Epigallocatechin-3-gallate local pre-exposure application prevents SHIV rectal infection of macaques (original) (raw)
. Author manuscript; available in PMC: 2018 Nov 30.
Published in final edited form as: Mucosal Immunol. 2018 May 31;11(4):1230–1238. doi: 10.1038/s41385-018-0025-4
Abstract
Epigallocatechin-3-gallate (EGCG), a natural and major ingredient of green tea, has been shown to have anti-inflammation and anti-HIV-1 properties. We demonstrated that the intrarectal administration of EGCG could protect rhesus macaques from repetitive, intrarectal challenges with low-dose SHIVSF162P3N. This protection has a per-exposure risk reduction of 91.5% (_P_=0.0009; log-rank test) and a complete protection of 87.5% (P<0.001; Fisher's exact test). All protected animals showed no evidence of systemic and mucosal SHIV infection as demonstrated by the absence of viral RNA, DNA and antibodies. In contrast, all controls became infected after repeated SHIV challenges (a median of 2.5 times, range of 1-8 times). Mechanistically, EGCG could block the binding of HIV-1 gp120 to CD4 receptor and suppress the macrophage infiltration/activation in the rectal mucosa of macaques. These data support further clinical evaluation and development of EGCG as a novel, safe and cost-effective microbicide for preventing sexual transmission of HIV-1.
Introduction
In the absence of a protective vaccine or a cure for HIV-1 infection, intervention with highly potent and cost-effective inhibitors that target at the initial sites of HIV-1 infection is an ideal strategy for slowing the AIDS pandemic. Currently, HIV-1 infection spreads predominantly through sexual transmission, in which the virus gains access to a new host through the penile, rectal or vaginal mucosal tissues. During sexual intercourse, HIV-1-infected men transmit the virus through their semen, which carries free-floating virus as well as the infected cells.1 Semen contains amyloid fibrils that potently enhance HIV-1 or SIV/SHIV infectivity in vitro.2-4 Therefore, to inhibit HIV-1 during sexual intercourse represents the most effective way in preventing transmission of the virus.
Microbicides targeting the early events in sexual transmission of HIV-1 have been extensively studied.5-8 Several recent studies reported that pre-exposure prophylaxis with the antiretrovirals (ARVs) was effective in the prevention of SHIV transmission in non-human primate (NHP) model.9, 10 These ARVs-containing microbicides were usually used in the formulation of topical gels.7 However, the topical gel microbicides are associated with rectal mucosal damage, manifested as histological abnormality and induction of proinflammatory response.11 In addition, ARVs-based microbicides are not effective on resistant HIV-1 strains and can induce viral resistance. Furthermore, semen can impair the antiviral efficacy of ARVs that act on intracellular targets at different steps of viral replication cycle.4 As compared with ARVs or other chemical drugs, the use of natural products for prevention of HIV-1 mucosal transmission might have some advantages, as they have low toxicity, minor side-effects, and are cost-effective.
The consumption of green tea has many physiological and pharmacological health benefits. Mounting evidence has shown that epigallocatechin-3-gallate (EGCG), a natural and major ingredient of green tea, not only has anti-inflammatory and anti-oxidative effects, but also possesses antiviral abilities against diverse families of viruses, including HIV-1.12 In vitro studies have demonstrated that EGCG at a physiologic concentration could inhibit HIV-1 infection/replication in peripheral blood mononuclear cells (PBMCs) and macrophages.13 In addition, EGCG could remodel seminal amyloid fibrils 14 and effectively suppress semen-mediated enhancement of HIV-1 replication.15 EGCG can interfere with several aspects of the HIV-1 life cycle, including the destruction of virions,16, 17 the inhibition of reverse transcriptase and integrase activities of the virus.18, 19 Most conclusively, several independent studies have confirmed that EGCG can bind to the same molecular pocket on CD4 receptor as does HIV-1 gp120, thus preventing the initial attachment of the viruses to CD4+ T cells.20-22 We recently reported that EGCG could also inhibit macaque SEVI (semen-derived enhancer of virus infection)-mediated enhancement of SIV/SHIV infection and degrade the formation of macaque semen-derived amyloid fibrils.23 A most recent clinical study showed that EGCG is a well-tolerated and non-toxic therapeutic agent that can upregulate protective CC chemokines against HIV-1. 24 Taken together, these observations strongly argue for further studies, particularly in vivo investigations on the anti-HIV-1 effect of EGCG as an entry inhibitor. In the present study, we evaluated whether mucosal pre-exposure application of EGCG can prevent SHIV rectal infection of rhesus macaques.
Results
EGCG inhibits HIV/SIV/SHIV in vitro
The major active ingredients of green tea are polyphenolic compounds, known as catechins, which include EGCG, EGC, ECG, and EC. EGCG accounts for 50% of the total green tea catechins. We first examined whether EGCG and other green tea catechins have the ability to inhibit infections with different strains of HIV-1, SHIV and SIV in TZM-bl cells. Among the green tea catechins, EGCG was the most potent inhibitor of the viruses (Figure 1a). In addition, EGCG dose-dependently inhibited HIV-1 Bal replication in primary human macrophages (Figure 1b). Furthermore, EGCG could inhibit SHIV infection of primary lymphocytes and macrophages of macaques (Figure 1c). Evaluation of cytotoxicity revealed that the treatment of TZM-bl and PBMCs of macaques with the catechins for as long as 48 h at the dose as high as 100 μM had little toxic effect (Supplementary Figures 1 and 2).
Figure 1.
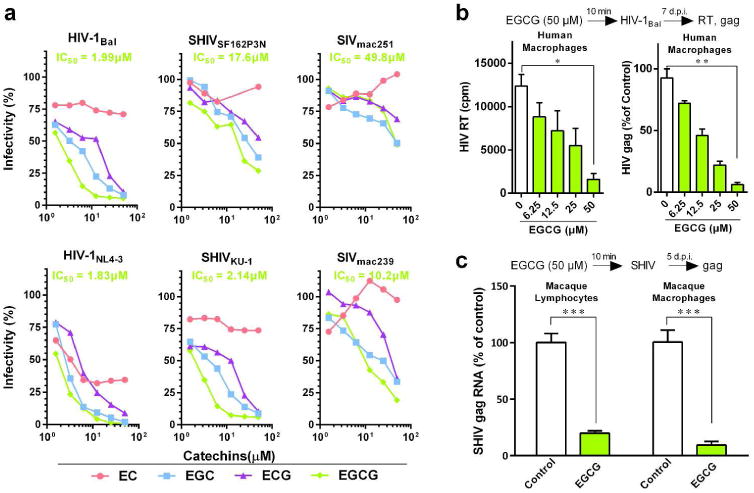
EGCG inhibits viral infectivity of a broad spectrum of AIDS-related viruses. (a) TZM-bl cells were treated with the indicated concentrations of green tea-derived catechins (EC, EGC, ECG and EGCG) for 10 min prior to infection with different strains of HIV-1 (Bal, NL4-3), SHIV (SF162P3N, KU-1), or SIV (mac239, mac251). Viral infectivity was assessed by luciferase activity, which is expressed as a percentage relative to that of the control (untreated). The half maximal inhibitory concentration (IC50) of EGCG is indicated, which was calculated based on the untreated control by the method of Reed and Muench. (b) Human peripheral blood monocyte-derived macrophages were incubated with the indicated doses of EGCG for 10 min prior to HIV-1Bal infection. Culture supernatant was collected on day 7 post-infection for HIV-1 reverse transcriptase (RT) assay. Cellular RNA was subjected to the real time RT-PCR for HIV-1 gag and GAPDH RNA. Data are expressed as HIV-1 RNA levels relative (%) to untreated control, which is defined as 100%. (c) Primary lymphocytes and macrophages from rhesus macaques were treated with or without EGCG (50 μM) for 10 min prior to SHIVSF162P3N infection. Intracellular gag RNA was measured by the real time PCR at day 5 post infection. Data are shown as mean ± SD, representative of three independent experiments with 3-4 replicates. *P<0.05, **P<0.01 and ***P<0.001.
EGCG has little toxicity on rectal mucosa
To determine the in vivo protective effect of EGCG on SHIV rectal infection of macaques, we first assessed the toxicity of EGCG on rectal mucosa of macaques and mice. We demonstrated that the rectal application of 5 mM EGCG for 10 min had little toxic effect on the rectal mucosa of macaques, as shown by the normal microstructures of epithelium, lamina propria, and intestinal glands in the rectum mucosa and submucosa (Supplementary Figure 3a) and lack of fecal blood (Supplementary Table 1). We also showed that EGCG had little effect on the expression of inflammatory cytokines and toxicity on the rectal mucosa of mice which is evidenced by normal intestinal microstructures, stool consistency, and lack of fecal blood (Supplementary Table 2 and Figure 3).
EGCG protects macaques from rectal SHIV infection
We next examined the protective effect of EGCG on repetitive and intra-rectal challenges with low-dose SHIVSF162P3N in macaques (Figure 2a). The assessment of the plasma SHIV RNA over the course of the study showed that all animals (8 out of 8) in the control group were infected, which required a median of 2.5 times of challenges (range from 1 to 8 times, Figure 2b). In contrast, only 1 out of 8 animals in the EGCG group became infected (Figure 2c). Evaluation of SHIV RNA and proviral DNA in PBMCs and the biopsied specimens (rectum and inguinal lymph nodes, LNs) at week 20 postinfection revealed infection in control animals, while no evidence of infection in 7 out of 8 EGCG-treated animals (Supplementary Figure 4).
Figure 2.
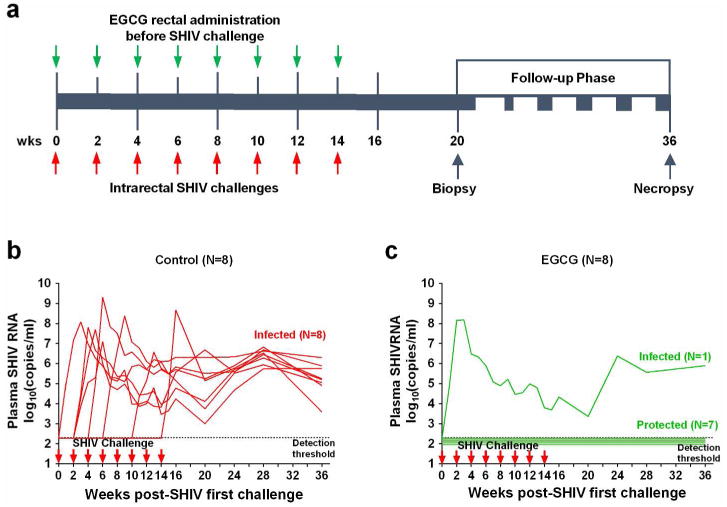
EGCG protects macaques from intra-rectal SHIV infection. (a) Experimental design of EGCG protective effect on macaques. Sixteen male macaques were administrated with 2 ml of 5 mM EGCG (8 animals) or 2 ml of PBS (8 animals) atraumatically in rectum 10 min prior to each SHIVSF162P3N challenge. All animals were rectally inoculated with SHIVSF162P3N (10 TCID50) for up to 8 times or until infection occurred. All animals were biopsied at week 20 and necropsied at week 36 postinfection for the evaluation of SHIV RNA and proviral DNA in the multiple tissues. (b, c) Longitudinal assessment of the plasma SHIV RNA (copies ml-1) levels in the animals with intrarectal pretreatment with PBS (control) or EGCG prior to SHIV challenges (up to 8 times or till infection occurred). Duplicate plasma samples were analyzed for SHIV RNA detection. Animals were considered infected and the virus challenges were stopped following two consecutive positive plasma SHIV RNA results.
Because gut-associated lymphoid tissue (GALT) is an important target organ of HIV-1 and may serve as a key viral reservoir, we also examined SHIV proviral DNA and RNA in the gastrointestinal tract (GI) tissues, lymph nodes (LNs) and intraepithelial lymphocytes (IELs) of study animals necropsied at week 36 postinfection. As shown in Figure 3 and Supplementary Table 3, SHIV RNA and proviral DNA were detected in all analyzed tissues (GI tissues, LNs and IELs) from control animals. In contrast, only one animal (WRE08) in EGCG group had detectable SHIV RNA and DNA in the GI tissues, LNs and IELs. In addition to the plasma viral loads, we also examined plasma antibody against SHIV in the study animals. Consistent with the viral load data, while all control animals were positive for SHIV-specific antibody, only one animal (WRE08) in EGCG group had detectable SHIV antibody (Supplementary Table 3). This animal had the highest CD69 and HLA-DR expression on CD4+ and CD8+ T cells in the first two weeks post-infection (Supplementary Figure 5). In addition, there was a significant decrease of CD4+ T cell numbers in blood of this animal at week 3 after SHIV infection (Supplementary Figure 6).
Figure 3.
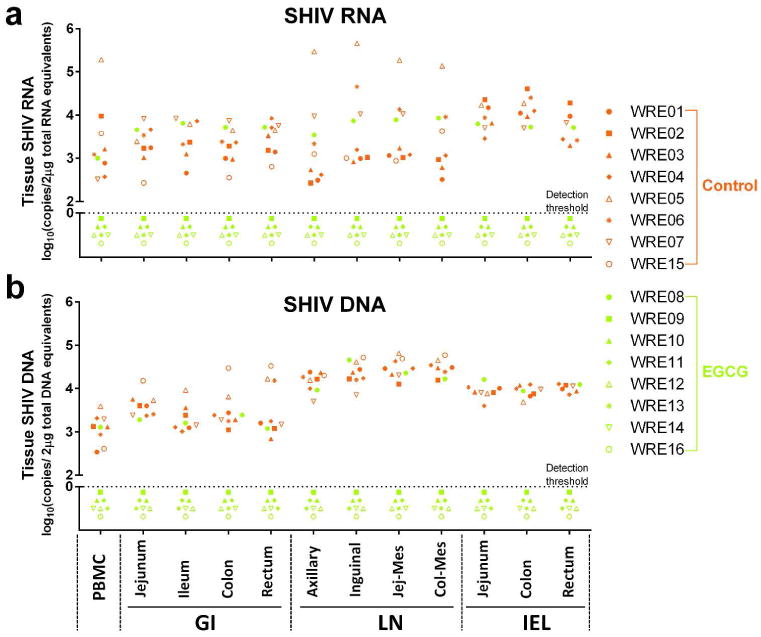
SHIV RNA and DNA detection in multiple tissues necropsied at week 36 post first SHIV challenge. SHIV RNA (a) and DNA (b) assays in the indicated tissues from the animals of PBS control (red symbols, n=6∼8) and EGCG group (green symbols, n=8) at necropsy. Log SHIV copies 2 μg-1 total genomic RNA or DNA equivalents are shown. Symbols represent individual animals and are pooled from three independent experiments. Triplication tissue samples were conducted in each independent experiment. The dot line: the detection threshold. GI: gastrointestinal tract; LN: lymph nodes; Jej-Mes: jejunal mesenteric; Col-Mes: colonal mesenteric; IEL: intraepithelial lymphocytes.
Statistically, the EGCG treatment afforded a protective efficacy of 91.5% [1-(hazard ratio), _P_=0.0009, log-rank test] reduction in the per-exposure acquisition risk as compared with control (Figure 4). The complete protection for macaques in the EGCG group was 87.5% (_P_=0.001, Fisher's exact test).
Figure 4.
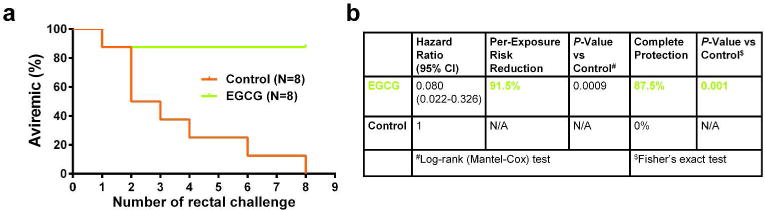
Protective efficacy of EGCG local pre-exposure application on SHIV rectal transmission in macaques. (a) Kaplan-Meier plot demonstrating the protection in EGCG-treated animals (n=8) relative to PBS-treated animals (n=8). (b) Statistical analyses include the hazard ratio with 95% confidence interval and the per-exposure reduction of acquisition risk in each group, with P values reflecting log-rank tests and Fisher's exact test.
Mechanisms of EGCG-mediated in vitro and in vivo anti-HIV activities
We first studied the mechanisms of the in vitro findings that the pre-treatment of the target cells (TZM-bl, primary macaque lymphocytes and macrophages) with EGCG potently inhibited HIV/SHIV/SIV infection. As shown in Figure 5a, b, EGCG pretreatment of T cells could block the binding of CD4 antibody to CD4 receptor. We also observed that the incubation of CD4+ T cells with EGCG blocked the binding of FITC-conjugated HIV-1 gp120 to the cells (Figure 5c). To determine in vivo mechanism of EGCG-mediated protection of macaques from SHIV rectal infection, we examined the effect of EGCG on the infiltration and activation of T cells and macrophages in rectal mucosa (Figure 6a). EGCG rectal administration significantly reduced the infiltration of CD68+ macrophages (P<0.01), but not CD3+ T cells in rectal mucosa (Figure 6b, c). In addition, EGCG pretreatment suppressed the local immune activation as shown by the diminished expression of CD163+ cells (P<0.05) and HLA-DR+ cells (P<0.001) in rectal mucosa (Figure 6d, e).
Figure 5.
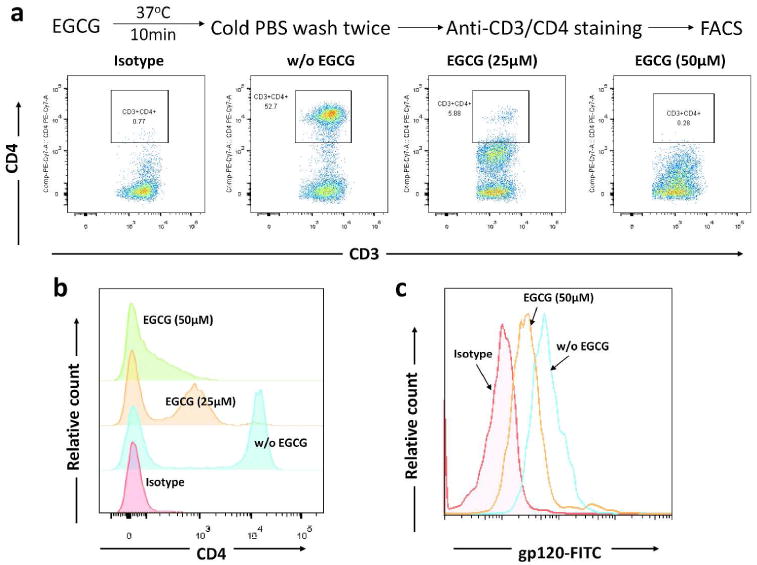
Effect of EGCG on binding of anti-CD4 antibody and gp120 to CD4 T cells. (a) Peripheral blood mononuclear cells were incubated with indicated doses of EGCG for 10 min. The reaction was then terminated with cold PBS. Cells were washed and then stained with anti-CD3 and anti-CD4 antibodies and subjected to flow cytometry analysis. The data shown are representative of three experiments with cells from three different donors. (b) Overlapping of the CD4 expression in PBMCs pretreated with indicated concentrations of EGCG. (c) Interference of binding of HIV-1 gp120 to CD4+ T cells. Purified CD4+ T cells were incubated with or without (w/o) 50 μM of EGCG. The cells were then washed with PBS and further incubated with recombinant HIV-1 gp120 protein conjugated with FITC for 2 h. The binding of gp120 to cells was indicated by intensity of FITC, which was examined by flow cytometry. Data are representative of three independent experiments.
Figure 6.
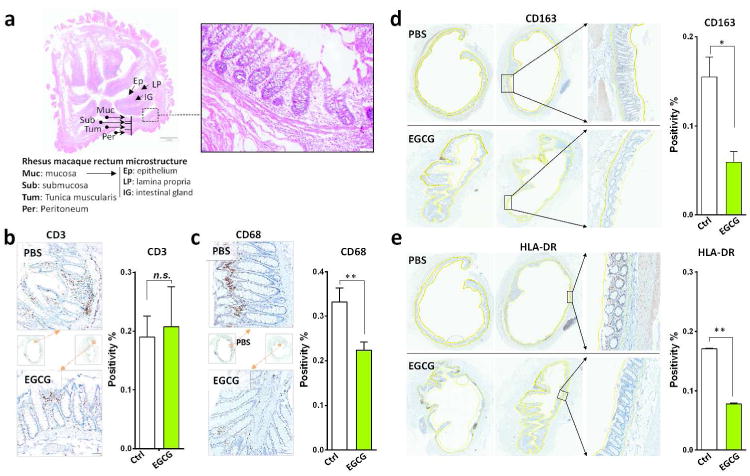
EGCG suppresses macrophage infiltration and immune activation in the rectal mucosa of SHIV-infected macaques. (a) Morphological observation of the microstructure of the rhesus macaque rectum. Architecture of the rectal mucosa of rhesus macaques as examined by hematoxylin and eosin staining. Magnification ×200. (b-e) Immunohistochemistry staining of the cell infiltration and chronic immune activation in rhesus macaques intra-rectally treated with PBS or EGCG. EGCG (5 mM, 2 ml) or PBS (2 ml) was delivered to the rectum of rhesus macaques for 10 min prior to the challenge with SHIVSF162P3N (10 TCID50). Rectal tissues from two macaques were collected at autopsy (96 h post-infection). Cell infiltrates were demonstrated by immunohistochemistry staining with anti-CD3 (b) or anti-CD68 (c) antibody. Tissue activation was examined by staining with anti-CD163 (d) or anti-HLA-DR (e) antibody. The positivity of mucosal tissue for CD3+, CD68+, CD163+, and HLA-DR+ cells in the rectum mucosa were quantified using the Aperio Image Scope software. The solid yellow lines (d, e) were used to designate regions, including the epithelium, lamina propria and intestinal glands of the rectal mucosa and submucosa, for algorithm analysis. Dotted yellow lines excluded the enclosed regions for the calculation. The positive cells within the mucosa and submucosa were counted per high-power field. At least two cross-sectioned rectal segments with different proximity to the anus were scanned and analyzed. Original magnification ×200. Data are shown as mean ± SD, which were analyzed using the 2-tailed Student's _t_-test. *P<0.05 and **P <0.01.
Discussion
Worldwide, sexual intercourse is currently the predominant mode of HIV-1 transmission, in which unprotected anal sex is riskier than unprotected vaginal sex.25 The rectum is a particularly susceptible entry site for HIV-1 or SHIV infection, as it is composed of only a single layer of columnar epithelium. In addition, the rectal mucosa contains numerous CD4-bearing target cells (CD4+ T cells, macrophages and DCs).26 The risk of HIV transmission during anal intercourse is estimated to be around 18 times greater than that in vaginal intercourse, according to the results of a meta-analysis.27 Therefore, the interventions to target the rectal site are critical in preventing sexual transmission of HIV-1. In this study, we demonstrate for the first time that EGCG can protect 7 out of 8 macaques from repeated intra-rectal challenges with SHIV, resulting in a per-exposure risk reduction of 91.5% (_P_=0.0009 by the log-rank test) and a complete protection of 87.5% (P<0.001 by Fisher's exact test). All protected animals showed no evidence of systemic and mucosal SHIV infection as evidenced by the absence of viral RNA, DNA and antibodies. Interestingly, EGCG failed to protect one animal from SHIV infection. This animal appeared to be highly susceptible to SHIV challenge, as it became infected after only one-time of exposure to the virus. In contrast, all control animals except one required 2 or more repeated SHIV challenges (Figure 2b and 2c). In examining the immune activation markers (CD69 and HLA-DR) on CD4+ and CD8+ T cells of all infected animals, we found that this unprotected animal had the highest CD69 expression on CD4+ and CD8+ T cells among all infected monkeys in the first two weeks post-infection (Supplementary Figure 5).
EGCG has been reported to have in vitro antiviral effect on different subtypes, strains and clinical isolates of HIV-1 in primary human CD4+ T cells and macrophages.13 We showed that EGCG had broad inhibitory effect on different strains of HIV-1, SIV and SHIV in TZM-bl cells, macaque lymphocytes and macrophages (Figure 1). Studies from different laboratories have demonstrated that EGCG exhibits antiviral activity at multiple steps of HIV-1 infection/replication cycle, including the inhibition of HIV-1 reverse transcriptase,18 protease,16 and proviral genome integration.19 Importantly, EGCG-mediated HIV-1 inhibition appears to be its interference with HIV-1 entry into the target cells. EGCG can directly disassemble HIV-1 virion,16, 17 block HIV-1 gp120 binding to CD4 molecule on T cells,20, 22, 28 and degrade the seminal amyloid fibrils (a semen-derived enhancer of virus infection, SEVI) that reportedly enhances HIV-1 infection/transmission.2, 4, 14, 29 We found that EGCG specifically reduced the surface expression of CD4 receptor, but not CD3 receptor on human T cells (Figure 5a), suggesting that EGCG blocks the binding of CD4 antibody to CD4 receptor (Figure 5b). Also we showed that EGCG could block the binding of FITC-conjugated HIV-1 gp120 to CD4 receptor (Figure 5c). More importantly, we demonstrated that in vivo application of EGCG could suppress the macrophage infiltration/activation in the rectal mucosa of macaques. The protective effect of EGCG was also observed in a recent human study, showing that EGCG oral administration upregulated the CC chemokines (MIP-1α/β and RANTES) against HIV-1. 24 These combined in vitro and in vivo observations provides a possible mechanism for EGCG-mediated rectal protection from SHIV infection.
These combined anti-HIV-1 actions of EGCG should reduce the risk of developing emergence of resistant viruses during the course of treatment. Furthermore, EGCG may confer an additional advantage over ARVs as it can counteract semen-mediated enhancement of HIV-1 infection. 23, 29 We recently reported that EGCG counteracted macaque SEVI-mediated enhancement of SIV/SHIV infection of macaque PBMCs.23 This finding is clinically relevant and important, as it has been reported that the anti-HIV-1 activity of a variety of potential microbicides could be compromised by the presence of semen or SEVI amyloid fibrils.4 Therefore, in order to identify the best candidates for the prevention of HIV-1 sexual transmission, it is necessary to evaluate the anti-HIV-1 efficacy of the ARVs-formulated microbicides in the presence of semen.30-32
EGCG is generally considered a natural product with minimal toxicity. Clinically, EGCG has been found to be safe and well tolerated by the study subjects and the reported adverse events were rated as mild events.33, 34 EGCG administration is safe in healthy individuals, at oral dose of 800 mg per day over a time period of 4 weeks, which is equivalent to about 8-16 cups of green tea a day.35 UIIman et al reported that purified EGCG at the doses up to 1600 mg per day were generally well-tolerated and safe to use clinically.36 Oral application of EGCG in mouthwash formulation at the dose of 35-87 mM for 2 min daily for 7 days had little systemic side effects.37 A recent phase I clinical trial showed that the drug formulation of EGCG, Polyphenon E, is a well-tolerated, non-toxic therapeutic agent with no significant adverse effects at up to 1600 mg per day for 14 days.24 Our evaluation of cytotoxicity in the cell cultures showed that EGCG at doses up to 50 μM had little cytotoxicity to TZM-bl cells and the primary macaque PBMCs. It was reported that the viability of HIV-infected human lymphocytes and macrophages was not significantly inhibited by EGCG over a range of 1– 100 μM.13 As compared to the concentrations of EGCG used in the cell cultures, higher dose (5 mM) of EGCG was administrated in the animal experiments. The rationale for use of this EGCG dose was based on the consideration that the macaque rectum contains multiple factors (feces, mucus, a number of microbial flora and metabolites) that could dilute and interfere with EGCG, reducing its antiviral activity. Nevertheless, we showed that 5 mM EGCG had little toxicity and inflammatory effects on rectal mucosa of macaques and mice, as evidenced by the normal rectal epithelium structure and lack of inflammatory cytokine induction after the EGCG application (Supplementary Figure 3, Tables 1 and 2). The dosage of the FDA approved green tea extract Polyphenon E ointment (Veregen) for topical treatment of genital warts is about 100∼300 mM.38, 39
Because sexual intercourse is a primary route of HIV-1 transmission worldwide and vaginal mucosal surface is a major site of the heterosexual transmission, future studies to determine the protective effect of EGCG on SHIV vaginal infection are also necessary. By extrapolating, it is likely that the same benefits of EGCG shown in the SHIV rectal infection model might apply to the prevention of vaginal SHIV infection. This supposition, however, will need to be confirmed experimentally given the differences between the rectal and vaginal transmission of HIV-1. 26 The fact that EGCG is very stable in acidic solution,41 a condition similar to vaginal environment (pH range of 3.8 to 4.5), supports the future studies on use of EGCG for prevention of intravaginal HIV-1 transmission. Importantly, it is necessary to develop EGCG formulations that have stable, effective and long-lasting action on inactivation of HIV-1 in vaginal lumen and rectum. For example, EGCG in a buffered gel or film should be able to maintain an acidic pH in the presence of semen.
Together with the known low toxicity, anti-inflammation and anti-viral properties of EGCG, our results argue in favor for more in vivo studies to examine the persistence, metabolism and chemical stability of EGCG at the mucosal exposure sites. These investigations are crucial for further clinical evaluation and development of EGCG as a safe, cost-effective and naturopathic microbicide for preventing sexual transmission of HIV-1, particularly in resource-poor settings.
Methods
Animals
Sixteen adult male rhesus macaques were used to examine the protective effect of EGCG on prevention of SHIV rectal infection. The animals were obtained from the Kunming Institute of Zoology of Chinese Academy of Science. The animals were individually housed at the Animal Biosafety Level-III Laboratory at the Center for Animal Experiment of Wuhan University with AAALAC International accreditation (001274). All the animal protocols were approved by the Institutional Animal Care and Use Committee (approval number: 2015024) of Wuhan University in accordance with the NIH “Guide for the Care and Use of Laboratory Animals”. All the details of animal welfare were carried out in accordance with the recommendations of the Weatherall report, “The Use of Nonhuman Primates in Research”. All the animals in this study were experimentally naïve at the beginning and negative for antibodies against the specific pathogens. All the procedures were performed under anesthesia with ketamine, and necessary efforts were taken to minimize suffering, improve housing conditions, and provide enrichment opportunities for the study animals.
Cells and viruses
TZM-bl cells with integrated firefly luciferase gene under the control of HIV-1 LTR were obtained from the NIH AIDS Research Program and grown in DMEM medium. Human and macaque PBMCs were prepared by Ficoll gradient centrifugation and cultured in RPMI medium containing 10% FBS, 100 units ml-1 penicillin, 0.1 mg ml-1 streptomycin, 2 mM L-glutamine and 20 U ml-1 interleukin-2. Human PBMCs were incubated with complete DMEM in gelatin flasks for 45 min at 37°C to collect monocytes. After detachment with 10 mM EDTA, monocytes were washed with DMEM and resuspended in complete DMEM and plated in 48-well plates. Monocytes differentiated into macrophages during in vitro culture. Macaque monocytes from PBMCs were cultured in vitro for 7 days when the cells became macrophages as described.42 SIVmac251, SIVmac239, SHIVKU-1, HIV-1Bal, and HIV-1NL4-3 strains were obtained from the NIH AIDS Research Program. The R5 SHIVSF162P3N derived from the HIV-1SF162 primary isolate 43, 44 was a generous gift from Dr. Cecilia Cheng-Mayer (Aaron Diamond AIDS Research Center, New York, NY) and was propagated in phytohemagglutinin (PHA)-activated macaque PBMCs. The 50% tissue culture infectious doses (TCID50) of SHIVSF162P3N was titrated in macaque PBMCs and the other virus stocks were titrated in TZM-bl cells.44 Briefly, PHA-activated PBMCs (2.5×105/well) were infected with a serial dilution of the virus stock in quadruplicate, starting with a 1/5 dilution in RPMI medium containing IL-2. The p27 antigens in culture supernatant were measured by SIV p27 ELISA (XpressBio, Frederick, MD). The TCID50 of the virus stocks were determined by the method of Reed and Muench.45
Rectal administration of EGCG and SHIV challenge
EGCG (Sigma-Aldrich, St. Louis, MO) was prepared as a stock solution (20 mM) in PBS. The protective efficacy of EGCG on the rectal SHIV infection was evaluated in a repeated exposure macaque model using the SHIVSF162P3N, an extensively used SHIV strain in NHP model of sexual transmission.46, 47 The animals were intramuscularly anesthetized with ketamine hydrochloride (10 mg kg-1) plus xylazine hydrochloride (1 mg kg-1). Anesthetized animals were maintained a ventral recumbent position and 2 ml of EGCG (5 mM) or PBS was administrated atraumatically into the rectal vault prior to the intrarectal inoculation with SHIVSF162P3N at a dose of 10 TCID50.44 Animals that were not infected after the first inoculation were treated with EGCG or PBS and inoculated with SHIV again once biweekly. All the experiments were performed under highly controlled conditions by the same personnel using the same virus stock, inoculum dose and methods. Animals were considered infected and the virus challenges were stopped following two positive plasma SHIV RNA results. The experiments were stopped when all control animals became infected.9
Plasma SHIV RNA determination
Plasma SHIV RNA was analyzed by real-time RT-PCR for SIV/SHIV gag gene as previously described.9 Primer used were: forward 5′-TGGAGAACAAAGAAGGATGTCAAA-3′, reverse 5′- CACCAGATGACGCAGACAGTATTAT-3′. Probe sequence was 6FAM-TTGGCACTAATGGAGCTAAGACCGAAAGTATT-BHQ1 (Gag-Btaq.2). Real-time PCR condition was: 95°C for 10 min, 40 cycles of 95°C for 30 sec and 60°C for 15 sec. Duplicate samples were analyzed and the limit of detection (LOD) was 200 SIV or SHIV RNA copies ml-1 plasma.
PCR amplification of proviral DNA and RNA
DNA and RNA was extracted from PBMCs, lymphocytes isolated from LNs and other GI tissues (Supplementary Table 3) with the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Valencia, CA). Specimens were analyzed in duplicate with 200 ng of DNA to amplify SHIV proviral DNA with the same primers and probes as for the plasma viral load assay described above. At week 20 after first SHIV challenge, inguinal LN and rectal mucosal were biopsied and subjected to RNA and proviral DNA detection. Intraepithelial lymphocytes from rectum or colon tissues were enriched to increase the sensitivity of the PCR as reported.9, 33
gp120 binding assay
CD4+ T cells were incubated with 50 μM of EGCG in RPMI-1640 medium for 10 min at 37°C. Large volume of cooled (4°C) PBS was then added immediately after incubation to halt the interaction between cells and EGCG. Cells were washed twice with PBS and aliquoted for two sets of experiments. In the first set of experiment, the cells were stained directly with PB-conjugated mAb against CD4 (Sk-3 clone) at room temperature subdue light for 45 min. PB-conjugated mouse IgG of unrelated specificity was used as a negative control. The stained cells were then examined by flow cytometry for CD4 expression. In the second set of experiments, the EGCG-treated CD4+ T cells were incubated with recombinant HIV gp120 protein conjugated with FITC (20 μg ml-1) for 1 h. The cells were washed with PBS and the fluorescence intensity of gp120-FITC bound to the surface of CD4+ T cells was measured by the FACSCanto flow cytometer (BD Biosciences). Data were analyzed using the FlowJo data analysis software package (TreeStar Inc., USA).
Serology
Plasma SHIV-specific antibodies (IgG and IgM) of the study animals were measured by enzyme immunoassay (EIA) as instructed by the manufacturer (Genscreen™ ULTRA HIV Ag-Ab; Bio-Rad).
Immunohistochemistry
Autopsied specimens from the rectal tissues were obtained 4 days after the rectal administration of PBS or EGCG and fixed in paraformaldehyde. The sections were incubated with mouse monoclonal antibodies against CD3 (F7.2.38) or CD68 (DK25; Dako; Carpinteria, CA), CD163 (EDHu-1; AbD Serotec; Raleigh, NC), HLA-DR (LN3; eBioscience; San Diego, CA) overnight at 4°C. Normal mouse serum was used as the negative antibody control for the specificity of the immunohistochemistry. The whole slides were digitally scanned using BX53 microscopy (Olympus, Japan) with ProScan III controller (Prior, UK). The tissue positivity for individual staining was assessed by Aperio ImageScope software (Leica Biosystems Inc., Buffalo Grove, IL).
EGCG toxicity analysis
The in vitro cytotoxicity of EGCG on the TZM-bl and PBMCs was determined by MTT assay or by flow cytometry using 7-amino actinomycin D (7-AAD). The in vivo toxicity of EGCG on the rectal mucosa was examined by assessing the mucosal pathology, inflammatory response, stool consistency and bleeding.48, 49 Briefly, EGCG was applied to rectum of the mice and the macaques. Fresh stools were then collected at 0, 24, 48, and 72 h post EGCG administration for occult blood test. Stool consistency of mice were graded accordingly as previously described.48 Inflammatory cytokines in the rectal tissues of the mice were measured by real time RT-PCR 72 h post-EGCG intrarectal administration. The impact of EGCG on rectal mucosal histology was analyzed by hematoxylin/eosin (HE) staining based on Obermeier's procedure.50 The rectum was divided into 3 equal segments with different proximity to the anus (proximal, middle, and distal), cross sectioned, and fixed in 10% formalin overnight. The tissues were then embedded in paraffin and serially sectioned at a thickness of 5 μm. Histological analysis was performed and evaluated in a blinded fashion. Each score represented the mean of nine sections.
Statistical analysis
The in vitro data were expressed as mean ± standard deviation (SD) of triplicate cultures and statistical significance was assessed by Student's t test. To protect the macaques from SHIVSF162P3N infection was analyzed using Cox proportional hazard models based on the exact partial likelihood for discrete time. The number of challenges was used as a discrete time scale. The hazard ratios with 95% confidence intervals (CI) for the per-exposure relative reductions of acquisition risk were calculated for EGCG group as compared to the control group. Protection against acquisition of infection was also analyzed by log-rank (Mantel-Cox) tests. The statistical analyses of complete protection for the study animals against SHIV challenge were performed by Fisher's exact tests. GraphPad Prism software (La Jolla, CA) was used for all statistical analyses. Significance was defined as *P<0.05, **P<0.01, and ***P<0.001.
Supplementary Material
Supplementary Figure 1. The cytotoxicity of the green tea-derived catechins on TZM-bl cells. The cytotoxicity of the green tea-derived catechins (EC, ECG, EGC, and EGCG) on TZM-bl cells. TZM-bl cells were incubated with the indicated concentrations of the catechins and the cell viabilities were measured by MTT assay at (a) 1 h, (b) 3 h or (c) 48 h post-treatment. Data are represented as mean ± SD and are representative of three independent experiments with 3 replicates.
Supplementary Figure 2. The cytotoxicity of green tea-derived catechins on macaque PBMC. The cytotoxicity of green tea-derived catechins on macaque PBMC. Cells were pretreated with the indicated concentrations of the catechins (EC, ECG, EGC, EGCG) for 1 h, 3 h or 48 h. The cells were then stained with membrane impermeant dye 7-AAD and the viability was determined by flow cytometry. Data are representative of three independent experiments.
Supplementary Figure 3. EGCG has little toxicity on the rectal mucosa of macaques and mice. EGCG has little cytotoxicity on the rectal mucosa of mice and rhesus macaques as assessed by inflammatory cytokine expression and pathological damage. (a) The rectal tissues from PBS or EGCG (5 mM)-intrarectal treated mice or rhesus macaques were fixed, sectioned and then stained with hematoxylin and eosin. Representative rectal sections from PBS or EGCG intrarectal treated mice or macaques showed normal architecture for 72 h post-treatment. Magnification ×200. (b) PBS or EGCG (2.5 or 5 mM) was delivered into the rectum of mice. The animals were necropsied 72 h post-treatment and the rectal tissues were collected. Rectal tissues (30 mg) were subjected to the RNA extraction using the AllPrep DNA/RNA/miRNA Universal Kit. Total RNA (1 μg) was revers-transcribed with an M-MLV RT system (Promega, Madison, WI). The expression of inflammatory cytokines was normalized with endogenous reference (GAPDH) and presented as the fold change relative to that of PBS-treated control mice. **P<0.01.
Supplementary Figure 4. SHIV RNA and DNA detection in PBMC and the biopsied tissues. Sixteen male macaques were administrated with 2 ml of 5 mM EGCG (8 animals) or 2 ml of PBS (8 animals) atraumatically in rectum 10 min prior to each SHIVSF162P3N challenge. All animals were rectally inoculated with SHIVSF162P3N (10 TCID50) for up to 8 times or until infection occurred. All animals were biopsied at week 20 (a, b) postinfection for the evaluation of SHIV RNA and proviral DNA in indicated tissues. (a, b) SHIV RNA and DNA assays in PBMC, rectum and inguinal lymph node (LN) at week 20 post first SHIV challenge. Inguinal LN and rectal mucosal tissues were biopsied from the animals of PBS control (red) and EGCG group (solid green circles), and subjected to (a) SHIV RNA and (b) proviral DNA detection. Log SHIV copies/2μg total genomic RNA or DNA equivalents are shown. Symbols represent individual animals and are pooled from three independent experiments. Triplication tissue samples were conducted in each independent experiment. The dot line: detection threshold.
Supplementary Figure 5. CD69 and HLA-DR expression on CD4+ and CD8+ T cells of SHIV-infected rhesus macaques. Rhesus macaques were administrated with 2 ml of 5 mM EGCG (n=8) or 2 ml of PBS (n=8) in rectum 10 min prior to each SHIVSF162P3N challenge. CD69 and HLA-DR expression on CD4+ and CD8+ T cells of all animals was monitored weekly by flow cytometry. Data are representative of the first 6 weeks post-SHIV infection of all infected animals. The number of infected macaques: N=8 (red lines) in control group; N=1 (green line) in EGCG.
Supplementary Figure 6. CD4+ T cells and CD4/CD8 ratio in whole blood of SHIV-infected rhesus macaques. Rhesus macaques were challenged with SHIVSF162P3N be-weekly. The number of CD4+ T cells and CD4/CD8 ratio were examined weekly by flow cytometry. Data are representative of the first 6 weeks post-SHIV infection of all infected animals. The number of infected macaques: N=8 (red lines) in control group; N=1 (green line) in EGCG.
Table 1. Effect of EGCG on fecal blood in rhesus macaques
Table 2. Effect of EGCG on stool consistency and fecal blood in mice
Table 3. Serology and virology analyses of SHIV infection of macaques
Acknowledgments
This work was funded by the National Science and Technology Major Projects of Infectious Disease (2012ZX10004501-001-004), the Mega-Projects of Science Research for the 12th Five-Year Plan, China (2014ZX10001003005), the National Natural Sciences Foundation of China (81271334, 81201261, 81301428), the Fundamental Research Funds for the Central Universities (2042017kf0030 to JBL) and in part by the National Institutes of Health Grants (DA041302 and DA022177 to WZH; DA040329 and MH109385 to JLL). HIV/AIDS Research Award from the Robert Mapplethorpe Foundation (New York, NY) to JLL is also acknowledged. We thank C. Cheng-Mayer at Aaron Diamond AIDS Research Center for providing SHIVSF162P3N.
Footnotes
Author Contributions: J.B.L., J.L.L. and W.Z.H. conceived of the study and experimental design and wrote the manuscript. J.B.L. and J.L.L. contributed equally to the work. J.B.L., J.L.L., H.L., Q.H.X., R.H.Z. and D.X.L. executed the macaque studies; X.W., K.Z., H.L., T.C.M., L.Z. and R.H.Z. performed viral loads and statistical analyses. M.Q.L. and W.Z. performed the SHIV antibody/plasma viral load measurement. J.B.L., J.L.L. and Q.H.X. performed IHC analyses and data analysis. All authors have read and approved the final version of this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- 1.Ceballos A, et al. Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells. J Exp Med. 2009;206:2717–2733. doi: 10.1084/jem.20091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munch J, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Usmani SM, et al. Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat Commun. 2014;5:3508. doi: 10.1038/ncomms4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zirafi O, et al. Semen enhances HIV infectivity and impairs the antiviral efficacy of microbicides. Sci Transl Med. 2014;6:262ra157. doi: 10.1126/scitranslmed.3009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cranage M, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008;5:e157. doi: 10.1371/journal.pmed.0050157. discussion e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lederman MM, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 7.Shattock RJ, Rosenberg Z. Microbicides: topical prevention against HIV. Cold Spring Harb Perspect Med. 2012;2:a007385. doi: 10.1101/cshperspect.a007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veazey RS, et al. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews CD, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014;343:1151–1154. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews CD, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med. 2015;7:270ra274. doi: 10.1126/scitranslmed.3010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patton DL, Sweeney YT, Paul KJ. A summary of preclinical topical microbicide rectal safety and efficacy evaluations in a pigtailed macaque model. Sex Transm Dis. 2009;36:350–356. doi: 10.1097/OLQ.0b013e318195c31a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168:1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nance CL, Siwak EB, Shearer WT. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J Allergy Clin Immunol. 2009;123:459–465. doi: 10.1016/j.jaci.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano LM, Hammond RM, Holmes VM, Weissman D, Shorter J. Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils. Biol Open. 2015;4:1206–1212. doi: 10.1242/bio.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartjen P, et al. Assessment of the range of the HIV-1 infectivity enhancing effect of individual human semen specimen and the range of inhibition by EGCG. AIDS Res Ther. 2012;9:2. doi: 10.1186/1742-6405-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fassina G, Buffa A, Benelli R, Varnier OE, Noonan DM, Albini A. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. Aids. 2002;16:939–941. doi: 10.1097/00002030-200204120-00020. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T. Inhibitory effects of (-)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1) Antiviral Res. 2002;53:19–34. doi: 10.1016/s0166-3542(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Hattori T, Kodama EN. Epigallocatechin gallate inhibits the HIV reverse transcription step. Antivir Chem Chemother. 2011;21:239–243. doi: 10.3851/IMP1774. [DOI] [PubMed] [Google Scholar]
- 19.Jiang F, et al. The evaluation of catechins that contain a galloyl moiety as potential HIV-1 integrase inhibitors. Clin Immunol. 2010;137:347–356. doi: 10.1016/j.clim.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Kawai K, et al. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J Allergy Clin Immunol. 2003;112:951–957. doi: 10.1016/s0091-6749(03)02007-4. [DOI] [PubMed] [Google Scholar]
- 21.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson MP, McCormick TG, Nance CL, Shearer WT. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. J Allergy Clin Immunol. 2006;118:1369–1374. doi: 10.1016/j.jaci.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhou RH, et al. Epigallocatechin gallate inhibits macaque SEVI-mediated enhancement of SIV or SHIV infection. J Acquir Immune Defic Syndr. 2017;75:232–240. doi: 10.1097/QAI.0000000000001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nance CL, et al. Translational Medicine in HIV-1 Infection: Preclinical and Clinical Development of the Green Tea Catechin, Epigallocatechin Gallate, as Therapy and Immunological Signatures. J Allergy Clin Immunol. 2017;139:AB209. [Google Scholar]
- 25.Jenness SM, Begier EM, Neaigus A, Murrill CS, Wendel T, Hagan H. Unprotected anal intercourse and sexually transmitted diseases in high-risk heterosexual women. Am J Public Health. 2011;101:745–750. doi: 10.2105/AJPH.2009.181883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamza A, Zhan CG. How can (-)-epigallocatechin gallate from green tea prevent HIV-1 infection? Mechanistic insights from computational modeling and the implication for rational design of anti-HIV-1 entry inhibitors. J Phys Chem B. 2006;110:2910–2917. doi: 10.1021/jp0550762. [DOI] [PubMed] [Google Scholar]
- 29.Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc Natl Acad Sci U S A. 2009;106:9033–9038. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roan NR, et al. The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. J Virol. 2009;83:73–80. doi: 10.1128/JVI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roan NR, Munch J. Improving preclinical models of HIV microbicide efficacy. Trends Microbiol. 2015;23:445–447. doi: 10.1016/j.tim.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neurath AR, Strick N, Li YY. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect Dis. 2006;6:150. doi: 10.1186/1471-2334-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow HH, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- 34.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen MM, et al. Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer Prev Res (Phila) 2012;5:290–298. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ullmann U, et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 37.Yoon AJ, et al. Topical Application of Green Tea Polyphenol (-)-Epigallocatechin-3-gallate (EGCG) for Prevention of Recurrent Oral Neoplastic Lesions. J Orofac Sci. 2012;4:43–50. doi: 10.4103/0975-8844.99891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyseng-Williamson KA, Hoy SM. Polyphenon E 10% ointment: a guide to its use in the treatment of external genital and perianal warts. Drugs Ther Perspect. 2012;28:6–9. [Google Scholar]
- 39.Stockfleth E, et al. Topical Polyphenon E in the treatment of external genital and perianal warts: a randomized controlled trial. Br J Dermatol. 2008;158:1329–1338. doi: 10.1111/j.1365-2133.2008.08520.x. [DOI] [PubMed] [Google Scholar]
- 40.Stalmach A, Troufflard S, Serafini M, Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res. 2009;53(Suppl 1):S44–53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Q, et al. Investigating the Stability of EGCg in Aqueous Media. Current Separations. 2003;20:83–86. [Google Scholar]
- 42.Sang M, Liu JB, Dai M, Wu JG, Ho WZ. Toll-like receptor 3 signaling inhibits simian immunodeficiency virus replication in macrophages from rhesus macaques. Antiviral Res. 2014;112:103–112. doi: 10.1016/j.antiviral.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shakirzyanova M, Tsai L, Ren W, Gettie A, Blanchard J, Cheng-Mayer C. Pathogenic consequences of vaginal infection with CCR5-tropic simian-human immunodeficiency virus SHIVSF162P3N. J Virol. 2012;86:9432–9442. doi: 10.1128/JVI.00852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 45.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. The American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- 46.Ren W, et al. Mucosal transmissibility, disease induction and coreceptor switching of R5 SHIVSF162P3N molecular clones in rhesus macaques. Retrovirology. 2013;10:9. doi: 10.1186/1742-4690-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai L, Tasovski I, Leda AR, Chin MP, Cheng-Mayer C. The number and genetic relatedness of transmitted/founder virus impact clinical outcome in vaginal R5 SHIVSF162P3N infection. Retrovirology. 2014;11:22. doi: 10.1186/1742-4690-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp. 2012 doi: 10.3791/3678. doi:3610.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabet SR, et al. Safety and toxicity of nonoxynol-9 gel as a rectal microbicide. Sex Transm Dis. 1999;26:564–571. doi: 10.1097/00007435-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The cytotoxicity of the green tea-derived catechins on TZM-bl cells. The cytotoxicity of the green tea-derived catechins (EC, ECG, EGC, and EGCG) on TZM-bl cells. TZM-bl cells were incubated with the indicated concentrations of the catechins and the cell viabilities were measured by MTT assay at (a) 1 h, (b) 3 h or (c) 48 h post-treatment. Data are represented as mean ± SD and are representative of three independent experiments with 3 replicates.
Supplementary Figure 2. The cytotoxicity of green tea-derived catechins on macaque PBMC. The cytotoxicity of green tea-derived catechins on macaque PBMC. Cells were pretreated with the indicated concentrations of the catechins (EC, ECG, EGC, EGCG) for 1 h, 3 h or 48 h. The cells were then stained with membrane impermeant dye 7-AAD and the viability was determined by flow cytometry. Data are representative of three independent experiments.
Supplementary Figure 3. EGCG has little toxicity on the rectal mucosa of macaques and mice. EGCG has little cytotoxicity on the rectal mucosa of mice and rhesus macaques as assessed by inflammatory cytokine expression and pathological damage. (a) The rectal tissues from PBS or EGCG (5 mM)-intrarectal treated mice or rhesus macaques were fixed, sectioned and then stained with hematoxylin and eosin. Representative rectal sections from PBS or EGCG intrarectal treated mice or macaques showed normal architecture for 72 h post-treatment. Magnification ×200. (b) PBS or EGCG (2.5 or 5 mM) was delivered into the rectum of mice. The animals were necropsied 72 h post-treatment and the rectal tissues were collected. Rectal tissues (30 mg) were subjected to the RNA extraction using the AllPrep DNA/RNA/miRNA Universal Kit. Total RNA (1 μg) was revers-transcribed with an M-MLV RT system (Promega, Madison, WI). The expression of inflammatory cytokines was normalized with endogenous reference (GAPDH) and presented as the fold change relative to that of PBS-treated control mice. **P<0.01.
Supplementary Figure 4. SHIV RNA and DNA detection in PBMC and the biopsied tissues. Sixteen male macaques were administrated with 2 ml of 5 mM EGCG (8 animals) or 2 ml of PBS (8 animals) atraumatically in rectum 10 min prior to each SHIVSF162P3N challenge. All animals were rectally inoculated with SHIVSF162P3N (10 TCID50) for up to 8 times or until infection occurred. All animals were biopsied at week 20 (a, b) postinfection for the evaluation of SHIV RNA and proviral DNA in indicated tissues. (a, b) SHIV RNA and DNA assays in PBMC, rectum and inguinal lymph node (LN) at week 20 post first SHIV challenge. Inguinal LN and rectal mucosal tissues were biopsied from the animals of PBS control (red) and EGCG group (solid green circles), and subjected to (a) SHIV RNA and (b) proviral DNA detection. Log SHIV copies/2μg total genomic RNA or DNA equivalents are shown. Symbols represent individual animals and are pooled from three independent experiments. Triplication tissue samples were conducted in each independent experiment. The dot line: detection threshold.
Supplementary Figure 5. CD69 and HLA-DR expression on CD4+ and CD8+ T cells of SHIV-infected rhesus macaques. Rhesus macaques were administrated with 2 ml of 5 mM EGCG (n=8) or 2 ml of PBS (n=8) in rectum 10 min prior to each SHIVSF162P3N challenge. CD69 and HLA-DR expression on CD4+ and CD8+ T cells of all animals was monitored weekly by flow cytometry. Data are representative of the first 6 weeks post-SHIV infection of all infected animals. The number of infected macaques: N=8 (red lines) in control group; N=1 (green line) in EGCG.
Supplementary Figure 6. CD4+ T cells and CD4/CD8 ratio in whole blood of SHIV-infected rhesus macaques. Rhesus macaques were challenged with SHIVSF162P3N be-weekly. The number of CD4+ T cells and CD4/CD8 ratio were examined weekly by flow cytometry. Data are representative of the first 6 weeks post-SHIV infection of all infected animals. The number of infected macaques: N=8 (red lines) in control group; N=1 (green line) in EGCG.
Table 1. Effect of EGCG on fecal blood in rhesus macaques
Table 2. Effect of EGCG on stool consistency and fecal blood in mice
Table 3. Serology and virology analyses of SHIV infection of macaques