Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation (original) (raw)
. Author manuscript; available in PMC: 2018 Feb 3.
Published in final edited form as: N Engl J Med. 2017 Jun 23;377(5):454–464. doi: 10.1056/NEJMoa1614359
Abstract
Background
Patients with acute myeloid leukemia (AML) and a FLT3 mutation have poor outcomes. We conducted a phase 3 trial to determine whether the addition of midostaurin — an oral multitargeted kinase inhibitor that is active in patients with a FLT3 mutation — to standard chemotherapy would prolong overall survival in this population.
Methods
We screened 3277 patients, 18 to 59 years of age, who had newly diagnosed AML for FLT3 mutations. Patients were randomly assigned to receive standard chemotherapy (induction therapy with daunorubicin and cytarabine and consolidation therapy with high-dose cytarabine) plus either midostaurin or placebo; those who were in remission after consolidation therapy entered a maintenance phase in which they received either midostaurin or placebo. Randomization was stratified according to subtype of FLT3 mutation: point mutation in the tyrosine kinase domain (TKD) or internal tandem duplication (ITD) mutation with either a high ratio (>0.7) or a low ratio (0.05 to 0.7) of mutant to wild-type alleles (ITD [high] and ITD [low], respectively). Allogeneic transplantation was allowed. The primary end point was overall survival.
Results
A total of 717 patients underwent randomization; 360 were assigned to the midostaurin group, and 357 to the placebo group. The FLT3 subtype was ITD (high) in 214 patients, ITD (low) in 341 patients, and TKD in 162 patients. The treatment groups were well balanced with respect to age, race, FLT3 subtype, cytogenetic risk, and blood counts but not with respect to sex (51.7% in the midostaurin group vs. 59.4% in the placebo group were women, P = 0.04). Overall survival was significantly longer in the midostaurin group than in the placebo group (hazard ratio for death, 0.78; one-sided P = 0.009), as was event-free survival (hazard ratio for event or death, 0.78; one-sided P = 0.002). In both the primary analysis and an analysis in which data for patients who underwent transplantation were censored, the benefit of midostaurin was consistent across all FLT3 subtypes. The rate of severe adverse events was similar in the two groups.
Conclusions
The addition of the multitargeted kinase inhibitor midostaurin to standard chemotherapy significantly prolonged overall and event-free survival among patients with AML and a FLT3 mutation. (Funded by the National Cancer Institute and Novartis; ClinicalTrials.gov number, NCT00651261.)
Acute myeloid leukemia (AML) is a heterogeneous disease that remains challenging to treat because of patient factors (age and coexisting diseases) and intrinsic biologic factors.1 Cytogenetic2 and mutational3 data are used to divide patients into subgroups defined according to prognostic factors4-7 and factors that dictate whether allogeneic hematopoietic stem-cell transplantation should be performed during an initial remission.8 Mutations in the fms-related tyrosine kinase 3 gene (FLT3) are present in 30% of adults with newly diagnosed AML.9 Approximately three quarters of these patients have a FLT3 internal tandem duplication mutation (ITD subtype), which results in duplication of between 3 and more than 100 amino acids located in the juxtamembrane region; ITD mutations are associated with a poor prognosis owing to a high relapse rate,10 particularly when there is a high ratio of mutant to wild-type FLT3 alleles.11,12 Approximately 8% of patients with newly diagnosed AML have a FLT3 point mutation in the tyrosine kinase domain (TKD subtype); the effect of TKD mutations on prognosis is uncertain.13,14 Both subtypes of FLT3 mutation yield proteins that spontaneously dimerize (bypassing ligand-mediated activation), cause factor-independent growth when they are transfected into murine cell lines, and lead to a fatal myeloproliferative neoplasm in a mouse model.15,16 Small-molecule inhibitors of activated FLT3 specifically inhibit proliferation of leukemia cells in preclinical models. However, clinical trials investigating the use of single-agent, first-generation FLT3 inhibitors in patients with relapsed–refractory AML and a FLT3 mutation showed transient reductions in the number of blasts in blood, marrow, or both but rarely showed complete remission.17,18 More specific FLT3 inhibitors, such as quizartinib19 and gilteritinib,20 yielded higher response rates than the first-generation inhibitors among patients with advanced disease.
Midostaurin, a multitargeted kinase inhibitor, was originally developed as a protein kinase C inhibitor for treatment of patients with solid tumors.21 On the basis of preclinical studies, which showed synergy between chemotherapy and midostaurin, a phase 1b study involving patients with newly diagnosed AML was conducted; the study established that oral midostaurin could be administered safely (with an acceptable side-effect profile) at a dose of 50 mg twice daily for 14 days, beginning on the eighth day after the start of treatment during courses of induction and consolidation chemotherapy, and that this regimen had encouraging efficacy in patients with a FLT3 mutation.22
To determine the effect of the addition of midostaurin to standard chemotherapy in patients with AML and a FLT3 mutation, we conducted the Cancer and Leukemia Group B (CALGB) 10603 (RATIFY) trial, a multi-institutional, multinational, randomized, double-blind, placebo-controlled trial. The CALGB is now part of the Alliance for Clinical Trials in Oncology.
Methods
Patients
Patients 18 to 59 years of age who had newly diagnosed AML and had not previously received antineoplastic therapy (except for limited urgent treatment for the current disease) were screened for FLT3 mutations. The patients provided written informed consent that allowed preregistration, and then a diagnostic bone marrow sample was obtained and submitted to one of nine academic laboratories for testing for FLT3 mutations.
Patients were registered in the trial if they had a FLT3 mutation and met the following other eligibility criteria: a diagnosis of AML (excluding acute promyelocytic leukemia) that was not therapy-related, a bilirubin level of less than 2.5 times the upper limit of the normal range, and the absence of other major coexisting illnesses. Hydroxyurea therapy was allowed for 5 days before the start of the trial therapy.
Screening for FLT3 Mutations
The presence of a FLT3 mutation of either the TKD subtype or the ITD subtype was reported to investigators within 48 hours after the sample was received in the laboratory. A ratio of mutant to wild-type alleles of at least 0.05 indicated that the patient was positive for FLT3. (For further details about assay validation and performance, see the Supplementary Appendix, available with the full text of this article at NEJM.org.)
Randomization and Treatments
Enrolled patients were randomly assigned, in a 1:1 ratio, to receive standard chemotherapy plus either midostaurin or placebo. Randomization was performed with a block size of 6 and was stratified according to the subtype of FLT3 mutation: TKD, or ITD with either a high ratio (>0.7) or a low ratio (0.05 to 0.7) of mutant to wild-type alleles (ITD [high] and ITD [low], respectively).
Therapy consisted of induction therapy with daunorubicin (at a dose of 60 mg per square meter of body-surface area per day, administered by rapid intravenous injection on days 1, 2, and 3) and cytarabine (at a dose of 200 mg per square meter, administered by continuous intravenous infusion on days 1 through 7). Midostaurin or placebo was administered in a double-blind fashion, at a dose of 50 mg orally twice daily, on days 8 through 21. Midostaurin or placebo was not administered if the patient had a corrected QT interval above 500 msec or a grade 3 or 4 non-hematologic toxic effect (for further details, see the Supplementary Appendix). A missed dose of midostaurin or placebo was not made up. A bone marrow examination was to be performed on day 21. If there was definitive evidence of clinically significant residual leukemia, a second cycle of induction therapy that was identical to the first, including midostaurin or placebo, was administered.
Patients who achieved complete remission after induction therapy received four 28-day cycles of consolidation therapy with high-dose cytarabine (at a dose of 3000 mg per square meter, administered over a period of 3 hours every 12 hours on days 1, 3, and 5). Midostaurin or placebo was administered at a dose of 50 mg orally twice daily on days 8 through 21. Patients who remained in remission after completion of consolidation therapy entered a maintenance phase in which they received midostaurin or placebo, administered at a dose of 50 mg orally twice daily, for twelve 28-day cycles. Complete remission was defined as the presence of less than 5% blasts in the marrow or extramedullary leukemia, an absolute neutrophil count of more than 1000 per microliter, a platelet count of more than 100,000 per microliter, and the absence of blasts in the peripheral blood; in addition, per protocol, the complete remission had to have occurred by day 60. Transplantation was not mandated in the protocol but was performed at the discretion of the investigator.
Trial Design and Oversight
The trial was conducted at 225 sites in 17 countries. The institutional review board at each participating center reviewed and approved the trial protocol, available at NEJM.org. The trial was conducted in accordance with the provisions of the Declaration of Helsinki.
The trial was funded at North American sites by the Cancer Therapy Evaluation Program of the National Cancer Institute and at non–North American sites (i.e., sites in Europe and Australia) by Novartis. The trial was designed by the CALGB and approved by the Cancer Therapy Evaluation Program and Novartis. The data were gathered by the investigators, and case-report forms were sent to the Alliance Statistics and Data Center for data analysis. Novartis performed monitoring of data at the non–North American sites and performed monitoring of data in a limited fashion at the North American sites; the CALGB performed audits at the North American sites (not specifically for this trial). Data collection and monitoring procedures are fully described in the Supplementary Appendix. The investigators had full access to the data. The authors vouch for the accuracy and completeness of the data and the fidelity of the study to the protocol. The principal investigator reviewed complete case-report forms for 250 patients, in accordance with CALGB policy, and wrote the manuscript without assistance from nonauthors. All the authors made the decision to submit the manuscript for publication.
Statistical Analysis
The primary end point was overall survival, which was defined as the time from randomization to death from any cause. The original enrollment goal was 514 patients with 374 events; however, the trial was expanded to 714 patients in 2010, after the proportion of patients who had undergone allogeneic hematopoietic stem-cell transplantation was higher than expected (anticipated rate of transplantation, 15%; rate observed at the time of amendment, 25%) and the proportion of patients who had a FLT3 mutation of the TKD subtype was also higher than expected (anticipated rate of TKD mutation, 14%; rate observed at the time of amendment, 26%). Assuming a hazard ratio for death (midostaurin vs. placebo) of 0.71 among patients who did not undergo transplantation and 1.0 among patients who underwent transplantation, we expected that the median overall survival would be 20.9 months in the midostaurin group and 16.3 months in the placebo group, corresponding to an overall hazard ratio for death of 0.78. We estimated that a total sample size of 714 patients with an expected 509 deaths (with a constant enrollment rate and a follow-up of 19 months after the end of enrollment) would give the trial 84% power, at a onesided significance level of 0.025 by a stratified log-rank test, to detect a hazard ratio for death of 0.78. One planned interim efficacy analysis was to be performed after 50% of the events (255) had occurred; this analysis took place in May 2012, and the Alliance data and safety monitoring board made the decision to continue the trial. We anticipated that 396 of the 509 deaths would occur in patients who did not undergo transplantation; thus we estimated that an overall survival analysis in which data for patients who underwent transplantation were censored would have 88% power to detect a hazard ratio for death of 0.71. P values for the primary efficacy analyses of overall and event-free survival are one-sided, in accordance with the trial design. Event-free survival was defined as the time from randomization to relapse, death from any cause, or failure to achieve protocol-specified complete remission. Disease-free survival was defined as the time from protocol-specified complete remission to relapse or death from any cause. P values for all secondary analyses are two-sided. Information about the secondary end points, as well as the complete statistical analysis plan and the amended plan to account for a lower-than-expected event rate, are provided in the Supplementary Appendix.
Results
Enrollment and Patient Characteristics
Data for this analysis were locked as of March 7, 2016. From May 2008 through October 2011, a total of 3277 patients were preregistered for the trial. Of the 896 patients who had a FLT3 mutation, 717 were enrolled in the trial. The reasons that patients with AML and a FLT3 mutation were not enrolled in the trial were not addressed prospectively but probably included patient decision and rapid disease progression. The nonenrollment rate among patients who had a FLT3 mutation was similar across FLT3 subtypes, including the ITD (high) subtype. At the time of this analysis, no patients were receiving the trial treatment; the trial treatment was discontinued in the last patient in August 2013.
Patients had high white-cell counts (median, 34,900 per microliter), and 375 of the 547 patients for whom the results of a cytogenetic analysis were available (68.6%) had normal karyotypes. The percentage of patients who had a FLT3 mutation of the TKD subtype was 22.6%. Patient age, race, white-cell count, and European Leukemia-Net classification were well balanced between the two groups, but sex was not; 51.7% of the midostaurin group versus 59.4% of the placebo group were women (P = 0.04 by chi-square test) (Table 1).
Table 1.
Baseline Characteristics of the Patients.
| Characteristic | All Patients (N = 717) | Midostaurin Group (N = 360) | Placebo Group (N = 357) | P Value* |
|---|---|---|---|---|
| Age at trial entry — yr | 0.22 | |||
| Median | 47.9 | 47.1 | 48.6 | |
| Range | 18.0–60.9 | 19.0–59.8 | 18.0–60.9 | |
| Female sex — no. (%) | 398 (55.5) | 186 (51.7) | 212 (59.4) | 0.04 |
| Race — no./total no. (%)† | 0.74 | |||
| White | 275/309 (89.0) | 147/165 (89.1) | 128/144 (88.9) | |
| Other | 34/309 (11.0) | 18/165 (10.9) | 16/144 (11.1) | |
| Subtype of FLT3 mutation — no. (%)‡ | 1.00 | |||
| TKD | 162 (22.6) | 81 (22.5) | 81 (22.7) | |
| ITD with low allelic ratio | 341 (47.6) | 171 (47.5) | 170 (47.6) | |
| ITD with high allelic ratio | 214 (29.8) | 108 (30.0) | 106 (29.7) | |
| Modified European LeukemiaNet classification — no./total no. (%)§ | 0.15 | |||
| Favorable | 29/547 (5.3) | 16/269 (5.9) | 13/278 (4.7) | |
| Normal | 375/547 (68.6) | 172/269 (63.9) | 203/278 (73.0) | |
| Intermediate II | 104/547 (19.0) | 59/269 (21.9) | 45/278 (16.2) | |
| Adverse | 39/547 (7.1) | 22/269 (8.2) | 17/278 (6.1) | |
| White-cell count per _μ_l¶ | 0.72 | |||
| Median | 34,900 | 35,600 | 33,000 | |
| Range | 600–421,800 | 600–421,800 | 800–329,800 | |
| Platelet count per _μ_l‖ | 0.58 | |||
| Median | 50,000 | 50,000 | 50,000 | |
| Range | 2000–461,000 | 2000–461,000 | 8000–444,000 | |
| Absolute neutrophil count per mm3** | 0.65 | |||
| Median | 2.2 | 2.2 | 2.3 | |
| Range | 0–55.9 | 0–55.9 | 0–55.9 |
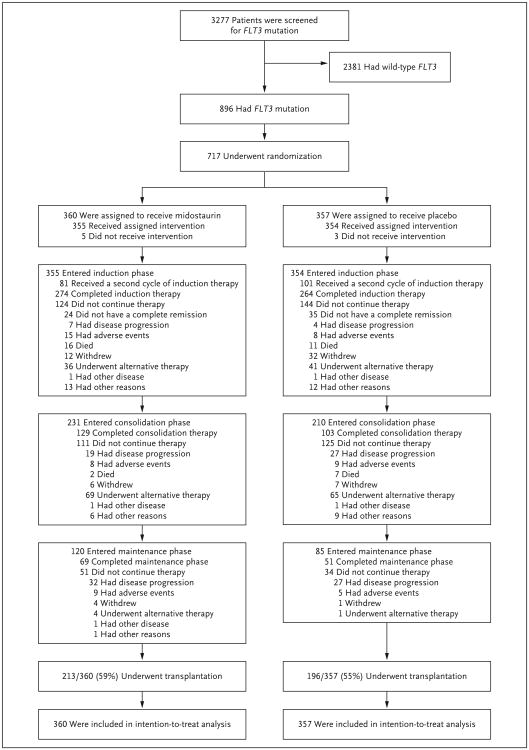
Figure 1 shows details regarding patient disposition throughout the trial. A second course of induction therapy was administered in 182 patients overall; the administration of a second course was more common among patients in the placebo group than among those in the midostaurin group (occurring in 101 and 81 patients, respectively). In 38 patients, trial treatment was discontinued immediately after a complete remission was achieved, for the following reasons: receipt of alternative therapy (in 11 patients), early disease progression (6), an adverse event (10), patient withdrawal from the trial (8), and other (3). Of these 38 patients, 29 underwent transplantation, including the 11 who received alternative therapy. A maintenance regimen was administered in more patients in the midostaurin group than in the placebo group (120 and 85 patients, respectively) and was administered for the full 12 cycles in 120 patients (69 in the midostaurin group, and 51 in the placebo group).
Figure 1. Screening, Randomization, and Treatment.
Four patients who had wild-type FLT3 at screening were registered in the trial, underwent randomization, and received the trial treatment because of a site error. In accordance with the rules for intention-to-treat analysis, these patients were included in all analyses.
Adverse Events
No unexpected adverse events were observed, although we noted adverse events that are typically associated with intensive chemotherapy for AML. Few significant differences were observed between the two treatment groups in the rates of adverse events of grade 3, 4, or 5 (combined) (Table 2). The rate of grade 3, 4, or 5 anemia was higher in the midostaurin group than in the placebo group (92.7% vs. 87.8%, P = 0.03), as was the rate of grade 3, 4, or 5 rash (14.1% vs. 7.6%, P = 0.008). The rate of nausea was higher in the placebo group than in the midostaurin group (9.6% vs. 5.6%, P = 0.05). Among patients who had a protocol-defined complete remission, the median time to recovery of the absolute neutro-phil count (to >500 per microliter) was 26 days (interquartile range, 24 to 30) in the midostaurin group and 26 days (interquartile range, 22 to 31) in the placebo group, and the median time to recovery of the platelet count (to >100,000 per microliter) was 21 days (interquartile range, 19 to 23) in the midostaurin group and 21 days (interquartile range, 19 to 24) in the placebo group.
Table 2.
Summary of Grade 3, 4, or 5 Adverse Events.
| Adverse Event | Midostaurin Group (N = 355) | Placebo Group (N = 354) | P Value* |
|---|---|---|---|
| no. of patients (%) | |||
| Hematologic | |||
| Thrombocytopenia | 346 (97) | 342 (97) | 0.52 |
| Neutropenia | 338 (95) | 339 (96) | 0.86 |
| Anemia | 329 (93) | 311 (88) | 0.03 |
| Leukopenia | 93 (26) | 105 (30) | 0.32 |
| Lymphopenia | 68 (19) | 78 (22) | 0.35 |
| Other blood or bone marrow event | 1 (<1) | 4 (1) | 0.22 |
| Bone marrow hypocellularity | 0 | 1 (<1) | 0.50 |
| Nonhematologic | |||
| Febrile neutropenia | 290 (82) | 292 (82) | 0.84 |
| Infection | 186 (52) | 178 (50) | 0.60 |
| Lymphopenia | 68 (19) | 78 (22) | 0.35 |
| Diarrhea | 56 (16) | 54 (15) | 0.92 |
| Hypokalemia | 49 (14) | 60 (17) | 0.25 |
| Pain | 47 (13) | 44 (12) | 0.82 |
| Increased alanine aminotransferase | 45 (13) | 33 (9) | 0.19 |
| Rash or desquamation | 50 (14) | 27 (8) | 0.008 |
| Fatigue | 32 (9) | 37 (10) | 0.53 |
| Pneumonitis or pulmonary infiltrates | 28 (8) | 29 (8) | 0.89 |
| Nausea | 20 (6) | 34 (10) | 0.05 |
| Hyponatremia | 31 (9) | 23 (6) | 0.32 |
| Hyperbilirubinemia | 25 (7) | 28 (8) | 0.67 |
| Mucositis or stomatitis | 22 (6) | 28 (8) | 0.38 |
| Hypophosphatemia | 19 (5) | 29 (8) | 0.14 |
| Hypocalcemia | 24 (7) | 21 (6) | 0.76 |
Efficacy Outcomes
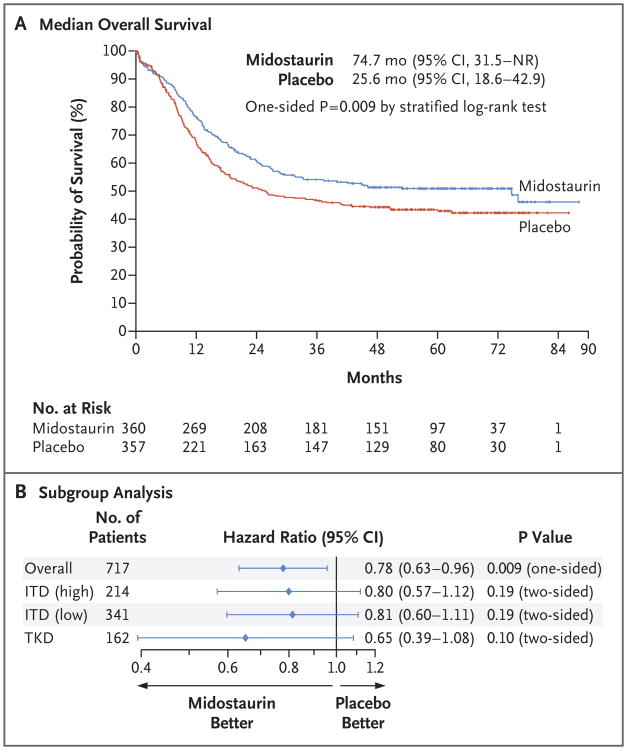
Among the 359 patients who survived, the median follow-up was 59 months. Median overall survival was 74.7 months (95% confidence interval [CI], 31.5 to not reached) in the midostaurin group and 25.6 months (95% CI, 18.6 to 42.9) in the placebo group (one-sided P = 0.009 by stratified log-rank test). The difference between groups in median overall survival may be large because of the inflection points on the Kaplan–Meier curves; however, the corresponding hazard ratio for death of 0.78 (95% CI, 0.63 to 0.96; one-sided P = 0.009 by stratified score test) more accurately reflects the magnitude of benefit (Fig. 2A). The 4-year overall survival rate was 51.4% in the midostaurin group and 44.3% in the placebo group. Analyses of subgroups according to FLT3 subtype showed that midostaurin had some benefit, but overall survival did not differ significantly according to trial regimen within each subgroup (Fig. 2B). The rate of complete remission was 58.9% (95% CI, 53.6 to 64.0) in the midostaurin group and 53.5% (95% CI, 48.2 to 58.8) in the placebo group (P = 0.15 by Fisher's exact test) (Table 3).
Figure 2. Overall Survival.
Panel A shows Kaplan–Meier curves for median overall survival in the midostaurin group and the placebo group. Tick marks indicate censoring of data. Panel B shows the between-group comparison of overall survival with stratification according to subtype of FLT3 mutation: point mutation in the tyrosine kinase domain (TKD) or internal tandem duplication (ITD) mutation with either a high ratio (>0.7) or a low ratio (0.05 to 0.7) of mutant to wild-type alleles (ITD [high] and ITD [low], respectively). NR denotes not reached.
Table 3.
Summary of Complete Remission.*
| Variable | Midostaurin Group (N = 360) | Placebo Group (N = 357) | P Value† |
|---|---|---|---|
| Protocol-specified complete remission — no. (%) | 212 (59) | 191 (54) | 0.15 |
| Kaplan–Meier estimate of time to complete remission — days | |||
| Median | 35 | 35 | |
| Range | 20–60 | 20–60 |
For the analysis of event-free survival, 536 events were observed: 298 failures to achieve protocol-specified complete remission, 181 relapses, and 57 deaths without relapse. Median event-free survival was 8.2 months (95% CI, 5.4 to 10.7) in the midostaurin group and 3.0 months (95% CI, 1.9 to 5.9) in the placebo group (one-sided P = 0.002 by stratified log-rank test). Patients assigned to the midostaurin group had a 21.6% lower likelihood of having an event than patients assigned to the placebo group (hazard ratio, 0.78; 95% CI, 0.66 to 0.93; one-sided P = 0.002 by stratified score test), with 4-year event-free survival rates of 28.2% in the midostaurin group and 20.6% in the placebo group. The benefit of midostaurin with respect to event-free survival was consistent across the FLT3 subtypes. Many patients were classified as having an early event because they did not achieve protocol-specified complete remission; 213 (29.7%) never achieved complete remission, and 101 (14.0%) achieved complete remission after more than 60 days of follow-up. Median disease-free survival was 26.7 months (95% CI, 19.4 to not reached) in the midostaurin group and 15.5 months (95% CI, 11.3 to 23.5) in the placebo group (P = 0.01 by stratified log-rank test), a difference that is due in part to a lower risk of relapse in the midostaurin group. (For more details, see Table S1 and Figs. S1 and S2 in the Supplementary Appendix.)
Transplantation was performed at some point during the disease course in 57.0% of the patients; it was performed during the first complete remission in 28.1% of the patients in the midostaurin group and in 22.7% in the placebo group (P = 0.10 by Fisher's exact test). At North American sites, transplantation was performed in 48.3% of the patients, whereas at non–North American sites, transplantation was performed in 61.3% of the patients (P = 0.001 by Fisher's exact test). A total of 101 patients in the midostaurin group and 81 patients in the placebo group underwent allogeneic transplantation during the first complete remission; median overall survival was not reached in either group, with a 95% confidence interval of 69.8 months to not reached in the midostaurin group and 21.8 months to not reached in the placebo group (P = 0.07 by log-rank test). In addition, among the 227 patients who underwent transplantation after the first complete remission, no treatment effect was observed (P = 0.85). Because allogeneic transplantation was an important alternative therapy, we performed a sensitivity analysis of the primary end point in which data were censored at the time patients underwent transplantation. In this analysis, there was a 24.3% lower risk of death in the midostaurin group than in the placebo group; the 4-year overall survival rate was 63.7% in the midostaurin group and 55.7% in the placebo group, but the difference between groups was not significant (P = 0.08 by log-rank test). (For further details, including results of post-hoc analyses, see Tables S2 and S3 and Figs. S3 and S4 in the Supplementary Appendix.)
Discussion
The CALGB 10603 (RATIFY) trial showed that among patients 18 to 59 years of age who had AML and a FLT3 mutation, the addition of midostaurin to chemotherapy resulted in a 22% lower risk of death than that among patients who received chemotherapy plus placebo. Although the trial was not powered for subgroup analyses, overall survival was longer in the midostaurin group than in the placebo group among patients with a FLT3 mutation of the TKD subtype and among those with a FLT3 mutation of the ITD subtype with either a high ratio or a low ratio of mutant to wild-type alleles. Since exposure to the FLT3 inhibitor was relatively brief (median duration of trial treatment, 3 months), it is probable that the major effect of the inhibitor was the early reduction of disease burden, although other potential explanations are possible. The trial was not designed to determine the independent effect of maintenance therapy. Given that there was a benefit among patients with an ITD mutation with a low allelic burden and that the disease in such patients might be largely due to mutations other than FLT3, it is possible that the benefit of midostaurin, which is a multitargeted kinase inhibitor, might lie beyond its ability to inhibit FLT3. For example, midostaurin is known to inhibit KIT and has activity as a single agent in patients with wild-type FLT323 and in patients with systemic mastocytosis.24 Treatment with another nonspecific FLT3 inhibitor, sorafenib, resulted in longer event-free survival (but not overall survival) when it was added to chemotherapy in unselected younger adults with AML.25
After trial enrollment began, an increasing number of investigators decided, on the basis of data from a retrospective series,8 that the best way to treat patients with a FLT3 mutation of the ITD subtype was to perform allogeneic transplantation during the first remission. This strategy, coupled with the knowledge that the only way to achieve long-term survival in a patient who had a relapse was to perform transplantation,26 probably led to the overall transplantation rate in this trial of 57%. Although rates of transplantation varied by region, transplantation was performed during the first remission in more patients in the midostaurin group than in the placebo group. The trial therapy was discontinued at the time of transplantation. Thus, an early transplantation could have limited exposure to midostaurin and thus limited its effect. However, in an analysis of the primary end point (overall survival) that was performed after censoring of data at the time of transplantation, a lower risk was nonetheless observed among patients in the midostaurin group than among those in the placebo group. The benefit of midostaurin was observed among patients who underwent transplantation during the first remission but not among those who underwent transplantation at a later time. After an overt relapse and additional chemotherapy, particularly if that treatment is followed by effective transplantation, there is probably little effect remaining from early randomization to an active drug versus placebo. However, there may be a role for FLT3 inhibitors after trans-plantation.27-29
The biologic and logistic challenges of this trial are further described in the Supplementary Appendix. Despite these challenges, in this large collaborative effort, we determined that midostaurin, a multitargeted kinase inhibitor, led to improved outcomes among younger adults with AML and a FLT3 mutation, a population with a poor prognosis that represents approximately one fourth of all patients with AML. It remains unclear whether agents with different target profiles, including more specific FLT3 inhibitors, would also improve outcomes if they were added to usual therapy for younger adults with AML and a FLT3 mutation and whether chemotherapy plus midostaurin might be beneficial for older adults or for those with wild-type FLT3.
Supplementary Material
Supplement1
Acknowledgments
Supported by grants from the National Cancer Institute, National Institutes of Health (U10CA031946, U10CA033601, U10CA180821, and U10CA180882 [to the Alliance for Clinical Trials in Oncology]; and U10CA032291, U10CA041287, U10CA077651, U10CA077658, U10CA180791, U10CA180836, U10CA180850, and U10CA180867), and in part by funding from Novartis.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 3.The Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 5.Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–9. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- 6.Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 9.Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116:5089–102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- 10.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 11.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a Cancer and Leukemia Group B study. Cancer Res. 2001;61:7233–9. [PubMed] [Google Scholar]
- 12.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 13.Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1, 262–70. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- 14.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cyto-genetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–9. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99:310–8. doi: 10.1182/blood.v99.1.310. [DOI] [PubMed] [Google Scholar]
- 16.Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–43. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 17.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 18.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 19.Cortes JE, Kantarjian H, Foran JM, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-in-ternal tandem duplication status. J Clin Oncol. 2013;31:3681–7. doi: 10.1200/JCO.2013.48.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perl AE, Altman JK, Cortes JE, et al. Final results of the Chrysalis trial: a first-in-human phase 1/2 dose-escalation, dose-expansion study of gilteritinib in patients with relapsed/refractory acute myeloid leukemia. Blood. 2016;128:1069. abstract. [Google Scholar]
- 21.Propper DJ, McDonald AC, Man A, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19:1485–92. doi: 10.1200/JCO.2001.19.5.1485. [DOI] [PubMed] [Google Scholar]
- 22.Stone RM, Fischer T, Paquette R, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia. 2012;26:2061–8. doi: 10.1038/leu.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer T, Stone RM, DeAngelo DJ, et al. Phase IIB trial of oral midostaurin, the FMS-like tyrosine kinase receptor and multi-targeted inhibitor in patients with acute myeloid leukemia and high-risk my-elodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–45. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530–41. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 25.Röllig C, Serve H, Hüttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multi-centre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16:1691–9. doi: 10.1016/S1470-2045(15)00362-9. [DOI] [PubMed] [Google Scholar]
- 26.Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood. 2015;126:319–27. doi: 10.1182/blood-2014-10-551911. [DOI] [PubMed] [Google Scholar]
- 27.Chen YB, Li S, Lane AA, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20:2042–8. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–71. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 29.Schlenk R, Dohner K, Salih H, et al. Midostaurin in combination with intensive induction and as single agent maintenance therapy after consolidation therapy with allogeneic hematopoietic stem cell transplantation or high-dose cytarabine. Blood. 2015;126:322. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1