1 | Fraccaro et al. [1977] |
| 45,XX,t(15q,15q) dicentric | 1 | Fleischnick et al. [1979] |
| 45,XX,t(14;15)(p11;q11) | 1 | Smith and Nöel [1980] |
| 45,XX,t(13;15)(p11;q11) or (q11;p11) | 1 | Berry et al. [1981] |
| 45,XX,t(15;15)(p11;q11) | 1 | Berry et al. [1981] |
| 45,XY,t(13q;15q) | 1 | Wu et al. [1981] |
| t(15;15)(p11;q12) | 1 | D. Ledbetter (personal communication as cited in Kousseff [1982]) |
| 45,XX,t(15;15)(p11;p11) | 1 | Ledbetter et al. [1982] |
| 45,XX,−15,− 15,t(15;15)(p11;q11) | 1 | Tylki et al. [1982] |
| 45,XX,t(15q;15q) | 1 | Winsor and Welch [1983] |
| 46,XX,t(15q;15q) | 1 | Niikawa and Ishikiriyama [1985] |
| Small additional chromosomes | ||
| 47,XX, + mar | 1 | Ridler et al. [1971] |
| 46,XY/47,XY, + mar | 2 | Michaelsen et al. [1979] |
| 47,XX, + idic(15p)(pter → q11::q11 → pter) | 1 | Fujita et al. [1980] |
| 47,XY, + inv dup(15)(pter→q11 or q12::p11 or q11 → pter) | 1 | Wisniewski et al. [1980] |
| 46,XY/47,XY, + del(15)(pter → q1.3:) | 1 | Kousseff [1982] |
| 47,XY,+ 15(pter→q21:) | 1 | M. Lubinsky (personal communication as cited by Kousseff [1982]) |
| 46,XX/47,XX, + idic(15)(pter→ q11::q11 → pter) | 1 | Ledbetter et al. [1982] |
| 46,XX/47,XX, + mar | 1 | Tajara et al. [1982] |
| 45.XX,− 1,− 15, + der(1),t(1;15)(pter;q11)/46,XX,− 1,− 15, +der(1), + idic(15)(q11) | 1a | Wulfsberg et al. [1982] |
| 47,XY, + mar | 1 | Fraccaro et al. [1983] |
| 47,XY, + idic(15)(q11) | 1 | Mattei et al. [1983] |
| 46,XX/47,XX, + mar | 1 | Goh et al. [1984] |
| 46,XY,−5,−15, + der(5),t(5;15)(5pter→ 5q35::15q13→ 15qter), + idic(15)(pter → q1?3:q1?3 → pter) | 1a | Murdock and Wurster-Hill [1986] |
| 46,XX or XY/47,XX or XY, + idic(15)(q11) | 2 | Wenger et al. [1987] |
| 47,XY,idic(15)(pter → q11::q11 → pter) | 1 | Zellweger et al. [1987] |
| Pericentric inversions | ||
| 46,XY,inv(15)(p13q13) | 1 | Winsor et al. [1982] |
| 48,XXXY,inv(15)(p11.2 q12 or q13) | 1 | Winsor et al. [1982] |
| Duplications | ||
| 46,XY,dup(15)(q12 or q13 → q15) | 1 | de France et al. [1984] |
| 46,XX,dup(15)(q11q13) | 1 | Fuhrmann-Rieger et al. [1984] |
| 46,XY,dup(15)(q11q12) | 1 | Pettigrew et al. [1987] |
| 46,XX,der(15),dup(15)(q12q13) | 1 | Pettigrew et al. [1987] |
Since 1980 at least 280 PWS patients have been reported to have undergone high-resolution chromosome analysis, and a chromosome 15 abnormality was identified in 168 individuals [Ledbetter et al., 1981, 1982; Mattei et al., 1983; Fraccaro et al., 1983; Charrow et al., 1983; Cassidy et al., 1984; Fukushima et al., 1984; Fear et al., 1985; Niikawa and Ishikiriyama, 1985; Takano et al., 1986; Butler et al., 1986; Labidi and Cassidy, 1986; Pettigrew et al., 1987; Wenger et al., 1987; Wiesner et al., 1987]. Table III shows the summary data of the high-resolution chromosome findings in published surveys of PWS patients. Sixty percent of the PWS patients studied with high-resolution analysis have a chromosome 15 abnormality, and 94% of the abnormalities are interstitial deletions (15q11–13). In most surveys more than 50% of PWS patients have an interstitial deletion of chromosome 15, but some investigators have reported that most, if not all, of their PWS patients had the 15q11–13 deletion [Mattei et al., 1983; Cassidy et al., 1984; Niikawa and Ishikiriyama, 1985]. Investigators of PWS individuals with lower frequencies of normal chromosomes apparently tend to use stricter diagnostic criteria for inclusion in their studies.
TABLE III.
High-Resolution Chromosome Findings in Surveys of Individuals With Prader-Willi Syndrome
| Reference | del(15)(q11q13) | Chromosome 15 abnormality | idic(15)(q11) | Other | Normal | Total | |
|---|---|---|---|---|---|---|---|
| Robertsonian translocation t(15;15) | Reciprocal translocation t(15;?) | ||||||
| Ledbetter et al. [1981, 1982] | 23 | 1 | — | 1 | — | 20 | 45 |
| Wyandt et al. [1981] | 7 | — | — | — | — | 4 | 11 |
| Mattei et al. [1983] | 8 | — | — | 1 | — | 8 | 17 |
| Cassidy et al. [1984] | 11 | — | 1a | — | — | — | 12 |
| Fukushima et al. [1984]b | 9 | — | — | — | — | — | 9 |
| Fear et al. [1985] | 9 | — | 1c | — | — | 5 | 15 |
| Niikawa and Ishikiriyama [1985] | 11 | 1 | — | — | — | 6 | 18 |
| Labidi and Cassidy [1986] | 9 | — | — | — | — | 4 | 13 |
| Butler et al. [1986] | 21 | — | — | — | — | 18 | 39 |
| Takano et al. [1986] | 12 | — | — | — | — | 6 | 18 |
| Wiesner et al. [1987] | 13 | — | — | — | 1d | 10 | 24 |
| Wenger et al. [1987] | 25 | — | — | 2 | 1e | 31 | 59 |
| Totals | |||||||
| No. | 158 | 2 | 2 | 4 | 2 | 112 | 280 |
| Percent | 56.4 | 0.7 | 0.7 | 1.4 | 0.7 | 40.0 |
Deletions of proximal 15q have also been reported in non-PWS patients [Schwartz et al., 1985; Kaplan et al., 1987; Reynolds et al., 1987; Greenberg and Ledbetter, 1987], in Angelman syndrome [Kaplan et al., 1987; Magenis et al., 1987], in Williams syndrome [Kaplan et al., 1987], and in hypomelanosis of Ito [Turleau et al., 1986]. Interestingly, these three syndromes also present with hypopigmentation (e.g., fair hair and skin, lightly pigmented irides). Williams syndrome individuals may also present with obesity [Morris et al., 1987]. Therefore, perturbations of this region of chromosome 15 may yield a wide spectrum of phenotypic manifestations.
Chromosome 15 has specific cytochemical properties in that the constitutive heterochromatin contains large amounts of 5-methylcytosine–rich DNA in comparison with other acrocentric chromosomes. Also, it has been proposed that inverted DNA repeats may be present in high numbers in the 15q11–13 region and may play a role in producing the chromosome changes seen in this region [Donlon et al., 1985]. More research is needed to determine the lability of the proximal long arm of chromosome 15.
Several DNA probes subcloned from an inverted duplicated proximal 15q cell line have been reported in nine PWS patients, in whom molecular rearrangements were also seen [Donlon et al., 1986; Nicholls et al., 1987; Gregory and Hamerton, 1987; Donlon, 1988]. Molecular rearrangements included deletion-duplications in patients with cytogenetically similar deletions (15q11–q13), a duplication in an apparently chromosomally normal patient, and a deletion-duplication in a patient with a chromosome 5; 15 translocation. Six probes (designated D15S9–D15S14) have been found to detect DNA fragments that have been assigned to 15q11.1 – 15q12, and five other probes (designated D15S15–D15S19) detect fragments that map within 15q11–15q13 but outside the 15q11.1–15q12 deletion area (see Fig. 1) [Nicholls et al., 1987; Donlon, 1988]. DNA deletions of PWS patients with apparently normal chromosomes were found for eight of nine probes tested, including D15S9 through D15S14, adding the potential for qualitative as well as quantitative studies of deletions and molecular determination of the parental source of the deleted chromosome [Donlon et al., 1986 Nicholls et al., 1987, 1989; Donlon, 1988; Tantravahi et al., 1989].
Cytogenetic studies to explain the cause of the chromosome 15 abnormalities in PWS are limited. To determine if chromosome instability or early chromosome changes exist in PWS, Hummel et al. [1986] studied sister chromatid exchanges (SCE) in seven PWS patients with the 15q12 deletion and reported differences compared with controls, while Butler and Jenkins [1987] found no SCE differences in 18 PWS patients, their parents, and age-matched controls. Recently, Wenger and Rauch [1988] found a significantly higher number of SCEs in the proximal long arm of chromosome 15 in nondeletion PWS individuals compared with deletion PWS and control individuals.
Cytogenetic analysis with mitomycin C, a bifunctional alkylating agent, and folate-deficient culture conditions was recently undertaken to identify if chromosome instability or fragility exit in PWS individuals [Butler and Jenkins, 1989]. No increased chromosome breakage was detected in 18 PWS patients and their parents when compared with age-matched controls, and no clustering of chromosome/chromatid breaks or SCEs were observed in the proximal one-third region of the long arm of the D group chromosomes when compared with the middle or distal one-third regions.
High-resolution parental chromosomes have also been studied in several PWS families and found to be normal [Ledbetter et al., 1982; Niikawa and Ishikiriyama, 1985; Butler et al., 1986], although Winsor and Welch [1983] reported a chromosome 15 inversion in a PWS child and his unaffected father. With the use of chromosome polymorphisms to study the parental origin of the chromosome 15 deletion in 22 informative PWS families, it was determined that the chromosome donated by the father was involved in the deletion of the child in 19 of the 22 families [Butler and Palmer, 1983; Mattei et al., 1983; Niikawa and Ishikiriyama, 1985; Butler et al., 1986]. Recently, DNA studies with the use of restriction fragment length polymorphisms with probes on chromosome 15 have confirmed the paternal origin of the deleted chromosome in several families [Nicholls et al., 1989]. Therefore, paternal origin of the chromosome deletion may suggest that an environmental agent is involved whose effect is greater on paternal than maternal gametogenesis.
To determine if paternal factors could be identified that may affect spermatogenesis and predispose to chromosomal rearrangements, an epidemiologic study was undertaken of paternal occupations in PWS and control families [Strakowski and Butler, 1987]. A significantly higher frequency of 652 PWS fathers (21%) than 334 fathers (12%) of children with Down or fragile X syndromes were employed in hydrocarbon-related occupations at the time of conception. In another study, the fathers’ occupations in 81 PWS individuals (53 deletion, 28 nondeletion) were analyzed. Forty-five percent of the fathers of deletion PWS individuals and 54% of fathers from nondeletion PWS individuals were employed in hydrocarbon-exposed occupations, which also supports an environmental cause of chromosome abnormalities in the sperm of these fathers [Cassidy et al., 1989]. Additional research is needed to determine if a causal relationship exists between hydrocarbon-exposed occupations and PWS.
FUTURE DIRECTIONS
Although great advances in diagnosis, causation determination, and management of PWS individuals have been made in the past 30 years, additional clinical, cytogenetic, and molecular studies are necessary to gain a better understanding of this syndrome. Studies of environmental factors (e.g., occupations, chemicals, viruses) that may influence male gametogenesis and DNA analysis to determine the frequency of microscopic and sub-microscopic deletions or rearrangements should be expanded. Several DNA probes on chromosome 15 have been isolated, and new probes and restriction fragment length polymorphisms are under investigation in PWS that should clarify the molecular structure and stability status of the proximal part of 15q. With the use of DNA technology and chromosome 15 probes, a better understanding of the molecular genetics of PWS should be achieved that will have direct application in the understanding of the molecular biology and predisposition of obesity in individuals from the general population.
New studies are necessary to describe the body composition, energy expenditure status (e.g., thermic effects of food; resting and 24 hour metabolic rates), and metabolic derangements (e.g., amino acid and steroid metabolism; insulin resistance; fat storage and mobilization) in serum and fat biopsy material from PWS patients. Additional behavior and psychological studies are needed to characterize further and to determine the frequency of personality, behavioral, and psychological findings in PWS individuals. Neuroendocrinological studies should be undertaken with endorphins, serotonin, catecholamines, and other neurotransmitters to determine if there are other abnormalities that can provide useful information on brain function, obesity, behavioral problems, and hormone status in PWS individuals. A better understanding of growth and metabolism may allow for better treatment of obesity (understanding the causation and treatment of obesity in PWS may have direct application to obesity in the general population) and allow for hormone therapy for hypogonadism and growth abnormalities in PWS individuals. Research should be continued with nutritional approaches and behavioral modification programs with emphasis on increased physical activity and pharmacological agents to suppress appetite.
Additional surveys are needed with correlation studies of cytogenetic and DNA findings with the clinical manifestations (e.g., hypopigmentation). This research should allow for better delineation of the syndrome and for better determination of the frequency of uncommon findings (e.g., precocious puberty, thyroid disease). Early diagnosis, particularly during the early stage of PWS, may be achieved through these studies and will be important to avoid morbid obesity.
No consistent neuroanatomical lesions have been identified in PWS individuals; additional detailed necropsy studies of the central nervous system should be undertaken. New diagnostic procedures such as magnetic resonance imaging and positron emission tomography should be used to study the brain of PWS patients. New studies will yield useful information on the neuropathophysiology of PWS individuals and will allow for potential treatment.
Acknowledgments
I thank Pam Phillips for expert preparation of the manuscript and Judy Haynes and Andy Allen for their technical assistance.
Footnotes
NOTE ADDED IN PROOF
Recent DNA studies with four loci from the proximal 15q region from 12 PWS patients predicted the order to be: centromere-D15S9-D15S12-D15Sll-D15S10-qter [Gregory CA, Kirkilionis AJ, Greenberg CR, Chudley AE, Hamerton JL (1989): Detection of molecular rearrangements in Prader-Willi syndrome patients using genomic probes recognizing four loci within PWCR. Am J Med Genet, in press]. Additionally, cloned DNA markers specific for the 15q11–13 region were used to determine the parental contribution of PWS and Angelman syndrome individuals, both syndromes associated with indistinguishable deletions of 15q11–13 [Knoll J, Nicholls R, Magenis R, Graham J Jr. (1989): Angelman and Prader-Willi syndrome share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet _32:_285–290]. The chromosome deletions in PWS are paternally inherited while in Angelman syndrome, a very different clinical disorder characterized by mental retardation, ataxic gait, inappropriate laughter, seizures and a characteristic face, the deletions are maternally inherited [Knoll et al., 1989]. Apparently, the parental origin of the chromosome 15 deletion plays a significant role in the development of the different phenotypes in these two syndromes possibly through a process of genetic imprinting of the chromosome 15s. With the use of DNA markers from the 15q11–13 region, maternal heterodisomy was recently determined from two PWS patients with apparently normal 15q11–13 chromosome regions [Nicholls RD, Knoll JHM, Butler MG, Karam S, Lalande M (1989): Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature, in press]. The presence of maternal heterodisomy for two different chromosome 15s demonstrate that such an anomaly can be associated with a human genetic disorder. Thus, these data indicate that the absence of paternal genetic information from the 15q11–13 region by maternal heterodisomy or by deletion of this region may result in the PWS phenotype. More research is needed to better explain the role of molecular genetics in the causation of PWS and Angelman syndrome.
References
- Afifi AK, Zellweger H. Pathology of muscular hypotonia in the Prader-Willi syndrome. J Neurol Sci. 1969;9:49–61. doi: 10.1016/0022-510x(69)90058-6. [DOI] [PubMed] [Google Scholar]
- Aurias A, Prieur M, Dutrillaux B, Lejeune J. Systemic analysis of 95 reciprocal translocations of autosomes. Hum Genet. 1978;45:250–282. doi: 10.1007/BF00278725. [DOI] [PubMed] [Google Scholar]
- Berry AC, Whittingham AJ, Neville BGR. Chromosome 15 in floppy infants. Arch Dis Child. 1981;56:882–885. doi: 10.1136/adc.56.11.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier DM, Kaplan SL, Havel RJ. The Prader-Willi syndrome: Regulation of fat transport. Diabetes. 1977;26:874–881. doi: 10.2337/diab.26.9.874. [DOI] [PubMed] [Google Scholar]
- Bonuccelli CM, Stetten G, Levitt RC, Levin LS, Pyeritz RE. Prader-Willi syndrome associated with an interstitial deletion of chromosome 15. Johns Hopkins Med J. 1982;151(5):237–42. [PubMed] [Google Scholar]
- Bray GA, Dahms WT, Swerdloff RS, Fiser RH, Atkinson RL, Carrel RE. The Prader-Willi syndrome. Medicine. 1983;62:59–80. [PubMed] [Google Scholar]
- Burke CM, Kousseff BG, Gleeson M, O’Connell BM, Devlin JG. Familial Prader-Willi syndrome. Arch Intern Med. 1987;147:673–675. [PubMed] [Google Scholar]
- Butler MG. Hypopigmentation: A common feature of Prader-Labhart-Willi syndrome. Am J Hum Genet. 1989;45:140–146. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Butler RI, Meaney FJ. The use of skinfold measurements to judge obesity during the early phase of Prader-Labhart-Willi syndrome. Int J Obes. 1988;12:417–422. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Haynes JL, Meaney FJ. Intra-familial and mid-parental-PWS child correlations and heritability estimates of anthropometric measurements in Prader-Willi syndrome families. 1989a Submitted for publication. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Hill JO, Spetalnick BM, Kaler ME. Resting metabolic rates in Prader-Willi syndrome and obese individuals. Dysmorph & Clin Genet. 1989b in press. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Hill JO, Swift LL. Plasma lipid, insulin and glucose levels in Prader-Willi syndrome and obese individuals. Dysmorph & Clin Genet. 1989c in press. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Jenkins BB. Sister chromatid exchange analysis in the Prader-Labhart-Willi syndrome. Am J Med Genet. 1987;28:821–827. doi: 10.1002/ajmg.1320280406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Jenkins BB. Analysis of chromosome breakage in Prader-Labhart-Willi syndrome. Am J Med Genet. 1989;32:514–519. doi: 10.1002/ajmg.1320320418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Jenkins BB, Orth DN. Plasma immunoreactive (β-melanocyte stimulating hormone (lipotropin) levels in individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1987;28:839–844. doi: 10.1002/ajmg.1320280408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Kaler SG, Yu PL, Meaney FJ. Metacarpophalangeal pattern profile analysis in Prader-Labhart-Willi syndrome. Clin Genet. 1982;22:315–320. doi: 10.1111/j.1399-0004.1982.tb01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney FJ. Metacarpophalangeal pattern profile analysis in Prader-Labhart-Willi syndrome. Clin Genet. 1985;28:27–30. doi: 10.1111/j.1399-0004.1985.tb01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney FJ. An anthropometric study of 38 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1987;26:445–455. doi: 10.1002/ajmg.1320260224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986;23:793–809. doi: 10.1002/ajmg.1320230307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Murrell JE, Greene HL. Amino acid profiles in Prader-Willi syndrome and obese individuals. Dysmorph & Clin Genet. 1989d in press. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Palmer CG. Parental origin of chromosome 15 deletion in Prader-Willi syndrome. Lancet. 1983;1:1285–1286. doi: 10.1016/s0140-6736(83)92745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB. Prader-Willi syndrome. Curr Prob Pediatr. 1984;14:1–55. doi: 10.1016/0045-9380(84)90043-4. [DOI] [PubMed] [Google Scholar]
- Cassidy SB. Recurrence risk in Prader-Willi syndrome. Am J Med Genet. 1987;28:59–60. doi: 10.1002/ajmg.1320280109. [DOI] [PubMed] [Google Scholar]
- Cassidy SB, Gainey AJ, Butler MG. Paternal hydrocarbon exposure at conception of Prader-Willi syndrome patients with and without deletions of chromosome 15q. Am J Hum Genet. 1989;44:806–810. [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Holm AV, Thuline HC. Does chromosome 15 proximal long arm deletion predict Prader-Willi syndrome? Clin Res. 1981;29:114A. [Google Scholar]
- Cassidy SB, Thuline HC, Holm VJ. Deletion of chromosome 15 (q11–13) in a Prader-Willi syndrome clinic population. Am J Med Genet. 1984;17:485–495. doi: 10.1002/ajmg.1320170211. [DOI] [PubMed] [Google Scholar]
- Cavalli IJ, Sbalqueiro IJ, Wajntal A, Freire-Maia N. A 15/17 translocation in a patient with Prader-Labhart-Willi syndrome. Hum Hered. 1982;32:149–151. doi: 10.1159/000153279. [DOI] [PubMed] [Google Scholar]
- Charrow J, Balkin N, Cohen MM. Translocations in Prader-Willi syndrome. Clin Genet. 1983;23:304–307. doi: 10.1111/j.1399-0004.1983.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Chasalow FI, Blethen SL, Tobash JG, Myles D, Butler MG. Steroid metabolic disturbances in Prader-Labhart-Willi syndrome. Am J Med Genet. 1987;28:857–864. doi: 10.1002/ajmg.1320280410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DW, Gedde-Dahl T. Report of the committee on the genetic constitution of chromosome 14 and 15. Interim Human Gene Mapping Workshop 9.5. Cytogenet Cell Genet. 1988;49:90–93. doi: 10.1159/000132656. [DOI] [PubMed] [Google Scholar]
- Creel DJ, Bendel CM, Wiesner GL, Wirtschafter JD, Arthur DC, King RA. Abnormalities of the central visual pathways in Prader-Willi syndrome associated with hypopigmentation. N Engl J Med. 1986;314:1606–1609. doi: 10.1056/NEJM198606193142503. [DOI] [PubMed] [Google Scholar]
- de France HF, Beemer FA, Ippel PF. Duplication in chromosome 15q in a boy with the Prader-Willi syndrome; further cytogenetic confusion. Clin Genet. 1984;26:379–382. doi: 10.1111/j.1399-0004.1984.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Donlon TA. Similar molecular deletions on chromosome 15q11.2 are encountered in both the Prader-Willi and Angelman syndromes. Hum Genet. 1988;80:322–328. doi: 10.1007/BF00273644. [DOI] [PubMed] [Google Scholar]
- Donlon TA, Lalande M, Wyman A, Bruns G, Latt SA. Molecular diagnosis and analysis of chromosome 15 microdeletion and lability in Prader-Willi syndrome. Am J Hum Genet. 1985;37:91A. [Google Scholar]
- Donlon TA, Lalande M, Wyman A, Bruns G, Latt SA. Isolation of molecular probes associated with the chromosome 15 instability in the Prader-Willi syndrome. Proc Natl Acad Sci USA. 1986;83:4408–4412. doi: 10.1073/pnas.83.12.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett DP, Roberts SH, Davies P. Unbalanced reciprocal translocations in cases of Prader-Willi syndrome. Hum Genet. 1984;67:156–161. doi: 10.1007/BF00272991. [DOI] [PubMed] [Google Scholar]
- Dunn HG. The Prader-Willi syndrome: Review of the literature and the report of nine cases. Acta Pediatr Scand [Suppl] 1968;186:1–38. doi: 10.1111/j.1651-2227.1968.tb06038.x. [DOI] [PubMed] [Google Scholar]
- Dunn HG, Tze WJ, Alisharan RM, Schulzer M. Clinical experience with 23 cases of Prader-Willi syndrome. In: Holm VA, Sulzbacher S, Pipes PL, editors. Prader-Willi Syndrome. Baltimore: University Park Press; 1981. pp. 69–88. [Google Scholar]
- Elder FFB, Nichols MM, Hood OJ, Harrison WR. Unbalanced translocation (15;17)(q13;p13.3) with apparent Prader-Willi syndrome but without Miller-Dieker syndrome. Am J Med Genet. 1985;20:519–524. doi: 10.1002/ajmg.1320200312. [DOI] [PubMed] [Google Scholar]
- Emberger JM, Rodiere M, Astruc J, Brunnel D. Syndrome de Prader-Willi et translocation 15–15. Ann Genet. 1977;20:297–300. [PubMed] [Google Scholar]
- Fear CN, Multon DE, Berry AC, Heckmatt JZ, Dubowitz V. Chromosome 15 in Prader-Willi syndrome. Dev Med Child Neurol. 1985;27:305–311. doi: 10.1111/j.1469-8749.1985.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Berry C, Mutton D. Prader-Willi syndrome in siblings, due to unbalanced translocation between chromosomes 15 and 22. Arch Dis Child. 1987;62:841–843. doi: 10.1136/adc.62.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesseler WH, Bierich JR. Untersuchungen beim Prader-Lab-hart-Willi syndrom. Monatsschr Kinderheilkd. 1983;131:844–847. [PubMed] [Google Scholar]
- Fleischnick E, Cone TE, Greer G, Wood JW. Prader-Willi syndrome with 15/15 translocation. Am J Hum Genet. 1979;31:94A. [Google Scholar]
- Fracarro M, Zuffardi O, Bühler E, Schinzel A, Simoni G, Witkowski R, Bonifaci E, Caufin D, Cignacco G, Delendi N, Gargantini L, Losanowa T, Marca L, Ullrich E, Vigi V. Deficiency, transposition, and duplication of one 15q region may be alternatively associated with Prader-Willi (or a similar) syndrome. Analysis of seven cases after varying ascertainment. Hum Genet. 1983;64:388–394. doi: 10.1007/BF00292373. [DOI] [PubMed] [Google Scholar]
- Fracarro M, Zuffardi O, Bühler EM, Yurik LP. 15/15 translocation in Prader-Willi syndrome. J Med Genet. 1977;14:275–276. doi: 10.1136/jmg.14.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann-Rieger A, Kohler A, Fuhrmann W. Duplication or insertion in 15q11–13 associated with mental retardation, short stature and obesity—Prader-Willi or Cohen syndrome? Clin Genet. 1984;25:347–352. doi: 10.1111/j.1399-0004.1984.tb02002.x. [DOI] [PubMed] [Google Scholar]
- Fujita H, Sakamoto Y, Hamamoto Y. An extra idic(15)(q11) chromosome in Prader-Willi syndrome. Hum Genet. 1980;55:409–411. doi: 10.1007/BF00290227. [DOI] [PubMed] [Google Scholar]
- Fukushima Y, Niikawa N, Kuroki Y. The Prader-Willi syndrome and interstitial deletion of chromosome 15: High-resolution chromosome analyses of 14 patients with the Prader-Willi syndrome and of 5 suspected infants. Jpn J Hum Genet. 1984;29:1–6. doi: 10.1007/BF01876751. [DOI] [PubMed] [Google Scholar]
- Garau A, Lixi ML, Melis P, Costa G, Nurchi AM. Cytogenetic and clinical aspects of Prader-Willi syndrome. Pediatr Med Chir. 1986;8(6):847–852. [PubMed] [Google Scholar]
- Ginsberg-Fellner F. Growth of adipose tissue in infants, children and adolescents. Int J Obes. 1981;5:605–611. [PubMed] [Google Scholar]
- Goh K, Herrmann MA, Campbell RG, Thompson D. Abnormal chromosome in Prader-Willi syndrome. Clin Genet. 1984;26:597–601. doi: 10.1111/j.1399-0004.1984.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Golden WL, Hanchett JM, Breslin N, Steele MW. Prader-Willi syndrome in black females. Clin Genet. 1984;26:161–163. doi: 10.1111/j.1399-0004.1984.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Greenberg F, Elder FFB, Ledbetter DH. Neonatal diagnosis of Prader-Willi syndrome and its implications. Am J Med Genet. 1987;28:845–856. doi: 10.1002/ajmg.1320280409. [DOI] [PubMed] [Google Scholar]
- Greenberg F, Ledbetter DH. Deletions of proximal 15q without Prader-Willi syndrome. Am J Med Genet. 1987;28:813–820. doi: 10.1002/ajmg.1320280405. [DOI] [PubMed] [Google Scholar]
- Greenswag LR. Adults with Prader-Willi syndrome: A survey of 232 cases. Dev Med Child Neurol. 1987;29:145–152. doi: 10.1111/j.1469-8749.1987.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Hamerton JL. Evidence of different molecular rearrangements of proximal regions of 15q in Prader-Willi syndrome patients. Am J Hum Genet. 1987;41:99A. [Google Scholar]
- Guanti G. A new case of rearrangement of chromosome 15 associated with Prader-Willi syndrome. Clin Genet. 1980;17:423–427. doi: 10.1111/j.1399-0004.1980.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Gurr MI, Jung RT, Robinson MP. Adipose tissue cellularity in man. Int J Obes. 1982;6:419–436. [PubMed] [Google Scholar]
- Gustavson KH, Anneren G, Jagell S. Prader-Willi syndrome in a child with a balanced (X;15) de novo translocation. Clin Genet. 1984;26:245–247. [Google Scholar]
- Hall BD, Smith DW. Prader-Willi syndrome. J Pediatr. 1972;81:286–293. doi: 10.1016/s0022-3476(72)80297-x. [DOI] [PubMed] [Google Scholar]
- Hamilton CR, Scully RE, Kliman B. Hypogonadotropinism in Prader-Willi syndrome. Am J Med. 1972;52:322–329. doi: 10.1016/0002-9343(72)90019-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Hara M, Ando M, Osawa M, Fukuyamay, Takahashi M, Yamada K. Cytogenetic studies of familial Prader-Willi syndrome. Hum Genet. 1984;65:325–330. doi: 10.1007/BF00291556. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ, Smithies A. The Prader-Willi syndrome with a 15/15 translocation. J Med Genet. 1976;13:152–156. doi: 10.1136/jmg.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins LA, Chasalow FI, Blethen SL. Lack of hyperen-dorphinemia in Prader-Willi syndrome. Dysmorph & Clin Genet. 1989 in press. [Google Scholar]
- Holm VA. The diagnosis of Prader-Willi syndrome. In: Holm VA, Sulzbacher S, Pipes PL, editors. Prader-Willi Syndrome. Baltimore: University Park Press; 1981. pp. 27–44. [Google Scholar]
- Hummel M, Neu R, Kousseff BG. Sister chromatid exchanges (SCE) in lymphocytes of Prader-Willi syndrome patients. Am J Hum Genet. 1986;39:343A. [Google Scholar]
- Jaskulsky SR, Stone NN. Hypogonadism in Prader-Willi syndrome. Urology. 1987;29(2):207–208. doi: 10.1016/0090-4295(87)90155-5. [DOI] [PubMed] [Google Scholar]
- Kadotani T, Watanabe Y, Kanata S, Kumada T, Takemura I. A case of the Prader-Willi syndrome having the interstitial deletion of No. 15 chromosome. Proc Jpn Acad. 1986;62:405–407. [Google Scholar]
- Kaplan LC, Wharton R, Elias E, Mandell F, Donlon T, Latt SA. Clinical heterogeneity associated with deletions in the long arm of chromosome 15: Report of 3 new cases and their possible genetic significance. Am J Med Genet. 1987;28:45–53. doi: 10.1002/ajmg.1320280107. [DOI] [PubMed] [Google Scholar]
- Kousseff BG. The cytogenetic controversy in the Prader-Lab-hart-Willi syndrome. Am J Med Genet. 1982;13:431–439. doi: 10.1002/ajmg.1320130412. [DOI] [PubMed] [Google Scholar]
- Kozma N, Scribanu N, Baumiller R, Cochran W, Kenessey S. Prader-Willi syndrome with 14/15 translocation. Am J Hum Genet. 1980;32:116A. [Google Scholar]
- Kucerova M, Strakova M, Polikova Z. The Prader-Willi syndrome with a 15/3 translocation. J Med Genet. 1979;16:234–253. [PMC free article] [PubMed] [Google Scholar]
- Kyriakides M, Silverstone T, Jeffcoate WJ, Laurance B. Effect of naloxone on hyperphagia in Prader-Willi syndrome. Lancet. 1980;1:876–877. doi: 10.1016/s0140-6736(80)91373-2. [DOI] [PubMed] [Google Scholar]
- Labidi F, Cassidy SB. A blind prometaphase study of Prader-Willi syndrome: Frequency and consistency in interpretation of del 15q. Am J Hum Genet. 1986;39:452–460. [PMC free article] [PubMed] [Google Scholar]
- Laurance BM. Hypotonia, mental retardation, obesity and cryptorchidism associated with dwarfism and diabetes in children. Arch Dis Child. 1967;42:126–139. doi: 10.1136/adc.42.222.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxova R, Gilderdale S, Ridler MAC. An aetiological study of 53 female patients from subnormality hospital and of their offspring. J Ment Defic Res. 1973;17:193–225. doi: 10.1111/j.1365-2788.1973.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter DH, Mascarello JT, Riccardi VM, Harper VD, Airhart SD, Strobel RJ. Chromosome 15 abnormalities and the Prader-Willi syndrome. Am J Hum Genet. 1982;34:278–285. [PMC free article] [PubMed] [Google Scholar]
- Ledbetter DH, Riccardi VM, Airhart SD, Strobel RJ, Keenan SB, Crawford JD. Deletions of chromosome 15 as a cause of the Prader-Willi syndrome. N Engl J Med. 1981;304:325–329. doi: 10.1056/NEJM198102053040604. [DOI] [PubMed] [Google Scholar]
- Ledbetter DH, Riccardi VM, Youngbloom SA, Strobel RJ, Keenan BS, Crawford JD, Louro JM. Deletion (15q) as a cause of the Prader-Willi syndrome (PWS) Am J Hum Genet. 1980;32:77A. [Google Scholar]
- Lee PDK, Wilson DM, Rountree L, Hintz RL, Rosenfeld RG. Linear growth response to exogenous growth hormone in Prader-Willi syndrome. Am J Med Genet. 1987;28:865–871. doi: 10.1002/ajmg.1320280411. [DOI] [PubMed] [Google Scholar]
- Lejeune J, Maunoury C, Prieur M, Van den Akker J. Translocation sauteuse (5p:15q), (8q:15q), (12q:15q) Ann Genet (Paris) 1979;22:210–213. [PubMed] [Google Scholar]
- Lubinsky M, Zellweger H, Greenswag L, Larson G, Hansmann I, Ledbetter D. Familial Prader-Willi syndrome with normal chromosomes. Am J Med Genet. 1987;28:37–43. doi: 10.1002/ajmg.1320280106. [DOI] [PubMed] [Google Scholar]
- Magenis RE, Brown MG, Lacy DA, Budden S, LaFranchi S. Is Angelman syndrome an alternate result of del(15)(q11q13)? Am J Med Genet. 1987;28:829–838. doi: 10.1002/ajmg.1320280407. [DOI] [PubMed] [Google Scholar]
- Markovic VD, Cox DW, Wilkinson J. X;14 translocation: An exception to the critical region hypothesis on the human X chromosome. Am J Med Genet. 1985;20:87–96. doi: 10.1002/ajmg.1320200111. [DOI] [PubMed] [Google Scholar]
- Mattei JF, Mattei MG, Giraud F. Prader-Willi syndrome and chromosome 15: A clinical discussion of 20 cases. Hum Genet. 1983;64:356–362. doi: 10.1007/BF00292367. [DOI] [PubMed] [Google Scholar]
- Mattei MG, Souiah N, Mattei JF. Chromosome 15 anomalies and the Prader-Willi syndrome: Cytogenetic analysis. Hum Genet. 1984;66:313–334. doi: 10.1007/BF00287636. [DOI] [PubMed] [Google Scholar]
- Meaney FJ, Butler MG. Craniofacial variation and growth in the Prader-Labhart-Willi syndrome. Am J Phys Anthropol. 1987;74:459–464. doi: 10.1002/ajpa.1330740405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney FJ, Butler MG. Assessment of body composition in Prader-Labhart-Willi syndrome. Clin Genet. 1989a;35(4):300. [Google Scholar]
- Meaney FJ, Butler MG. Characterization of obesity in Prader-Labhart-Willi syndrome: Fatness patterning. Med Anthropol Quart. 1988b;3(3):294–305. doi: 10.1525/maq.1989.3.3.02a00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen KF, Lundsteen C, Hansen FJ. Prader-Willi syndrome and chromosomal mosaicism 46,XY/47,XY,+mar in two cases. Clin Genet. 1979;16:147–150. doi: 10.1111/j.1399-0004.1979.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Miike T, Ogata T, Ohtani Y, Yamaguchi H, Yokoyama Y. Atypical Prader-Willi syndrome with severe developmental delay and emaciation. Brain Dev. 1988;10(3):186–188. doi: 10.1016/s0387-7604(88)80026-3. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Dyggve H. 6;15 translocation with loss of chromosome material in the patient and various chromosome aberrations in family members. Humangenetik. 1973;18:195–202. doi: 10.1007/BF00290596. [DOI] [PubMed] [Google Scholar]
- Morgner KD, Geisthovel W, Neidergerke U, von zur Muhlen A. Hypogonadismus infolge Mangels an Luteotropin-Releasing Hormon (LH-RH) bei Prader-Willi-Syndrom. Dtsch Med Wochenschr. 1974;99:1196–1198. doi: 10.1055/s-0028-1107917. [DOI] [PubMed] [Google Scholar]
- Moric-Petrovic S, Laca Z, Krstic A, Zivkov M. A new case of Prader-Willi syndrome with chromosomal aberration. J Med Genet. 1981;18:481–483. doi: 10.1136/jmg.18.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, Demsey SA, Leonard CO, Dills C. The natural history of Williams syndrome: Growth patterns and morbidity. Clin Res. 1987;35:226A. [Google Scholar]
- Murdock RL, Wurster-Hill DH. Non-reciprocal translocation (5;15), isodicentric (15), and Prader-Willi syndrome. Am J Med Genet. 1986;25:61–69. doi: 10.1002/ajmg.1320250108. [DOI] [PubMed] [Google Scholar]
- Nardella MT, Sulzbacher SI, Worthington-Roberts BS. Activity levels of persons with Prader-Willi syndrome. Am J Ment Defic. 1983;87:498–505. [PubMed] [Google Scholar]
- Nelson RA, Huse DM, Holman RT, Kimbrough BO, Wahner HW, Callaway CW, Hayles AB. Nutrition, metabolism, body composition, and response to the ketogenic diet in Prader-Willi syndrome. In: Holm VA, Sulzbacher S, Pipes PL, editors. Prader-Willi Syndrome. Baltimore: University Park Press; 1981. pp. 105–120. [Google Scholar]
- Nicholls RD, Knoll JH, Glatt K, Hersh JH, Brewster TD, Graham JM, Wurster-Hill D, Wharton R, Latt SA. Restriction fragment length polymorphisms within proximal 15q and their use in molecular cytogenetics and the Prader-Willi syndrome. Am J Med Genet. 1989;33:66–77. doi: 10.1002/ajmg.1320330109. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Tantravahi U, Fuller R, Stroh H, Wharton R, Latt SA. Molecular studies of the proximal long arm of human chromosome 15 and Prader-Willi syndrome. Am J Hum Genet. 1987;41:104A. [Google Scholar]
- Niikawa N, Ishikiriyama S. Clinical and cytogenetic studies of the Prader-Willi syndrome. Hum Genet. 1985;69:22–27. doi: 10.1007/BF00295524. [DOI] [PubMed] [Google Scholar]
- Parra A, Cervantes C, Schultz RB. Immunoreactive insulin and growth hormone responses in patients with Prader-Willi syndrome. J Pediatr. 1973;83:587–593. doi: 10.1016/s0022-3476(73)80219-7. [DOI] [PubMed] [Google Scholar]
- Pauli RM, Meisner LF, Szmanda RJ. “Expanded” Prader-Willi syndrome in a boy with an unusual 15q chromosome deletion. Am J Dis Child. 1983;137:1087–1089. doi: 10.1001/archpedi.1983.02140370047015. [DOI] [PubMed] [Google Scholar]
- Pettigrew AL, Gollin SM, Greenberg F, Riccardi VM, Ledbetter DM. Duplication of proximal 15q as a cause of Prader-Willi syndrome. Am J Med Genet. 1987;28:791–802. doi: 10.1002/ajmg.1320280403. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RA, Tschech L, Irle U, Wundisch GF. Chromosomenaberationen bei Prader-Willi-Labhart-Syndrom-Kritische übersicht, dokumentiert durch vier ungeulöhnliche fälle. Klin Pädiatr. 1987;199:329–335. doi: 10.1055/s-2008-1026814. [DOI] [PubMed] [Google Scholar]
- Phelan MC, Albiez KL, Flannery DB, Stevenson RE. The Prader-Willi syndrome and albinism in a black infant. Proc Greenwood Genet Center. 1988;7:27–29. [Google Scholar]
- Pipes PL, Holm VA. Weight control of children with Prader-Willi syndrome. J Am Diet Assoc. 1973;62:520–524. [PubMed] [Google Scholar]
- Prader A, Labhart A, Willi H. Ein Syndrome von Adipositas, Kleinwuchs, Kryptochismus und Oligophrenie nach myatonieartigem Zustand in Neugeborenenalter. Schweiz Med Wochenschr. 1956;86:1260–1261. [Google Scholar]
- Reed T, Butler MG. Dermatoglyphic features in Prader-Willi syndrome with respect to chromosomal findings. Clin Genet. 1984;25:341–346. doi: 10.1111/j.1399-0004.1984.tb02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JF, Daniel A, Fitzgerald J. Brief clinical report: Atypical phenotype associated with deletion (15)(pter→ q11::q13 → qter) Am J Med Genet. 1987;28:55–58. doi: 10.1002/ajmg.1320280108. [DOI] [PubMed] [Google Scholar]
- Ridler MAC, Garrod O, Berg JM. A case of Prader-Willi syndrome in a girl with small extra chromosome. Acta Paediatr Scand. 1971;60:222–226. doi: 10.1111/j.1651-2227.1971.tb06646.x. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Levitsky LL, Bandini LG, Dietz WW, Walczak A. Energy expenditure and body composition in Prader-Willi syndrome. Metabolism. 1988;37:115–120. doi: 10.1016/s0026-0495(98)90003-8. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Brunzell JD, Bierman EL. Elevated adipose tissue lipoprotein lipase in the pathogenesis of obesity in Prader-Willi syndrome. In: Holm VA, Sulzbacher S, Pipes PL, editors. Prader-Willi Syndrome. Baltimore: University Park Press; 1981. pp. 137–143. [Google Scholar]
- Schwartz S, Max SR, Panny SR, Cohen MM. Deletions of proximal 15q and non-classical Prader-Willi syndrome phenotypes. Am J Med Genet. 1985;20:255–263. doi: 10.1002/ajmg.1320200208. [DOI] [PubMed] [Google Scholar]
- Smith A, Noël M. A girl with the Prader-Willi syndrome and Robertsonian translocation 45,XX,t(14;15)(p11;q11) which was present in three normal family members. Hum Genet. 1980;55:271–273. doi: 10.1007/BF00291777. [DOI] [PubMed] [Google Scholar]
- Soper RT, Mason EE, Printen KJ, Zellweger H. Surgical treatment of morbid obesity in Prader-Willi syndrome. In: Holm VA, Sulzbacher S, Pipes PL, editors. Prader-Willi Syndrome. Baltimore: University Park Press; 1981. pp. 121–135. [Google Scholar]
- Strakowski SM, Butler MG. Paternal hydrocarbon exposure in Prader-Willi syndrome. Lancet. 1987;2:1458. doi: 10.1016/s0140-6736(87)91152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styles SM, Popkin JS. Proximal 15q13 → cen→ 15p deletion causing partial Prader-Willi syndrome (PPWS) Am J Hum Genet. 1981;33:123A. [Google Scholar]
- Tajara EH, Gagliardi ART, Varella-Garcia M. The Prader-Willi syndrome and mosaicism of an extra chromosome. Rev Brasil Genet. 1982;5(1):209–216. [Google Scholar]
- Takano T, Nakagome Y, Nagafuchi S, Tanaka F, Nakamura Y, Nagano T, Tanae A, Hibi I. High-resolution cytogenetic studies in patients with Prader-Willi syndrome. Clin Genet. 1986;30:241–248. doi: 10.1111/j.1399-0004.1986.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Tantravahi U, Nicholls RD, Stroh H, Ringer S, Neve RL, Kaplan L, Wharton R, Wurster-Hill D, Graham JM, Cantu ES, Frias JL, Kousseff B, Latt SA. Quantitative calibration and use of DNA probes for investigating chromosome abnormalities in Prader-Willi syndrome. Am J Med Genet. 1989;33:78–87. doi: 10.1002/ajmg.1320330110. [DOI] [PubMed] [Google Scholar]
- Tasset EM, Hartz JA, Kao F-T. Isolation and analysis of DNA markers specific to human chromosome 15. Am J Hum Genet. 1988;42:854–866. [PMC free article] [PubMed] [Google Scholar]
- Temperani P, Forabosco A. Two complex rearrangements involving X chromosome. Proceedings of the 6th International Congress of Human Genetics; Jerusalem. 1981. p. 179. [Google Scholar]
- Turleau C, Taillard F, Dousseau de Bazignan M, Delepine N, Desbois JC, de Grouchy J. Hypomelanosis of Ito (incontinentia pigmenti achromians) and mosaicism for a microdeletion of 15ql. Hum Genet. 1986;74:185–187. doi: 10.1007/BF00282090. [DOI] [PubMed] [Google Scholar]
- Tylki A, Lech H, Gurkau M, Parcheta B. Syndrome de Prader-Willi et translocation 15/15. Ann Genet (Paris) 1982;25:183–184. [PubMed] [Google Scholar]
- Wajntal A, Setian N, Gonzalez CH, Billerbeck AEC, Ruiz G., Jr The Prader-Willi syndrome and chromosome 15. Proceedings of the 6th International Congress of Human Genetics; Jerusalem. 1981. p. 183. [Google Scholar]
- Wenger SL, Hanchett JM, Steele MW, Maier BV, Golden WL. Clinical comparison of 59 Prader-Willi patients with and without the 15(ql2) deletion. Am J Med Genet. 1987;28(4):881–887. doi: 10.1002/ajmg.1320280413. [DOI] [PubMed] [Google Scholar]
- Wenger SL, Rauch S. Sister chromatid exchange (SCE) on chromosome 15 in Prader-Willi syndrome (PWS) Pediatr Res. 1988;23:335A. [Google Scholar]
- Wiesner GL, Bendel CM, Olds DP, White JG, Arthur DC, Ball DW, King RA. Hypopigmentation in the Prader-Willi syndrome. Am J Hum Genet. 1987;40:431–442. [PMC free article] [PubMed] [Google Scholar]
- Winsor EJT, Welch JP. Prader-Willi syndrome associated with inversion of chromosome 15. Clin Genet. 1983;24:456–461. doi: 10.1111/j.1399-0004.1983.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Winsor EJT, Welch JP, Raftus RA. Prader-Willi syndrome associated with inv(15) Am J Hum Genet. 1982;34:151A. [Google Scholar]
- Wisniewski KP, Witt ME, Ginsberg-Fellner F, Wilner J, Desnick RJ. Prader-Willi syndrome and a bisatellited derivative of chromosome 15. Clin Genet. 1980;18:42–47. doi: 10.1111/j.1399-0004.1980.tb01363.x. [DOI] [PubMed] [Google Scholar]
- Wu RH, Hasen J, Warburton D. Primary hypogonadism and 13/15 chromosome translocation in Prader-Labhart-Willi syndrome (hypogonadism and chromosome translocation in PLW) Horm Res. 1981;15:148–158. doi: 10.1159/000179444. [DOI] [PubMed] [Google Scholar]
- Wulfsberg EA, Sparkes RS, Klisak IJ, Gurfield WB. A (15 → 1) translocation in a patient mosaic for presence or absence of an isodic(15p)(q11) Am J Med Genet. 1982;13:417–421. doi: 10.1002/ajmg.1320130410. [DOI] [PubMed] [Google Scholar]
- Wyandt HE, Patil S, Shah HO, Hanson JW, Zellweger H, Kelly TE, Dolan LM, Wilson WG. Problems in the detection of 15q deletion in patients with Prader-Willi syndrome. Am J Hum Genet. 1981;33:127A. [Google Scholar]
- Zellweger H. Diagnosis and therapy in the first phase of Prader-Willi syndrome. In: Holm VA, Sulzbacher S, Pipes PL, editors. Prader-Willi Syndrome. Baltimore: University Park Press; 1981. pp. 55–68. [Google Scholar]
- Zellweger H, Jayasekara R, Superneau D, Wertelecki W. Prader-Willi syndrome (PWS) with rare chromosome anomaly (47,XY,idic)(15)(pter → q11::q11 → pter) Am J Med Genet. 1987;28:915–916. [Google Scholar]
- Zellweger H, Schneider HJ. Syndrome of hypotonia-hypomentia-hypogonadism-obesity (HHHO) or Prader-Willi syndrome. Am J Dis Child. 1968;115:588–598. doi: 10.1001/archpedi.1968.02100010590009. [DOI] [PubMed] [Google Scholar]
- Zellweger H, Soper RT. The Prader-Willi syndrome. Med Hyg. 1979;37:3338–3345. [Google Scholar]
- Zuffardi O, Bühler EM, Fraccaro M. Chromosome 15 andPrader-Willi syndrome. Clin Genet. 1978;14:315–316. [Google Scholar]