The NH2-terminus of Norepinephrine Transporter Contains a Basolateral Localization Signal for Epithelial Cells (original) (raw)
Abstract
When expressed in epithelial cells, dopamine transporter (DAT) was detected predominantly in the apical plasma membrane, whereas norepinephrine transporter (NET) was found in the basolateral membrane, despite 67% overall amino acid sequence identity. To identify possible localization signals responsible for this difference, DAT–NET chimeras were expressed in MDCK cells and localized by immunocytochemistry and transport assays. The results suggested that localization of these transporters in MDCK cells depends on their highly divergent NH2-terminal regions. Deletion of the first 58 amino acids of DAT (preceding TM1) did not change its apical localization. However, the replacement of that region with corresponding sequence from NET resulted in localization of the chimeric protein to the basolateral membrane, suggesting that the NH2-terminus of NET, which contains two dileucine motifs, contains a basolateral localization signal. Mutation of these leucines to alanines in the context of a basolaterally localized NET/DAT chimera restored transporter localization to the apical membrane, indicating that the dileucine motifs are critical to the basolateral localization signal embodied within the NET NH2-terminal region. However, the same mutation in the context of wild-type NET did not disrupt basolateral localization, indicating the presence of additional signals in NET directing its basolateral localization within the plasma membrane.
INTRODUCTION
Transporters localized near sites of neurotransmitter release terminate the action of these transmitters by reuptake into neurons and glia (Uhl, 1992; Borowsky and Hoffman, 1995; Rudnick, 1997). The transporters for dopamine, norepinephrine, and serotonin are high-affinity targets for drugs of abuse such as cocaine and amphetamines and for therapeutic drugs used to treat depression, obsessive-compulsive disorder, and other mental diseases (Ritz et al., 1987; Koe, 1990; Barr et al., 1992; Boyer and Feighner, 1992; Giros and Caron, 1993; Gu et al., 1994; Seeman and Madras, 1998; Smith et al., 1998). Successful cloning efforts have established a family of Na+- and Cl−-dependent transporters for neurotransmitters, amino acids, and other substrates (Guastella et al., 1990; Pacholczyk et al., 1991; Amara and Kuhar, 1993; Rudnick and Clark, 1993; Nelson and Lill, 1994). Like other members of this family, the biogenic amine transporters, including dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT), were predicted from hydropathy analysis to contain 12 transmembrane domains with cytoplasmic NH2- and COOH-termini (Blakely et al., 1991; Giros et al., 1991; Hoffman et al., 1991; Kilty et al., 1991; Pacholczyk et al., 1991). This topological model has been experimentally verified (Chen et al., 1998). Although much effort has been focused on elucidating the structures and functions of these transporters, little is known about the determinants that allow them to be sorted to their proper domains within the plasma membranes of neurons and other cells. In addition to their neuronal localizations, NET and SERT are also found in the syncytiotrophoblast cells of the placenta (Ramamoorthy et al., 1993).
The plasma membranes of polarized cells are divided into functionally and morphologically distinct domains with different lipid and protein compositions (Caplan and Matlin, 1989; Rodriguez-Boulan and Nelson, 1989). The neuronal plasma membrane is composed of axolemmal and somatodendritic domains, whereas epithelial cells contain apical and basolateral plasma membrane domains. These two types of cells may share some mechanisms for sorting proteins to target membranes. Both epithelial cells and cultured neurons have been used to study the sorting behaviors of neuronal proteins (Dehoop and Dotti, 1993; Pietrini et al., 1994; Cidarregui et al., 1995; Ahn et al., 1996; Rongo et al., 1998; Poyatos et al., 2000). On the basis of results of sorting studies with viral glycoproteins in both neurons and epithelial cells, Dotti and Simons (1990) proposed that the mechanisms responsible for axonal targeting in neurons may lead to apical sorting of the same proteins in epithelial cells. Several studies provided support for this model. For example, at least one GPI-linked protein (Thy1) that is apically targeted in epithelial cells was localized to axons when expressed in hippocampal neurons in culture (Dotti et al., 1991) Similarly, previous studies of neurotransmitter transporter localization found that the axonal γ-aminobutyric acid (GABA) transporters GAT-1 and GAT-3 were localized apically when expressed in the polarized epithelial MDCK cell line, whereas the nonneuronal GAT-2 and betaine transporters were sorted basolaterally (Pietrini et al., 1994; Ahn et al., 1996). These results are consistent with the proposal that epithelial cells target axonal proteins to the apical plasmalemma. However, several counterexamples have also been described, including the amyloid precursor protein (Haass et al., 1994) and a subset of GPI-linked proteins exogenously expressed in neurons (Lowenstein et al., 1994). Furthermore, the basolateral targeting of axonal transporters DAT, NET, and SERT in LLC-PK1 cells and of SERT and NET in MDCK cells suggested that this simple model is not sufficient (Gu et al., 1996).
Studies of the endogenous expression of biogenic amine transporters support their polarized expression in neurons. With the use of selective radioligands combined with autoradiography, DAT and NET were localized to brain regions containing high densities of release sites for each transmitter. For example, DAT ligands were found to bind to regions of the striatum known to contain dense innervation by dopaminergic neurons (Graybiel and Moratalla, 1989; Richfield, 1991). The selective NET ligand nisoxetine (Wong et al., 1982) was used in autoradiographic studies to localize NET to regions of high noradrenergic innervation such as the nucleus locus coeruleus, the dorsal raphe nuclei and the paraventricular nucleus of the hypothalamus (Charnay et al., 1995; Ordway et al., 1997). These studies have been extended by the use of selective antibodies against NET and DAT for immunohistochemistry at the light and electron microscopic level (Ciliax et al., 1995; Nirenberg et al., 1996b; Hersch et al., 1997; Schroeter et al., 2000). These studies demonstrated that DAT and NET found in cell bodies were almost exclusively intracellular and that plasma membrane transporters were mostly found near sites of neurotransmitter release. These sites, however, were found not only at axon varicosities and terminals but also at dendritic locations where other elements of the release apparatus, such as synaptic vesicle proteins, were located (Nirenberg et al., 1996a; Schroeter et al., 2000). Extraneuronal expression of NET has been reported in placental syncitiotrophoblast cells (Balkovetz et al., 1989; Ramamoorthy et al., 1993). The transporter was enriched in apical plasma membrane vesicles isolated from these polarized cells. Thus, NET and DAT are selectively targeted to specific regions of neurons, although those regions are not exclusively axonal.
Epithelial cells provide a convenient expression system with which to identify the signals used for localization of neurotransmitter transporters and other proteins. The identity of signals responsible for localization of GABA transporters was addressed with the use of deletion constructs and chimeric transporters composed of complimentary portions of GAT-2 and GAT-3 (Muth et al., 1998). These studies identified a sequence of 22 amino acids at the COOH-terminus of GAT-2 that was required for the transporter's basolateral distribution and was capable of directing GAT-3 to the basolateral surface when appended to the COOH-terminus of this normally apical polypeptide (Muth et al., 1998). Other basolateral targeting signals used in epithelial cells include tyrosine and dileucine based motifs (Trowbridge et al., 1993; Matter and Mellman, 1994). Poyatos et al. (2000) demonstrated that two dileucine motifs in the COOH-terminal region of glycine transporter (GLYT-1) served as a basolateral localization signal in MDCK cells.
We reported previously that the distribution of DAT expressed in MDCK cells was predominantly apical, whereas NET was sorted to basolateral membranes (Gu et al., 1996). The two transporters are very closely related with an overall identity of 67% in their amino acid sequences. They share many similar mechanistic properties but have distinct drug inhibition profiles. Previously, DAT–NET chimeric transporters had been constructed to identify domains responsible for the differences in transport properties and drug sensitivity between the two transporters (Buck and Amara, 1994; Giros et al., 1994). To identify possible sorting signals in neurotransmitter transporters, we expressed several of these chimeric proteins as well as additional chimeras constructed from the two transporters in MDCK cells.
MATERIALS AND METHODS
Materials
Human cDNA encoding the norepinephrine transporter was a gift from Dr. Susan Amara (Vollum Institute, Portland, OR). Rat mAb raised against antigens from the large extracellular loop of human DAT (Hersch et al., 1997) was generously provided by Dr. Allan Levey (Emory University, Atlanta, GA). Antibody 43411 recognizing NET N-terminal (residues 585–602; Schroeter et al., 2000) was kindly supplied by Dr. Randy Blakely (Vanderbilt University, Nashville, TN). A mouse mAb against Na,K-ATPase α-subunit was described in a separate publication (Pietrini et al., 1992). Secondary antibodies against mouse or rat IgG were purchased from Sigma (St. Louis, MO). Vector pRC/CMV was obtained from Invitrogen (San Diego, CA). Radiolabeled substrate 3,4-[7-3H]dihydroxyphenylethylamine (DA) was purchased from Du Pont NEN Research Products (Boston, MA). All other reagents were purchased from commercial sources.
Transfection and Cell Culture
The transporter cDNAs were subcloned into vector pRC/CMV carrying the neomycin resistance gene for selection. The transfection procedure is similar to the one previously described (Gu et al., 1994). After transfection of plasmid DNA into MDCK cells, G418 was added to the culture medium at 0.9 g/l for 10–15 d to select cells that had integrated the plasmid DNA into the cell genome. Surviving cells were pooled and sorted into 96-well plates by a fluorescence-activated cell sorter so that each well received only one cell. The clonal cell lines from each well were then tested for their DA transport activity. The parental MDCK cells were maintained in DMEM supplemented with 10% FBS and 2 mM l-glutamine at 37°C, 5% CO2. MDCK cell lines expressing transporters were maintained in the same medium plus 0.9 g/l of G418.
Immunocytochemistry
The procedure was similar to that described in a earlier article (Gu et al., 1996). Parental or transfected MDCK cells expressing the transporters were plated at 50% confluence on Transwell tissue culture inserts (0.4-μm filter pore size; Costar Co., Cambridge, MA), grown for 6 d and then grown 1 more day with fresh medium. Cells were first rinsed with PBS plus 1 mM MgCl2 and 0.1 mM CaCl2 (PBS/CM), fixed for 10 min in methanol at −20°C. After rehydration for 5 min in PBS/CM, the cells were permeabilized for 15 min in PBS/CM plus 0.3% Triton X-100 and 0.1% bovine serum albumin (permeabilization buffer) and then blocked for 30 min in GSDB buffer (16% goat serum [Sigma], 0.3% Triton X-100, 20 mM sodium phosphate, pH 7.4, 0.45 M NaCl; Gottardi and Caplan, 1993; Cameron and Williams, 1994). The cells were then incubated for 1 h in GSDB with the rat anti-DAT antibody and the mouse anti–Na,K-ATPase α-subunit antibody. After three 5-min washes with permeabilization buffer, goat anti-rat IgG conjugated with FITC and goat anti-mouse IgG conjugated with rhodamine were added to the cells at 1:100 dilution and incubated for 1 h. At the end of the incubation, the cells were washed again three times with permeabilization buffer for 5 min each and once with 5 mM sodium phosphate, pH 7.5, for 15 min. The cells on filters were mounted on slides with coverslips in Vectashield mounting solution (Vector Laboratories, Burlingame, CA). Immunofluorescence was observed and analyzed with a Zeiss laser scanning confocal microscope (Thornwood, NY).
Transport Assay
Cells were plated at 50% confluence on 0.4-μm pore size, 6.5-mm Transwell cell culture filter inserts and grown for 7 d. A cell monolayer growing on the porous membrane of the cell culture filter insert effectively separates each well into two chambers. The apical membranes of epithelial cells plated on these filters face the chamber above the cells, and the basolateral membranes face the lower chamber through the filter. After one wash each of the apical (upper chamber) and basolateral (lower chamber) sides of the monolayer with PBS/CM, the cells were incubated in PBS/CM containing [3H]DA either in the upper or the lower chamber at 22°C. A 100-fold excess of unlabeled DA was added to the chamber not containing the labeled substrate. After 8 min, cells were washed three times by dipping the filters sequentially into three beakers containing PBS/CM buffer. After the washes, the filters with cells attached were excised from the insert cups, submerged in 3 ml Optifluor scintillation fluid (Packard Instrument Company, Downers Grove, IL) and counted in a Beckman LS-3801 liquid scintillation counter (Fullerton, CA). In separate experiments we measured the amount of radioactive substrate present on the contralateral side of the monolayer at the conclusion of a typical transport incubation. In agreement with previous results (Pietrini et al., 1994; Ahn et al., 1996; Gu et al., 1998; Muth et al., 1998), the amount of leakage was minimal (<0.4%).
RESULTS
To search for the signals responsible for localization of NET or DAT to opposite poles of epithelial cells, we generated stable MDCK cell lines expressing the previously constructed DAT–NET chimeras (Giros et al., 1994). Cell lines expressing some of these chimeras had very low transport activity. Some of the chimeric proteins, particularly chimeras L and M (Giros et al., 1994), were found in intracellular organelles or dispersed throughout the cell, with relatively little on the cell surface (our unpublished results). MDCK cell lines expressing two chimeric constructs, ND-A and DN-F showed robust activity for [3H]DA uptake, relative to the other chimeras. Figure 1 shows diagrammatic representations of the predicted structures for DAT, NET, ND-A, and DN-F. ND-A contains about one quarter NET sequence, from the NH2-terminus through transmembrane domains TM1 and TM2 up to residue 133, with the remaining sequence from DAT (Giros et al., 1994). DN-F, conversely, is composed of DAT sequence from the NH2-terminus through residue 434 and then NET sequence from TM9 through the COOH-terminus. We visualized the transporters by immunocytochemistry with the use of a rat mAb that recognizes the large loop between TM3 and TM4 (Ciliax et al., 1995). The immunofluorescence micrographs of DAT and NET are shown in Figure 2, A and C, for comparison. Figure 2, panels B and D, shows fields identical to panels A and C, which were stained with an antibody against the Na,K-ATPase α-subunit, a basolateral membrane marker. The results show that DN-F was predominantly located in apical membrane, with some lateral staining (Figure 2F) similar to wild-type DAT. This result suggests that sequences unique to the COOH-terminal portion of DAT may not be required for its ability to reach apical membranes in MDCK cells. In contrast, replacement of the NH2-terminal portion of DAT with NET sequence targeted the chimeric protein ND-A to basolateral membranes (Figure 2E). This result suggests that either the NH2-terminal portion of DAT contains a required apical localization signal or that the NH2-terminal portion of NET contains a signal sufficient to localize the chimeric protein to basolateral membranes. We also attempted to assess the behavior of chimeric transporter DN-B, the reciprocal construct of ND-A (Giros et al., 1994). Unfortunately, this chimeric construct did not express well enough in MDCK cells to give reliable information.
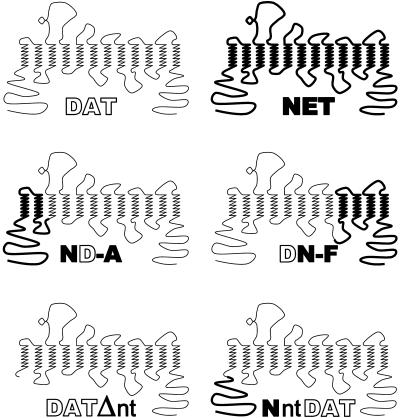
Figure 1.
Schematic representation of DAT, NET, and their chimeric constructs. DAT, NET, and their chimeric constructs are presented schematically as having 12 putative transmembrane domains with their COOH and NH2 termini on the cytoplasmic side of the plasma membrane. Bold and thin lines represent sequences from NET and DAT, respectively. ND-A is a chimeric protein consisting residues 1–128 from NET and 133–620 from DAT. DN-F consists amino acid 1–434 from DAT and the rest from NET (432–617). DATΔnt was constructed by deleting amino acid residues 2–59 from DAT. NntDAT was constructed by attaching the corresponding NET NH2-terminus (55 residues) to the deletion mutant DATΔnt.
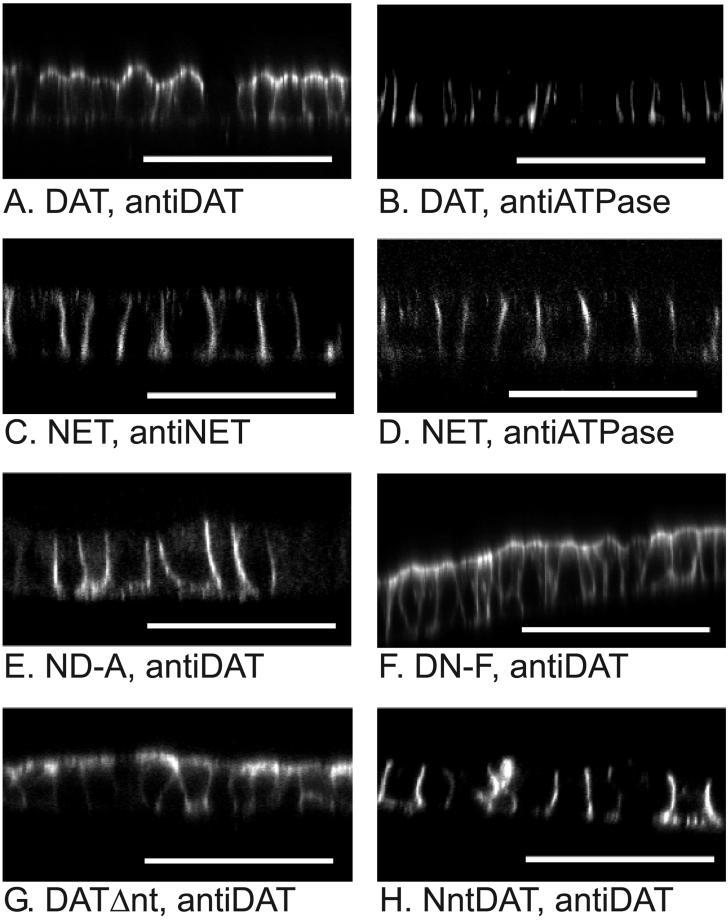
Figure 2.
The NH_2_ _-_terminal portion of NET contains a basolateral sorting signal. _X_-Z cross sections of MDCK cells expressing the transporters were generated by laser scanning confocal microscopy. Each panel is labeled for the transporter construct expressed and the primary antibody used for immunocytochemistry. A diagrammatic representation of the predicted structure of each transporter construct is illustrated in Figure 1. Micrographs for NET and DAT (A–D) are included for reference. Panels B and D are exactly the same fields as A and C, respectively, but cells shown in B and D were stained with an antibody recognizing the Na,K-ATPase α-subunit, a basolateral marker. Bars, 50 μM. Several clonal cell lines or mixtures of cells expressing each transporter construct were examined. The figure shows representative results.
To investigate possible sorting signals in the NH2-terminal region of NET and DAT, we generated two additional mutant transporters, DATΔnt and NntDAT. There is a sequence of nine residues just before TM1 that is identical in DAT and NET, but the NH2-terminus of the two transporters preceding this stretch has little significant homology. This divergent NH2-terminus seemed likely to contain signals responsible for the sorting of NET, DAT, and the chimeras DN-F and ND-A. Therefore, we deleted residues from the DAT NH2-terminus up to the conserved residues preceding TM1 (amino acids 1–58) to make the deletion mutant DATΔnt (Figures 1 and 3). Figure 2G shows that DATΔnt is found predominantly in the apical membrane of MDCK cells as with wild-type DAT. This result argues against the presence of essential apical sorting signals in the NH2-terminal region of DAT.
Figure 3.
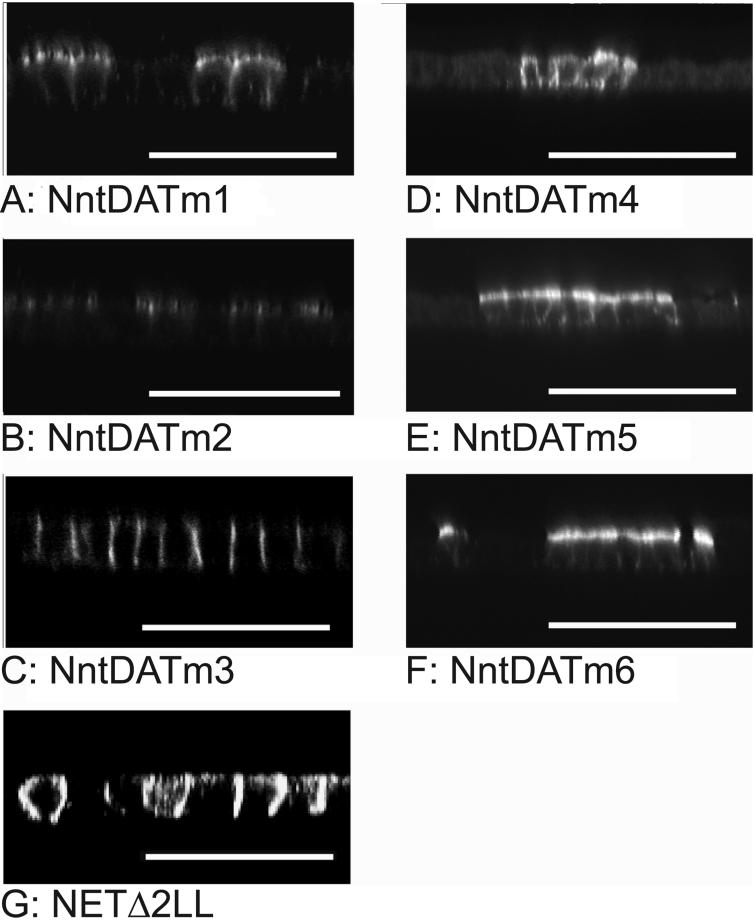
Alignment of the NH2 termini from NET and DAT, their chimeric constructs and mutants_._ The NH2 termini of DAT, DATΔnt, NntDAT, NETΔ2LL, NntDATm1, NntDATm2, NntDATm3, NntDATm4, NntDATm5, and NntDATm6 are aligned. The amino acid sequence from NET is in bold font and that from DAT is in plain font. The italic font represents regions where NET and DAT have the same sequence. Dashes represent the gaps in the sequence. The last three residues shown are from the first putative transmembrane domain. NETΔ2LL is a NET mutant with two dileucine motifs changed to alanines. DATΔnt was constructed by deleting 59 amino acid residues from DAT. NntDAT was constructed by attaching the corresponding NET NH2-terminus (55 residues) to the deletion mutant DATΔnt. NntDATm1, NntDATm2, and NntDATm3, are similar to NntDAT but with different portions of the 55-residue NET NH2-terminus added (residues 1–28, 1–19, and 27–55, respectively). NntDATm1, NntDATm2, and NntDATm3 were generated by site-directed mutagenesis.
In contrast, when residues 1–58 of DAT were replaced with the corresponding sequence from NET, the resulting chimeric transporter NntDAT (Figures 1 and 3) was targeted to basolateral membranes (Figure 2H). This result suggests that the first 55 residues of NET contain basolateral localization information used by MDCK cells. Next we constructed three additional chimeric proteins, NntDATm1, NntDATm2, and NntDATm3 (Figure 3), each of which contained different parts of the NET NH2-terminus attached to the NH2-terminus of the apically sorted DAT deletion mutant DATΔnt. Immunocytochemistry showed that addition of the first half (residues 1–28 in NntDATm1 and 1–19 in NntDATm2) of the NET NH2-terminus did not change the apical sorting of DATΔnt (Figure 4, A and B). In contrast, addition of the second half (residues 29–58, NntDATm3) to DATΔnt prevented accumulation of the transporter in the apical plasma membrane domain (Figure 4C). These results suggest that the 30 amino acid sequence region (NET 29–58) contains basolateral localization information used by MDCK cells. In Figure 2, some basal staining is apparent for chimeras ND-A and NntDAT. This was not a consistent finding but was observed sporadically for all basolaterally located mutants and does not represent a significant change in distribution from that of NET.
Figure 4.
Thirty residues from the NH2-terminus of NET contain a basolateral sorting signal. X_–_Z cross sections of MDCK cells expressing NET/DAT chimeras and their mutants were generated with a laser scanning confocal microscope. The primary antibody used was the rat mAb raised against the large extracellular loop of human DAT. The secondary antibody was a rabbit anti-rat IgG antibody conjugated to FITC. For NETΔ2LL, the primary antibody was an rabbit antiserum (43411) raised against mouse NET 585–602 (Schroeter et al., 2000), and the secondary antibody was a goat anti-rabbit IgG antibody conjugated to FITC. The transporter constructs are indicated below each panel and their sequences are shown in Figure 3.
Within this 30-residue NET 29–58 region, there are two pairs of adjacent leucine residues. Dileucine motifs have been implicated in surface delivery and localization of other proteins (Aiken et al., 1994; Matter and Mellman, 1994; Haney et al., 1995; Marsh et al., 1995; Gabilondo et al., 1997; Schulein et al., 1998; Poyatos et al., 2000). To test the role of the dileucine motifs in the localization of NET in epithelial cells, we mutated the first dileucine motif (NntDATm4), the second dileucine motif (NntDATm5), or both dileucine motifs (NntDATm6) to pairs of adjacent alanine residues (Figure 3). When expressed in MDCK cells, all three of these mutants were found in the apical and basolateral membrane (Figure 4, D–F), suggesting that both dileucine motifs are important for excluding the polypeptide from the apical plasmalemmal domain. A significant level of intracellular fluorescence was apparent with NntDATm4, and to a lesser extent NntDATm5, suggesting that the distribution between internal and surface membranes was altered in these constructs. Although replacement of first dileucine motif (NntDATm4) noticeably altered the distribution between apical and basolateral plasma membrane domains, replacement of the second dileucine (NntDATm5) had a more dramatic effect, and replacing both (NntDATm6) led to a predominantly apical localization.
To evaluate the role that these dileucine motifs play in the localization of NET, we also mutated the same residues to alanine in native NET (NETΔ2LL). If these two dileucine motifs constitute the only basolateral localization signal in NET, then we would expect that the mutation would lead to mislocalization of the mutant. The results in Figure 4, however, contradict this expectation, showing that NETΔ2LL is predominantly lateral in distribution, like native NET.
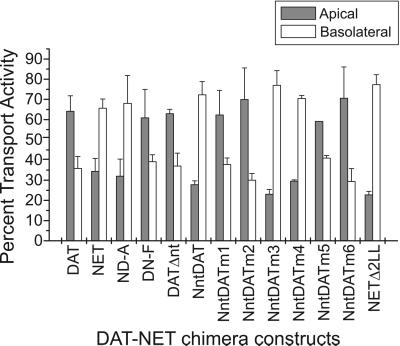
The immunofluorescence images clearly show the locations of these transporters and chimeras in transfected cells, but they do not indicate whether the proteins are functionally inserted in the plasma membrane. As an independent test for functional localization, we measured active DA transport from apical and basolateral sides of the epithelial cell monolayer. MDCK cells expressing DAT, NET, and the chimeric and deletion mutants were grown on tissue culture filter inserts. The cells grow as a monolayer that effectively separates each well in the cell culture plate into the apical and basolateral chambers. [3H]DA was added either to the apical or the basolateral chamber, and the rate of DA accumulation was determined. Figure 5 shows the transport activities at the apical and basolateral surfaces of MDCK cells expressing DAT, NET, or the chimeric constructs and mutants. They are expressed as a relative percentage of the total transport activity from both membrane domains. The total transport rate varied with each construct, but most of the variation was due to the relative number of transfected cells in the population, as judged by fluorescence. The results show that most of the transport activity in DN-F, DATΔnt, NntDATm1, and NntDATm2 was located in the apical membrane of transfected MDCK cells, similar to DAT. In contrast, the transport activity of cells expressing ND-A, NntDAT, and NntDATm3 was present mainly in the basolateral membrane, similar to NET. Removal of the dileucine motifs in NntDATm4, NntDATm5, and NntDATm6 led to a progressive increase in the percentage of DA transport activity found in the apical membrane. As with the immunofluorescence results (Figure 4), replacement of the first dileucine motif (NntDATm4) was less effective than replacement of the second motif (NntDATm5). Also, mutant NETΔ2LL, which was distributed laterally by immunofluorescence (Figure 4), was similar to NET in that transport of basolateral dopamine was much faster than for apical dopamine. Thus, the active transport data largely confirm the findings from immunocytochemistry.
Figure 5.
Transport measurements from apical and basolateral surfaces of cell monolayers. MDCK cells expressing DAT, NET, and the chimeric and deletion constructs were incubated for 8 min with [3H]DA added to either the apical side or the basolateral side of the cell monolayer. The amount of [3H]DA accumulated in the cells from each side are compared and shown as a relative percentage with the sum of the DA accumulation from both sides as 100%. The open bars and filled bars represent DA accumulation from the apical and basolateral sides, respectively. The transporter constructs expressed are indicated under the bars. The experiments were performed in triplicate, and the data shown are from representative experiments. SDs are shown as error bars. The absolute value of DA transport (100%) varied from 7.2 to 13.6 pmol/min/mg protein except for mutants m4, m5, and m6. Many of the cell lines were not clonal and represented a mixture of transfected and nontransfected cells. The 100% values for m4, m5, and m6 were 0.12, 5.4, and 5.6 pmol/min/mg protein, respectively, which was in approximate agreement with the percentage of transfected cells in the population.
Despite the general agreement between the two methods, there are instances where the transport results imply less polarized expression than the immunofluorescence results (e.g., DATΔnt, NntDATm6). This results from the fact that the two assays detect related but not identical features of transporter distribution. Immunofluorescence reports on surface density in a given plasma membrane domain. In contrast, transport studies reveal the total quantity of functional transporter exposed to a given side of the monolayer. Because the basolateral surface area of MDCK cells grown on filter supports exceeds their apical surface area by a factor of 4–8, we believe that the relatively enhanced basolateral polarity detected by the uptake assay is consistent with the immunofluorescence pattern. Finally, it is worth noting that the apical-to-basolateral polarity ratio of other apical membrane proteins, such as influenza HA, as determined by biochemical methods, rarely exceeds 4:1, which is similar to the values reported here.
DISCUSSION
Epithelial cells provide an informative setting in which to test the sorting properties of membrane proteins. Both neurons and epithelial cells have distinct plasma membrane domains. In epithelia, the basolateral and apical membranes are separated by tight junctions and have different protein and lipid compositions (Gottardi et al., 1994). Similarly, neurons have distinct axonal, somatic, and dendritic plasma membrane domains. Dotti and Simons (1990) put forward a proposal that the apical domain of epithelial cells corresponds to the axonal plasma membrane of neurons, and the basolateral domain corresponds to the somatic and dendritic plasma membrane. Results with expression of some GABA and glycine transporters (Pietrini et al., 1994; Ahn et al., 1996; Poyatos et al., 2000) and a glutamate receptor (Rongo et al., 1998) in epithelial cells apparently support this proposal. However, results obtained with biogenic amine transporters (Gu et al., 1996) and other proteins (Haass et al., 1994; Lowenstein et al., 1994) indicate that the situation is more complex.
Biogenic amine transporters are known to be axonal in neurons on the basis of autoradiographic studies with high-affinity ligands (De Souza and Kuyatt, 1987; Richfield, 1991; Tejani-Butt, 1992; Little et al., 1993) and from more recent immunocytochemical studies at the electron microscopic level (Ciliax et al., 1995; Qian et al., 1995; Pickel and Chan, 1999; Schroeter et al., 2000). When transfected into LLC-PK1 cells, however, these same transporters were found in the basolateral membrane of transfected cells, and in MDCK cells, NET and SERT were localized basolaterally, whereas DAT was also found on the apical surface (Gu et al., 1996).
Given that NaCl-coupled transporters for GABA, NE, and 5-HT are endogenously expressed in various epithelia (Balkovetz et al., 1989; Borden et al., 1992; Ramamoorthy et al., 1992; Yamauchi et al., 1992; Ramamoorthy et al., 1993), it is appropriate to use cultured epithelial cell lines, such as MDCK, as model systems to identify determinants for the cellular localization of these proteins. It must be noted, of course, that information gleaned from the study of transporter sorting in epithelia may not be directly applicable to the neuronal setting. Although neurons also demonstrate polarized expression of these transporters, the signals utilized in the two cell types may not be the same. Similarly, the same sorting signals may be used but interpreted differently in the two cell types (depending on the compliment of accessory factors), leading to the possible misreading, in epithelial cells, of structural determinants that may be used as localization signals in neurons. These caveats notwithstanding, however, the identification of the signals that mediate a transporter's epithelial localization provides an extremely useful insight into the family of mechanisms that are likely to participate in establishing that protein's neuronal distribution (Ahn et al., 1996; Gu et al., 1996; Muth et al., 1998; Poyatos et al., 2000).
Support for this contention can be found in the case of GAT-1 expression in MDCK cells, in which an apical localization signal was shown to reside in this protein's NH2-terminal region (Perego et al., 1997). Localization in MDCK cells correlated with axonal localization in neurons. The GABA transporters GAT-2 and GAT-3 were found to sort to opposite poles of transfected MDCK cells, and the sorting signals responsible for this phenomenon were identified in the extreme COOH-terminus of both transporters (Muth et al., 1998). Similarly, in the case of GLYT-1 and -2 dileucine motifs in the COOH-terminal region were identified as the signal utilized in both MDCK cells and cultured neurons (Poyatos et al., 2000).
In this work we suggest that a basolateral localization signal is present in the hydrophilic region just before the first transmembrane domain of NET. This contrasts with the results obtained with GAT-2 and GAT-3, which contained COOH-terminal signals that directed these proteins to the basolateral and apical plasma membrane, respectively (Muth et al., 1998) and the COOH-terminal basolateral signal in GLYT-1 (Poyatos et al., 2000). Deleting the terminal three residues from GAT-3, or modifying the COOH-terminus by addition of a c-myc epitope tag led to a form that was distributed to both apical and basolateral membranes of transfected MDCK cells (Muth et al., 1998). However, in GAT-1, an apical localization signal was found in the NH2-terminal region (Perego et al., 1997). Moreover, in GAT-3, we obtained preliminary evidence indicating that the NH2-terminus may contain targeting information. In the GAT-3 mutant where a COOH-terminal c-myc epitope masked the apical sorting information, deletion of the NH2-terminal hydrophilic region resulted in exclusive basolateral localization (Gu, Muth and Caplan, unpublished observations). This result suggests that a cryptic apical signal may be present at the NH2-terminus of this protein and argues that multiple, hierarchical signals may be involved in establishing transporter distribution. As further evidence for this point, we observed here that although the two dileucine motifs were essential for the NET N-terminus to serve as a basolateral localization signal when attached to DAT, replacement of these residues with alanine in NETΔ2LL did not alter the basolateral localization of NET. Thus, NET also contains additional signals responsible for its basolateral localization.
The basolateral sorting of NET and SERT in MDCK cells and the lack of a basolateral sorting signal in the DAT NH2-terminus may be related to the endogenous expression of these biogenic amine transporters in various tissues. SERT and NET have been identified in at least one epithelium, the placental syncytiotrophoblast (Balkovetz et al., 1989; Ramamoorthy et al., 1993), but DAT expression has been observed only in neurons. It is possible that NET contains the sorting signal identified here as a consequence of its expression in epithelial cells and the absence of that signal in DAT reflects the fact that DAT is not normally expressed in epithelia. Furthermore, the localization of any particular membrane protein may depend on the cell type in which it is expressed. For example, NET and SERT were found in the basolateral membranes of MDCK and LLC-PK1 cells (Gu et al., 1996) but are apical in the placental syncytiotrophoblast (Balkovetz et al., 1989; Ramamoorthy et al., 1993). The reverse behavior was seen with the H,K-ATPase β-subunit, which contains a tyrosine-based sorting signal that localizes the protein to the basolateral membrane of MDCK cells and the apical face of LLC-PK1 cells (Roush et al., 1998).
Evidence from studies with NET, DAT, and SERT suggests that regulation of these transporters by PKC involves endocytic removal from and reinsertion in the plasma membrane (Apparsundaram et al., 1998; Blakely et al., 1998; Pristupa et al., 1998; Melikian and Buckley, 1999; Ramamoorthy and Blakely, 1999). It is quite possible that the motifs identified here play a role in that process and that their effect on transporter distribution are exerted at the level of recycling endosomes.
It has been reported that dileucine motifs play important roles in surface delivery and localization of other proteins (Aiken et al., 1994; Matter and Mellman, 1994; Haney et al., 1995; Marsh et al., 1995; Gabilondo et al., 1997; Schulein et al., 1998; Poyatos et al., 2000; Sandoval et al., 2000). In some cases, acidic or basic residues upstream from the dileucine motif have been implicated in their ability to serve as localization signals (Sandoval et al., 2000). In the NET N-terminus, the first dileucine follows an Arg/Lys pair at i-4. However, the second dileucine is not similarly associated with charged residues, although this second dileucine motif exerts a greater influence than the first as a basolateral localization signal. Thus, association of charged residues with the dileucine motif does not play an influential role in this case.
Among transport proteins, the dileucine motif in the GAT-2 COOH-terminus was shown not to be required for basolateral sorting (Muth et al., 1998), although the dileucine motif in GLUT4 was necessary for intracellular targeting (Haney et al., 1995). In GLYT1, basolateral localization in MDCK cells required two dileucine motifs separated by 16 residues in the COOH-terminal region. Moreover, removal of either dileucine was found to alter the distribution of GLYT1 in cultured hippocampal neurons (Poyatos et al., 2000). The results presented here show that two dileucine motifs separated by 10 residues also function in basolateral transporter localization when present in the NH2-terminal region and suggest that a pair of dileucine motifs may represent a general signal for basolateral transporter localization in epithelial cells.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute on Drug Abuse to H.H.G. and G.R. M.G.C. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations used:
MDCK
Madin-Darby Canine Kidney
NET
norepinephrine transporter
DAT
dopamine transporter
SERT
serotonin transporter
DA
dopamine
TM
transmembrane domain
GABA
γ-aminobutyric acid
GAT
γ-aminobutyric acid transporter
GLYT
glycine transporter
PBS/CM
PBS plus 1 mM MgCl2 and 0.1 mM CaCl2
GSDB buffer
16% goat serum, 0.3% Triton X-100, 20 mM sodium phosphate, pH 7.4, 0.45 M NaCl
REFERENCES
- Ahn J, Mundigl O, Muth TR, Rudnick G, Caplan MJ. Polarized expression of GABA transporters in Madin-Darby canine kidney cells and cultured hippocampal neurons. J Biol Chem. 1996;271:6917–6924. doi: 10.1074/jbc.271.12.6917. [DOI] [PubMed] [Google Scholar]
- Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Amara S, Kuhar M. Neurotransmitter transporters—recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport. II. Pkc-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998;287:744–751. [PubMed] [Google Scholar]
- Balkovetz DF, Tirruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J Biol Chem. 1989;264:2195–2198. [PubMed] [Google Scholar]
- Barr I, Boodman W, LH P, McDouble C, Charney D. The serotonin hypothesis of obsessive compulsive disorder—implications of pharmacologic challenge studies. J Clin Psychiatry. 1992;53:17–28. [PubMed] [Google Scholar]
- Blakely R, Berson H, Fremeau R, Caron M, Peek M, Prince H, Bradely C. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry. 1998;44:169–178. doi: 10.1016/s0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Borden L, Smith K, Hartig P, Branchek T, Weinshank R. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system—cloning of 2 novel high affinity GABA transporters from rat brain. J Biol Chem. 1992;267:21098–21104. [PubMed] [Google Scholar]
- Borowsky B, Hoffman BJ. Neurotransmitter transporters: molecular biology, function, and regulation. Int Rev Neurobiol. 1995;38:139–199. doi: 10.1016/s0074-7742(08)60526-7. [DOI] [PubMed] [Google Scholar]
- Boyer WF, Feighner JP. An overview of paroxetine. J Clin Psychiatry. 1992;53(suppl):3–6. [PubMed] [Google Scholar]
- Buck K, Amara S. Chimeric dopamine norepinephrine transporters delineate structural domains influencing selectivity for catecholamines and 1-methyl-4-phenylpyridinium. Proc Natl Acad Sci USA. 1994;91:12584–12588. doi: 10.1073/pnas.91.26.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D, Williams J. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci. 1994;14:6763–6767. doi: 10.1523/JNEUROSCI.14-11-06763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan M, Matlin K. The sorting of membrane and secretory proteins in polarized epithelial cells. In: Matlin K, Valentich J, editors. Functional Epithelial Cells in Culture. New York: Alan R. Liss; 1989. pp. 71–127. [Google Scholar]
- Charnay Y, Leger L, Vallet PG, Hof PR, Jouvet M, Bouras C. [3H]Nisoxetine binding sites in the cat brain: an autoradiographic study. Neuroscience. 1995;69:259–270. doi: 10.1016/0306-4522(95)00257-j. [DOI] [PubMed] [Google Scholar]
- Chen JG, Liu-Chen S, Rudnick G. Determination of external loop topology in the serotonin transporter by site-directed chemical labeling. J Biol Chem. 1998;273:12675–12681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- Cidarregui A, Dehoop M, Dotti C. Mechanisms of neuronal polarity. Neurobiology Aging. 1995;16:239–243. doi: 10.1016/0197-4580(94)00190-c. [DOI] [PubMed] [Google Scholar]
- Ciliax B, Heilman C, Demchyshyn L, Pristupa Z, Ince E, Hersch S, Niznik H, Levey A. The dopamine transporter—immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza E, Kuyatt B. Autoradiographic localization of [3H]-paroxetine-labeled serotonin uptake sites in rat brain. Synapse. 1987;1:488–496. doi: 10.1002/syn.890010513. [DOI] [PubMed] [Google Scholar]
- Dehoop, M., and Dotti, C. (1993). Membrane traffic in polarized neurons in culture. J. Cell Sci. 85–92. [DOI] [PubMed]
- Dotti CG, Parton RG, Simons K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature. 1991;349:158–161. doi: 10.1038/349158a0. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Simons K. Polarized sorting of viral glycoproteins to axons and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Gabilondo AM, Hegler J, Krasel C, Boivin-Jahns V, Hein L, Lohse MJ. A dileucine motif in the C terminus of the beta2-adrenergic receptor is involved in receptor internalization. Proc Natl Acad Sci USA. 1997;94:12285–12290. doi: 10.1073/pnas.94.23.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Giros B, el Mestikawy S, Bertrand L, Caron MG. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- Giros B, Wang Y, Suter S, Mcleskey S, Pifl C, Caron M. Delineation of discrete domains for substrate, cocaine, and tricyclic antidepressant interactions using chimeric dopamine-norepinephrine transporters. J Biol Chem. 1994;269:15985–15988. [PubMed] [Google Scholar]
- Gottardi C, Pietrini G, Shiel M, Caplan M. Synthesis and sorting of ion pumps in polarized cells. Curr Top Membr. 1994;41:143–168. [Google Scholar]
- Gottardi CJ, Caplan MJ. An ion-transporting ATPase encodes multiple apical localization signals. J Cell Biol. 1993;121:283–293. doi: 10.1083/jcb.121.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R. Dopamine uptake sites in the striatum are distributed differentially in striosome and matrix compartments. Proc Natl Acad Sci USA. 1989;86:9020–9024. doi: 10.1073/pnas.86.22.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Caplan MJ, Rudnick G. Cloned catecholamine transporters expressed in polarized epithelial cells: sorting, drug sensitivity, and ion-coupling stoichiometry. Adv Pharmacol. 1998;42:175–179. doi: 10.1016/s1054-3589(08)60721-8. [DOI] [PubMed] [Google Scholar]
- Gu HH, Ahn J, Caplan MJ, Blakely RD, Levey AI, Rudnick G. Cell-specific sorting of biogenic amine transporters expressed in epithelial cells. J Biol Chem. 1996;271:18100–18106. doi: 10.1074/jbc.271.30.18100. [DOI] [PubMed] [Google Scholar]
- Gu HH, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in ion dependence and inhibitor sensitivity. J Biol Chem. 1994;269:7124–7130. [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel M, Davidson N, Lester H, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Haass C, Koo E, Teplow D, Selkoe D. Polarized secretion of beta-amyloid precursor protein and amyloid beta-peptide in MDCK cells. Proc Natl Acad Sci USA. 1994;91:1564–1568. doi: 10.1073/pnas.91.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney PM, Levy MA, Strube MS, Mueckler M. Insulin-sensitive targeting of the GLUT4 glucose transporter in L6 myoblasts is conferred by its COOH-terminal cytoplasmic tail. J Cell Biol. 1995;129:641–658. doi: 10.1083/jcb.129.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol. 1997;388:211–227. [PubMed] [Google Scholar]
- Hoffman BJ, Mezey E, Brownstein MJ. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Kilty J, Lorang D, Amara S. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991;254:578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- Koe B. Preclinical pharmacology of sertraline—a potent and specific inhibitor of serotonin reuptake. J Clin Psychiatry. 1990;51:13–17. [PubMed] [Google Scholar]
- Little K, Kirkman J, Carroll F, Breese G, Duncan G. [125I]RTI-55 binding to cocaine-sensitive dopaminergic and serotonergic uptake sites in the human brain. J Neurochem. 1993;61:1996–2006. doi: 10.1111/j.1471-4159.1993.tb07435.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR, Bain D, Morrison EE, Preston CM, Clissold P, Fournel S, Epstein A, Castro MG. HSV1 vectors to study protein targeting in neurones: are glycosyl-phosphatidylinositol anchors polarized targeting signals in neurones? Gene Ther. 1994;1:S32–S35. [PubMed] [Google Scholar]
- Marsh BJ, Alm RA, McIntosh SR, James DE. Molecular regulation of Glut-4 targeting in 3t3–L1 adipocytes. J Cell Biol. 1995;130:1081–1091. doi: 10.1083/jcb.130.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity—sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth TR, Ahn J, Caplan MJ. Identification of sorting determinants in the C-terminal cytoplasmic tails of the gamma-aminobutyric acid transporters Gat-2 and Gat-3. J Biol Chem. 1998;273:25616–25627. doi: 10.1074/jbc.273.40.25616. [DOI] [PubMed] [Google Scholar]
- Nelson N, Lill H. Porters and neurotransmitter transporters. J Exp Biol. 1994;196:213–228. doi: 10.1242/jeb.196.1.213. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu YJ, Edwards RH, Pickel VM. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons—potential sites for somatodendritic storage and release of dopamine. J Neurosci. 1996a;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996b;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway GA, Stockmeier CA, Cason GW, Klimek V. Pharmacology and distribution of norepinephrine transporters in the human locus coeruleus and raphe nuclei. J Neurosci. 1997;17:1710–1719. doi: 10.1523/JNEUROSCI.17-05-01710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely R, Amara S. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Perego C, Bulbarelli A, Longhi R, Caimi M, Villa A, Caplan MJ, Pietrini G. Sorting of two polytopic proteins, the gamma-aminobutyric acid and betaine transporters, in polarized epithelial cells. J Biol Chem. 1997;272:6584–6592. doi: 10.1074/jbc.272.10.6584. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Ultrastructural localization of the serotonin transporter in limbic and motor compartments of the nucleus accumbens. J Neurosci. 1999;19:7356–7366. doi: 10.1523/JNEUROSCI.19-17-07356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini G, Matteoli M, Banker G, Caplan MJ. Isoforms of the Na,K-ATPase are present in both axons and dendrites of hippocampal neurons in culture. Proc Natl Acad Sci USA. 1992;89:8414–8418. doi: 10.1073/pnas.89.18.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini G, Suh YJ, Edelmann L, Rudnick G, Caplan MJ. The axonal GABA transporter is sorted to the apical membrane of polarized epithelial cells. J Biol Chem. 1994;269:4668–4674. [PubMed] [Google Scholar]
- Poyatos I, Ruberti F, Martinez-Maza R, Gimenez C, Dotti CG, Zafra F. Polarized distribution of glycine transporter isoforms in epithelial and neuronal cells. Mol Cell Neurosci. 2000;15:99–111. doi: 10.1006/mcne.1999.0807. [DOI] [PubMed] [Google Scholar]
- Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJS, Wang YT, Niznik HB. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Qian Y, Melikian H, Rye D, Levey A, Blakely R. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Leibach FH, Mahesh VB, Ganapathy V. Active transport of dopamine in human placental brush-border membrane vesicles. Am J Physiol. 1992;262:C1189–C1196. doi: 10.1152/ajpcell.1992.262.5.C1189. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Prasad P, Kulanthaivel P, Leibach FH, Blakely RD, Ganapathy V. Expression of a cocaine-sensitive norepinephrine transporter in the human placental syncytiotrophoblast. Biochemistry. 1993;32:1346–1353. doi: 10.1021/bi00056a021. [DOI] [PubMed] [Google Scholar]
- Richfield E. Quantitative autoradiography of the dopamine uptake complex in rat brain using [3H]GBR 12935: binding characteristics. Brain Res. 1991;540:1–13. doi: 10.1016/0006-8993(91)90486-f. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998;94:751–759. doi: 10.1016/s0092-8674(00)81734-1. [DOI] [PubMed] [Google Scholar]
- Roush DL, Gottardi CJ, Naim HY, Roth MG, Caplan MJ. Tyrosine-based membrane protein sorting signals are differentially interpreted by polarized Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem. 1998;273:26862–26869. doi: 10.1074/jbc.273.41.26862. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Mechanisms of biogenic amine neurotransmitter transporters. In: Reith M, editor. Neurotransmitter Transporters: Structure, Function, and Regulation. Totowa, NJ: Humana Press; 1997. pp. 73–100. [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Sandoval IV, Martinez-Arca S, Valdueza J, Palacios S, Holman GD. Distinct reading of different structural determinants modulates the dileucine-mediated transport steps of the lysosomal membrane protein LIMPII and the insulin-sensitive glucose transporter GLUT4. J Biol Chem. 2000;275:39874–39885. doi: 10.1074/jbc.M006261200. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l- norepinephrine transporter. J Comp Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Schulein R, Hermosilla R, Oksche A, Dehe M, Wiesner B, Krause G, Rosenthal W. A dileucine sequence and an upstream glutamate residue in the intracellular carboxyl terminus of the vasopressin V2 receptor are essential for cell surface transport in COS.M6 cells. Mol Pharmacol. 1998;54:525–535. doi: 10.1124/mol.54.3.525. [DOI] [PubMed] [Google Scholar]
- Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998;3:386–396. doi: 10.1038/sj.mp.4000421. [DOI] [PubMed] [Google Scholar]
- Smith BH, Pelham WE, Gnagy E, Yudell RS. Equivalent effects of stimulant treatment for attention-deficit hyperactivity disorder during childhood and adolescence. J Am Acad Child Adolesc Psychiatry. 1998;37:314–321. doi: 10.1097/00004583-199803000-00017. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt S. [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther. 1992;260:427–436. [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Neurotransmitter transporters (plus)—a promising new gene family. Trends Neurosci. 1992;15:265–268. doi: 10.1016/0166-2236(92)90068-j. [DOI] [PubMed] [Google Scholar]
- Wong DT, Threlkeld PG, Best KL, Bymaster FP. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther. 1982;222:61–65. [PubMed] [Google Scholar]
- Yamauchi A, Uchida S, Kwon H, Preston A, Robey R, Garcia-Perez A, Burg M, Handler J. Cloning of a Na+-dependent and Cl−-dependent betaine transporter that is regulated by hypertonicity. J Biol Chem. 1992;267:649–652. [PubMed] [Google Scholar]