Cross-regulation of TNF and IFN-α in autoimmune diseases (original) (raw)
Abstract
Cytokines, most particularly TNF and type I IFN (IFN-αβ), have been long considered essential elements in the development of autoimmunity. Identification of TNF in the pathogenesis of rheumatoid arthritis and TNF antagonist therapy represent successes of immunology. IFN-αβ plays a major role in systemic lupus erythematosus (SLE), a prototype autoimmune disease characterized by a break of tolerance to nuclear components. Here, we show that TNF regulates IFN-α production in vitro at two levels. First, it inhibits the generation of plasmacytoid dendritic cells (pDCs), a major producer of IFN-αβ, from CD34+ hematopoietic progenitors. Second, it inhibits IFN-α release by immature pDCs exposed to influenza virus. Neutralization of endogenous TNF sustains IFN-α secretion by pDCs. These findings are clinically relevant, as five of five patients with systemic juvenile arthritis treated with TNF antagonists display overexpression of IFN-α-regulated genes in their blood leukocytes. These results, therefore, might provide a mechanistic explanation for the development of anti-dsDNA antibodies and lupus-like syndrome in patients undergoing anti-TNF therapy.
Keywords: autoimmunity, cytokines, dendritic cells
Cytokines are key players in immunity and autoimmunity. Type I interferons (IFN-αβ), major effectors in response to viral infection (1), play a role in systemic lupus erythematosus (SLE), a prototype autoimmune disease characterized by a break of tolerance to nuclear components (2, 3). In 1979, Notkins and colleagues (4) reported the presence of IFN activity in the serum of patients with autoimmune diseases including SLE, arthritis, and scleroderma. The findings were subsequently confirmed, mainly in SLE (5). Further confirmation of the role of IFN-αβ in SLE came from the studies demonstrating induction of autoimmunity during IFN-αβ therapy (6) and existence of circulating inducers of IFN-αβ in SLE patients' blood (7).
We have shown previously that unabated activation of myeloid dendritic cells (mDCs) driven by high IFN-α may underlie immune alterations in SLE. In SLE, the plasmacytoid dendritic cells (pDCs) release large amounts of IFN-α (8, 9), which induces mDC maturation. The mature DCs that have captured apoptotic cells activate, rather than silence, autoreactive T cells and B cells, leading to the increased production of autoantibodies by plasma cells, the generation of which might also be explained by overexpression of IFN-α (10). All patients with active SLE display an IFN-α biosignature in their blood (9, 11), thus underscoring the role of this cytokine in SLE pathogenesis (12, 13). Furthermore, crossing both NZB and B6 lpr/lpr mice with a type I IFN receptor knockout strain significantly decreases morbidity and prolongs mouse survival (13, 14), thus demonstrating the importance of IFN-αβ in mouse models of SLE as well.
TNF, a major factor in response to bacterial infection, contributes to the pathogenesis of several autoimmune diseases, including rheumatoid arthritis (RA) and Crohn's disease (15). TNF antagonists have proven to be the most efficient therapy for RA thus far (15). Despite a remarkable safety profile, clinical complications such as reactivation of tuberculosis (16) and reversible SLE (17) have been observed. Indeed, an increased titer of anti-dsDNA antibodies has been found in up to 15% of RA patients on anti-TNF therapy (15). About 0.2% of RA patients treated with anti-TNF antibody develop clinical symptoms of SLE that are reversible upon discontinuation of the treatment. Conversely, anti-TNF therapy is not effective in pediatric SLE patients. A recent study of six adult SLE patients treated with TNF blockade showed increased titers of anti-dsDNA antibodies in four patients (18). However, improvement in clinical symptoms of arthritis and decreased proteinuria were also observed (18). Based on these observations, we surmised that there exists a reciprocal regulation between TNF and IFN-α in human autoimmunity. Accordingly, we report here that TNF blockade is consistently associated with IFN-α signature in blood cells. To understand the mechanism of this, we analyzed pDCs because they are considered major IFN-αβ producing cells (19, 20). We report that TNF regulates IFN-α production by pDCs, thus providing a potential mechanistic explanation for lupus-like complications of TNF blockade.
Materials and Methods
Patients and Controls. After informed consent [Institutional Review Board (IRB) 0199017], blood was obtained from pediatric patients who satisfied diagnostic criteria of the American College of Rheumatology for SLE and systemic onset juvenile idiopathic arthritis (SOJIA) and controls (children visiting the clinic for reasons other than autoimmunity or infectious diseases). SLE patients were newly diagnosed and untreated at the time of sampling. Their SLE disease activity index ranged from 4–20. SOJIA patients were selected based on whether they were receiving i.v. anti-TNF therapy (infliximab) (n = 5) or not (n = 8). All patients in the infliximab treatment group were active, as defined by the presence of daily fever and/or active arthritis (Table 1). Only two patients in the non-infliximab group were inactive at the time of blood sampling: one patient was in complete remission (no symptoms and on no medication); another patient was asymptomatic but on long-term nonsteroidal antiinflammatory drugs and s.c. methotrexate (Table 1). All SOJIA patients had negative antinuclear antibody titers at the time of diagnosis and before starting infliximab therapy. Blood leukocytes were isolated on Ficoll gradient and either used fresh for cytokine release experiments or immediately processed for RNA extraction for microarray analysis. CD34+ hematopoietic progenitor cells (HPCs) were obtained from healthy volunteers (IRB 097-053) who received recombinant granulocyte colony-stimulating factor (Neupogen, Amgen Biologicals) 10 μg/kg per day s.c. for 5 consecutive days for HPC mobilization and then underwent leukapheresis to collect CD34+ HPCs. The cells were processed by using the ISOLEX cell-separator system (Baxter, Deerfield, IL) to obtain an enriched population of CD34+ HPCs.
Table 1. Patient characteristics.
| Patient no. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient data | SYS1 | SYS2 | SYS3 | SYS4 | SYS5 | SYS11 | SYS12* | SYS13* | SYS6 | SYS7 | SYS8 | SYS9 | SYS10 |
| Age, years | 12 | 13 | 7 | 2 | 14 | 5 | 4 | 9 | 2 | 10 | 3 | 8 | 16 |
| Sex | F | M | M | F | F | F | M | F | F | F | F | M | F |
| Fever | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes |
| Arthritis | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Nonsteroidal antiinflammatory drugs | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| s.c. methotrexate | Yes | No | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| Oral prednisone | Yes | No | No | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| i.v. solumedrol | No | No | No | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| Infliximab | No | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| No. of infusions† | 3 | 11 | 2 | 5 | 3 | ||||||||
| Dose, mg/kg | 5 | 7 | 5 | 5 | 5 | ||||||||
| Seroconversion | Yes | No | Yes | No | No |
Generation of pDCs. CD34+45RA- HPCs were sorted by using CD34-FITC and CD45RA-PE and were cultured in 96-well plates, 5 × 104 cells per 200 μl of Yssel's medium (Irvine Scientific) with 2% heat-inactivated pooled human AB serum and the recombinant human cytokines FLT3-ligand (FLT3-L) (100 ng/ml; R & D Systems) and thrombopoietin (TPO) (35 ng/ml; R & D Systems). TNF was added at the onset of culture (100 ng/ml unless otherwise indicated; R & D Systems); the cells were washed at day 8 and recultured in fresh medium with FLT3-L and TPO. Cultures were refreshed every 7 days by removing half a volume of spent media and adding fresh media with cytokines. Wells were split when necessary.
Quantification of Cytokine Production. Cells [sorted pDCs or total peripheral blood mononuclear cells (PBMCs)] were cultured overnight in RPMI medium 1640 and 10% FCS and triggered in vitro with live influenza virus (A/PR/8/34; Charles River Laboratories) and TNF (100 ng/ml; R & D Systems) in 96-microwell plates. After 24 h, the plates were centrifuged to collect the supernatants. The cells were incubated for a further 24 h in RPMI medium 1640 and 10%FCS with live influenza virus. Cell counts were performed at 24 and 48 h. Supernatants were analyzed by a human IFN-α kit (BioSource International, Camarillo, CA) by using a sandwich immunoassay (precoated microtiter strip plates). The kit is based on an ELISA with antisecondary antibody conjugated to horseradish peroxydase. Tetramethyl-benzidine is the substrate.
Microarray Analysis. Total RNA extracted from samples was used to generate cRNA targets, subsequently hybridized to human U95Av2 or U133A oligonucleotide probe arrays (Affymetrix, Santa Clara, CA) according to standard protocols provided by the manufacturer. An absolute expression analysis was carried out by using Affymetrix mas 5.0, and the data were imported into genespring software (Silicon Genetics, Santa Clara, CA) for further analyses.
Results
Patients on Anti-TNF Therapy Display Increased Transcription of IFN-α-Regulated Genes. Because anti-TNF therapy can induce SLE and because SLE appears to be driven by IFN-α, we wondered whether patients undergoing anti-TNF treatment would demonstrate signs of increased IFN-α expression/activity. To this end, we performed microarray analysis of Ficoll-separated PBMCs of two age-matched pediatric cohorts: eight SOJIA patients without anti-TNF therapy and five SOJIA patients undergoing therapy with infliximab (Table 1). Six of eight patients without anti-TNF therapy had active disease, and two were newly diagnosed (Table 1). SOJIA patients were analyzed and compared with a cohort of 13 healthy children and 10 children with active, untreated SLE (9). Among SOJIA patients on anti-TNF therapy, one patient had been treated for 1 month (no. 8 in Table 1), three patients for 4 months (nos. 6, 9, and 10), and one patient for 12 months (no. 7). All SOJIA patients had negative antinuclear antibody and anti-dsDNA titers at the time of diagnosis and before starting infliximab therapy.
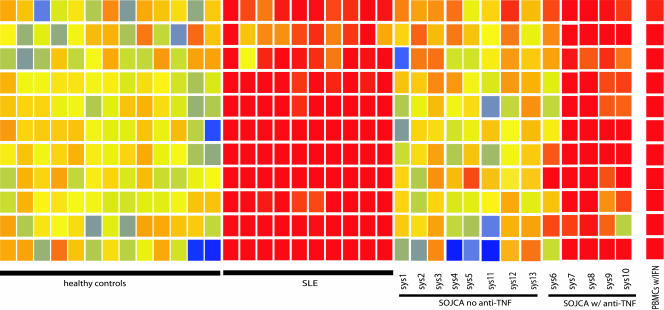
We have monitored a set of 11 genes that we earlier found to be highly up-regulated by IFN-α either in vitro or in vivo in blood of patients with active SLE (9). As shown in Fig. 1, SOJIA patients who did not receive anti-TNF therapy displayed basal expression levels of IFN-α-regulated genes, consistent with our preliminary observation (9). In contrast, five SOJIA patients analyzed at various time points during anti-TNF therapy (Table 1) displayed significantly increased (P < 0.005 in ANOVA) transcription of IFN-α-regulated genes (Fig. 1 and Table 2, which is published as supporting information on the PNAS web site). None of the patients had any indication of viral infection at the time of analysis. Furthermore, two patients (nos. 6 and 8) seroconverted and displayed increased titers of anti-dsDNA antibodies (Table 1). Thus, a pharmacological TNF inhibition in SOJIA patients is associated with an increased transcription of IFN-α-regulated genes.
Fig. 1.
PBMCs from SOJIA patients treated with anti-TNF therapy display increased transcription of IFN-α-regulated genes. Gene expression was analyzed by using U95Av2 and U133 Affymetrix chips and was normalized to the median of healthy controls. A set of IFN-α-regulated genes, identified by the analysis of SLE patients as described in ref. 9, was compared in patients with or without anti-TNF therapy.
TNF Inhibits Virus-Induced IFN-α Release by Healthy and SLE PBMCs. We next analyzed the mechanisms through which TNF antagonists might lead to increased transcription of IFN-α-regulated genes. First, we tested whether TNF might inhibit the ability of PBMCs to produce IFN-α. As shown in Fig. 2_a_, PBMCs from healthy donors produced up to 30 ng/ml IFN-α (mean ± SD, 22.5 ± 7.2 ng/ml, n = 5) when cultured overnight with influenza virus (5 × 103 viral particles). Adding TNF at the onset of the culture inhibited the production of IFN-α (8.4 ± 2 ng of IFN-α/ml, 58 ± 21% inhibition at 100 ng/ml TNF, P < 0.01). Maximum inhibition was seen already at 10 ng/ml, and adding increased amounts of TNF (up to 1,000 ng/ml) did not result in further inhibition (data not shown). The IFN-α secretion from healthy-donor PBMCs exposed in vitro to SLE Ig was also found to be partially inhibited by TNF (21).
Fig. 2.
Exogenous TNF inhibits IFN-α release from PBMCs exposed to influenza virus. PBMCs obtained from healthy volunteers (a) or SLE patients (b) were cultured overnight with live influenza virus with or without TNF. IFN-α release to supernatants (y axis) was measured by ELISA. Average ± SD of IFN-α release in cultures with or without addition of TNF. n = 5, healthy volunteer samples; n = 9, SLE patient samples.
SLE patients' PBMCs that were cultured overnight with influenza virus (5 × 103 viral particles by hemagglutinin titer) produced significantly less IFN-α (8.9 ± 5 ng/ml, 1–16 ng/ml, P < 0.0006) than did PBMCs from healthy donors (Fig. 2_b_). This result is consistent with an earlier demonstration of decreased numbers of circulating pDCs (8) and natural IFN-α-producing cells (22) in SLE patients. As shown in Fig. 2_b_, TNF inhibited IFN-α release (P < 0.01). Taken together, these data suggest that TNF inhibits the production of IFN-α by PBMCs exposed to influenza virus.
Exogenous TNF Inhibits IFN-α Release by Targeting pDCs. pDCs are a major source of IFN-α (19, 20), and IFN-α release in response to virus is abolished in healthy PBMCs depleted of CD123+ and/or BDCA-4+ cells (8, 10). Therefore, we wondered whether TNF was inhibiting influenza virus-induced IFN-α secretion from PBMCs by targeting pDCs.
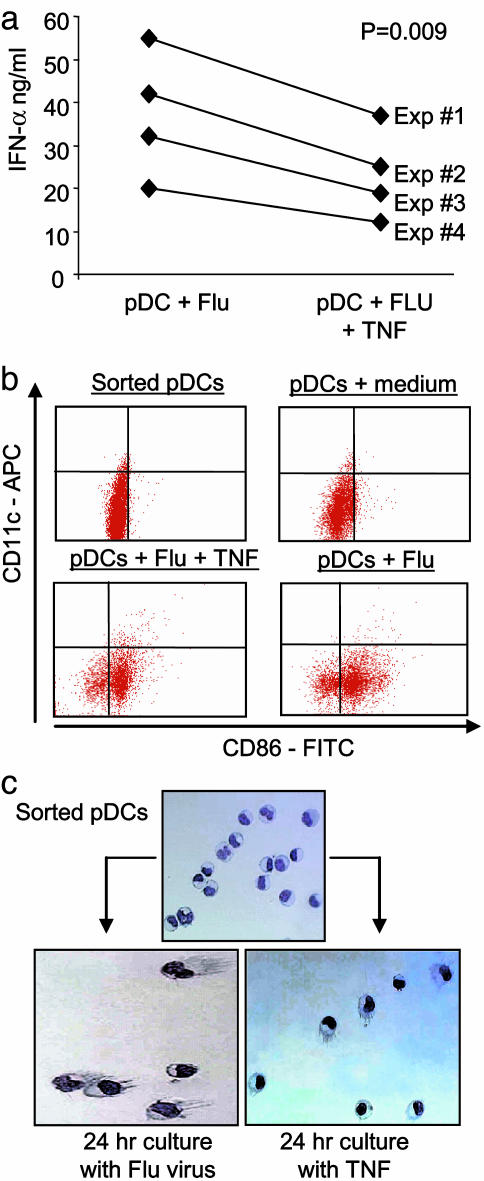
To this end, pDCs were generated in vitro by culturing CD34+CD45RA- HPCs with FLT3-L and TPO (23). Influenza virus triggered sorted HLA-DR+CD123+CD11c- pDCs to release up to 60 ng/ml IFN-α (37 ± 15 ng/ml, mean of four experiments). Adding TNF at the onset of pDC/influenza virus cultures inhibited the production of IFN-α by up to 40% (P = 0.009; Fig. 3_a_). The inhibition of IFN-α production was due not to decreased pDC survival (not shown) but to the cells' differentiation. Indeed, TNF-treated pDCs acquired the phenotype of mature DCs, including the expression of CD86 and CD83 (Fig. 3_b_) and the presence of dendrites (Fig. 3_c_), consistent with earlier results (24). As mature pDCs cease to secrete IFN-α, it is likely that TNF inhibits IFN-α production by virally triggered pDCs through induction of their maturation.
Fig. 3.
Exogenous TNF inhibits IFN-α release from pDCs exposed to influenza virus. pDCs were generated from CD34+ HPCs and sorted based on HLA-DR+CD123+CD11c- phenotype. (a) IFN-α release (y axis) after overnight culture with influenza virus with or without exogenous TNF. Paired t test was used. (b) Flow cytometry analysis of pDC phenotype after sorting or overnight culture with medium (these two conditions are from one experiment) or influenza virus with or without TNF (these two conditions are from another experiment). Four log decade scales are shown. (c) Giemsa staining of pDCs just after sorting, 24-h culture with influenza virus, or with TNF. Note the presence of prominent cytoplasmic protrusions (dendrites) on cells cultured with TNF or virus.
Neutralization of Endogenous TNF Sustains IFN-α Release by pDCs. Because TNF promotes pDC maturation, we surmised that neutralization of endogenous TNF might increase/sustain IFN-α release. Thus, pDCs were exposed to influenza virus without or with a neutralizing anti-TNF monoclonal antibody. As shown in Fig. 4_a_, after 24 h of culture, neither control mAb nor anti-TNF mAb affected the levels of secreted IFN-α. However, after resuspension in fresh medium, anti-TNF pretreated pDCs reexposed for 24 h to influenza virus produced about three times as much as IFN-α as did pDCs pretreated with control antibody (Fig. 4_b_). The anti-TNF antibody pretreatment also inhibited the virus-induced maturation of pDCs, as shown by the limited increase of surface HLA-DR expression (Fig. 4_c_). Interestingly, kinetics studies indicated that near maximum levels of TNF were reached 5 h after exposure to virus, when IFN-α levels represent ≈20% of maximal levels that are reached at 20–24 h (not shown).
Fig. 4.
Blocking endogenous TNF sustains IFN-α release from pDCs exposed to influenza virus. pDCs were generated and purified as described in Fig. 3 and cultured with influenza virus. (a) Primary cultures were carried out in the presence or absence of isotype control or TNF-neutralizing mAb. Supernatants were harvested at 24 h after centrifugation of culture plates, and IFN-α release (y axis) was measured by ELISA. (b) Cell pellets were resuspended in fresh medium (without mAbs), influenza virus was added, and cultures were carried out for an additional 24 h. Supernatants from secondary cultures were analyzed by ELISA (y axis). (c) Flow cytometry analysis of HLA-DR expression by pDCs cultured for 24 h with influenza virus with or without TNF-neutralizing mAb (Right). Fluorescence intensity is shown on the x axis. Data are representative of three experiments.
Thus, endogenous TNF controls IFN-α production by pDCs.
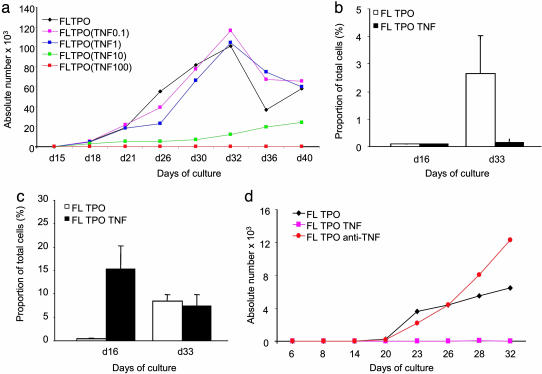
TNF Blocks pDC Generation from CD34+ HPCs in Vitro. Given that TNF plays a major role in the regulation of hematopoiesis (25), we surmised that TNF might also affect pDC ontogeny. To this end, CD34+CD45RA- HPCs were cultured with FLT3-L and TPO with or without TNF (100 ng/ml) during the first 7 days of culture. Cells were then washed of TNF and cultured for 3 additional weeks with FLT3-L, either alone or with TPO. A phenotypic analysis at day 28 revealed the complete absence of CD123+CD11c- pDCs in TNF-primed cultures (data not shown). The inhibition of differentiation was specific to pDCs; CD123-CD11c+ mDCs were not affected. Kinetics analysis further confirmed that TNF-primed CD34+ HPCs shut down their potential to generate pDCs both in relative (not shown) and absolute (Fig. 5_a_) numbers. In contrast, mDCs appear much earlier in cultures of TNF-primed CD34+ HPCs (Fig. 5 b and c), consistent with our earlier studies (26). Conversely, in two of three experiments, adding anti-TNF mAb in the first week of HPC culture resulted in a >2-fold increase in the percentage and number of cells with a pDC phenotype (Fig. 5_d_). Neither IFN-α nor IL-6, other major pDC products, was found to inhibit pDC ontogeny (data not shown). Thus, TNF blocks pDC differentiation at the early stages of hematopoiesis.
Fig. 5.
TNF blocks differentiation of pDCs from CD34+ HPCs. CD34+ HPCs isolated from granulocyte colony-stimulating factor mobilized blood of healthy volunteers were cultured with FLT3-L and TPO with or without TNF. (a) Representative experiment of three performed that demonstrate the kinetics of pDC differentiation and dose response to TNF. Absolute numbers of pDCs are shown on the y axis. (b and c) Average ± SD percentage of pDCs (b, y axis) and mDCs (c, y axis) at three time points (x axes) in CD34+ HPC cultures with FLT3-L plus TPO with (filled bars) or without (open bars) TNF at 100 ng/ml. Data shown are for three experiments. (d) Absolute numbers of pDCs in cultures of CD34+ HPCs with or without addition of anti-TNF mAb. Data are representative of three experiments.
Discussion
It is now well accepted that TNF plays a critical role in the pathogenesis of certain autoimmune diseases, such as RA (15) and psoriasis (27). Likewise, there is ample evidence that IFN-α plays a pivotal role in another set of autoimmune diseases, including SLE (8, 9, 11), thyroiditis (28, 29), and diabetes (30, 31).
The lupus-like syndrome observed in a fraction of patients undergoing therapy with TNF antagonists led us to hypothesize that TNF might actually act as an antagonist of the type I IFN pathway. Accordingly, the results from the current study demonstrate that (i) TNF inhibits pDC ontogeny, (ii) neutralization of endogenous TNF sustains IFN-α secretion by pDCs, and (iii) treatment with TNF antagonists is associated with the increased transcription of IFN-α-regulated genes in blood leukocytes of patients with SOJIA.
TNF has a major inhibitory impact on pDC ontogeny. Consistent with earlier studies in both humans and mice (23, 32), the inhibition of pDC differentiation is complete and cannot be rescued by the presence of TPO, a potent stimulator of HPC growth that synergizes with FLT3-L. pDC development is regulated by transcription factors Id-2 and Id-3, and their overexpression in HPCs blocks pDC differentiation (33). Because TNF has been shown to increase Id1–3 expression in astrocytes and microglia of rat brains (34), its inhibitory effect on pDC ontogeny might be explained by up-regulation of Id protein expression in HPCs. Although TNF inhibits FLT3-L-induced pDC development, it increases the generation of mDCs, a finding that is in line with earlier demonstration of the enhancing effect of TNF on mDC differentiation driven by granulocyte-macrophage colony-stimulating factor and/or IL-3 (25). Thus, TNF acts as a major regulator of DC ontogeny.
Exogenous TNF partially inhibits IFN-α release from immature pDCs exposed to influenza virus. This inhibition might be due to accelerated pDC maturation, a stage when pDCs cease secreting IFN-α (24). Indeed, the neutralization of endogenous TNF results in an increased IFN-α secretion, which is associated with a decreased maturation of pDCs. Therefore, decreased TNF bioavailability might lead to the increased IFN-α bioavailability in SLE and in patients under therapy with TNF antagonists. The present study demonstrates that SOJIA patients treated with TNF antagonists display increased transcription of IFN-α-regulated genes in their PBMCs. Thus, sustained release of IFN-α due to TNF blockade might provide a mechanistic explanation for the increased anti-dsDNA antibody titers, mostly IgM, and reversible lupus-like syndrome observed in arthritis patients treated with TNF antagonists. Development of lupus-like syndrome seems to be associated with the presence of IgG anti-dsDNA antibodies (35, 36). Such increased titers could also be ascribed to sustained bioactivity of IFN-α, because its role in the Ig isotype switch has been demonstrated in both humans (37) and mice (38).
With regard to SLE, it has been demonstrated that SLE patients have circulating soluble TNF receptors, the levels of which correlate with disease activity (39). These soluble receptors are likely to block the biological activity of TNF (40), either autocrine or produced by other cells, thereby leading to uncontrolled IFN-α release.
TNF and IFN-αβ are regarded as essential elements in the development of autoimmunity. It is striking, though, that they lead to different autoimmune diseases. Indeed, overexpression of TNF in mice not prone to autoimmunity promotes multiorgan inflammation, experimental autoimmune encephalomyelitis (EAE), arthritis, and inflammatory bowel disease (41). Interestingly, lupus-prone NZB/W mice have a genetic deficiency of TNF (42). Consequently, these mice benefit from replacement therapy with recombinant TNF with regard to the development of nephritis (43). Our results would suggest that this beneficial effect in lupus-prone mice might be due to TNF-mediated down-regulation of the type I IFN pathway. Likewise, there is evidence that IFN-αβ may regulate TNF. For example, IFN-β knockout mice seem more susceptible to EAE induction than wild-type mice (44), and administration of IFN-β to mice with EAE inhibits disease progression (45). In humans, IFN-β can inhibit in vitro TNF production by microglia, either directly or by attenuating the ability of T cells to trigger TNF secretion by microglial cells (46). This regulatory mechanism might partially explain the beneficial effect of IFN-β therapy in this disease (46). Indeed, PBMCs from healthy volunteers injected with IFN-β show markedly decreased secretion of TNF and lymphotoxin compared with placebo-treated volunteers (47).
Thus, the cross-regulation between TNF and type I IFN provides a framework that could explain their role in the pathogenesis of different autoimmune diseases.
Supplementary Material
Supporting Table
Acknowledgments
We thank Drs. Joseph Fay, Dorothee Stichweh, and Michael Ramsay for continuous support. J.B. is the recipient of the Caruth Chair for Transplantation Immunology Research, and A.K.P. is the recipient of the Michael A. Ramsay Chair for Cancer Immunology Research. This work was supported by the Baylor Health Care Systems Foundation, the Tietze Foundation, the Histiocytosis Association of America, and National Institutes of Health Grant CA 78846 (to J.B.).
Author contributions: A.K.P., V.P., and J.B. designed research; A.K.P., J.-P.B., L.B., and V.P. performed research; A.K.P., J.-P.B., L.B., V.P., and J.B. analyzed data; and A.K.P., V.P., and J.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: pDC, plasmacytoid dendritic cell; mDC, myeloid dendritic cell; SLE, systemic lupus erythematosus; HPC, hematopoietic progenitor cell; RA, rheumatoid arthritis; SOJIA, systemic onset juvenile idiopathic arthritis; PBMC, peripheral blood mononuclear cell; FLT3-L, FLT3-ligand; TPO, thrombopoietin.
References
- 1.Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. (1999) Annu. Rev. Immunol. 17**,** 189-220. [DOI] [PubMed] [Google Scholar]
- 2.Shlomchik, M. J., Craft, J. E. & Mamula, M. J. (2001) Nat. Rev. Immunol. 1**,** 147-153. [DOI] [PubMed] [Google Scholar]
- 3.Crow, M. K. & Kirou, K. A. (2001) Curr. Opin. Rheumatol. 13**,** 361-369. [DOI] [PubMed] [Google Scholar]
- 4.Hooks, J. J., Moutsopoulos, H. M., Geis, S. A., Stahl, N. I., Decker, J. L. & Notkins, A. L. (1979) N. Engl. J. Med. 301**,** 5-8. [DOI] [PubMed] [Google Scholar]
- 5.Preble, O. T., Black, R. J., Friedman, R. M., Klippel, J. H. & Vilcek, J. (1982) Science 216**,** 429-431. [DOI] [PubMed] [Google Scholar]
- 6.Ronnblom, L. E., Alm, G. V. & Oberg, K. E. (1991) Ann. Intern. Med. 115**,** 178-183. [DOI] [PubMed] [Google Scholar]
- 7.Vallin, H., Blomberg, S., Alm, G. V., Cederblad, B. & Ronnblom, L. (1999) Clin. Exp. Immunol. 115**,** 196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. (2001) Science 294**,** 1540-1543. [DOI] [PubMed] [Google Scholar]
- 9.Bennett, L., Palucka, A. K., Arce, E., Cantrell, V., Borvak, J., Banchereau, J. & Pascual, V. (2003) J. Exp. Med. 197**,** 711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jego, G., Palucka, A. K., Blanck, J. P., Chalouni, C., Pascual, V. & Banchereau, J. (2003) Immunity 19**,** 225-234. [DOI] [PubMed] [Google Scholar]
- 11.Baechler, E. C., Batliwalla, F. M., Karypis, G., Gaffney, P. M., Ortmann, W. A., Espe, K. J., Shark, K. B., Grande, W. J., Hughes, K. M., Kapur, V., et al. (2003) Proc. Natl. Acad. Sci. USA 100**,** 2610-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardin, J. A. (2003) J. Exp. Med. 197**,** 681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santiago-Raber, M. L., Baccala, R., Haraldsson, K. M., Choubey, D., Stewart, T. A., Kono, D. H. & Theofilopoulos, A. N. (2003) J. Exp. Med. 197**,** 777-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kono, D. H., Baccala, R. & Theofilopoulos, A. N. (2003) Autoimmunity 36**,** 503-510. [DOI] [PubMed] [Google Scholar]
- 15.Feldmann, M. & Maini, R. N. (2001) Annu. Rev. Immunol. 19**,** 163-196. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Reino, J. J., Carmona, L., Valverde, V. R., Mola, E. M. & Montero, M. D. (2003) Arthritis Rheum. 48**,** 2122-2127. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers, S. (2003) Ann. Rheum. Dis. 62**,** Suppl. 2, ii37-ii42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aringer, M., Graninger, W. B., Steiner, G. & Smolen, J. S. (2004) Arthritis Rheum. 50**,** 3161-3169. [DOI] [PubMed] [Google Scholar]
- 19.Siegal, F. P., Kadowaki, N., Shodell, M., Fitzgerald-Bocarsly, P. A., Shah, K., Ho, S., Antonenko, S. & Liu, Y. J. (1999) Science 284**,** 1835-1837. [DOI] [PubMed] [Google Scholar]
- 20.Cella, M., Jarrossay, D., Facchetti, F., Alebardi, O., Nakajima, H., Lanzavecchia, A. & Colonna, M. (1999) Nat. Med. 5**,** 919-923. [DOI] [PubMed] [Google Scholar]
- 21.Bave, U., Vallin, H., Alm, G. V. & Ronnblom, L. (2001) J. Autoimmun. 17**,** 71-80. [DOI] [PubMed] [Google Scholar]
- 22.Ronnblom, L. & Alm, G. V. (2001) J. Exp. Med. 194**,** F59-F63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, W., Antonenko, S., Sederstrom, J. M., Liang, X., Chan, A. S., Kanzler, H., Blom, B., Blazar, B. R. & Liu, Y. J. (2004) Blood 103**,** 2547-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadowaki, N., Antonenko, S., Lau, J. Y. & Liu, Y. J. (2000) J. Exp. Med. 192**,** 219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caux, C., Saeland, S., Favre, C., Duvert, V., Mannoni, P. & Banchereau, J. (1990) Blood 75**,** 2292-2298. [PubMed] [Google Scholar]
- 26.Caux, C., Dezutter-Dambuyant, C., Schmitt, D. & Banchereau, J. (1992) Nature 360**,** 258-261. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb, A. B. (2003) J. Am. Acad. Dermatol. 49**,** S112-S117. [DOI] [PubMed] [Google Scholar]
- 28.Conlon, K. C., Urba, W. J., Smith, J. W., II, Steis, R. G., Longo, D. L. & Clark, J. W. (1990) Cancer 65**,** 2237-2242. [DOI] [PubMed] [Google Scholar]
- 29.Prummel, M. F. & Laurberg, P. (2003) Thyroid 13**,** 547-551. [DOI] [PubMed] [Google Scholar]
- 30.Foulis, A. K., Farquharson, M. A. & Meager, A. (1987) Lancet 2**,** 1423-1427. [DOI] [PubMed] [Google Scholar]
- 31.Stewart, T. A., Hultgren, B., Huang, X., Pitts-Meek, S., Hully, J. & MacLachlan, N. J. (1993) Science 260**,** 1942-1946. [DOI] [PubMed] [Google Scholar]
- 32.Gilliet, M., Boonstra, A., Paturel, C., Antonenko, S., Xu, X. L., Trinchieri, G., O'Garra, A. & Liu, Y. J. (2002) J. Exp. Med. 195**,** 953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spits, H., Couwenberg, F., Bakker, A. Q., Weijer, K. & Uittenbogaart, C. H. (2000) J. Exp. Med. 192**,** 1775-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzeng, S. F., Kahn, M., Liva, S. & De Vellis, J. (1999) Glia 26**,** 139-152. [DOI] [PubMed] [Google Scholar]
- 35.Charles, P. J., Smeenk, R. J., De Jong, J., Feldmann, M. & Maini, R. N. (2000) Arthritis Rheum. 43**,** 2383-2390. [DOI] [PubMed] [Google Scholar]
- 36.Swale, V. J., Perrett, C. M., Denton, C. P., Black, C. M. & Rustin, M. H. (2003) Clin. Exp. Dermatol. 28**,** 604-607. [DOI] [PubMed] [Google Scholar]
- 37.Litinskiy, M. B., Nardelli, B., Hilbert, D. M., He, B., Schaffer, A., Casali, P. & Cerutti, A. (2002) Nat. Immunol. 3**,** 822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkelman, F. D., Svetic, A., Gresser, I., Snapper, C., Holmes, J., Trotta, P. P., Katona, I. M. & Gause, W. C. (1991) J. Exp. Med. 174**,** 1179-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattorno, M., Picco, P., Barbano, G., Stalla, F., Sormani, M. P., Buoncompagni, A., Gusmano, R., Borrone, C. & Pistoia, V. (1998) J. Rheumatol. 25**,** 361-365. [PubMed] [Google Scholar]
- 40.Porteu, F. & Nathan, C. (1990) J. Exp. Med. 172**,** 599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kollias, G. & Kontoyiannis, D. (2002) Cytokine Growth Factor Rev. 13**,** 315-321. [DOI] [PubMed] [Google Scholar]
- 42.Jacob, C. O., Fronek, Z., Lewis, G. D., Koo, M., Hansen, J. A. & McDevitt, H. O. (1990) Proc. Natl. Acad. Sci. USA 87**,** 1233-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob, C. O. & McDevitt, H. O. (1988) Nature 331**,** 356-358. [DOI] [PubMed] [Google Scholar]
- 44.Teige, I., Treschow, A., Teige, A., Mattsson, R., Navikas, V., Leanderson, T., Holmdahl, R. & Issazadeh-Navikas, S. (2003) J. Immunol. 170**,** 4776-4784. [DOI] [PubMed] [Google Scholar]
- 45.Yu, M., Nishiyama, A., Trapp, B. D. & Tuohy, V. K. (1996) J. Neuroimmunol. 64**,** 91-100. [DOI] [PubMed] [Google Scholar]
- 46.Chabot, S., Williams, G. & Yong, V. W. (1997) J. Clin. Invest. 100**,** 604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothuizen, L. E., Buclin, T., Spertini, F., Trinchard, I., Munafo, A., Buchwalder, P. A., Ythier, A. & Biollaz, J. (1999) J. Neuroimmunol. 99**,** 131-141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table