Immune hyporesponsiveness to amyloid β-peptide in amyloid precursor protein transgenic mice: Implications for the pathogenesis and treatment of Alzheimer's disease (original) (raw)
Abstract
Alzheimer's disease is a dementia that involves progressive deposition of amyloid β-protein (Aβ) in brain regions important for memory and cognition, followed by secondary inflammation that contributes to the neuropathologic process. Immunization with Aβ can reduce cerebral Aβ burden and consequent neuropathologic changes in the brains of mice transgenic for the β-amyloid precursor protein (APP). We found that transgenic expression of human APP in B6SJL mice, under the prion promoter, results in immune hyporesponsiveness to human Aβ, in terms of both antibody and cellular immune responses. The decreased antibody responses were related not to B cell tolerance but rather to the inability of Aβ-specific T cells to provide help for antibody production. The immune hyporesponsiveness could be overcome if T cell help was provided by coupling an Aβ B cell epitope to BSA. Our results suggest that expression of APP in transgenic mice is associated with an Aβ-specific impaired adaptive immune response that may contribute to the neuropathology. Moreover, humans with life-long elevation of brain and peripheral Aβ (e.g., patients with presenilin mutations or Down syndrome) could have reduced immune responses to Aβ vaccination.
Alzheimer's disease (AD) is a highly prevalent dementia that is associated with the abnormal accumulation and aggregation of amyloid β-peptide (Aβ), a process that precedes neuronal injury (reviewed in ref. 1). Accumulating evidence suggests that aggregated forms of Aβ extracellularly, and perhaps also intracellularly, have neurotoxic properties (1–4). Moreover, the autosomal dominant familial forms of AD involve mutations in the genes encoding β-amyloid precursor protein (APP), presenilin 1 (PS1), or PS2, and these mutations all cause increased production and accumulation of Aβ, a process that begins many years before clinical symptoms (1). Aβ is also overproduced in trisomy 21 (Down syndrome) patients, who overexpress APP from birth, thus making them more susceptible to AD (5, 6). Although there are numerous unresolved issues regarding the molecular and cellular cytotoxic events mediating AD, it is likely that Aβ plays a very early and central role in both the inherited and sporadic forms of the disease.
The immune system also appears to participate in AD pathogenesis (7–11). Moreover, immune-based strategies have been shown to be effective in clearing Aβ from the brains of APP transgenic (Tg) mice (12–15). Aβ deposition causes activation of microglia and astrocytes at sites of its accumulation, followed by the induction of an inflammatory response (9, 10, 16). This inflammatory response may represent in part an attempt by the immune system to clear excess amounts of Aβ. The proinflammatory environment in the brain may become chronic probably because of progressive accumulation of Aβ and its aggregation into forms that are less efficiently cleared and/or more cytotoxic to neurons (7, 10). We hypothesized that chronic exposure of the immune system to Aβ in humans and mouse models might lead to hyporesponsiveness in terms of cellular and humoral immune responses to Aβ itself, which could contribute to the disease process. On the basis of this hypothesis, we investigated the immune response to Aβ in Tg mice expressing human APP throughout life.
Materials and Methods
Mice.
C57BL6 and B6SJLF1 mice were purchased from The Jackson Laboratory. APP-Tg line 2576 expressing APP under the prion promoter was described by Hsiao et al. (17) and was obtained from the Mayo Clinic (Rochester, MN). B6SJL APP-Tg+ mice were bred with C57BL6 mice, and the N1 generation was used for all of the experiments described herein. APP-Tg mice were analyzed by PCR as described (18) and were also confirmed by measuring serum levels of human Aβ, as indicated.
Antigens.
For immunization, Aβ1–40 and -42 peptides (synthesized in the Biopolymer Laboratory, Center for Neurological Diseases, Brigham and Women's Hospital) were dissolved in DMSO (40 mg/ml) and then diluted immediately at 1:20 to12.5 mM Tris⋅HCl, pH 7.4. Aβ1–15 and BSA were dissolved in PBS to final concentrations of 2 mg/ml each. Aβ1–15 was also covalently coupled to BSA by using the crosslinker EDC (Pierce), followed by overnight dialysis against PBS in dialysis cassettes having a 10-K molecular weight cutoff (Pierce) and then dilution to a final concentration of 2 mg/ml. For in vitro stimulation of lymphocytes, Aβ was dissolved in 5 mg/ml DMSO (Sigma) before final dilution in X-vivo media (BioWhittaker). Purified protein derivative (PPD) was purchased from Accurate Chemicals and dissolved at 1 mg/ml. Final concentrations are indicated in the legend to Fig. 4.
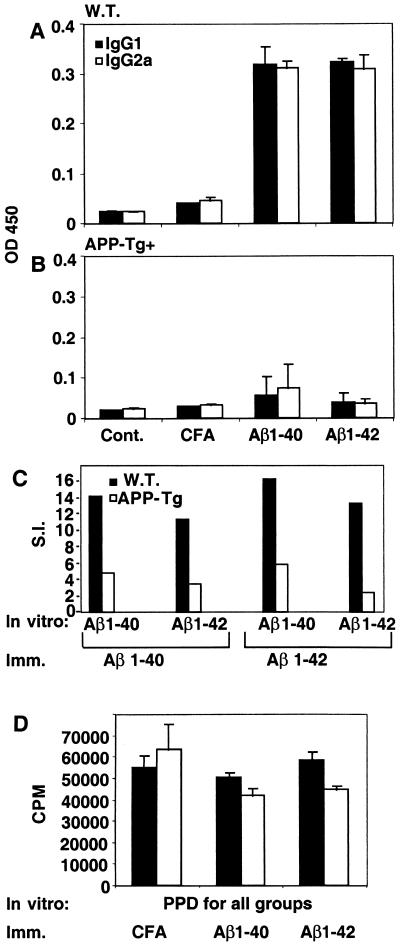
Figure 4.
Decreased antibody production and T cell proliferation persists in old APP-Tg+ mice. Old (14–18 months) APP-Tg+ mice (A) and control adult (wild-type) mice (B) were immunized with Aβ1–40 or -42. Mice were either not immunized or injected with CFA only. Anti-Aβ antibodies (IgG1 and IgG2a) were measured by ELISA at day 10 postimmunization. Spleens were excised at day 10 postimmunization, and their lymphocytes were stimulated in vitro with 50 μg/ml of either Aβ1–40 or -42 (C) or the bacterial antigen PPD (D) for 72 h, followed by incubation with 3H-thymidine for 12 h. The results for the antigen PPD are shown in cpm because of relative very low background.
Immunization.
Mice were immunized by footpad injection. Each mouse received 100 μl of antigen (1 μg/μl), emulsified in an equal volume of complete Freund's adjuvant containing 50 μg of Mycobacterium tuberculosis. Ten days later, mice were bled, and popliteal draining lymph nodes (PLN) were excised. PLN-derived lymphocytes were then cultured in X-vivo serum-free medium in U-bottom 96-well plates and tested in vitro for antigen-induced proliferation and cytokine production. For cytokine measurement by ELISA, supernatants were collected at 24 h for IL-2 and -4, at 40 h for IL-10 and INFγ, and at 72 h for transforming growth factor-β (TGF-β). For proliferation measurements, cells were pulsed with 1 μCi 3H-thymidine/well 72 h after stimulation and harvested 12 h later, followed by measuring 3H-thymidine incorporation. Anti-Aβ antibodies were measured in serum by ELISA.
ELISA.
Antigen-induced cytokine production was measured by sandwich ELISA, as described (19). Recommended pairs of antibodies (coating and detecting) for IL-2, -4, -10, and INFγ were purchased from PharMingen, TGF-β antibodies were purchased from Promega.
To measure titers of anti-Aβ antibodies in serum of immunized mice, flat-bottom ELISA plates were coated overnight at 4°C with Aβ1–40, 4 μg/ml in 0.1 M NaHCO3, pH 8.3. Identical concentrations were used for the negative control antigens, Aβ 42–1 and growth hormone releasing factor (GRF) (Bachem). Plates were then washed twice with washing buffer (PBS, 0.05% Tween 20) and blocked with 1% BSA and 2% normal rat serum in PBS for 2 h at room temperature (RT). Plates were washed ×2 and incubated with serum samples diluted 1:100 in blocking buffer for 2 h at RT while shaking. Plates were washed ×4 and incubated with rat–anti-mouse biotinylated IgG (specific isotypes are indicated in figure legends 1, 4, 5) for 2 h at RT. Plates were washed ×4 and incubated with avidin–horseradish peroxidase for 1 h at RT. Plates were washed ×4 and analyzed colorimetrically after incubation with the chromogen substrate 3,3′,5,5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories) for 1–2 min at RT. Nonspecific titers that were measured for the negative control antigen, GRF, were subtracted from the titers measured for Aβ1–40 in the BSA-conjugated Aβ1–15 group.
To measure Aβ levels in serum samples and brain homogenates of APP-Tg mice, we used an Aβ-detection kit according to manufacturer's instructions (Biosource International, Camarillo, CA).
Immunohistochemistry.
Cryosections (6 μm) from brains of APP-Tg+ mice were placed onto glass slides and fixed with ice-cold methanol for 2 min followed by 2% paraformaldehyde at RT for 4 min. Aβ plaque-containing sections were stained with mouse serum samples by using a mouse on mouse (M.O.M.) kit (Vector, Burlingame, CA), according to the manufacturer's instructions. Sample dilutions are indicated in the legend to Fig. 3.
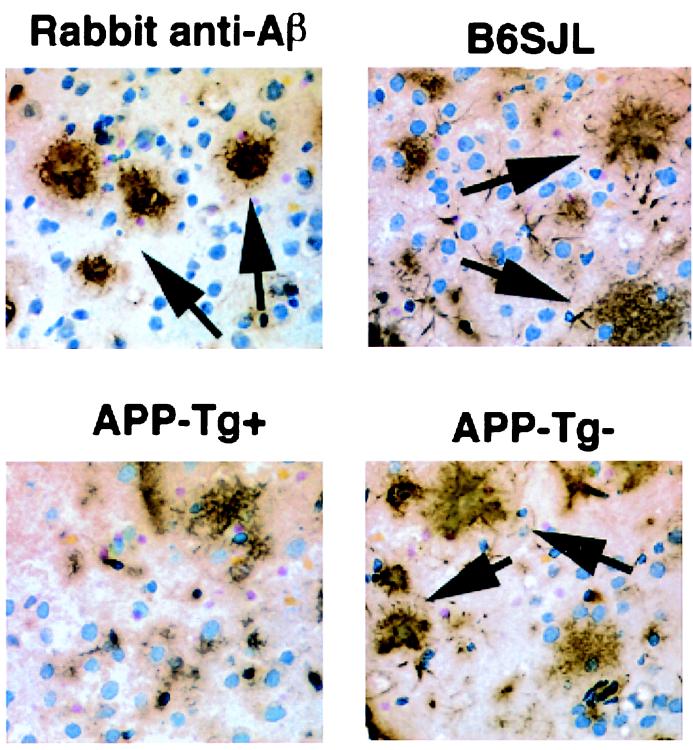
Figure 3.
Reactivity of sera from Aβ -immunized mice when examined on central nervous system sections with neuritic plaques. Sera from young Aβ-immunized mice were tested for Aβ binding on sections of amyloid plaques taken from an old APP-Tg+ mouse. The following mouse sera (diluted 1:100) are shown: B6SJL, APP-Tg+ and APP-Tg-. A high-titer polyclonal rabbit anti-Aβ antiserum (R1282) was used as a positive control staining. Some of the numerous immunopositive Aβ plaques are indicated by arrows.
Results
APP-Tg+ Mice Have a Decreased Antibody and Cellular Immune Response to Aβ.
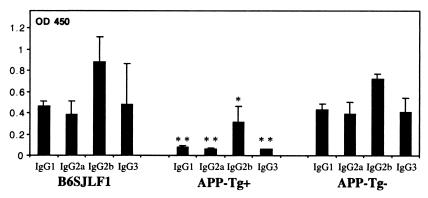
Control B6SJLF1 mice and APP-Tg+ and -Tg− mice [i.e., non-Tg (wild-type) littermates of the APP-Tg+ mice] were immunized at 5 weeks of age with synthetic human Aβ1–40, and anti-Aβ antibody production was measured at day 10 postimmunization. The levels of IgG1, IgG2a, IgG2b, and IgG3 anti-Aβ antibodies were significantly reduced in APP-Tg+ mice as compared with B6SJLF1 and wild-type littermates (P < 0.0001) (Fig. 1). Among the isotypes tested, only IgG2b was detected in significant levels in the APP-Tg+ mice. When 3-month-old mice were immunized, titers of anti-Aβ antibodies (including those of IgG2b) in the APP-Tg+ mice were even lower at both 10 and 18 days after immunization (not shown). Aβ-specific antibodies were not detected in mice immunized with complete Freund's adjuvant (CFA) only. As a further control, sera from Aβ1–40-immunized APP-Tg− mice were tested for antibodies to Aβ42–1 (the Aβ peptide synthesized in reverse sequence) and to an irrelevant peptide of similar size (growth hormone-releasing factor), and no such antibodies were detected.
Figure 1.
Suppressed anti-Aβ antibody production in immunized APP-Tg+ mice. Control B6SJL, APP-Tg+ and APP-Tg- mice were immunized with Aβ1–40 at 5 weeks of age and analyzed 10 days later for anti-Aβ antibody production. IgG1, IgG2a, IgG2b, and IgG3 anti-Aβ antibodies were measured by ELISA for APP-Tg+ (n = 7) and APP-Tg- (n = 7) littermates as well as for control wild-type mice (n = 6) (see Methods). The data shown are for sera dilution of 1:100, as a higher dilution did not yield detection of significant anti-Aβ antibodies in the APP-Tg+ animals. All isotypes are significantly decreased in the APP-Tg+ mice compared with APP-Tg- (*, P < 0.0001; **, P < 0.0005).
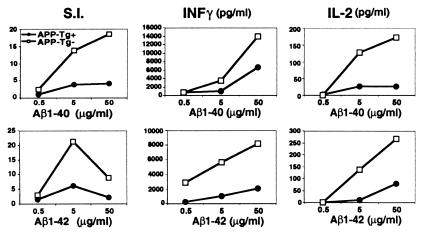
We also measured Aβ-induced T cell proliferation and cytokine production. T cell proliferation and secretion of INFγ and IL-2 were decreased in the APP-Tg+ vs. the APP-Tg− littermates (Fig. 2). Of note, the proliferation dose response to Aβ1–40 vs. -42 was different, with maximal proliferation occurring at 5 μg/ml for Aβ1–42 and at 50 μg/ml for Aβ1–40. Aβ1–42 is more prone to aggregation than Aβ1–40, which may decrease antigen availability for in vitro T cell stimulation at high concentrations. IL-10, IL-4, and transforming growth factor-β were also measured but were not detected in either APP-Tg+ or APP-Tg− animals. T cell proliferative responses to PPD (5 μg/ml) were similar in both groups: 83,113 ± 8,750 cpm for APP-Tg+ and 63,124 ± 2,864 cpm for the APP-Tg− (means ± SD, n = 3).
Figure 2.
Decreased Aβ-induced T cell proliferation and cytokine production. APP-Tg+ and APP-Tg- mice were immunized with Aβ1–40 as described in Fig. 1. In vitro T cell proliferation induced by either Aβ1–40 or -42 were measured after incubating the lymph node-derived lymphocytes with the respective Aβ peptide for 3 days, followed by 3H-thymidine incorporation for 12 h (see Methods). Stimulation index (S.I.) represents the cpm in the presence of antigen divided by the cpm in the absence of antigen. T cells from the various groups were also tested for Aβ-mediated cytokine secretion. Results are shown for Aβ-induced IL-2 and INFγ production measured at 24 and 40 h after Aβ stimulation, respectively.
Substantial Amounts of Aβ Occur in the Serum of APP-Tg+ Mice.
A possible immunologic mechanism for the suppressed immune response to Aβ described above is that the overexpression of human APP from birth induces a form of immunologic tolerance to human Aβ, as has been described for hen egg lysozyme (HEL) (20, 21). High levels of Aβ in the serum could penetrate the thymus and induce anergy or deletion of Aβ-reactive T cells. To address this possibility, we measured human Aβ levels with an Aβ1–40-specific ELISA in both sera and brain homogenates of APP-Tg+ mice at 5 weeks of age; APP-Tg− mice served as a negative control. Human Aβ1–40 was detected only in samples from the APP-Tg+ mice. By 5 weeks of age, mean Aβ1–40 concentration in the serum of these mice was 643 ± 166 pg/ml (mean ± SD, n = 12) and Aβ1–40 concentration in the brain was 48 ± 3 ng/g tissue (n = 3). By 3 months of age, mean Aβ1–40 concentration in the serum was 970 ± 418 pg/ml (n = 5).
Serum from Aβ-Immunized APP-Tg− Mice React with Central Nervous System Neuritic Plaques.
To establish that the anti-Aβ antibodies detected by ELISA in immunized APP-Tg mice reflect true reactivity with amyloid plaques in the brain, we carried out immunohistochemistry on brain cryosections from mature APP-Tg+ mice. Aβ-plaques were stained by sera from APP-Tg− or B6SJLF1 mice immunized with Aβ in an indistinguishable manner to the Aβ-plaque staining by rabbit polyclonal anti-Aβ antibodies (Fig. 3). Aβ plaques were not stained with serum derived from nonimmunized APP-Tg+ mice. Fewer numbers of stained plaques were observed with serum from immunized APP-Tg+ mice compared with serum from APP-Tg− mice, as expected from the lower anti-Aβ antibody titers in the former.
Decreased Antibody Production and T Cell Proliferation in Old APP-Tg+ Mice.
Because amyloid deposition rises with age in APP-Tg+ mice, we tested whether the decreased immune response to Aβ we observed in young APP-Tg+ mice persisted in older animals (14–18 months). APP-Tg+ mice and control (B6SJLF1) adult mice were immunized with Aβ1–40 or -42 in CFA or with CFA alone. Anti-Aβ antibodies (IgG1 and IgG2a) were measured at day 10 postimmunization. We observed a decrease in anti-Aβ antibody production for both IgG1 and IgG2a isotypes in old APP-Tg+ mice as compared with B6SJLF1 animals (Fig. 4 A and B). In addition, T lymphocytes from APP-Tg+ mice had a decreased proliferative response to Aβ1–40 and -42 (Fig. 4C). However, both APP-Tg+ and control mice immunized with Aβ1–40 or -42 in CFA had similar T cell proliferative responses to PPD in vitro as shown in (Fig. 4D).
Immunization with BSA-Conjugated Aβ1–15 Induces High Titers of Anti-Aβ Antibodies in APP-Tg+ Mice.
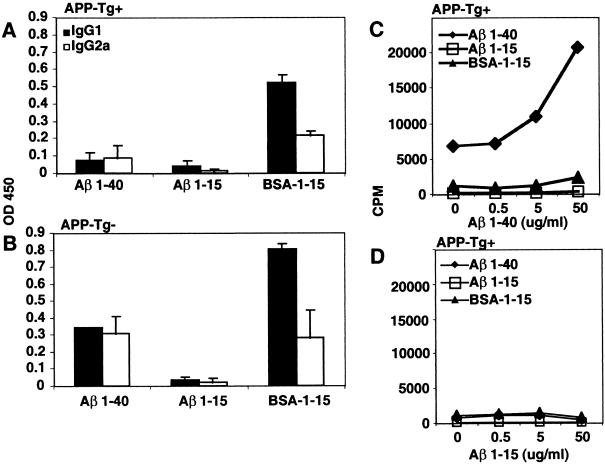
Because antibody production depends on T cell help, we postulated that the decreased antibody production we observed in both young and old APP-Tg+ mice could result from defective T cell help and that this could be overcome if T cell help was provided. To test this hypothesis, in the APP-Tg+ mice, we provided T cell help by coupling a B cell epitope of Aβ to BSA as a carrier antigen. We first analyzed the Aβ B cell epitopes in the sera of APP-Tg− mice by coating ELISA plates with a series of nested Aβ peptides (1–15, 5–20, 10–25, 15–30, 20–35, 25–42). This analysis revealed that the dominant peptide to which anti-Aβ antibodies were made was Aβ1–15 (not shown). We then immunized APP-Tg+ mice with Aβ1–15 conjugated with BSA, and as controls we immunized mice with either Aβ1–40 alone, Aβ1–15 alone, or BSA alone. Anti-Aβ1–40 antibody production was still reduced (both IgG1 and IgG2a) when APP-Tg+ mice were immunized with Aβ1–40 or -15 alone (Fig. 5A). However, high titers of antibodies against Aβ1–40 were detected when APP-Tg+ mice were immunized with BSA-conjugated Aβ1–15 (Fig. 5A).
Figure 5.
Immunization with BSA-conjugated Aβ1–15 induces high titers of anti-Aβ antibodies in APP-Tg+ mice. APP-Tg littermates (both + and −) were immunized with one of the following antigens: Aβ1–40, Aβ1–15, or BSA-conjugated Aβ1–15. At day 10 postimmunization, mice were bled, and popliteal draining lymph nodes (PLN) were excised. Titers of IgG1 and IgG2a anti-Aβ1–40 antibodies were measured in the serum of the APP-Tg+ (A) and APP-Tg− (B) littermates by ELISA. In addition, PLN-derived cells from APP-Tg+ mice were stimulated in vitro at day 10 postimmunization with either Aβ1–40 (C) or Aβ1–15 (D) for a T cell proliferation assay. Note that levels of Aβ were measured by ELISA in preimmune sera of the APP-Tg+ mice tested, and the concentrations were found to be similar for the Aβ1–40 group (576.6 ± 117 pg/ml), the Aβ1–15 group (540 ± 198 pg/ml), and the BSA-conjugated Aβ1–15 group (616.6 ± 231 pg/ml).
Immunization of APP-Tg− littermates with the same regimen led to higher antibody titers to Aβ1–40 in the BSA-conjugated Aβ1–15 group and in the Aβ1–40 group, as compared with the APP-Tg+ littermates (Fig. 5B). Immunization with BSA alone did not induce anti-Aβ1–40 antibody production in either APP-Tg+ or APP-Tg− animals. We also observed that immunization with BSA-conjugated Aβ1–15 induced higher titers of IgG1 than IgG2a. T cell proliferative responses were also tested in the APP-Tg+ mice. Immunization with Aβ1–40 induced measurable Aβ1–40-specific T cell proliferative responses (Fig. 5C), similar to that shown in Fig. 2 (stimulation index < 5). No proliferative responses to Aβ1–40 were seen in animals immunized with Aβ1–15 or BSA-conjugated Aβ1–15 (Fig. 5C). No proliferative responses were seen in all groups when Aβ1–15 was used for the in vitro stimulation (Fig. 5D). These results demonstrate that T cell proliferation to Aβ depends on other regions of the Aβ molecule, not Aβ1–15, and that immunization with BSA-conjugated Aβ1–15 induces antibody responses in the absence of Aβ-specific T cell proliferation.
Discussion
In the present study, we investigated whether the chronic expression of human APP in APP Tg+ mice is associated with a decreased immune response to human Aβ. We found highly significantly decreased antibody production and T cell responses in (B6 × SJL) × B6 APP-Tg+ mice immunized with Aβ1–40, compared with APP-Tg− littermates or controls. These differences were associated with substantial levels of human Aβ peptide in the sera of the APP-Tg+ mice. The decreased immune response persisted in old APP-Tg+ mice and was antigen specific for Aβ, as these animals maintained normal responses to PPD. This hyporesponsiveness could be partially overcome by providing T cell help by coupling Aβ1–15 to BSA; the APP-Tg+ animals had normal humoral immune response to BSA alone.
A growing body of evidence has demonstrated that self-reactive lymphocytes may escape negative selection or may even be positively selected to be part of the normal immune repertoire (22). Immunologic tolerance involves multiple mechanisms by which self-reactive lymphocytes do not cause harm to the host. Moreover, it has been demonstrated that self-reactive T cells may also be of benefit to the host (23, 24). Antigen itself is the primary driving factor in immune tolerance, and it has been shown that exposure to soluble antigen in the thymus, either as a result of its expression in the thymus or secondary to high levels in the blood, induces efficient tolerance (20, 21). In APP-Tg+ mice, there is high expression of human APP in the brain (17, 25) and substantial levels of human Aβ in the serum. In the model of APP-Tg mice we used, the APP gene is expressed under the prion promoter (17). Recent work has shown that the expression of a reporter gene under the control of the bovine prion promoter occurs in additional tissues besides the brain, including the thymus (26). It is likely that chronic expression of APP in the brain and other organs and especially the substantial levels of Aβ in the blood lead to hyporesponsiveness of Aβ-reactive T or B cells. The immune system is first exposed to Aβ as a soluble antigen at a time when no cerebral deposition has yet taken place, and at this relatively early stage in life, Aβ may be tolerogenic. Later in life, deposition of Aβ in the brain is associated with an inflammatory response that could potentially induce the activation of Aβ-specific T and B cells and perhaps reduce the subsequent pathology. However, because the T cells may be tolerized, the inflammation may become chronic, and further neuronal damage ensues.
The decreased immune response we observed involved both humoral and cellular immunity. Nonetheless, it appears that the primary defect is related to T cells, as we could partially overcome the defective antibody production by providing T cell help by coupling BSA to Aβ1–15, which is a B cell epitope within Aβ1–40. Immunization with Aβ1–15 by itself did not elicit significant T cell responses or antibody production in either Tg+ and Tg− mice. However, coupling of Aβ1–15 to BSA induced anti-Aβ antibody production with no Aβ-specific T cell proliferation. Thus, our results suggest that there are different T- and B cell epitopes in the Aβ1–40 molecule, and the reduced antibody responses we observed are not principally related to B cell tolerance but to the inability of T cells to provide help for antibody production. This observation is in line with the results of Adelstein et al. (20) and Peterson et al. (21), who showed that T cell tolerance in the HEL Tg model occurs at low serum concentration of HEL (500 pg/ml), whereas B cell tolerance was achieved only at 1.5 ng/ml. At lower concentrations, B cells could be rescued if HEL was conjugated to a carrier protein before immunization. Studies by Yule et al. (27) have shown that 100 times more HEL was required to evoke an immune response in HEL-Tg mice; however, the clones isolated responded to the same epitopes as in non-Tg mice. Therefore, the HEL-reactive T cells were not deleted but rather anergized, and on immunization with high concentrations of HEL, they partially recovered. These results are consistent with our present data that Aβ-reactive T cells, although hyporesponsive, did proliferate in the APP-Tg+ mice in response to Aβ immunization. Also, the pattern of cytokine secretion showed a more profound decrease in IL-2 than in INFγ in APP–Tg+ mice, suggesting a mechanism of anergy rather than deletion (28). Further studies are ongoing to elucidate the mechanisms of Aβ-mediated immune hyporesponsiveness we observed.
Immunization with Aβ1–40 in APP-Tg− mice primarily induces production of antibodies against the Aβ1–15 portion of the molecule. The dominant T cell epitope for Aβ is not 1–15, because immunization with Aβ1–15 alone does not lead to T cell-mediated antibody production; rather, the coupling of Aβ1–15 to BSA is required to induce anti-Aβ antibody production. This segregation of T and B cell epitopes within the Aβ molecule allows the opportunity to induce anti-Aβ antibodies in a diminished of an Aβ-specific T cell response.
Recent studies have demonstrated that repeated peripheral or nasal Aβ immunization can generate anti-Aβ antibodies in APP-Tg+ mice, and this treatment is associated with decreased Aβ deposition (12, 13). These studies indicate that T cell hyporesponsiveness, if it exists in these mice, can be partially overcome by repeated immunization. However, in the PDAPP mouse line used in these two studies, APP was expressed under the platelet-derived growth factor promoter, and thus the blood concentration of Aβ as well as its tolerogenicity at various ages may differ from the mice we used. It was recently shown that repeated Aβ immunization reduces memory loss in a Tg mouse model that expresses APP under the prion promoter, and anti-Aβ antibody titers were lower in the Tg mice as compared with non-Tg animals (15). Furthermore, clearance of Aβ was not as efficient in these animals, compared with Aβ immunization in the PDAPP mice. This difference may be not only because of differences in plasma levels of Aβ but also because of different genetic backgrounds of the mice tested, which may affect immune responsiveness. Expression of APP under the same promoter but on a different genetic background may result in different immunogenicity of Aβ. It is likely, however, that in old APP-Tg+ mice, as a result of life-long Aβ accumulation and aging, the immune hyporesponsiveness might be even more pronounced, and therefore an immune approach to prevent Aβ deposition in the brain or to induce its clearance may be less effective in such animals.
An important question is how our finding of a decreased immune response in APP-Tg+ mice applies either to the pathogenesis of AD or to strategies for its treatment. In terms of pathogenesis, it is known that Down syndrome patients, who are highly susceptible to developing β-amyloid deposition and AD neuropathology, overexpress APP from before birth and have increased levels of Aβ1–40 and -42 in the plasma (29). In both familial and sporadic forms of AD, there are markedly increased levels of Aβ in the brain (30). Such increased levels are very likely to occur well before the first clinical symptoms, and the progression of the disease and could result in a defective T cell immune response to Aβ that, as we have shown in mice, may lead to decreased antibody responses. T cell responses to Aβ have been reported in humans, and it has been suggested that there are defective T cell immune responses to Aβ in AD patients (31). We are currently investigating whether correlation exists in AD patients between disease severity and immune reactivity to Aβ.
In terms of therapy for AD, immunization of mice with Aβ peptide reduced plaques and associated neuropathologic sequelae in a human APP-Tg mouse line (12). This effect could also be obtained by passive transfer of anti-Aβ antibodies systemically or by direct injection into the plaque region (32, 33). We have obtained similar results by using an immunologic approach in which Aβ was administrated nasally, and the therapeutic effect was associated with the induction of anti-Aβ antibodies and the infiltration of regulatory T cells into the central nervous system (13), which may also play a role in the protective effect. Human trials of Aβ immunization are currently in progress. The results of our study suggest that chronic presymptomatic accumulation of Aβ in the brain (and perhaps in serum and other tissues) in AD patients [e.g., in individuals having Down syndrome or APP or presenilin mutations (34)] could make it more difficult to induce antibody responses. Indeed, this decrease in responsiveness may have blunted the beneficial immune response in APP-Tg mice. We show here that one way to overcome this problem is to couple the dominant B cell epitope, Aβ1–15, to a carrier protein such as BSA. Such an immunization paradigm would preferentially induce antibody responses and yield minimal Aβ-reactive T cell responses. Another approach would be to identify a subset of Aβ-reactive T cells that are not tolerized, as has been demonstrated by using the HEL Tg mice (21), and use them to evoke an immune response to Aβ.
Autoreactive T cells may be harmful to the host and mediate certain autoimmune diseases such as multiple sclerosis or autoimmune diabetes (35–38). Nevertheless, it is also now clear that T cell autoreactivity may have beneficial effects for the host and that self-reactive T cells for nervous system proteins may have neuroprotective properties (39). For example, oral administration of myelin basic protein (MBP) can protect the brain in animal models of stroke (40), and the administration of MBP-reactive T cells results in a neuroprotective effect in a model involving secondary degeneration of neurons after trauma (41). It is therefore possible that the inefficient adaptive T cell immune response we have demonstrated in APP-Tg+ mice and that may also occur in AD patients could result in less neuroprotection in the patients.
In summary, our results demonstrate that chronic accumulation of Aβ peripherally and centrally may be associated with an impaired adaptive immune response to Aβ. This finding has important implications for both the pathogenesis and immune-based treatment of AD.
Acknowledgments
We thank Vijay Kuchroo, Arnon Karni, and Amit Bar-Or for helpful discussions. This work was supported by grants from the National Institutes of Health and the Foundation for Neurologic Diseases.
Abbreviations
Aβ
amyloid β-peptide
APP
amyloid precursor protein
AD
Alzheimer's disease
CFA
complete Freund's adjuvant
HEL
hen egg lysozyme
Tg
transgenic
PPD
purified protein derivative
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Selkoe D J. Nature (London) 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 2.Chui D H, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirotani K, et al. Nat Med. 1999;5:560–564. doi: 10.1038/8438. [DOI] [PubMed] [Google Scholar]
- 3.Hsia A Y, Masliah E, McConlogue L, Yu G Q, Tatsuno G, Hu K, Kholodenko D, Malenka R C, Nicoll R A, Mucke L. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geula C, Wu C K, Saroff D, Lorenzo A, Yuan M, Yankner B A. Nat Med. 1998;4:827–831. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- 5.Teller J K, Russo C, DeBusk L M, Angelini G, Zaccheo D, Dagna-Bricarelli F, Scartezzini P, Bertolini S, Mann D M, Tabaton M, Gambetti P. Nat Med. 1996;2:93–95. doi: 10.1038/nm0196-93. [DOI] [PubMed] [Google Scholar]
- 6.Lemere C A, Blusztajn J K, Yamaguchi H, Wisniewski T, Saido T C, Selkoe D J. Neurobiol Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 7.Bradt B M, Kolb W P, Cooper N R. J Exp Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meda L, Cassatella M A, Szendrei G I, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Nature (London) 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 9.Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson M P, Flavell R A, Mullan M. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- 10.Cooper N R, Kalaria R N, McGeer P L, Rogers J. Neurobiol Aging. 2000;21:451–453. doi: 10.1016/s0197-4580(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 11.McGeer P L, McGeer E G. J Neural Transm Suppl. 1998;54:159–166. doi: 10.1007/978-3-7091-7508-8_15. [DOI] [PubMed] [Google Scholar]
- 12.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Nature (London) 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 13.Weiner H L, Lemere C A, Maron R, Spooner E T, Grenfell T J, Mori C, Issazadeh S, Hancock W W, Selkoe D J. Ann Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- 14.Janus C, Pearson J, McLaurin J, Mathews P M, Jiang Y, Schmidt S D, Chishti M A, Horne P, Heslin D, French J, et al. Nature (London) 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 15.Morgan D, Diamond D M, Gottschall P E, Ugen K E, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, et al. Nature (London) 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole G M, Cooper N R, Eikelenboom P, Emmerling M, Fiebich B L, et al. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao K K, Borchelt D R, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, et al. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- 19.Maron R, Hancock W W, Slavin A, Hattori M, Kuchroo V, Weiner H L. Int Immunol. 1999;11:1573–1580. doi: 10.1093/intimm/11.9.1573. [DOI] [PubMed] [Google Scholar]
- 20.Adelstein S, Pritchard-Briscoe H, Anderson T A, Crosbie J, Gammon G, Loblay R H, Basten A, Goodnow C C. Science. 1991;251:1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- 21.Peterson D A, DiPaolo R J, Kanagawa O, Unanue E R. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 22.Janeway C A., Jr Curr Biol. 1999;9:R342–R345. doi: 10.1016/s0960-9822(99)80209-7. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz M, Cohen I R. Immunol Today. 2000;21:265–268. doi: 10.1016/s0167-5699(00)01633-9. [DOI] [PubMed] [Google Scholar]
- 24.Faria A M, Weiner H L. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 25.Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, et al. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaire-Vieille C, Schulze T, Podevin-Dimster V, Follet J, Bailly Y, Blanquet-Grossard F, Decavel J P, Heinen E, Cesbron J Y. Proc Natl Acad Sci USA. 2000;97:5422–5427. doi: 10.1073/pnas.080081197. . (First Published May 2, 2000; 10.1073/pnas.080081197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yule T D, Basten A, Allen P M. J Immunol. 1993;151:3057–3069. [PubMed] [Google Scholar]
- 28.Fields P E, Gajewski T F, Fitch F W. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 29.Tokuda T, Fukushima T, Ikeda S, Sekijima Y, Shoji S, Yanagisawa N, Tamaoka A. Ann Neurol. 1997;41:271–273. doi: 10.1002/ana.410410220. [DOI] [PubMed] [Google Scholar]
- 30.Naslund J, Haroutunian V, Mohs R, Davis K L, Davies P, Greengard P, Buxbaum J D. J Am Med Assoc. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 31.Trieb K, Ransmayr G, Sgonc R, Lassmann H, Grubeck-Loebenstein B. Neurobiol Aging. 1996;17:541–547. doi: 10.1016/0197-4580(96)00068-1. [DOI] [PubMed] [Google Scholar]
- 32.Bard F, Cannon C, Barbour R, Burke R L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 33.Bacskai B J, Kajdasz S T, Christie R H, Carter C, Games D, Seubert P, Schenk D, Hyman B T. Nat Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- 34.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 35.Schloot N C, Batstra M C, Duinkerken G, De Vries R R, Dyrberg T, Chaudhuri A, Behan P O, Roep B O. J Autoimmun. 1999;12:289–296. doi: 10.1006/jaut.1999.0280. [DOI] [PubMed] [Google Scholar]
- 36.Ota K, Matsui M, Milford E L, Mackin G A, Weiner H L, Hafler D A. Nature (London) 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Gonzalez A, Benoist C, Mathis D. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 38.Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu H C, Lassmann H, Wekerle H. Eur J Immunol. 1993;23:1364–1372. doi: 10.1002/eji.1830230627. [DOI] [PubMed] [Google Scholar]
- 39.Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H. J Neuroimmunol. 2000;107:161–166. doi: 10.1016/s0165-5728(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 40.Becker K J, McCarron R M, Ruetzler C, Laban O, Sternberg E, Flanders K C, Hallenbeck J M. Proc Natl Acad Sci USA. 1997;94:10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen I R, Schwartz M. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]