Lipid Rafts Act as Specialized Domains for Tetanus Toxin Binding and Internalization into Neurons (original) (raw)
Abstract
Tetanus (TeNT) is a zinc protease that blocks neurotransmission by cleaving the synaptic protein vesicle-associated membrane protein/synaptobrevin. Although its intracellular catalytic activity is well established, the mechanism by which this neurotoxin interacts with the neuronal surface is not known. In this study, we characterize p15s, the first plasma membrane TeNT binding proteins and we show that they are glycosylphosphatidylinositol-anchored glycoproteins in nerve growth factor (NGF)-differentiated PC12 cells, spinal cord cells, and purified motor neurons. We identify p15 as neuronal Thy-1 in NGF-differentiated PC12 cells. Fluorescence lifetime imaging microscopy measurements confirm the close association of the binding domain of TeNT and Thy-1 at the plasma membrane. We find that TeNT is recruited to detergent-insoluble lipid microdomains on the surface of neuronal cells. Finally, we show that cholesterol depletion affects a raft subpool and blocks the internalization and intracellular activity of the toxin. Our results indicate that TeNT interacts with target cells by binding to lipid rafts and that cholesterol is required for TeNT internalization and/or trafficking in neurons.
INTRODUCTION
Tetanus (TeNT) and botulinum neurotoxins (BoNTs) block neurotransmitter release and are responsible for tetanus and botulism, respectively. These toxins share a common structure comprised of a heavy (H, 100 kDa) and a light (L, 50 kDa) chain linked by a disulfide bond. The H chain mediates binding and internalization in neurons, whereas the L chain is a metallo-protease that selectively cleaves synaptic proteins (Niemann et al., 1994; Schiavo et al., 2000). TeNT and BoNTs bind to the neuromuscular junction, but their intracellular actions take place at different levels of the nervous system. TeNT undergoes retrograde transport to the cell body of spinal cord motor neurons (MNs), is transcytosed, and cleaves the synaptic vesicle protein vesicle-associated membrane protein (VAMP)/synaptobrevin in inhibitory synapses. Instead, BoNTs act at a peripheral level by blocking acetylcholine release at the motor nerve terminal. This differential sorting has been interpreted as a consequence of binding to different surface receptors (Habermann and Dreyer, 1986; Herreros et al., 1999).
TeNT and BoNTs bind to polysialogangliosides of the G1b series (Halpern and Neale, 1995). However, the fact that their binding is sensitive to proteases (Lazarovici and Yavin, 1986; Pierce et al., 1986; Yavin and Nathan, 1986) suggests the existence of specific protein receptors. Thus, a model in which TeNT and BoNTs interact with a complex constituted by both lipid and protein receptors has been proposed (Montecucco, 1986). Despite several efforts, these protein receptors have not been conclusively identified. Several BoNT serotypes interact with synaptotagmins (Nishiki et al., 1994; Li and Singh, 1998), but the role of these proteins as physiological BoNT receptors remains controversial (Evans et al., 1986; Bakry et al., 1997). We have previously followed a cross-linking approach (Schiavo et al., 1991) to demonstrate that the binding domain of TeNT (TeNT HC) interacts with a glycoprotein of ∼15 kDa (p15) in nerve growth factor (NGF)-differentiated PC12 cells and spinal cord MNs (Herreros et al., 2000a). In these neuronal cell types p15 showed the behavior of an integral membrane protein (Herreros et al., 2000a).
Several toxins, including cholera toxin (CT) (Orlandi and Fishman, 1998; Wolf et al., 1998; Shogomori and Futerman, 2001) and all the known pore-forming toxins (Fivaz et al., 1999; Gordon et al., 1999), bind to lipid raft components. Furthermore, some bacteria and viruses enter cells via lipid rafts (Dehio et al., 1995; Baorto et al., 1997; Parton and Lindsay, 1999; Shin et al., 2000). Lipid rafts are microdomains of the plasma membrane enriched in sphingolipids (including gangliosides), cholesterol, and glycosylphosphatidylinositol (GPI)-anchored proteins (Brown and London, 2000; Simons and Toomre, 2000). They have been implicated in vesicular sorting, trafficking to the apical membrane, and signaling (reviewed in Simons and Ikonen, 1997; Simons and Toomre, 2000). Recently, several lines of evidence have supported the role of cholesterol in the control of intracellular membrane trafficking (Grimmer et al., 2000; Hoekstra and van Ijzendoorn, 2000; Mukherjee and Maxfield, 2000; Simons and Gruenberg, 2000).
Here, we characterize the cross-linking products containing TeNT HC and p15 to show that p15s are GPI-anchored proteins in different neuronal cell types. Immunoprecipitation experiments identify p15 as Thy-1, a GPI-anchored neuronal raft protein (Williams and Gagnon, 1982; Madore et al., 1999) in NGF-differentiated PC12 cells. The association of TeNT HC with Thy-1 at the plasma membrane is confirmed by fluorescence resonance energy transfer (FRET) measurements with the use of fluorescence lifetime imaging microscopy (FLIM). Cell-bound TeNT HC and Thy-1 are found in detergent-insoluble glycolipid-enriched (DIGs) fractions, which represent in vitro isolated lipid rafts (Brown and London, 2000). Furthermore, we show that cholesterol depletion causes the displacement of TeNT HC from a DIG subpool and protects neurons from the toxic activity of TeNT in vivo.
MATERIALS AND METHODS
Materials
Recombinant TeNT HC fragments were produced and radiolabeled with [γ-32P]ATP as previously described (Lalli et al., 1999; Herreros et al., 2000a). TeNT was purified and biotinylated as in Arribas et al. (1993). Aerolysin and anti-aerolysin antibodies were kindly provided by Dr. G. van der Goot (University of Geneva, Geneva, Switzerland). Monoclonal antibody against GAP-43 (clone NM4) was from Autogen Bioclear (Calne, Wilshire, United Kingdom) and anti-HPC-1/syntaxin-1 and anti-SNAP-25 antibodies were a kind gift from T.H. Söllner (Memorial Sloan-Kettering Cancer Center, New York, NY).
Binding, Cross-linking, and Immunoprecipitation
Rat pheochromocytoma (PC12) cells were differentiated with 75 ng/ml 7S NGF (Alomone, Jerusalem, Israel) for 6–7 d. Spinal cord MNs were purified from E14 rat embryos and mouse spinal cord cells were isolated from E13 mice embryos (Lalli et al., 1999; Herreros et al., 2000a).
For phosphoinositol-phospholipase C (PI-PLC) treatment, cells were washed in serum-free medium, pretreated (1 h, 37°C) with different amounts of PI-PLC (Sigma, Poole, Dorset, United Kingdom) and extensively washed. Cells were cooled on ice, washed with Hanks' buffer (Herreros et al., 2000a), and incubated (1 h, 4°C) with 300 pM 32P-labeled TeNT HC in Hanks' buffer containing 0.2% bovine serum albumin. After binding, cells were washed and cross-linked (10 min, 4°C) with 0.22 mM bis[2(succinimidyloxycarbonyloxy)ethyl]sulfone (Perbio-Science, Tatten Hall, Cheshire, United Kingdom) in Hanks' buffer. The reaction was stopped and cells were solubilized as previously described (Herreros et al., 2000a). Proteins were analyzed in 6–12% acrylamide gradient gels followed by autoradiography.
In immunoprecipitation experiments, selected samples were incubated with 1 nM CT (1 h, 4°C) after 32P-labeled TeNT HC binding and cross-linking. Cells were scraped in Hanks' buffer containing 1 mM Pefabloc (Roche Molecular Biochemicals, Mannheim, Germany), 1 mM iodoacetamide, 1 mM benzamidine, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and phosphatase inhibitors cocktail (all from Sigma). Samples were pelleted down and solubilized (30 min, 4°C) with 4% octyl-β-d-glucopyranoside, 0.5% Triton X-100 (TX-100) in Hanks' buffer plus inhibitors. After centrifugation (10,000 rpm, 10 min), supernatants were incubated (2 h, 4°C) with protein G or protein A beads precoupled with monoclonal anti-vesicular stomatitis virus protein G (VSV-G) (Herreros et al., 2000a), anti-Thy-1 (clone OX7), anti-CT (Biogenesis, Pool, Dorset, United Kingdom), and mock antibodies or affinity-purified polyclonal anti-PrP antibodies (Affinity Bioreagents, Golden, CO), respectively. Beads were extensively washed with detergent-containing Hanks' buffer. In some cases, an additional wash with 1 M urea was performed after the detergent washes. Beads were resuspended in SDS-sample buffer and analyzed as described above.
Isolation of DIGs and Western Blot
PC12 cells (seeded at 110 cells/mm2) were treated with NGF for 6–7 d. A total of 3.9 × 106 mouse spinal cord neurons was cultured for 2 wk before the experiment. Cells were washed and in selected cases pretreated (1 h, 37°C) with 4.5 mM methyl-β-cyclodextrin (MCDX; Sigma) in serum-free medium. After extensive washing, 300 pM TeNT 32P-HC or 1 nM biotinylated-TeNT (b-TeNT) was bound as mentioned above. Cells were washed with Hanks' buffer and resuspended in 1 ml (PC12) or 0.25 ml (spinal cord cells) of 1% TX-100 in Hanks' buffer plus inhibitors. Cells were solubilized (30 min, 4°C), the cell lysate was adjusted to 41% sucrose in Hanks' buffer, and overlaid with 8.5 ml of 35% sucrose and 2.5 ml of 16% sucrose. DIGs were isolated by ultracentrifugation (35,000 rpm, 18 h, 4°C; Abrami et al., 1998). Then 11–12 fractions of 1 ml were collected, precipitated with 6.5% trichloroacetic acid in the presence of 0.05% sodium deoxycholate (Sigma), and washed with 80% cold acetone. Samples were analyzed by SDS-PAGE followed by Western blot and autoradiography. b-TeNT was detected with the use of streptavidin-peroxidase (1.25 μg/ml; Sigma). Western blots were developed with the use of the enhanced chemiluminescence method (Amersham Pharmacia Biotech UK, Little Chalfont, Buckinghamshire, United Kingdom). Alternatively, in the case of mouse spinal cord cells, gels were rinsed for 20 min with 50 mM Tris-HCl, pH 7.4, 20% glycerol and transferred to nitrocellulose (5 h, 150 mA) in 10 mM NaHCO3, 3 mM Na2CO3, pH 9.8 (Abrami et al., 1998). Blots were rinsed with binding buffer (50 mM NaH2PO4, pH 7.5, 0.3% Tween 20) and incubated with aerolysin (2 nM, 2 h), followed by washing and Western blot with the use of anti-aerolysin antibodies.
For cross-linking experiments, fractions 2–5 (DIGs) and 9–12 (soluble) were pooled and cross-linked with 0.5 mM bis[2(succinimidyloxycarbonyloxy)ethyl]sulfone (10 min, 4°C, under shaking). The reaction was stopped with 30 mM glycine and samples were analyzed as described above.
Immunofluorescence
Fab fragment from the OX7 clone was purified with the use of the ImmunoPure Fab Preparation kit, according to manufacturer's instructions (Perbio-Science). Direct conjugation of IgG and Fab to Cy3 and Cy5 fluorophores (Amersham Pharmacia Biotech UK) was performed at pH 8.5 (IgG) or pH 9.0 (Fab) as described previously (Bastiaens and Jovin, 1998).
Cells were incubated with either TeNT HC (80–120 nM at 4 or 37°C) or CT (2 nM, 1 h, 4°C) and fixed with 3.7% paraformaldehyde (10 min at room temperature). TeNT HC was immunodetected with the use of purified monoclonal antibodies against the VSV-G epitope as previously described (Lalli et al., 1999). CT in Figure 4 was detected by with the use of anti-CT polyclonal antibodies (Sigma). When permeabilization was needed, cells were incubated with 0.1% TX-100 for 5 min. Texas Red- (Amersham Pharmacia Biotech UK) or Alexa 488-coupled secondary antibodies (Molecular Probes, Eugene, OR) were used according to manufacturer's instructions. Cells were visualized through a Plan-APOCHROMAT, 63×/1.4 numerical aperture phase 3 oil objective with the use of a laser scanning confocal microscope (Zeiss LSM 510; Zeiss, Jena, Germany). Images correspond to selected optical sections (collected in the _z_-axis at intervals of 0.4 μm).
Figure 4.
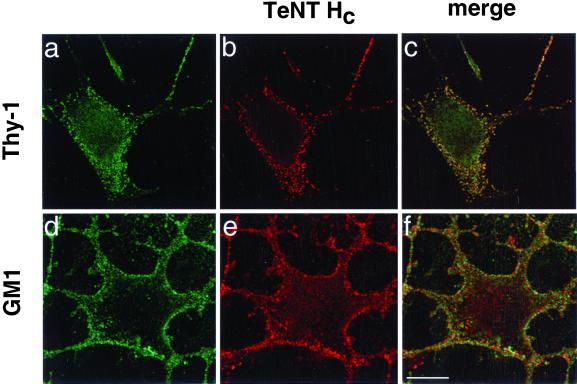
TeNT HC binding colocalizes with the raft markers Thy-1 and GM1. Binding of TeNT HC (1 h, 4°C) was revealed with the use of Cy3 labeled-monoclonal anti-VSV-G antibodies (b) or Texas Red-coupled secondary antibodies (e) in nonpermeabilized NGF-differentiated PC12 cells. Thy-1 immunoreactivity was detected with the use of Cy5-labeled anti-Thy-1 Fab (a) (staining is shown in green pseudocolor). GM1 was detected by CT binding (d; 1 h, 4°C) followed by polyclonal anti-CT and Alexa 488-coupled secondary antibodies. Overlaps are shown in c and f (colocalization appears in yellow). Confocal images correspond to single z-sections taken at a similar distance from the substrate. Bar, 10 μm.
FRET Determination by FLIM
For FLIM measurements, immunocytochemical staining was performed as described above, without cell permeabilization, and included an additional fixation with 3.7% paraformaldehyde before mounting. A detailed description of the FLIM apparatus used for FRET determination can be found elsewhere (Squire and Bastiaens, 1999). The lifetime instrument performs phase- and modulation-based imaging fluorometry by microscopy. All images were taken with the use of a Zeiss Plan-APOCHROMAT 100×/1.4 numerical aperture phase 3 oil objective and the homodyne phase-sensitive images recorded at a modulation frequency of 80.224 MHz. Donors (Cy3-labeled monoclonal anti-VSV-G or anti-CT IgGs) were excited with the use of the 414 nm line of an argon/krypton laser and the resultant fluorescence separated with the use of a combination of dichroic beamsplitter (HQ 565 LP; Chroma, Brattleboro, VT) and emission filter (HQ 610/75; Chroma). Acceptor images (Cy5-anti Thy-1 Fab) were recorded with the use of a 100-W mercury arc lamp (Zeiss Attoarc) as a source of sample illumination combined with a high Q Cy3 filter set (exciter, HQ 620/60; dichroic, HQ 660 LP; emitter, HQ 700/75 LP; Chroma).
TeNT Intoxication and VAMP Cleavage
Mouse spinal cord cells (10–12 d in culture) were pretreated with 2 or 4.5 mM MCDX (1 h, 37°C) in serum-free medium and washed. The effect of adding cholesterol–MCDX complexes could not be assessed because this treatment was toxic in spinal cells. Treated and untreated cells were incubated with 200 pM TeNT (20 h, 37°C) in serum-free medium. Cells were scraped in Hanks' buffer plus Pefabloc and proteins recovered by trichloroacetic acid precipitation. Samples were analyzed by SDS-PAGE containing urea and Western blot with the use of anti-VAMP-2 monoclonal antibodies (Edelmann et al., 1995). VAMP/synaptobrevin immunoreactivity was quantified with the use of NIH Image 1.61 and normalized to syntaxin-1 for equal loading.
Transferrin Uptake
MCDX-treated and untreated spinal cord cells were assessed for 125I-transferrin uptake (human diferric form; 670 ng/ml; PerkinElmer Life Science Products, Boston, MA) in serum-free medium at 4°C or 37°C for different times. After two rounds of acid wash (0.2 M acetic acid, 0.5 M NaCl, pH 2.5) (Hopkins and Trowbridge, 1983) for 2 min on ice followed by a wash in medium, cell lysates were recovered in 1 M NaOH and counted in a gamma counter (Packard Instrument, Meriden, CT). Counts represent endocytosed transferrin. Competition was tested with the use of 200× excess of human holotranferrin (Sigma).
RESULTS
p15s Are GPI-anchored TeNT HC Binding Proteins
We recently demonstrated that cross-linking products very similar in size are formed after binding of 32P-labeled TeNT HC (Figure 1A) to NGF-differentiated PC12 cells, rat MNs, and mouse spinal cord cells (Herreros et al., 2000a). These results indicated the interaction of TeNT HC (48 kDa) with a protein of an apparent molecular weight of 15 kDa (p15) in different cell types. p15s are _N_-glycosylated and behave as integral membrane proteins in detergent-partitioning experiments (Herreros et al., 2000a). This last property of p15 could be mediated by the presence of transmembrane domain(s) or by various forms of covalent lipid modification.
Figure 1.
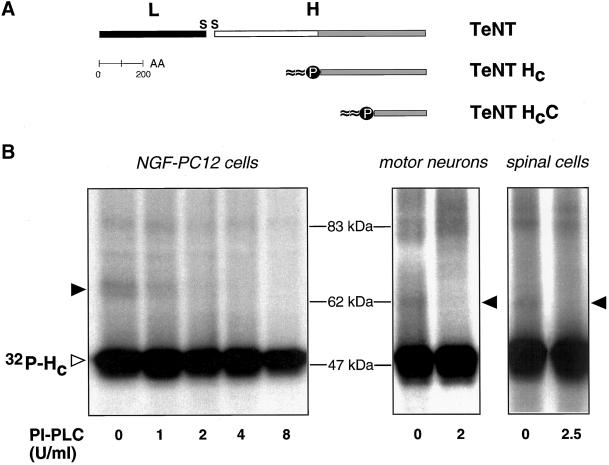
p15s are GPI-anchored proteins. (A) Schematic representation of TeNT, the binding fragment HC and the carboxyl-terminal subdomain HCC. (B) Binding and cross-linking of 32P-labeled TeNT HC after pretreatment with increasing doses of PI-PLC in NGF-differentiated PC12 cells, rat MNs, and mouse spinal cord cells. Empty arrowhead indicates cell-bound TeNT-HC and filled arrowheads point to the corresponding cross-linking products, which do not form after PI-PLC treatment.
GPI-anchored proteins are abundant components of the plasma membrane, behaving as integral membrane proteins. Their biochemical analysis is simplified by the use of PI-PLC, an enzyme that causes their selective release from the membrane. We therefore tested the possibility that p15s are GPI-anchored proteins. Pretreatment with PI-PLC before TeNT HC binding and cross-linking inhibited the formation of the previously characterized ∼65 kDa cross-linking product in NGF-differentiated PC12 cells in a dose-dependent manner (Figure 1B, filled arrowhead), whereas total binding was not significantly affected (Figure 1B, empty arrowhead). Formation of the corresponding cross-linking products in MNs and spinal cord cells (Figure 1B, filled arrowheads) was also inhibited by PI-PLC pretreatment. These results indicate that p15s, proteins interacting with TeNT HC in our cross-linking assays, are GPI-anchored proteins in all three neuronal cell types.
p15 Is Thy-1 in NGF-differentiated PC12 Cells
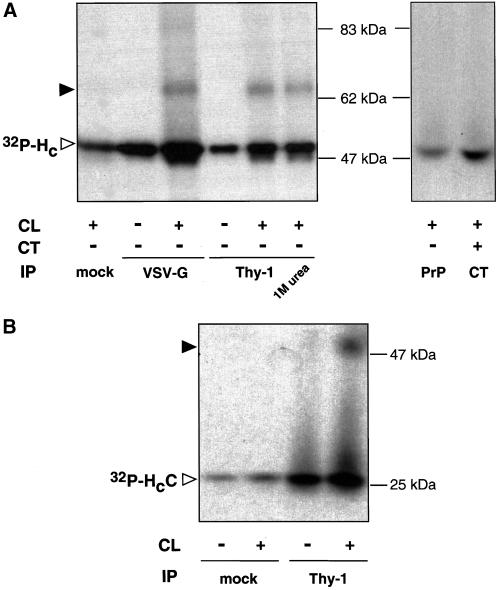
In our attempts to identify p15s, we searched for known proteins with the properties described above. Among others, Thy-1 (Williams and Gagnon, 1982), a major cell-surface GPI-anchored glycoprotein of 25–29 kDa present in thymocytes and brain and expressed in PC12 cells (Jeng et al., 1998), represented a likely candidate. To test whether p15 is Thy-1, immunoprecipitation experiments with a panel of different antibodies was performed in NGF-differentiated PC12 cells (Figure 2A). As expected, antibodies against the VSV-G tag of TeNT HC pulled down both the unmodified binding fragment and the ∼65-kDa cross-linking product (Figure 2A). The ∼65-kDa cross-linking band was specifically recovered with monoclonal antibodies against Thy-1, but not with mock antibodies or with antibodies against PrP, a GPI-anchored protein similar in size to Thy-1 (Figure 2A). Antibodies against CT, which binds to GM1 and is used as a raft marker, did not immunoprecipitate the cross-linking product (Figure 2A), indicating that lipid rafts had been disrupted by solubilization with octyl-β-d-glucopyranoside under our experimental conditions (Arni et al., 1998; Simons et al., 1999; Simons and Toomre, 2000). Furthermore, incubation of cells with both CT and TeNT HC followed by cross-linking did not result in the appearance of additional cross-linking products (our unpublished results). These findings suggest that the cross-linking of TeNT HC to Thy-1 is not a consequence of high local concentration or proximity but rather due to their direct interaction. Interestingly, anti-Thy-1 antibodies coimmunoprecipited a fraction of TeNT HC that could not be dissociated from the beads with 1 M urea (Figure 2A), suggesting a strong interaction between Thy-1 and TeNT HC. Moreover, the decrease in Thy-1 immunoreactivity after PI-PLC treatment of NGF-differentiated PC12 cells strictly correlated with the inhibition of the formation of the ∼65-kDa cross-linking product (our unpublished results). The apparent molecular weight of the cross-linking product is lower that the sum of the individual components (48 and 25 kDa). Anomalous migration of cross-linking adducts in SDS-PAGE has been reported (Tate and Khadse, 1987) and is dependent on the nature of the interacting proteins surfaces and the generation of conformational constrains stable in denaturing conditions.
Figure 2.
Thy-1 is p15 in NGF-differentiated PC12 cells. Immunoprecipitation (IP) after binding and cross-linking (CL) of 32P-labeled TeNT-HC (A) or HCC (B) with the use of mock antibodies (anti-myc or anti-HA) or antibodies against VSV-G, Thy-1, CT, or PrP in NGF-differentiated PC12 cells. Filled arrowheads indicate the position of the corresponding cross-linking products.
We have previously demonstrated that the carboxyl-terminal subdomain of TeNT HC (HCC; Figure 1A) is necessary and sufficient for binding to p15 (Herreros et al., 2000b). Consistently, anti-Thy-1 but not mock monoclonal antibodies immunoprecipitated the cross-linking product obtained after binding of 32P-labeled HCC to NGF-differentiated PC12 cells (Figure 2B).
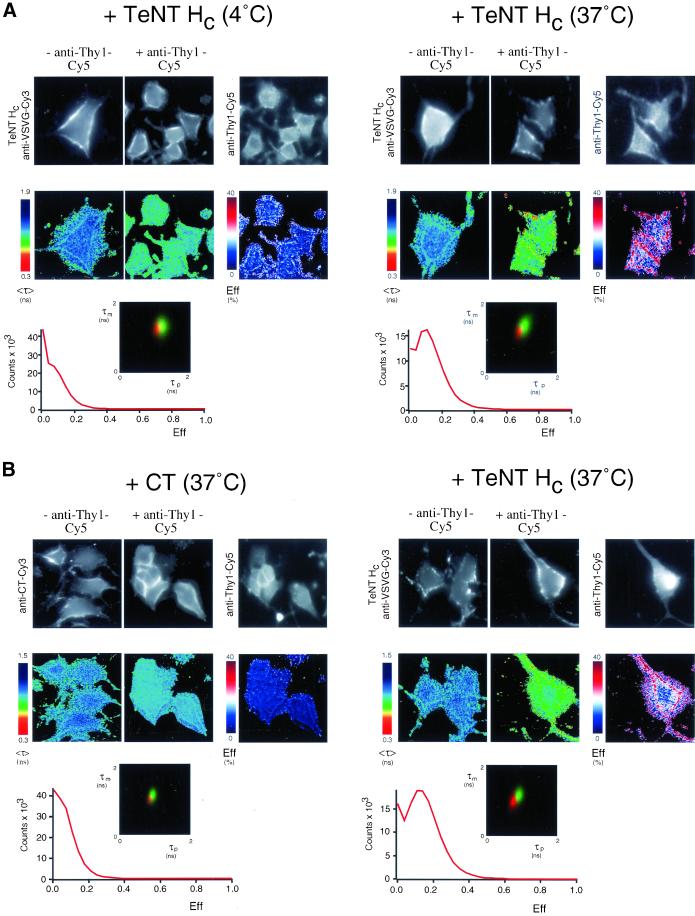
The interaction of TeNT HC with Thy-1 in its membrane environment was further analyzed by FLIM (Figure 3). With the use of FLIM, we determined the extent of FRET between a Cy3-labeled IgG directed against the VSV-G epitope of TeNT HC (donor) and a Cy5-conjugated IgG Fab fragment recognizing Thy-1 (acceptor). FRET results in a shortening of the donor fluorescence lifetime, which is measured by two independent parameters, the phase shift (τp) and relative modulation depth (τm). FRET is only detected between two proteins that are closely associated or complexed in vivo, typically within 10 nm (Selvin, 2000). NGF-differentiated PC12 cells were stained with a Cy3-VSV-G IgG to visualize cell-bound TeNT HC after incubation with the toxin for 1 h at 4°C or 30 min at 37°C either alone, or together with a Cy5-labeled anti-Thy-1 Fab fragment. The fluorescence lifetime, <τ>, measured as the average phase shift and relative modulation depth [(τp + τm)/2] for Cy3-VSV-G antibodies was decreased at punctate structures (see below) of the plasma membrane (Figure 3, A and B, right). This was particularly evident when TeNT HC had been incubated with the cells at 37°C. The presence of FRET was confirmed by photobleaching the Cy5 acceptor, resulting in a lengthening of the lifetime of the donor (our unpublished results). The FRET efficiency (Eff)1 pseudocolor plots also indicate that FRET occurs at the plasma membrane. Statistical analysis of cumulative results from all the cells analyzed demonstrates that TeNT HC interacts with Thy-1 at 4°C and that their association is enhanced at 37°C (see two-dimensional τp vs. τm lifetime profiles and pixel counts vs. FRET efficiency plots in the lower part of Figure 3A). Permeabilized cells (allowing staining of putative intracellular structures) showed the same FRET efficiency (our unpublished results), indicating that FRET was due to the interaction of the TeNT HC and Thy-1 at the plasma membrane. Parallel experiments with the use of a Cy3-labeled anti-CT IgG to visualize cell-bound CT either alone or together with a Cy5-labeled anti-Thy-1 Fab fragment showed no significant FRET, compared with the positive control cells (stained with Cy3-labeled anti-VSVG IgG against cell-bound TeNT HC/Cy5-labeled anti-Thy-1 Fab fragment) (Figure 3B), indicating that localization to lipid rafts per se is not sufficient to produce FRET under our experimental conditions. Taken together, these results identify p15 as Thy-1 in NGF-differentiated PC12 cells and indicate that TeNT HC specifically associates with Thy-1 on the surface of these cells.
Figure 3.
TeNT HC and Thy-1 association in NGF-differentiated PC12 cells detected by FLIM. NGF-differentiated PC12 cells were incubated with TeNT HC (1 h at 4°C or 30 min at 37°C) or CT (30 min at 37°C), washed, and then fixed in paraformaldehyde. Cells were stained with a Cy3-labeled anti-VSV-G IgG (donor) alone (−anti-Thy1-Cy5) or together with a Cy5-conjugated anti-Thy1 IgG Fab fragment (+anti-Thy1-Cy5) (A and right panels of B). In parallel experiments, cells were stained with Cy3-labeled anti-CT IgG (donor) alone (−anti-Thy1-Cy5) or together with a Cy5-conjugated anti-Thy-1 IgG Fab fragment (+anti-Thy1-Cy5) (B, left). The fluorescence images from the donor (TeNT HC anti-VSV-G-Cy3 or anti-CT-Cy3) and acceptor (anti-Thy1-Cy5), the donor fluorescence lifetime <τ> and its corresponding pseudocolor scales are shown. Eff is the pixel-by-pixel FRET efficiency represented on a pseudocolor scale (see details in the text) and the average lifetime of the donor in the absence of acceptor (τD) used to calculate Eff has been taken by averaging six anti-VSVG or anti-CT-Cy3 donor (alone without acceptor) measurements. The cumulative lifetimes of the donor alone (green) and those measured in the presence of the acceptor fluorophore (red) are plotted on the two-dimensional histograms (n = 7 in A and n = 6 in B). The pixel counts versus Eff profiles summarize all the FRET efficiency data observed. FRET between TeNT HC and Thy-1 is greatly enhanced by incubation at 37°C and is not significant between CT and Thy-1.
TeNT Interacts with Lipid Rafts
GPI-anchored proteins and gangliosides, together with cholesterol and other sphingolipids, concentrate in microdomains of the plasma membrane called lipid rafts (Jacobson and Dietrich, 1999; Simons and Toomre, 2000). The interaction of TeNT HC with Thy-1, which is considered a neuronal raft marker (Aarts et al., 1999; Madore et al., 1999), therefore suggested that the binding of TeNT to the cell surface could be mediated by lipid rafts. To test this hypothesis, we first looked at the distribution of Thy-1 and TeNT HC in NGF-differentiated PC12 cells. Bound TeNT HC displays a punctate pattern on the plasma membrane (Lalli et al., 1999; Herreros et al., 2000a), which is reminiscent of lipid rafts (Mayor et al., 1994; Harder et al., 1998) and is shared by Thy-1 (Jeng et al., 1998) (Figure 4, a–c). Furthermore, GM1, another raft marker (Orlandi and Fishman, 1998; Wolf et al., 1998), showed a partial colocalization with TeNT HC (Figure 4, d–f).
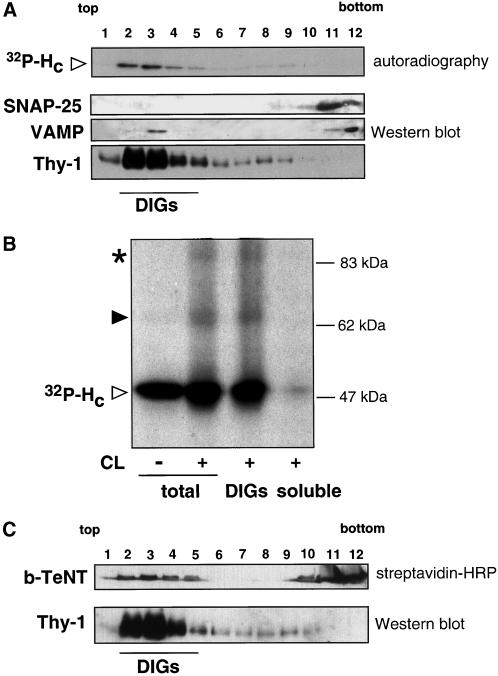
To investigate more directly whether TeNT HC binds to lipid microdomains, we prepared DIG-enriched membranes. Isolation of DIGs is one of the most widely used methods for studying lipid rafts (Brown and London, 2000). DIGs were purified from NGF-differentiated PC12 cells after binding of 300 pM 32P-labeled TeNT HC and radioactivity was followed along the gradient fractions. TeNT HC concentrated in fractions 2–4 at the top of the gradient (Figure 5A), corresponding to DIGs. DIG fractions were defined by their enrichment in Thy-1 (Figure 5A) and other raft markers such as the ganglioside GM1 or PrP (our unpublished results). SNAP-25, a palmitoylated plasma membrane protein, remained concentrated in the bottom fractions of the gradient (Figure 5A), which contain the soluble material and the majority of proteins. Similarly, only a very small amount of VAMP/synaptobrevin, an integral membrane protein of synaptic vesicles, was found in DIGs (Figure 5A; Chamberlain et al., 2001). The ability of TeNT HC to reach the top of the gradient is dependent on the presence of cell lysate (our unpublished results), indicating that the flotation of TeNT HC to the lighter fractions is not due to the presence of detergent and the centrifugation procedure.
Figure 5.
TeNT HC and TeNT are found in DIGs isolated from NGF-differentiated PC12 cells. (A) Bound 32P-labeled TeNT HC (300 pM) concentrates in the lighter fractions (2–4; DIGs) of the gradient (see MATERIALS AND METHODS), which contain the majority of Thy-1. In contrast, SNAP-25 and VAMP are mainly found in soluble fractions (11 and 12). (B) TeNT HC and Thy-1 interact in isolated DIGs. The cross-linking (CL) pattern observed in intact NGF-differentiated PC12 cells (total) is reproduced when DIGs (pooled fractions 2–5) but not the soluble fractions (9–12) were used for cross-linking. Filled arrowhead indicates the ∼65-kDa cross-linking product representing the interaction of TeNT HC with Thy-1 and the asterisk points to the ∼83-kDa cross-linking band corresponding to TeNT HC homodimers. (C) b-TeNT (1 nM) is found in the lighter fractions (2–5) of the gradient where it comigrates with Thy-1 and also in fractions 11–12. The band showed corresponds to the H chain of b-TeNT.
To assess whether the interaction of TeNT HC and Thy-1 is stable in the conditions used for DIG isolation, TeNT HC was bound to NGF-differentiated PC12 cells and then DIGs or soluble fractions were isolated and used for cross-linking. DIGs showed the same pattern of cross-linking seen in intact cells (Figure 5B). Both the ∼65-kDa cross-linking product representing the interaction of TeNT HC with Thy-1 and the ∼85-kDa adduct, which is likely to reflect the formation of HC homodimers (Herreros et al., 2000a), were obtained in purified DIGs (Figure 5B). In contrast, no cross-linking products were observed in the soluble fraction.
In parallel experiments, b-TeNT was bound to NGF-differentiated PC12 cells and DIGs were isolated. As shown for TeNT HC, b-TeNT was found in DIG fractions, although in this case the association with DIGs was not complete and some of the bound toxin remained with the soluble material (Figure 5C, fractions 10–12). A possible explanation for this result would be that TeNT binding is more sensitive to TX-100 solubilization than that of HC due to steric hindrance caused by the translocation domain of the holotoxin. The fact that both TeNT HC and TeNT were found in DIGs is supported by their very similar punctate distribution on the plasma membrane (Herreros et al., 2000a).
These results indicate that the interaction of TeNT and its binding fragment HC with the plasma membrane of NGF-differentiated PC12 cells is mediated by lipid microdomains. The two identified TeNT ligands, polysialogangliosides and Thy-1, are clustered in lipid rafts, which could therefore serve as concentration platforms for the toxin binding. However, Thy-1 appears to be a dispensable component of the machinery involved in TeNT binding to the neuronal surface. In fact, anti-Thy-1 antibodies were not able to immunoprecipitate the cross-linking product of TeNT HC with a GPI-anchored protein present on the surface of MNs (our unpublished results), indicating that p15 is not Thy-1 in these cells (see DISCUSSION). TeNT interaction with Thy-1 and other GPI-anchored proteins could therefore be interpreted as an indication of the entry of the toxin in a raft environment and used as a probe to follow the recruitment of TeNT into lipid microdomains.
TeNT Binds to Lipid Rafts in Spinal Neurons
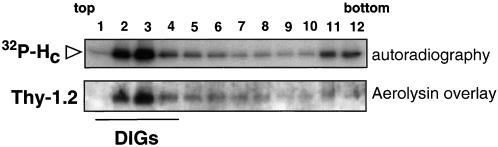
Our observations on the interaction of TeNT HC with GPI-anchored proteins in all the different cell types tested prompted us to investigate TeNT recruitment to lipid microdomains in neurons. We prepared DIGs from mouse spinal cord cells, a mixed culture that has been extensively used for the study of TeNT binding and internalization (Lalli et al., 1999; Williamson et al., 1999). After binding of TeNT HC, isolated DIGs contained ∼60% of the total bound toxin and the majority of Thy-1.2 (the mouse allotype for Thy-1; Figure 6) as detected by overlay with the GPI-binding toxin aerolysin (Abrami et al., 1998). A small subpool of TeNT HC was found in fractions containing soluble proteins at the bottom of the gradient, possibly reflecting differences in the lipid composition of spinal cord and NGF-differentiated PC12 cells (Figure 6).
Figure 6.
TeNT HC fragment associate with DIGs in spinal cord cells. TeNT HC (300 pM) concentrates in DIGs and only a small portion is found in the soluble fractions. Thy-1.2, the mouse Thy-1 allotype (which is not recognized by OX7 anti-Thy-1 antibodies), was detected with the use of the GPI-binding toxin aerolysin (Abrami et al., 1998; see MATERIALS AND METHODS) and its identification among other GPI-anchored proteins was inferred from the comparison with samples prepared from Thy-1 knockout cells. Thy-1.2 comigrates with TeNT HC in DIG fractions.
Cholesterol Depletion Protects Neurons from TeNT Intoxication
The use of cholesterol-sequestering drugs to disrupt membrane rafts is well established (reviewed in Simons and Toomre, 2000). Studies with these drugs led to the conclusion that cholesterol plays a key structural role in the lipid microdomain architecture (Schroeder et al., 1998), although some raft components appear resistant to these drugs in nonneuronal cells (Abrami and van der Goot, 1999; Lipardi et al., 2000).
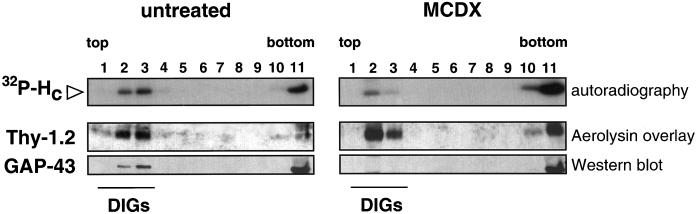
Mouse spinal cord cells were pretreated with MCDX, a drug that extracts cholesterol from the membranes (Neufeld et al., 1996), before binding of TeNT HC and isolation of DIGs. Under these conditions GAP-43, a palmitoylated protein of the neuronal membrane, was completely extracted from DIGs (Laux et al., 2000) and represents a positive control for the treatment (Figure 7; compare left and right, bottom panels). Interestingly, preincubation with MCDX induced a moderate, but consistent, shift of TeNT HC from DIGs to the soluble fractions of the gradient (Figure 7). The increase in the solubility of TeNT HC correlates with an increase in Thy-1.2 in the same fractions. These results indicate that distinct raft components are differently affected by cholesterol depletion and suggest that in spinal cord cells TeNT HC binds to distinct lipid rafts subpools, which display different sensitivities to MDCX.
Figure 7.
MCDX treatment partially extracts TeNT HC from DIGs in spinal cord cells. TeNT HC is partially solubilized from DIGs (lanes 2 and 3) and its recovery in soluble fractions (lanes 11 and 12) increases accordingly upon MCDX treatment (right). Thy-1.2 follows the same pattern of TeNT HC before and after MCDX, whereas GAP-43 is completely extracted from DIGs (lanes 2 and 3) by MCDX (bottom right).
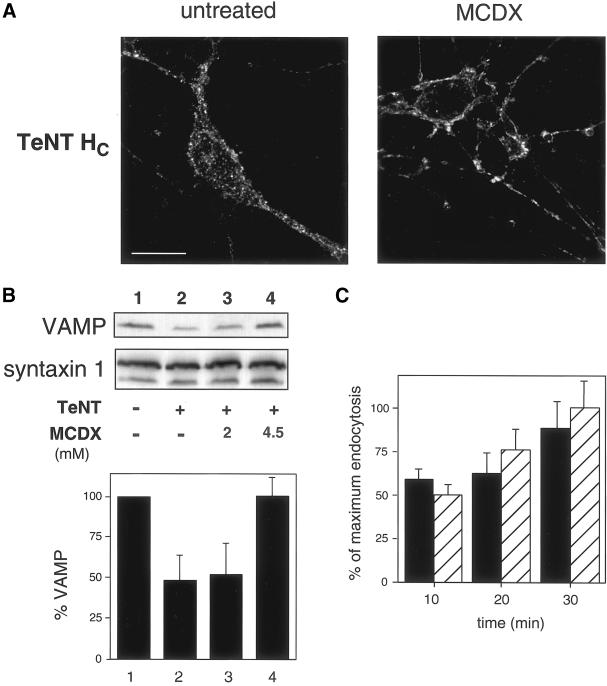
After the effect of MCDX on the recruitment of TeNT HC to DIGs, we investigated whether cholesterol plays a role in the internalization or intracellular sorting of TeNT. Pretreatment of spinal cord cells with MCDX inhibited the internalization of TeNT HC as shown by immunofluorescence (Figure 8A). Endocytic structures labeled with TeNT HC in control cells (which are internal in single confocal z-sections; Figure 8A) were greatly reduced in MCDX-treated neurons, where TeNT HC was concentrated on the plasma membrane (Figure 8A). The limited intracellular staining present in MCDX-treated cells closely resembled the background levels of control samples. These observations suggest that cholesterol depletion inhibits TeNT HC internalization in neurons.
Figure 8.
MCDX treatment blocks TeNT HC internalization and the toxic activity of TeNT in spinal cord cells. (A) After internalization of TeNT HC (100 nM, 1 h, 37°C) and permeabilization, untreated cells show a dotted intracellular staining, detected with the use of monoclonal anti-VSV-G antibodies. MCDX-treated (4.5 mM) cell bodies and neurites exhibit membrane staining but internalized TeNT HC is not observed. Confocal images corresponding to single z-sections of 0.4 μm were taken at the same distance from the substrate. Bar, 10 μm. (B) TeNT-induced cleavage of VAMP (compare lane 2 vs. lane 1) is slightly (lane 3) or completely protected (lane 4) by pretreatment with 2 or 4.5 mM MCDX, respectively. Syntaxin-1 (isoforms 1a and 1b) is used as a loading control. Results from four independent experiments (±SD), normalized for syntaxin-1, are plotted in the panel below. (C) 125I-Transferrin endocytosis at 37°C is similar in untreated (▪) and MCDX-treated (4.5 mM, ▨) spinal cord cells. Results from two independent experiments (±SD; p > 0.05) are shown. 125I-Transferrin binding (20 min, 4°C) and residual endocytosis upon competition with unlabeled transferrin accounts for 12 ± 3 and 25 ± 3% of maximal endocytosis, respectively (our unpublished results).
Because MCDX blocked TeNT internalization, we next asked whether MDCX-treated cells would be protected from the intracellular zinc-endopeptidase activity of TeNT. We followed the effect of MDCX preincubation on the proteolysis of VAMP/synaptobrevin, the intracellular target of TeNT (Schiavo et al., 2000). In spinal cord neurons, ∼50% of VAMP is cleaved upon overnight incubation with 200 pM TeNT, as detected by Western blot with an anti-VAMP antibody (Figure 8B) (Lalli et al., 1999). This partial cleavage is due both to the resistance of VAMP to TeNT proteolysis when engaged in preformed soluble _N_-ethylmaleimide-sensitive fusion protein-attachment receptor complexes (SNARE) (Schiavo et al., 2000) and to the contamination of the neuronal culture with VAMP-containing glial cells (Parpura et al., 1995), which are not competent to TeNT intoxication. Cell pretreatment with 4.5 mM MCDX completely protected VAMP from TeNT cleavage (Figure 8B). The effect of MDCX is dose-dependent as demonstrated by the lack of protection seen with 2 mM MCDX (Figure 8B). We did not observe any significant change in the recovery of VAMP after treatment with the drug alone (our unpublished results). Transferrin uptake in untreated and MCDX-treated spinal cells was not significantly different (Figure 8C), indicating that under our experimental conditions, this treatment does not affect unspecifically cell functionality or viability (Figure 8C) (see DISCUSSION). Taken together, these results demonstrate that cholesterol plays a key role in the internalization of TeNT in spinal cord neurons.
DISCUSSION
In this study, we demonstrate that TeNT HC interacts with GPI-anchored proteins, p15s, in PC12 cells, spinal cord neurons, and purified MNs. In NGF-differentiated PC12 cells, we identify p15 as Thy-1 and show by immunoprecipitation and FLIM experiments that Thy-1 interacts with TeNT HC and HCC, the HC subdomain sufficient for binding, in this neuronally differentiated cell line. Thy-1 is a major component of neurons and T lymphocytes, where it is expressed in several distinct glycoforms (Parekh et al., 1987). Thy-1 has been implicated in multiple processes, including neurite outgrowth (Tiveron et al., 1992), long-term potentiation (Nosten-Bertrand et al., 1996), and T-cell receptor signaling (Hueber et al., 1997). Despite the strong similarities observed between p15s in different neuronal cell types (Herreros et al., 2000a), several lines of evidence indicate that Thy-1 is not the neuronal receptor for TeNT. Spinal cord cells isolated from Thy-1 knockout mice (Nosten-Bertrand et al., 1996) show TeNT HC binding and internalization similar to those isolated from wild-type animals (our unpublished results). Together with the inability of an anti-Thy-1 antibody to immunoprecipitate the TeNT HC cross-linking product in rat MNs (our unpublished results), our findings indicate that Thy-1 is not an essential component of the TeNT binding and internalization machinery and suggest that a still unidentified glycosylated GPI-anchored protein could act as cellular acceptor for TeNT in MNs.
The identification of GPI-anchored proteins (including Thy-1) as specific TeNT binding partners and the concentration of these proteins in lipid rafts led us to investigate the association of TeNT with lipid microdomains. Several experiments suggest that TeNT binds to lipid rafts. First, the punctate plasma membrane staining obtained after TeNT HC binding to NGF-differentiated PC12 cells, spinal cord cells, and purified MNs is reminiscent of lipid rafts (Mayor et al., 1994; Harder et al., 1998). Second, TeNT as well as TeNT HC associates with DIG fractions both in NGF-differentiated PC12 cells and spinal cord neurons. Third, the interaction of TeNT HC with Thy-1 occurs in DIGs. Finally, binding of anti-TeNT HC antibody induces clustering in unfixed cells (our unpublished results), a feature of lipid raft components (Mayor et al., 1994). In this light, the molecular interaction between Thy-1 and TeNT HC shown by cross-linking and FLIM in NGF-differentiated PC12 cells could be enhanced by the association of both proteins to lipid rafts (Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998). FRET efficiency was higher at 37°C than at 4°C, suggesting that physiological temperatures promote a closer interaction between the two proteins directly or via changes in the lateral mobility of raft components. We did not however detect FRET between CT and Thy-1, suggesting that under our experimental conditions lipid raft clustering is not sufficient to account for the observed FRET between the TeNT HC and Thy-1 (Kenworthy et al., 2000).
Some BoNTs have also been found to bind to neuronal DIGs (Herreros and Schiavo, unpublished results), increasing the possibility that the interaction with lipid microdomains represents a general mechanism for the recruitment of TeNT and BoNTs to the neuronal membrane.
What is the physiological relevance of TeNT binding to lipid rafts? These lipid domains have been implicated in membrane trafficking and signaling (Simons and Ikonen, 1997; Simons and Toomre, 2000). Interestingly, lipid rafts have recently emerged as membrane domains essential for the binding and uptake of pathogens and virulence factors into cells (Dehio et al., 1995; Baorto et al., 1997; Orlandi and Fishman, 1998; Wolf et al., 1998; Fivaz et al., 1999; Gordon et al., 1999; Parton and Lindsay, 1999; Ricci et al., 2000; Shin et al., 2000). A possible explanation for this common mechanism is that binding to lipid rafts promotes a local increase of the pathogen/toxin's concentration (Abrami and van der Goot, 1999), which is exploited by pore-forming and multivalent toxins (Fivaz et al., 1999). TeNT binds to polysialogangliosides (Montecucco, 1986; Halpern and Neale, 1995), which are likely to be concentrated in lipid rafts as demonstrated for most sphingolipids (Prinetti et al., 2000). PI-PLC treatment does not affect TeNT HC total binding, suggesting a role of gangliosides as primary low-affinity TeNT acceptors (Williamson et al., 1999) that trap TeNT on the neuronal surface. Thus lipid rafts, by clustering polysialogangliosides, GPI-anchored binding proteins, and possibly other proteins involved in TeNT binding (Lazarovici and Yavin, 1986; Pierce et al., 1986) could act as concentrating platforms for TeNT at the plasma membrane. This multiple binding within lipid rafts could confer to TeNT the extreme high affinity and neurospecificity observed in vitro and in vivo (Montecucco, 1986). Furthermore, binding to raft components could target CNTs to hot spots on the plasma membrane that would give access to the signaling cascades emerging from these microdomains. In agreement with this view, it has been reported that TeNT binding stimulates phosphorylation of trk A and mitogen-activated protein kinase activity (Gil et al., 2000, 2001). Similar to the signaling cascades triggered by NGF binding (Huang et al., 1999), these events are likely to involve lipid rafts.
Depletion of the cellular cholesterol by treatment with cholesterol-sequestering drugs (MDCX, filipin) or detergents (saponin) disrupts lipid rafts (Neufeld et al., 1996; Simons and Toomre, 2000) and consequently reduces the recovery of typical raft components in DIGs (Abrami and van der Goot, 1999; Huang et al., 1999; Martens et al., 2000). MDCX treatment of spinal cord cells causes the displacement of a discrete fraction of the bound TeNT HC and other raft markers from DIGs. These findings suggest the existence of heterogeneous raft pools on the neuronal membrane (Madore et al., 1999) that could be differently affected by changes in the cholesterol content. This moderate increase in the solubility of TeNT HC correlates with a blockade of its internalization in MCDX-treated spinal cord cells, suggesting that a specialized subpool of lipid rafts is responsible for the productive binding and internalization of TeNT. In this regard, specialized lipid microdomains have been implicated in the internalization of proteins and pathogens (Parton et al., 1994; Baorto et al., 1997) and endocytosis of CT is inhibited by MCDX in nonneuronal cells (Orlandi and Fishman, 1998).
Strikingly, MDCX treatment causes the complete protection of VAMP from TeNT proteolytic activity, indicating an essential role of cholesterol in the internalization and intracellular trafficking of the toxin. Although alternative explanations are possible (see below), these findings could now explain some unique physiological features of TeNT and BoNTs. One of these properties is the apparent irreversibility of the binding of these toxins to the neuronal surface (Schmitt et al., 1981; Habermann and Dreyer, 1986). The association of TeNT with lipid rafts would be characterized by a very low dissociation constant due to the multivalent binding nature of both lipid rafts (containing polysialogangliosides and protein acceptors) and TeNT. Structural analysis of TeNT HC reveals the presence of multiple binding sites for oligosaccharides at the extreme carboxy terminus (Emsley et al., 2000), which is necessary and sufficient for the interaction with the neuronal surface (Herreros et al., 2000b). Moreover, TeNT has been described to form homodimers (Ledoux et al., 1994; Herreros et al., 2000a), a process that would further strengthen the multimeric nature of these interactions. In addition, the existence of at least two subpopulations of TeNT HC that are differently affected by MDCX treatment could explain the observation of a nonproductive and productive neurotoxin binding (Daniels-Holgate and Dolly, 1996). The fraction of TeNT interacting with MDCX-sensitive lipid rafts could thus represent the productive subpool of TeNT, which is internalized, cleaves VAMP, and leads to the inhibition of neurotransmitter release.
Recently, MCDX has been reported to inhibit clathrin-dependent internalization of transferrin in nonneuronal cells (Rodal et al., 1999; Subtil et al., 1999). The blockade of TeNT internalization by cholesterol depletion could therefore suggest that TeNT is internalized by a clathrin-dependent pathway in spinal cord neurons (Parton et al., 1987). MCDX-treated and control cells showed a similar transferrin uptake, suggesting that the endosomal recycling pathway is not affected in our experimental conditions. However, we cannot completely rule out a possible inhibition of clathrin-mediated endocytosis in a subset of cells in our mixed spinal culture. Cholesterol-sequestering drugs also inhibit endocytosis from lipid rafts/caveolae in nonneuronal cells (Schnitzer et al., 1994; Deckert et al., 1996; Orlandi and Fishman, 1998) and GPI-anchored proteins can be internalized by clathrin-dependent and -independent routes (Makiya et al., 1992; Mayor et al., 1994; Parton et al., 1994; Skretting et al., 1999). Thus, TeNT internalization in neurons could involve both coated and uncoated pathways (Schwab and Thoenen, 1978; Parton et al., 1987).
In conclusion, we demonstrate that TeNT interacts with GPI-anchored proteins and binds to lipid rafts. Cholesterol depletion causes the displacement of a subpool of TeNT from DIGs and blocks the internalization and intracellular activity of TeNT. This finding highlights a key role of cholesterol in the trafficking of TeNT in neurons.
ACKNOWLEDGMENTS
We are indebted to Dr. R. Morris (Guy's Hospital, London, United Kingdom) for kindly providing Thy-1 knockout mice, Dr. G. van der Goot (University of Geneva, Geneva, Switzerland) for aerolysin reagents, G. Lalli for purified MNs and valuable help in preparing spinal cord cultures, and Dr. P. Bastiaens for the FLIM microscope setup. We thank G. van der Goot, T. Iglesias, H. McNeil, C. Montecucco, R. Morris, G. Stenbeck, and members of our laboratory for critical reading of the manuscript and useful discussion. This work was supported by the Imperial Cancer Research Fund and the Human Frontier Science Program (to J.H.).
Abbreviations used:
BoNT
botulinum neurotoxin
b-TeNT
biotinylated-TeNT
CT
cholera toxin
DIG
detergent-insoluble glycolipid-enriched membrane
FLIM
fluorescence lifetime imaging microscopy
FRET
fluorescence resonance energy transfer
GPI
glycosylphosphatidylinositol
H
heavy chain
HC
binding domain
L
light chain
MCDX
methyl- β-cyclodextrin
MN
motor neuron
p15
TeNT binding protein of ∼15 kDa
PI-PLC
phosphoinositol-phospholipase C
TeNT
tetanus neurotoxin
TX-100
Triton X-100
VSV-G
vesicular stomatitis virus protein G
Footnotes
1
Eff = 1 − τDA/τD, where τDA is the lifetime map of the donor in the presence of acceptor, and τD is the average lifetime of the donor in the absence of acceptor.
REFERENCES
- Aarts LHJ, Verkade P, van Dalen JJW, van Rozen AJ, Gispen WH, Schrama LH, Schotman P. B-50/GAP-43 potentiates cytoskeletal reorganization in raft domains. Mol Cell Neurosci. 1999;14:85–97. doi: 10.1006/mcne.1999.0775. [DOI] [PubMed] [Google Scholar]
- Abrami L, Fivaz M, Glauser P-E, Parton RG, van der Goot FG. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1998;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L, van der Goot FG. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J Cell Biol. 1999;147:175–184. doi: 10.1083/jcb.147.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arni S, Keilbaugh SA, Ostermeyer AG, Brown DA. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J Biol Chem. 1998;273:28478–28485. doi: 10.1074/jbc.273.43.28478. [DOI] [PubMed] [Google Scholar]
- Arribas M, Blasi J, Egea G, Fariñas I, Solsona C, Marsal J. High resolution labeling of cholinergic nerve terminals using a specific fully active biotinylated botulinum neurotoxin type A. J Neurosci Res. 1993;36:635–645. doi: 10.1002/jnr.490360604. [DOI] [PubMed] [Google Scholar]
- Bakry NM, Kamata Y, Simpson LL. Expression of botulinum toxin binding sites in Xenopus oocytes. Infect Immun. 1997;65:2225–2232. doi: 10.1128/iai.65.6.2225-2232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, Abraham SN. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389:636–639. doi: 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- Bastiaens PIH, Jovin TM. Fluorescence resonance energy transfer microscopy. In: Celis JE, editor. Cell Biology: A Laboratory Manual. San Diego, CA: Academic Press; 1998. pp. 136–146. [Google Scholar]
- Brown DA, London E. Structure, and function of sphingolipid-, and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells. Implications for the spatial control of exocytosis. Proc Natl Acad Sci USA. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels-Holgate PU, Dolly JO. Productive and non-productive binding of botulinum neurotoxin A to motor nerve endings are distinguished by its heavy chain. J Neurosci Res. 1996;44:263–271. doi: 10.1002/(SICI)1097-4547(19960501)44:3<263::AID-JNR7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehio C, Prevost MC, Sansonetti PJ. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signaling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Hanson PI, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Fotinou C, Black I, Fairweather NF, Charles IG, Watts C, Hewitt E, Isaacs NW. The structures of the HC fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. J Biol Chem. 2000;275:8889–8894. doi: 10.1074/jbc.275.12.8889. [DOI] [PubMed] [Google Scholar]
- Evans DM, Williams RS, Shone CC, Hambleton P, Melling J, Dolly JO. Botulinum neurotoxin type B. Its purification, radioiodination and interaction with rat-brain synaptosomal membranes. Eur J Biochem. 1986;154:409–416. doi: 10.1111/j.1432-1033.1986.tb09413.x. [DOI] [PubMed] [Google Scholar]
- Fivaz M, Abrami L, van der Goot FG. Landing on lipid rafts. Trends Cell Biol. 1999;9:212–213. doi: 10.1016/s0962-8924(99)01567-6. [DOI] [PubMed] [Google Scholar]
- Friedrichson T, Kurzchalia TV. Micromains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- Gil C, Chaib-Oukadour I, Blasi J, Aguilera J. HC fragment of tetanus toxin activates protein kinase C isoforms, and phosphoproteins involved in signal transduction. Biochem J. 2001;356:97–103. doi: 10.1042/0264-6021:3560097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil C, Chaib-Oukadour I, Pelliccioni P, Aguilera J. Activation of signal transduction pathways involving trkA, PLCγ1, PKC isoforms, and ERK-1/2 by tetanus toxin. FEBS Lett. 2000;481:177–182. doi: 10.1016/s0014-5793(00)02002-0. [DOI] [PubMed] [Google Scholar]
- Gordon VM, Nelson KM, Buckley JT, Stevens VL, Tweten RK, Elwood PC, Leppla SH. Clostridium septicum alpha toxin uses glycosylphosphatidylinositol-anchored protein receptors. J Biol Chem. 1999;274:27274–27280. doi: 10.1074/jbc.274.38.27274. [DOI] [PubMed] [Google Scholar]
- Grimmer S, Iversen T-G, van Deurs B, Sandvig K. Endosome to Golgi transport of ricin is regulated by cholesterol. Mol Biol Cell. 2000;11:4205–4216. doi: 10.1091/mbc.11.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E, Dreyer F. Clostridial neurotoxins: handling and action at the cellular and molecular level. Curr Top Microbiol Immunol. 1986;129:93–179. doi: 10.1007/978-3-642-71399-6_2. [DOI] [PubMed] [Google Scholar]
- Halpern JL, Neale EA. Neurospecific binding, internalization, and retrograde axonal transport. Curr Top Microbiol Immunol. 1995;195:221–241. doi: 10.1007/978-3-642-85173-5_10. [DOI] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain-structure of the plasma-membrane revealed by patching of membrane-components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros J, Lalli G, Montecucco C, Schiavo G. Pathophysiological properties of clostridial neurotoxins. In: Freer JH, Alouf JE, editors. The Comprehensive Source Book of Bacterial Protein Toxin. London, UK: Academic Press, London; 1999. pp. 202–228. [Google Scholar]
- Herreros J, Lalli G, Montecucco C, Schiavo G. Tetanus toxin fragment C binds to a protein present in neuronal cell lines, and motor neurons. J Neurochem. 2000a;74:1941–1950. doi: 10.1046/j.1471-4159.2000.0741941.x. [DOI] [PubMed] [Google Scholar]
- Herreros J, Lalli G, Schiavo G. C-Terminal half of tetanus toxin fragment C is sufficient for neuronal binding, and interaction with a putative protein receptor. Biochem J. 2000b;347:199–204. [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D, van Ijzendoorn SCD. Lipid trafficking, and sorting. how cholesterol is filling gaps. Curr Opin Cell Biol. 2000;12:496–502. doi: 10.1016/s0955-0674(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Trowbridge T. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-S, Zhou J, Feng AK, Lynch CC, Klumperman J, DeArmond SJ, Mobley WC. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem. 1999;274:36707–36714. doi: 10.1074/jbc.274.51.36707. [DOI] [PubMed] [Google Scholar]
- Hueber A-O, Bernand A-M, Langlet-El Battari C, Marguet D, Massol P, Foa C, Brun N, Garcia S, Stewart C, Pierres M, He HT. Thymocytes in Thy −/− mice show augmented TCR signaling and impaired differentiation. Curr Biol. 1997;7:705–708. doi: 10.1016/s0960-9822(06)00300-9. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Dietrich C. Looking at lipid rafts? Trends Cell Biol. 1999;9:87–91. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- Jeng C-J, McCarroll SA, Martin TFJ, Floor E, Adams J, Krantz D, Butz S, Edwards R, Schweitzer ES. Thy-1 is a component common to multiple populations of synaptic vesicles. J Cell Biol. 1998;140:685–698. doi: 10.1083/jcb.140.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Petranova N, Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit, and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli G, Herreros J, Osborne SL, Montecucco C, Rossetto O, Schiavo G. Functional characterization of tetanus and botulinum neurotoxins binding domains. J Cell Sci. 1999;112:2715–2714. doi: 10.1242/jcs.112.16.2715. [DOI] [PubMed] [Google Scholar]
- Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P2 at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000;149:1455–1471. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovici P, Yavin E. Affinity-purified tetanus neurotoxin interaction with synaptic membranes: properties of a protease-sensitive receptor component. Biochemistry. 1986;25:7047–7054. doi: 10.1021/bi00370a044. [DOI] [PubMed] [Google Scholar]
- Ledoux DN, Be XH, Singh BR. Quaternary structure of botulinum and tetanus neurotoxins as probed by chemical cross-linking and native gel electrophoresis. Toxicon. 1994;32:1095–1104. doi: 10.1016/0041-0101(94)90393-x. [DOI] [PubMed] [Google Scholar]
- Li L, Singh BR. Isolation of synaptotagmin as a receptor for type A and type E botulinum neurotoxin and analysis of their comparative binding using a new microtiter plate assay. J Nat Toxins. 1998;7:215–226. [PubMed] [Google Scholar]
- Lipardi C, Nitsch L, Zurzolo C. Detergent-insoluble GPI-anchored proteins are apically sorted in Fisher rat thyroid cells, but interference with cholesterol or sphingolipids differentially affects detergent insolubility, and apical sorting. Mol Biol Cell. 2000;11:531–542. doi: 10.1091/mbc.11.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore N, Smith KL, Graham CH, Jen A, Brady K, Hall S, Morris R. Functionally different GPI proteins are organized in different domains on the neuronal surface. EMBO J. 1999;18:6917–6926. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makiya R, Thornell LE, Stigbrand T. Placental alkaline phosphatase, a GPI-anchored protein, is clustered in clathrin-coated vesicles. Biochem Biophys Res Commun. 1992;183:803–808. doi: 10.1016/0006-291x(92)90554-x. [DOI] [PubMed] [Google Scholar]
- Martens JR, NavarroPolanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski TD, Tamkun MM. Differential targeting of shaker-like potassium channels to lipid rafts. J Biol Chem. 2000;275:7443–7446. doi: 10.1074/jbc.275.11.7443. [DOI] [PubMed] [Google Scholar]
- Mayor S, Rothberg KG, Maxfield FR. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- Montecucco C. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem Sci. 1986;11:315–317. [Google Scholar]
- Mukherjee S, Maxfield FR. Role of membrane organization, and membrane domains in endocytic lipid trafficking. Traffic. 2000;1:203–211. doi: 10.1034/j.1600-0854.2000.010302.x. [DOI] [PubMed] [Google Scholar]
- Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem. 1996;271:21604–21613. doi: 10.1074/jbc.271.35.21604. [DOI] [PubMed] [Google Scholar]
- Niemann H, Blasi J, Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Nishiki T, Kamata Y, Nemoto Y, Omori A, Ito T, Takahashi M, Kozaki S. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J Biol Chem. 1994;269:10498–10503. [PubMed] [Google Scholar]
- Nosten-Bertrand M, Errington ML, Murphy KPSJ, Tokugawa Y, Barboni E, Kozlova E, Michalovich D, Morris RG, Silver J, Stewart CL, Bliss TV, Morris RJ. Normal spatial learning despite regional inhibition of LTP in mice lacking Thy-1. Nature. 1996;379:826–829. doi: 10.1038/379826a0. [DOI] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera-toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh RB, Tse AGD, Dweek RA, Williams AF, Rademacher TW. Tissue-specific N-glycosylation, site-specific oligosaccharide patterns and lentil lectin recognition of rat Thy-1. EMBO J. 1987;6:1233–1244. doi: 10.1002/j.1460-2075.1987.tb02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- Parton RG, Lindsay M. Exploitation of major histocompatibility complex class I molecules and caveolae by simian virus 40. Immunol Rev. 1999;168:23–31. doi: 10.1111/j.1600-065x.1999.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Parton RG, Ockleford CD, Critchley DR. A study of the mechanism of internalization of tetanus toxin by primary mouse spinal cord cultures. J Neurochem. 1987;49:1057–1068. doi: 10.1111/j.1471-4159.1987.tb09994.x. [DOI] [PubMed] [Google Scholar]
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EJ, Davison MD, Parton RG, Habig WH, Critchley DR. Characterization of tetanus toxin binding to rat brain membranes. Evidence for a high-affinity proteinase-sensitive receptor. Biochem J. 1986;236:845–852. doi: 10.1042/bj2360845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinetti A, Chigorno V, Tettamanti G, Sonnino S. Sphingolipid-enriched membrane domains from rat cerebellar granule cells differentiated in cultures. J Biol Chem. 2000;275:11658–11665. doi: 10.1074/jbc.275.16.11658. [DOI] [PubMed] [Google Scholar]
- Ricci V, Galmiche A, Doye A, Necchi V, Solcia E, Boquet P. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein, and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol Biol Cell. 2000;11:3897–3909. doi: 10.1091/mbc.11.11.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Ferrari G, Rossetto O, Montecucco C. Specific cross-linking of tetanus toxin to a protein of NGF-differentiated PC12 cells. FEBS Lett. 1991;290:227–230. doi: 10.1016/0014-5793(91)81266-b. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Dreyer F, John C. At least three sequential steps are involved in the tetanus toxin-induced block of neuromuscular transmission. Naunyn-Schemiedeberg's Arch Pharmacol. 1981;317:326–330. doi: 10.1007/BF00501314. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Selective binding, uptake, and retrograde transport of tetanus toxin by nerve terminals in the rat iris. An electron microscope study using colloidal gold as a tracer. J Cell Biol. 1978;77:1–13. doi: 10.1083/jcb.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin PR. The renaissance of fluorescence resonance energy transfer. Nat Struct Biol. 2000;7:730–734. doi: 10.1038/78948. [DOI] [PubMed] [Google Scholar]
- Shin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells. Science. 2000;289:785–788. doi: 10.1126/science.289.5480.785. [DOI] [PubMed] [Google Scholar]
- Shogomori H, Futerman AH. Cholera toxin is found in detergent-insoluble rafts/domains at the cell surface of hippocampal neurons but is internalized via a raft-independent mechanism. J Biol Chem. 2001;276:9182–9188. doi: 10.1074/jbc.M009414200. [DOI] [PubMed] [Google Scholar]
- Simons M, Friedrichson T, Schulz JB, Pitto M, Masserini M, Kurzchalia TV. Exogenous administration of gangliosides displaces GPI-anchored proteins from lipid microdomains in living cells. Mol Biol Cell. 1999;10:3187–3196. doi: 10.1091/mbc.10.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Gruenberg J. Jamming the endosomal system. Lipid rafts and lysosomal storage diseases. Trends Cell Biol. 2000;10:459–462. doi: 10.1016/s0962-8924(00)01847-x. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts, and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Skretting G, Torgersen ML, van Deurs B, Sandvig K. Endocytic mechanisms responsible for uptake of GPI-linked diphtheria toxin receptor. J Cell Sci. 1999;112:3899–3909. doi: 10.1242/jcs.112.22.3899. [DOI] [PubMed] [Google Scholar]
- Squire A, Bastiaens P. Three dimensional image restoration in fluorescence lifetime imaging microscopy. J Microsc. 1999;193:36–49. doi: 10.1046/j.1365-2818.1999.00427.x. [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate SS, Khadse V. Intramolecular crosslinking of gamma-glutamyl transpeptidase. Arch Biochem Biophys. 1987;255:304–308. doi: 10.1016/0003-9861(87)90397-3. [DOI] [PubMed] [Google Scholar]
- Tiveron M-C, Barboni E, Pliego Rivero FB, Gormley AM, Seeley PJ, Grosveld F, Morris R. Selective inhibition of neurite outgrowth on mature astrocytes by Thy-1 glycoprotein. Nature. 1992;355:745–748. doi: 10.1038/355745a0. [DOI] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Williams AF, Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982;216:696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Williamson LC, Bateman KE, Clifford JCM, Neale EA. Neuronal sensitivity to tetanus toxin requires gangliosides. J Biol Chem. 1999;274:25173–25180. doi: 10.1074/jbc.274.35.25173. [DOI] [PubMed] [Google Scholar]
- Wolf AA, Jobling MG, Wimermackin S, Fergusonmaltzman M, Madara JL, Holmes RK, Lencer WI. Ganglioside structure dictates signal-transduction by cholera-toxin and association with caveolae-like membrane domains in polarized epithelia. J Cell Biol. 1998;141:917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavin E, Nathan A. Tetanus toxin receptors on nerve cells contain a trypsin-sensitive component. Eur J Biochem. 1986;154:403–407. doi: 10.1111/j.1432-1033.1986.tb09412.x. [DOI] [PubMed] [Google Scholar]