Highly Efficient and Versatile Plasmid-Based Gene Editing in Primary T Cells (original) (raw)
Abstract
Adoptive cell transfer is an important approach for basic research and emerges as an effective treatment for various diseases, including infections and blood cancers. Direct genetic manipulation of primary immune cells opens up unprecedented research opportunities and could be applied to enhance cellular therapeutic products. In this article, we report highly efficient genome engineering in primary murine T cells using a plasmid-based RNA-guided CRISPR system. We developed a straightforward approach to ablate genes in up to 90% of cells and to introduce precisely targeted single nucleotide polymorphisms in up to 25% of the transfected primary T cells. We used gene editing–mediated allele switching to quantify homology-directed repair, systematically optimize experimental parameters, and map a native B cell epitope in primary T cells. Allele switching of a surrogate cell surface marker can be used to enrich cells, with successful simultaneous editing of a second gene of interest. Finally, we applied the approach to correct two disease-causing mutations in the Foxp3 gene. Repairing the cause of the scurfy syndrome, a 2-bp insertion in Foxp3, and repairing the clinically relevant Foxp3K276X mutation restored Foxp3 expression in primary T cells.
Introduction
Lymphocytes are among the best understood mammalian cells. A plethora of genetically modified mice has yielded deep insight into the molecular and cellular processes underlying lymphocyte, but also more generally, mammalian, development and function. Inbred mouse strains enable adoptive transfer experiments without immunological rejection; however, although genetically modified murine models are very powerful, a major practical limitation is the time required to generate genetically altered mice. Moreover, intercrossing mice with combinations of mutations and/or transgenes requires extensive breeding. Finally, for immunologic reasons, a given genetic alteration often needs to be introduced on a particular genetic background or mice need to be backcrossed for multiple generations to change the genetic background. Thus, systems to directly genetically edit murine lymphocytes would be highly desirable to reduce the need for breeding.
Although previous efforts to establish clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9)-mediated gene editing in primary T cells have mainly focused on human T cells (1–4), to our knowledge, only two reports describe successful CRISPR/Cas9 gene editing in primary murine T cells (5, 6). Both approaches depend on mice expressing transgenic Cas9 and either a second transgenic construct to express the guide RNA (gRNA) (5) or viral transduction of T cells followed by antibiotic selection (6). As a result, although these approaches constitute significant advances, they still require breeding of a constitutive Cas9 transgene, which may be genotoxic and might lead to immunologic rejection after adoptive transfer of transgenic cells.
In an earlier report, transfection of plasmids for CRISPR/Cas9-mediated genome editing in human hematopoietic stem cells (hHSCs) and CD4+ T cells was successfully used (1). However, gene ablation was less efficient in T cells than in hHSCs, with efficiencies in primary human CD4+ T cells mostly <10%, even with a strategy using two gRNAs targeting the same gene simultaneously. Subsequently, it was reported that CRISPR/Cas9 genome engineering could be improved in primary human T cells by replacing plasmids with chemically modified gRNAs combined with Cas9-encoding mRNA (3). Nucleofection with a plasmid encoding the single gRNA (sgRNA) and Cas9 did not demonstrate editing efficiencies above background, whereas cotransfection of a chemically modified sgRNA with a Cas9-expressing plasmid yielded deletion in <10% of T cells. This is in comparison with high double-digit deletion efficiencies with sgRNA and Cas9 mRNA (3). Similarly, electroporation of recombinant Cas9/gRNA ribonucleoprotein (RNP) complexes results in moderate to high double-digit deletion efficiencies (2). More recently, multiplexed high-efficiency CRISPR/Cas9 editing was reported using mRNA and multiple in vitro–transcribed sgRNAs (4). Thus, there is a growing notion that, in comparison with other approaches, DNA-based techniques work poorly, if at all, for CRISPR/Cas9 gene editing in primary T cells (2, 3, 7, 8). However, because plasmids have been the workhorse of molecular biology for decades as a result of their ease of use, versatility, low production costs, and wide availability to the scientific community, they would have specific merits were they to be successfully used. Importantly, thousands of CRISPR-related products are already available as plasmids, and the latest CRISPR nuclease variants are rapidly being deposited into a growing publically available resource (https://www.addgene.org/crispr). In addition, in contrast to viral transduction and/or transgenic Cas9 expression, transient expression of nuclease and gRNA is sufficient, and even preferred, as a method to reduce off-target genome editing. Furthermore, technical advances make electroporation a clinically useful approach (9). Finally, RNPs are more difficult to produce than plasmids, particularly for complex constructs, such as base editors, and are more expensive than plasmids, thus preventing access to low-budget research groups.
We report a highly efficient method to ablate genes in primary murine T cells. The approach is based on plasmids that are commonly accessible through publicly available resources (https://www.addgene.org). Combining two plasmids enables efficient multiplexed gene ablation. Gene-edited T cells are viable, home to lymphoid organs, expand in a lymphophenic environment, and differentiate normally in response to a viral infection in lymphoreplete mice. Using a novel assay, we additionally increased the efficiency of homology-directed repair (HDR) in primary CD4+ T cells, reaching up to 25% HDR using commonly available reagents. Through the application of this approach, we mapped a native B cell epitope directly in primary cells and corrected a defective Foxp3 gene. We demonstrate that multiplexed HDR editing of a cell surface molecule can serve as a surrogate marker to enrich for cells undergoing HDR at a second locus for which no marker may exist, similar to previous findings (10). This principle was applied to enrich gene-corrected Foxp3-deficient cells. Thus, we report a versatile T cell–editing approach and provide proof of principle that gene-edited Foxp3-deficient T cells can produce the repaired gene product, Foxp3 protein.
Materials and Methods
Gene editing in primary murine CD4+ T cells
Naive CD4+ T cells were purified (>96% purity) from C57BL/6N mouse spleen (SP) and lymph nodes (LNs) using an EasySep Mouse Naive CD4+ T Cell Isolation Kit (STEMCELL Technologies). Alternatively, total CD4+ T cells were purified (>96% purity) from C57BL/6 SMARTA+ CD45.1 mouse SPs and LNs using an EasySep Mouse CD4+ T Cell Isolation Kit (STEMCELL Technologies). Complete RPMI medium (CM RPMI 1640) was generated by supplementing RPMI 1640 (Sigma) with 10% heat-inactivated FCS (Atlanta Biologicals), 2 mM GlutaMAX (Life Technologies), 50 μM 2-ME (Life Technologies), 10 mM HEPES (Sigma), and nonessential amino acids (Life Technologies). For T cell activation, 2 × 106 naive CD4+ T cells were plated in a 24-well plate (Corning) coated with anti-CD3 mAb (hybridoma clone 2C11, 1 μg/ml) and anti-CD28 mAb (hybridoma clone PV-1, 0.5 μg/ml; both from Bio X Cell) for 24 h at 37°C with 5% CO2 in the presence of 50 IU/ml recombinant human IL-2 (rhIL-2) (R&D Systems). Twenty-four hours later, T cells were harvested and washed with PBS. A total of 2 × 106 activated T cells was electroporated with a Neon Transfection System (Invitrogen) under the following conditions: voltage (1550 V), width (10 ms), pulses (three), 100-μl tip, and Buffer R. Cells were transfected with 6.5 μg of empty plasmid px458 (plasmid number 48138; Addgene) or with the plasmids described in the figure legends and Supplemental Table I (plasmid numbers 82670, 82672–82675, 82677, and 102910; Addgene). For HDR, cells were cotransfected with 12 μg of HDR template (if plasmid: Supplemental Table III; 82661–82665, 82667–82669, 104988; Addgene) or with 10 μl of 10 μM stock ssDNA template (Supplemental Table II) purchased from IDT. After electroporation, cells were plated in a 24-well plate in 650 μl of CM RPMI 1640 with 50 IU rhIL-2/ml in the presence of plate-bound mAbs at half of the concentration used for the initial activation (i.e., anti-CD3 [0.5 μg/ml] and anti-CD28 [0.25 μg/ml]). GFP+ and GFP− cells were sorted 24 h posttransfection using a FACSAria cell sorter to >98% purity (BD Biosciences). Immediately after sorting, cells were plated in 96-well flat-bottom plates, without activating Abs, in 250 μl of CM RPMI 1640 supplemented with 50 IU rhIL-2/ml. For HDR experiments, sorted cells were cultured in the presence of nonhomologous end joining (NHEJ) inhibitors or HDR enhancers for the following 24 h to enhance HDR (as indicated in the figure legends). Cells were reactivated with plate-bound anti-CD3 (0.5 μg/ml) and anti-CD28 (0.25 μg/ml) on day 4 post–GFP sorting and expanded for the following 9 d in culture (until the end of the experiment). For in vivo–editing experiments, cells were injected immediately into the recipient mouse.
Gene editing in EL-4 cells
EL-4 cells were grown in RPMI 1640 (Sigma) supplemented with 10% heat-inactivated FBS (Atlanta Biologicals), 2 mM GlutaMAX (Life Technologies), and 50 μM 2-ME (Life Technologies). FACS analysis confirmed homozygous CD90.2 and CD45.2 expression by EL-4 cells comparable to that of primary T cells. A total of 2 × 106 EL-4 cells was electroporated with a Neon Transfection System (Invitrogen) under the following conditions: voltage (1080 V), width (50 ms), number of pulses (one), 100-μl tip, and Buffer R. The amount of plasmids and concentrations of HDR templates were the same as for the primary T cells described above. After electroporation, cells were plated in 24-well plates in 650 μl of CM RPMI 1640. GFP+ and GFP− cells were sorted 24 h posttransfection using a FACSAria Cell Sorter (BD Biosciences) to a purity > 98%. Immediately after sorting, cells were plated in 96-well flat-bottom plates. For HDR experiments, sorted cells were cultured in the presence of NHEJ inhibitors or HDR enhancers for the following 24 h to enhance HDR, and cells were expanded for the next 7–9 d in culture.
Foxp3 repair protocol
Although the majority of T cells from Foxp3K276X C57BL/6 mice are highly activated, they had to be reactivated in vitro for electroporation, otherwise we could not obtain reasonable transfection efficiencies. We adjusted the protocol used to electroporate primary T cells from healthy mice by reducing the TCR stimulation to obtain a good balance between cell viability and transfection rate. In addition, we used total CD4+ T cells as a starting population because of the low numbers of naive T cells (data not shown). Total CD4+ T cells were purified (>96% purity) from Foxp3K276X C57BL/6 pooled SPs and LNs using an EasySep CD4+ T Cell Isolation Kit (STEMCELL Technologies). For T cell activation, 2 × 106 CD4+ T cells were plated in a 24-well plate coated with anti-CD3 (clone 2C11; 0.5 μg/ml) and anti-CD28 (clone PV-1; 0.25 μg/ml) (Bio X Cell) for 24 h at 37°C, 5% CO2, with 50 IU rhIL-2/ml (R&D Systems). Twenty-four hours later, T cells were harvested and washed with PBS. A total of 2 × 106 activated T cells was electroporated with a Neon Transfection System (Invitrogen) under the following conditions: voltage (1550 V), width (10 ms), number of pulses (three), 100-μl tip, and Buffer R. Cells were transfected with 6.5 μg of plasmid (p240_LTJ_sgRNAFoxp3K276X; number 82675; Addgene) and 12 μg of the dsDNA wild-type (wt) Foxp3 repair template (number 82664; Addgene). After electroporation, cells were plated in a 24-well plate with 50 IU/ml of rhIL-2 in the presence of plate-bound mAbs at half of the concentrations used for the initial activation (i.e., 0.25 μg/ml anti-CD3 and 0.12 μg/ml anti-CD28) in 650 μl of CM RPMI 1640. GFP+ and GFP− cells were sorted 24 h posttransfection using a FACSAria cell sorter to a purity > 98% (BD Biosciences). Immediately after cell sorting, the purified cells were reactivated with plate-bound anti-CD3 (0.5 μg/ml) and anti-CD28 (0.25 μg/ml) and expanded until the end of the experiment in the presence of rhIL-2 (250 IU/ml), TGF-β (5 ng/ml; R&D Systems), anti–IFN-γ (10 mg/ml; Bio X Cell), and anti–IL-4 (10 mg/ml; Bio X Cell), with or without retinoic acid (RA; 10 mM; Sigma), as indicated in the figure legends.
Mice
C57BL/6N (stock number 027) were purchased from The Charles River Laboratory. Foxp3K276X C57BL/6 mice (stock number 019933; The Jackson Laboratory) were a generous gift from E. Palmer (Basel University Hospital). C57BL/6 SMARTA CD45.1+ mice were obtained from the Swiss Immunological Mouse Repository. B6.129S7-Rag1tm1Mom/J mice (stock number 002216; The Jackson Laboratory) were obtained from the Swiss Immunological Mouse Repository. All animal work was done in accordance with the federal and cantonal laws of Switzerland. The Animal Research Commission of the Canton of Basel-Stadt, Switzerland, approved the animal research protocols.
Adoptive T cell transfers and lymphocytic choriomeningitis virus immunization
Edited SMARTA+ CD45.1+ CD4+ T cells were transferred into C57BL/6 CD45.2+ recipient mice by i.v. tail injection immediately after GFP+ cell sorting. Mice were infected i.p. with lymphocytic choriomeningitis virus (LCMV) Armstrong (2 × 105 PFU) 5 d after the initial T cell transfer and were euthanized 3, 5, or 7 d after virus administration.
Flow cytometry and Abs
Cells were stained, acquired on a BD Fortessa (BD Biosciences), and analyzed with FlowJo software (TreeStar). Surface phenotype staining was done with the following fluorochrome-conjugated mAbs: anti-CD90.2 (clone 53-2.1), anti-CD90.1 (clone OX7), anti-CD45.2 (clone 104), anti-CD45.1 (clone A20) (all from eBioscience), anti-CD4 (clone RM4-5), anti-CD25 (clone PC61; both from BioLegend), anti-ICOS (clone 7E.17G9), anti–PD-1 (clone RMP1-14), and anti-CXCR5 (clone L138D7) (all from BioLegend). The expression of Foxp3 (clone FJK-16s; eBioscience) was determined by intracellular staining performed according to the manufacturers’ protocols. Prior to staining of the surface Abs, cells were stained for live/dead discrimination with Zombie UV dye (BioLegend).
Design of sgRNA
DNA sequences of all sgRNA encoding plasmids, primers, and HDR templates used in this study are listed as 5′–3′ sequences in Supplemental Tables 1–3. sgRNAs were designed using the CRISPRtool (http://crispr.mit.edu) and sgRNA Scorer 1.0 (https://crispr.med.harvard.edu). The sgRNA sequences and their respective scores are listed in Supplemental Table I. For CD45 epitope mapping, two sgRNAs were designed per candidate region. Results obtained with the ones closest to the single nucleotide polymorphism (SNP) of interest are shown in the figures. However, all six tested sgRNAs cut efficiently, and region R1 switched epitopes with both sgRNAs (data not shown). The cut-to-mutation difference did not play a role.
Cloning of sgRNAs into px458 plasmid
pSpCas9(BB)-2A-GFP (px458) (plasmid number 48138; Addgene) was a gift from Feng Zhang. Cloning into px458 was modified from Ran et al. (11). The px458 plasmid was digested with BbsI for 1.5 h at 37°C, followed by heat inactivation for 20 min at 65°C. The digested plasmid was gel purified using a NucleoSpin Gel and PCR Clean-up purification kit, according to the manufacturer’s recommendations (Macherey-Nagel). The forward and reverse oligonucleotides (oligos) of each sgRNA were diluted at 100 μM in H2O. To phosphorylate and anneal the oligos, 2 μl of each oligo was mixed with T4 ligation buffer and T4 PNK to a final volume of 20 μl and incubated for 30 min at 37°C (phosphorylation), followed by 5 min at 95°C and then ramping down the temperature to 20°C at −1°C/min (annealing). The annealed and phosphorylated oligos were diluted 1:200 in H2O. Ligation reactions for each sgRNA were performed by mixing 100 ng of the digested and purified px458 plasmid with 2 μl of the diluted phosphorylated and annealed oligos, T4 ligation buffer, and T4 ligase in a final volume of 20 μl. Ligation was carried out for 1 h at 22°C. Bacterial transformation was performed by mixing 5 μl of the ligation reaction with 50 μl of ice-cold chemically competent JM109 bacteria. The mixture was incubated on ice for 30 min, followed by a heat shock at 42°C for 30 s and a subsequent 2-min incubation on ice. Then, 200 μl of SOC medium (Sigma) was added, and bacteria were grown for 1 h at 37°C. The transformation reaction was plated on LB plates containing 50 μg/ml ampicillin, and plates were incubated overnight at 37°C. Colonies were checked for correct insertion of the sgRNA by PCR colony screening, followed by sequencing. Plasmids are available from https://www.addgene.org (plasmid numbers 82670, 82672–82675, 82677, and 102910; Addgene).
PCR colony screening for cloning into Addgene plasmid px458
Bacteria from two colonies per plate were picked with a pipette tip and mixed in PCR tubes with H2O, REDTaq ReadyMix PCR Reaction Mix (Sigma), and specific primers (forward primer 5′-GAGGGCCTATTTCCCATGATTCC-3′ and reverse primer 5′-TCTTCTCGAAGACCCGGTG-3′). PCR was performed using an annealing temperature of 64.9°C and 35 cycles. Positive colonies (with sgRNA insertion) display no PCR amplicon, whereas negative colonies show a 264-bp amplicon.
Plasmid sequencing
Two colonies were picked from each LB plate using a pipette tip and inoculated into a 5-ml culture of LB medium supplemented with 50 μg/ml ampicillin. The cultures were grown overnight at 37°C. Plasmid DNA from the culture was isolated using a GenElute Plasmid Miniprep Kit (Sigma), following the manufacturer’s recommendations. Correct insertion of the sgRNA was verified by sequencing the plasmid DNA using a U6 forward primer (5′-ACTATCATATGCTTACCGTAAC-3′).
HDR templates
DNA repair templates were designed as homologous genomic DNA sequences flanking the sgRNA binding sites. Unless noted otherwise, the sgRNAs were centered as much as possible with respect to the repair templates, resulting in symmetric arms of homology. Silent mutations (i.e., not altering the amino acid sequence) were introduced into the protospacer adjacent motif (PAM) sequences. Short ssDNA templates were purchased from IDT. Lyophilized ssDNA oligos were reconstituted to 10 μM in double-distilled H2O (for specific sequences see Supplemental Table II). dsDNA templates for CD90.1, CD45.1, and Foxp3 (180 bp, 1, 2, and/or 4 kb) were purchased from GenScript as synthetic DNA cloned into pUC57 (for specific sequences see Supplemental Table III). Maxi preps (Sigma) were prepared for each of the plasmids prior to their use in experiments. For all HDR experiments, circular HDR template plasmids were used because we obtained better results compared with the use of linearized plasmids (data not shown). Plasmids containing HDR templates are available at https://www.addgene.org (plasmid numbers 82661–82665, 82667–82669, and 104988; Addgene).
Small molecules
The following NHEJ inhibitors were used to enhance HDR: vanillin (12) reconstituted in H2O, 300 μM final concentration (product number V1104; Sigma) and SCR7-X in DMSO, 1 μM final concentration (catalog number M60082; Xcess Biosciences). Because we purchased SCR7-X from Xcess Biosciences, we refer to this compound as SCR7-X, as recently suggested (13). Rucaparib/AG-014699/PF-01367338 was reconstituted in DMSO, 1 μM final concentration (catalog number S1098; Selleck Chemicals); veliparib/ABT-888 in DMSO, 5 μM final concentration (catalog number S1004; Selleck Chemicals); RS-1 (14) in DMSO, 7.5 μM final concentration (catalog number 553510; Merck Millipore); RS-1 in DMSO, 7.5 μM final concentration (product number R9782; Sigma); luminespib/AUY-922/NVP-AUY922 in DMSO, 1 μM final concentration (catalog number S1069; Selleck Chemicals); L-755,507 in DMSO, 5 μM final concentration (catalog number 2197; Tocris); and vanillin derivatives (12) 6-nitroveratraldehyde in DMSO, 3 μM final concentration (catalog number 11427047), 4,5-dimethoxy-3-iodobenzaldehyde in DMSO, 3 μM final concentration (catalog number 11328426); and 6-bromoveratraldehyde in DMSO, 3 μM final concentration (catalog number 11480124; all from Maybridge).
Genomic DNA sequencing
Genomic DNA was extracted from different sorted cell populations (e.g., CD45.2+/CD45.1−, CD45.2+/CD45.1+, CD45.2−/CD45.1+, and CD45.2−/CD45.1−) by incubating the cells with extraction buffer (100 mM Tris [pH 8.5], 5 mM Na-EDTA, 0.2% SDS, 200 mM NaCl, and 100 μg/ml Proteinase K; all from Sigma) for 1 h at 56°C. After a 15-min heat inactivation of proteinase K at 95°C, the samples were mixed with an equal volume of isopropanol and inverted several times to facilitate DNA precipitation. After a 2-min centrifugation, the supernatant was removed, and the pellet was washed with 70% ethanol. DNA was pelleted by centrifugation, air dried, and resuspended in Milli-Q water, and the concentration was measured with a NanoDrop device (Witec). PCR primers, including BamHI (forward 5′-TAAGCAGGATCCATTCCTTAGGACCACCACCTG-3′) and SalI (reverse 5′-TGCTTAGTCGACACACCGCGATATAAGATTTCTGC-3′) overhangs, were purchased (Microsynth) to amplify a 2-kb region for the HDR experiment in which the sgRNA was centered within the PCR product. PCRs with 2–6 ng of the different genomic DNA samples were performed using Phusion polymerase (Thermo Scientific). For the 2-kb fragment, the optimal annealing temperature used was 68.1°C. The PCR products were loaded on a 1.5% agarose gel, and the bands were purified using a NucleoSpin gel and PCR Clean-up Purification Kit, according to the manufacturer’s recommendations (Macherey-Nagel). The purified PCR products (160 ng) were digested with BamHI and SalI using BamHI buffer for 1.5 h at 37°C. The digested PCR products were loaded on a 1.5% agarose gel and the bands were purified using a NucleoSpin Gel and PCR Clean-up Purification Kit, according to the manufacturer’s recommendations. Ninety nanograms of the digested and purified 2-kb PCR amplicons were ligated for 1 h at 22°C with 50 or 100 ng of pGEM3Z plasmid that had been digested with BamHI/SalI and purified (Promega). Transformation was performed by mixing 10 μl of the ligation reaction with 50 μl of ice-cold chemically competent JM109 bacteria (purchased from Promega or made using the RbCl protocol [https://openwetware.org/wiki/RbCl_competent_cell]). The mixture was incubated on ice for 30 min, followed by a heat shock at 42°C for 30 s and a subsequent 2-min incubation on ice. Then, 200 μl of SOC medium (Sigma) was added, and bacteria were grown for 1 h at 37°C. The transformation reaction was plated on LB plates containing 50 μg/ml ampicillin, 0.1 mM IPTG (Promega), and 35 μg/ml X-Gal (Promega). The plates were incubated overnight at 37°C. Twelve (22 for HDR) white colonies were picked from each plate using a pipette tip and inoculated into a 5-ml culture of LB medium supplemented with 50 μg/ml ampicillin. The cultures were grown overnight at 37°C. Plasmid DNA from the culture was isolated using a GenElute Plasmid Miniprep Kit (Sigma), following the manufacturer’s recommendations. DNA was sent for sequencing using T7, SP6, and an internal primer (5′-GAGAAAGCAACCTCCGGTGT-3′) for the 2-kb fragments. Sequences were analyzed using Lasergene (DNASTAR).
Cas9 RNP assembly and transfection
The delivery of a Cas9 RNP complex, containing an Alt-R CRISPR RNA (crRNA) and Atto 550–labeled _trans_-activating crRNA (tracrRNA) (both from IDT) and a Cas9 nuclease (QB3 MacroLab, University of California, Berkeley), into primary mouse T cells or EL-4 cells using a Neon Transfection System (Invitrogen) was adapted from a protocol provided by IDT. In brief, the RNA oligos (crRNA and tracrRNA) were resuspended in Nuclease-Free IDTE Buffer at a final concentration of 200 μM each. The two RNA oligos were mixed in equimolar concentrations to a final complex concentration of 44 μM. The complexes were heated at 95°C for 5 min and then cooled to room temperature. Cas9 protein (36 μM) was mixed slowly with the crRNA:tracrRNA complex and incubated at room temperature for 10–20 min before the transfection. Fresh crRNA:tracrRNA complexes were made for each experiment, as per IDT’s recommendations.
EL-4 cells were transfected with RNPs using a Neon Transfection System (Invitrogen) under the following conditions: voltage (1380 V), width (50 ms), pulses (one), 100-μl tip, and Buffer R (for RNPs). Primary T cells were transfected with RNPs using a Neon Transfection System (Invitrogen) under the following conditions: voltage (1550 V), width (10 ms), pulses (three), 100-μl tip, and Buffer R (for RNPs).
Results
Efficient plasmid-based gene ablation in a murine T cell line
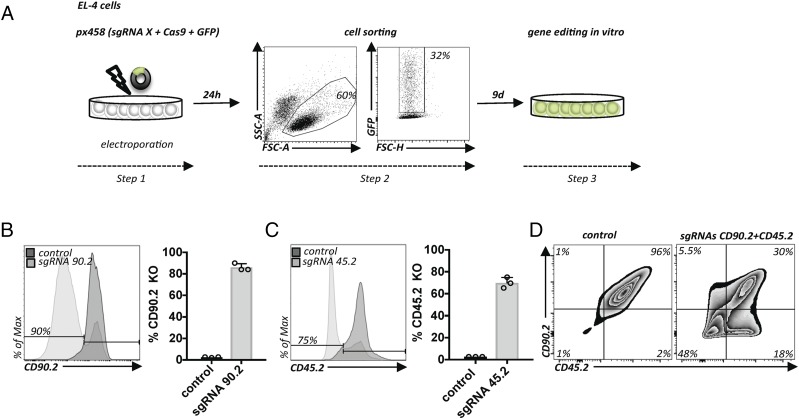
We set out to develop a plasmid-based approach for CRISPR/Cas9 editing in primary T cells. Based on a successful T cell electroporation protocol (15), we optimized experimental conditions for murine EL-4 cells using a commonly available plasmid expressing an sgRNA, SpCas9, and GFP (11) (Fig. 1A). One day after electroporation, we purified the successfully electroporated cells based on GFP expression. We quantified the efficiency of gene editing in single cells for genes encoding cell surface proteins using flow cytometry (Fig. 1B). After systematic optimization of experimental conditions, taking into account electroporation parameters, concentration, choice of plasmid (size, promoter), cell number, and timing, we achieved very high deletion efficiencies for CD90.2 and CD45.2, which were lost in the vast majority of cells compared with control conditions (Fig. 1B, 1C). Next, we asked whether this protocol allows multiplexed gene editing by combining CD45.2 and CD90.2 gene targeting. Almost half of the cells lost CD90.2 and CD45.2 expression simultaneously, indicating homozygous deletion of both genes (Fig. 1D). Thus, we successfully established very simple conditions for high-efficiency gene editing in EL-4 cells.
FIGURE 1.
Efficient plasmid-based gene ablation in EL-4 cells. (A) Protocol for plasmid-based gene editing in EL-4 cells. Electroporation of a plasmid encoding an sgRNA targeting the gene X, Cas9, and GFP (Step 1). After 24 h, successfully transfected cells are purified by flow cytometry based on GFP expression (Step 2). Subsequent cell expansion for 9 d for gene editing in vitro (Step 3). (B) Flow cytometry of EL-4 cells transfected as in (A), with a plasmid encoding a CD90.2-targeting sgRNA (sgRNA90.2) or empty vector px458 (control). Flow cytometry graph (left panel). Quantification of multiple experiments (n = 3); error bars represent SD (right panel). (C) The same conditions as in (B), but with sgRNA45.2 or empty vector (control). Representative data from three experiments; error bars represent SD. (D) EL-4 cells transfected as in (A) but with two plasmids encoding two sgRNAs (sgRNA90.2 and sgRNA45.2). Flow cytometry of cells transfected with empty px458 vector (left panel) or cells transfected with plasmids encoding sgRNAs targeting CD90.2 and CD45.2 (right panel). Representative data from two experiments.
Efficient plasmid-based gene ablation in primary T cells
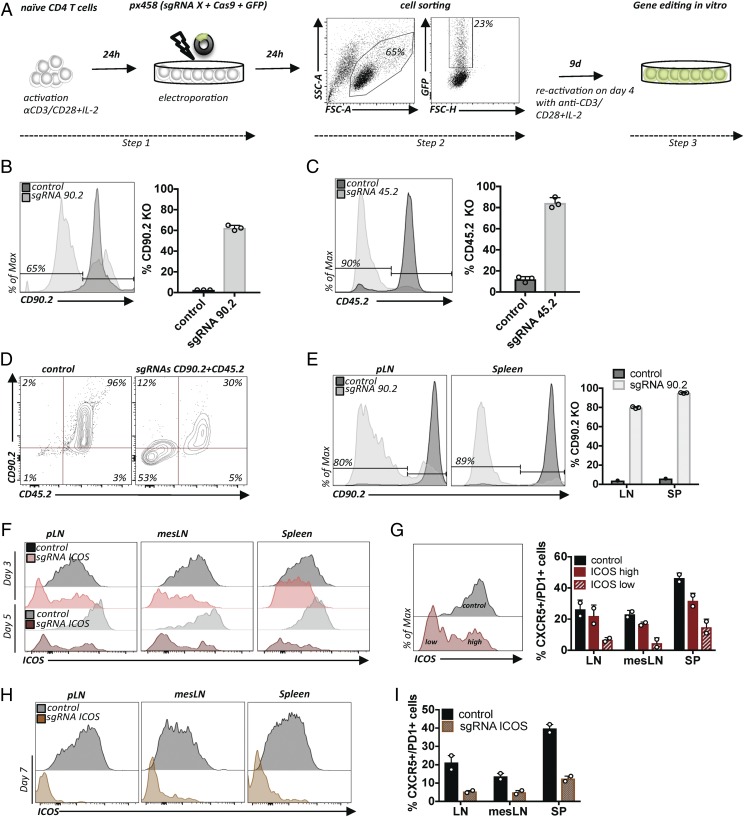
Encouraged by these results, we tested whether the same plasmids could be used to edit primary mouse CD4+ T cells. To this end, we added a T cell–activation step with plate-bound anti-CD3 and anti-CD28 mAbs required for electroporation (Fig. 2A) (15). Careful titration of the concentration of mAbs used to coat the plates and timing were necessary; ultimately, we found conditions allowing good viability and transfection efficiencies up to 20% (Fig. 2A). Using sgRNAs targeting CD90.2 or CD45.2, we found 60–90% deletion efficiencies among purified GFP+ cells (Fig. 2B, 2C). A second reactivation step with half of the concentration of mAb was necessary for efficient editing. Remarkably, despite the notion that DNA-based CRISPR/Cas editing does not work in T cells, the gene-ablation efficiencies are equal to alternative protocols using in vitro–transcribed sgRNA/Cas9 mRNA or RNPs (2, 3) and even to the protocol using viral transduction of transgenic Cas9-expressing T cells (6). Next, we combined the plasmids targeting CD90.2 and CD45.2. Similar to the efficiency in EL-4 cells, we found that more than half of the GFP-expressing cells lost both surface proteins (Fig. 2D).
FIGURE 2.
Efficient plasmid-based gene ablation in primary T cells. (A) Protocol for plasmid-based gene editing in primary CD4+ T cells. Activation of T cells prior to the transfection step with px458 plasmid (Step 1). Twenty-four hours later, successfully transfected cells are purified based on GFP expression (Step 2) and expanded for 9 d in vitro (Step 3). (B) Primary T cells transfected as in (A), with a plasmid encoding an sgRNA90.2 or empty vector (control). Flow cytometry graph (left panel). Quantification of three experiments; error bars represent SD (right panel). (C) Same conditions as in (B), but with sgRNA45.2 or empty vector (control). Representative data from three experiments; error bars represent SD. (D) Primary T cells were transfected as in (A) but with two plasmids encoding two sgRNAs (sgRNA90.2 and sgRNA45.2) simultaneously. Flow cytometry of cells transfected with empty px458 vector (left panel) or transfected with plasmids encoding sgRNAs targeting CD90.2 and CD45.2 (right panel). (E) Primary CD4+ T cells transfected as in (A), with sgRNA90.2 or empty vector (control). Flow cytometry graphs for CD90.2 expression compared with the control on live CD4+ T cells in LN and SP (left panel) and quantification of multiple recipients (right panel). Representative data from three experiments; error bars represent SD. (F) Primary CD4+ T cells transfected as in (A), except that CD4+ total T cells were used as the initial population, with sgRNAICOS or empty vector (control). Flow cytometry graphs for ICOS on live CD45.1+ donor T cells compared with controls in LN, mesLN, and SP 3 and 5 d posttransfer. (G) Frequencies of CXCR5+PD-1+ (TFH) cells in LN, mesLN, and SP within ICOSlow and ICOShigh populations (day 5) compared with the nonedited control. (H) Flow cytometry graphs for ICOS on live CD45.1+ donor T cells compared with controls in LNs, mesLNs, and SPs 7 d posttransfer. (I) Frequencies of CXCR5+PD-1+ (TFH) cells in LN, mesLN, and SP within cells that were transfected with sgRNAICOS compared with the nonedited control. Representative data from two experiments; error bars represent SD.
As mentioned previously, a powerful aspect of studying murine T cells is the possibility of adoptive cell transfer (ACT) of T cells to immunologically matched recipients. Therefore, we wondered whether the edited T cells functioned in vivo. To investigate this, we adoptively transferred T cells electroporated with sgRNA for CD90.2 into lymphodeficient RAG-knockout (KO) mice immediately after GFP sorting. Cells were harvested from LNs and SPs 10 d after ACT. We observed deletion of CD90.2 in up to 80% of CD4+ T cells recovered from LNs and in up to 95% of CD4+ T cells recovered from SPs (Fig. 2E). Thus, overall, deletion efficiencies were comparable to the ones observed in vitro. The recovered cells were viable and had expanded substantially (data not shown). Furthermore, we wanted to examine whether CRISPR/Cas9-mediated editing could be used to study gene function in primary mouse CD4+ T cells during a viral infection that induces T follicular helper (TFH) cell differentiation. We optimized experimental conditions based on a previously established protocol for acute infection with LCMV (16). We electroporated CD4+ T cells bearing an LCMV-specific transgenic TCR (SMARTA) with plasmid encoding an sgRNA for ICOS, a marker that is highly expressed by, and required for, TFH cell differentiation (17). Sorted GFP+ T cells were transferred immediately to C57BL/6N mice. Transferred T cells were given 5 d to migrate and then rest in host mice. Thereafter, host mice were infected with LCMV to induce TFH cell differentiation. Transferred cells were harvested from LNs, mesenteric LNs (mesLNs), and SPs at 3, 5, and 7 d post–virus administration. Compared with control cells, we observed a progressive decrease in ICOS mean fluorescence intensity of SMARTA+ CD4+ T cells recovered from all organs at all examined time points, with a near-complete absence of ICOS expression on day 7 (Fig. 2F, 2H). Importantly, cells that had lost ICOS expression (ICOSlow) due to gene editing demonstrated impaired TFH cell differentiation based on expression of the TFH cell markers CXCR5 and PD1 compared with the cells that maintained high ICOS (ICOShigh) expression in the same host (Fig. 2G). Of note, on day 7, CXCR5 and PD1 expression was only evaluated in the ICOSlow population and compared with cells electroporated with empty plasmid because of the insufficient number of cells within the ICOShigh population (Fig. 2H, 2I). Overall, this demonstrates successful gene editing and confirms the importance of ICOS signals for TFH cell differentiation (17).
Thus, this plasmid-based approach enables efficient gene ablation in primary T cells and can be used to study T cell biology and gene function after ACT in vivo in lymphophenic hosts, as well as during viral infection of lymphoreplete hosts.
Efficient introduction of targeted point mutations in EL-4 and primary T cells
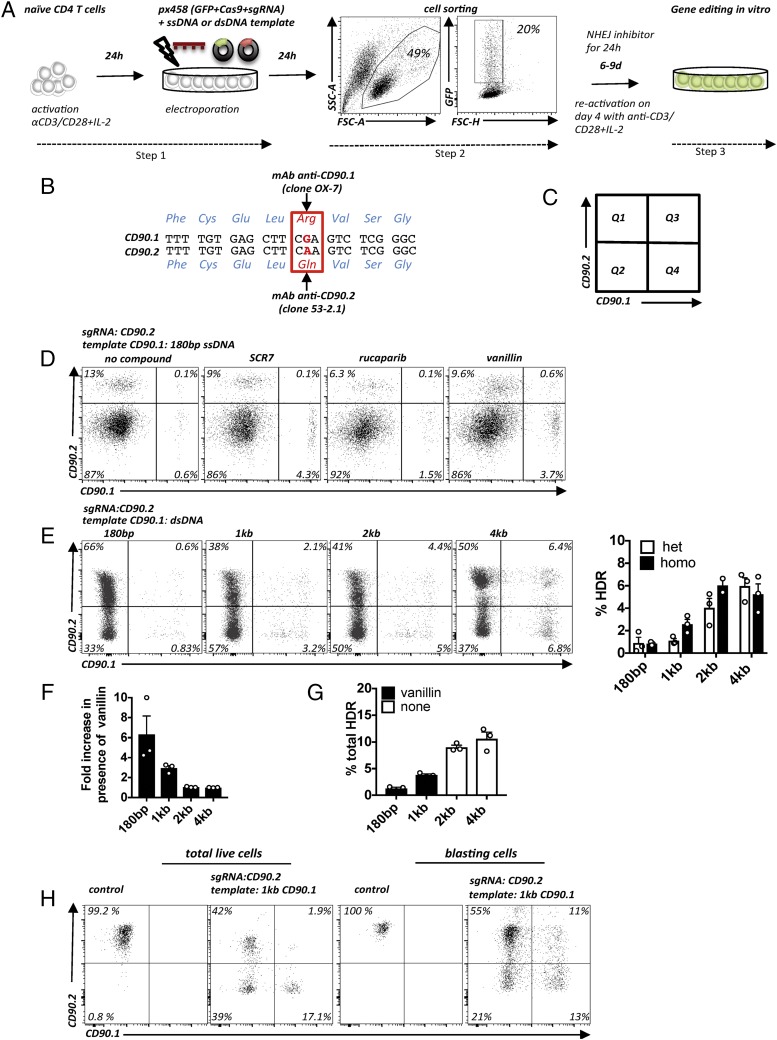
Gene editing–induced DNA double-strand breaks (DSBs) are mostly repaired by NHEJ that results in random indels. In contrast, DSB repair by HDR allows controlled genome editing and, therefore, is desirable for more sophisticated experimental questions, as well as for clinical applications. Unfortunately, because HDR occurs much more rarely, it remains challenging to establish efficient HDR protocols (18). The absence of suitable assays to readily quantify HDR events hinders improvement of HDR efficiencies in cells, in general, and particularly in primary cells. To allow rapid assessment of HDR efficiencies in T cells, we designed a novel assay (Fig. 3A–C). Two naturally occurring alleles of murine CD90 (CD90.1 and CD90.2) differ by a single nucleotide, resulting in a single amino acid difference (CD90.1: arginine [Arg]; CD90.2 glutamine [Gln]) (Fig. 3B) that can be distinguished by two mAbs (19). We hypothesized that successful DNA editing from one allelic variant to the other could be quantified using the two allele-specific mAbs (Fig. 3B, 3C). To establish the allele-switching assay (ASA), we tested whether we could switch the CD90.2 allele to CD90.1 in EL-4 cells by cutting with sgRNACD90.2 and providing a 180-bp ssDNA template encoding CD90.1 and a silent PAM mutation (Fig 3D, far left panel). Given the low HDR efficiency that we observed in initial experiments (<1% CD90.1+ cells), we sought ways in which to increase it. Because it was demonstrated previously that interfering with the DNA repair pathways could increase HDR efficiencies (20), we compared several small molecules known to interfere with the NHEJ pathway, or that directly enhance HDR, to find the best HDR-enhancing strategy for T cells. Along with SCR7-X, the DNA-PK inhibitor vanillin and the PARP1 inhibitor rucaparib yielded the strongest increases in HDR frequency (Fig. 3D, right three panels). Other compounds, such as veliparib, L75507 (21), luminespib, RS-1 (14), and the vanillin derivatives A14415, A1359, and L17452 (12) increased HDR less or were toxic (data not shown). Because vanillin resulted in the strongest increase in HDR and, additionally, was the only water-soluble compound, we focused on it in subsequent experiments. Thus, we demonstrate that allele switching of an endogenous gene can be used to quantify HDR, as well as NHEJ, in a population of cells with single-cell resolution.
FIGURE 3.
Targeted introduction of point mutations in primary T cells. (A) Protocol for plasmid-based HDR in CD4 T cells. Activation and electroporation of primary T cells (Step 1). Purification of GFP+ cells by flow cytometry (Step 2). Cell incubation for 24 h with NHEJ inhibitor. Subsequent in vitro cell expansion for gene editing for 6–9 d with reactivation at 4 d postsorting (Step 3). EL-4 cells are transfected in the same way, with the exceptions that they do not require TCR activation prior to the electroporation or on day 4 postsorting, and the electroporation parameters are different (see Materials and Methods). (B) Genomic CD90.1 and CD90.2 nt and aa sequences. The CGA (CD90.1)/CAA (CD90.2) SNP leading to Arg108Gln is highlighted in red. (C) Graphic representation of the experimental readout: Q1, unedited cells or cells with mutations that do not abolish protein expression (e.g., in-frame mutations); Q2, cells after NHEJ; Q3, edited CD90.2/CD90.1 heterozygous cells; and Q4, edited homozygous CD90.1 cells or cells with one KO allele and one HDR edited allele. (D) EL-4 cells coelectroporated with a plasmid encoding an sgRNACD90.2 and a 180-bp CD90.1 ssDNA template. Flow cytometry for CD90.2 and CD90.1 expression in untreated (far left panel) and treated samples (right three panels). Representative data from three experiments. (E) EL-4 cells coelectroporated with plasmid sgRNACD90.2 and a circular plasmid including a CD90.1 dsDNA template of various lengths (180 bp; 1, 2, and 4 kb). Flow cytometry for CD90.2 and CD90.1. Representative flow cytometry plots (left panel) and quantification of multiple experiments of the average frequency of cells that underwent HDR (heterozygous and homozygous) (right panel). Representative data from three experiments; error bars represent SD. (F) Quantification of the effect of vanillin on the relative enrichment of HDR frequency (fold change) as a function of dsDNA template length. Experiment as in (E). Fold increase in HDR frequency of cells treated with vanillin relative to the absence of vanillin for each template. Representative data from three experiments; error bars represent SD. (G) Long templates without NHEJ inhibitor result in higher HDR frequencies than do short templates with NHEJ inhibitor. Quantification of HDR frequency obtained with short templates (180 bp, 1 kb) plus NHEJ inhibitor (vanillin) and long templates (2, 4 kb) without vanillin. Experiment as in (E). Representative data from three experiments; error bars represent SD. (H) Bead-enriched naive CD4+ T cells from C57BL/6N mice activated and electroporated with empty px458 plasmid (control), with plasmid encoding for sgRNACD90.2 and a plasmid including a 1-kb CD90.1 dsDNA template. Flow cytometry for CD90.2 and CD90.1 expression. Flow cytometry plots demonstrate gating on total live cells (left panels) and blasting cells (right panels). Representative data from two experiments.
The next parameter that we evaluated was the length of the repair template. Although recent gene editing studies often used relatively short ssDNA templates (usually <200 bp), the arms of homology for gene targeting in embryonic stem cells are usually much longer (several kilobases). Indeed, increasing the arms of homology of a circular dsDNA (plasmid) CD90.1 HDR template correlated positively with HDR efficiency (Fig. 3E). The largest increase was found between 1 and 2 kb total homology (Fig. 3E). Notably, the HDR-enhancing effect of vanillin was more pronounced for shorter templates (180 bp, 1 kb) than for the long templates (2, 4 kb) (Fig. 3F). Therefore, we wondered whether a long template without NHEJ inhibition could yield a comparable HDR frequency as shorter templates with NHEJ inhibitors. A direct comparison showed that 2- and 4-kb templates without vanillin outperformed the 180-bp and the 1-kb template in the presence of vanillin (Fig. 3G). Thus, despite the notion in the field that ssDNA templates yield higher HDR efficiencies, it is worth considering long arms of homology to increase HDR efficiency. Importantly, the optimized conditions also yielded high HDR frequencies in primary mouse CD4+ T cells, in which ∼20% of the total cells had switched one or both alleles (Fig. 3H, left panels). Interestingly, we noticed even higher HDR frequencies in large blasting cells, in which 25% had undergone HDR (Fig. 3H, right panels). Thus, plasmids with long arms of homology are much more efficient for introducing point mutations to primary T cells than are short ssDNA templates.
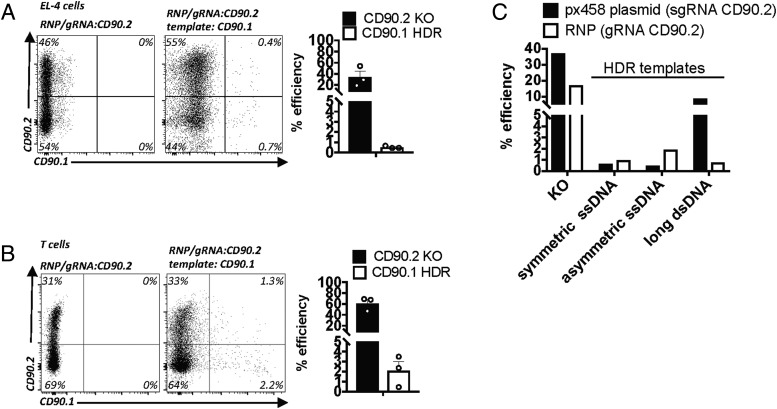
Given the many reports attributing low toxicity and high editing efficiencies to Cas9 RNPs (NHEJ and HDR) in various cell types, we compared RNP performance with the results obtained with plasmids. Electroporation of EL-4 cells with recombinant Cas9 complexed with tracrRNA/crRNA targeting the same CD90.2 sequence as the one targeted by the plasmid-encoded sgRNA led to comparable KO frequencies (Fig. 4A, left panel, Fig. 1B). In contrast, in comparison with plasmid-mediated HDR (Fig. 3E), we observed much lower HDR efficiencies when RNP was combined with the plasmid HDR template (Fig. 4A, right panel). Similar results were obtained with primary T cells (Figs. 2B, 3H, 4B). Because high HDR efficiencies were reported for RNPs combined with short ssDNA templates, we compared HDR templates provided as long dsDNA or as symmetric and asymmetric short ssDNA templates, as described before (22). Compared with the all-plasmid approach, HDR efficiencies were much lower with RNPs, independent of template design (Fig. 4C).
FIGURE 4.
ASAs using Cas9 RNPs. (A) EL-4 cells transfected with Cas9 RNP targeting CD90.2 alone or with a 2-kb dsDNA CD90.1 DNA template. Flow cytometry for CD90.2 and CD90.1 (left panel). Quantification of two experiments; error bars represent SD (right panel). (B) Primary CD4+ T cells transfected with Cas9 RNP targeting CD90.2 alone or with a 2-kb dsDNA CD90.1 DNA template. Flow cytometry for CD90.2 and CD90.1 (left panel). Quantification of two experiments; error bars represent SD (right panel). (C) EL-4 cells transfected with Cas9 RNP or px458 plasmid targeting CD90.2 alone or with CD90.1 DNA templates provided as symmetric and asymmetric 180-bp ssDNA templates or as a plasmid including a 2-kb dsDNA template. Quantification of KO efficiency and HDR in the presence of symmetric or asymmetric short ssDNA or long dsDNA template between the plasmid-based and RNP approaches.
Foreign DNA can integrate into the genome through homology-directed mechanisms or through nonhomologous or “illegitimate” insertion (23). Although rare, we tested whether our protocol resulted in detectable genomic plasmid integration. GFP expression used to purify successfully transfected cells peaked at 24 h posttransfection and rapidly decreased in the following days, becoming undetectable by flow cytometry after 5–7 d in EL-4 and primary T cells. We followed EL-4 cells for up to 30 d in vitro and primary T cells for up to 16 wk in vivo and were unable to detect any GFP in these cells. However, using two PCR primer sets detecting Cas9 and Cas9-GFP, we observed faint bands in EL-4 and primary T cells at all examined time points (data not shown). We cannot tell whether this represents persisting extrachromosomal episomal DNA or true genomic integration. Future studies are required to thoroughly assess genomic plasmid integration.
Thus, the ASA described in this article is a simple, rapid, and cost-effective system to quantify HDR efficiency with an endogenous marker in primary cells. Various HDR-enhancing small molecules and HDR templates with long arms of homology (>1 kb) are important parameters to consider when optimizing HDR efficiency.
Epitope mapping of CD45.2- and CD45.1-binding Abs in primary T cells
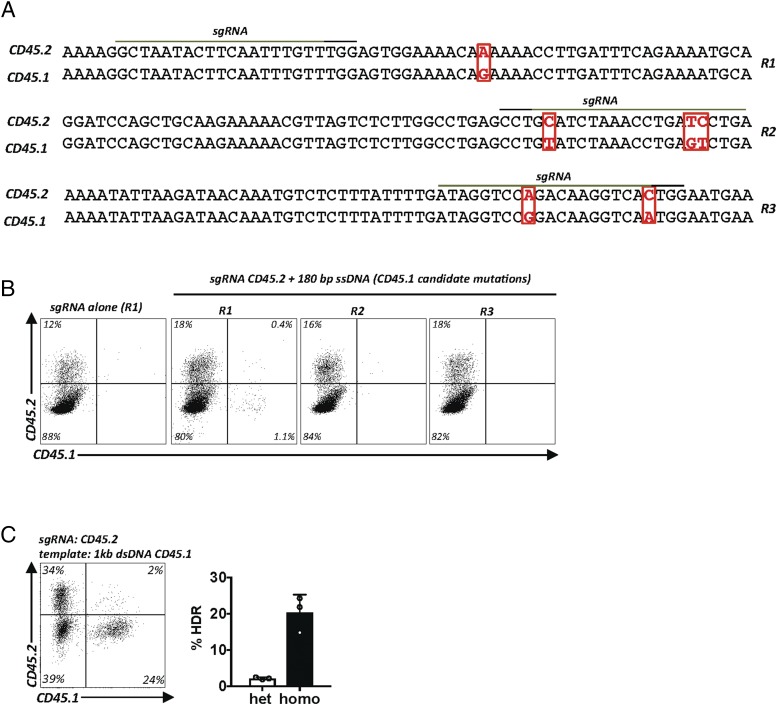
To test whether the optimized conditions found with the CD90 ASA are more universally applicable, we turned to Ptprc, another gene with two naturally occurring alleles, whose products CD45.1 and CD45.2 can be discriminated by two mAbs. However, in contrast to CD90.1 and CD90.2, the precise epitopes recognized by anti-CD45.1 (clone A20) mAb and anti-CD45.2 (clone 104) mAb are unknown. The genomic sequence encoding the extracellular domain of CD45.1 and CD45.2 differs by 6 nt, but it is not known which epitope is being recognized as an allelic difference (Fig. 5A) (24, 25). One nucleotide substitution is silent, whereas the other five change the amino acid sequence. Therefore, we hypothesized that editing the five candidate nucleotide substitutions individually, or as combinations, directly in primary T cells could be used to fine map the epitopes being recognized by the two known mAbs. We grouped the five candidate nucleotides into three genomic regions (R1–R3) that could be covered by three ssDNA templates. Each HDR template encoded partial CD45.1 sequences and was matched to CD45.2 cutting sgRNAs binding as closely as possible to the candidate mismatches (Fig. 5A). Using the T cell HDR protocol, we found that all three sgRNAs led to efficient cuts (Fig. 5B). Exchange of a single nucleotide within region R1 enabled binding of anti-CD45.1 mAb and prevented binding of anti-CD45.2 mAb in some cells. In contrast, editing R2 and R3 did not result in anti-CD45.1 binding (Fig. 5B). A longer repair template increased HDR efficiency and confirmed this result (Fig. 5C). Sanger sequencing of all four purified populations (Supplemental Fig. 1A) confirmed correct editing (Supplemental Fig. 1B). Thus, the Lys302Glu substitution is necessary and sufficient to explain reactivity of the CD45.1 epitope with anti-CD45.1 mAb clone A20 (Supplemental Fig. 1C). These results demonstrate the feasibility of epitope mapping in primary cells (i.e., in the native context of an endogenous Ag using the CRISPR/Cas9 system). Furthermore, they validate the robustness of this HDR protocol and the ASA as a rapid and versatile assay to quantify single-nucleotide editing.
FIGURE 5.
Epitope mapping of CD45.2 and CD45.1 binding Abs in primary T cells. (A) Alignment of select regions of the genomic murine C57BL/6 CD45.1 and CD45.2 gene sequences. The extracellular domains of CD45.1 and CD45.2 differ by 6 nt (indicated in red) in three different regions (designated R1, R2 and R3). sgRNA binding sites (green line), PAM sequence (black line). (B) High-resolution gene editing–based mapping of the native CD45.1 epitope. Experimental setup as in Fig. 3A. The three candidate regions were cut in primary CD4+ T cells using three sgRNAs targeting the CD45.2 gene as close as possible to the SNP of interest (sgRNACD45.2_R1, sgRNACD45.2_R2, and sgRNACD45.2_R3) and repaired with three 180-bp ssDNA CD45.1 templates (R1, R2, R3). Flow cytometry for CD45.2 and CD45.1 expression. The experiment was carried out once with EL-4 cells (data not shown) and once with primary CD4+ T cells. (C) Validation of results obtained in (B) using a longer 1-kb CD45.1 dsDNA template. The Lys302Glu mutation is necessary and sufficient to switch CD45.2 reactivity to CD45.1 reactivity. Data are displayed as a representative flow cytometry plot (left panel) and quantification of multiple experiments (right panel). Representative data from three experiments; error bars represent SD.
Gene correction of Foxp3-deficient primary T cells
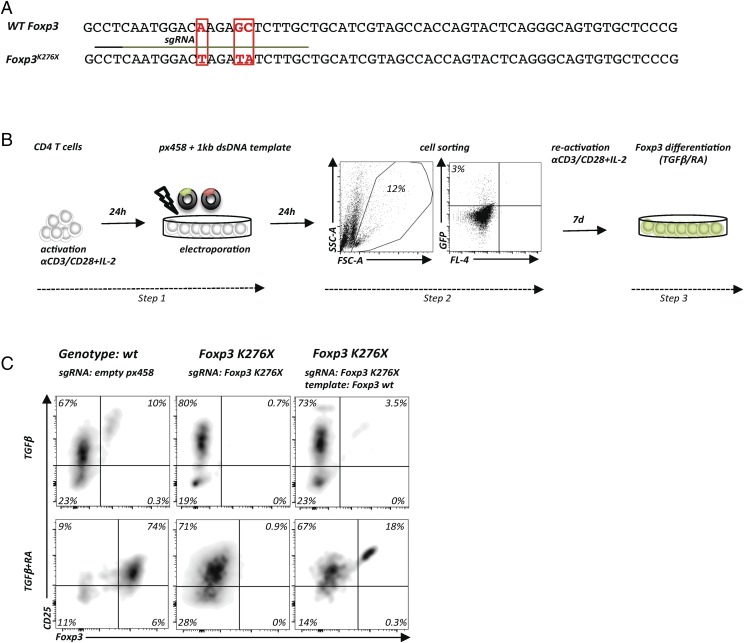
Next, we sought to apply the newly developed T cell–editing protocol to correct a monogenic disease. The prototypic mutations causing human immunodysregulation polyendocrinopathy enteropathy X-linked syndrome are mutations in the FOXP3 gene, which encodes a transcription factor critical for T regulatory cell (Treg) function and maintenance of immune regulation (26, 27). Mutations in murine Foxp3 lead to a very similar syndrome termed scurfy (26). A 2-bp insertion in Foxp3 exon 8 results in a frameshift leading to the scurfy phenotype (27). Affected mice die within weeks after birth due to multiorgan failure caused by a complete breakdown of immune tolerance, resulting in uncontrolled activation of the immune system, tissue infiltration, and immune-mediated destruction of multiple organs (28). Foxp3-deficient mice with a genetically marked Foxp3 locus contain Treg “wannabes,” indicating that cells destined to become Foxp3+ cells are actively transcribing the Foxp3 locus and are present in scurfy mice; however, due to the absence of Foxp3, they cannot be identified as Tregs, and they lack suppressive function (29). Thus, we hypothesized that gene correction of Foxp3-mutated T cells should lead to restoration of Foxp3 protein expression, a prerequisite for Treg function.
To test our hypothesis, we used T cells from gene-targeted mice that bear a Foxp3K276X mutation that abolishes Foxp3 protein expression (“Foxp3 KO”) and recapitulates a known human immunodysregulation polyendocrinopathy enteropathy X-linked disease–causing Foxp3 mutation (26, 30) (Fig. 6A). We adjusted the HDR-based gene repair approach to T cells from diseased mice and examined the in vitro Treg-differentiation potential of gene-corrected Foxp3-KO cells by providing the Foxp3-inducing signals TGF-β alone or RA plus TGF-β (31) (Fig. 6B). After gene repair and stimulation with TGF-β alone, 10% of wt T cells became CD25+Foxp3+, whereas no Foxp3+ cells were detected in Foxp3K276X CD4+ T cells transfected with sgRNAFoxp3K276X alone. In contrast, the Foxp3 wt repair template restored Foxp3 expression in 3.5% of the cells (Fig. 6C, upper panels). Exposing electroporated T cells to combined TGF-β and RA resulted in Foxp3 expression in 74% of wt T cells, no detectable Foxp3 expression in Foxp3K276X CD4+ T cells without HDR template, and 18% Foxp3+ T cells among Foxp3K276X CD4+ T cells repaired with the wt Foxp3 HDR template (Fig. 6C, lower panels). Comparable results were obtained with scurfy cells (data not shown). Thus, the plasmid-based HDR protocol described in this article works to repair primary T cells from severely sick mice.
FIGURE 6.
Gene correction of Foxp3-deficient cells. (A) Alignment of genomic DNA sequences of wt Foxp3 (C57BL/6) and the Foxp3 locus with a targeted mutation Foxp3K276X that introduces a premature stop codon. sgRNA binding site (green line) and PAM sequence (black line). (B) Protocol for gene editing of total CD4+ T cells from Foxp3K276X C57BL/6 mice. In vitro activation and electroporation (Step 1) with plasmids encoding sgRNA targeting the Foxp3K276X mutation and a circular plasmid containing a 1-kb wt Foxp3 repair template. Successfully transfected cells are isolated based on GFP expression (Step 2). Cell expansion in vitro for gene editing in the presence of rhIL-2, TGF-β alone, or TGF-β in combination with RA and cytokine-neutralizing Abs (anti–IL-4 and anti–IFN-γ) for 7 d (Step 3). (C) Experimental setup as in (B) with total CD4+ T cells from wt control or Foxp3K276X mice. Flow cytometry of CD25 and Foxp3 expression (gated on live CD4+ T cells). Differentiation of wt cells electroporated with empty px458 plasmid into CD4+Foxp3+CD25+ T cells (left panels), absence of Foxp3 differentiation in Foxp3K276X cells electroporated with sgRNAFoxp3K276X alone (middle panels), and restoration of Foxp3 protein expression in Foxp3K276X cells electroporated with sgRNAFoxp3K276X and 1-kb Foxp3 dsDNA repair template (right panels). Foxp3 induction with TGF-β alone (upper panels). Foxp3 induction with TGF-β combined with RA (lower panels). Representative data from two experiments with Foxp3K276X cells.
Two HDR events are linked in a given cell
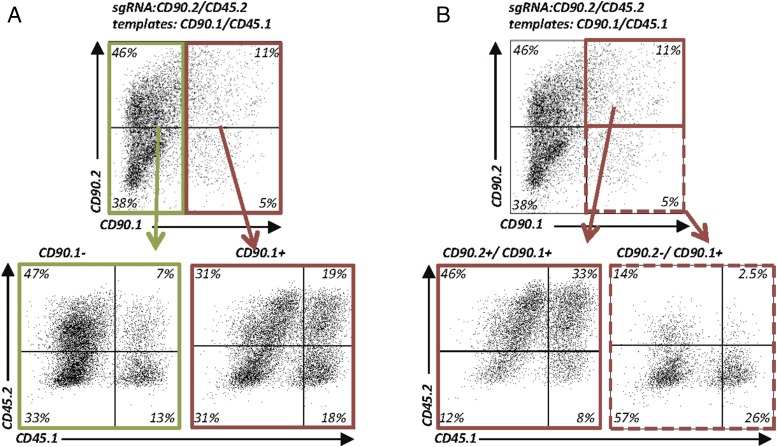
Because we now had two unique assays at hand, we wondered whether the CD90 ASA and CD45 ASA could be combined to quantify multiplexed HDR in single cells. To address this question, we electroporated EL-4 cells with plasmids encoding sgRNAs targeting CD90.2 and CD45.2, along with repair templates for CD90.1 and CD45.1. Cutting efficiency under these conditions was slightly lower than with fewer plasmids (data not shown), but HDR for CD90 and CD45 individual alleles was very efficient. We then sought to determine whether two HDR events at two separate loci in the same cell are independent from each other or linked. We found a 2-fold enrichment of cells switching CD45.2 to CD45.1 in cells that had switched CD90.2 to CD90.1 compared with cells that remained CD90.1− (Fig. 7A). Importantly, one third of the CD90.2+/CD90.1+ heterozygous cells were also heterozygous for CD45.2+/CD45.1+ (Fig. 7B). Similarly, the highest relative frequency of homozygous CD45.1+ cells was found among cells that were also homozygous for CD90.1+ (Fig. 7B). Thus, DNA repair by the HDR pathway is more likely to occur in cells that concurrently repair a second DNA break by HDR.
FIGURE 7.
Enrichment of HDR-edited cells through monitoring of allele switching of a surrogate cell surface marker. (A) Enrichment of HDR-edited cells using allele switching of a surrogate cell surface marker. EL-4 cells electroporated with plasmids encoding sgRNACD90.2 and sgRNACD45.2_R1 and 2-kb dsDNA templates (CD90.1 and CD45.1) for multiplexed HDR. Flow cytometry for CD90.2, CD90.1, CD45.2, and CD45.1 expression. Pregating on CD90.1− (green) and CD90.1+ (red) (i.e., allele switched cells) demonstrates that HDR events at a second locus (Ptprc) are linked within the same cell (upper panel). CD45 allele-switched cells are more frequent in cells that also switched the CD90 allele (lower panels). Representative data from two experiments. (B) Selection of zygosity of HDR-edited cells. Experimental data as in (A). Pregating on heterozygous CD90.1+/CD90.2+ cells (upper panel; solid red line) enriches CD45.1+/CD45.2+ heterozygous cells (lower left panel). Pregating on homozygous CD90.1+/CD90.1+ cells (upper panel, dashed red line) enriches homozygous CD45.1+/CD45.1+ cells (lower right panel).
Allele switching of a surrogate surface receptor enriches gene-repaired cells
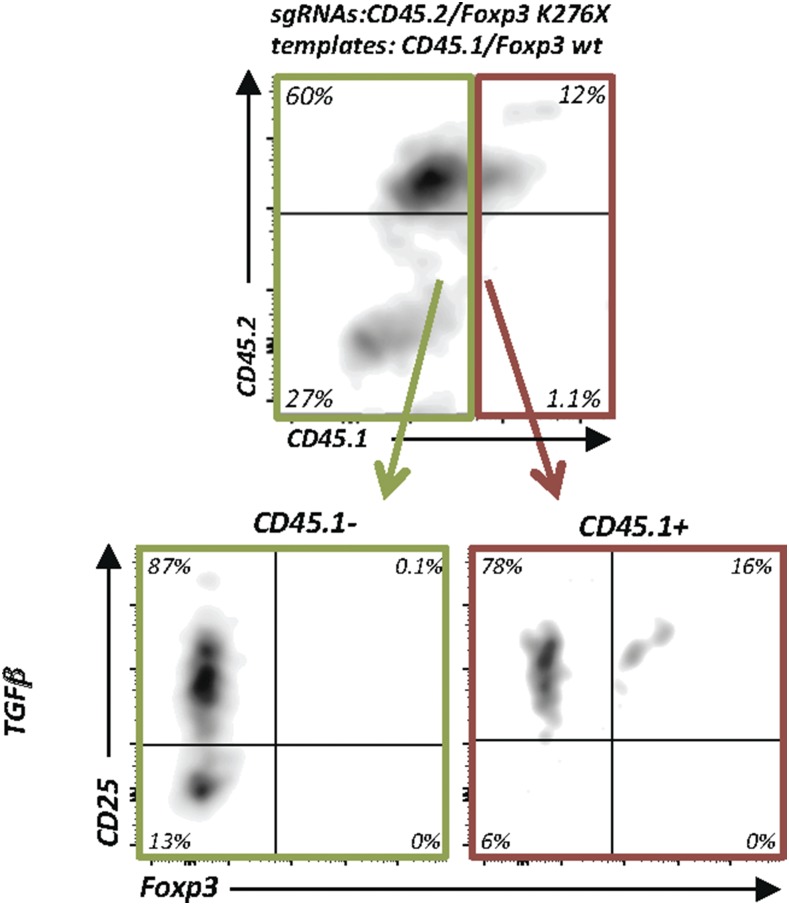
Finally, we wondered whether linked HDR could be exploited to enrich correctly repaired cells using multiplexed HDR. In a clinical context, it will be desirable to select correctly edited cells before transfusion to patients. However, identification of these cells (without killing them) is difficult if the repaired gene is not expressed at the cell surface. Because Foxp3 is an intracellular transcription factor, we sought to repair primary Foxp3-KO CD4+ T cells and combine the repair process with CD45.2 to CD45.1 allele switching as a surrogate cell surface marker to monitor allele switching. As described in Fig. 6, total CD4+ T cells were isolated from Foxp3K276X mice and electroporated with four plasmids to cut CD45.2 and mutant Foxp3 and repair them with CD45.1 and wt Foxp3 templates. Thereafter, cells were exposed to TGF-β to induce Foxp3 expression. Indeed, CD25+Foxp3+ cells were substantially enriched among CD45.1+ cells (16%) compared with CD45.1− cells (0.1%) (Fig. 8). Compared with Foxp3 repair without allele switching, which, under these conditions (TGF-β alone), resulted in 3.5% Foxp3-expressing cells (Fig. 6C, upper panels), monitoring allele switching of a surrogate surface marker resulted in ∼4-fold enrichment. In summary, we established conditions to repair Foxp3 in primary T cells and demonstrate the applicability of multiplexing HDR to enrich gene-corrected cells. Therefore, we propose that assessment of a surrogate marker HDR gene-editing event could be exploited to enrich and/or select for zygosity of HDR gene editing at a second gene locus of interest for which no marker is available.
FIGURE 8.
Enrichment of Foxp3 repaired cells through monitoring of allele switching of a surrogate cell surface marker. Enrichment of gene-repaired Foxp3-expressing primary T cells using multiplexed CD45 allele switching as a surrogate marker. Experimental setup as in Fig. 6B, but with simultaneous electroporation of plasmids encoding two sgRNAs (sgRNAFoxp3K276X and sgRNACD45.2_R1) and two 1-kb dsDNA templates (Foxp3 wt and CD45.1). Flow cytometry of CD45.2, CD45.1, CD25, and Foxp3 (gated on live CD4+ cells). Pregating on CD45.1− cells (green line) and CD45.1+ cells (red line) (upper panel). Enrichment of CD25+Foxp3+ cells in allele-switched CD45.1+ cells (lower panels). Representative data from two experiments.
Discussion
Efficient protocols to genetically engineer T cells will likely contribute to a better understanding of the architecture of our genome and the wiring of genetic networks guiding cellular behavior and will, therefore, ultimately lead to safer and more efficient cellular therapies. In contrast to recent results suggesting that plasmid-based genome engineering is inefficient in T cells, our results demonstrate that plasmids are well-suited, very powerful, and versatile vectors to edit a T cell’s genome. Plasmids are commonly available, inexpensive, and transiently expressed vectors that can be used to edit the many existing genetically modified mouse models, including TCR-transgenic mice. Importantly, the method described in this article does not rely on importing and intercrossing mice, but it can be immediately applied to cells of existing mouse models, independent of genetic backgrounds. Thus, we expect that this method will accelerate research in areas as diverse as genome biology, developmental biology, lymphocyte biology, immunology, and animal models of cellular therapy. It will be important to investigate potential genomic plasmid integration and off-target mutations. Because plasmids tend to persist longer in cells than RNPs and because we detect plasmid sequences in some cells up to several weeks after electroporation by PCR, the frequency of off-target cleavage could be increased compared with the use of RNPs. This may not be relevant for many basic research questions, because using different sgRNAs can control for off-target cleavage. In contrast, these questions are highly relevant for potential clinical translation and warrant thorough investigation.
Our results go beyond the field of immunology, however. Although targeted HDR is often preferred over NHEJ, achieving high-efficiency HDR is still challenging. The results from the ASAs suggest that HDR templates with long arms of homology should be revisited to increase HDR efficiency. In addition, although NHEJ inhibitors can be useful to increase HDR, in cases in which NHEJ inhibitors are preferentially avoided (e.g., clinical gene editing), long arms of homology can prove effective and do not necessarily have to be delivered virally. Although we used dsDNA HDR templates, it will be worthwhile to investigate whether increasing the length of the arms of homology of ssDNA templates leads to comparably increased HDR efficiency. Using long ssDNA templates, rather than dsDNA templates, might also decrease the risk for off-target genomic HDR template integration. Moreover, we provide proof-of-concept data that genome editing can be applied for rapid and precise epitope mapping in primary cells. This approach could be particularly powerful to characterize the majority of B cell epitopes, which are discontinuous (i.e., conformational). The approach presented in this article is unique, because the mapping was achieved in primary cells (i.e., in the native context with all endogenous posttranslational modifications). Knowing precise epitopes is particularly important for the many therapeutic Abs, as well as to define the binding sites of chimeric AgRs (CARs) that are derived from mAbs. Because tumors can escape attacks by the highly efficient CAR T cells, gene editing–based epitope mapping could be applied to tumor cells to investigate escape mutants or to define tumor Ags.
Finally, cell-based therapeutics constitute the next “pillar” of medicine (32). Genome modification of the cellular product can repair genetic defects before subsequent autologous transplantation and allows the cell to be equipped with designer features to increase safety and efficacy (32, 33). Thus, protocols to efficiently introduce precise and targeted genetic modifications in hematopoietic cells, particularly T cells, are key to the success of adoptive T cell therapy. Targeted insertion of a CAR into the endogenous TCR locus is likely safer and results in more efficient CAR T cells compared with virally delivered randomly integrated CAR constructs (7, 34). To further optimize cellular products and to thoroughly investigate novel experimental synthetic genetic networks, mouse models will remain important animal models for T cell therapy. To this end, this plasmid-based protocol enables efficient nonviral-targeted cellular engineering to investigate new concepts for cellular therapies in immunocompetent mouse models (35). However, we would like to caution that adaptation of this protocol for human T cells, particularly in clinical use, would require a thorough investigation of potential genomic plasmid integration, as well as other possible effects, such as triggering of TLR9.
Supplementary Material
Data Supplement
Acknowledgments
We thank the University of Basel, the Basel University Hospital, and the Department of Biomedicine for institutional support; laboratory members, Pawel Pelczar, and members of the Department of Biomedicine genome editing club for discussions; Marianne Dölz, Oliver Gorka, Jeffrey Bluestone, Xuyu Zhou, and Tony Nguyen for critical comments on the manuscript; and Regan Geissman for editorial assistance. We also thank Angelika Offinger, Ulrich Schneider, and the team from the Department of Biomedicine animal facility for animal husbandry; Ed Palmer and Carolyn King for kindly sharing mice; Georg Holländer for kindly sharing EL-4 cells; Shane Crotty and Simon Bélanger for sharing ICOS sgRNA sequences; Annaise Jauch for providing LCMV; Danny Labes, Emmanuel Traunecker, and Lorenzo Raeli of the Department of Biomedicine flow cytometry core for support; and Marco Amsler and Caroline Schwenzel for technical support.
This work was supported by grants from the Swiss National Science Foundation (Professorship PP00P3_144860 to L.T.J.) and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (R56/R01AI106923 to L.T.J.). M.K. received a fellowship from the Fonds de Recherche Santé Québec (Canada).
The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this article:
ACT
adoptive cell transfer
ASA
allele-switching assay
CAR
chimeric AgR
Cas9
CRISPR-associated protein 9
CM RPMI 1640
complete RPMI medium
CRISPR
clustered regularly interspaced short palindromic repeat
crRNA
CRISPR RNA
DSB
double-strand break
gRNA
guide RNA
HDR
homology-directed repair
hHSC
human hematopoietic stem cell
KO
knockout
LCMV
lymphocytic choriomeningitis virus
LN
lymph node
mesLN
mesenteric LN
NHEJ
nonhomologous end joining
oligo
oligonucleotide
PAM
protospacer adjacent motif
RA
retinoic acid
rhIL-2
recombinant human IL-2
RNP
ribonucleoprotein
sgRNA
single gRNA
SNP
single nucleotide polymorphism
SP
spleen
TFH
T follicular helper
tracrRNA
_trans_-activating crRNA
Treg
T regulatory cell
wt
wild-type.
Disclosures
M.K. and L.T.J. have filed provisional patent applications related to this work. The other author has no financial conflicts of interest.
References
- 1.Mandal P. K., Ferreira L. M., Collins R., Meissner T. B., Boutwell C. L., Friesen M., Vrbanac V., Garrison B. S., Stortchevoi A., Bryder D., et al. 2014. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15: 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumann K., Lin S., Boyer E., Simeonov D. R., Subramaniam M., Gate R. E., Haliburton G. E., Ye C. J., Bluestone J. A., Doudna J. A., Marson A. 2015. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl. Acad. Sci. USA 112: 10437–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendel A., Bak R. O., Clark J. T., Kennedy A. B., Ryan D. E., Roy S., Steinfeld I., Lunstad B. D., Kaiser R. J., Wilkens A. B., et al. 2015. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 33: 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren J., Liu X., Fang C., Jiang S., June C. H., Zhao Y. 2016. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23: 2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beil-Wagner J., Dössinger G., Schober K., vom Berg J., Tresch A., Grandl M., Palle P., Mair F., Gerhard M., Becher B., et al. 2016. T cell-specific inactivation of mouse CD2 by CRISPR/Cas9. Sci. Rep. 6: 21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu V. T., Graf R., Wirtz T., Weber T., Favret J., Li X., Petsch K., Tran N. T., Sieweke M. H., Berek C., et al. 2016. Efficient CRISPR-mediated mutagenesis in primary immune cells using CrispRGold and a C57BL/6 Cas9 transgenic mouse line. Proc. Natl. Acad. Sci. USA. 113: 12514–12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornu T. I., Mussolino C., Cathomen T. 2017. Refining strategies to translate genome editing to the clinic. Nat. Med. 23: 415–423. [DOI] [PubMed] [Google Scholar]
- 8.Ren J., Zhang X., Liu X., Fang C., Jiang S., June C. H., Zhao Y. 2017. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 8: 17002–17011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fesnak A. D., June C. H., Levine B. L. 2016. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 16: 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agudelo D., Duringer A., Bozoyan L., Huard C. C., Carter S., Loehr J., Synodinou D., Drouin M., Salsman J., Dellaire G., et al. 2017. Marker-free coselection for CRISPR-driven genome editing in human cells. Nat. Methods 14: 615–620. [DOI] [PubMed] [Google Scholar]
- 11.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. 2013. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8: 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durant S., Karran P. 2003. Vanillins--a novel family of DNA-PK inhibitors. Nucleic Acids Res. 31: 5501–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greco G. E., Matsumoto Y., Brooks R. C., Lu Z., Lieber M. R., Tomkinson A. E. 2016. SCR7 is neither a selective nor a potent inhibitor of human DNA ligase IV. DNA Repair 43: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J., Yang D., Xu J., Zhu T., Chen Y. E., Zhang J. 2016. RS-1 enhances CRISPR/Cas9- and TALEN-mediated knock-in efficiency. Nat. Commun. 7: 10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steiner D. F., Thomas M. F., Hu J. K., Yang Z., Babiarz J. E., Allen C. D., Matloubian M., Blelloch R., Ansel K. M. 2011. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity 35: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nance J. P., Bélanger S., Johnston R. J., Takemori T., Crotty S. 2015. Cutting edge: T follicular helper cell differentiation is defective in the absence of Bcl6 BTB repressor domain function. J. Immunol. 194: 5599–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi Y. S., Kageyama R., Eto D., Escobar T. C., Johnston R. J., Monticelli L., Lao C., Crotty S. 2011. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34: 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., La Russa M., Qi L. S. 2016. CRISPR/Cas9 in genome editing and beyond. Annu. Rev. Biochem. 85: 227–264. [DOI] [PubMed] [Google Scholar]
- 19.Williams A. F., Gagnon J. 1982. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science 216: 696–703. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama T., Dougan S. K., Truttmann M. C., Bilate A. M., Ingram J. R., Ploegh H. L. 2015. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. [Published erratum appears in 2016 Nat. Biotechnol. 34: 210.] Nat. Biotechnol. 33: 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu C., Liu Y., Ma T., Liu K., Xu S., Zhang Y., Liu H., La Russa M., Xie M., Ding S., Qi L. S. 2015. Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell 16: 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson C. D., Ray G. J., DeWitt M. A., Curie G. L., Corn J. E. 2016. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 34: 339–344. [DOI] [PubMed] [Google Scholar]
- 23.Würtele H., Little K. C., Chartrand P. 2003. Illegitimate DNA integration in mammalian cells. Gene Ther. 10: 1791–1799. [DOI] [PubMed] [Google Scholar]
- 24.Raschke W. C., Hendricks M., Chen C. M. 1995. Genetic basis of antigenic differences between three alleles of Ly5 (CD45) in mice. Immunogenetics 41: 144–147. [DOI] [PubMed] [Google Scholar]
- 25.Zebedee S. L., Barritt D. S., Raschke W. C. 1991. Comparison of mouse Ly5a and Ly5b leucocyte common antigen alleles. Dev. Immunol. 1: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsdell F., Ziegler S. F. 2014. FOXP3 and scurfy: how it all began. Nat. Rev. Immunol. 14: 343–349. [DOI] [PubMed] [Google Scholar]
- 27.Brunkow M. E., Jeffery E. W., Hjerrild K. A., Paeper B., Clark L. B., Yasayko S. A., Wilkinson J. E., Galas D., Ziegler S. F., Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27: 68–73. [DOI] [PubMed] [Google Scholar]
- 28.Khattri R., Cox T., Yasayko S.-A., Ramsdell F. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4: 337–342. [DOI] [PubMed] [Google Scholar]
- 29.Gavin M. A., Rasmussen J. P., Fontenot J. D., Vasta V., Manganiello V. C., Beavo J. A., Rudensky A. Y. 2007. Foxp3-dependent programme of regulatory T-cell differentiation. Nature 445: 771–775. [DOI] [PubMed] [Google Scholar]
- 30.Lin W., Truong N., Grossman W. J., Haribhai D., Williams C. B., Wang J., Martín M. G., Chatila T. A. 2005. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J. Allergy Clin. Immunol. 116: 1106–1115. [DOI] [PubMed] [Google Scholar]
- 31.Chen W., Jin W., Hardegen N., Lei K. J., Li L., Marinos N., McGrady G., Wahl S. M. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischbach M. A., Bluestone J. A., Lim W. A. 2013. Cell-based therapeutics: the next pillar of medicine. Sci. Transl. Med. 5: 179ps7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C. Y., Rupp L. J., Roybal K. T., Lim W. A. 2015. Synthetic biology approaches to engineer T cells. Curr. Opin. Immunol. 35: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S. J. C., Hamieh M., Cunanan K. M., Odak A., Gönen M., Sadelain M. 2017. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davila M. L., Kloss C. C., Gunset G., Sadelain M. 2013. CD19 CAR-targeted T cells induce long-term remission and B cell aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS One 8: e61338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement