Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial (original) (raw)
. Author manuscript; available in PMC: 2017 Aug 10.
Abstract
Importance
The presence of tumor-infiltrating lymphocytes (TILs) is associated with improved outcomes in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer treated with adjuvant trastuzumab and chemotherapy. The prognostic associations in the neoadjuvant setting of other anti-HER2 agents and combinations are unknown.
Objective
To determine associations between presence of TILs, pathological complete response (pCR), and event-free survival (EFS) end points in patients with early breast cancer treated with trastuzumab, lapatinib, or the combination.
Design, Setting, and Participants
The NeoALTTO trial (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization) randomly assigned 455 women with HER2-positive early-stage breast cancer between January 5, 2008, and May 27, 2010, to 1 of 3 neoadjuvant treatment arms: trastuzumab, lapatinib, or the combination for 6 weeks followed by the addition of weekly paclitaxel for 12 weeks, followed by 3 cycles of fluorouracil, epirubicin, and cyclophosphamide after surgery. The primary end point used in this study was pCR in the breast and lymph nodes, with a secondary end point of EFS. We evaluated levels of percentage of TILs using hematoxylin-eosin–stained core biopsy sections taken at diagnosis (prior to treatment) in a prospectively defined retrospective analysis.
Main Outcomes and Measures
Levels of TILs were examined for their associations with efficacy end points adjusted for prognostic clinicopathological factors including PIK3CA genotype.
Results
Of the 455 patients, 387 (85.1%) tumor samples were used for the present analysis. The median (interquartile range [IQR]) level of TILs was 12.5% (5.0%-30.0%), with levels lower in hormone receptor–positive (10.0% [5.0%-22.5%]) vs hormone receptor–negative (12.5% [3.0%-35.0%]) samples (P = .02). For the pCR end point, levels of TILs greater than 5% were associated with higher pCR rates independent of treatment group (adjusted odds ratio, 2.60 [95% CI, 1.26-5.39]; P = .01). With a median (IQR) follow-up time of 3.77 (3.50-4.22) years, every 1% increase in TILs was associated with a 3% decrease in the rate of an event (adjusted hazard ratio, 0.97 [95% CI, 0.95-0.99]; P = .002) across all treatment groups.
Conclusions and Relevance
The presence of TILs at diagnosis is an independent, positive, prognostic marker in HER2-positive early breast cancer treated with neoadjuvant anti-HER2 agents and chemotherapy for both pCR and EFS end points.
Trial Registration
clinicaltrials.gov Identifier: NCT00553358
The presence of tumor-infiltrating lymphocytes (TILs) has been shown to have significant positive prognostic relevance for certain subtypes of breast cancer.1-5 Tumor-infiltrating lymphocytes have also been reported to be associated with improved distant metastases–free survival in patients with human epidermal growth factor receptor 2 (HER2)-positive early breast cancer, as well as increased rates of pathological complete response (pCR) with neoadjuvant trastuzumab and chemotherapy.1-3 Increasingly, oncogenic addiction, in which tumors become dependent on a sole oncogenic pathway for growth, is thought to also promote a tumor microenvironment conducive to immune escape.6,7 Although this has not been shown yet for HER2 oncogenic signaling, one could speculate that anti-HER2 therapy may not only work in a cell-intrinsic manner but may also reverse HER2-induced immunosuppression as a mechanism of action.
In this study, we tested the hypothesis that higher levels of TILs would be associated with better clinical outcomes in patients treated with lapatinib alone, trastuzumab alone, or the combination of both drugs with standard cytotoxic chemotherapy. In other words, the prognostic effect of TIL levels would not be specific to the anti-HER2 agent trastuzumab but would also occur with other inhibitors of HER2 signaling when combined with chemotherapy.6 We also hypothesized that the dual inhibitor strategy would achieve more potent blockade of the HER2 pathway, resulting in more robust release of on-cogene-mediated immunosuppression. This in turn would correspond to better outcomes for those patients with lower levels of TILs at diagnosis; ie, we would observe a TILs by treatment (single vs dual) interaction. To this end, we evaluated TILs in baseline core biopsy samples from the Neo-ALTTO study (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization) at a median event follow-up of 3.77 years and studied their associations with rates of pCR and event-free survival (EFS).8
Methods
NeoALTTO Study Design
Study design and patient eligibility have previously been published.8,9 In brief, 455 women were randomized to 1 of 3 treatment groups: oral lapatinib (1500 mg/d), intravenous trastuzumab (4 mg/kg loading dose followed by 2 mg/kg), or the combination of lapatinib (1000 mg/d) plus the same dose of trastuzumab for 6 weeks. After 6 weeks of receiving the anti-HER2 agents, patients started weekly paclitaxel therapy (80 mg/m2) for a total of 12 weeks. Lapatinib doses were reduced during the paclitaxel administration.9 Definitive surgery was performed within 4 weeks of administration of the last dose of paclitaxel. Anthracycline-based chemotherapy was given after surgery (3 cycles of standard intravenous fluorouracil, epirubicin hydrochloride, and cyclophosphamide). Thereafter, patients received the anti-HER2 therapy to which they were randomized to complete a total of 52 weeks duration. Endocrine therapy and radiotherapy were given in accordance with institutional guidelines.
Patients were recruited between January 5, 2008, and May 27, 2010, and were required to have HER2-positive breast cancer, tumor size greater than 2 cm, adequate organ function, and adequate cardiac function. The ethics committee and relevant health authorities at each participating site approved the study prior to start of recruitment and all patients gave written informed consent, which also covered future biomarker research.
Results for the analyses of EFS and overall survival (OS), including a landmark analysis to assess the effect of achieving pCR, have also been previously reported for this phase III study.8 Compared with the control group of trastuzumab alone, the combination of lapatinib and trastuzumab resulted in significantly higher pCR rates in the breast (National Surgical Adjuvant Breast and Bowel Project criteria), as well as considering both breast and lymph nodes (Food and Drug Administration criteria). The results for pCR rates using Food and Drug Administration criteria (ypT0/is ypN0) are presented in this article. Event-free survival is defined as time from randomization to first event. At a median (interquartile range [IQR]) follow-up of 3.77 (3.50-4.22) years, the 3-year EFS was not significantly different between groups (trastuzumab 76%, lapatinib 78%, and combination 84%; P = .81); nor was OS; however, the study was not powered to detect differences between the groups for EFS or OS. In contrast, pCR was significantly associated with better EFS and OS compared with those who did not achieve pCR (EFS: hazard ratio, 0.38 [95% CI, 0.22-0.63]; P < .001; OS: hazard ratio, 0.33 [95% CI, 0.15-0.70]; P = .005). Evaluation of PIK3CA genotype has previously been reported (n = 318).10 Patients were hormone receptor (HR) positive if they had positive expression of estrogen and/or progesterone receptor as defined locally.
TIL Evaluation
Of the 455 patients randomized, slides from core biopsies were available for 424 (93.2%) patients. Two pathologists (R.S., C.D.) evaluated TIL levels independently between August and November 2013. Of the 424 biopsies, 395 samples (from 86.8% of the 455 randomized patients) had enough tumor tissue available for TIL assessment and 387 (85.1%) had both TILs and clinical data available for the presented analyses (Table 1). Recent guidelines suggest that evaluation of TILs in the surrounding stroma is more reproducible than intratumoral evaluation. The measurement of stromal TILs varies less between pathologists and hence is preferred for prognostic biomarker analyses.11 Tumor-infiltrating lymphocytes were evaluated according to previously published methods.3,11 The mean of the 2 independent assessments was used in subsequent analyses. The correlation coefficient between pathologists was 0.74 (P < .001), which was consistent with previous studies.
Table 1. Baseline Characteristics of Patients in the Tumor-Infiltrating Lymphocyte (TIL) Cohort Compared With the Entire NeoALTTO Trial Population.
| Characteristic | Cohort | P Value Between TILs and No-TILs Cohorts | ||
|---|---|---|---|---|
| All NeoALTTO (N = 455) | TILs (n = 387) | No TILs (n = 68) | ||
| Age, median (range), y | 50 (23-80) | 50 (23-80) | 49 (23-72) | .65 |
| Tumor size, median (range), cm | 4.0 (2.1-9.5) | 4.0 (2.1-9.0) | 4.8 (2.1-9.5) | .09 |
| Nodal status, No. (%) | ||||
| N > 2, Nx, or missing | 72 (16) | 63 (16) | 9 (13) | .53 |
| N0 or N1 | 383 (84) | 324 (84) | 59 (87) | |
| Hormone receptor status, No. (%) | ||||
| Positive (>1%) | 232 (51) | 195 (50) | 37 (54) | .54 |
| Negative | 223 (49) | 192 (50) | 31 (46) | |
| Treatment group, No. | ||||
| Trastuzumab | 154 | 130 | 24 | .46 |
| Lapatinib | 149 | 131 | 18 | |
| Trastuzumab + lapatinib | 152 | 126 | 26 | |
| Clinical outcome, No. | ||||
| pCR events | 138 | 120 | 18 | .50 |
| EFS events | 105 | 90 | 15 | .83 |
| Deaths | 54 | 43 | 11 | .24 |
| Outcome for trastuzumab + lapatinib vs trastuzumab alone, odds ratio (95% CI)a | ||||
| pCR benefit | 2.40 (1.44-4.00) | 2.88 (1.65-5.02) | 0.84 (0.22-3.20) | .72 |
| EFS difference | 0.78 (0.49-1.28) | 0.75 (0.43-1.30) | 0.97 (0.28-3.44) | .32 |
Statistical Analysis
The Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria were followed for this study.12 We examined associations of levels of percentage infiltrating stromal TILs with pCR and EFS. The primary hypothesis was that higher levels of TILs would be associated with a better prognosis overall, independent of anti-HER2 therapy. Two-sided 5% level tests were used throughout, with no adjustment for multiplicity. The association between presence of TILs and clinicopathological factors was evaluated by t test or Pearson correlations. A t test/binomial test was used to test for differences between the TILs and no-TILs cohorts (Table 1). Tumor-infiltrating lymphocytes were included in a multivariate model with known prognostic clinicopatho-logical factors. As prespecified in the NeoALTTO protocol, these clinicopathological factors were treated as binary variables: clinical tumor size (T2 [2-5 cm] vs T3 [≥5 cm]), nodal status (N0-1 vs ≥N2), and suitability for breast-conserving surgery (yes vs no). For the pCR analysis, a logistic regression was used; for the survival analyses, Cox proportional hazards models were used. The impact of attaining pCR on EFS was assessed using a Cox model–based landmark analysis. Two models were used, one with only clinicopathological characteristics of patients at baseline (the time when TILs were evaluated) and the second with the inclusion of pCR (landmark analysis). The effect of the presence of TILs, treatment, and other factors on EFS was assessed by fitting a standard Cox proportional hazards model. The TIL by treatment interaction was tested but excluded from the final model if its P value exceeded .20. The additional impact of attaining pCR on EFS was assessed by fitting a Cox model including pCR status to a landmark population (the landmark time used was 60 days from randomization, by which time surgery was complete for almost all patients). Event-free survival is defined as the time from randomization to first event, which included breast cancer relapse after surgery, second primary cancer, or death without recurrence. Analyses presented in the main text did not include the lapatinib-treated patients because the lapatinib group was inferior in the adjuvant ALTTO study13 and is unlikely to be of any further interest in this setting. Analyses including this group are presented in the eTable in the Supplement for completeness. Overall survival results are not presented here because of the small number of events so far seen.8
The linearity of the relationship between TILs and efficacy end points of pCR and EFS was assessed using cubic spline fits (eFigure 2 in the Supplement). If the TILs variable was found to be linear, TILs were evaluated as a 1% percentage increase as a continuous variable in accordance with our previous analyses.3,4
In an exploratory analysis, we attempted to define TILs value in a population of patients with an EFS of greater than 90% at 3 years by using visual inspection of the survival curves. Our rationale was that this good prognostic group defined by high TIL levels may only require single-agent trastuzumab with their chemotherapy, and this concept could be validated in future adjuvant trials in HER2-positive disease. We did not use the lymphocyte-predominant breast cancer binary variable in this analysis because this subgroup represented only 10.6% (41 of 387) of the trial population (too few events) and this definition includes intratumoral TIL evaluations, which was not included in these analyses.
Results
Baseline Patient Characteristics
In this study, there were no significant differences in patient characteristics of the populations evaluated and not evaluated for TILs (Table 1). Sixty-eight samples (14.9%) had no evaluable invasive tumor in the core biopsy.
Associations Between the Quantity of TILs and Clinicopathological Factors
In the overall cohort, the median (IQR) for presence of stromal TILs was 12.5% (5.0%-30.0%). Hormone receptor–negative (12.5% [3.0%-35.0%]) compared with HR-positive tumors (10.0% [5.0%-22.5%]) had significantly higher median (IQR) levels of TILs at diagnosis (P = .02) (eFigure 1 in the Supplement). There was no significant difference between levels of TILs according to PIK3CA genotype (P = .17), age (P = .92), more involved lymph nodes (P = .26), tumor size (P = .74), or if the patient was a candidate for planned breast conservation surgery (P = .97).
Associations Between the Quantity of TILs and Rates of pCR
In this analysis, we found that the relationship between pCR and presence of TILs was nonlinear (eFigure 2A in the Supplement). Rates of pCR increased sharply when levels of stromal TILs were greater than 5%, regardless of treatment group (Figure 1), with an adjusted odds ratio of 2.60 (95% CI, 1.26-5.39; P = .01) (Table 2). There was no significant interaction between presence of TILs and the combination group of trastu-zumab and lapatinib vs trastuzumab alone in predicting pCR (P = .51).
Figure 1. Rates of Pathological Complete Response According to Levels of Tumor-Infiltrating Lymphocytes Binned by Quartile to Illustrate the Nonlinear Effect.
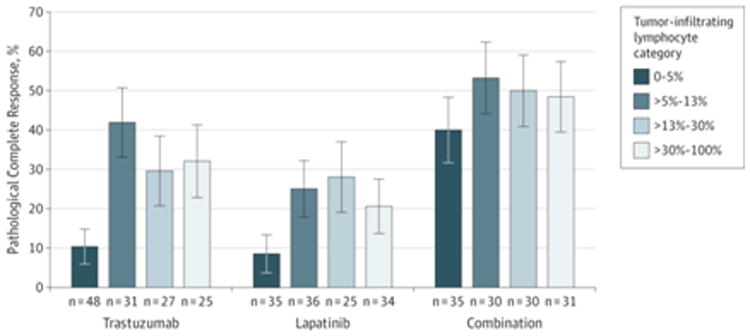
Table 2. Multivariate Analysis Using the Pathological Complete Response (Breast and Lymph Nodes) End Point (n=250).
| Effect | Odds Ratio (95% CI) | P Value for Effect |
|---|---|---|
| Treatment (combination vs trastuzumab) | 2.88 (1.65-5.03) | <.001 |
| Nodal status | 1.35 (0.64-2.82) | .43 |
| Hormone receptor status | 2.78 (1.59-4.86) | <.001 |
| Planned conservative surgery | 1.14 (0.60-2.18) | .69 |
| Tumor size | 0.97 (0.55-1.72) | .91 |
| >5% Tumor-infiltrating lymphocytes | 2.60 (1.26-5.39) | .01 |
Associations Between the Quantity of TILs and EFS
We then assessed the association between the levels of TILs and EFS after a median (IQR) follow-up of 3.77 (3.50-4.22) years. Here the relationship between presence of TILs and EFS was found to be linear, similar to our previously published data in the adjuvant setting (eFigure 2B in the Supplement). When the clinicopathological prognostic variables were entered as potential regression candidates, only stromal TILs percentage and planned conservative surgery were significant predictors of EFS in the multivariate model in analyses using both baseline characteristics and the landmark analysis that included pCR (Table 3 and eTable in the Supplement). Every 1% increase in TILs was associated with a 3% decrease in the rate of an event (adjusted hazard ratio, 0.97 [95% CI, 0.95-0.99]; P = .002)—in other words, the higher the level of TILs present in the primary tumor at diagnosis, the better the EFS after use of trastuzumab, lapatinib, or the combination. This effect is also shown in Figure 2A and 2B using the median TIL value and a high TIL value, which was associated with a 3-year EFS of approximately more than 90%.
Table 3. Event-Free Survival According to Levels of Stromal Tumor-Infiltrating Lymphocytes (TILs): Univariate and Multivariate Analyses With and Without Including Pathological Complete Response (pCR) in the Model.
| Characteristic | P Value | Hazard Ratio (95% CI) |
|---|---|---|
| Univariate Effect Without pCR (n = 387)a | ||
| Stromal TILs, per unit increase, % | <.001 | 0.97 (0.96-0.99) |
| Positive hormone receptor status | .16 | 0.74 (0.48-1.12) |
| Nodal statusb | .09 | 1.57 (0.91-2.58) |
| Tumor sizec | .39 | 1.20 (0.79-1.83) |
| Planned conservative surgeryd | .02 | 0.56 (0.33-0.91) |
| Combination treatmente | .26 | 0.74 (0.43-1.24) |
| Multivariate Effect Without pCR (n = 257)a | ||
| Stromal TILs, per unit increase, % | .002 | 0.97 (0.95-0.99) |
| Positive hormone receptor status | .66 | 0.89 (0.52-1.51) |
| Nodal statusb | .67 | 0.85 (0.37-1.71) |
| Tumor sizec | .85 | 1.05 (0.60-1.81) |
| Planned conservative surgeryd | .04 | 0.46 (0.21-0.91) |
| Combination treatmente | .53 | 0.84 (0.49-1.44) |
| Univariate Effect Including pCR (n = 257)f | ||
| Stromal TILs, per unit increase, % | <.001 | 0.97 (0.96-0.99) |
| Positive hormone receptor status | .24 | 0.78 (0.51-1.18) |
| Nodal statusb | .11 | 1.53 (0.89-2.51) |
| Tumor sizec | .53 | 1.14 (0.74-1.74) |
| Planned conservative surgeryd | .04 | 0.57 (0.33-0.94) |
| Combination treatmente | .21 | 0.71 (0.41-1.21) |
| pCR | .001 | 0.42 (0.24-0.70) |
| Multivariate Effect Including pCR (n = 250)f | ||
| Stromal TILs, per unit increase, % | .005 | 0.97 (0.95-0.99) |
| Positive hormone receptor status | .50 | 0.83 (0.48-1.44) |
| Nodal statusb | .78 | 0.90 (0.39-1.82) |
| Tumor sizec | .96 | 0.99 (0.56-1.70) |
| Planned conservative surgeryd | .048 | 0.46 (0.20-0.94) |
| Combination treatmente | .79 | 0.93 (0.53-1.61) |
| pCR | .04 | 0.51 (0.25-0.96) |
Figure 2. Kaplan-Meier Event-Free Survival (EFS) Curves Showing That Higher Levels of Tumor-Infiltrating Lymphocytes (TILs) Result in Better Survival Outcomes and Provide Information Independently of Pathological Complete Response (pCR).
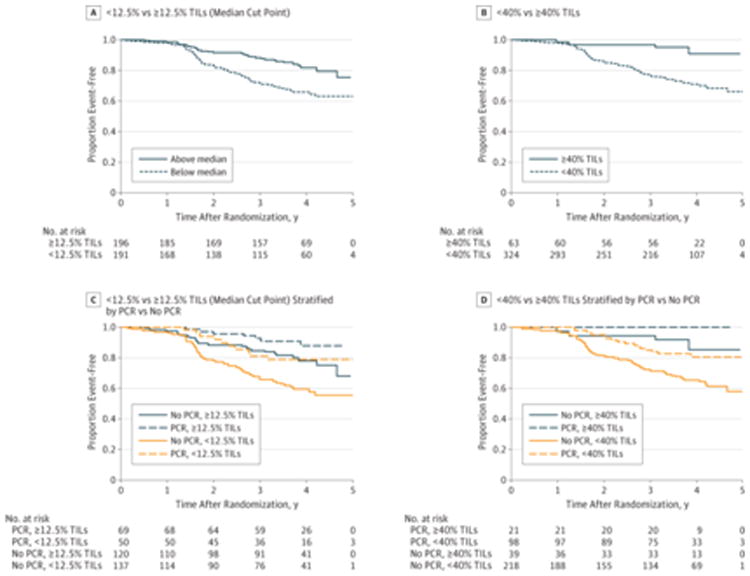
A, Three-year EFS was 88% (95% CI, 82%-92%) for patients whose tumors contained at least 12.5% TILs (the median level of TILs) and 71% (95% CI, 64%-78%) for patients with less than 12.5% TILs (log-rank P < .001). B, Three-year EFS was 97% (95% CI, 88%-99%) for patients whose tumors contained more than 40% TILs and 77% (95% CI, 72%-83%) for those with less than 40% TILs (log-rank P < .001). C, Three-year EFS was 67% (95% CI, 57%-74%) for patients with no pCR and less than 12.5% TILs; 85% (95% CI, 77%-90%) for patients with no pCR and at least 12.5% TILs; 81% (95% CI, 67%-90%) for patients with pCR and less than 12.5% TILs; and 92% (95% CI, 83%-97%) for patients with pCR and at least 12.5% TILs. D, Three-year EFS was 72% (95% CI, 65%-77%) for patients with no pCR and less than 40% TILs; 95% (95% CI, 80%-99%) for patients with no pCR and more than 40% TILs; 85% (95% CI, 76%-91%) for patients with pCR and less than 40% TILs; and 100% for patients with pCR and more than 40% TILs.
Of interest, patients who had higher TIL levels (greater than the median) and did not achieve pCR had EFS similar to those who did achieve pCR (Figure 2C). Patients with a baseline TIL level of at least 40% had excellent 3-year EFS (97% [95% CI, 88%-99%]) regardless of pCR outcome (of those patients with ≥40% TILs, only 21 of 60 [35%] achieved a pCR) (Figure 2D). Patients who did not achieve pCR and had low levels of TILs had the poorest survival (Figure 2C and 2D). There was no significant interaction between presence of TILs and the combination group of trastuzumab and lapatinib vs trastuzumab alone and EFS (P = .38 for the full population and P = .38 for the landmark population).
Discussion
In this prospectively planned analysis of a neoadjuvant clinical trial of HER2-positive early-stage breast cancer, we have shown for the first time, to our knowledge, that higher levels of stromal TILs were associated with good outcomes, independent of the anti-HER2 agent given (trastuzumab or lapatinib or the combination) with standard anthracycline and tax-ane-based chemotherapy.
These results add to our previous data in trastuzumab-treated patients with survival information,3 and hence we believe that this analysis firmly establishes the stromal TILs variable as a positive prognostic marker in primary HER2-positive breast cancer treated with anti-HER2 therapy and chemotherapy and provides new prognostic data regarding TILs in the neoadjuvant setting with pCR and the more robust EFS end point. Given the data regarding TILs and better outcomes in primary triple-negative breast cancer, we cannot exclude the possibility that the prognostic effect of TILs could predominantly be the result of the chemotherapy. Notably here, the TILs effect was found to be nonlinearly associated with pCR but was linear for EFS. We hypothesize that the anthracycline given after surgery (fluorouracil, epirubicin, and cyclophosphamide) could explain the further improvement in clinical outcomes in the presence of higher TIL levels (eFigure 2 in the Supplement), supporting the importance of anthracycline therapy for HER2-positive disease and similar to results from the BIG 2-98 trial analysis,4 as well as preclinical data.14
In this study, information on TIL levels and pCR provided independent prognostic information: we noted that patients with high levels of TILs at baseline had better outcomes independent of whether they achieved pCR in the neoadjuvant phase. Hence, as we move forward into an era of dual anti-HER2 therapy, it is possible that we can identify a subgroup of patients defined by their TIL level at diagnosis who may not require more than the current adjuvant standard of trastuzumab and chemotherapy. Although previously we had arbitrarily chosen a TIL level cutoff of 50% to 60% to describe the category of “lymphocyte-predominant breast cancers,”1,3 this value had not been derived from a data set of contemporary HER2-positive early breast cancers treated with standard anti-HER2 therapy and also included intratumoral TIL evaluations, which we have since found to have lower interobserver reproducibility.11 Whereas we did not observe any treatment interaction with TIL levels as originally hypothesized, analysis using the adjuvant trial samples13 will better answer this question because it is likely that we lacked statistical power to reveal an interaction in this data set.
At present, we do not understand why patients either do or do not have TILs present in their tumors at diagnosis. Exome and RNA sequencing of the NeoALTTO samples is ongoing and may shed light on this issue because mutant peptides have been hypothesized to initiate a T-cell response.15 Tumor PIK3CA genotype has been associated with poorer outcomes in HER2-positive disease,10,16 but here we did not observe any significant difference in levels of TILs between patients with mutant and wild-type PIK3CA. We have previously shown a correlation between levels of TILs and mRNA levels of genes representing T-cell activation and suppression.17 Because levels of T-cell checkpoint markers at the mRNA level are highly correlated with levels of TILs, T-cell checkpoint inhibition could be effective in combination with anti-HER2 therapy, particularly in patients with lower levels of TILs (ie, 5%-40%). This concept remains to be tested in future clinical trials.
This study's findings are strengthened by the use of a large phase III neoadjuvant clinical trial evaluating anti-HER2 treatments with both pCR and EFS end points and the fact that recent international guidelines were followed for evaluation of TILs.11 However, we acknowledge that pCR is not a validated surrogate end point for improvement in EFS18 and that the number of events for the survival end point (EFS) is low. Efforts are currently ongoing in an attempt to improve the re-producibility and consistency of TIL measurement among pathologists globally. Another limitation of our findings is that because the lapatinib with trastuzumab combination was not statistically superior to the control group in the adjuvant study,13 it is unlikely that this combination will be used in the future. Studies of the prognostic effect of TIL levels in clinical trials evaluating other dual anti-HER2 strategies are planned. At this stage, data suggest that the “TILs effect” will be similar with trastuzumab plus pertuzumab and chemotherapy,19 although this remains to be definitively shown in the adjuvant setting.
Conclusions
Finally, in conclusion we propose the concept that a group of patients with “high” TIL levels can have an excellent out-come with the current standard of therapy alone with chemotherapy, although we acknowledge the difficulty of proposing such cutoffs for clinical implementation.20 If a TIL cutoff can be identified and robustly validated using the large adjuvant HER2-positive data sets, future clinical trials of anti-HER2 therapy may be better placed to test the efficacy of new therapies in the poor prognostic group.
Supplementary Material
Supplemental Content
At a Glance.
- Tumor-infiltrating lymphocytes (TILs) have been shown to be associated with better survival outcomes in HER2-positive early breast cancer treated with trastuzumab and chemotherapy.
- In the NeoALTTO study, levels of TILs present at diagnosis were found to be significantly associated with improved pathological complete response rates as well as event-free survival across all treatments arms of lapatinib, trastuzumab, or the combination.
- Every 1% increment in TILs was associated with a 3% decrease in the rate of an event; adjusted hazard ratio, 0.97 (95% CI, 0.95-0.99); P = .002.
- We did not observe a significant interaction between TILs and treatment arm (dual vs trastuzumab alone) and pCR nor EFS.
- PIK3CA status was not significantly associated with different TIL levels at diagnosis.
Acknowledgments
Funding/Support: Dr Loi is supported by Cancer Council Victoria and the National Health and Medical Research Council of Australia (NHMRC). The NeoALTTO study was funded and sponsored by GlaxoSmithKline.
Role of the Funder/Sponsor: GlaxoSmithKline had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript. GlaxoSmithKline reviewed, commented on, and approved the manuscript prior to submission for publication.
Footnotes
Author Contributions: Drs Campbell and Bradbury had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Salgado and Denkert served as co–first authors, each with equal contribution to the manuscript. Drs Sotiriou and Loi served as co–senior authors.
Study concept and design: Salgado, Denkert, Baselga, Piccart-Gebhart, Sotiriou, Loi.
Acquisition, analysis, or interpretation of data: Salgado, Denkert, Campbell, Savas, Nuciforo, Aura, de Azambuja, Eidtmann, Ellis, Baselga, Michiels, Bradbury, Loi.
Drafting of the manuscript: Salgado, Michiels, Loi.
Critical revision of the manuscript for important intellectual content: Denkert, Campbell, Savas, Nuciforo, Aura, de Azambuja, Eidtmann, Ellis, Baselga, Piccart-Gebhart, Michiels, Bradbury, Sotiriou, Loi.
Statistical analysis: Denkert, Campbell, Michiels, Bradbury, Loi.
Obtained funding: Denkert, Loi.
Administrative, technical, or material support: Nuciforo, Loi.
Study supervision: Salgado, Eidtmann, Baselga, Piccart-Gebhart, Loi.
Conflict of Interest Disclosures: Dr de Azambuja has received grants from GlaxoSmithKline and Roche and personal fees from Roche outside this work. Dr Ellis is an employee of GlaxoSmithKline, the funder of the NeoALTTO study. Drs Baselga and Piccart-Gebhart have received personal fees from Roche. No other disclosures are reported.
References
- 1.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 2.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes (TILs) indicate trastuzumab benefit in early-stage HER2-positive breast cancer (HER2+ BC) Cancer Res. 2013;73(24 suppl) Abstract S1-05. [Google Scholar]
- 3.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 4.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 5.Adams S, Gray R, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakhra K, Bachireddy P, Zabuawala T, et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18(5):485–498. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137–1146. doi: 10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Bradbury I, Eidtmann H, et al. NeoALTTO Study Team. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. published correction appears in Lancet 2012;379(9816):616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majewski I, Nuciforo P, Mittempergher L, et al. A mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. :IK3C. doi: 10.1200/JCO.2014.55.2158. published online ahead of print January 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of NCI-EORTC Working Group on Cancer Diagnostics. Reporting recommendations for tumor marker prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100(2):229–235. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart MJ, Holmes AP, Baselga J, et al. First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC) J Clin Oncol. 2014;32(5s) Abstract LBA4. [Google Scholar]
- 14.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 15.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1-2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loibl S, von Minckwitz G, Schneeweiss A, et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (HER2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014;32(29):3212–3220. doi: 10.1200/JCO.2014.55.7876. [DOI] [PubMed] [Google Scholar]
- 17.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 18.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 19.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15(2):e58–e68. doi: 10.1016/S1470-2045(13)70477-7. [DOI] [PubMed] [Google Scholar]
- 20.Polley MY, Leung SC, McShane LM, et al. International Ki67 in Breast Cancer Working Group of the Breast International Group and North American Breast Cancer Group An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105(24):1897–1906. doi: 10.1093/jnci/djt306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Content