The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P (original) (raw)
Abstract
The surface glycoprotein of the Lassa virus, a member of the_arenaviridae_ family, is synthesized as a 76-kDa precursor (GP-C) that is posttranslationally cleaved into an N-terminal 44-kDa subunit and a C-terminal membrane-anchored 36-kDa subunit. Cleavage occurs at the C-terminal end of the unusual recognition motif R-R-L-L. We show here that GP-C is cleaved in the endoplasmic reticulum by the cellular subtilase SKI-1/S1P, an enzyme that has so far been observed to be involved in cholesterol metabolism. Furthermore, we present evidence that only cleaved glycoprotein is incorporated into virions and that this is necessary for the formation of infectious virus. To our knowledge, there have been no previous reports of this type of viral glycoprotein processing, one that may be an interesting target for antiviral therapy.
Lassa virus is a member of the large family of arenaviridae, comprising also the closely related lymphocytic choriomeningitis virus (LCMV) as well as other important human pathogens like the viruses causing Argentinian and Bolivian hemorrhagic fever. Lassa virus causes annually up to 100,000 cases of clinically apparent Lassa fever in West Africa, with 10% to 20% of the patients developing hemorrhagic manifestations and a total mortality of around 15% (1, 2). The disease is also increasingly exported from endemic regions to other parts of the world (3).
Lassa virus is an enveloped virus with glycoprotein spikes on its surface, and a nucleoprotein containing a bisegmented ambisense genome (4). The Lassa virus glycoprotein is synthesized as a 76-kDa glycosylated precursor protein (GP-C), which is posttranslationally cleaved into the N-terminal 44-kDa subunit GP-1 and the membrane bound 36-kDa subunit GP-2 (5). GP-1 of LCMV and Lassa virus interacts with the cellular receptor α-dystroglycan, whereas GP-2 presumably mediates pH-dependent fusion of the viral envelope with the cellular target membrane (6–8). We have recently shown that the Lassa virus glycoprotein GP-C is cleaved at the peptide bond between leucine259 and glycine260and that the amino acid motif R-X-L/I/V-L259is responsible for cleavage by a yet unidentified protease (9). This cleavage site and similar consensus motifs appear to be typical for arenaviruses (Table 1). However, they are different from those of most other viral glycoproteins, which are often cleaved by furin at arginine-lysine clusters or by proteases recognizing a monobasic cleavage site (10). The consensus sequence of Lassa virus glycoprotein was found to be homologous to the recognition motif of the recently discovered SKI-1/S1P protease belonging to the pyrolysin branch of subtilases. This enzyme has been identified in human, rat, mouse, and hamster cells, with the human and the hamster form showing 97% identity (11–13). In the present study, we have identified SKI-1/S1P as the cleavage enzyme of the Lassa virus glycoprotein.
Table 1.
Alignment of the amino acid sequences around confirmed amd presumed cleavage sites of GP-C of different arenaviruses
| Lassa (Josiah) | RTRDIYISRRLL | GTFTWTLSDSEG |
|---|---|---|
| Lassa (AV) | ........RRLL | ............ |
| Mopeia | ...NF...RRLL | L........... |
| LCMV (Armstrong) | K.K--FFTRRLA | ..........S. |
| Pichinde | ..AYSSVSRKLL | .F...D....S. |
| Junin | GKN-.QLPRRSL | KAF.S.S.T..S. |
| Tacaribe | QKS-.AVGRTLK | AF.S.S.T.PL. |
Materials and Methods
Cell Cultures and Viruses.
Vero, baby hamster kidney (BHK)-21 and primary chicken embryo cells were maintained in DMEM (GIBCO) supplemented with 10% FCS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. Chinese hamster ovary (CHO)-K1 cells were grown in DMEM nutrient mixture F12 Ham (GIBCO) supplemented with 10% FCS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin, whereas SRD-12B cells were maintained in the same medium as the CHO cells with the addition of 5 μg/ml cholesterin (Sigma), 1 mM sodium mevalonate (Sigma), and 20 μM sodium oleate (Sigma; ref. 14). The modified vaccinia Ankara strain containing the phage T7 RNA polymerase (MVA-T7) was kindly provided by R. Sutter (National Research Center for Environment and Health, Oberschleissheim, Germany), and seed stocks were grown in primary chicken embryo cells. Vaccinia virus titers were obtained by using a standard plaque assay procedure with subsequent 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) staining. All experiments performed with Lassa virus were done under biosafety level 4 biocontainment conditions. Lassa virus, strain Josiah, was grown on Vero cells. Lassa virus was titered by a plaque assay in Vero cell cultures that were overlaid with DMEM, 1% low-melting agarose, and 2% FCS, incubated at 37°C for 4–5 days, and counterstained with 0.012% neutral red.
Antibodies and Western Blotting.
A peptide comprising amino acids 53 to 75 of the nucleoprotein (NP) of Lassa virus, strain Josiah, with an additional C-terminal cysteine was prepared by chemical synthesis (M. Krause, University of Marburg). The oligopeptide was covalently linked to keyhole limpet hemocyanin (KLH; Pierce) as a carrier protein by a_N-_(-α-maleimide)-succimide ester and used for immunization of rabbits. The obtained antisera were tested by using peptide-based ELISA and Western blot analyses of lysates of Lassa virus-infected Vero cells. A monospecific antiserum raised against GP-2 that also detects GP-C was described recently (9). The mAb HA-2A11 raised against influenza virus hemagglutinin H7 was used in control experiments (15).
Western blotting was done following standard procedures (16). Proteins were electrophoretically transferred to nitrocellulose, which was subsequently incubated with monospecific antisera in 3% BSA and 0.1% Tween 20 in PBS for 1 h at room temperature. The detection system consisted of horseradish peroxidase-labeled anti-rabbit IgG from swine (Dako), and Super Signal system (Pierce).
Calcium Depletion.
Subconfluent BHK-21 cells were infected with modified vaccinia virus strain Ankara T7 (MVA-T7) in DMEM or in calcium-free DMEM at a multiplicity of infection (moi) of 10. At 1 h postinfection (p.i.) the cells were transfected with the T7-driven pTM1-GP-C (9) by using calcium-free DMEM or DMEM with calcium, respectively. The calcium specific ionophore A23187 (Calbiochem) solubilized in DMSO was added to a final concentration of 0.3 μM or 0.5 μM immediately and again 3h posttransfection (p.t.). Cells were lysed in sample buffer for SDS/PAGE and analyzed by Western blotting (9).
Pulse–Chase, Glycosidase Treatment, and Immunoprecipitation Analysis.
Vero cells were infected with MVA-T7 at a moi of 10. The inoculum was removed, and cells were Lipofectin transfected (GIBCO) with recombinant DNA of Lassa virus GP-C and fowl plague virus hemagglutinin (FPV-HA), cloned in a pTM1 vector, respectively (17). At 4 h posttransfection about 106 cells were starved for 1 h with DMEM lacking methionine and cysteine before they were labeled for 30 min with [35S]methionine and [35S]cysteine (100 μC [35S]Promix; Amersham Pharmacia). Labeling medium was removed from cells and replaced by DMEM for a 3-h chase. Brefeldin A (BFA; Sigma) was added from a stock solution (5 mg/ml stock solution in ethanol) to some samples at a final concentration of 15 μg/ml to the medium during starving, labeling, and chase periods. Cells were lysed in 500 μl CoIP buffer [20 mM Tris, pH 7.6/100 mM NaCl/5 mM EDTA/1% Nonidet P40/0.4% sodium deoxycholate/25 mM iodine acetamide/5% (vol/vol) Trasylol (Bayer)/1 mM PMSF]. The cell lysate was sonicated (40 Watt, Branson sonifier), freed from insoluble material by centrifugation at 14,000 rpm for 30 min (Minicentrifuge, Eppendorf), and incubated with protein A Sepharose and antibodies against GP-C or FPV-HA, respectively. Immuncomplexes were kept untreated or were treated either with EndoH or PNGaseF (New England Biolabs) at 37°C for 1 h and subsequently subjected to SDS/PAGE followed by autoradiography.
Transfection and Lassa Virus Infection.
CHO-K1, BHK, Vero, or SRD-12B cells were transfected with pcDNA3.1-hSKI-1 or empty vector by using Lipofectin (GIBCO) 12 to 16 h before infection with Lassa virus at a moi of 1. Transfection with Lipofectin resulted in an efficacy of 20% to 30% as judged by standard immunofluorescence procedure by using recombinant expressed Lassa GP-C as a reporter molecule. On day 4 p.i., supernatants of cells were centrifuged at 2000 rpm for 10 min (Minicentrifuge, Eppendorf) and pelleted through a 20% sucrose-cushion by using a SW28 Rotor (Beckman) at 20,000 rpm for 2 h at 5°C. Virus pellets and cells were lysed in protein sample buffer for SDS/PAGE and Western blot analysis.
Protease Protection Assay.
Supernatants of CHO and SRD-12B cells infected with Lassa virus at a moi of 1 were collected 4 days p.i., and the virus was pelleted through a 20% sucrose-cushion by ultracentrifugation (see above) and resuspended in 120 μl PBS. Aliquots (40 μl) were treated for 30 min at 37°C with either 0.1 μg/μl proteinase K or 0.1 μg/μl proteinase K in the presence of 1% Triton X-100 or were left untreated. Proteinase K was thereafter inactivated by addition of an excess of PMSF (10 μg/μl final concentration).
Results
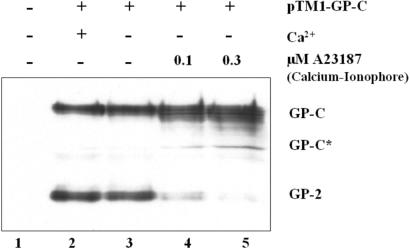
Because cleavage of proteins by SKI-1/S1P was shown to be calcium dependent (13), the role of calcium for the processing of Lassa virus GP-C was studied by use of the calcium-specific ionophore A23187 (18). GP-C of Lassa virus was vectorially expressed in Vero cells in the presence or absence of calcium and the ionophore, by using a T7-based recombinant vaccinia virus system (9). Cleavage was analyzed on Western-blots showing the precursor GP-C and the cleavage product GP-2 by detection with a GP-2-specific antiserum (Fig.1). Cleavage was observed in normal and calcium-free medium without the ionophore (lanes 2 and 3), whereas it decreased in a dose-dependent fashion when intracellular calcium was depleted by A23187 (lanes 4 and 5).
Figure 1.
Cleavage of Lassa virus GP-C is calcium dependent. Lassa virus glycoprotein precursor GP-C was expressed in BHK cells incubated with calcium free and normal Dulbecco's medium (GIBCO) by using pTM1-GP-C and the MVA-T7 system (31). The calcium-specific ionophore A23187 (Calbiochem; ref. 18) was added to the transfection medium in concentrations as indicated. Cells were lysed in sample buffer 6 h posttransfection, and the proteins were subjected to electrophoresis on an 11% polyacrylamide gel and electrophoretically blotted onto a nitrocellulose membrane. Cleaved GP-2 and the uncleaved precursor GP-C were identified by immunodetection by using rabbit anti-GP2 immune serum (9). GP-C* representing unglycosylated GP-C is due to overexpression in the vaccinia T7 system(9).
We then investigated the subcellular localization of GP-C cleavage. SKI-1/S1P cleaves its substrates in the endoplasmic reticulum or an early Golgi compartment (13, 19, 20). We therefore analyzed GP-C cleavage in the presence and absence of BFA, which causes disassembly of the cis and medial Golgi compartment and inhibits the anterograde transport of proteins along the secretory pathway (21). We also analyzed the glycosylation patterns of GP-C and GP-2 by glycosidase treatment to further confirm the block imposed by BFA on the transport of GP-C. Because N-glycosylated proteins acquire EndoH resistance in the medial-Golgi by gaining the complex carbohydrates, EndoH sensitivity is characteristic for glycoproteins that have not yet reached the medial-Golgi. Cleavage and glycosylation of vectorially expressed GP-C were analyzed in pulse–chase experiments, with hemagglutinin (HA) of fowl plague virus (FPV) known to be cleaved in the trans-Golgi network (TGN) serving as a control. As shown in Fig.2A (lanes 1 and 4), BFA treatment had no effect on proteolytic processing of the Lassa virus glycoprotein precursor, whereas it prevented cleavage of FPV-HA under the same conditions (Fig. 2B, lane 2 and 3), implying that Lassa GP-C is cleaved before it enters the TGN. Without BFA treatment, GP-2 displayed complex type sugars that were partially resistant to Endo H and sensitive to PNGase F (Fig.2A, lanes 2 and 3). When expressed in the presence of BFA, GP-C and GP-2 remained sensitive to Endo H and PNGase F (Fig.2A, lanes 5 and 6). Thus, cleavage of GP-C occurs already in the endoplasmic reticulum or the cis-Golgi compartment. This finding is in contrast to the glycoproteins of most other enveloped viruses, which are cleaved in a late Golgi compartment or the TGN.
Figure 2.
BFA does not prevent cleavage of Lassa virus GP-C. Lassa virus GP-C and FPV-HA were expressed in Vero cells by using the MVA-T7 system. (A) Four hours posttransfection cells were incubated with medium lacking methionine and cysteine for 1 h before they were labeled with [35S]methionine and [35S]cysteine for 30 min. The radioactive label was chased for 3 h in the presence of normal DMEM, the cells were solubilized in lysis buffer, and GP-C and GP-2 were immunoprecipitated with GP-2-specific rabbit-immune serum. Precipitated GP-C was treated with endoglycosidase H (New England Biolabs; lanes 2 and 5), with endoglycosidase F (New England Biolabs; lanes 3 and 6), or was left untreated (lanes 1 and 4) and subjected to SDS/PAGE and fluorography. BFA was added at a final concentration of 15 μg/ml to the medium as indicated. (B) As a control, cleavage of FPV-HA was analyzed by using mAb HA-2A11 (15).
To directly prove the role of the subtilase SKI-1/S1P in GP-C processing, a CHO cell line lacking this enzyme (SRD-12B; ref. 14), and its parental CHO-K1 cells were transfected with the plasmid pTM1-GP-C by using the vaccinia-T7 expression system. Cleavage was completely abolished in the protease-deficient cells, whereas the parental CHO cells efficiently cleaved GP-C (Fig.3A, lanes 1 and 2). However, when SRD-12B cells were cotransfected with pTM1-GP-C and human pcDNA3.1-SKI-1/S1P, cleavage was restored (Fig. 3A, lane 3). The slightly lower cleavage efficacy of Lassa GP-C in SRD-12B cells transfected with pCDNA3.1-SKI/S1P in comparison with CHO cells is due to the transfection efficiency of cells, which is about 30% and results in uncleaved GP-C. Furthermore, the ratio of cleaved to uncleaved GP-C varies for unknown reasons not only in the recombinant vaccinia system but also in Lassa virus-infected cells.
Figure 3.
SRD-12B cells cleave Lassa virus glycoprotein precursor GP-C only after transfection with recombinant SKI-1/S1P. (A) Nonconfluent cultures of SRD-12B cells and parental CHO-K1 cells were cotransfected with pCDNA3.1 encoding the human SKI-1/S1P (ref. 13; lane 3) or empty vector (lanes 1 and 2) 12 h before MVA-T7-mediated expression of Lassa virus GP-C. (B) Nonconfluent SRD-12B cells were transfected with pCDNA3.1-SKI-1/S1P (lane 4) or empty vector (lane 3) 12 h before infection with Lassa virus, strain Josiah, at a moi of 1. Parental CHO-K1 cells were infected with Lassa virus in parallel (lane 1). At 48 h p.i., cells were lysed in sample buffer, and cleavage of GP-C was analyzed on Western blots.
When SRD-12B cells were infected with Lassa virus, no cleavage was observed in SRD-12B cells 48 h p.i. (Fig. 3B, lane 3). Again, cleavage was fully restored after transfection with a plasmid encoding the human SKI-1/S1P (Fig. 3B, lane 4). These data show clearly that SKI-1/S1P is necessary for the cleavage of the Lassa virus GP-C. The remote possibility that GP-C is not cleaved by SKI-1/S1P directly but by another protease activated by SKI-1/S1P seems very unlikely in view of the identity of the recognition motif of SKI-1/S1P and the Lassa cleavage site (13).
To investigate whether the cleavage of GP-C is necessary for release of infectious viral particles, SRD-12B cells, pcDNA3.1-SKI-1/S1P-transfected SRD-12B cells, and Vero cells were infected with Lassa virus. Virus titers were determined in the supernatants of infected cells by plaque assays at various times after infection according to standard procedures (Fig.4A). Viral titers obtained from infected SRD-12B cells were consistently very low [102 plaque forming units (pfu)/ml], whereas supernatants from SKI-1/S1P-transfected SRD-12B cells contained almost 100,000-fold higher amounts of virus, similar to those seen in the supernatant of infected Vero cells (106 to 107 pfu/ml). To address the question whether noninfectious viral particles are released from SRD-12B cells, supernatants of Lassa virus-infected SRD-12B cells and pcDNA3.1-SKI-1/S1P-transfected SRD-12B cells were subjected to ultracentrifugation through a 20% sucrose cushion, and the presence of pelleted viral particles was detected by Western blotting by using GP-2 and NP-specific antisera, respectively. No viral glycoproteins, neither GP-C nor GP-2, were detectable in the supernatants of Lassa virus-infected SRD-12B cells at 48 h p.i. whereas NP was present in quantities as seen in pcDNA3.1-SKI-1/S1P-transfected SRD cells (Fig. 4B, lanes 2 and 3). Complete virus particles were released from infected SRD cells only after transfection with pcDNA3.1-SKI-1/S1P as indicated by the presence of GP-2 and NP (Fig.4B, lanes 4 and 5).
Figure 4.
SKI-1/S1P-deficient cells release noninfectious particles devoid of glycoprotein. (A) SRD-12B cells, SRD-12B cells transfected with pcDNA3.1-SKI-1/S1P, and Vero cells were infected with Lassa virus, strain Josiah. Supernatants of infected cells were analyzed by plaque titration at 0, 24, 48, 72, 96, and 144 h p.i. according to a standard protocol. (B) To detect release of noninfectious viral particles, SRD-12B cells and SRD-12B cells transfected with human SKI-1/S1P were infected with Lassa virus at a moi of 1, and virions in the supernatants were enriched and partially purified by centrifugation through a 20% sucrose cushion. These pellets (lanes 3 and 5; S) and lysates of transfected cells infected in parallel (lanes 2 and 4; C) were solubilized by treatment with sample buffer and analyzed by Western blot by using peptide sera raised against GP-2 (Upper) and against NP (Lower). (C) To study whether the NP is enclosed by a lipid envelope, supernatants of Lassa virus-infected CHO and SRD-12B cells were centrifuged as described above and treated for 30 min at 37°C with 0.1 μg/μl proteinase K (lanes 2 and 5) or with 0.1 μg/μl proteinase K and 1% Triton X-100 (lanes 3 and 6) or was left untreated (lanes 1 and 4). Samples were analyzed by using Western blots and staining with anti-NP immune serum.
To prove that NP-containing particles from the supernatant of infected SRD-12B cells are enveloped by a lipid membrane, the particles were either treated with proteinase K or proteinase K and Triton X-100 or left untreated (22). Treatment with proteinase K alone had no effect on NP whereas the addition of Triton X-100 destroyed the lipid envelope and enabled the proteinase K to degrade NP (Fig. 4C). These experiments demonstrate that noninfectious enveloped particles devoid of glycoprotein are released in the absence of cleaved GP-C. This finding means that only cleaved glycoproteins are efficiently incorporated into budding virus particles.
Discussion
Many enveloped viruses, such as influenza viruses, paramyxoviruses, HIV, and filoviruses, have fusion proteins that undergo posttranslational proteolytic processing by host proteases. In most cases, cleavage is an important biological control mechanism, because it triggers fusion activity and is, thus, essential for virus entry into the host cell. Cleavage of these fusion proteins is a late event in the replication cycle, occurring either during transit of the glycoproteins through the TGN or after their arrival at the cell surface, and it is not necessary for spike assembly and formation of virions. Although various endoproteases have been identified as activating enzymes, furin and related subtilases cleaving at multiple basic residues play a prominent role in this type of cleavage (23, 24). We demonstrate now that processing of GP-C to GP-2, which appears to be the fusion protein of Lassa virus (25), shows several important differences. First, GP-C is cleaved by a novel endoprotease, SKI-1/S1P, which, unlike furin, belongs to the pyrolysin group of subtilases and cleaves at non-basic residues. GP-C is the first viral glycoprotein known to be processed by SKI-1/S1P, and so far only three cellular proteins have been identified as substrates, the sterol regulatory element binding protein SREBP (26), the transcription factor ATF6 (27), and the neurotrophic factor BDNF (13, 20). Second, GP-C is cleaved in the endoplasmic reticulum or in the cis-Golgi stacks of which SKI-1/S1P is a resident protease (19, 28). It is therefore cleaved at an earlier stage of the exocytotic transport route than, for instance, the influenza virus hemagglutinin. Third, cleavage is necessary for incorporation of the glycoprotein into virions and presumably also for spike formation. However, we showed that noninfectious enveloped particles containing nucleocapsid but devoid of spikes are made in the absence of cleavage. Thus, spikes may not be necessary for formation of Lassa virus particles.
Analysis of the tissue expression pattern of SKI-I/S1P in rats revealed highest levels of activity in liver, spleen, and adrenal glands, and low levels in brain (13). In human Lassa fever cases, high viral titers are recovered from liver, spleen, lungs, kidneys, and adrenals, but not the brain (29). Further study of the tissue tropism of SKI-I/S1P in humans should contribute to the understanding of the pathology of Lassa virus infection in humans.
To date there is no vaccine available for the prevention of Lassa virus infections. Treatment with the guanosine analogue ribavarin is the only available therapy of Lassa fever in humans, but is effective only if started very early in the clinical course of the disease (30). The design of specific inhibitors for SKI-1/S1P-mediated cleavage of Lassa virus GP-C may lead to new therapeutical approaches for Lassa fever.
Acknowledgments
We thank Dr. J. L. Goldstein for generously providing the SRD-12B cells. We thank Prof. Dr. F. Fahrenholz, Institute of Biochemistry, University Mainz, Germany, and Dr. H. Feldmann, Canadian Science Center for Human and Animal Health, Winnipeg, Canada, for helpful discussions and technical cooperation. This work was done by O.L. in partial fulfillment of the requirements for a Ph.D. degree from the Philipps-University, Marburg, Germany. J.t.M. is an International Research Scholar of the Howard Hughes Medical Institute (HHMI) in the Infectious Disease and Parasitology Program. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 286 and Ga282/4-1), INCO-DEV grant ERBIC18CT980382 of the European Community, and HHMI Grant 75301564301.
Abbreviations
CHO
Chinese hamster ovary
NP
nucleoprotein
BHK
baby hamster kidney
MVA-T7
modified vaccinia virus strain Ankara T7
moi
multiplicity of infection
FPVHA
fowl plague virus hemagglutinin
BFA
Brefeldin A
TGN
trans-Golgi network
p.i.
postinfection
References
- 1.McCormick J B, Webb P A, Krebs J W, Johnson K M, Smith E S. J Infect Dis. 1987;155:437–444. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- 2.McCormick J B, King I J, Webb P A, Johnson K M, O'Sullivan R, Smith E S, Trippel S, Tong T C. J Infect Dis. 1987;155:445–455. doi: 10.1093/infdis/155.3.445. [DOI] [PubMed] [Google Scholar]
- 3.ter Meulen J, Lenz O, Koivogui L, Magassouba N, Kaushik S K, Lewis R, Aldis W. Trop Med Int Health. 2001;6:83–84. doi: 10.1046/j.1365-3156.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 4.Southern P J. In: Fields Virology. Fields B N, Knipe P M, Howley, editors. Philadelphia: Lippincott; 1996. pp. 1505–1519. [Google Scholar]
- 5.Burns J W, Buchmeier M J. In: The Arenaviridae. Salvato M S, editor. New York: Plenum; 1993. pp. 17–33. [Google Scholar]
- 6.Cao W, Henry M D, Borrow P, Yamada H, Elder J H, Ravkov E V, Nichol S T, Compans R W, Campbell K P, Oldstone M B. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Oldstone M B. Virology. 1994;198:1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- 8.Glushakova S E, Omelyanenko V G, Lukashevitch I S, Bogdanov A A, Jr, Moshnikova A B, Kozytch A T, Torchilin V P. Biochim Biophys Acta. 1992;1110:202–208. doi: 10.1016/0005-2736(92)90360-x. [DOI] [PubMed] [Google Scholar]
- 9.Lenz O, ter Meulen J, Feldmann H, Klenk H D, Garten W. J Virol. 2000;74:11418–11421. doi: 10.1128/jvi.74.23.11418-11421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klenk H D, Garten W. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 11.Espenshade P J, Cheng D, Goldstein J L, Brown M S. J Biol Chem. 1999;274:22795–22804. doi: 10.1074/jbc.274.32.22795. [DOI] [PubMed] [Google Scholar]
- 12.Sakai J, Rawson R B, Espenshade P J, Cheng D, Seegmiller A C, Goldstein J L, Brown M S. Mol Cell. 1998;2:505–514. doi: 10.1016/s1097-2765(00)80150-1. [DOI] [PubMed] [Google Scholar]
- 13.Seidah N G, Mowla S J, Hamelin J, Mamarbachi A M, Benjannet S, Toure B B, Basak A, Munzer J S, Marcinkiewicz J, Zhong M, Barale J C, Lazure C, Murphy R A, Chretien M, Marcinkiewicz M. Proc Natl Acad Sci USA. 1999;96:1321–1326. doi: 10.1073/pnas.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawson R B, Cheng D, Brown M S, Goldstein J L. J Biol Chem. 1998;273:28261–28269. doi: 10.1074/jbc.273.43.28261. [DOI] [PubMed] [Google Scholar]
- 15.Garten W, Will C, Buckard K, Kuroda K, Ortmann D, Munk K, Scholtissek C, Schnittler H, Drenckhahn D, Klenk H D. J Virol. 1992;66:1495–1505. doi: 10.1128/jvi.66.3.1495-1505.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyhse-Andersen J. J Biochem Biophys Methods. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 17.Felgner J H, Kumar R, Sridhar C N, Wheeler C J, Tsai Y J, Border R, Ramsey P, Martin M, Felgner P L. J Biol Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- 18.Klenk H D, Garten W, Rott R. EMBO J. 1984;3:2911–2915. doi: 10.1002/j.1460-2075.1984.tb02231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBose-Boyd R A, Brown M S, Li W P, Nohturfft A, Goldstein J L, Espenshade P J. Cell. 1999;99:703–712. doi: 10.1016/s0092-8674(00)81668-2. [DOI] [PubMed] [Google Scholar]
- 20.Toure B B, Munzer J S, Basak A, Benjannet S, Rochemont J, Lazure C, Chretien M, Seidah N G. J Biol Chem. 2000;275:2349–2358. doi: 10.1074/jbc.275.4.2349. [DOI] [PubMed] [Google Scholar]
- 21.Lippincott-Schwartz J, Yuan L C, Bonifacino J S, Klausner R D. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giddings A M, Ritter G D, Jr, Mulligan M J. Virology. 1998;248:108–116. doi: 10.1006/viro.1998.9284. [DOI] [PubMed] [Google Scholar]
- 23.Garten W, Hallenberger S, Ortmann D, Schafer W, Vey M, Angliker H, Shaw E, Klenk H D. Biochimie. 1994;76:217–225. doi: 10.1016/0300-9084(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 24.Klenk H D, Garten W. In: Cellular Receptors for Animal Viruses. Wimmer E, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. , monograph 28, pp. 241–280. [Google Scholar]
- 25.Gallaher W R, DiSimone C, Buchmeier M J. BMC Microbiol. 2001;1:1. doi: 10.1186/1471-2180-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakai J, Nohturfft A, Goldstein J L, Brown M S. J Biol Chem. 1998;273:5785–5793. doi: 10.1074/jbc.273.10.5785. [DOI] [PubMed] [Google Scholar]
- 27.Ye J, Rawson R B, Komuro R, Chen X, Dave U P, Prywes R, Brown M S, Goldstein J L. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 28.Seidah N G, Benjannet S, Hamelin J, Mamarbachi A M, Basak A, Marcinkiewicz J, Mbikay M, Chretien M, Marcinkiewicz M. Ann N Y Acad Sci. 1999;885:57–74. doi: 10.1111/j.1749-6632.1999.tb08665.x. [DOI] [PubMed] [Google Scholar]
- 29.Walker D H, McCormick J B, Johnson K M, Webb P A, Komba-Kono G, Elliott L H, Gardner J J. Am J Pathol. 1982;107:349–356. [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick J B, King I J, Webb P A, Scribner C L, Craven R B, Johnson K M, Elliott L H, Belmont-Williams R. N Engl J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 31.Sutter G, Ohlmann M, Erfle V. FEBS Lett. 1995;371:9–12. doi: 10.1016/0014-5793(95)00843-x. [DOI] [PubMed] [Google Scholar]