Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling (original) (raw)
Abstract
In response to myocardial infarction (MI), time-dependent leukocyte infiltration is critical to program the acute inflammatory response. Post-MI leukocyte density, residence time in the infarcted area, and exit from the infarcted injury predict resolving or nonresolving inflammation. Overactive or unresolved inflammation is the primary determinant in heart failure pathology post-MI. Here, our review describes supporting evidence that the acute inflammatory response also guides the generation of healing and regenerative mediators after cardiac damage. Time-dependent leukocyte density and diversity and the magnitude of myocardial injury is responsible for the resolving and nonresolving pathway in myocardial healing. Post MI, the diversity of leukocytes, such as neutrophils, macrophages, and lymphocytes, has been explored that regulate the clearance of deceased cardiomyocytes by using the classic and reparative pathways. Among the innovative factors and intermediates that have been recognized as essential in acute the self-healing and clearance mechanism, we highlight specialized proresolving mediators as the emerging factor for post-MI reparative mechanisms—translational leukocyte modifiers, such as aging, the source of leukocytes, and the milieu around the leukocytes. In the clinical setting, it is possible that leukocyte diversity is more prominent as a result of risk factors, such as obesity, diabetes, and hypertension. Pharmacologic agents are critical modifiers of leukocyte diversity in healing mechanisms that may impair or stimulate the clearance mechanism. Future research is needed, with a focused approach to understand the molecular targets, cellular effectors, and receptors. A clear understanding of resolving and nonresolving inflammation in myocardial healing will help to develop novel targets with major emphasis on the resolution of inflammation in heart failure pathology.—Tourki, B., Halade, G. Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling.
Keywords: heart failure, inflammation, myocardial infarction, resolution of inflammation
In response to myocardial infarction (MI) injury, leukocytes synthesize a broad range of mediators that regulate the inflammatory response (1). Post-MI innate response-sensing mediators comprise chemokines, cytokines, peptides, and resolution factors. Experimental evidence that has been predominantly derived from large animal and clinical studies suggests that that control or expansion of leukocyte infiltration in ischemic areas is a hallmark of post-MI inflammation and the overwhelming infiltration of innate immune cells that has been shown to promote adverse remodeling and cardiac rupture (2). Pathologic remodeling is marked with the progressive change in size, shape, and function of the left ventricle (LV) to develop heart failure. Post-MI, acute inflammatory response, resolution of inflammation, and the return to homeostasis are active and highly orchestrated biochemical events that are now considered to be the programmed and physiologic resolution in local tissue as well as at the network and systemic level. Local resolution can be defined as the interval from maximum neutrophilic infiltration post-MI to the point when neutrophils exit or clear from the tissue (3), which implies the clearance of the proinflammatory neutrophil phenotype; recruitment of reparative neutrophils, monocyte subsets, and regulatory T (Treg) cells; and macrophage (Mϕ) differentiation (4, 5). This process leads to the resumption of normal physiologic recovery from the ischemic attack (6). A rapid and complete clearance of leukocytes from the injury site in minimal time is the ideal outcome of the successful resolution that seems to be a key event in the repair of tissue damage. In the clinical setting, the fate of this process is nonresolving in heart failure pathology determined by the imbalance between the presence of chemical lipid mediators and sensors (receptors) that amplify the inflammatory process, thereby, chronic inflammation (6, 7).
It is therefore important to understand the mechanisms that underlie the resolution of inflammation, particularly the regulators that interfere in resolving and nonresolving processes. Such an approach would mean developing modifiers that exert multiple effects at various phases of the inflammatory response, limiting leukocyte trafficking, hastening cell clearance, and helping to restore inflamed cardiac tissue to its prior state. This review discusses leukocyte diversity as the main cellular effector and provides an update of post–immune cell kinetics and pharmacologic and biologic modifiers that are implicated in the resolving and nonresolving inflammatory response post-MI.
Leukocytes in cardiac remodeling and heart failure pathology
Aggressive leukocytosis is a cardinal sign of acute inflammatory response post-MI, and sustained leukocytosis has emerged as a powerful predictor of morbidity and mortality in patients with advanced heart failure. In the hours immediately after MI, leukocytes—neutrophils and monocytes—rapidly infiltrate into the LV infarcted area. It has been proven that leukocytes activated in patients with MI as evidenced by being more active in transcription of inflammatory genes. There are also significant changes in leukocyte number, with various markers and status of different leukocyte subpopulations in the spleen and kidney and at the systemic level that indicate network dynamics of post-MI injury. Various cell types, including neutrophils, platelets, and Mϕs, are involved at different stages of infarct healing, which ultimately leads to on-time scar formation and adaptive remodeling to preserve LV function. Leukocytes are directed by apoptotic cardiomyocyte—the “find me” signal—for clearance—the “eat me” signal—of danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). The chemokine, lipokine, and cytokine milieu of activated or deceased cardiac cells induce a “get in” signal to neutrophils to enter the infarcted myocardium within the first few hours after the onset of ischemia (1). Differential leukocytes generate high levels of reactive oxygen species (ROS) and secrete proteases, which exacerbates local vascular and tissue injury (8). Subsequently, monocyte-derived Mϕs are expanded in the infarcted area to remove debris and apoptotic neutrophils, which leads to the activation of reparative pathways that are necessary for scar formation (9). In this review, we highlight the diversity of leukocyte subsets and their roles in the context of the cardiac ischemic disease, focusing on resolving and nonresolving inflammation post-MI.
Neutrophil diversity in myocardial infarction
In acute inflammation, neutrophils are vital not only for clearing the dead cardiomyocyte or post-MI debris but also for the resolution of inflammation and the coordination of LV tissue homeostasis (10). In the context of MI, neutrophils are traditionally considered detrimental. Neutrophils are acutely recruited within hours of the onset of MI and contribute to the tissue injury healing response, whereas their context- or time-dependent secretive role in infarct healing thus far remains unclear. Recent evidence suggests that neutrophils are required for resolving post-MI inflammation and cardiac healing by polarizing Mϕs toward a reparative phenotype. Indeed, neutrophil depletion modulates cardiac monocyte and Mϕ profiles, promotes excessive fibrosis, and may be a consequence of the impaired resolution of inflammation because of insufficient monocyte recruitment, thereby, unfavorable repair (4). Neutrophil diversity is a novel topic. Neutrophil polarization has recently been identified in cancer, bacterial infection, and stroke animal models (11, 12). In the setting of MI, it is for the first time; a study identified that neutrophil polarization is differentiated in 2 subsets: proinflammatory N1 and anti-inflammatory N2 in the MI heart similar to Mϕ phenotypes. N1 is the major neutrophil phenotype in the LV post-MI, with 80% of the total neutrophils, whereas the percentage of N2 increased over time post-MI, which supports its role in the resolution of inflammation and local wound repair (13). Neutrophils display a proinflammatory N1 subtype, which peaked at d 1 with higher expression of surface markers, Ly6G+CD206−, indicating proresolving signature genes, such as Ym1, and proinflammatory genes, such as Ccl3, IL-1β, IL-12α, TNF-α, with a gradual shift to the anti-inflammatory N2 phenotype from d 1 to 7 post-MI (13). The time-dependent shift to CD206+ N2 neutrophils is locally activated because circulating neutrophils are CD206− post-MI. In addition, N2 neutrophils negatively correlate with infarct wall thinning, which indicates that N2 neutrophils may help to prevent additional LV wall thinning, whereas N1 neutrophils contribute to wall thinning, possibly by generating high levels of matrix metalloproteinase-12 and -25 (14, 15). It is unclear how neutrophil subsets are differently polarized in the setting of MI. To date, DAMPs and PAMPs have been evaluated in neutrophil N1 polarization. DAMPs can initiate and perpetuate the inflammatory response by binding to their receptors on leukocytes (16). As such, DAMPs and PAMPs are early potentiators of MI injury (17). It is likely that DAMPs and PAMPs do not fully recapitulate the entire clinical MI setting or other unknown factors as a mixture of stimuli (milieu) to post-MI neutrophil N1 activation. Conversely, neutrophil density and clearance time are extended in an infarcted area, particularly in the setting of obesity combined with aging (18). Indeed, obese and aging mice show greater numbers of neutrophils in the infarct area, with higher VCAM-1 levels at d 1 post-MI. VCAM-1 is a primary mediator of neutrophil–endothelial adhesion and it plays a role in neutrophil arrival, coordinating adhesion, recruitment, and transmigration from the circulation to the site of myocardium injury (19). Increased VCAM-1 levels could explain the higher recruitment of neutrophils. Thus, VCAM-1–mediated increased endothelial dysfunction in obesity may serve as a trigger to increase neutrophil trafficking and recruitment in the infarcted area.
Future studies are warranted to define the individual components and highlight the combined effects on neutrophil polarization at the local LV site and the network level to understand acute and nonresolving chronic inflammation.
Role of neutrophils in ischemia and reperfusion injury
Current therapeutic strategies for ischemia and reperfusion (I/R) injury are mainly palliative and do not offer cardioprotection; therefore, there is an urgent need for the development of a therapeutic program that could prevent or reverse tissue damage caused by I/R. Neutrophils are the primary first responders after I/R and represent important components in the protracted inflammatory response. Extended survival of neutrophils and defective apoptosis indicate the severity associated with I/R pathology. Excessive neutrophils increase collateral myocardial necrosis during I/R-related injury or in nonreperfused infarction setting (8, 20). During the past 2 decades, experimental evidence has suggested that neutrophils may also directly contribute to collateral damage of cardiomyocytes via release of toxic products, such as oxidative radicals and proteolytic enzymes (21). In particular, compelling evidence for a key role for neutrophils in I/R-mediated myocardial damage stems from studies of pharmacologic—Abs and inhibitors—and genetic interventions that were aimed at preventing neutrophil recruitment or function with minimum emphasis on leukocyte diversity and chronic heart failure pathology. The strongest evidence of an anti-inflammatory role for these inflammatory phagocytes in I/R is derived from studies that employed neutrophil depletion strategies or the prevention of their adhesion to the endothelium by the use of immune-neutralizing Abs directed against adhesion molecules. For example, anti-CD18 Ab has been shown to reduce LV neutrophil numbers in a dog I/R model (22), but clinical trials failed to show a significant reduction in infarct size (23). Alternatively, mice that are genetically deficient in adhesion molecules (24), such as GPCRs, have displayed a range of metabolic defects and disorders that account for almost one third of all prescription drugs in current use (25). Furthermore, evidence to date suggests that GPCRs may also be future targets for the I/R injury clearance mechanism. Some exogenous and self-defensive endogenous agents have been identified at the site of injury to modulate the post-MI acute inflammatory response. In particular, adenosine has been shown to offer cardioprotection because of its anti-inflammatory properties (26). For instance, another GPCR, adenosine A2B receptor, has been studied in neutrophils during cardiac I/R (27). This report suggests that the adenosine A2B receptor is dominantly protective when neutrophils are activated and that it limits inflammatory responses after I/R injury (27).
Complement inhibition is another attractive target to prevent postischemic damage or cardiac myocyte salvation (28, 29). C5a protein is an important chemoattractant trigger for neutrophils, and, therefore, inhibition of C5 could reduce neutrophil infiltration in I/R to offer cardioprotective modality; however, experimental results from pexelizumab, a close analog to eculizumab that also inhibits the cleavage of C5, failed to reduce infarct size and speculative cardioprotection in a human MI trial (30). Now, however, it has been realized that infarct reduction alone is not the primary end point (31) on the basis of the multidimensional origin of MI event, comedication, and comorbidity in heart failure pathology. Many agents tested for reducing infarct or for cardiac protective actions are not reproducible and not translated to the clinic; therefore, a precise and personalized approach will add value in current research on inflammation and resolution of inflammation. In I/R-induced animal models, complement depletion significantly reduces neutrophil numbers (32). There is a strong possibility that the reduction of I/R-related collateral myocardium injury when inhibiting complement is actually a result of a decrease in neutrophil trafficking, which, specifically, could be the key to preventing I/R injury. At the same time, the context-dependent, long-term protective or detrimental role of neutrophils and the survival benefit need to be addressed to capitalize on research outcomes and to develop new therapies for cardiac protection post-MI in heart failure pathology (4, 13, 33).
Whereas the primacy of neutrophils in the process of post-MI healing is currently the nonconsensus view, recent studies have emphasized and appreciated the potential role of other inflammatory cells, such as Mϕs, lymphocytes, mast cells, eosinophils, and platelets, in the modulation of neutrophil recruitment and trafficking (34).
Monocyte/Mϕ diversity in post-MI healing
Traditionally, monocytes were thought to be a homogenous population that patrols the vasculature awaiting a damage or injury signal before entering tissue, differentiating into Mϕs, and subsequently performing effector functions; however, monocytes are the dominant cell type that infiltrates myocardium injury, and now we have begun to appreciate the novel and divergent role that monocytes play during infarct healing (35). Post-MI, ischemic tissue attracts inflammatory Ly6Chigh monocytes (the find me signal) as the immediate wave. Within 30 min postligation, these cells are abundantly recruited to the infarcted area, initially surpassing even neutrophils (36). Post-MI neutrophil tissue numbers peak 24 h after the onset of myocardium injury, whereas inflammatory monocytes peak around 24–72 h, depending on the magnitude of injury or the time of occlusion (37). Splenic and bone marrow–derived Ly6Chigh monocyte recruitment relies on monocyte chemoattractant protein-1 (MCP-1)/CCR2 chemokine/chemokine receptor interaction (37, 38). Once recruited to the site of injury, many monocytes differentiate into Mϕs, as the marker, F4/80, is expressed several days after MI. During the resolution of post-MI inflammation, Mϕs are important for both inhibitory actions—by secreting mediators that suppress inflammation—and proresolving actions to remove or clear inflammatory leukocytes. This dichotomy lies in time- or milieu-dependent alterations in the phenotype and function of Mϕ subpopulations. In a multiple-injury setting, for simplicity, Mϕs are classified as classic (M1) and reparative (M2). M1 Mϕs can be induced in vitro by IFN-γ, LPS, or the combination of both. They are classified as IL-12++/IL-23++/IL-10+, have a high capacity for antigen presentation (TLR2/4, CD80, CD86), are efficient producers of toxic intermediates (NO, ROS) and inflammatory cytokines (TNF-α, IL-1β, IL-6), and stimulate and sustain polarized T helper (Th)1 responses (39). In contrast, M2 Mϕs are time sensitive and more heterogeneous in myocardial healing. Several subsets have been described in vitro, depending on the stimulating signal–monocyte polarized to Mϕs by exposure to IL-4; those formed by stimulation with immune complexes, and Mϕs differentiated by exposure to IL-10, glucocorticoids, or glucocorticoids with TGF-β (39). All M2 Mϕs share an IL-12+/IL-23+/IL-10++ phenotype; have high phagocytic activity and high expression of scavenger (CD204, CD163, CD36), mannose receptor (CD206, CD209), and galactose-type receptors; participate in polarized Th2 responses; and mediate type 2 inflammation, tissue remodeling, angiogenesis, and immunoregulation (40, 41). Overactivation or early activation of M2 Mϕs is increasingly associated with fibrotic and chronic inflammatory disorders, including pulmonary fibrosis and hepatic fibrosis (42–44). Both monocytes and Mϕs generate inflammatory cytokines, lipokines, chemokines, oxygenases, cathepsins, lipoxygenases, and matrix metalloproteinases. Presumably, the main function of infiltrated monocytes is to replenish Mϕs to remove the debris of dead myocytes, whereas the main function of apoptotic neutrophils is to prepare LV tissue rebuilding and regeneration (45, 46). Monocytes and Mϕs initially accumulate in the infarct border zone (47), likely because microcirculatory vascular access is still operative in the ischemic myocardium adjacent to the infarcted tissue. The first clinical study described a biphasic monocyte pattern in the blood of patients after MI, with inflammatory CD14+CD162 monocytes dominating first (48). A recent autopsy study confirmed the kinetics in infarct tissue, with an early abundance of CD14+CD162 monocytes, whereas CD14+CD16+ monocytes were found only in the infarcts of patients who died at later time points (46, 47). The heterogeneity of monocytes/Mϕs is a result of the plasticity and flexibility of the milieu around the leukocytes, which are the primary features of Mϕ phenotypes and their activation states (49). Monocytes that are recruited into the tissue at different times encounter different microenvironments with different signals that can polarize them in M1 during early phases and in M2 during late phases (50). In this case, cytokines, growth factors, PAMPs, and other microenvironmental lipid mediators in the tissue direct the functional phenotypes of Mϕs. Infarcted milieu dictates a transcriptional response that shapes the phenotype and function of Mϕs on the basis of the physiologic or pathologic context (49). Blood monocytes’ diurnal rhythm is linked to the fluctuation of several clock genes, such as Bmal1, Nrld1, and Dbp (9). The circadian rhythm (sleep and wake cycle), overnutrition, undernutrition, or action (exercise) and inaction plays an important role that underlies the inflammatory recruitment that is key for infarct healing and remodeling (51). There is a crucial sequence of humoral and cellular factors in LV infarct remodeling. Like monocyte fluctuation, circadian rhythms influence mortality and morbidity post-MI. Disrupted diurnal rhythms, obesity, and aging aggravate myocardial remodeling and function post-MI (9, 18, 52). This theory is also supported by a clinical study (53) that reported that infarct size peaked at 1:00 am in patients with ST-segment elevation.
Role of monocyte/Mϕ subsets in I/R
Myocardium ischemic injury or I/R-mediated collateral damage demonstrates a biphasic monocyte/Mϕ response, which likely occurs in many sterile and nonsterile injuries and has also been described in rodent models of permanent coronary ligation (37, 54). A similar time-dependent leukocytic infiltration was subsequently observed for temporal ischemia followed by reperfusion injury (55). The I/R collateral myocardium injury model is a highly comparable system that simulates the contemporary clinical scenario, but whether it contributes to the study of chronic heart failure is unclear. Initially, there is a rapid influx of inflammatory Ly6Chigh monocytes, followed by Ly6Clow monocytes, and, contrary to the completed infarction model, there is a rapid resolution of cellular infiltrate within 5 to 7 d depending on the magnitude of the injury (35). Of importance, similar leukocyte subsets exist in humans, and excessive monocyte numbers in the circulation post-MI correlate with the limited recovery of LV function over time (48). Monocyte-to-Mϕ differentiation began within 24 h and peaked at 48 to 72 h, remaining at a significantly high level until d 7. A recent study has shown that the M1 subset population reached their peak at d 3 with a classic M1 (proinflammatory) surface as well as signature markers viz, Ly6Chigh, CD206low, and NOS2A and IL-6; however, M2 Mϕs (healing or resolving) demonstrated a biphasic pattern and remained temporary at the site of healing with M1 population, suggesting wound healing and angiogenic effects from d 1 through 7 after reperfusion (56, 57). The peak of dendritic cells, T cells, NK cells, and NK T cells also shifted from d 3 to 7, which indicated an early adaptive immune response (57). This embrace of early adaptive response in reperfusion results in the resolution of inflammation for balancing homeostasis after injury (6). Thus, the innate immune response is followed by M1 Mϕs, which leads to the resolution of inflammation by an adaptive immune response in the injured LV infarcted area (55). The mechanisms of bimodal response that regulate monocyte production, migration, and monocyte-to-Mϕ differentiation after heart attack have not yet been completely elucidated. Of particular interest, whether the biphasic infarct Mϕ phenotype regulation is a specific factors or is milieu dependent is unclear. Recruitment or infiltration of dendritic cells is a hallmark of the adaptive immune signal but is closely related to Mϕs, which actively collaborate with T cells in the setting of infection and tissue injury. Likewise, there may be a direct or indirect interaction of lymphocytes with monocytes and Mϕs after ischemic injury post-MI (46).
Recent studies suggest a role for Mϕs in the regulation of red blood cell generation as well as in the close relationship with pluripotent progenitor cells (58–60). An interesting question has arisen regarding how monocytes and Mϕs participate in tissue regeneration or the reparative mechanism after MI. Indeed, Mϕs can differentiate into endothelial, epithelial, and other cell types under specific culture conditions. An attractive hypothesis suggests that Mϕs can interact with cells that bear regenerative capacities, which contributes to myocardium regeneration at an early age. Some studies report the enhancement of blood and spleen hematopoietic progenitors and increased activity of bone marrow after MI (61–63); however, their function is not yet fully understood in cardiac pathology or cardioprotection.
Lymphocyte diversity in MI
Unlike neutrophils and monocytes, a lower lymphocyte count has been observed post-MI in survivors. A decreased lymphocyte count and a higher neutrophil-to-lymphocyte ratio were found to be related to more cardiovascular events during follow-up (64). Lymphocytes are a diverse group that consists of T cells, B cells, and NK cells and have a breadth of roles in both innate and adaptive immune responses post-MI; however, little attention has been paid to lymphocytes in the setting of myocardial healing, in part because numerically, they are in the minority of influx cell types. The role of B cells and other lymphocytes (e.g., CD8+, Treg and NK cells) in MI has received less attention; however, several studies that used mice that were deficient in either B cells or components of the complement system, which interacts with B cell receptors, have shown that these cells contribute to I/R injury (34, 65). Regulatory cells also often have potent effects, despite their relative scarcity (35).
T cells are a critical subset of lymphocytes and an inherent part of the adaptive immune system that is activated post-MI, and the proliferation of T cells can be divided into Th cells (CD4: Th1, -2, -17, etc.), cytotoxic T cells (CD8), and Foxp3+ regulatory CD4+ T cells in heart-draining lymph nodes (66). Post–myocardial injury, B- and T-cell numbers increase 5- to 10-fold, likely as a result of recruitment, because there is no reported lymphocyte proliferation in the myocardium. Both B- and T-cell levels peak around d 7 after MI (57); however, patients with MI had lower CD4+ but higher CD8+ T lymphocytes in both percentage and absolute number (67, 68) which leads to an inverted CD4-to-CD8 ratio (69, 70). A prolonged depressed CD4-to-CD8 ratio was a poor prognostic factor (70). CD4+ T lymphocytes can differentiate into Th1 and Th2 lineage in response to the local milieu of cytokines. Testing of anticytokine hypothesis confirmed that cytokines are relatively downstream from the actual origin of the pathology. In the clinical setting, high numbers of Th2 cells were independently associated with decreased carotid intima-media thickness and a reduced risk of MI, which suggests a protective role for Th2 cells in MI (71).
NK cells are a type of cytotoxic lymphocyte that is critical to the acute immune system. Reductions in both the total number and the cell fraction of NK cells were reported in patients with MI (69). Expression of NK cell sensors was also decreased in MI (68). A recent study reported that chronic NK cell deficiency was associated with low-grade chronic inflammation, which indicates a protective role for NK cells in atherosclerosis and MI healing (72).
Much less is known about B lymphocytes in MI. Unlike T lymphocytes, increased peripheral B lymphocytes in both percentage and absolute number were reported in patients with MI (67, 69). Flow cytometry demonstrates that myocardial B220+ B cells were CD19+IgD+IgMlow, which indicates that MI triggers the infiltration of circulating mature B cells (65).
Role of lymphocytes in I/R
There are ample experimental results that indicate lymphocytes play a significant role in response to ischemic cardiac injury. Vascular reperfusion reduces the total number of leukocytes that are recruited to the myocardium, likely because of a small infarct area, and temporarily polarize the entire array of infiltrated leukocytes, including lymphocytes, to earlier time points, with absolute cell numbers peaking around d 3 (57). Experimental mouse models of MI reveal that both T and B lymphocytes activate and modify myocardial I/R injury, wound healing, and LV remodeling (73). In fact, inhibitory lymphocyte subpopulations, such as Treg cells, can participate in the suppression of postinfarction inflammatory response (74). Specifically, CD4+ T cells add to immediate I/R injury but are also required for proper healing and also attenuate chronic remodeling post-MI.
Although the role of T cells in I/R cardiac injury has been studied in rodent models and the clinical setting, there are little data to explain how CD4+ T cells are activated post-I/R injury (75). There are solid experimental data to indicate that CD4+ T cells contribute to myocardial I/R injury. Outcomes of experiments suggest that CD4+ T cells—but rather unexpectedly, not CD8+ T cells—contribute to cardiac I/R injury that involves IFN-γ expression (73).
Mature B lymphocytes are a subset of immune cells that synchronize a variety of adaptive and chronic immune responses that are relevant to human diseases (65, 76) but that have been relatively ignored in the setting of ischemic injury. B cells seem to have detrimental effects—they both impair healing and potentially contribute to autoantibody formation in chronic ischemic cardiomyopathy. Recent studies have shown that B lymphocytes promote monocyte mobilization and recruitment and enhance tissue injury (65). In response to MI, circulating B cells produce the chemokine, Ccl7, which induces inflammatory monocytes from the bone marrow reservoir to enter the blood, after which they infiltrate the infarcted heart (77). In summary, these studies describe a fundamental role for systemic B cells during immune responses that lead to tissue damage after acute MI. B cells are also secondarily recruited to the infarcted area as a component of the acute or chronic inflammatory response. B cells are known to promote proinflammatory innate and link to adaptive immune responses (78). Recently, systemic depletion showed that B cell–derived CCL7 (MCP-3) drives monocyte trafficking, which leads to amplified tissue injury in a post-MI mouse model. Treatment with an anti-CD20 (rituximab) or anti–B-cell activating factor (belimumab) Abs leads to B-cell depletion and B cell–derived CCL7 reduction, reduced infarct size, and reduced cardiac pathology (65). Thus, we can speculated that rituximab- or belimumab-treated patients may also have a better outcome post-MI (79). Accordingly, reducing the systemic amplification of the inflammatory response post-MI is likely to further reduce T cell, Mϕ, and neutrophil-induced tissue damage.
As a result, B-cell depletion has a notable impact on postischemic injury. B-cell depletion substantially limits myocardial inflammation, reduces infarct size, and improves myocardial function (65); however, additional study must be performed to investigate the mechanism and associations between B lymphocytes and MI. Future experimental research should concentrate on the receptor of lymphocyte activation and identification of tissue autoantigens for immunotherapy. As well as the importance of the time-dependent role of different lymphocyte effector mechanisms in myocardial disease, and the regulatory crosstalk of lymphocytes with innate leukocytes.
Resolving and nonresolving modifiers in cardiac remodeling
Overactive and unresolved chronic inflammation is a major trigger of disease processes that contribute to advanced heart failure post-MI. The perpetuation of acute inflammatory response is an inherent risk factor, because inflammation can damage tissue and necrosis can provoke chronic inflammation. Many experimental studies have shown a significant improvement in infarcted myocardium by using anti-inflammatory treatment in preclinical models (80). In contrast, in clinical trials, the inhibition of inflammation approach is not a feasible target with which to decrease the risk of heart failure, as the inflammatory response plays a major role in the clearance of dead cells and promotes the reparative process (6). Nonetheless, multiple mechanisms normally ensure resolution.
Such cells as monocytes and Mϕs switch phenotypes, secreted molecules, such as reactive oxygen intermediates, switch impact from pro- to anti-inflammatory, and additional mediators of resolution arise, including proteins, peptides, lipids, and gasses, such as carbon monoxide, hydrogen sulfide, and NO. Aside from the persistence of the initial trigger, nonresolution may result from deficiencies in these mechanisms (81). The effectiveness or lack of monocyte and Mϕ-mediated clearance, in turn, is linked to active inflammation resolution signaling pathways when an inflammatory response begins either excessively or abnormally adverse post-MI cardiac remodeling, which leads to heart failure.
Resolving modifiers of monocytes and Mϕs
The resolution of inflammation is accomplished by time- and site-specific generation of proresolving mediators that limit the magnitude and the improve self-healing during the inflammatory response (82). In cardiac healing, the acute inflammatory response is required to discontinue the overactive priming of neutrophils to resolve inflammation. Thus, as part of the post-MI resolution process, several specialized proresolving lipid mediators (SPMs) are produced during neutrophil–monocyte trafficking and cell–cell interaction with platelets. Interactions of neutrophils, platelets, and Mϕs promote the generation of SPMs, including lipoxins, resolvins, protectins, and maresins, with their function being to limit neutrophil recruitment and promote the resolution process of acute inflammation (83) (Table 1). Among the resolving modifiers of monocytes and Mϕs are novel mediators that possess specific protective functions that have been demonstrated in animal models in a time-dependent manner. Once SPMs species, such as lipoxins, resolvins, protectins, and maresins, are produced, these SPMs stimulate Mϕs to clear apoptotic neutrophils and cellular debris. In addition, the corpses of apoptotic neutrophils can serve to bind chemokines and cytokines for their disposal. SPMs enhance Mϕ uptake and clearance so that Mϕs phagocytose apoptotic neutrophils. This process is stimulated by SPM and is an anti-inflammatory and nonphlogistic process. Rather than producing proinflammatory mediators during phagocytosis, such as LTB4, TNF-α, and IL-1, these Mϕs produce lipoxins, resolvins, and neuroprotectin (NPD1/PD1) that, in turn, control additional inflammation via a feedback and feed-forward mechanism (84). Indeed, resolvin E1 binds ChemR23 on monocytes, Mϕs, and dendritic cells to attenuate TNF-α–mediated NF-κβ activation, thereby forming an anti-inflammatory signaling pathway (85, 86). In addition, resolvin D1 promotes the resolution of inflammation initiated by MI by regulating lipoxygenase and cyclooxygenase enzymes, thereby delaying the onset of heart failure (87) (Table 1). The discovery of lipid mediators, such as resolvins and protectins, will serve as a platform for therapeutics on the basis of endogenous biotemplates for treating the inflammatory response via stimulation of resolution instead of inhibiting the inflammation (6).
TABLE 1.
Resolving and nonresolving modifiers of monocytes and Mϕs in cardiac remodeling
| Resolving modifier | Reference | Nonresolving regulator | Reference |
|---|---|---|---|
| SPMs | 83 | TLR, pentraxin-3, IL-1Ra | 97, 98, 101 |
| Lipoxins | LTB4, 12-HETE | 6, 18 | |
| D-series resolvins | MERTK, MFG-E8, Fas, C1q deficiency | 102 | |
| E-series resolvins | |||
| Protectins | |||
| Maresins |
The role of monocytes and Mϕs in cardiac I/R injury and postinfarction healing is a double-edged sword. In fact, they play detrimental roles in the pathophysiologic processes that are triggered by MI injury. In addition, monocytes and Mϕs attenuate the post-MI inflammatory response and have beneficial effects on cardiac remodeling (88, 89). Mϕs possess a striking functional and phenotypic plasticity that becomes apparent during the resolution phase of inflammation (90). On the basis of disease pathology, Mϕs play janitorial, patrolling, or regulatory roles, having a basal autophagy property, which is antiapoptotic in nature and protects them against various stressors (6). Upon apoptotic cell efferocytosis, Mϕs turn off production of proinflammatory cytokines and chemokines, respond to diverse stimuli by expressing an array of lipid mediators, eicosanoids, and scavenging receptors (6), and launch an anti-inflammatory transcriptional program that is characterized by the release of IL-10 and TGF-β (91), which is reminiscent of the M2 alternative Mϕ activation pattern. During reparative or resolution phases, Mϕs are enriched in bioactive molecules that are important for antigen processing and presentation and that secrete T- and B-cell chemoattractants (XCL1, CCL5, and CXCL13) (10). Resolution-phase Mϕs also express TIM4 and TGF-β, key molecules in the clearance of inflammatory cells, then return to original tissue homeostasis. Cardiac functional phenotypes of resolving Mϕs revealed lower levels of CD11b, an enhanced capacity of efferocytosis of dead neutrophils, and reduced responsiveness to TLR4 ligands, possibly leading to a clearance state with the ultimate departure via lymph (92). Several receptors have been described for Mϕs, including CD36, LDL receptor-related protein 1 and MER proto-oncogene, tyrosine kinase (MERTK) (93–95). The distinct presence of MERTK on Mϕs and its role in cardiac repair post-MI has been experimentally demonstrated (96). Mϕs that are positive for MERTK digest large cardiomyocytes, but size does not affect efferocytosis capacity. In addition, the big eater Mϕ receptor, MERTK, is critical for the clearance of dying adult cardiomyocytes and is important in the resolution of inflammation post-MI. Post-MI, MERTK is specifically induced on Ly6Clow proresolving (M2) Mϕs, which indicates a role in the resolution of inflammation.
Nonresolving regulators of monocytes and Mϕs
Persistent, nonresolving inflammatory condition is the major unifying component in the progression of chronic heart failure post-MI. Many cellular and molecular pathways have been identified in the repression of resolution, among which is the negative regulation of TLR signaling. Current evidence describes a role in preventing uncontrolled TLR signaling (97, 98). Recently, another compound, pentraxin-3, has been identified as a negative regulator of inflammation. In fact, C-reactive protein (CRP) and serum amyloid peptide are the classic prototypic acute phase proteinous molecules that are released by hepatocytes upon inflammatory injury. CRP injection in an experimental model of coronary occlusion reduces cardiac injury by activating the complement cascade. CRP blockade has been indicated as a promising therapeutic strategy in the MI setting (99). Conversely, the prototypic long pentraxin, PTX3, is increased in the infarcted heart and seems to play an important role in the negative regulation of the inflammatory reaction (100). Many SPMs act as natural endogenous cytokine inhibitors and may be up-regulated to suppress specific innate responses in a temporal manner. As with IL-1Ra, an endogenous competitive inhibitor of IL-1 cytokine-driven inflammation plays a major role in preventing autoinflammatory responses. Although the role of endogenous IL-1Ra in the suppression of inflammation and the resolution of the postinfarction inflammatory response has not been assessed, IL-1Ra overexpression exerts protective effects on the infarcted heart (101).
Several studies have linked the defective resolution axis to the nonclearance of apoptotic bodies, which contributes to chronic inflammation (102). The primary function of Mϕ is to engulf and digest necrotic cells within an injured tissue while facilitating wound healing to stimulate the resolution of inflammation. Mϕ clearance or apoptosis of Mϕs by itself will not trigger plaque necrosis in a steady-state or inflammatory setting. Instead, plaque necrosis or milieu around Mϕs will delay clearance or efferocytosis (103). Efficient efferocytosis of apoptotic Mϕs is effected via phagocytic (MERTK, LDL receptor-related protein 1) and apoptotic receptors (phosphatidylserine, C1q). It is likely that bridging molecules (Gas6) facilitate the recognition of apoptotic cells or cardiomyocyte by phagocytes (104).
Several possible cellular and molecular mechanisms might contribute to nonresolving efferocytosis, including ROS-induced efferocyte death that results from defective cholesterol efflux after apoptotic cell engulfment. Lipoprotein-associated phospholipase A2 mediated the hydrolysis of oxidized phosphatidylserine on the surface of apoptotic cells, which is a ligand for efferocytosis (105) and protease-mediated cleavage of the efferocytosis receptor, MERTK (106). Another mechanism that may be relevant to obesity is based on in vitro and in vivo studies that have shown that Mϕs exposed to saturated fatty acids have a defective phagocytic capacity of apoptotic cells, perhaps because of changes in plasma membrane structure (107). Obesity combined with aging induces defects in this process to generate a proresolving environment and, therefore, impair the resolution ability of the Mϕ, with marked increases in neutrophils (18). Genetic manipulation in various targets, such as tissue transglutaminase, milk-fat globule-EGF factor-8, complement C1q, lysophosphatidylcholines, and Fas, leads to the development of nonresolving inflammation in atherosclerosis plaques, which indicates defective efferocytosis (102). MERTK deficiency leads to an accumulation of necrotic cardiomyocytes independent of the phagocytosis of noncardiomyocytes, which leads to reduced efferocytosis (96). Excessive intake of ω-6 polyunsaturated fatty acids (PUFAs) promotes nonresolving post-MI inflammation, which leads to early LV dysfunction (6, 18).
What matters for leukocyte diversity in post-MI injury and healing?
For leukocyte diversity, what matters most is whether leukocyte diversity is highly influenced by genetic diversity, source, age, or milieu-dependent factors, which lead to the temporal separation of these cellular subsets in MI that could help to identify a novel mechanism by which large numbers of leukocytes are recruited and cleared in a time-dependent manner. It has been recognized that these processes can help design therapeutics that avoid inappropriate targeting (35). The interplay between monocytes and Mϕs and neutrophils in sterile inflammation is highly interactive, and the mechanisms are only now coming to light (108).
Genetic diversity
Intercellular heterogeneity within seemingly homogeneous cell populations has recently emerged as an important source of functional variation within uncontrolled inflammation (109). The genotypic and phenotypic diversity present in immune response disorders is also present in the immune response of healthy individuals (110, 111). The interindividual diversity of CD8 T- and B-cell subpopulations is mainly influenced by the environment, whereas CD4 T cells have shown a high degree of heritability (112). Eosinophil, neutrophil, and some NK cell features have also shown a high level of heritability, whereas monocytes have shown a low heritability (113); therefore, a clear understanding of the genetic basis of innate and adaptive system variation in healthy individuals and patients can improve our understanding of how diseases advance to pathology (114).
Leukocyte source
Although bone marrow is the major origin of blood cells under steady-state conditions, monocytes and Mϕs immediately infiltrate the heart from the spleen after MI (115). At d 1 post-MI after coronary ligation in mice, approximately one half of monocytes emigrate from the splenic reservoir, whereas the monocyte number in the bone marrow is unchanged (40). In addition, 40 to 75% of splenic monocytes enter the blood stream and mobilize to the infarct region to govern ischemic myocardium healing (116). In fact, splenectomy on d 1 or 3 after coronary ligation reduced the numbers of monocytes and Mϕs that were recruited to the infarct and led to impaired wound healing post-MI (117). These findings indicate the significance of the spleen as a supplier of inflammatory monocytes and a regulator of cardiac healing and remodeling post-MI. In contrast, splenectomy reversed pathologic cardiac remodeling and inflammation when performed 8 wk after coronary ligation (118).
Aging
When we consider age as an important parameter in leukocyte diversity in the post-MI translational setting, emphasis is placed on the dysregulation of the innate immune system induced by age (119). Innate immune response substantially changes with age, in particular via dysregulation of proinflammatory cytokines, such as IL-6, IL-1β, TNF-α, and TGF-β, which leads to chronic inflammation and thus contributes to the inflammaging phenotype that is often observed in the elderly (120). In a recent work in the context of MI, the leukocyte response in both young and aging groups demonstrated high levels of Ly6G+ and a Ly6Chigh population that resists clearing as a result of an altered chemokine microenvironment that is responsible for the expansion of the innate phase post-MI (116). Of interest, aging mice displayed a distinct Mϕ population (F4/80+) with high levels of CD11b expression, called CD11bhigh (116). Because CD11b expression is commonly associated with neutrophil recruitment and monocytes (121), the persistent presence of an F4/80+/CD11bhigh population in naive aged mice indicated that presence of a proinflammatory milieu and signs of low-grade inflammation, which suggests that the phenomenon of inflammaging (122) is the primary cause of recurrent MI in heart failure. Another recent work showed that thrombi from patients age 50 yr contained significantly more neutrophils and monocytes and Mϕs regardless of thrombus age. The composition of inflammatory and noninflammatory cells differs with thrombus age in the thrombosuction material of patients with ST elevation acute myocardial infarction that, in part, depends on patient age and comedication (123).
Microenvironment
In rodents, to heal the infarct post-MI, inhibitory and reparative monocyte subpopulations are recruited to the site of injury via poorly understood chemotactic mechanisms (37); however, the monocyte phenotype in the infarcted area is dynamically modulated by the cytokine- and growth factor–rich environment. Mϕ-colony stimulating factor is markedly up-regulated in the infarct area and may drive monocytes to Mϕ differentiation (124). The dynamic SPM-enriched environment of the infarct may lead to the generation of reparative Mϕ phenotypes with distinct functional properties that regulate fibroblast–myofibroblast activity, matrix metabolism, and vascularization as well as orchestrate the reparative response (125).
In vitro and in vivo studies suggest that the post-MI activation of TGF-β coordinates immune and inflammatory responses by modulating leukocyte phenotype and activity. The effects of TGF-β on lymphocytes, monocytes, and Mϕs can be either stimulatory or inhibitory, depending on the milieu, the state of cellular differentiation, and the tissue origin of the cells (126), which highlights the pleiotropic nature of the cytokine. TGF-β inhibits T- and B-lymphocyte proliferation (127), suppresses Th-cell differentiation, and induces the conversion of naive T cells to Tregs (128). Femtomolar concentrations of TGF-β stimulate chemotaxis in peripheral blood monocytes (129), which suggests that it may play a critical role in the recruitment of mononuclear cells in inflamed tissues. Picomolar concentrations of TGF-β activate monocytes, which stimulates the synthesis of a variety of proinflammatory cytokines, chemokines, and growth factors (130) and increases integrin expression. In contrast to the stimulating effects of TGF-β on peripheral blood monocytes, its actions on mature or differentiated Mϕs are predominantly suppressive. TGF-β has a diversified effect on Mϕs, suppressing proinflammatory cytokine and chemokine synthesis (131) and decreasing reactive oxygen generation. These TGF-β–mediated effects may be critical to the leukocyte clearance mechanism and the resolution of the inflammatory response.
Pharmacologic modifiers in leukocyte diversity and cardiac remodeling
For more than 50 yr, extensive preclinical science has explored the role of inflammatory signals and the diversity of the leukocyte subpopulation in MI. A number of quantitative and semiquantitative outcomes derived from rodent and clinical studies of ischemic or nonischemic cardiac disease has speculated that the inhibition of inflammatory cascades may attenuate myocardium injury or protect from pathologic remodeling. Unfortunately, translating promising experimental findings to the clinical setting failed (132). Indeed, post-MI cytokine inhibitory anti-inflammatory treatments have been unsuccessful in treating the defective resolution of inflammation. Unfortunately, with the failure of cyclooxygenase-2 inhibitors, rofecoxib and celecoxib (133, 134), and TNF-α inhibitor trials (135); therefore, current guidelines recommend against the use of broad-range anti-inflammatory therapy—corticosteroids and nonsteroidal anti-inflammatory drugs—in patients with acute MI (136). Therefore, novel treatments to resolve inflammation are necessary for post-MI heart failure pathology.
Several exciting, elegant studies have recently contributed to this expanding area of interest, focusing on proresolving lipid mediators that have shown their effectiveness in resolving inflammation (85, 87, 116). The example of the ω-3 PUFAs, which their beneficial effects are dependent on the formation of SPMs. Known SPMs include arachidonic acid–derived lipoxins, the docosahexaenoic acid–derived d-series resolvin, neuroprotectins, maresins, and the eicosapentaenoic acid–derived E-series resolvins (83). There are multiple reports to indicate that ω-3 fatty acids have positive effects on controlling inflammation, including the reduction of cytokines, endothelial cell activation and platelet aggregation, heart rate, and LV function. A clinical phase III trial demonstrated that long-term administration of 1 g/d of ω-3 PUFA resulted in a significant reduction in both all-cause mortality and cardiovascular readmissions in all predefined subgroups, including patients with heart failure (137); however, the origin and advancement of nonresolving inflammation is a challenge to physicians and heart failure specialists. Therefore, it is hoped precision or personal treatment will optimize medical therapy.
An alternative approach to targeting specific components of the inflammatory cascade is to employ one that inhibits the various components of the systemic inflammatory response. Given the dynamic interaction between the innate and adaptive immune systems that leads to progressive LV remodeling after acute MI, and that pathologic LV remodeling is driven by the aberrant activation of monocyte-derived Mϕs, dendritic cells, and CD4+ T cells that interact with cardiac autoantigen-loaded dendritic cells (118, 138), there has been active interest in developing broad immunomodulatory strategies for patients with heart failure, as with methotrexate, which was evaluated in a small prospective, randomized clinical trial of patients with heart failure (139). Compared with patients on optimal medical therapy, an addition of low-dose methotrexate resulted in a significant reduction in circulating levels of proinflammatory cytokines (TNF-α, IL-6, and MCP-1) and up-regulation of anti-inflammatory cytokines (IL-10 and soluble IL-1 receptor antagonist). Another potential compound, rosuvastatin, belongs to the statin family and has a variety of pleiotropic effects, including the inhibition of inflammatory responses (140). A phase III clinical trial demonstrated that rosuvastatin had beneficial effects among those patients with heart failure with evidence of increased inflammation at baseline, which was defined as a CRP >2.0 mg/dl (141). Of importance, treatment with rosuvastatin resulted in a significant decrease in heart failure hospitalizations; however, this result is contradicted by the fact that treatment with statins might lead to worsening heart failure.
CONCLUSIONS
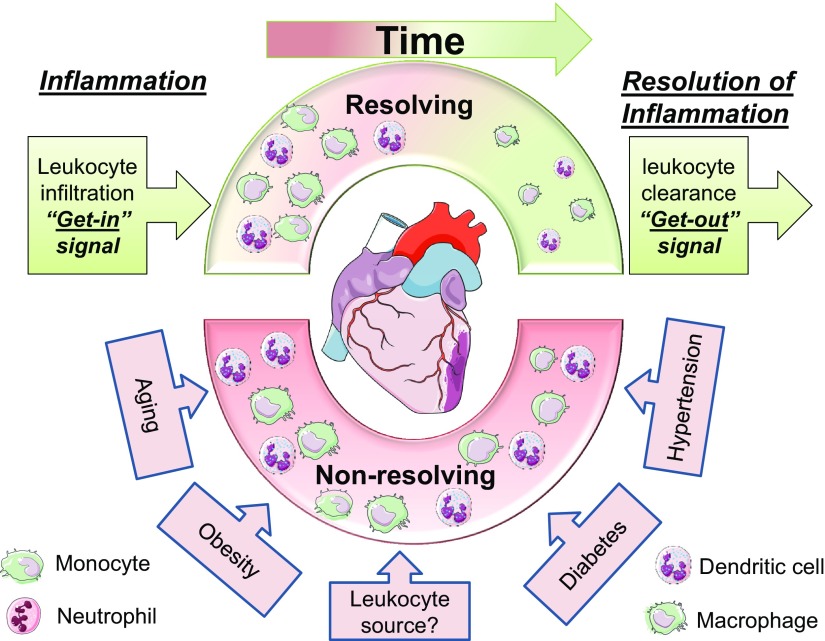
Persistent, nonresolving, and chronic inflammatory condition is the major unifying component in the progression of heart failure pathology post-MI. As a result of the failure of a panoply of pharmacologic modifiers as anti-inflammatory treatment for patients with acute MI, we are at the dawn of a new era of drug discovery in cardiac remodeling that finally exploits natural self-healing endogenous molecules that are generated in the body to terminate acute inflammatory response on time. The potential adverse effect of developing an aggressive proresolving-modifier is that acute inflammation could be prematurely cleared before it has time to deal with the pathogen or injured cells, the essence of the inflammatory response. This can be accomplished by the time- and site-specific generation of proresolving mediators that are secreted by leukocytes that control both the magnitude and the duration of the acute inflammatory response, monitoring the rate of infiltration and exit from the injured area (Fig. 1). To advance this specific goal for the resolution of inflammation in cardiac healing, it is necessary to understand leukocyte diversity and milieu around leukocytes in cardiac pathology, particularly in the setting of obesity, diabetes, and hypertension combined with aging. This will help to steer preclinical focus to understand the leukocyte-like source, age, and milieu that could help to develop novel therapeutics and optimize the resolution of inflammation with major translational interest. Thus, not only do Mϕs show reparative phenotypes, but the neutrophil resolving phenotype may also be a novel developing topic in cardiac recovery, which supports leukocyte diversity in the resolution of inflammation and post-MI cardiac remodeling.
Figure 1.
Schematic design of leukocyte diversity in resolving and nonresolving modifiers in the post-MI setting. In the clinical setting, phenotypic stressors, such as obesity, diabetes, hypertension, and aging, contribute to the inflammatory milieu and nonresolving inflammation.
LIMITATIONS AND CONTROVERSIES
Quantitative technology has advanced to measure leukocyte diversity in the clinical and preclinical setting. Likewise, the diagnostic application of big genomic, proteomic, metabolomic, or lipidomic data is unclear in the clinical and preclinical setting. First, we need to identify the diversity of leukocytes in the context of milieu around leukocytes and test the hypothesis of whether to allow the prediction of nonresponder vs. responder in a clinical setting. The second limitation is the heterogeneity of human samples. It is widely known that plasma or serum milieu is responsive to the diversity of different dietary ingredients, particularly ω-3 and -6 fatty acids, metabolite factors, and anti-inflammatory drugs, that alter the levels of SPMs. Third, diversified lipid processing enzymes—lipoxygenase, cyclooxygenase, and cytochrome P450—compete for the same fat substrate; therefore, the production of downstream SPMs that control the milieu is dependent on the complex substrate and enzyme interaction to form the downstream products in cardiac healing. Fourth, the biologic half-life of mediators is primary for healing, and many mediators are produced in a short-period of time and are active locally. Therefore, formulation and precise delivery studies are necessary to understand the pharmacokinetic and pharmacodynamic parameters of molecules at the site of injury and in the milieu.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants AT006704 (National Center for Complementary and Integrative Health) and HL132989 (National Heart, Lung, and Blood Institute) (to G.H.). The authors declare no conflicts of interest.
Glossary
CRP
C-reactive protein
DAMP
danger-associated molecular pattern
I/R
ischemia/reperfusion
LV
left ventricle
Mϕ
macrophage
MCP-1
monocyte chemoattractant protein-1
MERTK
MER proto-oncogene, tyrosine kinase
MI
myocardial infarction
PAMP
pathogen-associated molecular pattern
PUFA
polyunsaturated fatty acid
ROS
reactive oxygen species
SPM
specialized proresolving mediator
Th
T helper
Treg
regulatory T
AUTHOR CONTRIBUTIONS
G. Halade designed the outline and edited, revised, and approved the submission to the journal; and T. Bourki wrote the manuscript draft based on the outline.
REFERENCES
- 1.Frangogiannis N. G. (2012) Regulation of the inflammatory response in cardiac repair. Circ. Res. 110, 159–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cochain C., Auvynet C., Poupel L., Vilar J., Dumeau E., Richart A., Récalde A., Zouggari Y., Yin K. Y. H. W., Bruneval P., Renault G., Marchiol C., Bonnin P., Lévy B., Bonecchi R., Locati M., Combadière C., Silvestre J.-S. (2012) The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 32, 2206–2213 [DOI] [PubMed] [Google Scholar]
- 3.Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O’Neill L. A. J., Perretti M., Rossi A. G., Wallace J. L. (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horckmans M., Ring L., Duchene J., Santovito D., Schloss M. J., Drechsler M., Weber C., Soehnlein O., Steffens S. (2017) Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 38, 187–197 [DOI] [PubMed] [Google Scholar]
- 5.Van Dyke T. E., Kornman K. S. (2008) Inflammation and factors that may regulate inflammatory response. J. Periodontol. 79 (8 Suppl), 1503–1507 [DOI] [PubMed] [Google Scholar]
- 6.Kain V., Prabhu S. D., Halade G. V. (2014) Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res. Cardiol. 109, 444 [DOI] [PubMed] [Google Scholar]
- 7.Freire M. O., Van Dyke T. E. (2013) Natural resolution of inflammation. Periodontol. 2000 63, 149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucchesi B. R., Werns S. W., Fantone J. C. (1989) The role of the neutrophil and free radicals in ischemic myocardial injury. J. Mol. Cell. Cardiol. 21, 1241–1251 [DOI] [PubMed] [Google Scholar]
- 9.Dutta P., Nahrendorf M. (2015) Monocytes in myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 35, 1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortega-Gómez A., Perretti M., Soehnlein O. (2013) Resolution of inflammation: an integrated view. EMBO Mol. Med. 5, 661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neely C. J., Kartchner L. B., Mendoza A. E., Linz B. M., Frelinger J. A., Wolfgang M. C., Maile R., Cairns B. A. (2014) Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12- neutrophil polarization. PLoS One 9, e85623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amantea D. (2016) Polarizing the immune system towards neuroprotection in brain ischemia. Neural Regen. Res. 11, 81–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y., Yabluchanskiy A., Iyer R. P., Cannon P. L., Flynn E. R., Jung M., Henry J., Cates C. A., Deleon-Pennell K. Y., Lindsey M. L. (2016) Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 110, 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y., Yabluchanskiy A., Lindsey M. L. (2013) Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 6, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer R. P., Patterson N. L., Zouein F. A., Ma Y., Dive V., de Castro Brás L. E., Lindsey M. L. (2015) Early matrix metalloproteinase-12 inhibition worsens post-myocardial infarction cardiac dysfunction by delaying inflammation resolution. Int. J. Cardiol. 185, 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prince L. R., Whyte M. K., Sabroe I., Parker L. C. (2011) The role of TLRs in neutrophil activation. Curr. Opin. Pharmacol. 11, 397–403 [DOI] [PubMed] [Google Scholar]
- 17.De Haan J. J., Smeets M. B., Pasterkamp G., Arslan F (2013) Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm. 2013, 206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez E. F., Kabarowski J. H., Ingle K. A., Kain V., Barnes S., Crossman D. K., Lindsey M. L., Halade G. V. (2015) Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 308, H269–H280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross E. A., Douglas M. R., Wong S. H., Ross E. J., Curnow S. J., Nash G. B., Rainger E., Scheel-Toellner D., Lord J. M., Salmon M., Buckley C. D. (2006) Interaction between integrin alpha9beta1 and vascular cell adhesion molecule-1 (VCAM-1) inhibits neutrophil apoptosis. Blood 107, 1178–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta J., Dinerman J., Mehta P., Saldeen T. G., Lawson D., Donnelly W. H., Wallin R. (1989) Neutrophil function in ischemic heart disease. Circulation 79, 549–556 [DOI] [PubMed] [Google Scholar]
- 21.Vinten-Johansen J. (2004) Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 61, 481–497 [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M., Brooks S. E., Richard V. J., FitzHarris G. P., Stoler R. C., Jennings R. B., Arfors K. E., Reimer K. A. (1993) Effect of anti-CD18 antibody on myocardial neutrophil accumulation and infarct size after ischemia and reperfusion in dogs. Circulation 87, 526–535 [DOI] [PubMed] [Google Scholar]
- 23.Faxon D. P., Gibbons R. J., Chronos N. A. F., Gurbel P. A., Sheehan F.; HALT-MI Investigators (2002) The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J. Am. Coll. Cardiol. 40, 1199–1204 [DOI] [PubMed] [Google Scholar]
- 24.Granger D. N., Schmid-Schönbein G. W. (1995) Physiology and Pathophysiology of Leukocyte Adhesion, Oxford University Press, New York [Google Scholar]
- 25.Heilker R., Wolff M., Tautermann C. S., Bieler M. (2009) G-protein-coupled receptor-focused drug discovery using a target class platform approach. Drug Discov. Today 14, 231–240 [DOI] [PubMed] [Google Scholar]
- 26.Peart J. N., Headrick J. P. (2007) Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol. Ther. 114, 208–221 [DOI] [PubMed] [Google Scholar]
- 27.Koeppen M., Harter P. N., Bonney S., Bonney M., Reithel S., Zachskorn C., Mittelbronn M., Eckle T. (2012) Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury. Anesthesiology 116, 1245–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming S. D., Mastellos D., Karpel-Massler G., Shea-Donohue T., Lambris J. D., Tsokos G. C. (2003) C5a causes limited, polymorphonuclear cell-independent, mesenteric ischemia/reperfusion-induced injury. Clin. Immunol. 108, 263–273 [DOI] [PubMed] [Google Scholar]
- 29.Stahl G. L., Xu Y., Hao L., Miller M., Buras J. A., Fung M., Zhao H. (2003) Role for the alternative complement pathway in ischemia/reperfusion injury. Am. J. Pathol. 162, 449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin G. M., Li Y. H., Jaiteh L. E., Han C. L. (2012) Pexelizumab fails to inhibit assembly of the terminal complement complex in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Insight from a substudy of the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. Am. Heart J. 164, e19; author reply e21. [DOI] [PubMed] [Google Scholar]
- 31.Bolli R. (2015) Reflections on the irreproducibility of scientific papers. Circ. Res. 117, 665–666 [DOI] [PubMed] [Google Scholar]
- 32.Arumugam T. V., Magnus T., Woodruff T. M., Proctor L. M., Shiels I. A., Taylor S. M. (2006) Complement mediators in ischemia-reperfusion injury. Clin. Chim. Acta 374, 33–45 [DOI] [PubMed] [Google Scholar]
- 33.Halade G. V., Ma Y. (2016) Neutrophils: friend, foe, or contextual ally in myocardial healing. J. Mol. Cell. Cardiol. 97, 44–46 [DOI] [PubMed] [Google Scholar]
- 34.Kalogeris T., Baines C. P., Krenz M., Korthuis R. J. (2012) Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epelman S., Mann D. L. (2012) Communication in the heart: the role of the innate immune system in coordinating cellular responses to ischemic injury. J. Cardiovasc. Transl. Res. 5, 827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung K., Kim P., Leuschner F., Gorbatov R., Kim J. K., Ueno T., Nahrendorf M., Yun S. H. (2013) Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ. Res. 112, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nahrendorf M., Swirski F. K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J.-L., Libby P., Weissleder R., Pittet M. J. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dewald O., Zymek P., Winkelmann K., Koerting A., Ren G., Abou-Khamis T., Michael L. H., Rollins B. J., Entman M. L., Frangogiannis N. G. (2005) CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 96, 881–889 [DOI] [PubMed] [Google Scholar]
- 39.Glezeva N., Horgan S., Baugh J. A. (2015) Monocyte and macrophage subsets along the continuum to heart failure: misguided heroes or targetable villains? J. Mol. Cell. Cardiol. 89 (Pt B), 136–145 [DOI] [PubMed] [Google Scholar]
- 40.Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 [DOI] [PubMed] [Google Scholar]
- 42.Porcheray F., Viaud S., Rimaniol A.-C., Léone C., Samah B., Dereuddre-Bosquet N., Dormont D., Gras G. (2005) Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 142, 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pechkovsky D. V., Prasse A., Kollert F., Engel K. M., Dentler J., Luttmann W., Friedrich K., Müller-Quernheim J., Zissel G. (2010) Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin. Immunol. 137, 89–101 [DOI] [PubMed] [Google Scholar]
- 44.Li K., Xu W., Guo Q., Jiang Z., Wang P., Yue Y., Xiong S. (2009) Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ. Res. 105, 353–364 [DOI] [PubMed] [Google Scholar]
- 45.Aurora A. B., Porrello E. R., Tan W., Mahmoud A. I., Hill J. A., Bassel-Duby R., Sadek H. A., Olson E. N. (2014) Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frantz S., Nahrendorf M. (2014) Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc. Res. 102, 240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van der Laan A. M., Ter Horst E. N., Delewi R., Begieneman M. P., Krijnen P. A., Hirsch A., Lavaei M., Nahrendorf M., Horrevoets A. J., Niessen H. W., Piek J. J. (2014) Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur. Heart J. 35, 376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsujioka H., Imanishi T., Ikejima H., Kuroi A., Takarada S., Tanimoto T., Kitabata H., Okochi K., Arita Y., Ishibashi K., Komukai K., Kataiwa H., Nakamura N., Hirata K., Tanaka A., Akasaka T. (2009) Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J. Am. Coll. Cardiol. 54, 130–138 [DOI] [PubMed] [Google Scholar]
- 49.Italiani P., Boraschi D. (2014) From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front. Immunol. 5, 514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alibhai F. J., Tsimakouridze E. V., Chinnappareddy N., Wright D. C., Billia F., O’Sullivan M. L., Pyle W. G., Sole M. J., Martino T. A. (2014) Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ. Res. 114, 1713–1722 [DOI] [PubMed] [Google Scholar]
- 52.Halade G. V., El Jamali A., Williams P. J., Fajardo R. J., Fernandes G. (2011) Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp. Gerontol. 46, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiter R., Swingen C., Moore L., Henry T. D., Traverse J. H. (2012) Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ. Res. 110, 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Troidl C., Möllmann H., Nef H., Masseli F., Voss S., Szardien S., Willmer M., Rolf A., Rixe J., Troidl K., Kostin S., Hamm C., Elsässer A. (2009) Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J. Cell. Mol. Med. 13 (9B), 3485–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saxena A., Chen W., Su Y., Rai V., Uche O. U., Li N., Frangogiannis N. G. (2013) IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 191, 4838–4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nahrendorf M., Pittet M. J., Swirski F. K. (2010) Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan X., Anzai A., Katsumata Y., Matsuhashi T., Ito K., Endo J., Yamamoto T., Takeshima A., Shinmura K., Shen W., Fukuda K., Sano M. (2013) Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 62, 24–35 [DOI] [PubMed] [Google Scholar]
- 58.Chow A., Huggins M., Ahmed J., Hashimoto D., Lucas D., Kunisaki Y., Pinho S., Leboeuf M., Noizat C., van Rooijen N., Tanaka M., Zhao Z. J., Bergman A., Merad M., Frenette P. S. (2013) CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat. Med. 19, 429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehninger A., Trumpp A. (2011) The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 208, 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lo Celso C., Scadden D. T. (2011) The haematopoietic stem cell niche at a glance. J. Cell Sci. 124, 3529–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haubner B. J., Schneider J., Schweigmann U., Schuetz T., Dichtl W., Velik-Salchner C., Stein J.-I., Penninger J. M. (2016) Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 118, 216–221 [DOI] [PubMed] [Google Scholar]
- 62.Assmus B., Iwasaki M., Schächinger V., Roexe T., Koyanagi M., Iekushi K., Xu Q., Tonn T., Seifried E., Liebner S., Kranert W. T., Grünwald F., Dimmeler S., Zeiher A. M. (2012) Acute myocardial infarction activates progenitor cells and increases Wnt signalling in the bone marrow. Eur. Heart J. 33, 1911–1919 [DOI] [PubMed] [Google Scholar]
- 63.Massa M., Rosti V., Ferrario M., Campanelli R., Ramajoli I., Rosso R., De Ferrari G. M., Ferlini M., Goffredo L., Bertoletti A., Klersy C., Pecci A., Moratti R., Tavazzi L. (2005) Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 105, 199–206 [DOI] [PubMed] [Google Scholar]
- 64.Núñez J., Núñez E., Bodí V., Sanchis J., Miñana G., Mainar L., Santas E., Merlos P., Rumiz E., Darmofal H., Heatta A. M., Llàcer A. (2008) Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am. J. Cardiol. 101, 747–752 [DOI] [PubMed] [Google Scholar]
- 65.Zouggari Y., Ait-Oufella H., Bonnin P., Simon T., Sage A. P., Guérin C., Vilar J., Caligiuri G., Tsiantoulas D., Laurans L., Dumeau E., Kotti S., Bruneval P., Charo I. F., Binder C. J., Danchin N., Tedgui A., Tedder T. F., Silvestre J.-S., Mallat Z. (2013) B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 19, 1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hofmann U., Beyersdorf N., Weirather J., Podolskaya A., Bauersachs J., Ertl G., Kerkau T., Frantz S. (2012) Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125, 1652–1663 [DOI] [PubMed] [Google Scholar]
- 67.Liu L.-L., Lu J.-L., Chao P.-L., Lin L.-R., Zhang Z.-Y., Yang T.-C. (2011) Lower prevalence of circulating invariant natural killer T (iNKT) cells in patients with acute myocardial infarction undergoing primary coronary stenting. Int. Immunopharmacol. 11, 480–484 [DOI] [PubMed] [Google Scholar]
- 68.Yan W., Zhou L., Wen S., Duan Q., Huang F., Tang Y., Liu X., Chai Y., Wang L. (2015) Differential loss of natural killer cell activity in patients with acute myocardial infarction and stable angina pectoris. Int. J. Clin. Exp. Pathol. 8, 14667–14675 [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Ahmad R. S., Mahafzah A. M., Al-Mousa E. N. (2004) Immunological changes in acute myocardial infarction. Saudi Med. J. 25, 923–928 [PubMed] [Google Scholar]
- 70.Blum A., Yeganeh S. (2003) The role of T-lymphocyte subpopulations in acute myocardial infarction. Eur. J. Intern. Med. 14, 407–410 [DOI] [PubMed] [Google Scholar]
- 71.Fang L., Moore X.-L., Dart A. M., Wang L.-M. (2015) Systemic inflammatory response following acute myocardial infarction. J. Geriatr. Cardiol. 12, 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Backteman K., Ernerudh J., Jonasson L. (2014) Natural killer (NK) cell deficit in coronary artery disease: no aberrations in phenotype but sustained reduction of NK cells is associated with low-grade inflammation. Clin. Exp. Immunol. 175, 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hofmann U., Frantz S. (2015) Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ. Res. 116, 354–367 [DOI] [PubMed] [Google Scholar]
- 74.Dobaczewski M., Xia Y., Bujak M., Gonzalez-Quesada C., Frangogiannis N. G. (2010) CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am. J. Pathol. 176, 2177–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linfert D., Chowdhry T., Rabb H. (2009) Lymphocytes and ischemia-reperfusion injury. Transplant. Rev. (Orlando) 23, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin F., Chan A. C. (2006) B cell immunobiology in disease: evolving concepts from the clinic. Annu. Rev. Immunol. 24, 467–496 [DOI] [PubMed] [Google Scholar]
- 77.Kim N. D., Luster A. D. (2013) To B or not to B--that is the question for myocardial infarction. Nat. Med. 19, 1208–1210 [DOI] [PubMed] [Google Scholar]
- 78.Yanaba K., Bouaziz J.-D., Matsushita T., Magro C. M., St Clair E. W., Tedder T. F. (2008) B-lymphocyte contributions to human autoimmune disease. Immunol. Rev. 223, 284–299 [DOI] [PubMed] [Google Scholar]
- 79.Tsiantoulas D., Sage A. P., Mallat Z., Binder C. J. (2015) Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 35, 296–302 [DOI] [PubMed] [Google Scholar]
- 80.Silverman H. S., Pfeifer M. P. (1987) Relation between use of anti-inflammatory agents and left ventricular free wall rupture during acute myocardial infarction. Am. J. Cardiol. 59, 363–364 [DOI] [PubMed] [Google Scholar]
- 81.Nathan C., Ding A. (2010) Nonresolving inflammation. Cell 140, 871–882 [DOI] [PubMed] [Google Scholar]
- 82.Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serhan C. N. (2010) Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177, 1576–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keyes K. T., Ye Y., Lin Y., Zhang C., Perez-Polo J. R., Gjorstrup P., Birnbaum Y. (2010) Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 299, H153–H164 [DOI] [PubMed] [Google Scholar]
- 86.Arita M., Ohira T., Sun Y.-P., Elangovan S., Chiang N., Serhan C. N. (2007) Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178, 3912–3917 [DOI] [PubMed] [Google Scholar]
- 87.Kain V., Ingle K. A., Colas R. A., Dalli J., Prabhu S. D., Serhan C. N., Joshi M., Halade G. V. (2015) Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 84, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lavine K. J., Epelman S., Uchida K., Weber K. J., Nichols C. G., Schilling J. D., Ornitz D. M., Randolph G. J., Mann D. L. (2014) Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 111, 16029–16034Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaikita K., Hayasaki T., Okuma T., Kuziel W. A., Ogawa H., Takeya M. (2004) Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am. J. Pathol. 165, 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fujiu K., Wang J., Nagai R. (2014) Cardioprotective function of cardiac macrophages. Cardiovasc. Res. 102, 232–239 [DOI] [PubMed] [Google Scholar]
- 91.Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schif-Zuck S., Gross N., Assi S., Rostoker R., Serhan C. N., Ariel A. (2011) Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moore K. J., El Khoury J., Medeiros L. A., Terada K., Geula C., Luster A. D., Freeman M. W. A. (2002) A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J. Biol. Chem. 277, 47373–47379 [DOI] [PubMed] [Google Scholar]
- 94.Rohlmann A., Gotthardt M., Hammer R. E., Herz J. (1998) Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Invest. 101, 689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Camenisch T. D., Koller B. H., Earp H. S., Matsushima G. K. (1999) A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 162, 3498–3503 [PubMed] [Google Scholar]
- 96.Wan E., Yeap X. Y., Dehn S., Terry R., Novak M., Zhang S., Iwata S., Han X., Homma S., Drosatos K., Lomasney J., Engman D. M., Miller S. D., Vaughan D. E., Morrow J. P., Kishore R., Thorp E. B. (2013) Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ. Res. 113, 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Neill L. A. J. (2008) When signaling pathways collide: positive and negative regulation of Toll-like receptor signal transduction. Immunity 29, 12–20 [DOI] [PubMed] [Google Scholar]
- 98.Micera A., Balzamino B. O., Di Zazzo A., Biamonte F., Sica G., Bonini S. (2016) Toll-like receptors and tissue remodeling: the pro/cons recent findings. J. Cell. Physiol. 231, 531–544 [DOI] [PubMed] [Google Scholar]
- 99.Pepys M. B., Hirschfield G. M., Tennent G. A., Gallimore J. R., Kahan M. C., Bellotti V., Hawkins P. N., Myers R. M., Smith M. D., Polara A., Cobb A. J. A., Ley S. V., Aquilina J. A., Robinson C. V., Sharif I., Gray G. A., Sabin C. A., Jenvey M. C., Kolstoe S. E., Thompson D., Wood S. P. (2006) Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 440, 1217–1221 [DOI] [PubMed] [Google Scholar]
- 100.Salio M., Chimenti S., De Angelis N., Molla F., Maina V., Nebuloni M., Pasqualini F., Latini R., Garlanda C., Mantovani A. (2008) Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 117, 1055–1064 [DOI] [PubMed] [Google Scholar]
- 101.Suzuki K., Murtuza B., Smolenski R. T., Sammut I. A., Suzuki N., Kaneda Y., Yacoub M. H. (2001) Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation 104 (12 Suppl 1), I-308–I-313 [DOI] [PubMed] [Google Scholar]
- 102.Thorp E., Tabas I. (2009) Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J. Leukoc. Biol. 86, 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tabas I. (2010) Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henson P. M., Bratton D. L., Fadok V. A. (2001) Apoptotic cell removal. Curr. Biol. 11, R795–R805 [DOI] [PubMed] [Google Scholar]
- 105.Wilensky R. L., Macphee C. H. (2009) Lipoprotein-associated phospholipase A2 and atherosclerosis. Curr. Opin. Lipidol. 20, 415–420 [DOI] [PubMed] [Google Scholar]
- 106.Sather S., Kenyon K. D., Lefkowitz J. B., Liang X., Varnum B. C., Henson P. M., Graham D. K. (2007) A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109, 1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li S., Sun Y., Liang C.-P., Thorp E. B., Han S., Jehle A. W., Saraswathi V., Pridgen B., Kanter J. E., Li R., Welch C. L., Hasty A. H., Bornfeldt K. E., Breslow J. L., Tabas I., Tall A. R. (2009) Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ. Res. 105, 1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kreisel D., Nava R. G., Li W., Zinselmeyer B. H., Wang B., Lai J., Pless R., Gelman A. E., Krupnick A. S., Miller M. J. (2010) In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl. Acad. Sci. USA 107, 18073–18078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu S., Trapnell C. (2016) Single-cell transcriptome sequencing: recent advances and remaining challenges. F1000 Res. 5, F1000 Faculty Rev-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carr E. J., Dooley J., Garcia-Perez J. E., Lagou V., Lee J. C., Wouters C., Meyts I., Goris A., Boeckxstaens G., Linterman M. A., Liston A. (2016) The cellular composition of the human immune system is shaped by age and cohabitation. Nat. Immunol. 17, 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Jager P. L., Hacohen N., Mathis D., Regev A., Stranger B. E., Benoist C. (2015) ImmVar project: insights and design considerations for future studies of “healthy” immune variation. Semin. Immunol. 27, 51–57 [DOI] [PubMed] [Google Scholar]
- 112.Brodin P., Jojic V., Gao T., Bhattacharya S., Angel C. J. L., Furman D., Shen-Orr S., Dekker C. L., Swan G. E., Butte A. J., Maecker H. T., Davis M. M. (2015) Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hirayasu K., Arase H. (2015) Functional and genetic diversity of leukocyte immunoglobulin-like receptor and implication for disease associations. J. Hum. Genet. 60, 703–708 [DOI] [PubMed] [Google Scholar]
- 114.Vieira Braga F. A., Teichmann S. A., Chen X. (2016) Genetics and immunity in the era of single-cell genomics. Hum. Mol. Genet. 25 (R2), R141–R148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.-L., Kohler R. H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T. R., Libby P., Weissleder R., Pittet M. J. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Halade G. V., Kain V., Black L. M., Prabhu S. D., Ingle K. A. (2016) Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY) 8, 2611–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leuschner F., Rauch P. J., Ueno T., Gorbatov R., Marinelli B., Lee W. W., Dutta P., Wei Y., Robbins C., Iwamoto Y., Sena B., Chudnovskiy A., Panizzi P., Keliher E., Higgins J. M., Libby P., Moskowitz M. A., Pittet M. J., Swirski F. K., Weissleder R., Nahrendorf M. (2012) Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ismahil M. A., Hamid T., Bansal S. S., Patel B., Kingery J. R., Prabhu S. D. (2014) Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ. Res. 114, 266–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shaw A. C., Goldstein D. R., Montgomery R. R. (2013) Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 13, 875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 [DOI] [PubMed] [Google Scholar]
- 121.Funk C. D., FitzGerald G. A. (2007) COX-2 inhibitors and cardiovascular risk. J. Cardiovasc. Pharmacol. 50, 470–479 [DOI] [PubMed] [Google Scholar]
- 122.Baylis D., Bartlett D. B., Patel H. P., Roberts H. C. (2013) Understanding how we age: insights into inflammaging. Longev. Healthspan. 2, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fuijkschot W. W, Groothuizen W. E., Appelman Y., Radonic T., van Royen N., van Leeuwen M. A., Krijnen P. A., van der Wal A. C., Smulders Y. M., Niessen H. W. (2017) Inflammatory cell content of coronary thrombi is dependent on thrombus age in patients with ST-elevation myocardial infarction. J. Cardiol. 69, 394–400 [DOI] [PubMed] [Google Scholar]
- 124.Frangogiannis N. G., Mendoza L. H., Ren G., Akrivakis S., Jackson P. L., Michael L. H., Smith C. W., Entman M. L. (2003) MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am. J. Physiol. Heart Circ. Physiol. 285, H483–H492 [DOI] [PubMed] [Google Scholar]
- 125.Frantz S., Hofmann U., Fraccarollo D., Schäfer A., Kranepuhl S., Hagedorn I., Nieswandt B., Nahrendorf M., Wagner H., Bayer B., Pachel C., Schön M. P., Kneitz S., Bobinger T., Weidemann F., Ertl G., Bauersachs J. (2013) Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J. 27, 871–881 [DOI] [PubMed] [Google Scholar]
- 126.Fan K., Ruan Q., Sensenbrenner L., Chen B. (1992) Transforming growth factor-beta 1 bifunctionally regulates murine macrophage proliferation. Blood 79, 1679–1685 [PubMed] [Google Scholar]
- 127.Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. (1986) Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 163, 1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoshimura A., Wakabayashi Y., Mori T. (2010) Cellular and molecular basis for the regulation of inflammation by TGF-beta. J. Biochem. 147, 781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. (1987) Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. USA 84, 5788–5792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Letterio J. J., Roberts A. B. (1998) Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16, 137–161 [DOI] [PubMed] [Google Scholar]
- 131.Werner F., Jain M. K., Feinberg M. W., Sibinga N. E., Pellacani A., Wiesel P., Chin M. T., Topper J. N., Perrella M. A., Lee M. E. (2000) Transforming growth factor-beta 1 inhibition of macrophage activation is mediated via Smad3. J. Biol. Chem. 275, 36653–36658 [DOI] [PubMed] [Google Scholar]
- 132.Saxena A., Russo I., Frangogiannis N. G. (2016) Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl. Res. 167, 152–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saito T., Rodger I. W., Hu F., Robinson R., Huynh T., Giaid A. (2004) Inhibition of COX pathway in experimental myocardial infarction. J. Mol. Cell. Cardiol. 37, 71–77 [DOI] [PubMed] [Google Scholar]
- 134.Kimmel S. E., Berlin J. A., Reilly M., Jaskowiak J., Kishel L., Chittams J., Strom B. L. (2005) Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann. Intern. Med. 142, 157–164 [DOI] [PubMed] [Google Scholar]
- 135.Mann D. L., McMurray J. J. V., Packer M., Swedberg K., Borer J. S., Colucci W. S., Djian J., Drexler H., Feldman A., Kober L., Krum H., Liu P., Nieminen M., Tavazzi L., van Veldhuisen D. J., Waldenstrom A., Warren M., Westheim A., Zannad F., Fleming T. (2004) Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109, 1594–1602 [DOI] [PubMed] [Google Scholar]
- 136.Steg P. G., James S. K., Atar D., Badano L. P., Blömstrom-Lundqvist C., Borger M. A., Di Mario C., Dickstein K., Ducrocq G., Fernandez-Aviles F., Gershlick A. H., Giannuzzi P., Halvorsen S., Huber K., Juni P., Kastrati A., Knuuti J., Lenzen M. J., Mahaffey K. W., Valgimigli M., van ’t Hof A., Widimsky P., Zahger D.; Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology (ESC) (2012) ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 33, 2569–2619 [DOI] [PubMed] [Google Scholar]
- 137.Yancy C. W., Jessup M., Bozkurt B., Butler J., Casey D. E. Jr., Drazner M. H., Fonarow G. C., Geraci S. A., Horwich T., Januzzi J. L., Johnson M. R., Kasper E. K., Levy W. C., Masoudi F. A., McBride P. E., McMurray J. J., Mitchell J. E., Peterson P. N., Riegel B., Sam F., Stevenson L. W., Tang W. H., Tsai E. J., Wilkoff B. L.; Writing Committee members, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 128, e240–e327 [DOI] [PubMed] [Google Scholar]
- 138.Epelman S., Lavine K. J., Beaudin A. E., Sojka D. K., Carrero J. A., Calderon B., Brija T., Gautier E. L., Ivanov S., Satpathy A. T., Schilling J. D., Schwendener R., Sergin I., Razani B., Forsberg E. C., Yokoyama W. M., Unanue E. R., Colonna M., Randolph G. J., Mann D. L. (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gong K., Zhang Z., Sun X., Zhang X., Li A., Yan J., Luo Q., Gao Y., Feng Y. (2006) The nonspecific anti-inflammatory therapy with methotrexate for patients with chronic heart failure. Am. Heart J. 151, 62–68 [DOI] [PubMed] [Google Scholar]
- 140.Ramasubbu K., Estep J., White D. L., Deswal A., Mann D. L. (2008) Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 51, 415–426 [DOI] [PubMed] [Google Scholar]
- 141.McMurray J. J. V., Kjekshus J., Gullestad L., Dunselman P., Hjalmarson A., Wedel H., Lindberg M., Waagstein F., Grande P., Hradec J., Kamenský G., Korewicki J., Kuusi T., Mach F., Ranjith N., Wikstrand J.; CORONA Study Group (2009) Effects of statin therapy according to plasma high-sensitivity C-reactive protein concentration in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): a retrospective analysis. Circulation 120, 2188–2196 [DOI] [PubMed] [Google Scholar]