Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017 (original) (raw)
Abstract
Background
Cardiovascular disease (CVD) is a common comorbidity in type 2 diabetes (T2DM). CVD’s prevalence has been growing over time.
Purpose
To estimate the current prevalence of CVD among adults with T2DM by reviewing literature published within the last 10 years (2007–March 2017).
Methods
We searched Medline, Embase, and proceedings of major scientific meetings for original research documenting the prevalence of CVD in T2DM. CVD included stroke, myocardial infarction, angina pectoris, heart failure, ischemic heart disease, cardiovascular disease, coronary heart disease, atherosclerosis, and cardiovascular death. No restrictions were placed on country of origin or publication language. Two reviewers independently searched for articles and extracted data, adjudicating results through consensus. Data were summarized descriptively. Risk of bias was examined by applying the STROBE checklist.
Results
We analyzed data from 57 articles with 4,549,481 persons having T2DM. Europe produced the most articles (46%), followed by the Western Pacific/China (21%), and North America (13%). Overall in 4,549,481 persons with T2DM, 52.0% were male, 47.0% were obese, aged 63.6 ± 6.9 years old, with T2DM duration of 10.4 ± 3.7 years. CVD affected 32.2% overall (53 studies, N = 4,289,140); 29.1% had atherosclerosis (4 studies, N = 1153), 21.2% had coronary heart disease (42 articles, N = 3,833,200), 14.9% heart failure (14 studies, N = 601,154), 14.6% angina (4 studies, N = 354,743), 10.0% myocardial infarction (13 studies, N = 3,518,833) and 7.6% stroke (39 studies, N = 3,901,505). CVD was the cause of death in 9.9% of T2DM patients (representing 50.3% of all deaths). Risk of bias was low; 80 ± 12% of STROBE checklist items were adequately addressed.
Conclusions
Globally, overall CVD affects approximately 32.2% of all persons with T2DM. CVD is a major cause of mortality among people with T2DM, accounting for approximately half of all deaths over the study period. Coronary artery disease and stroke were the major contributors.
Keywords: Cardiovascular disease, Type 2 diabetes, Prevalence, Stroke, Ischemic heart disease, Myocardial infarction, Angina
Background
The International Diabetes Federation (IDF) estimates that worldwide, 415 million people have diabetes, 91% of whom have type 2 diabetes mellitus (T2DM) [1]. People with diabetes comprise 8.8% of the world’s population, and IDF predicts that the number of cases of diabetes will rise to 642 million by 2040 [1]. The prevalence of T2DM has been steadily increasing over time. Using data from the Framingham Heart Study, Abraham et al. [2] noted that the overall annualized incidence rates of the disease per 1000 persons increased from 3.0 in the 1970s to 5.5 in the first decade of the 2000s. That change represented an increase in the incidence of T2DM of 83.3% and was higher in males than females by a factor of 1.61.
Cardiovascular disease (CVD) is a major cause of death and disability among people with diabetes [1, 3]. Adults with diabetes historically have a higher prevalence rate of CVD than adults without diabetes [4], and the risk of CVD increases continuously with rising fasting plasma glucose levels, even before reaching levels sufficient for a diabetes diagnosis [5].
T2DM reduces life expectancy by as much as 10 years, and the main cause of death for patients with T2DM is CVD [1, 3]. Furthermore, people with T2DM are disproportionately affected by CVD compared with non-diabetic subjects [6]. Haffner et al. [6] reported death rates due to cardiovascular causes over a 7-year period in patients with and without T2DM. In persons with T2DM, the death rates were 15.4% for those with no prior myocardial infarction (MI) and 42.0% in patients having a history of MI. In contrast, patients who did not have T2DM, the death rates due to cardiovascular causes were 2.1 and 15.9%, respectively.
In the Framingham Heart Study, Fox [7] reported that, along with the increasing T2DM prevalence, the attributable risk of CVD due to T2DM increased from 5.4% in the period 1952–1974 to 8.7% in the period 1975 and 1998. In a longitudinal study of 881 patients with T2DM over 10 years, van Hateren et al. [8] indicated that the hazard ratio for death due to CVD was constantly increasing each year. Thus, an increasing burden of diabetes will likely be followed by an increasing burden of CVD.
Given the clinical burden that CVD complications have on T2DM patients, there has been an increased focus on the joint management of T2DM and CVD. Good glycemic control remains the main foundation for managing T2DM. Although the importance of intensive glycemic control for protection against microvascular complications and CVD in people with T1DM is well established [9, 10], its role for reducing cardiovascular risk has not been established as clearly in people with T2DM [11–13]. Hence, the most effective approach for prevention of macrovascular complications appears to be multifactorial risk factor reduction (glycemic control, smoking cessation, diet, exercise, aggressive blood pressure control, treatment of dyslipidemia).
As a result, diabetes treatment guidelines have been updated to provide guidance on how to prevent and manage the onset of CVD [14]. Furthermore, there is increasing pressure from regulatory agencies that antidiabetic treatments demonstrate cardiovascular safety and benefits, especially for major cardiovascular events such as cardiovascular mortality, non-fatal MI, and stroke [15, 16]. Following these regulatory requirements, several cardiovascular outcomes trials (CVOT) have been completed, which demonstrate that certain anti-diabetic treatments are associated with a lower risk of CVD [17–20].
The increased focus on adequately treating patients with both CVD and T2DM requires that we have updated prevalence rates of CVD among patients with T2DM. This is especially needed to inform clinical and policy level decision-making by healthcare providers, healthcare policy decision-makers, and health economic analysts. Reviews have been published on the epidemiology of type 1 diabetes (T1DM), and CVD [21], pre-diabetes and the risk of CVD [22], or reviews have focused on specific countries [23]. However, there is no recent global review on the prevalence of CVD among adults with T2DM. Therefore, the objective of this systematic literature review was to quantitatively summarize rates of prevalence of CVD in adults with T2DM in studies published during the past 10 years.
Although CVD is an umbrella term that includes coronary artery disease (CAD), cerebrovascular disease (CBV), and peripheral vascular disease, the focus of this review was on CVD outcomes that are relevant to major cardiovascular events. Therefore, the review specifically focused on the prevalence of CAD and CBV. CAD has many synonyms, including ischemic heart disease, coronary heart disease (CHD), atherosclerotic heart disease, and atherosclerotic CVD. Conditions within this category are stable angina pectoris, unstable angina pectoris, MI (also known as heart attack), and sudden cardiac death (SCD). CBV comprises mainly stroke (intracerebral hemorrhage, cerebral infarction, cerebral arterial disease), but also may include transient ischemic attacks.
Methods
This review was undertaken in adherence to the PRISMA Statement for systematic reviews [24].
Eligibility criteria
Criteria for eligibility were guided by the PICO reporting system (which describes the participants, interventions, comparisons, and outcome[s] of the systematic review), together with the specification of the type of study design (PICOS), from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [24].
Participants
Included in this research were adult patients ≥ 18 years old who had been diagnosed with T2DM.
Interventions
Not applicable in this research.
Comparisons
Prevalence rates of CVD between males and females, and between obese and non-obese patients were compared. It was acknowledged that, in the literature, authors often used different terms or combinations of terms to describe their patients. The aim was to be all-inclusive in order to capture all relevant patient populations. Broad definitions of acceptable diseases were CVD, CAD, CHD, ischemic heart disease (IHD), congestive heart failure (CHF), or CBV. Specific conditions of interest included stroke, MI/heart attack, angina pectoris, heart failure, and atherosclerosis as well as cardiovascular or cardiac death.
Excluded were other forms of CVD including peripheral artery disease (PAD), rheumatic heart disease, cardiac dysrhythmias (e.g., atrial or ventricular fibrillation), or requirement for surgery such as coronary artery bypass grafting (CABG)/coronary revascularization. Also excluded were intermediate states such as hypertension or metabolic syndrome or studies of carotid intima-media thickness (CIMT).
Outcome[s]
The outcome of interest was the prevalence of each of these diseases/outcomes, then aggregated by continent/IDF Region, by country, and by the country’s economic status.
Study design
The primary focus was on prevalence studies and cross-sectional surveys, including database studies or patient chart reviews. Incidence studies were accepted only if they provided population-based baseline and follow-up data. Included were peer-reviewed studies published in any language. Both published articles and abstracts from scientific meetings were eligible. However, any published studies from clinical trial programs or individual pharmaceutical products were excluded.
Information sources and search strategy
The search was undertaken between February 15 and March 6, 2017. Databases searched included Medline and Embase between January 2007 and March 2017. In addition, PubMed was searched from 2014 to identify articles that were “ahead of print” yet fully available. Evidence presented at selected conferences during the last 5 years were accessed, including the Annual Meetings of the International Society Pharmacoeconomic Outcomes and Research (ISPOR), American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD) and American Association of Clinical Endocrinologists (AACE). Keywords linked to MeSH terms specific to each database were used in the search including prevalence, OR epidemiology, AND acute coronary syndrome, OR cardiovascular disease, OR cardiovascular death, OR non-fatal myocardial infarction, OR non-fatal stroke, OR obesity AND type 2 diabetes mellitus. Other keywords were cerebrovascular disease, cerebral arterial disease, intracerebral hemorrhage, cerebral infarction, coronary artery disease, ischemic heart disease, atherosclerotic heart disease, coronary heart disease, angina pectoris. Identified articles and previous reviews were hand searched for articles that may have included data useful to this search.
Article identification and selection
Two reviewers independently searched Medline, Embase and the proceedings of major scientific meetings for suitable papers. Results were compared and adjudicated through consensus discussion. A third reviewer checked all results for quality assurance.
Data collection
Data extracted from articles included information concerning the publication, the patients involved, and outcomes of interest. Publication items included the first author, year of publication, the country in which the data were collected, and date of data collection. Patient data collected included the number of patients screened, percentages of males and females, average age, duration of T2DM, the proportion with obesity (or average body mass index (BMI) ± SD). Outcome data consisted of the numbers and percentages of patients having each cardiovascular outcome, overall and separately for males and females, where available. The same procedure (two independent reviewers plus a third judge) was followed for data collection as for article selection.
Data analysis
Data were analyzed descriptively, with sums, averages, and medians, and ranges reported. The primary outcome was the estimate of prevalence rates of CVD in patients with T2DM. No overall quantitative synthesis was undertaken. Weighted averages were calculated for individual countries and IDF regions. For patient characteristics, we calculated simple averages and medians across studies. Due to a single study with a sample size of more than three million people, which skewed the data, we calculated weighted averages for patient characteristics with and without that study. It should be noted that averages were based on the studies that reported the outcome, which may then represent a subgroup of the entire pool of studies.
The risk of bias was explored by applying the checklist from the STROBE initiative [25]. They have produced a validated checklist of items that should be addressed in reports of observational studies. There are 22 main items, each of which addresses an issue of research design and is presented in a list of recommendations. Items are scored as dichotomously as acceptable or not acceptable.
Results
Included studies
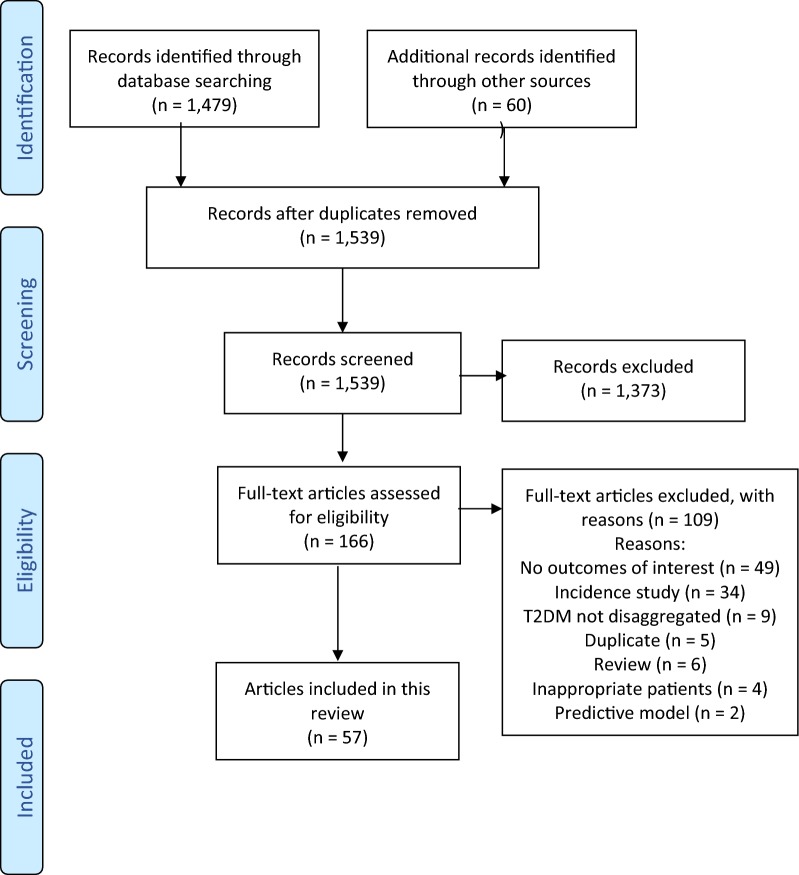
The flowchart in Fig. 1 depicts the article selection process. We initially identified 1539 papers that appeared to be suitable, but after examining them systematically, 57 studies were accepted. Three articles each presented two different sets of results [26–28]; therefore, there are 60 sets of analyses within these 57 articles. Table 1 lists these studies along with their descriptive variables. There were 51 full articles and six abstracts presented at scientific meetings. Data collectively represent more than 4.5 million people with T2DM from around the globe.
Fig. 1.
Flowchart of article selection. The flowchart depicts the article selection process
Table 1.
Overview of studies in the analysis
| Author (year) | Country | Patients | % obese or reported BMI | % males | Age (years) | Diabetes duration (years) | Follow-up (years) | Time of data collection |
|---|---|---|---|---|---|---|---|---|
| Alaboud (2016) [41] | Saudi Arabia | 748 | 64.3% | 42.4% | 57.9 | 13.3 | NR | Apr–Jun 2014 |
| Alonso-Moran (2014) [42] | Spain | 134,421 | NR | 54.0% | NR | NR | NR | 2007–2011 |
| Alwakeel (2008) [30] | Saudi Arabia | 1952 | 44.8% | 48.3% | 58.4 | 10.4 | 7.9 | Jan 1989–Jan 2004 |
| Bhatti (2016) [26]a | India | 1522 | BMI = 26.7 ± 4.4 | 58.3% | 58.1 | 7.2 | NR | 2011–2014 |
| Boonman-de Winter (2012) [38] | Netherlands | 581 | 28.1% | 53.4% | 71.6 | 5.5 | NR | Feb 2009–Mar 2010 |
| Cardoso (2008) [31] | Brazil | 471 | NR | 34.2% | 60.5 | 9.3 | 4.8 | 1994–1996, 2001 |
| Carnethon (2010) [32] | USA | 919 | BMI = 28.2 ± 4.9 | 53.4% | 72.8 | NR | 11.3 | (1989/92–93) through 2005 |
| Carrasco-Sánchez (2014) [43] | Spain | 490 | BMI = 31.4 ± 14.23 | 44.3% | 76.6 | NR | NR | 2008–2011 |
| Cheng (2014) [86] | China | 2834 | 91.6% | 51.8% | 58.5 | 7.0 | NR | Aug 2011–Mar 2012 |
| Collier (2015) [27]a | Scotland | 7385 | 51.0% | NR | 64.3 | NR | NR | NR |
| 6032 | 57.5% | NR | 66.4 | NR | NR | NR | ||
| Cortez-Dias (2010) [87] | Portugal | 3215 | 45.1% | 38.4% | 58.1 | NR | NR | Apr 2006–Nov 2007 |
| Daghash (2007) [88] | Qatar | 180 | BMI = 30.35 ± 4.9 | 43.0% | 51.3 | NR | NR | May–Oct 2004 |
| Doucet (2016) [89] | France | 987 | BMI = 29.7 ± 5.2 | 47.9% | 77.1 | 17.8 | NR | Jun 2009–Jul 2010 |
| Eeg-Olofsson (2010) [33] | Sweden | 18,334 | BMI = 28.8 ± 5 | 56.7% | 64.0 | 8.0 | 5.6 | 1997–1998–2003 |
| Farrell (2014) [90] | Ireland | 309 | NR | NR | NR | NR | NR | NR |
| Fu (2010) [91] | Spain, France, UK, Norway, Finland, Germany, Poland | 1942 | 52.9% | 64.4% | 64.5 | 6.2 | 2.8 | Jun 2006–Feb 2007 |
| Giallauria (2015) [92] | Italy | 475 | NR | 74% | 69.7 | NR | NR | Jan 28–Feb 10, 2008 |
| Glogner (2014) [40] | Sweden | 83,021 | BMI = 28.9 ± 5.04 | 55.3% | 65.8 | 7.6 | 7.2 | Enrolled: 1998–2003; through 2009 |
| Gobardhan (2017) [93] | Netherlands | 318 | 53.0% | 50.9% | 52.3 | 11.0 | 10.0 | NR |
| Gondim (2016) [94] | Brazil | 66 | BMI = 27.17 ± 4.62 | 43.9% | 64.6 | NR | NR | NR |
| Hermans (2016) [95] | Belgium | 711 | BMI = 29.5 ± 5.8 | 66% | 67.0 | 16.0 | NR | NR |
| Hunt (2014) [96] | USA | 1030 | BMI = 33.6 | 23.5% | 52.7 | 10.5 | NR | 1995–2003 |
| Jackson (2012) [97] | Scotland | 216,652 | NR | 53.6% | ≥40 | NR | 4.5 | 2001–2007 |
| Jurado (2009) [98] | Spain | 307 | 44.9% | 61.6% | 59.6 | 8.5 | NR | Nov 2001–Dec 2002 |
| Kucharska-Newton (2010) [99] | USA | 209 | BMI = 31.0 ± 6.0 | 43.5% | 55.5 | NR | NR | 1987–1989–2001 |
| Kwon (2014) [100] | Korea | 59 | NR | 59.3% | 64.5 | NR | 13.0 | Korea |
| Lin (2013) [45] | USA | 162,332 | NR | NR | ≥18 | ≥2 | ≥2 | USA |
| Liu (2015) [101] | China | 21,072 | NR | 53.9% | 63.7 | NR | NR | China |
| Luo (2014) [102] | China | 4836 | BMI: 24.3 | 57.6% | 64.9 | NR | 1.0 | China |
| MacDonald (2011) [103] | 247 countries | 669 | BMI = 31.2 ± 4.6 | 51.7% | 58.8 | 7.2 | 2.0 | 247 countries |
| Malik (2015) [34] | Scotland | 121,523 | BMI = 31.7 ± 6.6 | 52.0% | 63.0 | 4.2 | 4.8 | Scotland |
| Mansour (2013) [48] | Iraq | 1079 | 33.8% | 58.8% | 56.3 | 7.4 | NR | Iraq |
| Mazza (2007) [104] | Italy | 581 | 30.1% | 34.8% | 74.3 | 20.3 | 12.0 | Italy |
| Menghua (2014) [49] | China | 240 | NR | NR | NR | NR | NR | China |
| Menzaghi (2014) [36] | Italy | 2094 | BMI = 29.1 ± 5.3 | 51.3% | 61.9 | 10.4 | 12.9 | Italy |
| Mody (2007) [105] | USA | 4816 | NR | 34.4% | 50.9 | NR | NR | USA |
| Mundet (2012) [106] | Spain | 4298 | BMI = 29.34 ± 4.84 | 48.2% | 67.4 | 8.4 | 10.0 | Spain |
| Narksawat (2013) [107] | Thailand | 1505 | 32.2% | 30.4% | 63.3 | NR | NR | Thailand |
| Norhammar (2016) [50] | Sweden | 352,436 | NR | 56.1% | 67.1 | NR | NR | Sweden |
| Penno (2015) [108] | Italy | 11,538 | 34.5% | 52.9% | 65.5 | 12.5 | NR | Italy |
| Rodriguez-Poncelas (2014) [109] | Spain | 1141 | 46.5% | 60.6% | 66.8 | 9.1 | NR | Feb–Jul 2011 |
| Rossi (2011) [51] | Italy | 5181 | BMI = 29.8 ± 5.0 | 58.4% | 64.4 | 10.0 | 2.3 | (Jan 2006–Nov 2007), 2009 |
| Salinero-Fort (2016) [35] | Spain | 3407 | BMI = 30.1 ± 4.9 | 50.3% | 69.0 | 9.1 | 5.0 | 2007 (2008–2012) |
| Senthil (2014) [69] | India | 134 | NR | 72.1% | NR | NR | NR | NR |
| Shestakova (2016) [29] | Russian Federation | 3,060,517 | NR | 28.3% | NR | NR | 1.0 | 2014–2015 |
| Soetedjo (2014) [110] | Indonesia | 400 | 56.8% | 43.8% | 57.7 | 10.3 | NR | Dec 2013–Jun 2014 |
| Song (2009) [46] | UK | 2733 | BMI = 33.4 ± 6.7 | NR | 64.2 | 12.7 | NR | 2008 |
| Suh (2008) [111] | USA | 608 | 51.4% | 44.82% | 73.2 | 12.9 | 10.0 | 1999–2004 |
| Tamba (2013) [37] | Cameroon | 132 | 30.0% | 56% | 58.0 | 12.0 | 6.0 | 2000–2009 |
| Tan (2016) [28]a | Australia | 793 | 54.8% | 50.9% | 67.2 | 8.0 | 15.0 | 2008–2011 |
| 65 | 35.4% | 56.9% | 61.1 | 10.0 | 15.0 | |||
| Utrera-Lagunas (2013) [112] | Mexico | 160 | 33.8% | 45.0% | 69.2 | 18.3 | NR | Feb 2011–Jan 2012 |
| Vinagre (2012) [113] | Spain | 286,791 | 45.4% | 53.7% | 68.2 | 6.5 | NR | 2009 |
| Wentworth (2012) [39] | Australia | 711 | >50% | 55.1% | 53.0 | 11.4 | NR | 1998–2011 |
| Wong (2012) [114] | USA | 889 | NR | 46.2% | 60.6 | 13.3 | NR | 2003–2006 |
| Yan (2015) [115] | Hong Kong | 10,952 | 63.6% | 56.1% | 58.2 | 7.0 | NR | Nov 2007–Jul 2012 |
| Yang (2015) [47] | Korea | 595 | BMI = 24.29 ± 3.15 | 58.32% | 64.9 | 13.6 | NR | 2006–2010 |
| Zekry (2012) [116] | Switzerland | 83 | BMI = 27.2 ± 4.9 | 36.1% | 84.2 | NR | 4.0 | Jan 2004–Dec 2005 |
| 57 studies | Total | 4549,481 | ||||||
| Median | 1030 | 45.4% | 52.0% | 64.3 | 10.0 | 6.0 | ||
| Average | 77,110 | 47.0% | 50.5% | 63.6 | 10.4 | 7.3 | ||
| SD (range) | (59–3,060,517) | 14.7% | 10.3% | 6.9 | 3.7 | 4.5 |
In Table 2, results are presented geographically according to the classification system used by the IDF [1]. Studies from 25 countries were represented in this review: Australia, Belgium, Brazil, Cameroon, China, France, India, Indonesia, Iraq, Ireland, Italy, Korea, Mexico, Netherlands, Portugal, Qatar, Russian Federation, Saudi Arabia, Scotland, Spain, Sweden, Switzerland, Thailand, UK and USA. Details are provided in Table 3. Three areas were responsible for generating 80% of the studies. Europe produced most articles (46%), followed by the Western Pacific/China (21%), and North America (13%). The other 20% were from the rest of the world. There were no discernible patterns differentiating prevalence rates between countries, based on income status. Part of the problem is that there are few studies in low- and middle-income countries and none from those in the lowest income level.
Table 2.
Geographic distribution of prevalence studies of cardiovascular disease in type 2 diabetes mellitus
| Region | Populationa (millions) | Studies | N | Stroke (%) | MI | Angina | Heart failure | Atherosclerosis | CAD | CVD (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Africa | 441 | 1 | 132 | 5.0 | NR | NR | NR | NR | 23.6% | 28.6 |
| Europe | 660 | 29b | 4,327,503 | 7.2 | 10.0% | 14.6% | 19.0% | 33.0% | 15.4% | 30.0 |
| Middle East and North Africa | 387 | 4 | 3959 | 7.1 | 11.4% | NR | NR | NR | 27.4% | 26.9 |
| North America and Caribbean | 344 | 8 | 170,963 | 10.9 | 13.6% | 17.2% | 29.5% | NR | 20.1% | 46.0 |
| South and Central America | 315 | 2 | 537 | 5.5 | NR | NR | 4.2% | NR | 22.6% | 27.5 |
| Southeast Asia | 926 | 3b | 1656 | 3.1 | NR | NR | NR | NR | 29.4% | 42.5 |
| Western Pacific (includes China) | 1600 | 12b | 44,062 | 11.4 | NR | NR | 4.3% | 26.0% | 23.6% | 33.6 |
| Multiple countries | NR | 1 | 669 | 1.9 | 3.9% | 9.9% | 0.7% | NR | NR | 16.4 |
| Totalc | 4673 | 60 | 4,549,481 | 7.6 | 10.0% | 14.6% | 14.9% | 29.1% | 21.2% | 32.2 |
Table 3.
Number of studies and cardiovascular outcomes reported, by country
| Country | Income statusa | Studies | Patients | Stroke | MI | Angina | CHF | Atherosclerosis | CAD | CVD (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Australia | High | 3b | 1569 | 7.7% | NR | NR | NR | NR | 21.8% | 29.4 |
| Belgium | High | 1 | 711 | 8.0% | NR | NR | NR | NR | 22.0% | 30.0 |
| Brazil | Upper middle | 2 | 537 | 5.5% | NR | NR | 4.2% | NR | 22.6% | 27.5 |
| Cameroon | Low middle | 1 | 132 | 5.0% | NR | NR | NR | NR | 23.6% | 28.6 |
| China | Upper middle | 5 | 39,934 | 15.2% | NR | NR | 4.3% | 30.5% | 17.0% | 28.4 |
| France | High | 1 | 987 | 15.3% | NR | NR | 9.1% | NR | 29.5% | 53.9 |
| India | Low middle | 3b | 1656 | 3.1% | NR | NR | NR | NR | 29.4% | 42.6 |
| Indonesia | Low middle | 1 | 400 | 10.8% | NR | NR | NR | NR | 28.8% | 39.6 |
| Iraq | Upper middle | 1 | 1079 | NR | NR | NR | NR | NR | NR | 16.0 |
| Ireland | High | 1 | 309 | 5.2% | NR | NR | NR | NR | 17.8% | 23.0 |
| Italy | High | 5 | 19,869 | 2.4% | 6.2% | NR | NR | NR | 11.1% | 14.8 |
| Korea | High | 2 | 654 | 20.3% | NR | NR | NR | 21.5% | 37.0% | 47.2 |
| Mexico | Upper middle | 1 | 160 | NR | NR | NR | 57.5% | NR | NR | 57.5 |
| Netherlands | High | 3 | 899 | 6.1% | NR | NR | 30.6% | 33.0% | 28.2% | 44.5 |
| Portugal | High | 1 | 3215 | 5.0% | NR | NR | NR | NR | 12.0% | 17.0 |
| Qatar | High | 1 | 180 | NR | NR | NR | NR | NR | 31.7% | 31.7 |
| Russian Federation | Upper middle | 1 | 3,060,517 | 4.2% | 3.4% | NR | NR | NR | 13.4% | 21.0 |
| Saudi Arabia | High | 2 | 2700 | 7.1% | 11.4% | NR | NR | NR | 23.1% | 30.0 |
| Scotland | High | 3b | 351,592 | NR | NR | NR | NR | NR | 19.7% | 19.7 |
| Spain | High | 7 | 430,855 | 8.0% | 20.2% | NR | 30.3% | NR | 13.6% | 29.8 |
| Sweden | High | 3 | 453,791 | 10.3% | 9.3% | 14.6% | 9.5% | NR | NR | 31.3 |
| Switzerland | High | 1 | 83 | 15.7% | NR | NR | NR | NR | 33.7% | 49.4 |
| Thailand | Upper middle | 1 | 1505 | 2.5% | NR | NR | NR | NR | NR | 2.5 |
| UK | High | 1 | 2733 | 7.9% | NR | NR | NR | NR | 34.5% | 42.4 |
| USA | High | 7 | 170,803 | 10.9% | 13.6% | 17.2% | 15.5% | NR | 28.1% | 44.0 |
| 16 countries | High | 42 | 1,440,950 | 9.3% | 12.1% | 15.9% | 19.0% | 27.3% | 24.3% | 33.6 |
| 6 countries | Upper middle | 11 | 3,103,732 | 6.9% | 3.4% | 22.0% | 30.5% | 17.7% | 25.5 | |
| 3 countries | Low middle | 5 | 2188 | 6.3% | 27.3% | 36.9 | ||||
| 0 countries | Low | 0 | 0 | |||||||
| 25 countries | Overall | 58 | 4,546,870c | 8.4% | 10.7% | 15.9% | 20.1% | 28.3% | 23.7% | 32.1 |
Patient characteristics
In the 57 individual studies, data from 4.5 million people with T2DM were presented with nearly 3.1 million people coming from a single Russian study by Shestakova [29]. As presented in Table 1, using a simple average across studies, the average age was 63.6 ± 6.9 (median = 64.3 years; weighted average = 66.3 ± 6.9 years). The weighted average proportion of persons with obesity was 46.3 ± 15.0%, with a simple average of 47.0 ± 14.7% (median = 45.4%), defined as a BMI ≥ 30 kg/m2. The mean percentage of males across the studies was 50.5 ± 10.3% (median = 52.0%); the weighted average of the proportion of males was 36.0 ± 10.0%, including the study by Shestakova [29], and 54.1 ± 9.9% excluding that study. The patients had T2DM for an average duration of 10.4 ± 3.7 years (median = 10.0 years; weighted average = 6.6 ± 3.7 years). Among the 23 studies that reported duration of follow-up, the average was 7.3% ± 4.5 years (median = 6.0 years; weighted average = 5.2 ± 4.3 years).
Prevalence rates of cardiovascular comorbidities are summarized in Table 4 for all patients as well as separately for males and females. In studies reporting gender-specific prevalence rates, males had higher prevalence rates than females for all outcomes except overall CVD, where both sexes had an overall prevalence rate of approximately 27%. Overall, in studies that presented prevalence rates for males and females combined, the prevalence of CVD among persons with T2DM was 32.2%. CAD and atherosclerosis were the most prevalent CVD comorbidities, with prevalence rates of 21.2 and 29.1%, respectively, whereas stroke was the least prevalent with a prevalence rate of 7.6%. It is unclear why people with T2DM have different susceptibilities to these diseases. An explanation for the high prevalence rate for atherosclerosis could be that it is an artifact of patient selection. In the studies that examined atherosclerosis, most patients were enrolled if they had high-risk scores for atherosclerosis, resulting in a very high rate of disease detection.
Table 4.
Summary of prevalence rates of cardiovascular comorbidities in persons with type 2 diabetes
| Sex | Cardiovascular outcome | Studies | N | Ratea (%) | 95% confidence interval (%) |
|---|---|---|---|---|---|
| Both | Stroke | 39 | 3,901,505 | 7.6 | 6.6–8.6 |
| Myocardial infarction | 13 | 3,518,833 | 10.0 | 7.5–12.5 | |
| Angina pectoris | 4 | 354,743 | 14.6 | 12.0–17.3 | |
| Heart failure | 14 | 601,154 | 14.9 | 13.0–16.7 | |
| Atherosclerosis | 4 | 1153 | 29.1 | 21.7–36.4 | |
| Coronary artery disease | 42 | 3,833,200 | 21.2 | 20.3–22.2 | |
| Cardiovascular disease (any) | 53 | 4,289,140 | 32.2 | 30.0–34.4 | |
| Malesb | Stroke | 10 | 232,525 | 6.7 | 6.0–7.3 |
| Myocardial infarction | 2 | 1170 | 11.9 | 4.3–19.5 | |
| Angina pectoris | 1 | 454 | 21.1 | 16.3–26.9 | |
| Heart failure | 4 | 73,361 | 25.3 | 11.4–39.2 | |
| Coronary artery disease | 9 | 237,367 | 18.7 | 16.5–20.8 | |
| Cardiovascular disease | 16 | 241,406 | 27.6 | 25.3–29.9 | |
| Femalesb | Stroke | 10 | 202,348 | 5.9 | 5.1–6.7 |
| Myocardial infarction | 2 | 1812 | 9.8 | 3.5–16.0 | |
| Angina pectoris | 1 | 803 | 17.4 | 15.0–20.2 | |
| Heart failure | 4 | 62,690 | 24.0 | 11.2–36.8 | |
| Coronary artery disease | 10 | 205,493 | 14.3 | 12.4–16.1 | |
| Cardiovascular disease | 16 | 209,153 | 27.2 | 22.7–31.7 |
CVD mortality among patients with T2DM
Table 5 presents the data regarding the rates of mortality associated with CVD in persons with T2DM. The weighted average of death rates from the eight studies with 3,208,557 patients with T2DM was 9.9% (95% CI 8.6–11.3%) [29–36]. There were 6.3% who died due to CAD and another 1.5% from CBV. Comparing patients with both T2DM and CVD with patients having neither T2DM nor CVD, the odds ratio for death was 4.56 (95% CI 3.53–5.89) [32]. Using a weighted average from seven studies (N = 86,557) [29–35], the calculated deaths due to CVD comprised 50.3% (95% CI 37.0–63.7%) of all deaths in patients with T2DM. The major contributors were CAD, which was responsible for 29.7% (95% CI 25.1–34.4%) and stroke/CBV for 11.0% (95% CI 8.8–13.3%).
Table 5.
Mortality associated with cardiovascular disease in persons with type 2 diabetes
| Disease | Author (year) | Data collection period | Patients | n | All deaths | % | CVD Deaths | % Death rate | % CVD proportion of all deaths |
|---|---|---|---|---|---|---|---|---|---|
| CVD | Alwalkeel (2008) [30] | Jan 1989–Jan 2004 | T2DM adults | 1952 | 161 | 8.20% | 97 | 5.00% | 60.20% |
| CVD | Cardoso (2008) [31] | 1994–96 to 2001 | T2DM adults | 471 | 121 | 25.70% | 44 | 9.30% | 36.40% |
| CVD | Carnethon (2010) [32] | 1989–93 to 2005 | T2DM only | 659 | 468 | 71.00% | 211 | 32.0% | 45.10% |
| CVD | Carnethon (2010) [32] | 1989–93 to 2005 | CVD only | 868 | 620 | 71.40% | 304 | 35.0% | 49.00% |
| CVD | Carnethon (2010) [32] | 1989–93 to 2005 | T2DM + CVD | 260 | 219 | 84.20% | 129 | 49.60% | 58.90% |
| CVD | Carnethon (2010) [32] | 1989–93 to 2005 | No T2DM or CVD | 3997 | 2095 | 52.40% | 710 | 17.8% | 33.90% |
| CVD | Eeg-Olofsson (2010) [33] | 1997–98 to 2003 | T2DM adults | 18,334 | 1902 | 10.40% | 1456 | 7.90% | 76.60% |
| CVD | Malik (2015) [34] | 2005–2011 | T2DM adults | 121,523 | 17,637 | 14.50% | 3722 | 3.10% | 21.10% |
| CVD | Menzaghi (2014) [36] | 2001–2008 | GHS study—males | 242 | NR | – | 42 | 17.40% | – |
| CVD | Menzaghi (2014) [36] | 2001–2008 | GHS study—females | 117 | NR | – | 16 | 13.70% | – |
| CVD | Menzaghi (2014) [36] | 1993–1999 | HPFS study—males | 833 | NR | – | 146 | 17.50% | – |
| CVD | Menzaghi (2014) [36] | 1976–1990 | NMS study—females | 902 | NR | – | 144 | 16.00% | – |
| CVD | Salinero-Fort (2016) [35] | 2007–08 to 2012 | T2DM adults | 2442 | 203 | 8.30% | 96 | 3.90% | 47.30% |
| CVD | Salinero-Fort (2016) [35] | 2007–08 to 2012 | T2DM adults + kidney disease | 965 | 221 | 22.90% | 124 | 12.80% | 56.10% |
| CVD | Shestakova (2016) [29] | 2015 | T2DM adults | 3,060,516 | 66,093 | 2.20% | 30,560 | 1.00% | 46.20% |
| All CVD death | Patients with T2DM | 3,208,557 | 86,557a | 42.3%a | 36,576a | 9.9%a | 50.3%a | ||
| CAD | Cardoso (2008) [31] | 1994–1996 to 2001 | T2DM adults | 471 | 121 | 25.70% | 30 | 6.40% | 24.80% |
| CAD | Carnethon (2010) [32] | 1989–1993 to 2005 | T2DM only | 659 | 468 | 71.00% | 132 | 20.00% | 28.20% |
| CAD | Carnethon (2010) [32] | 1989–1993 to 2005 | CVD only | 868 | 620 | 71.40% | 213 | 24.50% | 34.40% |
| CAD | Carnethon (2010) [32] | 1989–1993 to 2005 | T2DM + CVD | 260 | 219 | 84.20% | 111 | 42.70% | 50.70% |
| CAD | Carnethon (2010) [32] | 1989–1993 to 2005 | No T2DM or CVD | 3997 | 2095 | 52.40% | 425 | 10.60% | 20.30% |
| CAD | Jackson (2012) [97] | 2001–2007 | Male diabetics | 116,145 | 22,033 | 19.00% | 6000 | 5.20% | 27.20% |
| CAD | Jackson (2012) [97] | 2001–2007 | Male non-diabetics | 2,433,748 | 36,801 | 1.50% | |||
| CAD | Jackson (2012) [97] | 2001–2007 | Female diabetics | 100,507 | 20,571 | 20.50% | 4554 | 4.50% | 22.10% |
| CAD | Jackson (2012) [97] | 2001–2007 | Female non-diabetics | 2,630,482 | 32,449 | 1.20% | |||
| All CAD death | Patients with T2DM | 218,462a | 42,944a | 24.9%a | 10,695a | 6.3%a | 29.7%a | ||
| CHF | Mazza (2007) [104] | 1983–1985 to 1997 | Male diabetics | 202 | – | – | 22 | 10.90% | – |
| CHF | Mazza (2007) [104] | 1983–1985 to 1997 | Female diabetics | 379 | – | – | 29 | 7.70% | – |
| CHF | Mazza (2007) [104] | 1983–1985 to 1997 | All | 581 | 369 | 63.50% | – | – | 13.80% |
| CHF | Shestakova (2016) [29] | 2015 | T2DM adults | 3,060,516 | 66,093 | 2.20% | 18,963 | 0.60% | 28.70% |
| All CHF deaths | Patients with T2DM | 3,061,097a | 66,093a | 2.2%a | 1,9104a | 6.1%a | 28.7%a | ||
| MI | Shestakova (2016) [29] | 2015 | T2DM adults | 3,060,516 | 66,093 | 2.20% | 3393 | 0.10% | 5.10% |
| SCD | Kucharska-Newton (2010) [99] | 1987–89 to 2001 | T2DM adults | 1550 | NR | – | 69 | 4.50% | – |
| SCD | Kucharska-Newton (2010) [99] | 1987–89 to 2001 | No T2DM | 12,428 | NR | – | 140 | 1.10% | – |
| Stroke | Jackson (2012) [97] | 2001–2007 | Male diabetics | 116,145 | 22,033 | 19.00% | 1942 | 1.70% | 8.80% |
| Stroke | Jackson (2012) [97] | 2001–2007 | Male non-diabetics | 2,433,748 | 13,191 | 0.50% | |||
| Stroke | Jackson (2012) [97] | 2001–2007 | Female diabetics | 100,507 | 20,571 | 20.50% | 2436 | 2.40% | 11.80% |
| Stroke | Jackson (2012) [97] | 2001–2007 | Female non-diabetics | 2,630,482 | 23,632 | 0.90% | |||
| Stroke | Shestakova (2016) [29] | 2015 | T2DM adults | 3,060,516 | 66,093 | 2.20% | 8204 | 0.30% | 12.40% |
| All CBV deaths | Patients with T2DM | 327,168a | 108,697a | 3.30% | 12,582a | 1.50% | 11.0%a |
CVD among obese vs. non-obese people with T2DM
About half of the patients included in this analysis had obesity. Three-quarters of the included studies reported on patients’ BMI or the percent of patients with obesity. While the definitions and BMI cut-off points of obesity varied across studies, the most commonly used definition of obesity was a BMI ≥ 30 kg/m2, which was employed by 16 studies (43% of those providing a definition).
Five papers reported prevalence rates of CVD according to obesity status, and all of them found a positive relationship between obesity and increased prevalence rates of CVD [26, 37–40]. Using logistic regression to control for multiple factors, Bhatti et al. [26] found a positive correlation between obesity and CAD (P = 0.021). Tamba et al. [37] reported positive correlations between obesity and both CAD (r = 0.3, P < 0.001) and stroke (r = 0.5, P < 0.001). Boonman-de Winter et al. [38] quantified the relationship between BMI and heart failure. The prevalence rate of heart failure was 38.7% (95% CI 31.2–46.1%) in patients with a BMI ≥ 30 kg/m2 and 23.4% (95% CI 19.4–27.5%) in those with a BMI < 30 kg/m2, which represents a 65% increase due to obesity.
Two studies explored the relationship between increasing BMI and risk of CVD [39, 40]. According to Wentworth et al. [39], for CAD in both males and females, the prevalence rate of CAD increased with each successive increase in BMI, with a five-fold increase between the lowest and highest categories [< 25 (normal), 25–30 kg/m2 (overweight), 30–35 kg/m2 (mild obesity), 35–40 kg/m2 (moderate obesity) and > 40 kg/m2 (severe obesity)]. The difference was that prevalence rates in males were about double those for females in every BMI category. For the outcome stroke/transient ischemic attack (TIA) in males, only the highest category (BMI > 40) had elevated prevalence rates, which were about double those for the lowest category (BMI < 25). For females, prevalence rates of stroke/TIA increased in those who were overweight and had mild or moderate obesity but decreased for those with severe obesity. Finally, Glogner et al. [40] had quite different results. They reported a steady increase in prevalence rates of MI from 6.86% in those with a BMI < 20–9.33% in patients who were overweight (BMI 25–30), a 36% increase. However, MI prevalence rates declined thereafter with each increasing category of obesity. The highest category (BMI ≥ 40) had a prevalence rate of 5.01%, which was 27% lower than those in the lowest category (BMI < 20). Thus, patterns vary quite widely, and studies often examined different outcomes.
Risk of bias in included studies
In the assessment of risk for bias, the studies addressed 80% of the STROBE checklist items (i.e., research design and data presentation), on average. The mean was 80 ± 12%, and the median was 81%, with a range of 54–100%. The two items that were addressed by 100% of the articles were reporting of outcome data and reporting of outcomes. The two items addressed the least were the statement of funding (56%) and indicating the study design with a commonly used term in the title or abstract (60%).
Discussion
In this systematic review of 4,549,481 persons with T2DM, we estimated the overall prevalence of CVD at 32.2%. The most frequent type of CVD reported was CAD (21.2%) and lowest was stroke (7.6%). Males had higher rates of prevalent disease than females. CVD was responsible for 50.3% of all deaths in T2DM patients over the period of the review. Along with diabetes, cardiovascular disease is associated with several risk factors, obesity, and age. We, therefore, evaluated the association between age and obesity among patients with CVD and T2DM in the selected articles.
Age as a risk factor for CVD
Age is a well-known risk factor for CVD. Out of the 57 articles, thirteen (25%) reported on the relationship between age and CVD and the results were quite mixed. Nine studies identified a significant relationship between age and CVD [30, 38, 41–47], but only two presented results across multiple age categories [38, 42]. Alonso-Moran [42] found that the odds ratio for IHD, stroke, heart failure and MI all increased sequentially with each increase in 5-year age category as compared with the age group 35–39 used as a reference. All of these individual outcomes achieved statistical significance (P < 0.001). Boonman-de Winter et al. [38] similarly reported a sequential increase in prevalence rates of heart failure for all patients in 5-year age categories from 60 to 64 to > 80 years of age. Other authors reported that older patients had higher prevalence rates than younger patients, but provided few details on age categories [30, 41, 43, 45, 46]. On the other hand, four studies reported no differences between age categories [26, 34, 48, 49]. Three other studies used age as a covariate in a logistic regression with no further details [28, 50, 51]. Therefore, few studies have quantified the effect of age on CVD prevalence rates among people with T2DM.
Obesity as a risk factor for CVD
Obesity has long been established as an independent risk factor for CVD [7, 52], and is associated with CAD [53, 54], atherosclerosis [51], and cardiac death [55, 56]. Furthermore, it has been shown that overweight and obesity are highly prevalent in T2DM patients with high CV risk and that BMI and waist circumference are related to major cardiometabolic risk factors such as hypertension and elevated low-density lipoprotein cholesterol (LDL-C) [57].
Obesity is usually defined by body mass index (BMI, calculated as body weight in kg divided by the square of height in meters), with the World Health Organization (WHO) classifying adults with a BMI 30 kg/m2 as obese [58]. However, BMI as a measure to stratify patients with obesity has limitations and does not account for the wide variation in body fat distribution nor the quality of fat, and may not account for associated health risk in different individuals and populations [58]. This has been shown to be true for South Asian populations [59]. In a study from Raji et al. [60] noted that compared with Caucasians, Asian Indians had significantly greater total abdominal and visceral fat matched with Caucasians of the same age, gender, and BMI, meaning that this population has an increased CVD risk. Besides, there is a weaker association between increasing BMI and T2DM in Asian populations compared with Caucasians due to the risk for T2DM begins increasing at comparatively normal BMI in Asian populations [61].
Seven of the included studies evaluated the relationship between obesity and/or BMI and CVD risk. Five of the studies included in this review identified a positive relationship between obesity and increased prevalence rates of CVD [26, 37–40]. One of these studies [26] used lower BMI cut-off points to account for Asian populations in accordance with WHO recommendations on BMI for Asian populations [62] and evaluated abdominal adiposity with waist circumference measurements to determine the prevalence of obesity. Overall, the studies found a positive relationship between increasing BMI and CVD; except in one study [39], where women with severe obesity had a reduced prevalence of stroke. While the authors do not explain the reduced prevalence of stroke/TIA, it may be explained by differences in vascular risk markers in men, such as pre-existing ischemic heart disease, age, and smoking [63]. Furthermore, the presence of gonadal steroids, most notably estrogen, may lend a protective effect against stroke/TIA in women and it has been shown that adiposity is associated with increased levels of estrogen [64].
Although obesity is identified as a risk factor for CVD, it is associated with a paradox in that mortality is lower in patients who are overweight or obese than in those whose BMI is normal or underweight [65]. Lee et al. [66] reported that obesity provided a survival benefit to patients with heart failure who did not have comorbid diabetes, but not in patients who did have concomitant diabetes. In contrast, a group led by Abi Khalil [67] examined a cohort of 2492 T2DM patients in seven countries in the Middle East, Gulf region, with acute heart failure. They reported that BMI was inversely correlated with the risk of mortality, with severe obesity associated with less mortality risk.
It is clear that the relationship between obesity and the risk of CVD and CVD-related deaths requires further exploration to identify these mechanisms and relationships.
CVD-related mortality in T2DM
In persons with T2DM, CVD is responsible for at least half of the mortality, as previously mentioned. Among the specific diseases within that term, CAD was most lethal, followed by stroke. Similar results have been demonstrated with other models. In an incidence-based study, Straka et al. [68] followed 29,863 patients (5501 with T2DM and 24,362 without T2DM) over a 1-year period. Four of the incident cardiovascular outcomes they reported were significantly higher in those with T2DM. Patients with T2DM had 10% greater risk of CAD, 53% of MI, 58% of stroke, and 112% increased risk of heart failure. Therefore, T2DM is a substantial risk factor for CVD and its consequences.
CVD prevalence rates across regions and countries
As this was a global review, studies from across the world were included. Given the variation in which diabetes and its macrovascular complications are treated and managed across countries and income levels, it is relevant to look at prevalence rates across regions and countries. However, almost half (46.0%) of the research was produced in Europe, and very little information was obtained from the less developed regions of the world such as Africa, Latin America, and the Asian subcontinent.
As shown in Table 2, the regions with the highest prevalence of overall CVD were North America and Caribbean (46.0%; N = 4,327,503), Southeast Asia (42.5%, N = 537) and Western Pacific (including China) (33.6%; N = 44,062). Southeast Asia stands out with a higher prevalence of CAD (29.4%) compared with other regions. The prevalence of CAD in this region is driven by one study from India [69], which specifically investigated the pattern of CAD in 134 symptomatic T2DM patients in India. However, epidemiological studies on people of South Asian origin have shown an increased likelihood of developing CAD that is up to two times higher than in Caucasians [70]. The higher risk is due to both pathophysiological and life course-related risk factors [70].
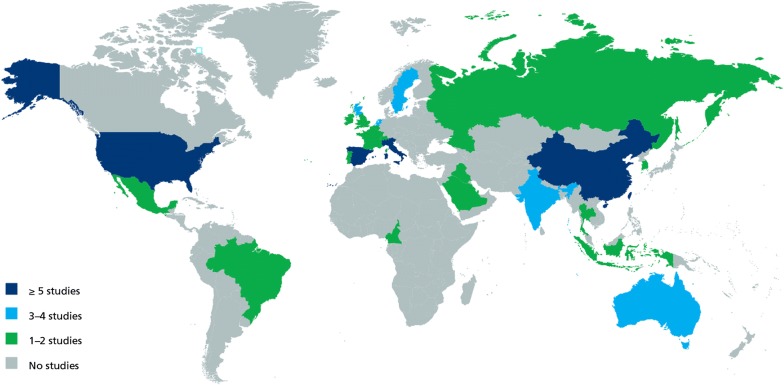
The summaries across countries and regions provide an overview of the geographic spread of research but should be interpreted with caution given the limited number of studies for some of the regions and countries. Figure 2 illustrates the distribution of studies across regions and countries and clearly shows that few studies exist for several regions. For example, only one study from one country in the African region was identified and therefore should not be seen to represent findings for the region’s entire T2DM population.
Fig. 2.
Distribution of studies across countries and regions. The figure illustrates the global distribution of studies across countries and regions
Treatment of both T2DM and CVD vary greatly between and within countries, and although much of the CVD risk in T2DM can be associated with the long-term complications of T2DM, there has been growing interest to determine whether certain antidiabetic drugs influence this risk. For example, sulfonylureas which are the second most commonly used antidiabetic drug after metformin, have been shown to be associated with an increased risk of cardiovascular events and mortality [71]. Newer antidiabetic drugs have been shown to lower the risk of CVD in T2DM patients [17–20]; however, these drugs are often intended to be used as second- to third-line treatments and many years may pass before patients can benefit.
Temporal trends in CVD risk assessment and management in T2DM
Encouragingly, CVD mortality is declining in high-income countries among the general population due to reductions in cardiovascular risk factors as well as to recent advances in prevention, treatment, and management [72]. This trend has also been observed in people with T2DM in some countries. Jung et al. [73] estimated trends in CVD in people with and without T2DM in South Korea using data from the national health insurance system. The results show a significant reduction in CVD risk among people with T2DM brought on by improvements in the care and management of patients. However, in many developing countries where the burden of T2DM is rapidly rising and lifestyle patterns changing an increase in CV risk factors among people with T2DM can be expected [3]. A study from China analyzed the relationship between lifestyle behaviors and multiple CV risk factors in 25,454 people with T2DM [74]. The researchers found that unhealthy lifestyles were common, especially among those who are non-elderly, and above-college educated. Furthermore, it was found that an unhealthy lifestyle was associated with poor blood, blood lipid, and blood pressure control. Decreasing the impact of T2DM and CVD in developing countries will require interventions aimed at changing risky lifestyle behaviors.
Screening people with T2DM for CV risk is an important strategy for reducing mortality and CVD events. A study from Denmark [75] found that a single round of diabetes screening and cardiovascular risk assessment in middle-aged adults in general practice was associated with a significant reduction in risk of all-cause mortality and CVD events in people with T2DM. The same researchers found that population-based stepwise screening for T2DM and CVD among all middle-aged adults was not associated with a reduction in mortality or CV events. Therefore, the benefits of population-based screening are limited in this context [76]. Kesall et al. [77] found that targeting specific occupational and industry groups with health checks could help identify individuals at high risk of both T2DM and CVD. In a study of 500,000 members of the Australian working population, they found that high T2DM and CVD risk was increased significantly in many occupational groups and industries.
Recent research points to an increasingly better understanding of the markers for identifying high CVD risk in people with T2DM. Li et al. [78] found that the combined application of carotid and lower extremity ultrasonography may be helpful to identify patients with T2DM who have a higher CVD risk. In a study of 2830 hospitalized patients with T2DM, they found that the concomitant presence of carotid and lower extremity atherosclerosis further increases the risk of CVD in patients with T2DM, compared with those who had either carotid or lower limb atherosclerosis and those without atherosclerosis. A study by Mohammedi et al. [79] found that major peripheral arterial disease (PAD) presenting as lower-extremity ulceration or amputation and peripheral revascularization is associated with increased risk of death and CV events in people with T2DM. The researchers conclude that screening for PAD along with active management are crucial for prevention of CVD in people with T2DM. In addition, coronary artery calcium (CAC) assessments have been found to significantly improve the risk classification for CHD and atherosclerotic CVD events in people with T2DM—regardless of the duration of diabetes [80]. Thus, a CAC assessment can be a useful tool for classifying people with T2DM into lower- or higher-risk groups for long-term CVD risk.
Lipid profile has long been considered among the most important risk factors for CVD in T2DM, and several trials have confirmed that lowering low-density lipoprotein cholesterol (LDL-C) via statins in T2DM was effective in reducing the risk of CVD [81, 82]. It is also well known that statins also have a triglyceride-lowering effect [83]. In a cross-sectional study of 223,612 patients with T2DM in China, researchers found that although lower triglyceride was associated with reduced CVD risk in the short-term, it was associated with increased risk in the long-term [84]. This paradox could mean that low triglyceride is not necessarily associated with good clinical outcomes in all people with T2DM and that there are subgroup associations with CVD in patients with different durations of T2DM. Furthermore, Clua-Espuny et al. [85] suggest that the relative importance of risk factors wanes in complex chronic patients with T2DM with advancing age. In a cohort study of almost 3500 complex chronic patients above the age of 80 of whom 53% had diabetes and a high prevalence of associated classical risk factors, the researchers found that all-cause mortality was more affected by aging factors than by specific complications of diabetes. The authors make the recommendation that, for these patients, the care strategy may need to be redefined and adapted to comorbidities and functional autonomy rather than being focused on treatment outcomes.
Limitations
As with all literature reviews, we were limited by the availability of the literature and the validity and quality of the articles. Some of the results appeared only in abstract form, and many were not subsequently published as full articles within the time horizon of this review. Abstracts had space limitations, restricting the amount of information they could present. As well, we noted that there was often incomplete reporting or selective reporting of specific outcomes of interest.
Furthermore, the findings of this literature review are limited to a select patient population. Specifically, in this research, we accepted only data from adults aged 18 or older. Therefore, our results may not apply to children or adolescents. As well, we dealt only with T2DM; therefore, outcomes may not apply to T1DM or secondary diabetes such as that associated with hemochromatosis or pancreatitis.
This study was also challenged by the fact that CVD and its associated conditions are described differently across the literature. For example, CHD was used interchangeably with CAD or ischemic heart disease. We made every effort to standardize definitions and to group like with like. Furthermore, the types of CVD conditions evaluated varied across articles. Some articles focused on a single outcome, whereas others focused on several outcomes. As a result, the calculated prevalence rates may represent underestimates, as not all studies reported all outcomes.
The types of studies included would have also impacted the overall results of this study. First, we analyzed only prevalence studies; incidence studies would have different results due to their different perspective. Second, the studies varied both in the method of data collection (e.g., national databases versus clinic records) and the length of time over which they collected data. It is plausible that time-period over which studies were conducted could have impacted the observed prevalence rate of CVD. For example, health status, lifestyle, and treatments have varied over time, which could impact the prevalence rates in the studies using older data.
Overall, it is possible that the prevalence estimates for CVD presented in this article overestimate the prevalence of CVD among patients with T2DM. First, studies in the medical literature tend to include a sicker population compared to the general T2DM population; therefore, due to self-selection bias, the sample may not be representative of the broader T2DM population and thus lead to an overestimate of the prevalence of CVD. Second, some of the studies included T2DM patients with an existing CVD diagnosis; therefore, the overall estimate of CVD within these studies could be higher compared to the broader T2DM population.
Finally, only 25 countries were represented in this analysis. Noticeably absent were such countries as Germany, Canada, and Denmark, which all have excellent electronic health data, yet no research studies have been published from them. Very little has appeared from Africa, the Asian subcontinent or Latin America. More studies from these areas would be welcome. While the scope of this study was to evaluate evidence from peer-reviewed literature, an alternative approach to estimating the prevalence of CVD among patients with T2DM could be to analyze data within existing registries.
Conclusions
This is the first systematic review to synthesize global prevalence rates of CVD, including stroke, MI, angina, heart failure, atherosclerosis and CAD among people with T2DM. The results show that CVD is a major cause of comorbidity and death among patients with T2DM with CAD having the highest prevalence. There is a paucity of research studies investigating both the prevalence of CVD and risk factors such as obesity among people with T2DM. Given the large burden that CVD exerts on healthcare systems, patients and families around the world, more evidence is needed, ideally in the form of registry studies, to more accurately quantify the global prevalence of CVD among people with T2DM.
Authors’ contributions
TRE, AA, CL and UHP made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; been involved in drafting the manuscript or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Acknowledgements
This research was funded by Novo Nordisk A/S, Bagsværd, Denmark.
TRE, AA, and CL all received consulting fees for undertaking this project.
Competing interests
TRE, AA, and CL all received consulting fees for undertaking this project. UHP is an employee of Novo Nordisk A/S.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This research was funded by Novo Nordisk A/S, Bagsværd, Denmark.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas R. Einarson, Email: t.einarson@rogers.com
Annabel Acs, Email: aa@last-mile.dk.
Craig Ludwig, Email: cl@last-mile.dk.
Ulrik H. Panton, Email: uhp@novonordisk.com
References
- 1.International Diabetes Federation . idf diabetes atlas. 7. Brussels: International Diabetes Federation; 2015. [PubMed] [Google Scholar]
- 2.Abraham TM, Pencina KM, Pencina MJ, Fox CS. Trends in diabetes incidence: the Framingham heart study. Diab Care. 2015;38:482–487. doi: 10.2337/dc14-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation . Diabetes and cardiovascular disease. Brussels: International Diabetes Federation; 2016. pp. 1–144. [Google Scholar]
- 4.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Angelantonio D, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. emerging risk factors collaboration. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS ONE. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 7.Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diab Care. 2008;31:1582–1584. doi: 10.2337/dc08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Hateren KJ, Landman GW, Kleefstra N, Logtenberg SJ, Groenier KH, Kamper AM, Houweling ST, Bilo HJ. The lipid profile and mortality risk in elderly type 2 diabetic patients: a 10-year follow-up study (ZODIAC-13) PLoS ONE. 2009;4:e8464. doi: 10.1371/journal.pone.0008464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study (UKPDS) Group: intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 13.Action to Control Cardiovascular Risk in Diabetes Study Group: effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes H-P, Huikuri H. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD-summary. Diab Vasc Dis Res. 2014;11:133–173. doi: 10.1177/1479164114525548. [DOI] [PubMed] [Google Scholar]
- 15.Schnell O, Rydén L, Standl E, Ceriello A. Updates on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol. 2017;16:128. doi: 10.1186/s12933-017-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration: Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. US Department of Health and Human Services. 2008.
- 17.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 18.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;2016:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanon VP, Patel S, Sanon S, Rodriguez R, Pham SV, Chilton R. Differential cardiovascular profiles of sodium-glucose cotransporter 2 inhibitors: critical evaluation of empagliflozin. Ther Clin Risk Manag. 2017;13:603. doi: 10.2147/TCRM.S97619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trujillo JM, Nuffer WA. Impact of sodium–glucose cotransporter 2 inhibitors on nonglycemic outcomes in patients with type 2 diabetes. Pharmacotherapy. 2017;37:481–491. doi: 10.1002/phar.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnell O, Cappuccio F, Genovese S, Standl E, Valensi P, Ceriello A. Type 1 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2013;12:156. doi: 10.1186/1475-2840-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease. J Am Coll Cardiol. 2010;55:1310–1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 23.Saquib N, Saquib J, Ahmed T, Khanam MA, Cullen MR. Cardiovascular diseases and type 2 diabetes in Bangladesh: a systematic review and meta-analysis of studies between 1995 and 2010. BMC Public Health. 2012;12:434. doi: 10.1186/1471-2458-12-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Group. P: preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Bhatti GK, Bhadada SK, Vijayvergiya R, Mastana SS, Bhatti JS. Metabolic syndrome and risk of major coronary events among the urban diabetic patients: North Indian Diabetes and cardiovascular disease study—NIDCVD-2. J Diab Complications. 2016;30:72–78. doi: 10.1016/j.jdiacomp.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Collier A, Ghosh S, Hair M, Waugh N. Impact of socioeconomic status and gender on glycaemic control, cardiovascular risk factors and diabetes complications in type 1 and 2 diabetes: a population based analysis from a Scottish region. Diab Metab. 2015;41:145–151. doi: 10.1016/j.diabet.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Tan ED, Davis WA, Davis TM. Changes in characteristics and management of Asian and Anglo-Celts with type 2 diabetes over a 15-year period in an urban Australian community: the fremantle diabetes study. J Diab. 2016;8:139–147. doi: 10.1111/1753-0407.12267. [DOI] [PubMed] [Google Scholar]
- 29.Shestakova M: Dynamics in prevalence of diabetes, diabetic complications and quality of diabetes care in Russian Federation in 2014–2015 by data of national diabetes register. In: Conference: 52nd annual meeting of the european association for the study of diabetes, EASD. 2016. p. 309.
- 30.Alwakeel JS, Sulimani R, Al-Asaad H, Al-Harbi A, Tarif N, Al-Suwaida A, Al-Mohaya S, Isnani AC, Alam A, Hammad D. Diabetes complications in 1952 type 2 diabetes mellitus patients managed in a single institution in Saudi Arabia. Ann Saudi Med. 2008;28:260–266. doi: 10.4103/0256-4947.51702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardoso C, Salles G. Gross proteinuria is a strong risk predictor for cardiovascular mortality in Brazilian type 2 diabetic patients. Braz J Med Biol Res. 2008;41:674–680. doi: 10.1590/S0100-879X2008000800006. [DOI] [PubMed] [Google Scholar]
- 32.Carnethon MR, Biggs ML, Barzilay J, Kuller LH, Mozaffarian D, Mukamal K, Smith NL, Siscovick D. Diabetes and coronary heart disease as risk factors for mortality in older adults. Am J Med. 2010;123:556. doi: 10.1016/j.amjmed.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjörnsdóttir S, Eliasson B. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR) J Intern Med. 2010;268:471–482. doi: 10.1111/j.1365-2796.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 34.Malik MO, Govan L, Petrie JR, Ghouri N, Leese G, Fischbacher C, Colhoun H, Philip S, Wild S, McCrimmon R, et al. Ethnicity and risk of cardiovascular disease (CVD): 4.8 year follow-up of patients with type 2 diabetes living in Scotland. Diabetologia. 2015;58:716–725. doi: 10.1007/s00125-015-3492-0. [DOI] [PubMed] [Google Scholar]
- 35.Salinero-Fort MA, Andres-Rebollo FJ, Burgos-Lunar C, Abanades-Herranz JC, Carrillo-de-Santa-Pau E, Chico-Moraleja RM, Jimenez-Garcia R, Lopez-de-Andres A, Gomez-Campelo P. Cardiovascular and all-cause mortality in patients with type 2 diabetes mellitus in the MADIABETES Cohort Study: association with chronic kidney disease. J Diab Complications. 2016;30:227–236. doi: 10.1016/j.jdiacomp.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Menzaghi C, Xu M, Salvemini L, De Bonis C, Palladino G, Huang T, Copetti M, Zheng Y, Li Y, Fini G. Circulating adiponectin and cardiovascular mortality in patients with type 2 diabetes mellitus: evidence of sexual dimorphism. Cardiovasc Diabetol. 2014;13:130. doi: 10.1186/s12933-014-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamba SM, Ewane ME, Bonny A, Muisi CN, Nana E, Ellong A, Mvogo CE, Mandengue SH. Micro and macrovascular complications of diabetes mellitus in Cameroon: risk factors and effect of diabetic check-up-a monocentric observational study. Pan African Med J. 2013;15:141. doi: 10.11604/pamj.2013.15.141.2104. [DOI] [Google Scholar]
- 38.Boonman-de Winter LJ, Rutten FH, Cramer MJ, Landman MJ, Liem AH, Rutten GE, Hoes AW. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55:2154–2162. doi: 10.1007/s00125-012-2579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wentworth JM, Fourlanos S, Colman PG. Body mass index correlates with ischemic heart disease and albuminuria in long-standing type 2 diabetes. Diab Res Clin Pract. 2012;97:57–62. doi: 10.1016/j.diabres.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Glogner S, Rosengren A, Olsson M, Gudbjörnsdottir S, Svensson AM, Lind M. The association between BMI and hospitalization for heart failure in 83,021 persons with Type 2 diabetes: a population-based study from the Swedish National Diabetes Registry. Diab Med. 2014;31:586–594. doi: 10.1111/dme.12340. [DOI] [PubMed] [Google Scholar]
- 41.Alaboud AF, Tourkmani AM, Alharbi TJ, Alobikan AH, Abdelhay O, Al Batal SM, Alkashan HI, Mohammed UY. Microvascular and macrovascular complications of type 2 diabetic mellitus in Central, Kingdom of Saudi Arabia. Saudi Med J. 2016;37:1408–1411. doi: 10.15537/smj.2016.12.17062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alonso-Moran E, Orueta JF, Fraile Esteban JI, Arteagoitia Axpe JM, Marques Gonzalez ML, Toro Polanco N, Ezkurra Loiola P, Gaztambide S, Nuno-Solinis R. The prevalence of diabetes-related complications and multimorbidity in the population with type 2 diabetes mellitus in the Basque Country. BMC Public Health. 1059;2014:14. doi: 10.1186/1471-2458-14-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrasco-Sánchez FJ, Gomez-Huelgas R, Formiga F, Conde-Martel A, Trullàs JC, Bettencourt P, Arévalo-Lorido JC, Pérez-Barquero MM. Association between type-2 diabetes mellitus and post-discharge outcomes in heart failure patients: findings from the RICA registry. Diabetes Res Clin Pract. 2014;104:410–419. doi: 10.1016/j.diabres.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Gregg EW, Cheng YJ, Saydah S, Cowie C, Garfield S, Geiss L, Barker L. Trends in death rates among US adults with and without diabetes between 1997 and 2006. Diab Care. 2012;35:1252–1257. doi: 10.2337/dc11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin PJ, Cohen JT, Kent WA, Neumann PJ. Patterns of comorbidity clusters among adults with diabetes. Value Health. 2013;16:A155–A156. doi: 10.1016/j.jval.2013.03.774. [DOI] [Google Scholar]
- 46.Song S, Hardisty C. Early onset type 2 diabetes mellitus: a harbinger for complications in later years—clinical observation from a secondary care cohort. QJM. 2009;102:799–806. doi: 10.1093/qjmed/hcp121. [DOI] [PubMed] [Google Scholar]
- 47.Yang HK, Kang B, Lee S-H, Yoon K-H, Hwang B-H, Chang K, Han K, Kang G, Cho JH. Association between hemoglobin A1c variability and subclinical coronary atherosclerosis in subjects with type 2 diabetes. J Diab Complications. 2015;29:776–782. doi: 10.1016/j.jdiacomp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Mansour AA, Ajeel NA. Atherosclerotic cardiovascular disease among patients with type 2 diabetes in Basrah. World J Diab. 2013;4:82. doi: 10.4239/wjd.v4.i3.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menghua Z. GW25-e1447 clinical significance of multislice coronary CT angiography in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol. 2014;64:C227. doi: 10.1016/j.jacc.2014.06.1062. [DOI] [Google Scholar]
- 50.Norhammar A, Bodegard J, Nystrom T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose-lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006–2013. Diabetologia. 2016;59:1692–1701. doi: 10.1007/s00125-016-3971-y. [DOI] [PubMed] [Google Scholar]
- 51.Rossi MC, Lucisano G, Comaschi M, Coscelli C, Cucinotta D, Di Blasi P, Bader G, Pellegrini F, Valentini U, Vespasiani G. Quality of diabetes care predicts the development of cardiovascular events: results of the AMD-QUASAR study. Diab Care. 2011;34:347–352. doi: 10.2337/dc10-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart study. Circulation. 1983;67:968–977. doi: 10.1161/01.CIR.67.5.968. [DOI] [PubMed] [Google Scholar]
- 53.Rabkin SW, Mathewson FA, Hsu PH. Relation of body weight to development of ischemic heart disease in a cohort of young North American men after a 26 year observation period: the Manitoba study. Am J Cardiol. 1977;39:452–458. doi: 10.1016/S0002-9149(77)80104-5. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Labbe D, Ruka E, Bertrand OF, Voisine P, Costerousse O, Poirier P. Obesity and coronary artery disease: evaluation and treatment. Can J Cardiol. 2015;31:184–194. doi: 10.1016/j.cjca.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Plourde B, Sarrazin JF, Nault I, Poirier P. Sudden cardiac death and obesity. Exp Rev Cardiovasc Ther. 2014;12:1099–1110. doi: 10.1586/14779072.2014.952283. [DOI] [PubMed] [Google Scholar]
- 56.Engeland A, Bjorge T, Sogaard AJ, Tverdal A. Body mass index in adolescence in relation to total mortality: 32-year follow-up of 227,000 Norwegian boys and girls. Am J Epidemiol. 2003;157:517–523. doi: 10.1093/aje/kwf219. [DOI] [PubMed] [Google Scholar]
- 57.Masmiquel L, Leiter L, Vidal J, Bain S, Petrie J, Franek E, Raz I, Comlekci A, Jacob S. Gaal Lv: LEADER 5: prevalence and cardiometabolic impact of obesity in cardiovascular high-risk patients with type 2 diabetes mellitus: baseline global data from the LEADER trial. Cardiovasc Diabetol. 2016;15:29. doi: 10.1186/s12933-016-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization . Obesity and Overweight. Factsheet No 311. Geneva: World Health Organization; 2017. [Google Scholar]
- 59.Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013;1281:51–63. doi: 10.1111/j.1749-6632.2012.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 61.Huxley R, James W, Barzi F, Patel J, Lear S, Suriyawongpaisal P, Janus E, Caterson I, Zimmet P, Prabhakaran D. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9:53–61. doi: 10.1111/j.1467-789X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 63.Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res. 2013;4:390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meseguer A, Puche C, Cabero A. Sex steroid biosynthesis in white adipose tissue. Horm Metab Res. 2002;34:731–736. doi: 10.1055/s-2002-38249. [DOI] [PubMed] [Google Scholar]
- 65.Hartrumpf M, Kuehnel RU, Albes JM. The obesity paradox is still there: a risk analysis of over 15,000 cardiosurgical patients based on body mass index. Interact Cardiovasc Thorac Surg. 2017;25:18–24. doi: 10.1093/icvts/ivx058. [DOI] [PubMed] [Google Scholar]
- 66.Lee KS, Moser DK, Lennie TA, Pelter MM, Nesbitt T, Southard JA, Dracup K. Obesity paradox: comparison of heart failure patients with and without comorbid diabetes. Am J Crit Care. 2017;26:140–148. doi: 10.4037/ajcc2017634. [DOI] [PubMed] [Google Scholar]
- 67.Abi Khalil C, Sulaiman K, Singh R, Jayyousi A, Asaad N, AlHabib KF, Alsheikh-Ali A, Al-Jarallah M, Bulbanat B, Al Mahmeed W, et al. BMI is inversely correlated to the risk of mortality in patients with type 2 diabetes hospitalized for acute heart failure: findings from the Gulf aCute heArt failuRE (Gulf-CARE) registry. Int J Cardiol. 2017;5273(0116):34386–34388. doi: 10.1016/j.ijcard.2017.02.119. [DOI] [PubMed] [Google Scholar]
- 68.Straka RJ, Liu LZ, Girase PS, DeLorenzo A, Chapman RH. Incremental cardiovascular costs and resource use associated with diabetes: an assessment of 29,863 patients in the US managed-care setting. Cardiovasc Diabetol. 2009;8:53. doi: 10.1186/1475-2840-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Senthil AN, Ravishankar G, Ravi MS, Meenakshi K, Muthu Kumar D, Swaminathan N, Paul J, Venkatesan S. Pattern of coronary artery disease in symptomatic Type 2 diabetic subjects in the contemporary era and the difference from past studies. Indian Heart J. 2014;66:S46. doi: 10.1016/j.ihj.2014.10.130. [DOI] [Google Scholar]
- 70.Nair M, Prabhakaran D. Why do South Asians have high risk for CAD? Global Heart. 2012;7:307–314. doi: 10.1016/j.gheart.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Azoulay L, Suissa S. Sulfonylureas and the risks of cardiovascular events and death: a methodological meta-regression analysis of the observational studies. Diab Care. 2017;40:706–714. doi: 10.2337/dc16-1943. [DOI] [PubMed] [Google Scholar]
- 72.World Health Organization . Global status report on noncommunicable diseases. Geneva: World Health Organization; 2014. pp. 1–302. [DOI] [PubMed] [Google Scholar]
- 73.Jung CH, Chung JO, Han K, Ko S-H, Ko KS, Park J-Y. Improved trends in cardiovascular complications among subjects with type 2 diabetes in Korea: a nationwide study (2006–2013) Cardiovasc Diabetol. 2017;16:1. doi: 10.1186/s12933-016-0482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Y, Li J, Zhu X, Sun J, Ji L, Hu D, Pan C, Tan W, Jiang S, Tao X. Relationship between healthy lifestyle behaviors and cardiovascular risk factors in Chinese patients with type 2 diabetes mellitus: a subanalysis of the CCMR-3B STUDY. Acta Diabetol. 2017;54:569–579. doi: 10.1007/s00592-017-0981-2. [DOI] [PubMed] [Google Scholar]
- 75.Simmons RK, Griffin SJ, Lauritzen T, Sandbæk A. Effect of screening for type 2 diabetes on risk of cardiovascular disease and mortality: a controlled trial among 139,075 individuals diagnosed with diabetes in Denmark between 2001 and 2009. Diabetologia. 2017;60:2192–2199. doi: 10.1007/s00125-017-4299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simmons RK, Griffin SJ, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbæk A. Effect of population screening for type 2 diabetes and cardiovascular risk factors on mortality rate and cardiovascular events: a controlled trial among 1,912,392 Danish adults. Diabetologia. 2017;60:2183–2191. doi: 10.1007/s00125-017-4323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelsall HL, Fernando PHS, Gwini SM, Sim MR. Cardiovascular disease and type 2 diabetes risk across occupational groups and industry in a statewide study of an Australian working population. J Occup Environ Med. 2018;60:286–294. doi: 10.1097/JOM.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 78.Li M-F, Zhao C-C, Li T-T, Tu Y-F, Lu J-X, Zhang R, Chen M-Y, Bao Y-Q, Li L-X, Jia W-P. The coexistence of carotid and lower extremity atherosclerosis further increases cardio-cerebrovascular risk in type 2 diabetes. Cardiovasc Diabetol. 2016;15:43. doi: 10.1186/s12933-016-0360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohammedi K, Woodward M, Marre M, Colagiuri S, Cooper M, Harrap S, Mancia G, Poulter N, Williams B, Zoungas S. Comparative effects of microvascular and macrovascular disease on the risk of major outcomes in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16:95. doi: 10.1186/s12933-017-0574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malik S, Zhao Y, Budoff M, Nasir K, Blumenthal RS, Bertoni AG, Wong ND. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the multi-ethnic study of atherosclerosis. JAMA Cardiol. 2017;2:1332–1340. doi: 10.1001/jamacardio.2017.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 82.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart J-C, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the treating to new targets (TNT) study. Diab Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 83.Stein EA, Lane M, Laskarzewski P. Comparison of statins in hypertriglyceridemia. Am J Cardiol. 1998;81:66B–69B. doi: 10.1016/S0002-9149(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 84.Ren Y, Ren Q, Lu J, Guo X, Huo X, Ji L, Yang X. Low triglyceride as a marker for increased risk of cardiovascular diseases in patients with long-term type 2 diabetes: a cross-sectional survey in China. Diab Metab Res Rev. 2018;34:e2960. doi: 10.1002/dmrr.2960. [DOI] [PubMed] [Google Scholar]
- 85.Clua-Espuny JL, González-Henares MA, Queralt-Tomas MLL. Mortality and cardiovascular complications in older complex chronic patients with type 2 diabetes. BioMed Res Int. 2017;2017:6078498. doi: 10.1155/2017/6078498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng Y, Zhang H, Chen R, Yang F, Li W, Chen L, Lin S, Liang G, Cai D, Chen H. Cardiometabolic risk profiles associated with chronic complications in overweight and obese type 2 diabetes patients in South China. PLoS ONE. 2014;9:e101289. doi: 10.1371/journal.pone.0101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cortez-Dias N, Martins S, Belo A, Fiuza M. em nome dos Investigadores do Estudio VALSIM: prevalência, tratamento e controlo da diabetes mellitus e dos factores de risco associados nos cuidados de saúde primários em Portugal. Rev Port Cardiol. 2010;29:509–537. [Google Scholar]
- 88.Daghash MH, Bener A, Zirie M, Dabdoob W, Al-Hamaq AO, Al-Arabi ZA. Lipoprotein profile in Arabian type 2 diabetic patients. relationship to coronary artery diseases. Int J Cardiol. 2007;121:91–92. doi: 10.1016/j.ijcard.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 89.Doucet JA, Bauduceau B, Le Floch JP, Verny C. Medical treatments of elderly, French patients with type 2 diabetes: results at inclusion in the GERODIAB Cohort. Fundam Clin Pharmacol. 2016;30:76–81. doi: 10.1111/fcp.12160. [DOI] [PubMed] [Google Scholar]
- 90.Farrell C, Moran J. Comparison of comorbidities in patients with pre-diabetes to those with diabetes mellitus type 2. IMJ. 2014;3:107. [PubMed] [Google Scholar]
- 91.Fu AZ, Qiu Y, Radican L, Yin DD, Mavros P. Impact of concurrent macrovascular co-morbidities on healthcare utilization in patients with type 2 diabetes in Europe: a matched study. Diab Obes Metab. 2010;12:631–637. doi: 10.1111/j.1463-1326.2010.01200.x. [DOI] [PubMed] [Google Scholar]
- 92.Giallauria F, Fattirolli F, Tramarin R, Ambrosetti M, Griffo R, Riccio C, De Feo S, Piepoli MF, Vigorito C. Clinical characteristics and course of patients with diabetes entering cardiac rehabilitation. Diab Res Clin Pract. 2015;107:267–272. doi: 10.1016/j.diabres.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 93.Gobardhan SN, Dimitriu-Leen AC, van Rosendael AR, van Zwet EW, Roos CJ, Oemrawsingh PV, Kharagjitsingh AV, Jukema JW, Delgado V, Schalij MJ. Prevalence by computed tomographic angiography of coronary plaques in South Asian and white patients with type 2 diabetes mellitus at low and high risk using four cardiovascular risk scores (UKPDS, FRS, ASCVD, and JBS3) Am J Cardiol. 2017;119:705–711. doi: 10.1016/j.amjcard.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 94.Gondim F, Caribé A, Vasconcelos KF, Segundo AD, Bandeira F. Vitamin D deficiency is associated with severity of acute coronary syndrome in patients with type 2 diabetes and high rates of sun exposure. Clin Med Insights. 2016;9:37. doi: 10.4137/CMED.S39427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hermans MP, Bouenizabila E, Ahn SA, Rousseau MF. How to transform a metabolic syndrome score into an insulin sensitivity value? Diab Metab Res Rev. 2016;32:87–94. doi: 10.1002/dmrr.2675. [DOI] [PubMed] [Google Scholar]
- 96.Hunt KJ, Kistner-Griffin E, Spruill I, Teklehaimanot AA, Garvey WT, Sale M, Fernandes J. Cardiovascular risk in Gullah African Americans with high familial risk of type 2 diabetes mellitus: project SuGAR. South Med J. 2014;107:607–614. doi: 10.14423/SMJ.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jackson CA, Jones NR, Walker JJ, Fischbacher CM, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, Morris AD, Petrie JR, et al. Area-based socioeconomic status, type 2 diabetes and cardiovascular mortality in Scotland. Diabetologia. 2012;55:2938–2945. doi: 10.1007/s00125-012-2667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jurado J, Ybarra J, Solanas P, Caula J, Gich I, Pou JM, Romeo JH. Prevalence of cardiovascular disease and risk factors in a type 2 diabetic population of the North Catalonia diabetes study. J Am Acad Nurse Pract. 2009;21:140–148. doi: 10.1111/j.1745-7599.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- 99.Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, Chakravarti A, Folsom AR, Siscovick DS, Rosamond WD. Diabetes and the risk of sudden cardiac death, the atherosclerosis risk in communities study. Acta Diabetol. 2010;47(Suppl 1):161–168. doi: 10.1007/s00592-009-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwon H, Lim J, Shin J, Son J, Lee S, Kim S, Yoo S. A relationship of asymptomatic coronary artery disease and type 2 diabetes in acute ischaemic stroke patients; cerebral angiography and coronary angiography study. Diabetoligia. 2014;57:S26. [Google Scholar]
- 101.Liu X, Liu Y, Lv Y, Li C, Cui Z, Ma J. Prevalence and temporal pattern of hospital readmissions for patients with type I and type II diabetes. BMJ Open. 2015;5:e007362. doi: 10.1136/bmjopen-2014-007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo Y, Wang X, Wang Y, Wang C, Wang H, Wang D, Liu L, Jia Q, Liu G, Zhao X, et al. Association of glomerular filtration rate with outcomes of acute stroke in type 2 diabetic patients: results from the China National Stroke Registry. Diab Care. 2014;37:173–179. doi: 10.2337/dc13-1931. [DOI] [PubMed] [Google Scholar]
- 103.MacDonald MR, Petrie MC, Home PD, Komajda M, Jones NP, Beck-Nielsen H, Gomis R, Hanefeld M, Pocock SJ, Curtis PS, McMurray JJ. Incidence and prevalence of unrecognized myocardial infarction in people with diabetes: a substudy of the rosiglitazone evaluated for cardiac outcomes and regulation of glycemia in diabetes (RECORD) study. Diab Care. 2011;34:1394–1396. doi: 10.2337/dc10-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mazza A, Zamboni S, Rizzato E, Pessina AC, Tikhonoff V, Schiavon L, Casiglia E. Serum uric acid shows a J-shaped trend with coronary mortality in non-insulin-dependent diabetic elderly people. The CArdiovascular STudy in the ELderly (CASTEL) Acta Diabetol. 2007;44:99–105. doi: 10.1007/s00592-007-0249-3. [DOI] [PubMed] [Google Scholar]
- 105.Mody R, Kalsekar I, Kavookjian J, Iyer S, Rajagopalan R, Pawar V. Economic impact of cardiovascular co-morbidity in patients with type 2 diabetes. J Diab Complications. 2007;21:75–83. doi: 10.1016/j.jdiacomp.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 106.Mundet X, Cano F, Mata-Cases M, Roura P, Franch J, Birules M, Gimbert R, Llusa J, Cos X. Trends in chronic complications of type 2 diabetic patients from Spanish primary health care centres (GEDAPS study): 10 year-implementation of St. Vincent recommendations. Prim Care Diab. 2012;6:11–18. doi: 10.1016/j.pcd.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 107.Narksawat K, Sujirarat D, Panket P. Combined effects of hypertension and diabetes mellitus with stroke among Thais in the central region of Thailand: a cross-sectional study. J Med Assoc Thai. 2013;5:S1–S7. [PubMed] [Google Scholar]
- 108.Penno G, Solini A, Zoppini G, Fondelli C, Trevisan R, Vedovato M, Cavalot F, Gruden G, Lamacchia O, Laviola L. Independent correlates of urinary albumin excretion within the normoalbuminuric range in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicentre study. Acta Diabetol. 2015;52:971–981. doi: 10.1007/s00592-015-0789-x. [DOI] [PubMed] [Google Scholar]
- 109.Rodriguez-Poncelas A, Coll-De Tuero G, Turro-Garriga O, Barrot-de la Puente J, Franch-Nadal J, Mundet-Tuduri X, Red GSG. Impact of chronic kidney disease on the prevalence of cardiovascular disease in patients with type 2 diabetes in Spain: PERCEDIME2 study. BMC Nephrol. 2014;15:150. doi: 10.1186/1471-2369-15-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soetedjo N, Permana H, Ruslami R, Livia R, Panduru N, Critchley J, Hulscher M, Tack C, Alisjahbana B, van Crevel R. PO035 High prevalence of macrovascular complications and insufficient cardiovascular management in indonesian diabetes patients; a hospital survey. Diab Res Clin Pract. 2014;106:S63. doi: 10.1016/S0168-8227(14)70330-4. [DOI] [Google Scholar]
- 111.Suh DC, Kim CM, Choi IS, Plauschinat CA. Comorbid conditions and glycemic control in elderly patients with type 2 diabetes mellitus, 1988 to 1994 to 1999 to 2004. J Am Geriatr Soc. 2008;56:484–492. doi: 10.1111/j.1532-5415.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 112.Utrera-Lagunas M, Orea-Tejeda A, Castillo-Martinez L, Balderas-Munoz K, Keirns-Davis C, Espinoza-Rosas S, Sanchez-Ortiz NA, Olvera-Mayorga G. Abnormal myocardial perfusion and risk of heart failure in patients with type 2 diabetes mellitus. Exp Clin Cardiol. 2013;18:e44–e46. [PMC free article] [PubMed] [Google Scholar]
- 113.Vinagre I, Mata-Cases M, Hermosilla E, Morros R, Fina F, Rosell M, Castell C, Franch-Nadal J, Bolibar B, Mauricio D. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain) Diab Care. 2012;35:774–779. doi: 10.2337/dc11-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wong K, Glovaci D, Malik S, Franklin SS, Wygant G, Iloeje U, Kan H, Wong ND. Comparison of demographic factors and cardiovascular risk factor control among US adults with type 2 diabetes by insulin treatment classification. J Diab Complications. 2012;26:169–174. doi: 10.1016/j.jdiacomp.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yan BP, Zhang Y, Kong AP, Luk AO, Ozaki R, Yeung R, Tong PC, Chan WB, Tsang C-C, Lau K-P. Borderline ankle–brachial index is associated with increased prevalence of micro-and macrovascular complications in type 2 diabetes: a cross-sectional analysis of 12,772 patients from the Joint Asia Diabetes Evaluation Program. Diab Vasc Dis Res. 2015;12:334–341. doi: 10.1177/1479164115590559. [DOI] [PubMed] [Google Scholar]
- 116.Zekry D, Frangos E, Graf C, Michel JP, Gold G, Krause KH, Herrmann FR, Vischer UM. Diabetes, comorbidities and increased long-term mortality in older patients admitted for geriatric inpatient care. Diab Metab. 2012;38:149–155. doi: 10.1016/j.diabet.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 117.World Bank. GNI ranking, atlas method. http://data.worldbank.org/data-catalog/GNI-per-capita-Atlas-and-PPP-table. Accessed 26 Apr 2017.
- 118.World Bank. The data blog: New country classifications by income level. https://blogs.worldbank.org/opendata/new-countryclassifications-2016. Accessed 26 Apr 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.