FTY720 protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway (original) (raw)
. Author manuscript; available in PMC: 2018 Dec 1.
Abstract
Background and Purpose
White matter (WM) ischemic injury, a major neuropathological feature of cerebral small vessel diseases, is an important cause of vascular cognitive impairment in later life. The pathogenesis of demyelination following WM ischemic damage are often accompanied by microglial activation. Fingolimod (FTY720) was approved for the treatment of multiple sclerosis for its immunosuppression property. In this study, we evaluated the neuroprotective potential of FTY720 in a WM ischemia model.
Methods
Chronic WM ischemic injury model was induced by bilateral carotid artery stenosis (BCAS). Cognitive function, WM integrity, microglial activation and potential pathway involved in microglial polarization were assessed after BCAS.
Results
Disruption of WM integrity was characterized by demyelination in the corpus callosum and disorganization of Ranvier’s Nodes using Luxol Fast Blue staining, immunofluorescence staining and electron microscopy. In addition, radial maze test demonstrated that working memory performance was decreased at one-month post BCAS-induced injury. Interestingly, FTY720 could reduce cognitive decline and ameliorate the disruption of WM integrity. Mechanistically, cerebral hypoperfusion induced microglial activation, production of associated pro-inflammatory cytokines and priming of microglial polarization toward the M1 phenotype; whereas FTY720 attenuated microglia mediated neuroinflammation after WM ischemia and promoted oligodendrocytogenesis by shifting microglia toward M2 polarization. FTY720’s effect on microglial M2 polarization was largely suppressed by selective STAT3 blockade in vitro, revealing that FTY720-enabled shift of microglia from M1 to M2 polarization state was possibly mediated by STAT3 signaling.
Conclusions
Our study suggested that FTY720 might be a potential therapeutic drug targeting brain inflammation by skewing microglia toward M2 polarization after chronic cerebral hypoperfusion.
Keywords: FTY720, white matter ischemic injury, microglial polarization, demyelination, STAT3
Introduction
White matter (WM) damage manifests as a core pathology in the progression of various diseases such as stroke, vascular dementia, and multiple sclerosis1, 2. Characterized by loss of axon-glial integrity and demyelination, WM injury is an important cause of cognitive deficits after cerebral ischemia and is often associated with microglial activation3. A persistent pro-inflammatory microenvironment after cerebral hypoperfusion is considered an underlying mechanism that hinders remyelination and white matter repair4.
The polarization of microglia has been described as a functional dichotomy: Classical (M1, pro-inflammatory) and alternative (M2, anti-inflammatory) activation5. Polarization of microglial populations toward various functional phenotypes can be beneficial or detrimental in response to specific microenvironmental signals following ischemic stroke, which contributes considerably to the regulation of inflammatory status in the injured WM and ultimately impacts the WM integrity2, 4, 5. Thus, therapeutic approaches targeting cerebral inflammation should shift from broad suppression of microglia towards the selective balance between their phenotypes in CNS repair after chronic injury.
Fingolimod (FTY720), a high-affinity agonist of sphingosine 1 phosphate (S1P) receptor, was the first FDA-approved drug for the treatment of relapsing–remitting multiple sclerosis through its immunosuppression effect4. Although cause and likewise clinical presentation set the two diseases apart, stroke shares certain common downstream mechanisms with multiple sclerosis in WM damage and recovery6. Indeed, FTY720 has been used in various models of stroke and neurological disorders4, 7. Some small clinical trials showed that oral FTY720 also have therapeutic efficacy in patients with acute stroke, including both ischemic stroke and intracerebral hemorrhage8, 9. However, the potential effect of FTY720 on WM ischemic damage is largely unknown. In this study, using a mouse model of WM ischemic damage caused by chronic hypoperfusion, we explored the cellular mechanism of FTY720 in WM injury and repair. Particularly, we focused on whether FTY720 exerted neuroprotective effects through modulating microglial polarization toward M2 type, a potential therapeutic strategy for patients with WM injury.
Materials and Methods
The data that support the findings of this study and analytic methods are available from the corresponding author upon reasonable request. The materials are available from commercial sources.
Animals and BCAS procedure
All animal studies were followed the instructions by the Institute of Animal Care Committee of Tongji Medical College, Huazhong University of Science and Technology, China. A total of 271 wild-type mice were used in the experiment. All the experimental groups were randomized and all outcome analysis was carried out by independent investigators blinded to the treatment condition.
Chronic cerebral hypoperfusion was induced by BCAS as microcoils (0.18 mm internal diameter, Sawane Spring Co, Shizuoka, Japan) were twined around both common carotid arteries as described by Shibata et al10.
Primary Cell Culture
Primary microglia and oligodendrocytes were isolated from the brain of neonatal C57BL/6J mice at P1–P2 as described11, 12. For M1 induction, lipopolysaccharide (LPS, 100ng/ml, L6529, Sigma–Aldrich, USA) and IFN-γ (20 ng/mL Sigma–Aldrich, USA) were added to the microglial cultures for 24 hours. For M2 induction, IL-4 (20 ng/mL) was added to the culture for 24 hours5. The study of OPCs apoptosis and differentiation in response to microglia consisted of 6 groups: OPCs were treated with unconditioned OPC culture medium/differentiation medium (OPC), or microglia CM (in a 1:1 ratio to OPC medium): untreated CM (control), LPS plus IFN-γ stimulated CM, LPS plus IFN-γ stimulated and FTY720 treated CM, IL-4 stimulated CM, and LPS plus IFN-γ stimulated CM added with 100nM FTY720.
FTY720 Treatment
For FTY720 treatment in vivo, FTY720 (10006292, 0.3 mg/kg, Cayman Chemical company, USA) was dissolved in PBS and administered intraperitoneally for 3, 10 or 30 consecutive days. For FTY720 treatment in vitro, cells were treated with 100 nM non-phosphorylated FTY720 for 1h ahead and then stimulated with LPS and IFN-γ for another 24h. The dose and duration of FTY720 was selected based on according to our preliminary experiments and previous studies13–15. Stattic (10 nM) was administered to block STAT3 pathway.
Eight-arm radial maze test, Luxol fast blue staining and Nissl staining, Immunofluorescence staining, Electron Microscopy, ELISA, Quantitative Real-Time PCR and Western Blot, TUNEL, and Immunomagnetic enrichment and Flow cytometry
A detailed description of experimental procedures was provided in the online-only Data Supplement.
Statistical analysis
Statistical analyses were performed using SPSS 19.0. All data were expressed as the mean ± SEM except for LFB staining, and significance was determined by two-way one-way analysis of variance (ANOVA) with repeated analysis, Mann–Whitney U test, or one-way ANOVA with Dunnett’s post-hoc test to compare differences between groups. All tests were considered statistically significant at P<0.05.
Results
Cerebral hypoperfusion-induced WM injury and cognitive dysfunction was partially reduced by FTY720
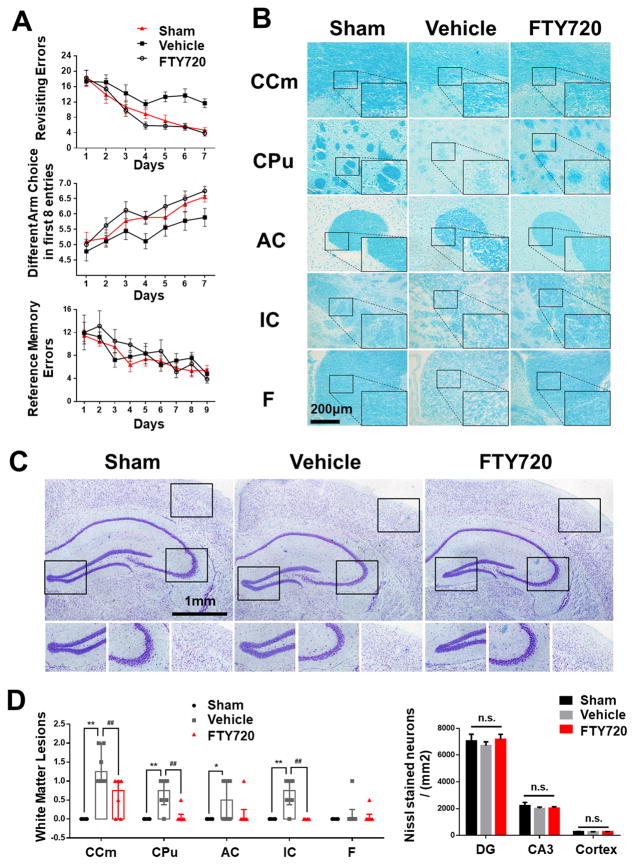
Cognitive impairment was detected in mice with cerebral hypoperfusion10. Indeed, sham-operated mice quickly improved their performance in the working memory task of the 8-arm radial maze, while mice at 1 month after BCAS took significantly more revisiting errors and less different arm choices in the first 8 entries (Figure 1A). Interestingly, we found that BCAS mice treated with FTY720 displayed improved performance in both revisiting sessions and different choices count, suggesting an improvement in short-term memory (Figure 1A). On the other hand, no spatial reference memory was compromised in this model, as there were no significant differences between groups in the reference memory task of the 8-arm radial maze (Figure 1A).
Figure 1. Working memory impairment and white matter lesions were partially reduced by FTY720 treatment.
(A) The working memory and reference memory of mice were assessed by the 8-arm maze test at 1 month post injury (n=8 for Sham, n=9 for Vehicle and FTY720). Vehicle mice made much more revisiting errors (P<0.001) and less different arm choices (P<0.001) comparing to the sham-operated mice. FTY720-treated group made much less revisiting errors and more different arm choices (P=0.016) comparing to Vehicle group. No impairment in spatial reference memory was revealed between different groups (P>0.05).
(B) White matter lesions were detected by LFB staining in different WM regions. Scale bar, 200μm.
(C) Representative images of Nissl stained coronal sections depicting the morphology of hippocampus and cerebral cortex. Scale bar, 1mm.
(D) Dot-plot with median and interquartile range of the severity of white matter lesions in CCm, CPu, AC, IC, and F in histogram. Quantitative analysis of Nissl stained neuron numbers in DG, CA3 region in hipocampus and cerebral cortex was performed. n.s. no significant changes between different groups. * P<0.05 ** P<0.01 versus Sham, # P<0.05 ## P<0.01 versus Vehicle. n=6 per group.
In previous studies, working memory deficits have been related either to frontal-subcortical circuits damage or compromised integrity of hippocampus10. To confirm that cognitive decline was a result of the difference in WM lesions in this model, we evaluated the WM lesion grades by LFB staining and hippocampus histological changes by Nissl staining at 1 month post hypoperfusion. We confirmed the disruption of white matter integrity by LFB stain of the medial part of corpus callosum (CCm), caudoputamen (CPu), anterior commissure (AC), the internal capsule (IC), and fimbria of hippocampus (F) at 1 month after hypoperfusion. The most severe rarefaction was noted in the medial part of CCm (Figure 1B and 1D). In contrast, we found no obvious histological damage in the hippocampus and the cortex in all groups (Figure 1C and 1D). Administration of FTY720 mitigated histological damage of WM, which was in agreement with its protective effect on cognitive impairment.
FTY720 treatment protected WM against chronic hypoperfusion induced demyelination and disorganization of Ranvier’s Nodes
Major components of the WM include neuronal axons, the surrounding myelin sheath and myelin-producing oligodendrocytes3. To further determine which components of WM suffered from the heaviest damage and the impact of FTY720, we assessed WM related markers in CCm using immunofluorescence and Western blot analysis. Myelin associated glycoprotein (MAG), a key myelin protein involved in the maintenance of axon-glial integrity, was compromised at 3 days after BCAS and greatly decreased as hypoperfusion prolonged (Figure I). Whereas, gross myelin marker myelin basic protein (MBP) tended to be lower in BCAS mice, but the difference between the groups did not reach statistical significance, and pan-axon marker neurofilament (NF) remained largely unchanged after hypoperfusion (Figure I).
Ultrastructural changes of myelination was detected by electron microscopy at 1 month after BCAS. The disruption of myelin was observed as vacuole or grid-liked changes in CCm of mice with BCAS, while axons in sham group were intact with well-defined lamellar myelin sheath. The g-ratio in BCAS mice increased significantly, suggesting a reduction in the average myelin thickness, while no significant difference in axon diameter or myelinated axon number was observed after hypoperfusion (Figure IIA). After FTY720 treatment, g-ratio was largely reduced approaching the levels seen in sham group. Collectively, the compromised integrity of WM by BCAS was largely attenuated by FTY720 treatment, both in MAG expression and the ultra-structure (Figure I and IIA), indicating FTY720’s protective effect on myelin status.
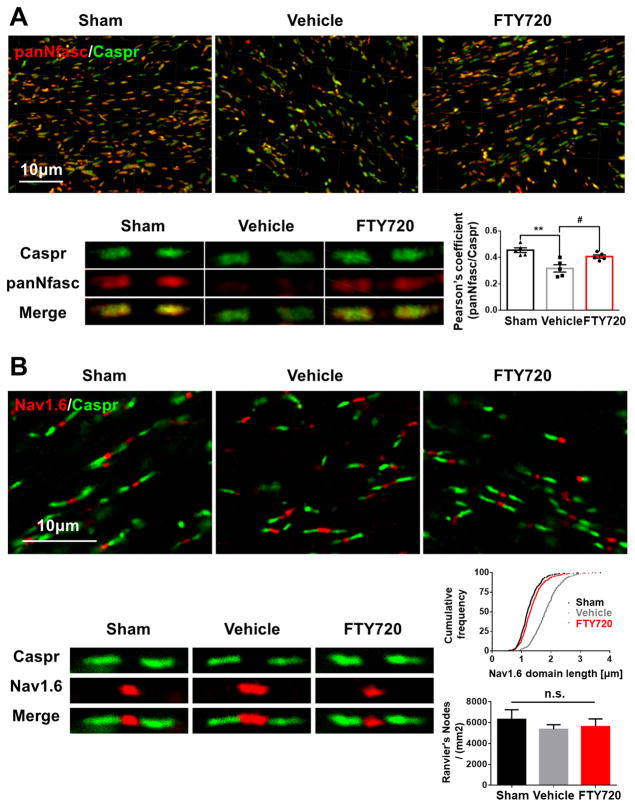
Figure 2. Disruption of Ranvier’s nodes due to hypoperfusion was reversed by FTY720 treatment.
(A) Representative 3 dimensional confocal images labeled with panNfasc/Caspr. Scale bar, 10μm. A higher magnification of representative single Ranvier’s node was shown below. Quantitative analysis of panNfasc/Caspr colocalization was shown in the histogram. **P<0.01 versus Sham, #P<0.05 versus Vehicle. n=5 per group. (B) Representative confocal images labeled with Nav1.6/Caspr. Scale bar, 10μm. A higher magnification of representative single Ranvier’s node was shown below. Summary of the length of Nav1.6 domain was shown in curve. P<0.0001 FTY720 versus Vehicle, Vehicle versus Sham. n=5 mice per group and 100 Nav1.6 domains for each mouse. Quantitative analysis of Ranvier’s nodes number is shown in the histogram. n.s. no significant changes between different groups. n=5 per group.
To further determine whether myelin stability is paralleled by alterations in oligodendrocyte myelination, we characterized the proliferation and differentiation of OPCs by BrdU labeling at 1 month post hypoperfusion. Both the Olig2+ oligodendrocyte lineage cells and their mature type APC+ cells were greatly reduced in BCAS group. After BCAS, the number of Olig2+/BrdU+ cells were slightly increased, while APC+/BrdU+ cells were significantly decreased, indicating compromised OPC differentiation after hypoperfusion. Administration of FTY720 reduced the oligodendrocyte loss after WM ischemic injury, as both proliferating Olig2+/BrdU+ OPCs and differentiating APC+/BrdU+ cells greatly increased (Figure IIB). Therefore, FTY720 might reverse WM ischemic injury by its protective effects via induction of oligodendrocyte proliferation and differentiation.
The structural organization of nodes of Ranvier is vulnerable to ischemia and is a sensitive indicator of WM ischemic injury3, 16. To test the integrity of the paranodal septate-like junctions and the nodal regions, we analyzed three key proteins (Caspr, Nav1.6 and panNfasc) of nodes of Ranvier at 1 month after BCAS. We found a significant reduction of colocalization of Caspr and panNfasc within the paranodes after chronic hypoperfusion, and FTY720 treatment profoundly reversed this reduction (Figure 2A). And there was a significant increase in the length of the Nav1.6 domain after hypoperfusion, indicating the injured status of Ranvier’s nodes. In FTY720-treated group, the damage of nodes was largely reversed (Figure 2B). Meanwhile, no difference in node number was detected between groups (Figure 2B). Together, our data suggests that FTY720 exerts its protective effect against structural derangement at the nodes of Ranvier following hypoperfusion.
FTY720 attenuated neuroinflammation after WM ischemic injury via altering microglial polarization
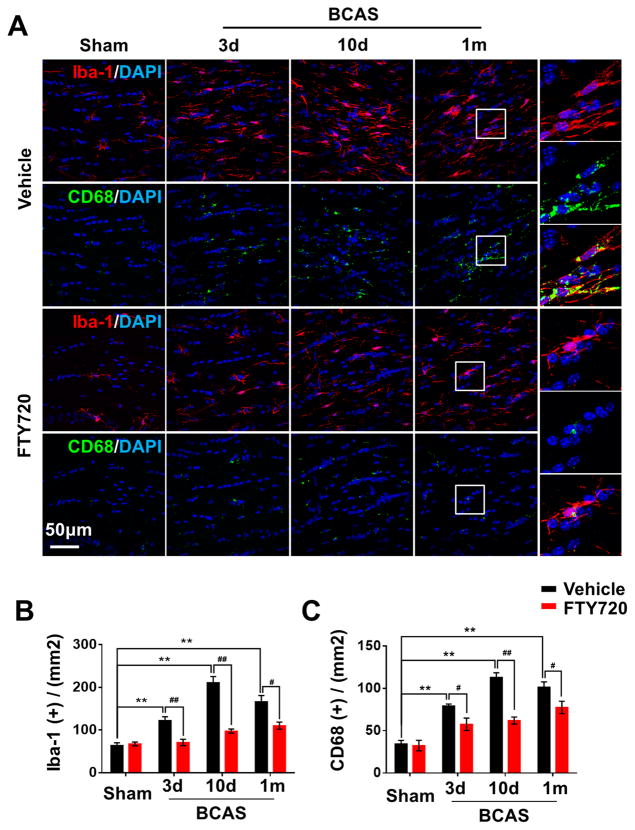
Microglial activation with enhanced release of inflammatory mediators is known to impair myelination in different models of WM injury2–4. Here, we found the number of cells positive for the general marker Iba-1 and the phagocytosis marker CD68 were remarkably elevated in BCAS mice, reached the peak on the 10th day after BCAS and then sustained the status of highly activated (Figure 3). Microglia were significantly activated after prolonged hypoperfusion in the higher magnification, characterized by hypertrophic morphology, with thickened and retracted processes. In contrast, FTY720-treated mice showed fewer Iba-1+ or CD68+ microglia displaying smaller cell bodies and thinner processes after hypoperfusion (Figure 3), indicating that FTY720 may attenuate neuroinflammation by suppressing microglial activation.
Figure 3. Activation of microglia due to hypoperfusion injury was retained by FTY720 treatment.
(A) Representative confocal images of coronal sections labeled with Iba-1 and CD68 at different time points post BCAS. Scale bar, 50μm. (B, C) Quantitative analysis of Iba-1+ and CD68+ cells was shown in the histogram. **P<0.01 versus Sham, #P<0.05 ##P<0.01 versus Vehicle. n=6 per group.
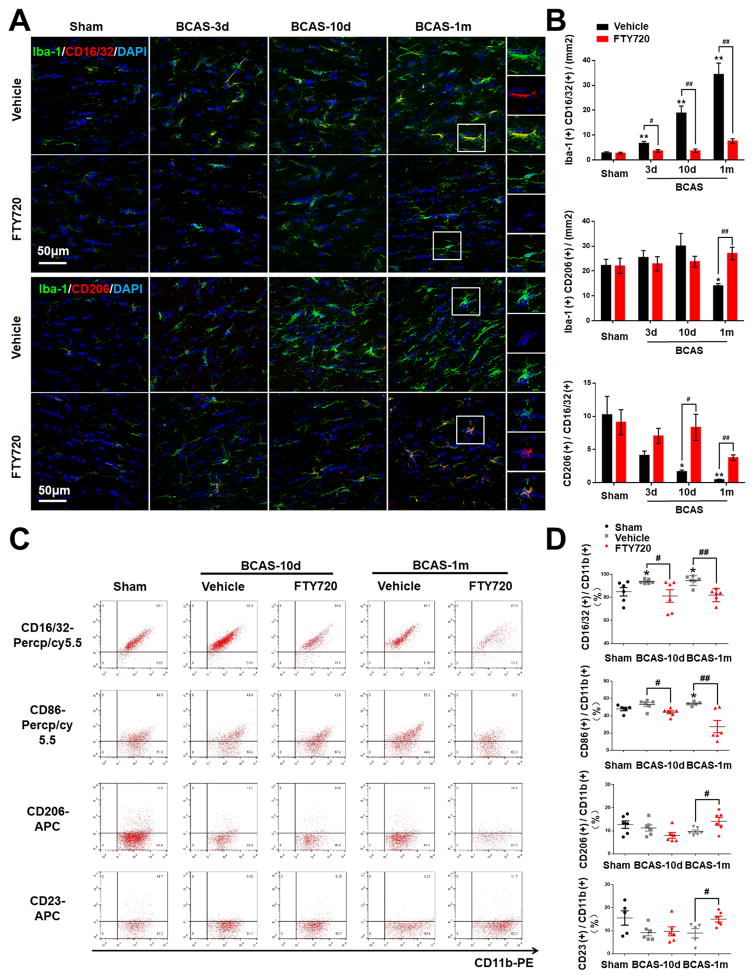
Activated microglia exist along a continuum of two functional states of polarization namely M1-type (classical/pro-inflammatory activation) and M2-type (alternative/anti-inflammatory activation). Microglia with M1 phenotype are characterized by upregulation of CD16 Fc receptors, CD32, CD86, interleukin (IL)-1β, IL-23, tumor necrosis factor (TNF)-α, and inducible nitric oxide synthase (iNOS), whereas microglia of M2 phenotype display the upregulation of arginase (Arg)-1, mannose receptor (CD206), IL-10, transforming-like growth factor (TGF)-β, and chitinase 3-like 3 (Ym-1). We explored the phenotype of microglia in WM ischemic injury by double labeling Iba-1 with M1 marker CD16/32 and M2 marker CD206. With prolonged hypoperfusion, the number of CD16/32+ Iba-1+ cells (M1) was substantially increased while CD206+ Iba-1+ cells (M2) were significantly decreased compared to sham-operated mice (Figure 4A and 4B). It was interesting that the number of M2 type cells remained almost unchanged in FTY720-treated mice, whereas the M2:M1 ratio was significantly upregulated in FTY720-treated mice compared to vehicle-treated group (Figure 4A and 4B), indicating that FTY720 switched microglia polarization from M1 to M2 type in WM ischemic injury. To further distinguish this differential polarization from simply suppressing microglia, CD11b-high microglia were isolated from WM 10 days or 1 month after surgery and analyzed by FACS. The populations of cells positive for M1 markers CD16/32 or CD86 in microglia were significantly increased while CD206 or CD23 positive cells in microglia slightly decreased as compared to sham-operated control (Figure 4C and 4D). After FTY720 treatment, this imbalance of microglia polarization was diminished, as CD16/32+ or CD86+ M1 cells ameliorated and CD206+ or CD23+ M2 cells elevated (Figure 4C and 4D). Together, our results consistently indicated that FTY720 not just prevented the activation of microglia subsequent to BCAS, but also intensified its protective effects through modulating microglia polarization toward M2 type.
Figure 4. Hypoperfusion induced microglia M1 polarization was partially retained by FTY720 treatment.
(A) Representative confocal images of coronal sections labeled with Iba-1, CD16/32 and CD206 at different time points post BCAS. Scale bar, 50μm. (B) Quantitative analysis of Iba-1, CD16/32 double positive cells and Iba-1, CD206 double positive cells was shown in the histogram. Ratio of CD206 positive cells to CD16/32 positive cells was performed. *P<0.05 **P<0.01 versus Sham, #P<0.05 ##P<0.01 versus Vehicle. n=6 per group.
(C) FACS analysis of microglia in WM after BCAS. Dot plots represent CD16/32 staining (upper panel), CD86 staining (second panel), CD206 staining (third panel), CD23 staining (lower panel) of CD11b positive microglia. (D) Percentage of CD16/32/CD86/CD206/CD23 positive cells in total CD11b positive microglia. *P<0.05 versus Sham, #P<0.05 ##P<0.01 versus Vehicle. n=5–6 mice for each group.
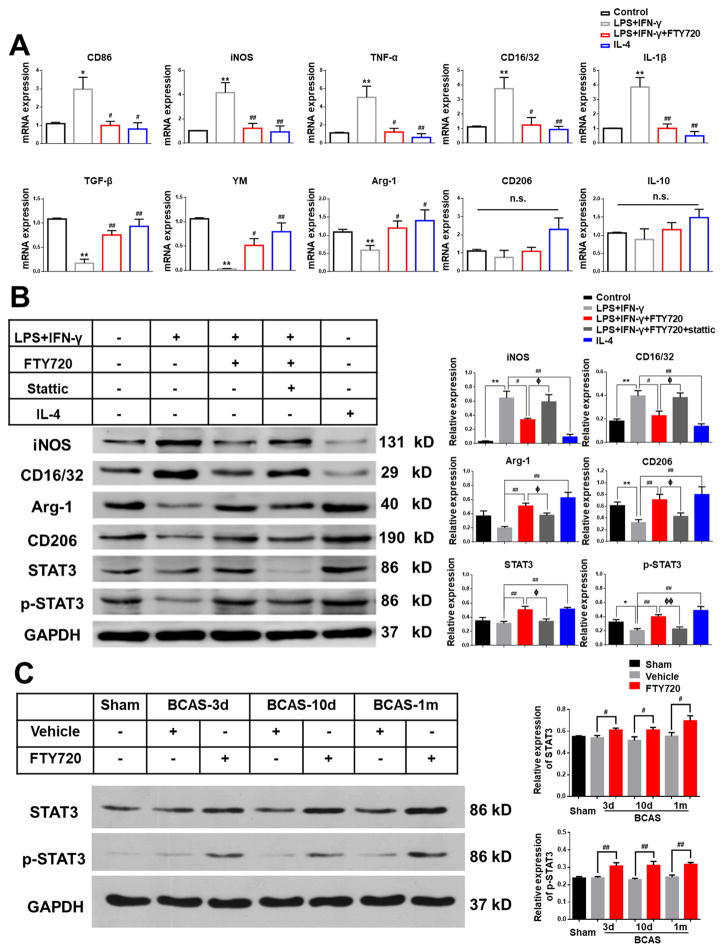
To further investigate whether FTY720 regulated microglial polarization directly, we used primary cultured microglia, induced M1/M2 polarization by LPS plus IFN-γ or IL-4 stimulation in vitro, and then measured the expression of various M1/M2 markers under different treatment. Quantitative real-time PCR revealed that M1 markers (CD86, TNF-α, iNOS, CD32 and IL-1β) were significantly increased by LPS plus IFN-γ stimulation and M2 markers (TGF-β, arginase1 and YM-1) were greatly suppressed, although several M2 markers (CD206 and IL-10) decreased without significance (Figure 5A). These alterations in M1 and M2 markers were significantly attenuated by FTY720 treatment (Figure 5A). Consistently, Western blot results showed similar alterations at protein level as M1 markers (iNOS and CD16/32) increased and M2 markers (arginase-1 and CD206) decreased by LPS plus IFN-γ stimulation (Figure 5B). FTY720 treatment attenuated these alterations significantly, indicating its direct modulation in microglial polarization (Figure 5B).
Figure 5. FTY720 modulated microglia toward M2 polarization via STAT3 pathway.
(A) Primary microglia were exposed to vehicle as Control, LPS plus IFN-γ stimulation, LPS plus IFN-γ stimulation and FTY720 treatment, and IL-4 stimulation. The mRNA expression of pro-inflammatory markers (CD86, TNF-α, iNOS, CD32 and IL-1β) and anti-inflammatory markers (TGF-β, YM, Arg-1, CD206 and IL-10) in response to different stimulation and treatment was detected by RT-PCR. n.s. no significant changes between different groups. *P<0.05 **P<0.01 versus Control, #P<0.05 ##P<0.01 versus LPS plus IFN-γ stimulation. n=6 per group.
(B) The protein expression of M1 markers (iNOS, CD16/32), M2 markers (Arg-1, CD206), STAT3 and p-STAT3 in primary microglia were detected by western-blots. Quantitative analysis was performed. **P<0.01 versus Control, #P<0.05 ##P<0.01 versus LPS plus IFN-γ stimulation, ΦP<0.05 ΦΦP<0.01 versus LPS plus IFN-γ stimulation and FTY720 treatment. n=6 per group.
(C) Total protein expression and phosphorylation level of STAT3 were detected by western-blots in mice from Sham, Vehicle and FTY720 groups at different time points after BCAS. Quantitative analysis was performed. #P<0.05 ##P<0.01 versus Vehicle. n=8 per group.
FTY720 modulated oligogenesis and OPCs maturation via M1 to M2 switch of microglia
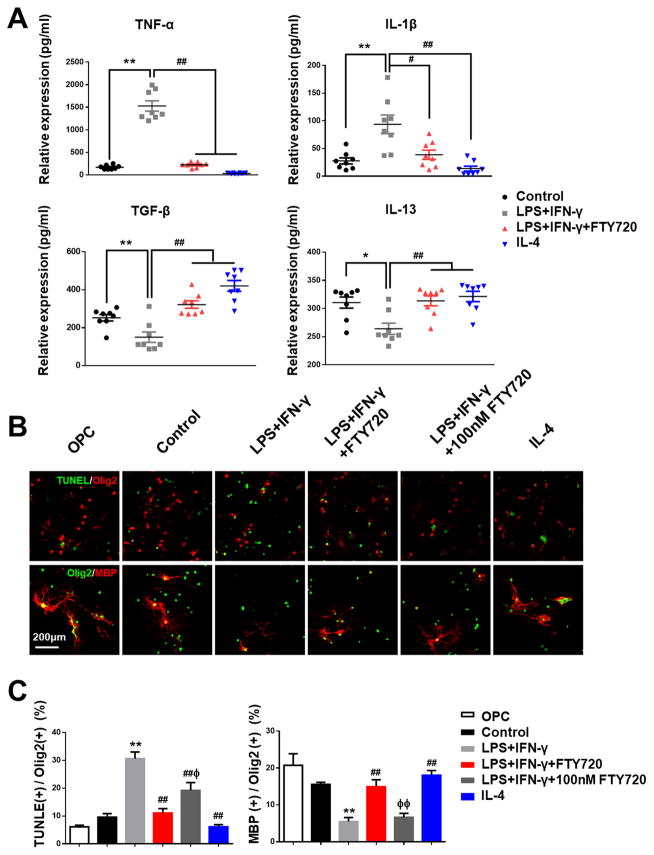
The above findings raised the questions about the possible relevance of microglial polarization with oligogenesis and myelination after WM ischemic injury. To directly address this question, we sought to determine the response of OPCs using microglia conditioned medium (CM). Firstly, cytokines IL-1β, IL-13, TNF-α and TGF-β in CM by different stimulation and treatment were analyzed by ELISA. LPS plus IFN-γ stimulation profoundly increased the levels of pro-inflammatory proteins (IL-1β and TNF-α), and downregulated anti-inflammatory proteins (IL-13 and TGF-β) (Figure 6A). FTY720 treatment attenuated these alterations (Figure 6A), which was consistent with its protective effect on microglial polarization. Next, primary cultured OPCs were incubated with CM from the different treatment groups for 3 days. TUNEL assay showed that inflammatory stimulus led to OPCs apoptosis with increased TUNEL+ Olig2+ cells, which was significantly reversed in FTY720-treated CM (Figure 6B and 6C). In addition to the increase of oligodendrocyte survival, FTY720 restored oligodendrocyte differentiation as demonstrated by an increased number of MBP+ differentiated oligodendrocytes in FTY720-treated CM (Figure 6B and 6C). In order to exclude the possibility of FTY720’s direct effect on OPCs, we set a separate group as LPS plus IFN-γ stimulated CM added with 100nM FTY720. Quantification of TUNEL+ OPCs showed that FTY720’s direct protection on OPCs reduced a few apoptotic cells, but not as good as FTY720-treated CM did (Figure 6B and 6C). And myelinating oligodendrocytes also revealed no significant differences with additional 100nM FTY720 in CM (Figure 6B and 6C), suggesting that the protective effect of FTY720 was likely attributed to its modulation on microglia polarization instead of the direct effect on OPCs.
Figure 6. FTY720 modulated OPCs apoptosis and maturation via M1 to M2 switch of microglia.
(A) Microglia supernatants under different stimulation and treatment were analyzed by ELISA. Quantitative analysis of expression level of cytokines TNF-α, IL-1β, TGF-β and IL-13 was performed. **P<0.01 *P<0.05 versus Control, #P<0.05 ##P<0.01 versus LPS plus IFN-γ stimulation. n=8 per group.
(B) Representative images of oligodendrocytes labeled with Olig2 and TUNEL (upper label), Olig2 and MBP (lower label) in response to different stimulation and treatment. Scale bar, 200 μm. (C) Quantitative analysis was performed as percentage of TUNEL+ and MBP+ in Olig2+ cells. **P<0.01 versus Control, ##P<0.01 versus LPS plus IFN-γ stimulation, ΦΦP<0.01 ΦP<0.05 versus LPS plus IFN-γ stimulation and FTY720 treatment. n=8 per group.
FTY720 facilitated M1 to M2 switch of microglia via STAT3 pathway
To further elucidate which signaling pathways were involved, we performed quantitative real-time PCR to screen various related markers (IRF-3, IRF-4, MSX3, PPARG, STAT3, STAT6, JAK1, JAK2 and JAK3) that have been previously reported17******. Compared with LPS plus IFN-γ stimulation, IL-4 incubation induced enhanced activation of PPAR-γ, STAT3, STAT6 and JAK3 (Figure III). Treatment of FTY720 upregulated the mRNA levels of STAT3, which was increased over two folds compared to LPS plus IFN-γ group (Figure III). Western blot further confirmed that the total protein expression and phosphorylation level of STAT3 were enhanced with FTY720 treatment, both in vivo and in vitro (Figure 5B, C). To seek deeper insight into the underlying role of STAT3 pathway in this situation, Stattic, reported previously to highly and selectively block STAT3 pathway18, was chosen to inhibit activation of STAT3. We found that FTY720’s effect on microglia M2 polarization was diminished after STAT3 activity was inhibited by Stattic (10 nM) (Figure 5B). Therefore, these results indicated that FTY720 was likely to shift microglia from M1 to M2 polarization state via STAT3 activation.
Discussion
Demyelination profoundly compromise the communication between brain regions and is thought to underlie long-term cognitive deficits in CNS disorders such as stroke and MS6. The present study showed that FTY720 significantly attenuated myelin loss, protected nodal structures, promoted OPCs proliferation and maturation and finally facilitated myelin-axon integrity in the WM following BCAS, paralleled by an improvement in working memory performance. The underlying mechanisms for neuroprotection by FTY720 were probably attributed to STAT3-mediated modulation of microglia toward M2 polarization. To the best of our knowledge, this study is the first to indicate a protective effect and the underlining mechanism of FTY720 in hypoperfusion-induced WM injury.
Previous studies suggested benefits of FTY720 in MS model through immunosuppression and direct protection on OPCs via S1P signaling19. FTY720 was also reported to reduce oxidative stress, microglia activation and associated pro-inflammatory cytokine expression in CNS injury12, 20. To further illustrate whether FTY720 exerts its protective effects on demyelination directly or not, we separately treated OPCs with CM from activated microglia. FTY720 significantly mitigated inflammation-induced OPCs apoptosis and promoted oligodendrocyte differentiation, but this phenomenon could not be duplicated by simply adding FTY720 to OPCs CM. In vivo, FTY720 could largely attenuate microglial activation, down-regulate the production of pro-inflammatory cytokines and promote OPCs proliferation and differentiation after BCAS. Our findings strongly suggested that FTY720’s efficacy on WM ischemic injury was likely attributed to modulation of OPCs survival microenviroment mediated by microglial inflammatory/anti-inflammatory balance state.
It has been demonstrated that the neuroinflammation response can contribute to the pathogenesis of ischemic stroke2, 4, 8, 21. M2 type microglia have been reported to be an essential part of the regenerative response in CNS demyelinating diseases by promoting oligodendrocyte differentiation22. We observed that prolonged cerebral hypoperfusion significantly induced microglia activation in preferentially M1 polarization state following BCAS. A switch from M1 to M2 polarization was stably detected after FTY720 treatment both in vivo and in vitro. Our in vitro data further illustrated that FTY720 could significantly ameliorate inflammatory microenvironment and promote repair of the injured oligodendrocytes. Therefore, these results further determined that FTY720’s direct modulation on microglia polarization, rather than general immunosuppressive effects, would be an important pathway involved in its efficacy on WM injury.
To date, how FTY720 modulates microglia toward M2 polarization remains uncharacterized. We screened several possible signaling pathways crucial for microglial M1/M2 polarization and found the obvious activation of STAT3 pathway in FTY720-treatment group. Our results demonstrated that FTY720 significantly increased both the expression and phosphorylation level of STAT3 compared to vehicle group. Consistently, previous in vitro data suggested that S1P could activate JNK/STAT3 signaling cascades23, which was associated with M2 polarization17. In addition, FTY720-induced the anti-inflammatory effects and polarization toward M2 type microglia were abolished by the STAT3 inhibitor, Stattic. The effect of Stattic towards STAT family members was reported to be temperature-dependent and highly STAT3-selective at 37°C, shown to inhibit cellular phosphorylation of STAT3 with little effect towards STAT1 phosphorylation or the phosphorylation of JAK1, JAK2, and c-Src18. Inspite of the possible lack of selectivity of FTY720 and Stattic, these results further illustrated that FTY720 manipulated microglia polarization after WM ischemic injury via STAT3 pathways.
There were several limitations of our study which may preclude the generalizability of our findings in human WM diseases. The murine BCAS model utilized in this study replicates some of the key features of ischemic WM injury, including WM pathology and cognitive decline, whereas other aspects of the disease, such as an underlying vascular pathology triggered by hypertension or diabetes and variability of age and gender, are lacking. Nonetheless, the post-mortem brain pathology of WM disease patients shares a number of features with the anatomic pathology noted in post-BCAS mice, including both microglia activation as well as frank demyelination24, 25. Therefore, our data suggested that FTY720 might exhibit a potential of clinical application in WM ischemic injury. Though we intended to identify the specific mechanism of FTY720’s protection on WM, the diverse subtypes of S1P receptors expressed on peripheral immune cells and brain-intrinsic cells preclude a better understanding of targeted cell types responsible for the mechanisms of FTY72019. Recent works in human cerebral ischemia have demonstrated that FTY720 restrains lymphocytes within the lymphoid organs resulting in reduced circulating lymphocyte counts8, 26. However, in neonatal hyperoxia it has been proved that brain lymphocyte counts were modulated neither by FTY720 nor by hyperoxia12. In the current study, we cannot exclude the possibility of peripheral immune cells and various brain-intrinsic cells involvement in protective effect of FTY720 following chronic cerebral hypoperfusion. In addition, we could not rule out whether the drug has direct effects on spatial learning and memory. Further pharmacological investigation of FTY720 on cognitive function is needed in the future. Nevertheless, our results demonstrate that FTY720 directly modulated microglia/macrophages-mediated immune-inflammatory state toward better microenvironment facilitated for myelin repair and regeneration in WM ischemic injury.
In conclusion, FTY720 has shown its neuroprotective effect in MS and acute stroke 4. The present study further proved its potential efficacy in preventing WM ischemic injury induced by chronic hypoperfusion model. Accordingly, we believe that the strategy of FTY720 treatment in patients with chronic cerebral hypoperfusion is promising and worth further evaluation.
Supplementary Material
Related Manuscript File
Visual Abstract
online supplement
Acknowledgments
We thank Peter J. Brophy (University of Edinburgh, Centre for Neuroregeneration, Edinburgh, United Kingdom) for kindly providing the antibody against panNeurofascin and technical assistance.
Sources of Funding
This work was supported by National Natural Science Foundation of China (81571132, 81171157 to D.S. Tian), the Fundamental Research Funds for the Central Universities (2017KFYXJJ107 to D.S. Tian, 2017KFYXJJ124 to C. Qin), Science Foundation of Tongji Hospital (2016A005 to C. Qin) and National Institutes of Health (R01NS088627 to L.J. Wu).
Footnotes
Disclosures
The authors declare that they have no conflict of interest.
References
- 1.Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, et al. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verden D, Macklin WB. Neuroprotection by central nervous system remyelination: Molecular, cellular, and functional considerations. Journal of neuroscience research. 2016;94:1411–1420. doi: 10.1002/jnr.23923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi BR, Kim DH, Back DB, Kang CH, Moon WJ, Han JS, et al. Characterization of white matter injury in a rat model of chronic cerebral hypoperfusion. Stroke. 2016;47:542–547. doi: 10.1161/STROKEAHA.115.011679. [DOI] [PubMed] [Google Scholar]
- 4.Fu Y, Liu Q, Anrather J, Shi FD. Immune interventions in stroke. Nature reviews. Neurology. 2015;11:524–535. doi: 10.1038/nrneurol.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 6.Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, et al. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochimica et biophysica acta. 2016;1862:461–471. doi: 10.1016/j.bbadis.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (fty720): Discovery and development of an oral drug to treat multiple sclerosis. Nature reviews Drug discovery. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: A pilot trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, et al. Fingolimod for the treatment of intracerebral hemorrhage: A 2-arm proof-of-concept study. JAMA neurology. 2014;71:1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 10.Shibata M, Yamasaki N, Miyakawa T, Kalaria RN, Fujita Y, Ohtani R, et al. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38:2826–2832. doi: 10.1161/STROKEAHA.107.490151. [DOI] [PubMed] [Google Scholar]
- 11.Fu P, Tang R, Yu Z, Huang S, Xie M, Luo X, et al. Bumetanide-induced nkcc1 inhibition attenuates oxygen-glucose deprivation-induced decrease in proliferative activity and cell cycle progression arrest in cultured opcs via p-38 mapks. Brain research. 2015;1613:110–119. doi: 10.1016/j.brainres.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Serdar M, Herz J, Kempe K, Lumpe K, Reinboth BS, Sizonenko SV, et al. Fingolimod protects against neonatal white matter damage and long-term cognitive deficits caused by hyperoxia. Brain, behavior, and immunity. 2016;52:106–119. doi: 10.1016/j.bbi.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, et al. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive cns inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:2012–2017. doi: 10.1073/pnas.1615413114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanc CA, Rosen H, Lane TE. Fty720 (fingolimod) modulates the severity of viral-induced encephalomyelitis and demyelination. Journal of neuroinflammation. 2014;11:138. doi: 10.1186/s12974-014-0138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cipriani R, Chara JC, Rodriguez-Antiguedad A, Matute C. Fty720 attenuates excitotoxicity and neuroinflammation. Journal of neuroinflammation. 2015;12:86. doi: 10.1186/s12974-015-0308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reimer MM, McQueen J, Searcy L, Scullion G, Zonta B, Desmazieres A, et al. Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:18185–18194. doi: 10.1523/JNEUROSCI.4936-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jha MK, Lee WH, Suk K. Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochemical pharmacology. 2016;103:1–16. doi: 10.1016/j.bcp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: A small-molecule inhibitor of stat3 activation and dimerization. Chemistry & biology. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Hunter SF, Bowen JD, Reder AT. The direct effects of fingolimod in the central nervous system: Implications for relapsing multiple sclerosis. CNS drugs. 2016;30:135–147. doi: 10.1007/s40263-015-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. Journal of neuroimmunology. 2013;256:13–18. doi: 10.1016/j.jneuroim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Kraft P, Gob E, Schuhmann MK, Gobel K, Deppermann C, Thielmann I, et al. Fty720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44:3202–3210. doi: 10.1161/STROKEAHA.113.002880. [DOI] [PubMed] [Google Scholar]
- 22.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during cns remyelination. Nature neuroscience. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garris CS, Wu L, Acharya S, Arac A, Blaho VA, Huang Y, et al. Defective sphingosine 1-phosphate receptor 1 (s1p1) phosphorylation exacerbates th17-mediated autoimmune neuroinflammation. Nature immunology. 2013;14:1166–1172. doi: 10.1038/ni.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Liu G, Hong D, Chen F, Ji X, Cao G. White matter injury in ischemic stroke. Progress in neurobiology. 2016;141:45–60. doi: 10.1016/j.pneurobio.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18315–18320. doi: 10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Related Manuscript File
Visual Abstract
online supplement