Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector (original) (raw)
Abstract
Ideally, somatic gene therapy should result in lifetime reversal of genetic deficiencies. However, to date, phenotypic correction of monogenic hyperlipidemia in mouse models by in vivo gene therapy has been short-lived and associated with substantial toxicity. We have developed a helper-dependent adenoviral vector (HD-Ad) containing the apolipoprotein (apo) E gene. A single i.v. injection of this vector completely and stably corrected the hypercholesterolemia in apoE-deficient mice, an effect that lasted the natural lifespan of the mice. At 2.5 years, control aorta was covered 100% by atherosclerotic lesion, whereas aorta of treated mice was essentially lesion-free. There was negligible toxicity associated with the treatment. We also developed a method for repeated HD-Ad vector administration that could be applied to organisms, e.g., humans, with life spans longer than 2–3 years. These studies indicate that HD-Ad is a promising system for liver-directed gene therapy of metabolic diseases.
There are some encouraging recent developments in gene therapy using nonviral vectors (1) as well as viral-derived vectors (2). In the latter approach, Cavazzana-Calvo et al. used a retrovirus to deliver the γc cytokine receptor gene to CD34+ cells in two patients with combined immunodeficiency-X1 and restored γc expression in T and NK cells that persisted for at least 10 months (3). Kay et al. used an adeno-associated viral vector (AAV) to deliver the blood coagulation factor IX into skeletal muscle of three patients with factor IX deficiency and observed persistence of the vector and suggestive evidence for expression 8–12 weeks after treatment (4). In these trials, the vector systems seemed to be especially suited to their respective target tissues, retrovirus for CD34+ stem cells and AAV for muscle cells. The liver is an important organ for many inborn errors of metabolism and was the target tissue in a gene therapy trial for the treatment of familial hypercholesterolemia, in which the LDL receptor gene was delivered ex vivo to LDL receptor-deficient patients by using a retroviral vector (5). Unfortunately, clinical benefit of liver-directed gene therapy through this approach was uncertain (5). In LDL receptor−/− mice, hepatic delivery of the LDL receptor gene in vivo by using adenovirus-mediated gene transfer was effective in reversing the hypercholesterolemia (6), but the lipid-lowering effect was transient with the first-generation adenoviral vector (FG-Ad) used. Recently, a more prolonged (lasting 6 months), but partial, amelioration of the hypercholesterolemia in the same mouse model was reported by two groups that delivered the VLDL receptor gene to the liver by using either an AAV (7) or a helper-dependent adenoviral vector (HD-Ad) devoid of all viral protein-coding genes (8). Phenotypic correction was better with the HD-Ad than with the AAV, possibly because transgene expression was much higher in the former.
Despite the apparently stronger transgene expression with HD-Ad over AAV in liver-targeted gene delivery, the lack of integration of HD-Ad is a potential drawback. Because AAV transgenes are integrated into the genome of the recipient, although at varying frequency, the potential for long-term transgene expression is high. In contrast, HD-Ad transgenes are not integrated and might eventually be eliminated from the host by cell division or cell turnover. To test whether HD-Ads are a suitable gene transfer vehicle for long-term correction of genetic diseases that require high-level transgene expression, we investigated the efficacy and monitored the durability of apoE transgene expression in mice with apoE deficiency. We found that, surprisingly, a single i.v. injection of a modest dose of apoE HD-Ad led to high-level stable expression of apoE that completely corrected the hypercholesterolemia of apoE−/− mice for their entire natural lifespan (until they died naturally at 2–2.5 years). We further showed that we could reinject the HD-Ad reinducing apoE expression, an important consideration for animals with life spans longer than 2–3 years.
Materials and Methods
Recombinant Adenovirus.
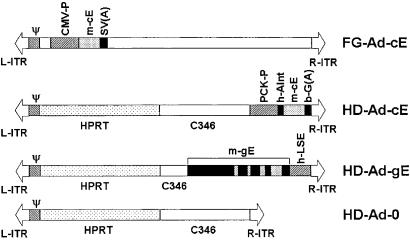
Four Ad vectors were produced (Fig. 1). Mouse apolipoprotein E (apoE) cDNA was inserted into a KS vector by reverse transcription–PCR cloning using total cellular RNA prepared from the liver of C57BL/6 mice. Primers used were 5′-GAAGGATCCACCATGAAGGCTCTGTGGGCCGTG-3′ and 5′-AAGGAATTCTCATTGATTCTCCTGGGCCAC-3′. The forward primer contained an artificial _Bam_HI site (underlined), and the reverse primer contained an _Eco_RI site for cloning purposes. The fully sequenced apoE cDNA was then subcloned into the pAvCvSv shuttle vector, and the FG-Ad-cE was produced by standard techniques (9). We used pΔ28 plasmid vector (8) as backbone to construct HD-Ad-cE. To construct the shuttle vector for HD-Ad-gE, we inserted into pΔ21 plasmid (a pΔ28 vector with 4.8 kb of the 11-kb C346) (8) an 8-kb _Eco_RI fragment of the mouse apoE gene ligated at its 3′ end to a 1.7-kb _Pst_I fragment containing the human apoE liver-specific enhancer (10). HD-Ad vectors were produced by the method of Parks et al. (11).
Figure 1.
Structures of Ad vectors. L-ITR and R-ITR, left and right Ad inverted terminal repeat sequence, respectively; HPRT, intron region of human genomic hypoxanthine phosphoribosyltransferase stuffer sequence; C346, cosmid C346 human genomic stuffer sequence (8); Ψ, Ad packaging sequence; CMV-P, cytomegalovirus promoter; PCK-P, phospho_enol_pyruvate carboxykinase promoter; _m_-cE, mouse apoE cDNA; _m_-gE, mouse genomic apoE DNA; h-Aint, human apoA-I intron 1; b-G(A), bovine β-globin polyadenylation signal; SV(A), SV40 polyadenylation signal.
Animals.
Female apoE−/− mice on a C57BL/6 background purchased from The Jackson Laboratory were maintained on a regular chow diet. Adenoviral vectors in dialysis buffer (DB) were injected via the tail vein. To measure plasma parameters, we anesthetized mice with either methoxyflurane or isoflurane after a 5-h fast and collected blood in EDTA.
Immunoblot Analysis.
One microliter of plasma was electrophoresed on a 12% denaturing SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and incubated with goat anti-apoE antibody (1:2,000 dilution, Chemicon). Immunoreactive apoE was detected by enhanced chemiluminescence (Amersham Pharmacia). Pooled plasma from 12–16-month-old female C57BL/6 wild-type mice (C57-wt) was used as positive control.
Plasma ApoE Levels.
We measured plasma apoE by sandwich ELISA using polyclonal rabbit anti-apoE antibody (1:500 dilution, Accurate Chemical & Scientific) as a capture antibody and polyclonal goat anti-apoE antibody (1:1000 dilution, Chemicon) as the detecting antibody. Anti-goat IgG antibody conjugated to horseradish peroxidase was used for detection with chromagen. Mouse apoE was purified by electroelution from SDS/PAGE of total lipoprotein fraction (d < 1.21 g/ml) of C57BL/6 wild-type mice and used to generate a standard curve in the assay.
Tissue Analyses.
DNA, RNA, and protein were extracted simultaneously from the same tissue by using TRIzol (Life Technologies, Grand Island, NY). For Southern blotting, 10 μg of DNA was digested with _Eco_RI and separated on a 1% agarose gel. After transfer to a nylon membrane, vector-derived DNA was detected by a 32P-labeled 1.9-kb _Eco_RI fragment of pΔ21-gE, which contained human liver-specific enhancer plus right ITR. Northern blot analysis was performed on 20 μg of cellular RNA by using a 32P-labeled 0.9-kb mouse apoE cDNA probe. Western blot analysis was performed by using 25 μg of protein.
Immunohistochemistry.
Paraffin sections (5 μm) of liver from virus-treated, DB control, or C57BL/6 wild-type (10-month-old female) mice were incubated at 4°C with a polyclonal goat anti-apoE antibody (1:800, Chemicon) in the presence of 10% horse serum. Sections were then incubated with rabbit anti-goat IgG conjugated to horseradish peroxidase (1:2,000 dilution, Bio-Rad), and subsequent peroxidase detection was performed by using metal-enhanced diaminobenzidine (Pierce).
Other Procedures.
FPLC analyses, plasma lipid and aspartate aminotransferase, and alanine aminotransferase activities were determined as previously described (8). Neutralizing antibody titers were measured as described by Morral et al. (12). Quantitative analysis of aortic atherosclerotic lesion areas was performed as described (8). Statistical analyses were performed by using ANOVA with SIGMASTAT (SPSS, Chicago), and statistical significance was assigned at P < 0.05. All results are expressed as mean ± SD.
Results
Production and Characterization of ApoE-Ad Vectors.
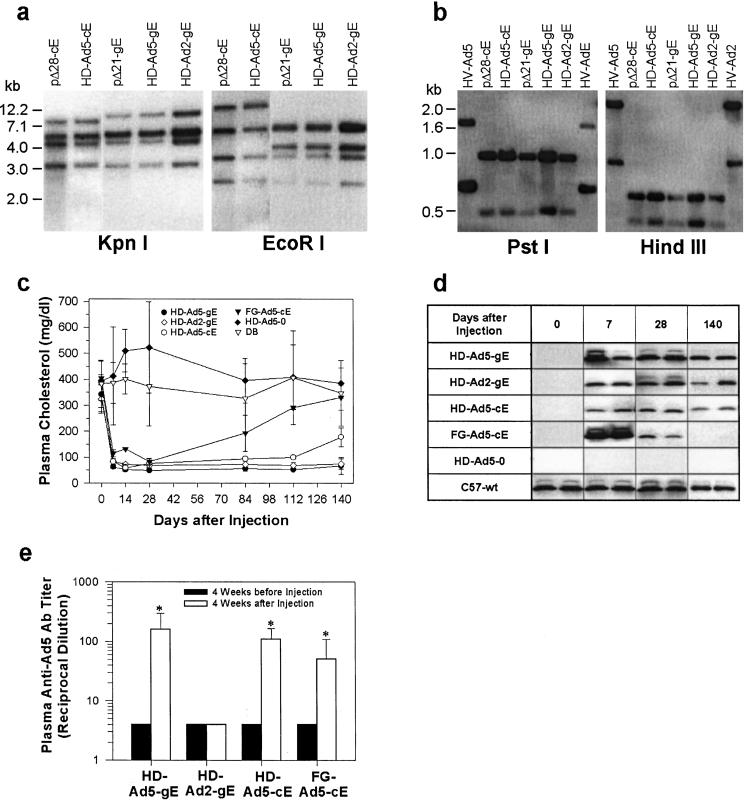
We constructed the following vectors: (i) FG-Ad5-cE, a serotype-5 FG-Ad containing an apoE cDNA driven by a cytomegalovirus promoter; (ii) HD-Ad-cE, an HD-Ad containing an apoE cDNA behind a phosphoenolpyruvate carboxykinase promoter; (iii) HD-Ad-gE, an HD-Ad containing the native apoE gene with a liver-specific enhancer at the 3′-end; and (iv) HD-Ad-0, an empty HD-Ad without any transgene insert (Fig. 1). Two different helper Ads were used that were based on Ad serotypes 5 and 2 (13); the vectors were designated HD-Ad5 and HD-Ad2, respectively. Southern blotting showed that there was no detectable DNA rearrangement (Fig. 2a) or helper virus in the HD-Ads (indicating ≤0.2% helper virus contamination, Fig. 2b).
Figure 2.
Comparison of FG-Ad and different HD-Ads. (a) Restriction mapping of shuttle and HD-Ad vectors. Lane 1, _Pme_I-digested pΔ28-cE; lane 2, HD-Ad-cE; lane 3, _Pme_I-digested pΔ21-gE; lane 4, HD-Ad5-gE (serotype 5); lane 5, HD-Ad2-gE (serotype 2). (b) Southern blot analysis of helper virus contamination. Lane 1, helper virus serotype 5; lane 2, _Pme_I-digested pΔ28-cE; lane 3, HD-Ad-cE; lane 4, _Pme_I-digested pΔ21-gE; lane 5, HD-Ad5-gE; lane 6, HD-Ad2-gE; lane 7, helper virus serotype 2. The upper and lower bands correspond to the left arm and the right arm, respectively. (c) Plasma cholesterol levels in apoE−/− mice following injections of Ad vectors. Cholesterol levels in apoE Ad-treated groups were significantly lower than those in the DB group with a P < 0.001 except the following: HD-Ad5-cE at 140 days (P < 0.01) and FG-Ad5-cE at 84 days (P < 0.05) and after 84 days (not significant). (d) Western blot of plasma apoE in different experimental groups. Treatment groups are identified in the left column. (e) Plasma anti-Ad5 neutralizing antibody titers. *, P < 0.001.
Efficacy and Hepatotoxicity of Different Ad Vectors in ApoE−/− Mice.
We injected the different vectors via tail vein into hypercholesterolemic 12–14-week-old apoE−/− mice. All apoE-Ads (1 × 1011 particles/mouse, or 5 × 1012 particles/kg), but not HD-Ad5–0 or DB, caused an immediate fall in plasma cholesterol (Fig. 2c). The effect of the FG-Ad5-cE was, however, short-lived, and plasma cholesterol started to return toward control levels after 28 days. At 7 days, FG-Ad5-cE-treated mice had the highest plasma apoE level, followed by HD-Ad5-gE and HD-Ad2-gE, and HD-Ad5-cE gave the lowest level (Fig. 2d). With FG-Ad treatment, plasma apoE levels fell rapidly and became undetectable on day 140. In contrast, in HD-Ad5-gE-treated mice, plasma apoE was maintained at a level similar to wild-type apoE+/+ mice; it remained easily detectable for >4 months following HD-Ad2-gE and HD-Ad5-cE treatment. In this experiment, we found that the genomic-based transgene HD-Ad vector was substantially more effective than the cDNA-based vector (14). Of the different vectors used, only FG-Ad5-cE induced liver enzyme elevation (data not shown).
Ad Vectors Stimulate Serotype-Specific Anti-Ad Neutralizing Antibodies.
HD-Ad5 and FG-Ad5 vectors stimulated plasma Ad5-specific antibody production, whereas HD-Ad2 did not change the titer of Ad5-specific antibody (Fig. 2e). Thus, anti-Ad antibody production was serotype-specific.
Efficacy of Readministration of HD-Ad Vectors.
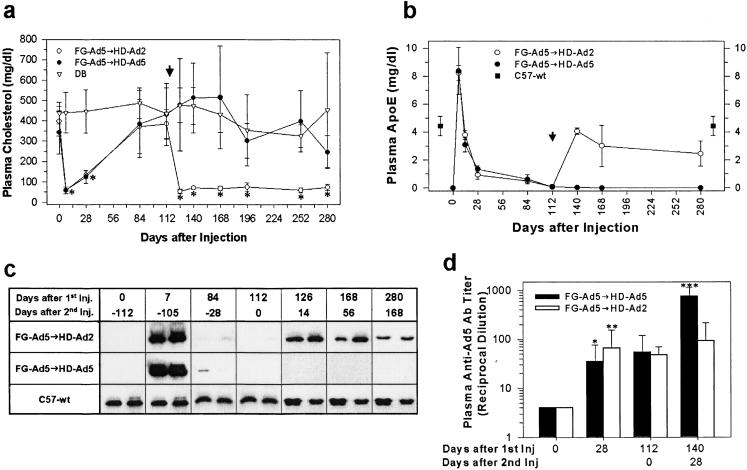
We next determined the feasibility of readministration of apoE-Ad vectors. As in the previous experiments, apoE−/− mice (n = 5/group) given a single injection of FG-Ad5-cE experienced a transient drop in plasma cholesterol, which started going up 4 weeks after treatment (Fig. 3a). At 112 days, when the plasma cholesterol returned to a pretreatment level of ≈385 mg/dl, we gave these animals another IV injection (same dose) of HD-Ad5-gE or HD-Ad2-gE (arrowhead, Fig. 3a). Reinjection with HD-Ad5-gE produced no change in plasma cholesterol, whereas reinjection with HD-Ad2-gE produced an immediate fall in plasma cholesterol to <100 mg/dl (Fig. 3_a_). These repeat injections produced no change in plasma liver enzyme activities (data not shown). The readministration of an HD-Ad of an alternate serotype stimulated plasma apoE level as assayed by ELISA (Fig. 3_b_) and by Western blotting (Fig. 3_c_). Plasma anti-Ad5 antibody titer was stimulated 10–100-fold 28 and 112 days following FG-Ad5 treatment (Fig. 3_d_). Reinjection using an HD-Ad2 vector did not change the antibody titer significantly, but reinjection using an HD-Ad5 vector produced a further >10-fold increase in the anti-Ad5 titer of these animals (Fig. 3d).
Figure 3.
Readministration of Ad vectors of different serotypes. (a) Plasma cholesterol levels following readministration of Ad vectors of different serotypes. *, P < 0.001. (b) Plasma apoE levels measured by ELISA. Arrowhead, vector readministration; ■, plasma apoE in C57BL/6 mice (4.4 ± 0.7 mg/dl). (c) Western blot analysis of plasma apoE in mice treated by different Ad vectors. (d) Plasma anti-Ad5 neutralizing antibody titer following readministration of HD-Ads of different serotypes. Closed bars, FG-Ad5-cE followed by HD-Ad5-gE (n = 5); open bars, FG-Ad5-cE followed by HD-Ad2-gE (n = 5). *, P < 0.005 (FG-Ad5, 0 vs. 28 days); **, P < 0.002 (FG-Ad5, 0 vs. 28 days); and ***, P < 0.01 (FG-Ad5 at 112 days vs. HD-Ad5–2nd injection at 140 days).
HD-Ads Effect Lifetime Correction of Hypercholesterolemia in ApoE−/− Mice.
The pilot experiments described above enabled us to develop a rational approach to produce prolonged correction of the hypercholesterolemia of apoE−/− mice. We decided on a slightly higher dose of HD-Ad for the initial treatment, although the dose selected (7.5 × 1012 particles per kg) was only 50% of that previously used (1.5 × 1013 particles per kg) to deliver the VLDL receptor in mice (8). We used HD-Ad5 vectors in the initial injection in case we needed to reinject with the HD-Ad2 vectors. As we did not expect FG-Ad5-cE to have a long-lasting effect, we kept the dose of this vector at the same level to minimize toxicity.
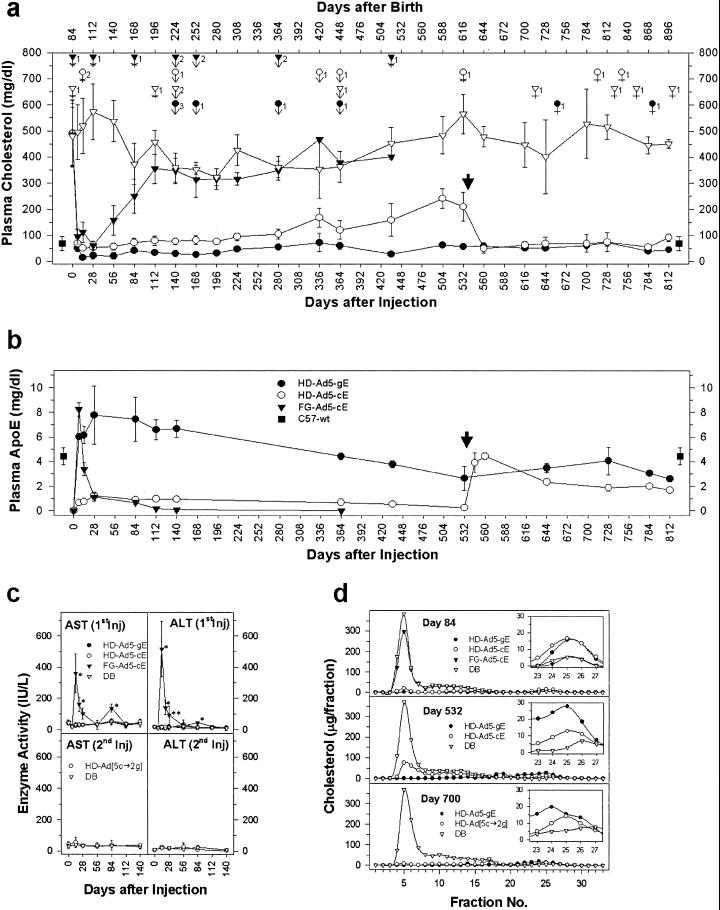
Twelve-week-old apoE−/− mice were treated with a single IV injection of FG-Ad5-cE (5 × 1012 particles per kg), HD-Ad5-cE, HD-Ad5-gE (both at 7.5 × 1012 particles per kg), or DB (Fig. 4a). Plasma liver enzymes were markedly (>10 to 20-fold) elevated after FG-Ad5 treatment but remained normal in HD-Ad-treated mice, despite the use of a higher dose in these animals (Fig. 4c). Plasma cholesterol (Fig. 4a) stayed elevated at ≈350–550 mg/dl throughout the lifetime of control (DB-treated apoE−/−) mice. As noted in previous experiments, FG-Ad5-cE produced an immediate but transient fall in plasma cholesterol. A single injection of HD-Ad5-cE or HD-Ad5-gE produced a complete, immediate, and sustained lowering in plasma cholesterol as opposed to FG-Ad5-cE. The normalization of cholesterol was the result of a normalization of the plasma lipoproteins back to a predominantly high-density lipoprotein pattern seen in wild-type mice as analyzed by FPLC (Fig. 4d). In HD-Ad5-cE-treated mice, plasma cholesterol remained normal (i.e., similar to that in wild-type apoE+/+ mice) for about a year before it gradually drifted upward. In HD-Ad5-gE-treated mice, it remained subnormal for about 9 months and stayed within the normal range for the rest of the natural lifespan of the mice. The plasma cholesterol lowering was correlated with the apoE response (Fig. 4b). In HD-Ad5-cE-injected mice, apoE appeared in plasma within a week and remained at a level ≈25% that of wild-type apoE+/+ mice. It had been shown previously that in mice <10% of the normal plasma apoE concentration is needed to maintain normal plasma cholesterol (15, 16). In contrast, plasma apoE in HD-Ad5-gE-injected mice reached the ≈200% wild-type apoE+/+ level within 4 weeks and stayed at supraphysiological levels for >4 months after a single injection of the vector. It slowly declined to the ≈100% wild-type apoE+/+ level at 1 year and remained at 60–90% physiological concentrations for the rest of the lifetime of the mice (≈2.5 years).
Figure 4.
Lifetime correction of hypercholesterolemia in apoE−/− mice by HD-Ads. (a) Plasma cholesterol levels in mice injected with different Ads. (Upper) Days after birth; (Lower) days after injection. ●, HD-Ad5-gE (1.5 × 1011 particles per mouse, n = 10); ○, HD-Ad[5-cE → 2-gE], HD-Ad5-cE (1.5 × 1011 particles per mouse) followed by HD-Ad2-gE (1.0 × 1011 particles per mouse) (n = 10); ▾, FG-Ad5-cE (1 × 1011 particles per mouse, n = 10); ▿, DB control (n = 10); ■, plasma cholesterol level of normal C57-wt mice (n = 10). Boldface arrow, reinjection of HD-Ad2-gE. During the 2.5-year experimental period, mice died of different causes as denoted by the symbols above the cholesterol values. Similar symbols were used to denote the mouse groups as those used for plasma cholesterol. Number of deaths are as indicated; causes of death were as follows: *, accidental death (accidents during handling/bleeding); small arrows, anesthetic death (especially in the course of changing anesthetic agent from methoxyflurane to isoflurane); and crosses, natural deaths. (b) Plasma apoE levels. ●, HD-Ad5-gE; ○, HD-Ad[5-cE → 2-gE]; ▾, FG-Ad5-cE; and ■, C57-wt. Bold arrow, reinjection of HD-Ad2-gE. (c) Plasma liver enzyme activities. (Upper) Plasma aspartate aminotransferase and alanine aminotransferase activities after an initial injection. ●, HD-Ad5-gE; ○, HD-Ad5-cE; ▾, FG-Ad5-cE; and ▿, DB control. (Lower) Plasma aspartate aminotransferase and alanine aminotransferase activities after readministration of HD-Ad2-gE (n = 5) in HD-Ad5-cE-injected mice. DB, mice reinjected with dialysis buffer (n = 5). *, P < 0.001; **, P < 0.005. (d) FPLC profile of total plasma cholesterol in apoE−/− mice after injection of different Ad vectors. The individual lipoprotein classes are very-low density lipoprotein and remnants (nos. 3–8), intermediate and low-density lipoproteins (nos. 9–18), and HDL (nos. 22–27). HDL profiles are magnified inside a window in each panel. ●, HD-Ad5-gE; ○, HD-Ad5-cE; ▾, FG-Ad5-cE; and ▿, DB control.
In HD-Ad5-cE-treated mice, plasma apoE concentration slowly declined to <10% normal after about 1.5 years, at which time plasma cholesterol concentration rose to ≈50% that in DB-treated mice (Fig. 4a) mainly because of a rise in the first lipoprotein (remnant) peak (Fig. 4d). At this point (532 days after primary injection), we gave these mice a repeat injection of HD-Ad2-gE (at 5 × 1012 particles per kg), a vector produced with a serotype 2 helper Ad (13). The second injection did not cause any liver enzyme elevation (Fig. 4c). Plasma apoE immediately rose to a normal range (Fig. 4b, arrowhead); at the same time, plasma cholesterol declined to wild-type apoE+/+ levels with normalization of the lipoprotein profile (Fig. 4 a and d). The normal plasma cholesterol concentration lasted the rest of the lifetime of these mice (Fig. 4a).
We had started the long-term experiment with 10 mice in each group. With time, some mice died as a result of accidents during handling/blood letting, and especially after the administration of anesthetics (Fig. 4a). After about 2 years, mice in both the control (DB) and treatment (HD-Ad5-gE, or HD-Ad5-cE → HD-Ad2-gE) groups started to die naturally. When all except one mouse in the DB group and two mice each in the HD-Ad-cE and HD-Ad-gE groups had died at 2–2.4 years, we killed the remaining mice at about 2.5 years of age to examine vector distribution and transgene expression. All HD-Ad5-gE-treated mice that died accidentally or naturally had normal plasma cholesterol as well as normal or near normal plasma apoE concentrations at the time of death.
A Single Treatment With HD-Ad Leads to Lifetime Liver-Specific Transgene Expression.
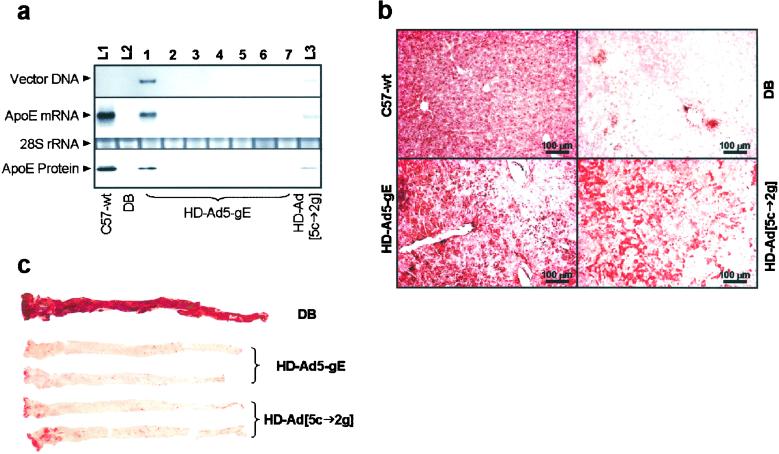
At 2.5 years of age (2.3 years after treatment), HD-Ad5-gE vector DNA was detected by Southern blotting only in the liver (Fig. 5a), although trace amounts could be detected by PCR also in lung and spleen, but not in any other tissues examined (kidney, brain, small intestine, or muscle; data not shown). ApoE mRNA was detected in the liver only and not in any other tissues by Northern blotting (Fig. 5a) or reverse transcription–PCR (data not shown). ApoE protein was present in the liver of HD-Ad5-gE-treated mice at ≈40–50% that in wild-type mice (Fig. 5a). Similarly, vector, apoE mRNA, and protein were detected in the liver of HD-Ad5-cE → HD-Ad2-gE mice, but the levels were substantially lower, probably a reflection of either the lower transduction efficiency of the HD-Ad2 vector or the lower (second injection) dose used (Fig. 5a). By immunohistochemical staining (Fig. 5b), >90% of the hepatocytes in wild-type apoE+/+ mice stained positive for apoE, compared with ≈50–60% in apoE−/− mice treated with HD-Ad5-gE. The corresponding value for the HD-Ad5-cE → HD-Ad2-gE mice was ≈30%.
Figure 5.
Tissue analyses at 817 days after HD-Ad injection (age 901 days). (a) Tissue distribution of vector-derived DNA, apoE mRNA, and protein. L1, liver of C57-wt; L2, liver of DB control; L3, liver of HD-Ad[5-cE → 2-gE]-treated mice. Lanes 1–7 are from the HD-Ad5-gE treated mice. Lane 1, liver; lane 2, spleen; lane 3, lung; lane 4, kidney; lane 5, brain; lane 6, small intestine; lane 7, muscle. (b) Immunohistochemistry of liver sections 817 days after HD-Ad treatment. (c) Aortas of HD-Ad-treated and DB control mice stained with Oil Red O.
A Single HD-Ad Injection Confers Lifetime Protection Against Aortic Atherosclerosis.
At necropsy, no hepatic neoplasms were noted in any of the mice. Mild to moderate inflammatory reactions with hepatic necrosis and occasional mitotic cells were noted in all mice analyzed, including the DB control and the two HD-Ad-treated pairs with no significant difference between them. We speculate that these changes may be related to the nonsterile conditions of the mouse facility. The aorta of the control DB-treated apoE−/− mouse was completely covered with atherosclerotic lesions, whereas HD-Ad5-gE aortas were essentially free of lesions and HD-Ad5-cE → HD-Ad2-gE aortas had small lesions localized in the aortic arch (Fig. 5c). The lesion areas determined by quantitative morphometry are DB, 91.45 mm2; HD-Ad5-gE, 0.81 and 0.13 mm2; and HD-Ad[5-cE → 2-gE], 5.89 and 1.74 mm2. Therefore, a single injection of HD-Ad5-gE at a young age conferred essentially complete lifetime protection against atherosclerosis to the apoE−/− mice.
Discussion
First-generation replication-defective E1a-inactivated Ad has been used for many years (17, 18). FG-Ad-mediated gene transfer is highly efficient, and Ads given IV have a propensity to transduce the liver, although, as shown in this study, many other tissues also take up the vector. A major drawback of FG-Ads is that they are associated with considerable toxicity, and FG-Ad-transgene expression is short-lived (19). Novel Ads were developed in an attempt to reduce their toxicity by the selective inactivation of one or two additional Ad genes. Unfortunately, neither toxicity nor duration of expression was much improved with the second- and third-generation Ads (19). Ads with all viral protein genes deleted were developed by a number of laboratories (20–26), and a Cre/loxP system was introduced by Parks et al. (11) for their efficient production. The experiments described in this report represent a lifetime study of the efficacy of an injection of HD-Ad for the treatment of a monogenic disease in a mouse model.
The data indicate that hepatic gene replacement using an HD-Ad results in lifelong and essentially complete correction of a genetic deficiency in mice. Of note is the fact that the HD-Ad dose used was modest, being half that of a nontoxic dose previously used in a short-term study (8). Just as important is the demonstration of the feasibility of retreating the animals with a repeat injection of HD-Ad. This was possible because neutralizing antibodies to Ad are serotype-specific, and we could circumvent the immune response by retreating with an HD-Ad with an alternate serotype (Figs. 2e and 3d) (13). We note that the production of HD-Ads of different serotypes does not require the engineering of new HD-Ads. Only the helper virus needs to be changed. Helper viruses are relatively easy to produce as they are FG-Ads. Because there are large numbers of human Ad serotypes, as well as nonhuman Ad viruses of different serotypes, one can readily assemble a library of serologically distinct helper Ads. With the relatively prolonged transgene expression following each injection, one can maintain a therapeutic level of transgene expression almost indefinitely by periodic treatments (e.g., once every 1–3 years, exact interval depending on the response) using HD-Ads produced with helper Ads having different serotypes. HD-Ads are relatively nontoxic in experimental animals. However, their toxicity and efficacy remain untested in humans, and further studies are needed to establish the safety and feasibility of this rotating-HD-Ad approach before it can be applied to human trials. Nevertheless, the data presented here suggest that further experiments along these lines are warranted.
Acknowledgments
We thank A. Merched, M. Merched-Sauvage, E. A. Nour, S. Billah, and R. Montgomery for their assistance in the study; A. Beaudet for collaboration and helpful comments on the manuscript; F. Graham for providing helper-virus; Merck & Co. for providing reagents developed by F. Graham; N. Maeda for providing mouse apoE gene clone; and J. Smith for human apoE genomic clone. This work was supported by National Institutes of Health Grant HL 59314.
Abbreviations
apo
apolipoprotein
AAV
adeno-associated viral vector
FG-Ad
first-generation adenoviral vector
HD-Ad
helper-dependent adenoviral vector
References
- 1.Roth D A, Tawa N E, Jr, O'Brien J M, Treco D A, Selden R F. N Engl J Med. 2001;344:1735–1742. doi: 10.1056/NEJM200106073442301. [DOI] [PubMed] [Google Scholar]
- 2.Kay M A, Glorioso J C, Naldini L. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 3.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova J L, et al. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 4.Kay M A, Manno C S, Ragni M V, Larson P J, Couto L B, McClelland A, Glader B, Chew A J, Tai S J, Herzog R W, et al. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 5.Grossman M, Rader D J, Muller D W, Kolansky D M, Kozarsky K, Clark B J, III, Stein E A, Lupien P J, Brewer H B, Jr, Raper S E, et al. Nat Med. 1995;1:1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi S, Brown M S, Goldstein J L, Gerard R D, Hammer R E, Herz J. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S J, Rader D J, Tazelaar J, Kawashiri M, Gao G, Wilson J M. Mol Ther. 2000;2:256–261. doi: 10.1006/mthe.2000.0122. [DOI] [PubMed] [Google Scholar]
- 8.Oka K, Pastore L, Kim I H, Merched A, Nomura S, Lee H J, Merched-Sauvage M, Arden-Riley C, Lee B, Finegold M, et al. Circulation. 2001;103:1274–1281. doi: 10.1161/01.cir.103.9.1274. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K, Oka K, Forte T, Ishida B, Teng B, Ishimura-Oka K, Nakamuta M, Chan L. J Biol Chem. 1996;271:6852–6860. doi: 10.1074/jbc.271.12.6852. [DOI] [PubMed] [Google Scholar]
- 10.Simonet W S, Bucay N, Lauer S J, Taylor J M. J Biol Chem. 1993;268:8221–8229. [PubMed] [Google Scholar]
- 11.Parks R J, Chen L, Anton M, Sankar U, Rudnicki M A, Graham F L. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra P A, Zhou H, Parks R J, Velji R, Aguilar-Cordova E, et al. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parks R, Evelegh C, Graham F. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- 14.Morral N, Parks R J, Zhou H, Langston C, Schiedner G, Quinones J, Graham F L, Kochanek S, Beaudet A L. Hum Gene Ther. 1998;9:2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- 15.Hasty A H, Linton M F, Swift L L, Fazio S. J Lipid Res. 1999;40:1529–1538. [PubMed] [Google Scholar]
- 16.Thorngate F E, Rudel L L, Walzem R L, Williams D L. Arterioscler Thromb Vasc Biol. 2000;20:1939–1945. doi: 10.1161/01.atv.20.8.1939. [DOI] [PubMed] [Google Scholar]
- 17.Stratford-Perricaudet L D, Levrero M, Chasse J F, Perricaudet M, Briand P. Hum Gene Ther. 1990;1:241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- 18.Gerard R D, Chan L. Curr Opin Lipidol. 1996;7:105–111. doi: 10.1097/00041433-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Brann T, Kayda D, Lyons R M, Shirley P, Roy S, Kaleko M, Smith T. Hum Gene Ther. 1999;10:2999–3011. doi: 10.1089/10430349950016401. [DOI] [PubMed] [Google Scholar]
- 20.Mitani K, Graham F L, Caskey C T, Kochanek S. Proc Natl Acad Sci USA. 1995;92:3854–3858. doi: 10.1073/pnas.92.9.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochanek S, Clemens P R, Mitani K, Chen H H, Chan S, Caskey C T. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemens P R, Kochanek S, Sunada Y, Chan S, Chen H H, Campbell K P, Caskey C T. Gene Ther. 1996;3:965–972. [PubMed] [Google Scholar]
- 23.Fisher K J, Choi H, Burda J, Chen S J, Wilson J M. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 24.Kumar-Singh R, Chamberlain J S. Hum Mol Genet. 1996;5:913–921. doi: 10.1093/hmg/5.7.913. [DOI] [PubMed] [Google Scholar]
- 25.Morsy M A, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks R J, Graham F L, et al. Proc Natl Acad Sci USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floyd S S, Jr, Clemens P R, Ontell M R, Kochanek S, Day C S, Yang J, Hauschka S D, Balkir L, Morgan J, Moreland M S, et al. Gene Ther. 1998;5:19–30. doi: 10.1038/sj.gt.3300549. [DOI] [PubMed] [Google Scholar]