Modulators of microglial activation and polarization after intracerebral haemorrhage (original) (raw)
. Author manuscript; available in PMC: 2017 Aug 30.
Published in final edited form as: Nat Rev Neurol. 2017 May 19;13(7):420–433. doi: 10.1038/nrneurol.2017.69
Abstract
Intracerebral haemorrhage (ICH) is the most lethal subtype of stroke but currently lacks effective treatment. Microglia are among the first non-neuronal cells on the scene during the innate immune response to ICH. Microglia respond to acute brain injury by becoming activated and developing classic M1-like (proinflammatory) or alternative M2-like (anti-inflammatory) phenotypes. This polarization implies as yet unrecognized actions of microglia in ICH pathology and recovery, perhaps involving microglial production of proinflammatory or anti-inflammatory cytokines and chemokines. Furthermore, alternatively activated M2-like microglia might promote phagocytosis of red blood cells and tissue debris, a major contribution to haematoma clearance. Interactions between microglia and other cells modulate microglial activation and function, and are also important in ICH pathology. This Review summarizes key studies on modulators of microglial activation and polarization after ICH, including M1-like and M2-like microglial phenotype markers, transcription factors and key signalling pathways. Microglial phagocytosis, haematoma resolution, and the potential crosstalk between microglia and T lymphocytes, neurons, astrocytes, and oligodendrocytes in the ICH brain are described. Finally, the clinical and translational implications of microglial polarization in ICH are presented, including the evidence that therapeutic approaches aimed at modulating microglial function might mitigate ICH injury and improve brain repair.
Intracerebral haemorrhage (ICH) accounts for 10–15% of all strokes, making it the second most common type of stroke1–3. ICH is estimated to affect over 1 million people worldwide each year1, and that number is expected to rise substantially as the population ages. Most patients with ICH die or become severely disabled, and supportive care is the mainstay of current therapy4,5. Primary brain damage develops within the first few hours after ICH as a result of haematoma or oedema, formation and expansion of which induces mass effects and increased intracranial pressure that can lead to herniation and death. Secondary brain damage results from activation of microglia, which drive proinflammatory, oxidative and cytotoxic cascades causing cell death and functional impairment6.
As key innate immune cells, microglia act as guardians of the brain and are recognized to be the first non-neuronal cells to respond to various acute brain injuries7, including ICH6. Increasing evidence indicates that activated microglia are the primary source of cytokines, chemokines, prostaglandins, proteases, ferrous iron and other immunomodulatory molecules in the brain6. These molecules combine to initiate secondary brain injury and the subsequent brain repair processes. In fact, cell therapy with bone marrow-derived mononuclear cells or macrophages ameliorates injury and improves outcome after ischaemic stroke8–11. In view of these findings, research into microglial activation and function, and on cell–cell interactions, is critical to understanding ICH pathophysiology.
To develop new and effective therapies for ICH, we also need to explore the cellular and molecular mechanisms that underlie ICH-induced brain injury and repair12. As inflammation contributes to ICH-induced secondary brain injury6, modulation of the microglial phenotype might not only accelerate the absorption of haematoma and oedema, but also improve white matter integrity, brain repair and functional recovery13. The fact that clinical trials of open surgical haematoma evacuation have not shown positive results14,15 emphasizes the need to investigate endogenous mechanisms of haematoma removal. This knowledge could lead to the development of improved treatments for patients with ICH.
In this Review, we summarize key advances in our understanding of microglial function after ICH, with a particular focus on microglial activation, migration, proliferation, secretion and phagocytosis. In addition, we discuss microglial polarization, modulators and interactions with other cells. We also address the clinical and translational implications of these observations and highlight new therapeutic directions for ICH.
Microglia
Microglia are the major phagocytes of the brain. In vitro, activated microglia develop into either classically activated (M1, proinflammatory) or alternatively activated (M2, anti-inflammatory) phenotypes, a process termed polarization16,17. Alternatively activated M2 microglia are divided into three subtypes — M2a, M2b and M2c — each with different cell surface markers and distinct biological functions18: M2a microglia mainly contribute to cell regeneration, whereas M2b and M2c cells participate in phagocytosis and removal of tissue debris.
In response to acute brain injury, microglia can dynamically and temporally change their phenotype19,20. Individual classically activated and alternatively activated microglia within brain lesions can contribute to either tissue damage or repair under conditions of spinal cord injury21–23, ischaemic stroke19,24 and traumatic brain injury (TBI)20,25–27. Although the supposed dichotomy between M1 and M2 phenotypes is now recognized as an oversimplification (these two extreme activation states are only observed in vitro), this classification remains useful for understanding the function of microglia in various brain diseases28. In a mouse model of spinal cord injury, for example, although global M1 and M2 marker levels both increased in the acute phase23, flow cytometry showed that microglia and macrophages had mainly developed an M1 phenotype21. In models of TBI20,29 and ischaemic stroke30, an M2 to M1 shift was observed, whereas a mixed M1-like and M2-like response was reported in an animal model of epilepsy31. However, controversy has begun to surround the traditional concept of M1 versus M2 microglial polarization. For example, in a mouse model of experimental auto-immune encephalomyelitis32, the classic M1 marker IL-6 was induced by IL-4 and exerted an anti-inflammatory effect. Additionally, in a model of TBI, canonical markers of both polarization states were highly co-expressed by the same cell33. Hence, whether microglia actually undergo M1 or M2 polarization has been called into question34. Researchers using animal models of ICH should, therefore, pursue functional studies of microglial activities such as phagocytosis and haematoma clearance, as discussed below. The functional properties of known M1-like and M2-like markers are summarized in TABLE 1.
Table 1.
Markers of M1 and M2 microglia
| Marker | Type | Function and characteristic |
|---|---|---|
| M1 | ||
| IL-1β | Cytokine | Proinflammatory |
| IL-6 | Cytokine | Proinflammatory |
| IL-12 p70 | Cytokine | Proinflammatory |
| TNF | Cytokine | Proinflammatory |
| IFNγ205 | Cytokine | Proinflammatory, M1 microglia and macrophage inducer |
| CCL5 (REF. 206) | Chemokine | Recruits immune cells |
| CCL20 (REF. 207) | Chemokine | Recruits immune cells |
| CXCL1 (REFS 208,209) | Chemokine | Recruits immune cells |
| CXCL10 (REFS 210,211) | Chemokine | Recruits immune cells |
| GM-CSF212 | Chemokine | Recruits immune cells, induces M1 microglia and macrophages |
| CD16 (REF. 213) | Immunoglobulin Fc receptor | Induces proinflammatory signalling |
| CD32 (REF. 213) | Immunoglobulin Fc receptor | Induces proinflammatory signalling |
| CD86 (REF. 214) | Surface receptor | Classic M1 microglia and macrophage marker |
| MHC-II215 | Surface receptor | Mediates T cell differentiation to TH1 |
| iNOS | Metabolic enzyme | Mediates nitric oxide synthesis |
| M2a | ||
| IL-1Ra216 | Cytokine | IL-1 receptor antagonist |
| IL-4 (REFS 78,217) | Cytokine | Anti-inflammatory, increases microglia and macrophage phagocytosis |
| TGFβ | Cytokine | Anti-inflammatory |
| CCL22 (REFS 218,219) | Chemokine | Recruits dendritic cells, TH2 cells and regulatory T cells |
| CD206 (REF. 213) | Mannose receptor | Mediates endocytosis and phagocytosis in response to microglia and macrophage activation |
| M2b, M2c | ||
| CD163 (REF. 220) | Scavenger receptor | Haemoglobin clearance |
| Arg1 (REFS 221,222) | Cytosolic enzyme | Suppresses inflammation; upregulated by IL-4 and IL-13 |
| Ym1 (REFS 223,224) | Secretory protein | Anti-inflammatory; induction depends on IL-4 and IL-13 |
| FIZZ1 (REF. 224) | Secretory protein | Anti-inflammatory; induction depends on IL-4 and IL-13 |
| IL-10 (REF. 225) | Cytokine | Anti-inflammatory, mediates microglia and macrophage phagocytosis |
| IL-4Rα226 | Cytokine | Binds to IL-4 and IL-13 |
| G-CSF227 | Cytokine | Mediates microglia and macrophage survival, proliferation and differentiation |
Changes in M1 markers
Proinflammatory cytokines
Activation of microglia usually results in production of proinflammatory cytokines. Currently, proinflammatory cytokines are thought to be produced specifically by classically activated M1 microglia, and alterations in proinflammatory cytokine profiles and levels are thought to explain the changes in microglial function after ICH.
Microglial activation can be assessed by immunohistochemical analysis of the ICH brain, using antibodies targeting cell surface markers shared by microglia and macrophages. Suitable markers include keratan sulfate (targeted by 5D4 antibody)35,36, cell surface glycoprotein F4/80 (targeted by F4/80 antibody)35,36, CD11b37,38, Iba1 (REFS 39,40) and CD68 (REFS 41,42). However, anti-CD11b antibody cannot distinguish microglia from neutrophils and monocytes in the ICH brain: although in the intact brain CD11b is expressed only by microglia, this marker is also expressed by neutrophils and monocytes in the ICH brain39,43. Myeloperoxidase, a specific marker of neutrophils, is used to discriminate microglia from neutrophils and monocytes44. Distinguishing activated microglia from macrophages in the ICH brain by immunohistochemistry is also challenging. CD45 is helpful in this scenario because it is more highly expressed in monocytes than in microglia. Thus, CD45 expression levels can be used to separate these two cell types using flow cytometry: CD45IntCD11b+ cells are microglia and CD45HiCD11b+ cells are macrophages45–47.
Changes in M1-signature (proinflammatory) cytokines are prominent after ICH. In a clinical study of perihaematomal brain tissue, nuclear factor-κB (NF-κB) was activated and migrated into the nucleus at 13–48 h after ICH, and IL-1β and tumour necrosis factor (TNF) levels increased within 1 day after ICH48,49. These findings suggest the presence of a proinflammatory state early after ICH. In both the collagenase-induced and autologous blood-induced models of ICH (TABLE 2), IL-1β, IL-6, TNF50,51 and inducible nitric oxide synthase (iNOS)52 mRNA levels are generally elevated in the acute phase, starting to rise as early as 3 h after ICH and peaking at 3 days53–54. Changes in the corresponding protein levels follow a similar time course52,53,55. In rodents, secretion of proinflammatory cytokines IL-1β, IL-6 and TNF increased in the first 3 days56, and then returned to within the normal range on day 7 in both colla-genase-induced37 and blood-induced37,54 models of ICH. Interestingly, IL-1β, but not TNF, macrophage mannose receptor 1 (CD206) or chitinase-like protein 3 (also known as Ym1), colocalized with amoeboid microglia in rats with collagenase-induced ICH57. This colocalization pattern suggests a phagocytic and secretory phenotype that might be associated with dynamic changes in microglial morphology57. Moreover, treatment with the TNF receptor antagonist R-7050 attenuated neurovascular injury and improved functional outcomes in mice with collagenase-induced ICH58.
Table 2.
Animal models of ICH
| Collagenase-induced model | Blood-induced model | Thrombin-induced model |
|---|---|---|
| Method | ||
| Collagenase injected into the brain breaks down small blood vessels and initiates bleeding | Autologous blood injected into the brain | Thrombin injected into the brain |
| Clinical features mimicked | ||
| Acute cerebrovascular rupture with blood–brain barrier breakdown | Blood toxicity and the resulting inflammation | Thrombin toxicity (thrombin released from haematoma is a main contributor to secondary brain damage in acute ICH228) |
| Advantages | ||
| Haematoma expansion and increased intracranial pressure resembles that in clinical ICH229 Brain infiltration by systemic immune cells is similar to that in blood-induced models when the blood volume is matched47 Simple procedure (requires less time than blood injection) | Haematoma size is easier to control than in collagenase-induced ICH230 Brain infiltration of systemic immune cells is similar to that in collagenase-induced models when the blood volume is matched47 | Used for investigating mechanisms of thrombin toxicity that cause neuroinflammation228 and cell death231,232 Simple procedure |
| Limitations | ||
| Excessive inflammation claimed by some (not all) researchers6,233,234 | Excessive inflammation claimed by some (not all) researchers6,50,233,235 Limited blood–brain barrier disruption and rapid haematoma resolution236,237 Blood reflux No small vessel rupture Not suitable for assessing long-term functional outcomes233,237,238 | No uses other than in thrombin toxicity |
| Adverse effects of ageing | ||
| Axonal damage in white matter before day 3 worse in aged versus young animals, despite similar grey matter losses239–241 Functional outcomes worse in aged versus young rats by day 3, attributed to increased microglial activation239–241 Increases in M1 markers (IL-1β, TNF, IL-6, iNOS) are delayed and higher in old versus young rats: levels increase within 24 h in young rats, but not until days 3–7 in aged rats64 Increases in M2 markers (arginase-1, CD163) delayed in old versus young rats; IL-4 increased only in aged rats64 TGFβ upregulated on day 3 in young and aged rats64 | Brain injury and swelling on day 3 worse in old versus young rats, attributed to increased microglial activation and suppression of reactive astrocytes130,132 Neurological deficits worse in old versus young rats on day 1 and throughout the study130 Increased expression of IL-1β and reduced expression of GFAP and ciliary neurotrophic factor in aged versus young rats132 | No data |
In experimental models, IFNγ levels did not change in the first 3 days (unlike IL-1β, IL-6 and TNF levels)50,59,60, but markedly increased at later stages after ICH50,60. A reduction in the IFNγ:IL-4 ratio contributed to long-term recovery after ICH61. These results suggest that the ratio of IFNγ to M2-like cytokine levels might be a more reliable marker than IFNγ levels alone for the assessment of inflammatory status in the chronic phase after ICH. Our group has shown that M1 markers such as CD16, CD32 and iNOS are highly expressed on microglia on days 1 and 3 after ICH62, suggesting that M1-like microglial polarization occurs early in the acute phase.
Although the specific proinflammatory cytokines produced vary according to the ICH model used and time point assessed (FIG. 1), the overall direction of changes in these classic M1 markers is consistent in the acute phase after ICH. By 14 days after ICH (during the chronic phase), levels of most proinflammatory cytokines have returned to baseline60. However, whether levels of these cytokines change during the chronic phase of ICH, and whether these changes affect brain repair processes, remains unclear.
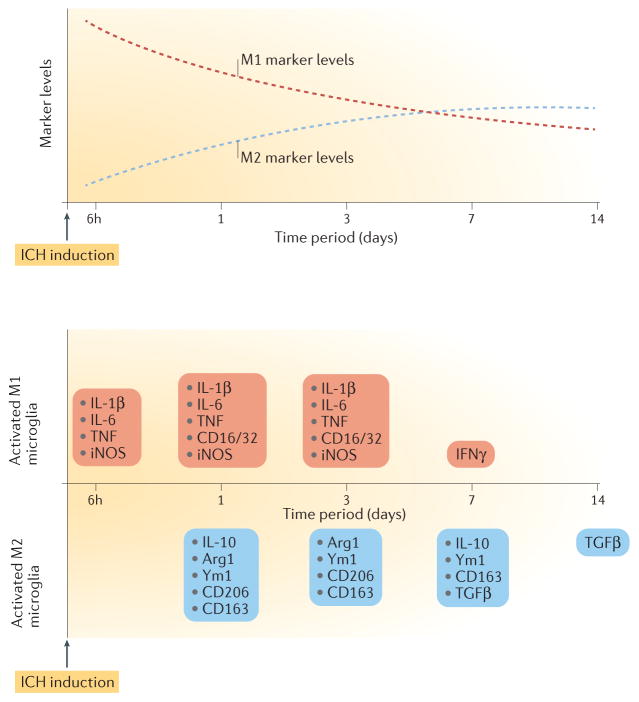
Figure 1. Dynamic changes in microglial marker levels and profiles over time after intracerebral haemorrhage.
Top panel, dashed red curve: microglial M1-like response exhibits a decreasing trend in the first 14 days. Top panel, dashed blue curve: microglial M2 like response exhibits an increasing trend in the first 14 days. Bottom panel: microglia exhibit an M1-like response as early as 6 h after intracerebral haemorrhage (ICH), as shown by upregulation of proinflammatory cytokines such as IL-1β, IL-6, tumour necrosis factor (TNF) and inducible nitric oxide synthase (iNOS). M2 markers, such as arginase-1 (Arg1), chitinase-like protein 3 (also known as Ym1), CD206, CD163 and IL-10, start to increase on day 1 after ICH. Transforming growth factor-β (TGFβ) is upregulated from day 7 until day 14 post-ICH. Although a mixed M1-like and M2-like microglial phenotype is evident during days 1 to 3, the balance of evidence supports an M1 to M2 phenotype switch in the first 7 days. IFNγ levels increase on day 7 after ICH, and levels of most proinflammatory cytokines return to baseline on day 14.
Toll-like receptors
Increased protein expression of Toll-like receptor (TLR)2 and TLR4 is associated with poor outcomes in patients with ICH63. In mice, expression of TLR2 (REFS 64,65) and TLR4 (REF. 62) proteins was upregulated (mainly in microglia) as early as 6 h after ICH and remained high throughout the first 3 days, in both collagenase-induced and blood-induced models of ICH54,62. In Tlr4−/− (REF. 54) and Tlr2−/− (REF. 65) mice after blood-induced ICH, IL-1β, IL-6 and TNF levels were substantially decreased compared with those in wild-type mice, a finding that supports a link between TLR2 and TLR4 activation and M1 microglia. Furthermore, TLR4 activates the NF-κB signalling pathway, via myeloid differentiation primary response protein MYD88 and TIR domain-containing adaptor molecule 2 (also known as TRIF-related adaptor molecule)66,67 (FIG. 2). TLR4 also upregulates M1-signature (proinflammatory) cytokines in the blood-induced model of ICH54,68–70. The same researchers demonstrated that TLR4 depletion reduced microglial autophagy, which contributes to the M1 phenotype of microglia71. In addition, treatment with a TLR4 antagonist reduced the production of proinflammatory cytokines (IL-1β, IL-6 and TNF) and inactivated microglia68. TLR2 exerts effects similar to those of TLR4 in the ICH brain, a fact that might be explained by the assembly of TLR2–TLR4 heterodimers, which trigger proinflammatory responses65.
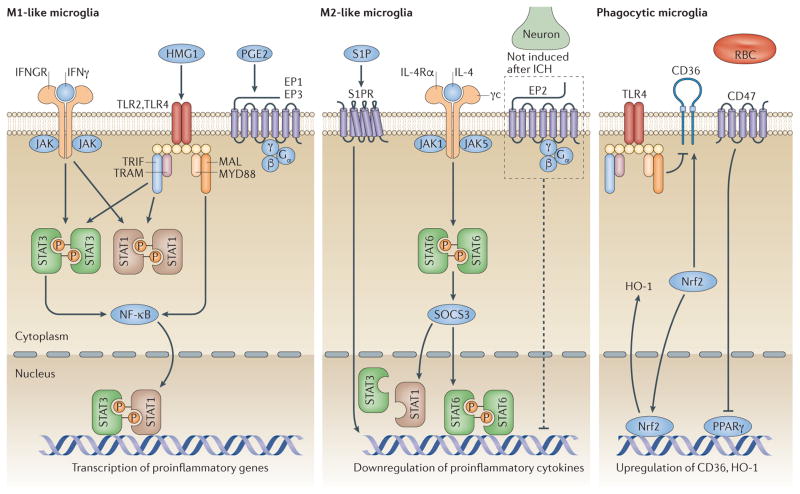
Figure 2. Modulators of microglial polarization and phagocytosis after intracerebral haemorrhage.
Several transcription factors modulate microglial polarization towards an M1-like (left panel) or an M2-like (centre panel) phenotype. Activation of high-mobility group protein 1 (HMG1) and Toll-like receptor (TLR)2 or TLR4 promotes microglial M1-like responses. Signal transducer and activator of transcription (STAT)1 is activated by TLRs and IFNγ, promoting microglial polarization to an M1 phenotype. After intracerebral haemorrhage (ICH), TLRs modulate STAT3 phosphorylation, which increases M1-like polarization. G-protein-coupled E prostanoid (EP) receptors EP1 and EP3 are mainly expressed on microglia and drive neuronal toxicity mediated by M1 upregulation after ICH. STAT6 accumulates in response to IL-4 and is responsible for the transcription of M2-related genes. Sphingosine-1-phosphate (S1P) receptor signalling contributes to the downregulation of proinflammatory cytokines and enhances M2-like responses after ICH. Although EP2 receptors are not expressed in microglia after ICH, EP2 deletion results in an increased microglial proinflammatory response. Other modulators regulate microglial phagocytosis (right panel). Activation of the transcription factor nuclear factor erythroid 2 related factor 2 (Nrf2) is associated with microglial phagocytosis after ICH and increases CD36 and haem oxygenase-1 (HO-1) expression. The CD36 gene is a target of peroxisome proliferator-activated receptor-γ (PPARγ), which belongs to the nuclear receptor family. CD47 expression in red blood cells (RBC) can decrease their phagocytosis by microglia after ICH. Although CD36 expression is increased in the ICH brain, TLR4 activation might negatively regulate its expression and delay haematoma clearance. IFGNR, IFNγ receptor; NF-κB, nuclear factor-κB; MAL, Toll/IL-1 receptor domain-containing adaptor protein (also known as MYD88 adapter-like protein); MYD88, myeloid differentiation primary response protein MYD88; SOCS3, suppressor of cytokine signalling 3; TRAM, TIR domain-containing adaptor molecule 2 (also known as TRIF-related adaptor molecule); TRIF, TIR domain-containing adaptor molecule 1.
Complement C3a and C5a receptors
Complement C3a and C5a are thought to be involved in neuro-inflammation72, neutrophil recruitment73 and blood–brain barrier disruption45. ICH-induced microglial activation, as assessed by immunoreactivity to an anti-CD11b and anti-CD11c dual-specificity antibody (OX42), was reduced in C3-deficient mice compared with wild-type controls74. In C5a receptor knockout mice, proinflammatory cytokine levels were decreased in the acute phase after ICH75. Moreover, treatment with the C5a receptor antagonist PMX53 in combination with the thrombin antagonist argatroban resulted in decreased mRNA expression of the M1 markers TNF, IL-6 and iNOS in the mouse ICH brain76. These data indicate a prominent role for activation of complement C3a and C5a receptors in the proinflammatory response to ICH.
Changes in M2 markers
Markers of M2 microglia in ICH have been considerably less well studied than M1 markers. Nevertheless, M2 microglia do have important functions in phagocytosis and toxicity clearance in other brain diseases77, such as ischaemic stroke17,78, TBI79 and epilepsy31, suggesting that the function of M2 microglia in ICH should be explored.
Anti-inflammatory cytokines
IL-10 is an anti- inflammatory M2-signature cytokine80 secreted by M2 macrophages and microglia. Treatment with IL-10 polarizes microglia and macrophages to the M2c subtype in vitro81,82, and enhances phagocytosis by monocytes83–85. IL-10 also induces production of suppressor of cytokine signalling (SOCS)1 and SOCS3, which suppress pro-inflammatory cytokine production via signal transducer and activator of transcription (STAT)3 in macrophages86,87. Interestingly, IL-10 levels in blood88–90 and brain tissue90,91 are increased in patients with ICH, as well as in preclinical models of ICH50,92,93. IL-10 levels are increased in patients during the early acute phase of spontaneous ICH, and this rise is associated with subsequent rebleeding89, although this relationship is not necessarily causal.
IL-4 (REF. 94) and transforming growth factor (TGF) β1 (REF. 60) exert anti-inflammatory effects, promote M2-like microglial responses, and improve functional recovery after ICH. Notably, the course of changes in levels of these M2 markers apparently differs between the two principal animal models of ICH. The mRNA levels of most M2 markers — such as IL-1 receptor antagonist53, IL-10 (REF. 50), arginase-1, Ym1 and CD206 (REF. 94) — increased within 1 day after collagenase-induced ICH, whereas mRNA levels of TGFβ53 and both mRNA and protein levels of IL-4 (REF. 56) were substantially increased on day 3 post-ICH. However, in mice with blood-induced ICH, IL-10 protein levels were unchanged and IL-4 was undetectable in the perihaematomal region in the first 2 weeks60. In the latter study, TGFβ1 protein levels increased from day 1 to day 10, and remained elevated up to day 14 after ICH; this time course was delayed compared with that of the M2 marker IL-13, protein levels of which increased from day 1 up to day 3 only60. Furthermore, intracerebral TGFβ1 treatment decreased microglial Il6 gene expression and improved functional outcomes in mice with blood-induced ICH60. Interestingly, an early increase in plasma levels of TGFβ1 was associated with improved functional outcome at 90 days in patients with ICH60. The authors of this study concluded that TGFβ1 is a potential target for ICH treatment.
Peroxisome proliferator-activated receptor-γ
Peroxisome proliferator-activated receptor-γ (PPARγ) belongs to a superfamily of nuclear receptors that are important contributors to antioxidant and anti- inflammatory responses95. In rats with blood-induced ICH, binding of PPARγ to DNA in nuclear extracts was suppressed at 1 h post-ICH, and treatment with PPARγ agonists had beneficial effects on ICH owing to their ability to reduce NF-κB activation96 and decrease M1 signature cytokine levels (specifically, iNOS, TNF and IL-1)97. In mice with blood-induced ICH, treatment with a PPARγ activator promoted microglial phagocytosis of red blood cells in vitro by inducing CD36 expression97,98. This evidence supports a potential role for PPARγ activation in haematoma clearance99. The mechanisms of microglial phagocytosis and haematoma resolution after ICH are discussed in more detail below.
Mammalian target of rapamycin
Dysregulation of serine/threonine-protein kinase mTOR (mammalian target of rapamycin) activation occurs in various brain diseases, notably Alzheimer disease100, Parkinson disease101,102 and TBI103. In a rat model of subarachnoid haemorrhage104, rapamycin and another mTOR inhibitor, AZD8055, ameliorated early brain injury by inhibiting production of IL-1β and TNF. Rapamycin and AZD8055 also decreased the CD16:CD206 ratio in oxyhaemoglobin-treated primary microglia in vitro104. In rats with collagenase-induced ICH, phosphorylation of mTOR was markedly increased at 30 min. Treatment with rapamycin(50–500 μg/kg) led to a dose-dependent increase in brain levels of the M2 cytokines IL-10 and TGFβ, as well as in the ratio of IL-10 to IFNγ, after ICH105. In a separate study that used the same model106, rapamycin treatment reduced protein levels of TNF, IL-1β and IL-6. This evidence suggests a link between mTOR inhibition and microglial M2 polarization.
Other M2 markers
Upregulation of the M2 marker CD163 is observed in the brains of patients with ICH within 3 days of onset91. In mice with blood-induced ICH, global CD206 and Ym1 protein expression (detected by western blot) was increased at day 1 (REF. 107). Although our group showed that Ym1 expression was increased in microglia at 24 h and 72 h in both the thrombin-induced and collagenase-induced models of ICH62,108, the dynamics of changes in microglial M2 markers in the collagenase-induced ICH model are still unclear. Data from the limited number of available studies reviewed here60,62,107 suggest that microglial M2 markers increase by day 1 — later than M1 markers, which start to increase by 3–6 h — and remain elevated longer (that is, beyond day 7) than do M1 markers (FIG. 1).
M1 to M2 transition
Very little is known about the dynamics of changes in microglial polarization after ICH13,59. Two studies revealed changes over time in M1-like and M2-like markers in models of ICH94,107. We62 and others60 also demonstrated a similar M1 to M2 transition in both the collagenase-induced62 and blood-induced60 models of ICH. In mice with collagenase-induced ICH, we observed an M1 to M2 microglial phenotype switch from day 1 to day 3 after ICH62, whereas in mice with blood-induced ICH, the microglial phenotype switch occurred within the first week60. However, this transition could result from phenotypic transformation of a single microglial population, from M2-like microglial migration, or from infiltration of M2-like circulating blood monocytes or macrophages. These possibilities deserve further study.
In summary, by about 3 days after ICH (FIG. 1), microglia polarize toward an M1-like phenotype associated with molecules involved in short-term brain damage. Indeed, M1 microglial polarization might be the main cause of microglial activation in the acute phase after ICH, whereas M2-like microglial responses and mediators might have an important role in long-term recovery, especially in aged animals (TABLE 2). Interestingly, ageing in the nondiseased brain is associated with an overall increase in the expression of microglial genes involved in neuroprotection109. Data relating to the M2-like response also need to be expanded; in particular, the role of M2-like microglia in the subacute and chronic phase of ICH requires clarification. As some M2 surface markers and cytokines are not specific to microglia and macrophages, they should be assessed by double or triple immunofluorescence staining with microglia-specific and macrophage-specific markers. The mRNA and protein expression of these markers can be detected using fluorescence-activated cell sorting of microglia and macrophages. Unbiased genome-wide approaches such as transcriptomics and proteomics can also be applied.
Phagocytosis and haematoma clearance
Under physiological conditions, microglia act as phagocytes to scavenge plaques, neurofibrillary tangles and cell debris in the CNS. Haematoma clearance by microglia might be a major mechanism of recovery from ICH13,110–112. We previously showed that tissue plasminogen activator in microglia assists in the clearance of haematoma36.
CD36 belongs to the class B scavenger receptor family and also contributes to the phagocytic ability of microglia and macrophages113–116; cells lacking phagocytic ability acquire this capacity after being transfected with CD36 (REF. 116). In both patients and animals with ICH, haematoma absorption is increased by CD36 expression97,98,111,115. CD36 is also a pivotal target of PPARγ-mediated downstream gene transcription98. As discussed above, activation of PPARγ increases CD36 expression and thereby promotes microglial phagocytic ability, whereas CD36 expression is downregulated by TLR4 signalling in microglia and monocytes117,118. In a comparison of TLR4-deficient and wild-type mice with blood-induced ICH, perihaematomal regions in the TLR4-deficient mice showed fewer activated microglia and higher expression of CD36 and fractalkine (also known as CX3C motif chemokine 1 (CX3CL1))69. In _CD36_−/− mice with blood-induced ICH, IL-1β and TNF mRNA expression levels were higher than those in wild-type mice. The capacity of CD36−/− microglia from these mice to phagocytose red blood cells in vitro was also decreased, along with a complementary increase in IL-10 levels115. The authors concluded that CD36 deficiency led to TLR4 upregulation, suggesting that mediators downstream of the TLR4 pathway reduced microglial phagocytosis and slowed haematoma absorption by decreasing CD36 expression and IL-10 production115. In an in vitro study, Tlr4−/− microglia exposed to lysed erythrocytes exhibited reductions in autophagy and inflammation compared with similarly treated wild-type microglia71. This finding suggests that TLR4-mediated autophagy contributes to microglial polarization and function. However, whether IL-10 regulates the expression of CD36 and TLR4, thereby modulating phagocytosis of red blood cells and promoting haematoma resolution, remains unclear.
CD47 is an integrin-associated protein expressed on erythrocytes119,120 that regulates phagocytosis in microglia and macrophages121,122. In a study that compared the effects of using blood from either wild-type mice or Cd47−/− mice to induce ICH in wild-type mice, the mice receiving CD47-depleted blood showed increased expression of haem oxygenase-1 (HO-1) and accelerated haematoma clearance, in a manner that correlated with M2 microglial activation123.
Interestingly, levels of the haemoglobin scavenger receptor CD163 increased over time in parallel with haematoma resolution in piglets with blood-induced ICH110, indicating a potential role for CD163 in haematoma clearance. These observations indicate that proteins capable of modulating microglial phagocytosis (for example, IL-10, CD36, CD47 and CD163) can affect haematoma resolution (FIG. 2) and deserve further research. Identification of new targets that can regulate microglial phagocytosis and haematoma clearance might lead to the development of future therapeutic interventions for ICH.
Modulators of microglial polarization
Transcription factors
Although markers of M1 and M2 microglia and macrophage polarization are readily detectable, these proposed activation states are complex and their relevance to macrophage function in vivo is controversial124. Additionally, the signalling pathways that cause secondary damage after ICH are complex. Investigating the activation of transcription factor responses to brain injury is, therefore, important to our understanding of microglial M1 to M2 transition after ICH. However, very few studies have investigated transcription factors other than NF-κB after ICH.
NF-κB
NF-κB is a traditional transcription factor that is activated by lipopolysaccharide and regulates the expression of most M1-signature genes (those encoding proinflammatory cytokines)125. In human brain tissue, NF-κB p65 expression was detected in the nuclei of glial cells by immunochemistry126 and was associated with increases in IL-1β and TNF48. As discussed above, upregulation of TLR2 and TLR4 (which are expressed mainly in microglia) induces NF-κB activation after ICH, and increases proinflammatory cytokine levels54,65.
Signal transducer and activator of transcription proteins
The STAT family of transcription factors (STAT1–STAT6) are, like NF-κB, activated by lipopolysaccharide. The Janus activated kinase (JAK)–STAT pathway is activated by both type I and type II interferons and thereby regulates cellular proliferation, immunity, apoptosis, and inflammation127,128. However, few studies have investigated the roles of STAT proteins in ICH.
STAT family members have various roles in microglial and macrophage polarization (FIG. 2). STAT1 controls M1 macrophage polarization129, and in vitro, STAT1 levels increase in TLR3-stimulated and TLR4-stimulated microglia130. STAT1 activation also induces expression of genes encoding IL-1β, TNF and CXC motif chemokine 10 (CXCL10) in _Cryptococcus neoformans_-infected macrophages131. Conversely, loss of STAT1 in macrophages led to an increase in polarization to the M2 phenotype131. SOCS3 is a feedback inhibitor that limits excessive cytokine release resulting from persistent activation of STAT3; in vitro, SOCS3-deficient primary microglia showed increases in STAT3 activation and proinflammatory cytokine signalling, which led to their pronounced polarization to the M1 phenotype132. This polarization was associated with upregulation of iNOS, IL-1β, IL-12 p40, IL-23 p9, IL-6, CC-motif chemokine 2 (CCL2), and CXCL10 (REF. 132). In mice with collagenase-induced ICH, phosphorylated STAT3 is found mainly in microglia and macrophages, and inhibition of this molecule is associated with decreases in iNOS and cyclooxygenase-2 expression133. In this same model, levels of phosphorylated STAT3 and IL-6 decreased in Tlr4−/− and MYD88−/− mice on day 3 after induction of ICH67, suggesting that STAT3 is an important transcription factor downstream of TLR4 that mediates M1 microglial polarization in ICH. However, STAT6 became activated in microglia challenged with IL-4, which promoted microglial M2 polarization134–136. Thus, the roles of STAT proteins in ICH pathology need additional clarification.
Nuclear factor erythroid 2-related factor 2
Activation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) and its related signalling pathway exerts a protective effect after ICH and also contributes to haematoma clearance137,138. _Nrf2_−/− mice show more brain damage than do wild-type mice at 24 h after collagenase-induced ICH, although microglial activation status in these mice, as shown by immunostaining for allograft inflammatory factor-1 (AIF1, also known as Iba1), is not altered by the loss of Nrf2 (REF. 137). In vitro, microglial phagocytosis of red blood cells labelled with a fluorescent dye was substantially increased by Nrf2 activators such as sulforaphane139. Moreover, mRNA level of the scavenger receptor CD36 was upregulated in microglia treated with an Nrf2 activator111.
Interestingly, mRNA levels of HO-1, CD36, CD163, IL-10 and IL-1β did not change appreciably in _Nrf2_−/− mice compared with their levels in wild-type mice at 48 h after blood-induced ICH111. HO-1 deletion decreased the phagocytic ability of microglia in vitro and delayed haematoma clearance in an in vivo model of subarachnoid haemorrhage140. Additionally, HO-1 activity is elevated in the cerebrospinal fluid of patients with subarachnoid haemorrhage140, suggesting a critical role for microglial Nrf2 and HO-1 in haematoma clearance. Moreover, induction of HO-1, one of the major downstream targets of Nrf2, polarizes macrophages to the M2 phenotype141. Whether Nrf2 transcriptional activation and downstream molecules also modulate microglial polarization and function after ICH deserves further investigation.
High mobility group protein 1
Though not itself a transcription factor, high mobility group protein B1 (HMG1) is a DNA-binding protein that regulates gene transcription through its interactions with nucleosomes, transcription factors and histones142–144. Crucially, HMG1 is involved in proinflammatory cytokine gene transcription143 and is secreted by haem-stimulated microglia in vitro145. Serum levels of HMG1 are increased in patients with ICH, and correlate with levels of IL-6 and TNF, as well as with NIH Stroke Scale scores145. This evidence indicates that microglial HMG1 has a role in ICH. In addition, treatment with the nonspecific HMG1 inhibitors glycyrrhizin146 and ethyl pyruvate147 attenuates ICH injury and improves early outcomes in rodent models of ICH. Our group has shown that expression of HMG1 protein is substantially increased at 24 h after ICH39,148. HMG1 also promotes macrophage M1 polarization via increased TLR2 and TLR4 signalling, which contributes to cardiac remodelling in mice prone to accelerated senescence144. Thus, the beneficial effects of HMG1 inhibitors in rodent models of ICH might result from attenuation of microglial activation and inhibition of TLR2 and TLR4 (REF. 149).
Prostaglandin E2 receptors
Prostaglandin E2 (PGE2) is a major product of cyclo-oxygenase and prostaglandin E synthase, and is thought to be a classic proinflammatory mediator within the brain. PGE2 activates its downstream signalling pathways via the G-protein-coupled E prostanoid (EP) receptors EP1–EP4. EP1 receptors are expressed in microglia, and EP1 deletion dampens microglial activity and phagocytosis150. Furthermore, treatment with the EP1 receptor antagonist SC51089 decreases microglial activation and attenuates grey and white matter injury in mouse models of ICH39. Interestingly, in mice with collagenase-induced ICH, deletion of EP2 and EP3 produces opposite effects on ICH outcomes that correlate with microglial activation status148. Although EP2 is expressed in neurons rather than in microglia, deletion of EP2 led to an increase in M1-like microglial activation, indicating a potential EP2-mediated interaction between neurons and microglia148. By contrast, EP3 receptors are expressed in microglia108, and EP3 deletion caused decreased micro-glial activation in mice with collagenase-induced ICH151. Similarly, treatment with an EP3 antagonist inhibited microglial activation in mice with thrombin-induced ICH108, although this treatment increased the number of M2 microglia (as shown by CD11b and Ym1 double immunostaining)108. Finally, the EP2 and EP4 dual agonist misoprostol provided protection and reduced the proinflammatory response in mice with either collagenase-induced or blood-induced ICH152. Together, these results support a potential role for PGE2 and EP receptors in modulation of microglial function after ICH.
Sphingosine-1-phosphate receptors
Sphingosine-1-phosphate (S1P) is a bioactive lipid mediator that exerts a wide range of effects on cells via its five known receptors (S1PR1–S1PR5) in the brain153,154. The S1PR agonist fingolimod was approved by the FDA in 2010 for the treatment of multiple sclerosis (MS). Microglia express all five S1PRs in vitro155,156, and fingolimod treatment reduced proinflammatory cytokine levels in lipopolysaccharide-induced M1-like micro-glia156,157. In vivo, fingolimod decreased the production of IL-1β and TNF in animal models of status epilepticus158 and neonatal hyperoxia159. In an animal model of MS, microglia exhibited a reduced M1-like response and an enhanced M2-like response at day 120 with fingolimod treatment160. In rodents with collagenase-induced ICH, fingolimod treatment improved functional outcomes161,162 and reduced levels of IFNγ after ICH162, and also inhibited M1-like responses by preventing T-lymphocyte infiltration into the brain162. Notably, fingolimod also accelerated haematoma clearance and improved clinical outcomes in patients with ICH163, strongly indicating the importance of S1PRs in clinical ICH. However, another group found no effects of fingolimod treatment on haematoma volume and inflammatory cells in rodents with collagenase-induced ICH164. Thus, additional studies are needed to determine the role of S1PRs and whether they mediate microglial polarization after ICH. Currently, fingolimod is being investigated in a phase II clinical trial as a treatment for ischaemic and haemorrhagic stroke165.
Microglial interactions with other cells
T lymphocytes
In patients with ICH, the frequency of both activated (CD3+CD9+) and regulatory (CD4+CD25+FOXP3+) T cells (Treg cells)166,167 was increased in the peripheral blood of patients from day 3 to day 7 after ICH90. In mice with blood-induced ICH, Treg cells45 and γδ T cells168 infiltrated the brain starting at day 4, and their levels remained elevated until day 7; furthermore, these Treg cells inhibited M1 microglia in vitro169 and in vivo170. In a mouse model of blood-induced ICH, our group found that these Treg cells promoted M2-like polarization of microglia and macrophages via the IL-10–glycogen synthase kinase-3β signalling pathway45. These data all support the premise that T-cell infiltration into the brain after ICH enhances M1-like responses of microglia and macrophages, whereas Treg cells in the brain contribute to the microglial M1 to M2 phenotype shift.
Neurons
The signalling pathway involving soluble CX3CL1, which is expressed on neurons, and its receptor CX3C chemokine receptor 1 (CX3CR1), which is expressed on microglia, mediates interactions between microglia and neurons, thereby modulating microglial activation171,172. Mice engineered to express CX3CR1 labelled with green fluorescent protein have been widely used to study microglial activation and function39,62,108,173–175. For example, after ischaemic stroke, CX3CL1 is released by neurons and participates in microglial activation via CX3CR1 (REFS 175–177), and mice deficient in CX3CL1 have a reduced susceptibility to cerebral ischaemia–reperfusion injury178. However, the roles of CX3CL1 and CX3CR1 in ICH have not been well studied.
Both CX3CL1 and CX3CR1 are expressed in the brains of patients with ICH179. In Tlr4−/− mice with blood-induced ICH, mRNA levels of both CX3CL1 and CD36 were increased compared with their levels in wild-type mice69. Moreover, mice with monocyte-specific CX3CR1 deficiency showed no defect in functional recovery after blood-induced ICH180, suggesting that neuron-secreted CX3CL1 is involved in microglial phagocytosis and functional recovery after ICH, but that monocyte recruitment after ICH is independent of signalling via CX3CR1 (REF. 180). Thus, interactions between neurons and microglia, mediated by CX3CL1 binding to CX3CR1, might be involved in the dynamic regulation of microglial phenotype after ICH.
Astrocytes
The initial observation, in 2005, that microglial processes and protrusions directly contact astrocytes, suggested an important link between these two cell types in the healthy brain181. In the CNS, astrocytes secrete proinflammatory (IL-6 and IL-1β) as well as anti-inflammatory (IL-10) cytokines182. Furthermore, astrocytes are major sources of many chemokines, such as CCL2, CXCL1, CXCL10 and CXCL12 (REFS 183,184), which might have roles in microglial differentiation. Several chemokines have been implicated in macrophage activation and polarization185. In patients with ICH, chemokines such as CCL20 (REF. 186), CXCL1 (REF. 186), CXCL2 (REF. 187), CXCL3 (REF. 187) and CCR1 (REF. 187) are upregulated in perihaematomal brain regions, indicating a potential role for these chemokines in ICH pathology. Some chemokines and their receptors have also been studied in animal models of ICH188. In rats with blood-induced ICH, gene expression microarray studies showed that, 24 h after ICH, Cxcl2 was highly upregulated in the striatum and cortex189. Moreover, the neuroprotective effect of retinoic acid receptor agonist AM80 in mice with collagenase-induced ICH is attributable to inhibition of Cxcl2 mRNA expression51.
Few studies have assessed M1 and M2 chemokine markers in ICH. However, in mice with collagenase-induced ICH, mRNA expression of the M1 marker Cxcl1 was increased at 6 h but not at 24 h after ICH, compared with its expression in control mice after sham ICH51. Astrocytes also participate in microglial M1 to M2 phenotype switching. In hyperammonaemic rats, sulforaphane promoted a switch in microglial polarization from the M1 to the M2 phenotype by decreasing membrane expression of sodium-dependent and chloride-dependent GABA transporter GAT3, mainly in activated astrocytes190.
In an in vitro study, CCL2 released from primary astrocytes contributed to M1 microglial polarization191. In mice with collagenase-induced ICH, a lack of CCL2 or CCR2 decreased the haematoma volume early after collagenase-induced ICH but resulted in delayed recovery from ICH112; these changes correlated with decreases in microglial activation and iNOS expression112. Ccr2−/− mice with blood-induced ICH also exhibited an early improvement in motor function and fewer M1 micro-glia180. A high CCL2 level at 24 h correlated with poor outcomes at day 7 in patients with ICH180.
The limited research available suggests that interactions between microglia and astrocytes can be either beneficial or harmful to neurons, but the crosstalk between microglia and astrocytes has not been fully investigated. Further study of astrocytic mediators that modulate microglial polarization, phagocytosis and other functions would improve our understanding of ICH pathology and facilitate translational research.
Oligodendrocytes
During the first 2 weeks after collagenase-induced ICH in rats, oligodendrocyte precursor cells and mature oligodendrocytes proliferate and differentiate within white matter tracts of the perihaematomal region192. In MS, M2 microglia promote oligodendrocyte proliferation and differentiation during the remyelination period, which is associated with functional recovery193. After ICH induced by injection of sonicated whole blood, haptoglobin was secreted from oligodendrocytes at 24 h and protected a mixed population of brain cells (neurons, astrocytes, and microglia) from the effects of haemoglobin toxicity194. However, our knowledge of how microglial polarization affects oligodendrocyte function is very limited.
Clinical and translational implications
Currently, several promising anti-ICH drugs are undergoing clinical trials (TABLE 3). Most exert neuroprotective effects by targeting M1-like microglia in the acute phase after ICH. Inhibition of M1-like microglial activation and enhancement of M2-like microglial anti-inflammatory responses are the major underlying mechanisms of these compounds. Additional drug candidates that target microglial polarization have demonstrated efficacy for ICH treatment in preclinical studies (TABLE 3). Although in this Review we have emphasized the importance of M2-like microglial responses (especially in the late recovery stage of ICH), treatment strategies for ICH that focus on modulating or enhancing M2-like microglia are still limited. As discussed above, rapamycin (at a low dose of 150 μg/kg) increased levels of M2-like anti-inflammatory cytokines IL-10 and TGFβ105. Sinomenine is a dextrorotatory morphinan analogue extracted from herbs used in traditional Chinese medicine195,196, and has long been used clinically for treating rheumatoid arthritis in China197. In vitro, sinomenine reduced levels of pro-inflammatory cytokines IL-1β, IL-6 and TNF in ICH-exposed BV-2 microglia198 and increased levels of M2-like markers IL-10 and Arg1 in primary microglia exposed to erythrocyte lysate199. Sinomenine increased levels of IL10 and Arg1 mRNA in mice with blood-induced ICH199. The complicated changes in M2 phenotype microglia, as discussed above, probably make M2-like microglia a more challenging target than M1 microglia.
Table 3.
Promising anti-ICH drugs that target microglial polarization
| Drug | Mode of action | Effects on microglia | Development phase |
|---|---|---|---|
| Fingolimod | S1PR activator | Decreases M1-like microglial responses156,157,162 | Phase II165 |
| Deferoxamine | Iron chelation and haematoma clearance | Decreases M1-like microglial responses121,200 | Phase II245 |
| Minocycline | Iron chelation and haematoma clearance | Decreases M1-like microglial responses53 | Phase I and II246 |
| Rosuvastatin | Lipid metabolism | Promotes M1-to-M2 phenotype shift247 | Phase II248 |
| Erythropoietin | Anti-inflammatory response | Decreases M1-like microglial responses249,250 | Phase II251 |
| Pinocembrin | TLR4 inhibitor | Decreases M1-like microglial responses62 | Preclinical |
| Resatorvid | TLR4 inhibitor | Decreases M1-like microglial responses68,252 | Preclinical |
| Sinomenine | Anti-inflammatory response | Enhances M2-like microglial responses199 | Preclinical |
| Rapamycin | mTOR activator | Enhances M2-like microglial responses105 | Preclinical |
The effects of stem cell transplantation on ICH are also now beginning to be explored in clinical trials. Preclinical studies in rats with collagenase-induced ICH showed that transplantation of mesenchymal stem cells (MSCs) obtained from umbilical cord blood improved ICH outcomes by inhibiting the M1 markers cyclooxygenase-2 and TNF200. Pneumostem, a novel agent based on human MSCs obtained from umbilical cord blood, is currently under investigation in a phase I clinical trial as a treatment for premature infants with intraventricular haemorrhage201.
Despite decades of research, effective drug treatments for ICH are still lacking. However, with the expanding investigation of microglial polarization and function in animal models of ICH, therapies that target microglial phenotype switching might soon become a clinical reality for patients affected by ICH.
Conclusions
Microglial activation has long been recognized as important in the pathology and progression of ICH6,202,203. The evidence indicates that after becoming activated, microglia undergo polarization into various phenotypes that might contribute differently to neuroinflammation in models of brain disease. Although very little is known about the dynamics of microglial polarization specifically after ICH, modulation of microglial function might be expected to mitigate ICH-related brain injury, thereby promoting tissue repair and functional recovery13. In this regard, some promising drugs strongly inhibit M1-like micro-glia, whereas others are thought to enhance microglial M2-like responses. However, both M1-like and M2-like microglia have critical roles in tissue repair at different stages after brain injury, suggesting that persistence of an M2-dominant state after ICH might impair innate immune responses and result in serious adverse effects, such as neoplasia204. Appropriate anti-ICH treatment, therefore, should aim to boost the correct microglial phenotype at the correct time, so as to maximize the natural processes of haematoma clearance and brain repair.
Considering the limitations of existing animal models of ICH and the numerous failed attempts to translate promising experimental treatments for ischaemic stroke into clinical use, studies of histopathological material from patients with ICH49 are critically needed to confirm the findings from studies in rodent models. Such a step is vitally important for future translational studies. Applications of transcriptomic, epigenomic and proteomic studies, and animal models employing conditional targeting of microglial genes, are expected to move the microglial research field forward and improve our understanding of the underlying mechanisms of ICH, potentially leading to effective treatments for affected patients.
Key points.
- Microglial polarization after intracerebral haemorrhage (ICH) modulates microglial phagocytic function and might affect haematoma clearance
- Activation of microglia to an M1-like phenotype occurs mainly in the acute phase after ICH
- M2-like microglial responses occur in the subacute and chronic phase and might contribute to phagocytosis of cell debris and haematoma clearance
- Microglial polarization can be regulated by transcription factors, chemokines, receptors and their signalling pathways, and interactions between microglia and other cells in the brain (T lymphocytes, neurons, astrocytes and oligodendrocytes)
- Data from clinical trials and preclinical studies suggest that targeting of microglial phenotype switching represents a new research direction for ICH treatment
Acknowledgments
The authors’ research work was supported by American Heart Association (AHA) Mid-Atlantic Affiliate Grant-in-Aid 13GRNT15730001, NIH grants R01NS078026 and R01AT007317, and a Stimulating and Advancing ACCM Research (StAAR) grant from the Department of Anesthesiology and Critical Care Medicine (ACCM), Johns Hopkins University (to J.W.), and an AHA Mid-Atlantic Affiliate Postdoctoral Fellowship Award 15POST25090114 (to X.L.). The authors thank Claire Levine, MS, ELS, for assistance with manuscript preparation.
Footnotes
Author contributions
X.L., X.H., and Q.L., researched the data for the article. X.L., Q.-W.Y., and J. W. provided substantial contributions to discussions of the content. X.L. and J.W. wrote and revised the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Steiner T, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, Bae HJ. Spontaneous intracerebral hemorrhage: management. J Stroke. 2017;19:28–39. doi: 10.5853/jos.2016.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thabet AM, Kottapally M, Hemphill JC., III Management of intracerebral hemorrhage. Handb Clin Neurol. 2017;140:177–194. doi: 10.1016/B978-0-444-63600-3.00011-8. [DOI] [PubMed] [Google Scholar]
- 5.Hemphill JC, III , et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 6.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan X, Liu R, Sun L, Zhang T, Du G. Methyl salicylate 2-O-β-D-lactoside, a novel salicylic acid analogue, acts as an anti-inflammatory agent on microglia and astrocytes. J Neuroinflammation. 2011;8:98. doi: 10.1186/1742-2094-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. Bone marrow mononuclear cells exert long-term neuroprotection in a rat model of ischemic stroke by promoting arteriogenesis and angiogenesis. Brain Behav Immun. 2013;34:56–66. doi: 10.1016/j.bbi.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balami JS, Fricker RA, Chen R. Stem cell therapy for ischaemic stroke: translation from preclinical studies to clinical treatment. CNS Neurol Disord Drug Targets. 2013;12:209–219. doi: 10.2174/1871527311312020007. [DOI] [PubMed] [Google Scholar]
- 10.Larochelle A, Bellavance MA, Michaud JP, Rivest S. Bone marrow-derived macrophages and the CNS: an update on the use of experimental chimeric mouse models and bone marrow transplantation in neurological disorders. Biochim Biophys Acta. 2016;1862:310–322. doi: 10.1016/j.bbadis.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Jiang C, et al. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behav Brain Res. 2013;250:222–229. doi: 10.1016/j.bbr.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, et al. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol Neurobiol. 2017;54:1874–1886. doi: 10.1007/s12035-016-9785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendelow AD, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzales NR. Ongoing clinical trials in intracerebral hemorrhage. Stroke. 2013;44:S70–S73. doi: 10.1161/STROKEAHA.111.000563. [DOI] [PubMed] [Google Scholar]
- 16.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni MG. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81. doi: 10.3389/fneur.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhor V, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroner A, et al. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa K, et al. Lipopolysaccharide preconditioning facilitates M2 activation of resident microglia after spinal cord injury. J Neurosci Res. 2014;92:1647–1658. doi: 10.1002/jnr.23448. [DOI] [PubMed] [Google Scholar]
- 23.Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han L, et al. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke. 2015;46:2628–2636. doi: 10.1161/STROKEAHA.115.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A, et al. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA. 2015;112:2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turtzo LC, et al. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflammation. 2014;11:82. doi: 10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X, et al. Microglial and macrophage polarization — new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Alvarez-Croda DM, Stoica BA, Faden AI, Loane DJ. Microglial/macrophage polarization dynamics following traumatic brain injury. J Neurotrauma. 2016;33:1732–1750. doi: 10.1089/neu.2015.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perego C, et al. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiol Dis. 2016;96:284–293. doi: 10.1016/j.nbd.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Benson MJ, Manzanero S, Borges K. Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia. 2015;56:895–905. doi: 10.1111/epi.12960. [DOI] [PubMed] [Google Scholar]
- 32.Casella G, et al. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. JNeuroinflammation. 2016;13:139. doi: 10.1186/s12974-016-0596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim CC, Nakamura MC, Hsieh CL. Brain trauma elicits non-canonical macrophage activation states. J Neuroinflammation. 2016;13:117. doi: 10.1186/s12974-016-0581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Tsirka SE. Tuftsin fragment 1–3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke. 2005;36:613–618. doi: 10.1161/01.STR.0000155729.12931.8f. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1–3 in an animal model of intracerebral hemorrhage. Ann Neurol. 2003;54:655–664. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- 37.Liew HK, et al. Systemic administration of urocortin after intracerebral hemorrhage reduces neurological deficits and neuroinflammation in rats. J Neuroinflammation. 2012;9:13. doi: 10.1186/1742-2094-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, et al. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav Immun. 2015;46:293–310. doi: 10.1016/j.bbi.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang C, et al. Progesterone exerts neuroprotective effects and improves long-term neurologic outcome after intracerebral hemorrhage in middle-aged mice. Neurobiol Aging. 2016;42:13–24. doi: 10.1016/j.neurobiolaging.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tischer J, et al. Inhomogeneous distribution of Iba-1 characterizes microglial pathology in Alzheimer’s disease. Glia. 2016;64:1562–1572. doi: 10.1002/glia.23024. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, et al. Thrombin-induced microglial activation impairs hippocampal neurogenesis and spatial memory ability in mice. Behav Brain Funct. 2015;11:30. doi: 10.1186/s12993-015-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammond MD, Ai Y, Sansing LH. Gr1+ macrophages and dendritic cells dominate the inflammatory infiltrate 12 hours after experimental intracerebral hemorrhage. Transl Stroke Res. 2012;3:s125–s131. doi: 10.1007/s12975-012-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng T, et al. Cerebroprotection of flavanol (–)-epicatechin after traumatic brain injury via Nrf2-dependent and -independent pathways. Free Radic Biol Med. 2016;92:15–28. doi: 10.1016/j.freeradbiomed.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou K, et al. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3β/PTEN axis. J Cereb Blood Flow Metab. 2016;37:967–979. doi: 10.1177/0271678X16648712. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Greter M, Lelios I, Croxford AL. Microglia versus myeloid cell nomenclature during brain inflammation. Front Immunol. 2015;6:249. doi: 10.3389/fimmu.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mracsko E, et al. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. 2014;45:2107–2114. doi: 10.1161/STROKEAHA.114.005801. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, et al. NF-κB activation and cell death after intracerebral hemorrhage in patients. Neurol Sci. 2014;35:1097–1102. doi: 10.1007/s10072-014-1657-0. [DOI] [PubMed] [Google Scholar]
- 49.Wu H, et al. Dynamic changes of inflammatory markers in brain after hemorrhagic stroke in humans: a postmortem study. Brain Res. 2010;1342:111–117. doi: 10.1016/j.brainres.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liesz A, et al. Comparison of humoral neuroinflammation and adhesion molecule expression in two models of experimental intracerebral hemorrhage. Exp Transl Stroke Med. 2011;3:11. doi: 10.1186/2040-7378-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsushita H, et al. Suppression of CXCL2 upregulation underlies the therapeutic effect of the retinoid Am80 on intracerebral hemorrhage in mice. J Neurosci Res. 2014;92:1024–1034. doi: 10.1002/jnr.23379. [DOI] [PubMed] [Google Scholar]
- 52.Yang S, et al. Hemoglobin-induced nitric oxide synthase overexpression and nitric oxide production contribute to blood–brain barrier disruption in the rat. J Mol Neurosci. 2013;51:352–363. doi: 10.1007/s12031-013-9990-y. [DOI] [PubMed] [Google Scholar]
- 53.Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007;1180:140–154. doi: 10.1016/j.brainres.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 54.Lin S, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9:46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie RX, et al. Carnosine attenuates brain oxidative stress and apoptosis after intracerebral hemorrhage in rats. Neurochem Res. 2016;42:541–551. doi: 10.1007/s11064-016-2104-9. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, et al. Quercetin promotes neuronal and behavioral recovery by suppressing inflammatory response and apoptosis in a rat model of intracerebral hemorrhage. Neurochem Res. 2015;40:195–203. doi: 10.1007/s11064-014-1457-1. [DOI] [PubMed] [Google Scholar]
- 57.Yang SS, et al. High morphologic plasticity of microglia/macrophages following experimental intracerebral hemorrhage in rats. Int J Mol Sci. 2016;17:E1181. doi: 10.3390/ijms17071181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King MD, Alleyne CH, Jr, Dhandapani KM. TNF-α receptor antagonist, R-7050, improves neurological outcomes following intracerebral hemorrhage in mice. Neurosci Lett. 2013;542:92–96. doi: 10.1016/j.neulet.2013.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao H, Garton T, Keep RF, Hua Y, Xi G. Microglia/macrophage polarization after experimental intracerebral hemorrhage. Transl Stroke Res. 2015;6:407–409. doi: 10.1007/s12975-015-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor RA, et al. TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest. 2017;127:280–292. doi: 10.1172/JCI88647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L, et al. Blocking B7-1/CD28 pathway diminished long-range brain damage by regulating the immune and inflammatory responses in a mouse model of intracerebral hemorrhage. Neurochem Res. 2016;41:1673–1683. doi: 10.1007/s11064-016-1883-3. [DOI] [PubMed] [Google Scholar]
- 62.Lan X, et al. Pinocembrin protects hemorrhagic brain primarily by inhibiting Toll-like receptor 4 and reducing M1 phenotype microglia. Brain Behav Immun. 2017;61:326–339. doi: 10.1016/j.bbi.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez-Yanez M, et al. Increased expression of Toll-like receptors 2 and 4 is associated with poor outcome in intracerebral hemorrhage. J Neuroimmunol. 2012;247:75–80. doi: 10.1016/j.jneuroim.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Lively S, Schlichter LC. Age-related comparisons of evolution of the inflammatory response after intracerebral hemorrhage in rats. Transl Stroke Res. 2012;3:132–146. doi: 10.1007/s12975-012-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang YC, et al. Toll-like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Ann Neurol. 2014;75:876–889. doi: 10.1002/ana.24159. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, et al. TLR4/MD-2 activation by a synthetic agonist with no similarity to LPS. Proc Natl Acad Sci USA. 2016;113:E884–E893. doi: 10.1073/pnas.1525639113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong XY, et al. Toll-like receptor 4/MyD88-mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation. 2016;134:1025–1038. doi: 10.1161/CIRCULATIONAHA.116.021881. [DOI] [PubMed] [Google Scholar]
- 68.Wang YC, et al. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke. 2013;44:2545–2552. doi: 10.1161/STROKEAHA.113.001038. [DOI] [PubMed] [Google Scholar]
- 69.Sansing LH, et al. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann Neurol. 2011;70:646–656. doi: 10.1002/ana.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teng W, Wang L, Xue W, Guan C. Activation of TLR4-mediated NFκB signaling in hemorrhagic brain in rats. Mediators Inflamm. 2009;2009:473276. doi: 10.1155/2009/473276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Z, et al. Toll-like receptor-4-mediated autophagy contributes to microglial activation and inflammatory injury in mouse models of intracerebral haemorrhage. Neuropathol Appl Neurobiol. 2015;41:e95–e106. doi: 10.1111/nan.12177. [DOI] [PubMed] [Google Scholar]
- 72.Lian H, et al. Astrocyte–microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s disease. J Neurosci. 2016;36:577–589. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garrett MC, et al. Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res. 2009;1298:171–177. doi: 10.1016/j.brainres.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang S, et al. The role of complement C3 in intracerebral hemorrhage-induced brain injury. J Cereb Blood Flow Metab. 2006;26:1490–1495. doi: 10.1038/sj.jcbfm.9600305. [DOI] [PubMed] [Google Scholar]
- 75.Yuan B, et al. C5a/C5aR pathway plays a vital role in brain inflammatory injury via initiating Fgl-2 in intracerebral hemorrhage. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0141-7. http://dx.doi.org/10.1007/s12035-016-0141-7. [DOI] [PubMed]
- 76.Li G, et al. Neuroprotective effects of argatroban and C5a receptor antagonist (PMX53) following intracerebral haemorrhage. Clin Exp Immunol. 2014;175:285–295. doi: 10.1111/cei.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Z, Trapp BD. Microglia and neuroprotection. J Neurochem. 2016;136(Suppl 1):10–17. doi: 10.1111/jnc.13062. [DOI] [PubMed] [Google Scholar]
- 78.Zhao X, et al. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci. 2015;35:11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zanier ER, et al. Fractalkine receptor deficiency is associated with early protection but late worsening of outcome following brain trauma in mice. J Neurotrauma. 2015;33:1060–1072. doi: 10.1089/neu.2015.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 81.Avdic S, et al. Human cytomegalovirus interleukin-10 polarizes monocytes toward a deactivated M2c phenotype to repress host immune responses. J Virol. 2013;87:10273–10282. doi: 10.1128/JVI.00912-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koscso B, et al. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J Leukoc Biol. 2013;94:1309–1315. doi: 10.1189/jlb.0113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 84.Capsoni F, et al. IL-10 up-regulates human monocyte phagocytosis in the presence of IL-4 and IFN-γ. J Leukoc Biol. 1995;58:351–358. doi: 10.1002/jlb.58.3.351. [DOI] [PubMed] [Google Scholar]
- 85.Leidi M, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than M1 cells in vitro. J Immunol. 2009;182:4415–4422. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 86.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 87.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 88.Dziedzic T, et al. Intracerebral hemorrhage triggers interleukin-6 and interleukin-10 release in blood. Stroke. 2002;33:2334–2335. doi: 10.1161/01.str.0000027211.73567.fa. [DOI] [PubMed] [Google Scholar]
- 89.Wang KW, et al. Molecular biomarker of inflammatory response is associated with rebleeding in spontaneous intracerebral hemorrhage. Eur Neurol. 2011;66:322–327. doi: 10.1159/000332027. [DOI] [PubMed] [Google Scholar]
- 90.Shi L, et al. Increased frequency of circulating regulatory T cells in patients with acute cerebral hemorrhage. Neurosci Lett. 2015;591:115–120. doi: 10.1016/j.neulet.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 91.Liu B, et al. CD163/hemoglobin oxygenase-1 pathway regulates inflammation in hematoma surrounding tissues after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:2800–2809. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 92.Ewen T, et al. Neuroprotective effect of atorvastatin involves suppression of TNF-α and upregulation of IL-10 in a rat model of intracerebral hemorrhage. Cell Biochem Biophys. 2013;66:337–346. doi: 10.1007/s12013-012-9453-z. [DOI] [PubMed] [Google Scholar]
- 93.Gao L, et al. Transplanted neural stem cells modulate regulatory T, γδ T cells and corresponding cytokines after intracerebral hemorrhage in rats. Int J Mol Sci. 2014;15:4431–4441. doi: 10.3390/ijms15034431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang J, et al. Interleukin-4 ameliorates the functional recovery of intracerebral hemorrhage through the alternative activation of microglia/macrophage. Front Neurosci. 2016;10:61. doi: 10.3389/fnins.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neve BP, Fruchart JC, Staels B. Role of the peroxisome proliferator-activated receptors (PPAR) in atherosclerosis. Biochem Pharmacol. 2000;60:1245–1250. doi: 10.1016/s0006-2952(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 96.Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15D-prostaglandin J2 activates peroxisome proliferator-activated receptor-γ, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006;26:811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- 97.Zhao X, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor γ in microglia/macrophages. Ann Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 98.Zhao X, Grotta J, Gonzales N, Aronowski J. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke. 2009;40:S92–S94. doi: 10.1161/STROKEAHA.108.533158. [DOI] [PubMed] [Google Scholar]
- 99.Chang CF, et al. Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol Dis. 2017;103:54–69. doi: 10.1016/j.nbd.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.An WL, et al. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan T, et al. Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis. 2008;32:16–25. doi: 10.1016/j.nbd.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen S, et al. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- 104.You W, et al. Inhibition of mammalian target of rapamycin attenuates early brain injury through modulating microglial polarization after experimental subarachnoid hemorrhage in rats. J Neurol Sci. 2016;367:224–231. doi: 10.1016/j.jns.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 105.Lu Q, et al. Inhibition of mammalian target of rapamycin improves neurobehavioral deficit and modulates immune response after intracerebral hemorrhage in rat. J Neuroinflammation. 2014;11:44. doi: 10.1186/1742-2094-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang JP, Zhang MY. Role for target of rapamycin (mTOR) signal pathway in regulating neuronal injury after intracerebral hemorrhage. Cell Physiol Biochem. 2017;41:145–153. doi: 10.1159/000455983. [DOI] [PubMed] [Google Scholar]
- 107.Wan S, et al. Microglia activation and polarization after intracerebral hemorrhage in mice: the role of protease-activated receptor-1. Transl Stroke Res. 2016;7:478–487. doi: 10.1007/s12975-016-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han X, et al. Inhibition of prostaglandin E2 receptor EP3 mitigates thrombin-induced brain injury. J Cereb Blood Flow Metab. 2016;36:1059–1074. doi: 10.1177/0271678X15606462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hickman SE, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao S, et al. Hematoma changes during clot resolution after experimental intracerebral hemorrhage. Stroke. 2016;47:1626–1631. doi: 10.1161/STROKEAHA.116.013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao X, et al. Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving Nrf2. Stroke. 2015;46:1923–1928. doi: 10.1161/STROKEAHA.115.009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yao Y, Tsirka SE. The CCL2–CCR2 system affects the progression and clearance of intracerebral hemorrhage. Glia. 2012;60:908–918. doi: 10.1002/glia.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 2000;96:3231–3240. [PubMed] [Google Scholar]
- 114.Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 115.Fang H, et al. CD36-mediated hematoma absorption following intracerebral hemorrhage: negative regulation by TLR4 signaling. J Immunol. 2014;192:5984–5992. doi: 10.4049/jimmunol.1400054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li X, et al. Prostaglandin E2 receptor subtype 2 regulation of scavenger receptor CD36 modulates microglial Aβ42 phagocytosis. Am J Pathol. 2015;185:230–239. doi: 10.1016/j.ajpath.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zamora C, et al. Functional consequences of CD36 downregulation by TLR signals. Cytokine. 2012;60:257–265. doi: 10.1016/j.cyto.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 119.Olsson M, Nilsson A, Oldenborg PA. Target cell CD47 regulates macrophage activation and erythrophagocytosis. Transfus Clin Biol. 2006;13:39–43. doi: 10.1016/j.tracli.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 120.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47–SIRPα signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 121.Olsson M, Nilsson A, Oldenborg PA. Dose-dependent inhibitory effect of CD47 in macrophage uptake of IgG-opsonized murine erythrocytes. Biochem Biophys Res Commun. 2007;352:193–197. doi: 10.1016/j.bbrc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 122.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ni W, Mao S, Xi G, Keep RF, Hua Y. Role of erythrocyte CD47 in intracerebral hematoma clearance. Stroke. 2016;47:505–511. doi: 10.1161/STROKEAHA.115.010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hume DA. The many alternative faces of macrophage activation. Front Immunol. 2015;6:370. doi: 10.3389/fimmu.2015.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taetzsch T, et al. Redox regulation of NF-κB p50 and M1 polarization in microglia. Glia. 2015;63:423–440. doi: 10.1002/glia.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang ZL, et al. Nuclear factor-κB activation in perihematomal brain tissue correlates with outcome in patients with intracerebral hemorrhage. J Neuroinflammation. 2015;12:53. doi: 10.1186/s12974-015-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arimoto KI, et al. STAT2 is an essential adaptor in USP18-mediated suppression of type I interferon signaling. Nat Struct Mol Biol. 2017;24:279–289. doi: 10.1038/nsmb.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jeon SB, et al. Galectin-3 exerts cytokine-like regulatory actions through the JAK–STAT pathway. J Immunol. 2010;185:7037–7046. doi: 10.4049/jimmunol.1000154. [DOI] [PubMed] [Google Scholar]
- 129.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 130.Das A, et al. Transcriptome sequencing of microglial cells stimulated with TLR3 and TLR4 ligands. BMC Genomics. 2015;16:517. doi: 10.1186/s12864-015-1728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leopold Wager CM, et al. STAT1 signaling within macrophages is required for antifungal activity against Cryptococcus neoformans. Infect Immun. 2015;83:4513–4527. doi: 10.1128/IAI.00935-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qin H, et al. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci USA. 2012;109:5004–5009. doi: 10.1073/pnas.1117218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim CK, et al. Detrimental effects of leptin on intracerebral hemorrhage via the STAT3 signal pathway. J Cereb Blood Flow Metab. 2013;33:944–953. doi: 10.1038/jcbfm.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Freilich RW, Woodbury ME, Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS ONE. 2013;8:e79416. doi: 10.1371/journal.pone.0079416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uhlemann R, et al. Actin dynamics shape microglia effector functions. Brain Struct Funct. 2016;221:2717–2734. doi: 10.1007/s00429-015-1067-y. [DOI] [PubMed] [Google Scholar]
- 136.Ferreira R, Lively S, Schlichter LC. IL-4 type 1 receptor signaling up-regulates KCNN4 expression, and increases the KCa3.1 current and its contribution to migration of alternative-activated microglia. Front Cell Neurosci. 2014;8:183. doi: 10.3389/fncel.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang J, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43:408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shang H, et al. Time course of Keap1–Nrf2 pathway expression after experimental intracerebral haemorrhage: correlation with brain oedema and neurological deficit. Free Radic Res. 2013;47:368–375. doi: 10.3109/10715762.2013.778403. [DOI] [PubMed] [Google Scholar]
- 139.Zhao X, et al. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem. 2015;133:144–152. doi: 10.1111/jnc.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schallner N, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–2625. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 142.Lee S, et al. A small molecule binding HMGB1 and HMGB2 inhibits microglia-mediated neuroinflammation. Nat Chem Biol. 2014;10:1055–1060. doi: 10.1038/nchembio.1669. [DOI] [PubMed] [Google Scholar]
- 143.Kim JB, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karuppagounder V, et al. Modulation of macrophage polarization and HMGB1–TLR2/TLR4 cascade plays a crucial role for cardiac remodeling in senescence-accelerated prone mice. PLoS ONE. 2016;11:e0152922. doi: 10.1371/journal.pone.0152922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou Y, et al. Elevation of high-mobility group protein box-1 in serum correlates with severity of acute intracerebral hemorrhage. Mediators Inflamm. 2010;2010:142458. doi: 10.1155/2010/142458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ohnishi M, et al. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011;61:975–980. doi: 10.1016/j.neuropharm.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 147.Lei C, et al. High-mobility group box 1 protein promotes neuroinflammation after intracerebral hemorrhage in rats. Neuroscience. 2013;228:190–199. doi: 10.1016/j.neuroscience.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 148.Wu H, et al. Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice. J Cereb Blood Flow Metab. 2017;37:39–51. doi: 10.1177/0271678X15625351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li D, et al. Blockade of high mobility group box-1 signaling via the receptor for advanced glycation end-products ameliorates inflammatory damage after acute intracerebral hemorrhage. Neurosci Lett. 2015;609:109–119. doi: 10.1016/j.neulet.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 150.Singh N, et al. Role of PGE2 EP1 receptor in intracerebral hemorrhage-induced brain injury. Neurotox Res. 2013;24:549–559. doi: 10.1007/s12640-013-9410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leclerc JL, Lampert AS, Diller MA, Doré S. Genetic deletion of the prostaglandin E2 E prostanoid receptor subtype 3 improves anatomical and functional outcomes after intracerebral hemorrhage. Eur J Neurosci. 2015;41:1381–1391. doi: 10.1111/ejn.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu H, et al. PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice. Neurobiol Aging. 2015;36:1439–1450. doi: 10.1016/j.neurobiolaging.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Echten-Deckert G, Hagen-Euteneuer N, Karaca I, Walter J. Sphingosine-1-phosphate: boon and bane for the brain. Cell Physiol Biochem. 2014;34:148–157. doi: 10.1159/000362991. [DOI] [PubMed] [Google Scholar]
- 154.Yung YC, Stoddard NC, Mirendil H, Chun J. Lysophosphatidic acid signaling in the nervous system. Neuron. 2015;85:669–682. doi: 10.1016/j.neuron.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tham CS, Lin FF, Rao TS, Yu N, Webb M. Microglial activation state and lysophospholipid acid receptor expression. Int J Dev Neurosci. 2003;21:431–443. doi: 10.1016/j.ijdevneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 156.Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J Neuroimmunol. 2013;256:13–18. doi: 10.1016/j.jneuroim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 157.Marfia G, et al. The adipose mesenchymal stem cell secretome inhibits inflammatory responses of microglia: evidence for an involvement of sphingosine-1-phosphate signalling. Stem Cells Dev. 2016;25:1095–1107. doi: 10.1089/scd.2015.0268. [DOI] [PubMed] [Google Scholar]
- 158.Gao F, et al. Fingolimod (FTY720) inhibits neuroinflammation and attenuates spontaneous convulsions in lithium-pilocarpine induced status epilepticus in rat model. Pharmacol Biochem Behav. 2012;103:187–196. doi: 10.1016/j.pbb.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 159.Serdar M, et al. Fingolimod protects against neonatal white matter damage and long-term cognitive deficits caused by hyperoxia. Brain Behav Immun. 2016;52:106–119. doi: 10.1016/j.bbi.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Rothhammer V, et al. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci USA. 2017;114:2012–2017. doi: 10.1073/pnas.1615413114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rolland WB, II , et al. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochir Suppl. 2011;111:213–217. doi: 10.1007/978-3-7091-0693-8_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rolland WB, et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55. doi: 10.1016/j.expneurol.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fu Y, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71:1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 164.Lu L, et al. Fingolimod exerts neuroprotective effects in a mouse model of intracerebral hemorrhage. Brain Res. 2014;1555:89–96. doi: 10.1016/j.brainres.2014.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.US National Library of Medicine. ClinicalTrials.gov. 2014 https://clinicaltrials.gov/ct2/show/NCT02002390.
- 166.Chen S, et al. Regulatory T cell in stroke: a new paradigm for immune regulation. Clin Dev Immunol. 2013;2013:689827. doi: 10.1155/2013/689827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gong G, et al. Phosphoantigen-activated Vγ2Vδ2 T cells antagonize IL-2-induced CD4+CD25+Foxp3+ T regulatory cells in mycobacterial infection. Blood. 2009;113:837–845. doi: 10.1182/blood-2008-06-162792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhong Q, et al. Interleukin-23 secreted by activated macrophages drives γδT cell production of interleukin-17 to aggravate secondary injury after intracerebral hemorrhage. J Am Heart Assoc. 2016;5:e004340. doi: 10.1161/JAHA.116.004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang Z, et al. Regulatory T cells inhibit microglia activation and protect against inflammatory injury in intracerebral hemorrhage. Int Immunopharmacol. 2014;22:522–525. doi: 10.1016/j.intimp.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 170.Mao LL, et al. Adoptive regulatory T-cell therapy attenuates perihematomal inflammation in a mouse model of experimental intracerebral hemorrhage. Cell Mol Neurobiol. 2016 doi: 10.1007/s10571-016-0429-1. http://dx.doi.org/10.1007/s10571-016-0429-1. [DOI] [PMC free article] [PubMed]
- 171.Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Limatola C, Ransohoff RM. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci. 2014;8:229. doi: 10.3389/fncel.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wolf Y, Yona S, Kim KW, Jung S. Microglia, seen from the CX3CR1 angle. Front Cell Neurosci. 2013;7:26. doi: 10.3389/fncel.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zabel MK, et al. Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1–CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia. 2016;64:1479–1491. doi: 10.1002/glia.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- 176.Tarozzo G, Campanella M, Ghiani M, Bulfone A, Beltramo M. Expression of fractalkine and its receptor, CX3CR1, in response to ischaemia–reperfusion brain injury in the rat. Eur J Neurosci. 2002;15:1663–1668. doi: 10.1046/j.1460-9568.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 177.Zhu J, Zhou Z, Liu Y, Zheng J. Fractalkine and CX3CR1 are involved in the migration of intravenously grafted human bone marrow stromal cells toward ischemic brain lesion in rats. Brain Res. 2009;1287:173–183. doi: 10.1016/j.brainres.2009.06.068. [DOI] [PubMed] [Google Scholar]
- 178.Soriano SG, et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia–reperfusion injury. J Neuroimmunol. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 179.Gaetani P, et al. Immunohistohemical expression of the chemokine fractalkine and its receptor in the human brain cortex after severe traumatic brain injury and brain hemorrhage. J Neurosurg Sci. 2013;57:55–62. [PubMed] [Google Scholar]
- 180.Taylor RA, Hammond MD, Ai Y, Sansing LH. CX3CR1 signaling on monocytes is dispensable after intracerebral hemorrhage. PLoS ONE. 2014;9:e114472. doi: 10.1371/journal.pone.0114472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 182.Nash B, et al. Functional duality of astrocytes in myelination. J Neurosci. 2011;31:13028–13038. doi: 10.1523/JNEUROSCI.1449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McKimmie CS, Graham GJ. Astrocytes modulate the chemokine network in a pathogen-specific manner. Biochem Biophys Res Commun. 2010;394:1006–1011. doi: 10.1016/j.bbrc.2010.03.111. [DOI] [PubMed] [Google Scholar]
- 184.Allaman I, Belanger M, Magistretti PJ. Astrocyte–neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 185.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 186.Rosell A, et al. Brain perihematoma genomic profile following spontaneous human intracerebral hemorrhage. PLoS ONE. 2011;6:e16750. doi: 10.1371/journal.pone.0016750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Carmichael ST, et al. Genomic profiles of damage and protection in human intracerebral hemorrhage. J Cereb Blood Flow Metab. 2008;28:1860–1875. doi: 10.1038/jcbfm.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yao Y, Tsirka SE. Chemokines and their receptors in intracerebral hemorrhage. Transl Stroke Res. 2012;3:70–79. doi: 10.1007/s12975-012-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lu A, et al. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;26:230–252. doi: 10.1038/sj.jcbfm.9600183. [DOI] [PubMed] [Google Scholar]
- 190.Hernandez-Rabaza V, et al. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J Neuroinflammation. 2016;13:83. doi: 10.1186/s12974-016-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.He M, et al. Astrocyte-derived CCL2 is associated with M1 activation and recruitment of cultured microglial cells. Cell Physiol Biochem. 2016;38:859–870. doi: 10.1159/000443040. [DOI] [PubMed] [Google Scholar]
- 192.Joseph MJ, Caliaperumal J, Schlichter LC. After intracerebral hemorrhage, oligodendrocyte precursors proliferate and differentiate inside white-matter tracts in the rat striatum. Transl Stroke Res. 2016;7:192–208. doi: 10.1007/s12975-015-0445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhao X, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci. 2009;29:15819–15827. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shukla SM, Sharma SK. Sinomenine inhibits microglial activation by Aβ and confers neuroprotection. J Neuroinflammation. 2011;8:117. doi: 10.1186/1742-2094-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Qian L, et al. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med. 2008;74:1423–1429. doi: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 198.Yang Z, et al. Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Mol Immunol. 2014;60:109–114. doi: 10.1016/j.molimm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 199.Shi H, et al. Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage. J Neuroimmunol. 2016;299:28–34. doi: 10.1016/j.jneuroim.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 200.Kim K, et al. The effect of human umbilical cord blood-derived mesenchymal stem cells in a collagenase-induced intracerebral hemorrhage rat model. Exp Neurobiol. 2015;24:146–155. doi: 10.5607/en.2015.24.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.US National Library of Medicine. ClinicalTrials.gov. 2017 https://clinicaltrials.gov/ct2/show/NCT02274428.
- 202.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 203.Wang J, Tsirka SE. Contribution of extracellular proteolysis and microglia to intracerebral hemorrhage. Neurocrit Care. 2005;3:77–85. doi: 10.1385/NCC:3:1:077. [DOI] [PubMed] [Google Scholar]
- 204.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Siddiqui TA, Lively S, Schlichter LC. Complex molecular and functional outcomes of single versus sequential cytokine stimulation of rat microglia. J Neuroinflammation. 2016;13:66. doi: 10.1186/s12974-016-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vogel DY, et al. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J Neuroinflammation. 2014;11:23. doi: 10.1186/1742-2094-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guedj K, et al. M1 macrophages act as LTβR-independent lymphoid tissue inducer cells during atherosclerosis-related lymphoid neogenesis. Cardiovasc Res. 2014;101:434–443. doi: 10.1093/cvr/cvt263. [DOI] [PubMed] [Google Scholar]
- 208.Zhong TY, et al. Hemocyanins stimulate innate immunity by inducing different temporal patterns of proinflammatory cytokine expression in macrophages. J Immunol. 2016;196:4650–4662. doi: 10.4049/jimmunol.1501156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tarassishin L, Suh HS, Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J Neuroinflammation. 2011;8:187. doi: 10.1186/1742-2094-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chong BF, et al. A subset of CD163+ macrophages displays mixed polarizations in discoid lupus skin. Arthritis Res Ther. 2015;17:324. doi: 10.1186/s13075-015-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hennessy E, Griffin EW, Cunningham C. Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1β and TNF-α. J Neurosci. 2015;35:8411–8422. doi: 10.1523/JNEUROSCI.2745-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sielska M, et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230:310–321. doi: 10.1002/path.4192. [DOI] [PubMed] [Google Scholar]
- 213.Suenaga J, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol. 2015;272:109–119. doi: 10.1016/j.expneurol.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wachholz S, et al. Microglia activation is associated with IFN-α induced depressive-like behavior. Brain Behav Immun. 2015;55:105–113. doi: 10.1016/j.bbi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 215.Okuneva O, et al. Abnormal microglial activation in the Cstb−/− mouse, a model for progressive myoclonus epilepsy, EPM1. Glia. 2015;63:400–411. doi: 10.1002/glia.22760. [DOI] [PubMed] [Google Scholar]
- 216.Stojakovic A, et al. Role of the IL-1 pathway in dopaminergic neurodegeneration and decreased voluntary movement. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9988-x. http://dx.doi.org/10.1007/s12035-016-9988-x. [DOI] [PMC free article] [PubMed]
- 217.Liu X, et al. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. 2016;47:498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Peferoen LA, et al. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2015;74:48–63. doi: 10.1097/NEN.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 219.Yamashita U, Kuroda E. Regulation of macrophage-derived chemokine (MDC, CCL22) production. Crit Rev Immunol. 2002;22:105–114. [PubMed] [Google Scholar]
- 220.Evans BJ, Haskard DO, Sempowksi G, Landis RC. Evolution of the macrophage CD163 phenotype and cytokine profiles in a human model of resolving inflammation. Int J Inflam. 2013;2013:780502. doi: 10.1155/2013/780502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Munder M, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–2556. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 222.Pesce JT, et al. Arginase-1-expressing macrophages suppress TH2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–180. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- 224.Raes G, et al. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 225.Durafourt BA, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 226.Lively S, Hutchings S, Schlichter LC. Molecular and cellular responses to interleukin-4 treatment in a rat model of transient ischemia. J Neuropathol Exp Neurol. 2016;75:1058–1071. doi: 10.1093/jnen/nlw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23:33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 228.Babu R, Bagley JH, Di C, Friedman AH, Adamson C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg Focus. 2012;32:E8. doi: 10.3171/2012.1.FOCUS11366. [DOI] [PubMed] [Google Scholar]
- 229.Yang J, et al. Multimodality MRI assessment of grey and white matter injury and blood–brain barrier disruption after intracerebral haemorrhage in mice. Sci Rep. 2017;7:40358. doi: 10.1038/srep40358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lei B, et al. Intrastriatal injection of autologous blood or clostridial collagenase as murine models of intracerebral hemorrhage. J Vis Exp. 2014;89:51439. doi: 10.3791/51439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bao L, et al. Thrombin-induced apoptosis in neurons through activation of c-Jun-N-terminal kinase. Toxicol Mech Methods. 2017;27:18–23. doi: 10.3109/15376516.2016.1172691. [DOI] [PubMed] [Google Scholar]
- 232.Hu S, et al. Thrombin-induced autophagy: a potential role in intracerebral hemorrhage. Brain Res. 2011;1424:60–66. doi: 10.1016/j.brainres.2011.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kirkman MA, Allan SM, Parry-Jones AR. Experimental intracerebral hemorrhage: avoiding pitfalls in translational research. J Cereb Blood Flow Metab. 2011;31:2135–2151. doi: 10.1038/jcbfm.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.MacLellan CL, Paquette R, Colbourne F. A critical appraisal of experimental intracerebral hemorrhage research. J Cereb Blood Flow Metab. 2012;32:612–627. doi: 10.1038/jcbfm.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kleinig TJ, et al. Hemoglobin crystals: a pro-inflammatory potential confounder of rat experimental intracerebral hemorrhage. Brain Res. 2009;1287:164–172. doi: 10.1016/j.brainres.2009.06.077. [DOI] [PubMed] [Google Scholar]
- 236.MacLellan CL, Davies LM, Fingas MS, Colbourne F. The influence of hypothermia on outcome after intracerebral hemorrhage in rats. Stroke. 2006;37:1266–1270. doi: 10.1161/01.STR.0000217268.81963.78. [DOI] [PubMed] [Google Scholar]
- 237.MacLellan CL, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- 238.Manaenko A, Chen H, Zhang JH, Tang J. Comparison of different preclinical models of intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:9–14. doi: 10.1007/978-3-7091-0693-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 240.Wasserman JK, Schlichter LC. White matter injury in young and aged rats after intracerebral hemorrhage. Exp Neurol. 2008;214:266–275. doi: 10.1016/j.expneurol.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 241.Lee JC, Cho GS, Choi BO, Kim HC, Kim WK. Aging exacerbates intracerebral hemorrhage-induced brain injury. J Neurotrauma. 2009;26:1567–1576. doi: 10.1089/neu.2008.0630. [DOI] [PubMed] [Google Scholar]
- 242.Okauchi M, et al. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41:375–382. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke. 2010;41:S95–S98. doi: 10.1161/STROKEAHA.110.594457. [DOI] [PubMed] [Google Scholar]
- 244.Hiploylee C, Colbourne F. Intracranial pressure measured in freely moving rats for days after intracerebral hemorrhage. Exp Neurol. 2014;255:49–55. doi: 10.1016/j.expneurol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 245.US National Library of Medicine. ClinicalTrials.gov. 2017 https://clinicaltrials.gov/ct2/show/NCT02175225.
- 246.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT01805895.
- 247.Kata D, et al. Rosuvastatin enhances anti-inflammatory and inhibits pro-inflammatory functions in cultured microglial cells. Neuroscience. 2016;314:47–63. doi: 10.1016/j.neuroscience.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 248.US National Library of Medicine. ClinicalTrials.gov. 2009 https://clinicaltrials.gov/ct2/show/NCT00364559.
- 249.Yu Z, et al. Erythropoietin reduces brain injury after intracerebral hemorrhagic stroke in rats. Mol Med Rep. 2013;8:1315–1322. doi: 10.3892/mmr.2013.1666. [DOI] [PubMed] [Google Scholar]
- 250.Chau M, Chen D, Wei L. Erythropoietin attenuates inflammatory factors and cell death in neonatal rats with intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:299–305. doi: 10.1007/978-3-7091-0693-8_50. [DOI] [PubMed] [Google Scholar]
- 251.US National Library of Medicine. ClinicalTrials.gov. 2015 https://clinicaltrials.gov/ct2/show/NCT00413946.
- 252.Lei C, Wu B, Cao T, Liu M, Hao Z. Brain recovery mediated by Toll-like receptor 4 in rats after intracerebral hemorrhage. Brain Res. 2016;1632:1–8. doi: 10.1016/j.brainres.2015.11.045. [DOI] [PubMed] [Google Scholar]