Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis (original) (raw)
Abstract
The proteinase-activated receptor 2 (PAR-2) is a member of a family of G protein-coupled receptors for proteases. Proteases cleave PARs within the extracellular N-terminal domains to expose tethered ligands that bind to and activate the cleaved receptors. PAR-2 is highly expressed in colon in epithelial and neuronal elements. In this study we show that PAR-2 activation prevents the development and induces healing of T helper cell type 1-mediated experimental colitis induced by intrarectal administration of 2,4,6-trinitrobenzene sulfonic acid (TNBS) in mice. A role for PAR-2 in the protection against colon inflammation was explored by the use of SLIGRL-NH2, a synthetic peptide that corresponds to the mouse tethered ligand exposed after PAR-2 cleavage. TNBS-induced colitis was dose-dependently reduced by the administration of SLIGRL-NH2, whereas the scramble control peptide, LSIGRL-NH2, was uneffective. This beneficial effect was reflected by increased survival rates, improvement of macroscopic and histologic scores, decrease in mucosal content of T helper cell type 1 cytokines, protein, and mRNA, and a diminished myeloperoxidase activity. SLIGRL-NH2, but not the scramble peptide, directly inhibited IFN-γ secretion and CD44 expression on lamina propria T lymphocytes. Protection exerted by PAR-2 in TNBS-treated mice was reverted by injecting mice with a truncated form of calcitonin gene-related peptide and by sensory neurons ablation with the neurotoxin capsaicin. Collectively, these studies show that PAR-2 is an anti-inflammatory receptor in the colon and suggest that PAR-2 ligands might be effective in the treatment of inflammatory bowel diseases.
The biological actions of certain serine proteases on cells are increasingly being attributed to the activation of a novel class of G protein-coupled receptors termed protease-activated receptors (PARs) (1–3). Proteases activate PARs by cleaving the NH2-terminal sequence of the extracellular exodomain. This cleavage event unmasks a amino terminal sequence, which in turn serves as a tethered ligand, binding intramolecularly to the body of the receptor to trigger transmembrane signaling. Molecular cloning (4–7) has identified four PARs: PAR-1 and PAR-3, which are both preferentially activated by thrombin; PAR-2, which is selectively activated by trypsin; and PAR-4, which is activated by both thrombin and trypsin. In addition to endogenous proteases, PARs can be selectively activated by short agonistic peptides (PAR-APs), such as SLIGRL-NH2, a synthetic peptide that corresponds to the rat/mouse tethered ligand exposed after PAR-2 cleavage (1–7).
PAR-2 is highly expressed in the gastrointestinal tract and is found in intestinal and colon epithelial cell, neuronal elements, and myocytes (6, 8–10). In general, PAR-2 activation has been linked to proinflammatory responses (1–3, 11–14). Inflammation caused by PAR-2 activation is mediated by sensory neurons (14). Indeed, PAR-2 activation releases calcitonin gene-related peptide (CGRP) and substance P (SP) from nerve endings, and CGRP and SP antagonists abolish inflammatory responses induced by PAR-2 AP (14). A firm conclusion for a proinflammatory role of PAR-2, however, is still lacking because the effect of PAR-2 AP in an animal model of inflammatory diseases has never been tested (15).
Inflammatory bowel diseases (IBDs) represent chronic, relapsing, and tissue-destructive diseases. Although the etiology of IBDs remains unknown, there is circumstantial evidence to link IBDs to the mucosal immune system's failure to attenuate immunity to endogenous antigen (16). In recent years various murine models of intestinal inflammation have been developed (16–20). These models were characterized by an imbalance of regulatory cytokines, most notably by an excessive production of T helper cell type 1 (Th-1) cytokines, IL-2, IL-12, and IFN-γ. T cell-derived cytokines play a major role also in the hapten-induced model of colonic inflammation in which 2,4,6-trinitrobenzene sulfonic acid (TNBS) is delivered intrarectally to BALB/c mice (16–20). Intestinal inflammation, in this model, develops as a result of covalent binding of the haptenizing agent to autologous host proteins with subsequent stimulation of delayed-type hypersensitivity to TNBS-modified self-antigens.
Activation of sensory neurons protects against colon inflammation induced by TNBS (21–23). Indeed, inhibition of CGRP activity achieved by blocking CGRP receptor with a truncated form of CGRP (CGRP8–37), CGRP immunoneutralization, or ablation of sensory nerve fibers with the neurotoxin capsaicin, significantly worsened inflammation induced by TNBS. Although the exact mechanism through which CGRP protects against colitis is unclear (24), it has recently been found that this neuropetide prevents nuclear translocation of NF-κB and inhibits proliferation and cytokine (IL-2, IL-7, and IFN-γ) production from concanavallin A-primed T lymphocytes (25–28).
Similarly to CGRP, human T lymphocyte cell lines (Jurkat cells) express PAR-2 (29). The functional role of PAR-2 on T cells in unknown, although there is evidence that exposure of T lymphocytes to trypsin inhibits lymphocyte homing in a murine model of cutaneous delayed hypersensitivity (30). Because all of these observations suggest that PAR-2 could negatively regulate hypersensitivity reactions in the gut we have designed the present study to investigate whether PAR-2 activation modulates T lymphocytes function and protects against colitis development in the TNBS model of colitis.
Materials and Methods
Induction of Colitis and Study Design.
BALB/c mice (6–8 weeks old) were obtained from Charles River Breeding Laboratories. After 2 days of fasting, mice were anesthetized with halothane and O2, and a 3.5F catheter was inserted into the colon such that the tip was 4 cm proximal to the anus. To induce colitis 100 μl of 1.0–2.5 mg of TNBS (Sigma) in 50% ethanol (to break the intestinal epithelial barrier) was slowly administered into the lumen via the catheter fitted onto a 1-ml syringe (17). Control mice received 100 μl of 50% ethanol.
In the first study we examined whether PAR-2 AP protects BALB/c mice against lethality caused by TNBS. In this protocol, mice (12 per group) were injected intrarectally with 50% ethanol or 1, 1.5, 2, or 2.5 mg/mouse TNBS and then injected s.c with 1.5 mg/kg per day SLIGRL-NH2 (tethered ligand of murine PAR-2) or LSIGRL-NH2 (scramble control peptide) (31–34) for 7 days, and the number of surviving animals in each treatment group was recorded.
In the next set of experiments we assessed whether PAR-2 activation protects against colitis development. After TNBS injection, BALB/c mice (12 per group) were randomized to receive one of the following: no treatment, SLIGRL-NH2, or GRLILS-NH2 at the daily dose of 0.3, 1, and 1.5 mg/kg s.c. for 7 days. Animals were monitored daily for appearance of diarrhea, loss of body weight, and survival. Animals were then killed 24 h after the last dose of peptides. Blood samples were collected by cardiac puncture, and a segment of the colon (7 cm long) was excised for macroscopic damage evaluation and weighed. Tissue segments were then immediately frozen in liquid nitrogen for histological and immuno-histochemical studies, cytokine determination (Endogen, Woburn, MA), myeloperoxidase (35, 36) activity measurement, and reverse transcription–PCR analysis (36, 37). To investigate whether CGRP-containing sensory neurons were involved in protection exerted by PAR-2 AP, TNBS-injected mice were treated with 1.5 mg/kg per day SLIGRL-NH2 in combination with CGRP8–37 (Sigma). CGRP8–37 was injected four times a day (10 μ/kg each time s.c.) for 7 days (14, 22). In another set of experiments, mice were pretreated with the sensory neurotoxin capsaicin (Sigma) to ablate sensory neurons (14, 35). Under halothane anesthesia, mice received three doses of capsaicin (25, 50, and 50 mg/kg s.c.) dissolved in vehicle (10% ethanol, 10% Tween 80, sterile physiological saline) over 32 h (at 0, 6, and 32 h, respectively; 125 mg/kg total dose) or an equivalent volume of vehicle. Mice were studied 7 days after the last injection. On day 7 colitis was induced by intrarectal instillation of TNBS. Mice were then left untreated or treated with SLIGRL-NH2, 1.5 mg/kg per day for 7 days.
Macroscopic and Histologic Grading of Colitis.
The colon was examined under a dissecting microscope (×5) to evaluate the macroscopic lesions according to previously published scores. Macroscopic colon lesions were graded on a scale from 0 to 10 based on criteria reflecting inflammation, such as hyperemia, thickening of the bowel, and the extent of ulceration (32). For histologic examination a colon specimen located precisely 2 cm above the anal canal was obtained, fixed in 10% buffered formalin phosphate embedded in paraffin, cut into sections, and stained with hematoxylin and eosin. The degree of inflammation on microscopic cross sections was graded semiquantitatively from 0 to 4 (score 0: no signs of inflammation; score 1: very low level of inflammation; score 2: low level of leukocyte infiltration; score 3: high level of leukocyte infiltration, high vascular density, thickening of the colon wall; score 4: transmural infiltrations, loss of goblet cells, high vascular density, thickening of the colon wall) (17, 20).
Isolation and Purification of Lamina Propria (LP) T Lymphocytes.
LP T cells (LPT) were isolated from freshly obtained colonic specimens by using a modification of the technique described by Van der Heijden and Stok (38). In brief, colons were washed in Hanks' balanced salt solution calcium and magnesium free medium, and then treated with 1 mM EDTA in PBS for 20 min to remove the epithelium. The tissue was then digested with type IV collagenase (Sigma) and released cells were layered on a 40–100% Percoll gradient (Amersham Pharmacia) and spun at 1,800 rpm to obtain the lymphocyte-enriched populations at the 40–100% interface. Enriched CD4+ T cell populations were obtained from these lymphocytes by positive selection by using MS-type columns and CD4+-labeled microbeads in a magnetic cell sorting system (Milteny Biotec, Florence, Italy). Flow cytometric analysis (Epics XL, Beckman Coulter) showed that the resulting cell population contained about 90% CD4+ T lymphocytes.
Culture of LPT for Assay of Cytokine Production.
LPT obtained from control, ethanol-treated, TNBS-treated, and TNBS/SLIGRL-NH2-treated mice were suspended in complete medium consisting of RPMI 1640 supplemented with 3 mM l-glutamine, 10 mM Hepes buffer, 10 μg/ml gentamycin, 100 units/ml each of penicillin and streptomycin, 0.05 mM 2-mercaptoethanol, and 10% heat-inactivated FCS and cultured at a concentration of 106 cells/ml. To measure cytokine production 106 LPT were loaded into uncoated culture wells (Costar) or wells coated with 10 μg/ml murine anti-CD3ɛ antibody (clone 145–2C11; PharMingen). Cytokine production was then stimulated by adding 1 μg/ml soluble anti-CD28 antibody (clone 37.51; PharMingen) and cells were cultured for 48 h. The culture supernatants were then harvested and assayed for cytokine concentration by ELISA kits (Endogen). In some experiments LPT obtained from control (ethanol-treated) and TNBS-treated mice were stimulated with anti-CD3/CD28 antibody in the presence of increasing concentrations of SLIGRL-NH2 or LSIGRL-NH2, and IFN-γ release in cell supernatant was measured.
LPT Proliferation.
LPT proliferation assays was performed by culturing purified LPT (5 × 105/well) in 96-well microtiter plates for 72 h. After incubation, cultures were pulsed for 7 h with [3H]thymidine (0.5 μCi/well) (New England Nuclear), harvested on glass fiber filters, and counted for radioactivity (in cpm) in a liquid scintillation system (39). Recombinant IL-2 (Endogen) was added to cultures as a stimulant.
CD44 Expression.
LPT obtained from animals treated in different ways were stained with 5–10 μg/ml with phycoerythrin-conjugated rat anti-mouse CD44 (clone IM7, PharMingen) or rat IgG2b Ig (PharMingen) as an isotype-matched control antibody. Cells were then fixed with 3.7% formaldehyde and analyzed on an Epic XL flow cytometer (Beckman-Coulter). (39) For the Western blot analysis, lysates were prepared from LPT obtained from TNBS-treated mice and incubated in vitro with 10 μM of PAR-2 AP or scramble peptide. Lysates were electrophoresed on a SDS-polyacrylamide gel and filters were incubated with the primary rat anti-mouse CD44 antibody (PharMingen), followed by the secondary antibody, goat anti-rat antibody (PharMingen). Precipitates were detected by using an enhanced chemiluminescence system (Amersham Pharmacia), and signals were quantified by using Bio-Rad Gel Doc 1000 molecular analyst software.
Data Analysis.
All values are expressed as mean ± SE of mice/experiments. The variation between data sets was tested with the Student's t test of unpaired data and ANOVA (40).
Results
PAR-2 Activation Protects Against TNBS-Induced Colitis.
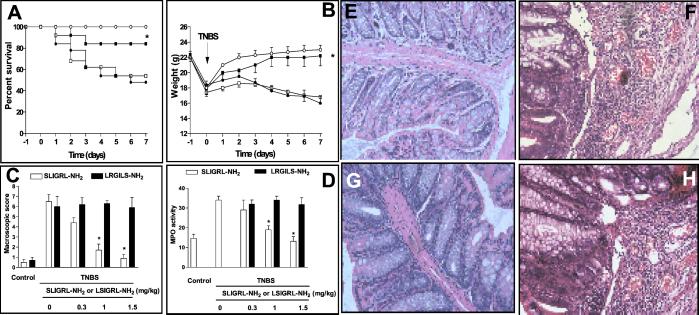
Although no mortality was observed in animals treated with 1 mg TNBS, higher doses of the haptenizing agent resulted in 15%, 40%, and 62% mortality 7 days after induction of colitis. A severe illness characterized by bloody diarrhea and severe wasting disease was observed in mice treated with 2 and 2.5 mg TNBS. Treating mice with 1.5 mg/kg of SLIGRL-NH2, but not with the scramble peptide, significantly reduced lethality caused by TNBS (Fig. 1A).
Figure 1.
(A) Effect of PAR-2 activation on survival rate of mice given TNBS enema. BALB/c mice were given 2.5 mg/mouse TNBS intracolonically on days 0 and then treated with saline (TNBS alone) or 1.5 mg/kg per day SLIGRL-NH2 or LSIGRL-NH2 for 7 days. Each group contained 12–16 mice. *, P < 0.01 versus mice treated with TNBS alone or TNBS plus LRGILS-NH2. (B) Time course of wasting disease in mice with TNBS colitis. Intrarectal administration of 1.5 mg/mouse TNBS alone induces bloody diarrhea and wasting disease. Shown are weight changes over a 7-day period occurring in BALB/c control mice treated with 50% ethanol alone (○), mice treated with TNBS alone (●), mice treated with 1.5 mg/kg SLIGRL-NH2 (■), or LSIGRL-NH2, (□). Each point represents average weight data pooled from 8–12 mice. *, P < 0.01 versus TNBS-treated mice. (C and D) Injection of SLIGRL-NH2 protects against development of TNBS colitis in a dose-dependently manner. Each bar is the mean ± SE of 8–12 mice. *, P < 0.01 versus TNBS alone or LSIGRL-NH2. (E_–_H) Histologic analysis (hematoxylin and eosin staining) of the colons from BALB/c mice with hapten-induced colitis. (E) Transparietal colon section of a vehicle-treated mouse (×200). (F) Colon section 7 days after the induction of colitis by TNBS, showing thickening of the colon wall and inflammatory infiltrate in the lamina propria (×200). (G) Transparietal colon section of a mouse, which received 1.5 mg/kg per day SLIGRL-NH2. Shown is subepithelial edema with no inflammatory infiltrate in the mucosa and submucosa (×200). (H) Transparietal colonic section of mice treated with PAR-2 reverse peptide (×200).
We examined whether PAR-2 activation protects against development of colitis induced with 1.5 mg/mouse TNBS. The colons of mice killed 7 days after colitis induction demonstrated a transmural inflammation involving all layers of the bowel wall with a marked increase in the thickness of muscular layer and adherence to surrounding tissues. In addition, a mixed inflammatory cell infiltrate consisting of lymphocytes and neutrophils was observed in the LP of the colon (Fig. 1F). Treating mice with LSIGRL-NH2, but not with the scramble peptide, resulted in a dose-dependent protection against colitis development, as measured by assessing animal weight loss, macroscopic and microscopic scores, and myeloperoxidase activity (Fig. 1).
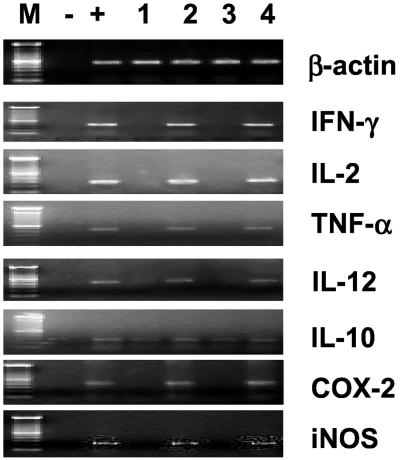
TNBS injection up-regulated Th-1 and Th-2 colonic cytokine mRNA expression, as determined by reverse transcription–PCR (Fig. 2). Daily administration of PAR-2 AP (1.5 mg/kg per day) prevented proinflammatory cytokine induction caused by TNBS. Furthermore, the PAR-2 AP was effective in preventing induction of cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) (Fig. 2) caused by TNBS (16). In contrast, the scramble peptide had no effect on cytokine, COX-2, and iNOS expression.
Figure 2.
PAR-2 activation prevents formation of proinflammatory mediators in the TNBS model of colitis. M, molecular masses; −, negative control (water); +, positive control (cytokine-positive cDNA); lane 1 colon for control (ethanol-treated) mice; lane 2, colon from a mouse treated with TNBS alone: lane 3, colon from a mouse treated with TNBS plus 1.5 mg/kg per day SLIGRL-NH2; and lane 4, colon from a mouse treated with TNBS plus LSIGRL-NH2. COX-2, cyclooxygenase 2.
PAR-2 Activation Inhibits Cytokine Production from LPT.
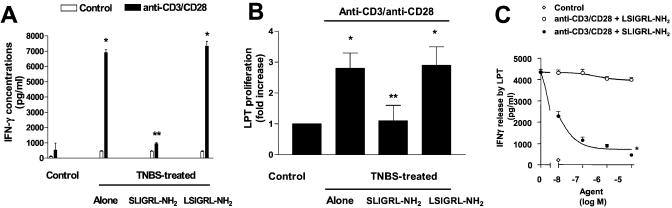
Anti-CD3/anti-CD28 stimulated cytokine production and proliferation of LPT obtained from control (ethanol-treated) and TNBS-treated mice. However, LPT obtained from mice treated with PAR-2 AP generated significantly lower amounts of IFN-γ (Fig. 3A) and IL-2 and IL-12 (data not shown) in response to anti-CD3/anti-CD28 crosslinking. Moreover, administration of PAR-2 AP in vivo significantly reduced proliferative response of LPT (Fig. 3B) induced by exposure to anti-CD3/anti-CD28. As illustrated in Fig. 3C, coincubating LPT with SLIGRL-NH2, but not to LSIGRL-NH2, caused a concentration-dependent inhibition of IFN-γ release induced by anti-CD3/anti-CD28 stimulation, that is consistent with the hypothesis that PAR-2 is localized on T lymphocyte cell surface.
Figure 3.
(A and B) In vitro cytokine production and proliferation of stimulated and unstimulated LPT. LPT were isolated 7 days after TNBS from control (ethanol-treated) mice and mice treated with TNBS alone or in combination with 1.5 mg/kg per day SLIGRL-NH2 or LRGILS-NH2. Data shown represent pooled values from four independent experiments. Standard errors are indicated. *, P < 0.001 versus control; **, P < 0.001 versus TNBS alone or TNBS plus LSIGRL-NH2. (C) IFN-γ production from LPT is inhibited by SLIGRL-NH2. Data are mean ± SE of six experiments carried out in duplicate. *, P < 0.001 versus LSIGRL-NH2.
CGRP8–37 and Capsaicin Reverts Protection Exerted by PAR-2 Activation in TNBS-Treated Mice.
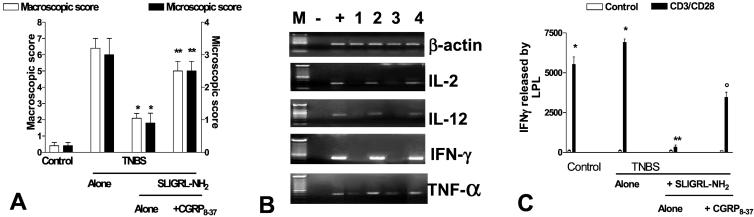
Coadministration of CGRP8–37 with SLIGRL-NH2 or capsaicin pretreatment caused a significant reduction (60–80%) of protective effect exerted by the PAR-2 AP on colitis development as assessed by measuring colitis score (macroscopic and microscopic) and colonic myeloperoxidase activity (Fig. 4 A and B). CGRP8–38 reverted inhibition of IL-2, IL-12, IFN-γ, and tumor necrosis factor α (TNF-α) production caused by PAR-2 activation. In line with this finding LPT isolated from animals treated with the CGRP antagonist secreted significantly more IFN-γ in response to anti-CD3/anti-CD28 antibody than LPT prepared from colitic mice treated with PAR-2 AP alone (P < 0.001).
Figure 4.
CGRP antagonist and sensory neuron ablation reverts protection exerted in vivo by PAR-2 activation in the TNBS model of colitis. (A) Effect of a CGRP receptor antagonist (CGRP8–37) on the macroscopic damage score. *, P < 0.01 versus ethanol treated groups. **, P < 0.01 versus mice treated with SLIGRL-NH2. Each group contained 8–12 mice. (B) Reverse transcription–PCR analysis of Th-1 cytokine mRNA expression 1 week after TNBS admnistration. M, molecular masses; −, negative control (water); +, positive control (cytokine-positive cDNA); lane 1, control (ethanol-treated) mice; lane 2, TNBS alone; lane 3, TNBS plus SLIGRL-NH2, 1.5 mg/kg per day; and lane 4, TNBS plus SLIGRL-NH2 in combination with CGRP8–37. (C) Cytokine production from LPT in TNBS colitis. LPT were isolated from control (ethanol-treated) mice and mice treated with TNBS alone or in combination with 1.5 mg/kg per day SLIGRL-NH2 and CGRP8–37. Data shown represent pooled values from four independent experiments. Standard errors are indicated. *, P < 0.001 versus control; **, P < 0.001 versus TNBS alone; ○, P < 0.01 versus TNBS plus SLIGRL-NH2.
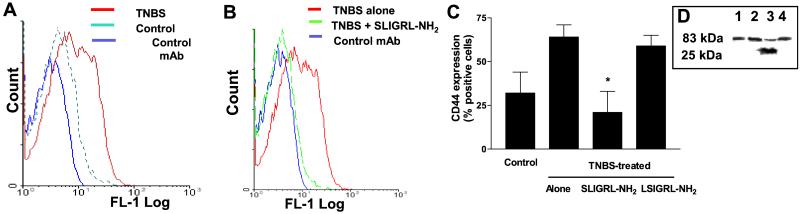
PAR-2 Activation Down-Regulates CD44 Expression on LPT.
Cell surface expression of CD44 was significantly increased on LPT prepared from TNBS-treated mice in comparison with LPT obtained from control mice (Fig. 5A). SLIGRL-NH2 administration completely reverted CD44 up-regulation caused by TNBS (Fig. 5B). This result prompted us to investigate whether PAR-2 AP directly down-regulates CD44 expression on LPT. As illustrated in Fig. 5 C and D, incubating LPT obtained from TNBS-treated mice with 100 μM SLIGRL-NH2, but not with scramble peptide, down-regulated CD44 expression and caused the appearance of CD44 cleavage products.
Figure 5.
TNBS administration increase CD44 expression on LPT cell surface. (A) Increased CD44 expression in LPT obtained from TNBS-treated mice. Increased CD44 expression is indicated by a left shift in fluorescence. (B) PAR-2 activation reverts CD44 up-regulation induced by TNBS. (C) Exposure to 100 μM SLIGRL-NH2, but no to LSIGRL-NH2, in vitro directly down-regulates CD44 expression on LPT obtrained from TNBS-treated mice. (D) Western blot analysis of SLIGRL-NH2-induced CD44 cleavage. Lane 1, control; lane 2, lysates obtained from LPL prepared from TNBS-treated mice; lanes 3 and 4, lysates obtained from LPL prepared from TNBS-treated mice and incubated in vitro with 10 μM SLIGRL-NH2 or LSIGRL-NH2, respectively. The results shown are representative of three independent experiments. Molecular masses of CD44 are shown.
Discussion
In the present study we provide experimental evidence that PAR-2 activation negatively regulates the inflammatory response in the TNBS model of colitis, implying that PAR-2 activation in the colon triggers a protective mechanism(s).
Intrarectal administration of TNBS in mice induces a colon inflammatory response that shares several macroscopic and histologic similarities with human IBD (16–20). These similarities include the presence of a wasting syndrome and a histologic feature of Crohn's disease such as granulomas and mucosal infiltration of neutrophils (16). The TNBS model of colitis is a Th1-driven process, depends highly on the production of IL-12, and requires the activation of the signal transducer and activator of transcription 1 and 4 signaling pathway (41–43).
To gain information on the mediator and the possible mechanism underlying the protective effect exerted in vivo by PAR-2 activation we monitored both expression and colon levels of Th-1 cytokines. Our results demonstrated that SLIGRL-NH2 administration prevented induction of IL-2, IL-12, IFN-γ, and TNF-α, mRNA and protein, caused by TNBS. In line with this effect PAR-2 activation inhibited up-regulation of IFN-γ, and TNF-α regulated inflammatory mediators such as cyclooxygenase 2 and iNOS (16). The inhibitory effect exerted by SLIGRL-NH2 administration in vivo was caused by direct inhibition of LPT activity as demonstrated by the finding that CD4+ LPT prepared from SLIGRL-NH2-treated mice not only released almost undetectable levels of IFN-γ (and IL-2 and IL-12) spontaneously, but were also refractory to stimulation with anti-CD3 and anti-CD28 agonistic antibodies. Moreover, our in vitro studies demonstrated that LPT obtained from TNBS-treated mice not only express PAR-2, as demonstrated by reverse transcription–PCR analysis (data not shown) but exposure of LPT prepared from TNBS-treated mice to SLIGRL-NH2 directly inhibits IFN-γ production and cell proliferation. On these bases, we suggest that protection exerted by SLIGRL-NH2 is caused by its effect on IL-12 induction/production and IFN-γ and TNF-α secretion (16).
A further insight into the mechanism of the action of PAR-2 AP is given by the finding that incubation of CD4+ LPT lymphocytes with SLIGRL-NH2 down-regulates CD44 expression. CD44 is a cell surface glycoprotein transmembrane receptor for hyaluronic acid, a key component of extracellular matrix, and is implicated in a wide variety of adhesion-dependent cellular processes including lymphocyte homing and activation, expression of other adhesion molecules, leukocyte activation, and apoptosis (44–46). CD44 provides costimulatory signals for T cell activation (39, 44–46), and stimulation through CD44 enhances CD28-independent T cell proliferation and production of proinflammatory mediators, including IL-2, IL-1β, and TNF-α. Signaling through the T cell receptor/CD3 complex is involved in the activation of CD44, whereas IL-4 and IL-10 as well as anti-IL-2, anti-IFN-γ, and anti-TNF-α antibodies down-regulate CD44 expression/activation (44–46). A role for CD44 in the regulation of immune responses in vivo has been shown by studies in which anti-CD44 treatment abrogates T lymphocyte-endothelial cell rolling and emigration into inflammatory sites and joint swelling in models of arthritis in mice (47, 48). More importantly, there is evidence that CD44 expression is increased in the colon of patients with Crohn's disease and that CD44 knockout mice are resistant to the TNBS-induced colitis (49–51). Because CD44 is required for T lymphocytes homing and activation in the TNBS model of colitis (49–51), it appears that one possible mechanism involved in inhibition of T lymphocytes recruitment and activation in SLIGRL-NH2-injected mice is the down-regulation of this adhesion molecule. In this respect, our data shed light on some old evidence demonstrating that trypsin reduced an hapten-mediated delayed type of hypersensitivity by inhibiting the homing of T lymphocytes (30).
Previous studies have demonstrated that SLIGRL-NH2 injection activates a capsaicin-sensitive component (26–28). Our results demonstrated that administration of CGRP8–37 and ablation of sensory nerves with capsaicin (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org) reverted, although not completely, the protection exerted by PAR-2 in the TNBS colitis, suggesting that neurogenic and non-neurogenic components are involved in protection exerted by SLIGRL-NH2 (14).
Protection exerted by CGRP against TNBS colitis has been attributed to its ability to induce mucosal hyperemia (24). However, several studies have illustrated the role for CGRP as a regulator of the immune response. Indeed, CGRP inhibits mitogen-stimulated T lymphocyte and thymocyte proliferation, IL-2 production by CD4+ T cells, and antigen presentation by dendritic cells (25, 28). Because of the similarity of their in vitro effects, it cannot be excluded that inhibition of cytokine generation documented in mice treated with SLIGRL-NH2 was, at least partially, mediated through CGRP, indicating that after PAR-2 activation neurogenic, CGRP-dependent, and non-neurogenic pathways are activated.
In summary, in this study we addressed the role of PAR-2 in a murine model of colitis. We showed that activation of PAR-2 in the TNBS model of colitis exerts potent anti-inflammatory activities. Further, we demonstrated that PAR-2 activation directly inhibits Th-1 cytokine production from LP CD4+ T lymphocytes in vitro. Overall these data demonstrate that PAR-2 represents a defensive latent mechanism that can be activated in the presence of inflammatory stimuli and suggest that failure of PAR-2 to limit colonic mucosal immune system reactivity plays a role in the pathogenesis of IBD.
Supplementary Material
Supporting Figure
Abbreviations
CGRP
calcitonin gene-related peptide
IBD
inflammatory bowel disease
iNOS
inducible NO synthase
LP
lamina propria
LPT
LP T cells
PAR
proteinase-activated receptor
PAR AP
PAR agonistic peptide
Th-1
T helper cell type 1
TNBS
2,4,6-trinitrobenzene sulfonic acid
TNF-α
tumor necrosis factor α
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dery O, Corvera C U, Steinhoff M, Bunnett N W. Am J Physiol. 1998;274:1429–1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 2.Cirino G, Bucci M, Cicala C, Napoli C. Trends Pharmacol Sci. 2000;21:170–172. doi: 10.1016/s0165-6147(00)01469-3. [DOI] [PubMed] [Google Scholar]
- 3.Vergnolle N, Wallace J L, Bunnett N W, Hollenberg M D. Trends Pharmacol Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- 4.Vu T K, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 5.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nystedt S, Emilsson K, Larsson A K, Strombeck B, Sundelin J. Eur J Biochem. 1995;232:84–89. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- 7.D'Andrea M R, Derian C K, Leturcq D, Baker S M, Brunmark A, Ling P, Darrow A L, Santulli R J, Brass L F, Andrade-Gordon P. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 8.Böhm S K, Kong W, Brömme D, Smeekens S P, Anderson D C, Connolly A, Kahn M, Nelken N A, Coughlin S R, Payan D G, Bunnett N W. Biochem J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergnolle N. Aliment Pharmacol Ther. 2000;14:257–266. doi: 10.1046/j.1365-2036.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 10.Corvera C U, Dery O, McConalogue K, Bohm S K, Khitin L M, Caughey G H, Payan D G, Bunnett N W. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nystedt S, Ramakrishnan V, Sundelin J. J Biol Chem. 1996;271:14910–14914. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- 12.Vergnolle N. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- 13.Hou L, Kapas S, Cruchley A T, Macey M G, Harriott P, Chinni C, Stone S R, Howells G L. Immunology. 1998;94:356–362. doi: 10.1046/j.1365-2567.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinhoff M, Vergnolle N, Young S H, Tognetto M, Amadesi S, Ennes H S, Trevisani M, Hollenberg M D, Wallace J L, Caughey G H, et al. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 15.Kawabata A, Kinoshita M, Nishikawa H, Kuroda R, Nishida M, Araki H, Arizono N, Oda Y, Kakehi K. J Clin Inv. 2001;107:1443–1450. doi: 10.1172/JCI10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiocchi C. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 17.Blumberg R S, Saubermann L J, Strober W. Curr Opin Immunol. 1999;11:648–656. doi: 10.1016/s0952-7915(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 18.Neurath M F, Fuss I, Kelsall B L, Stüber E, Strober W. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuber W, Strober R, Neurath M. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohi T, Fujihashi K, Rennert P D, Iwatani K, Kiyono H, McGhee J R. J Exp Med. 1999;189:1169–1180. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinshagen M, Rohm H, Steinkamp M, Lieb K, Geerling I, Von Herbay A, Flamig G, Eysselein V E, Adler G. Gastroenterology. 2000;119:368–376. doi: 10.1053/gast.2000.9307. [DOI] [PubMed] [Google Scholar]
- 22.Reinshagen M, Patel A, Sottili M, French S, Sterniniand C, Eysselein V E. J Pharmacol Exp Ther. 1998;286:657–661. [PubMed] [Google Scholar]
- 23.Reinshagen M, Flamig G, Ernst S, Geerling I, Wong H, Walsh J H, Eysselein V E, Adler G. Am J Physiol. 1996;270:G79–G86. doi: 10.1152/ajpgi.1996.270.1.G79. [DOI] [PubMed] [Google Scholar]
- 24.Collins S M. Gastroenterology. 1996;111:1683–1699. doi: 10.1016/s0016-5085(96)70034-3. [DOI] [PubMed] [Google Scholar]
- 25.Xing L, Guo J, Wang X. J Immunol. 2000;165:4359–4366. doi: 10.4049/jimmunol.165.8.4359. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura N, Tamura H, Obana S, Wenner M, Ishikawa T, Nakata A, Yamamoto H. Neuroimmunomodulation. 1998;5:9–15. doi: 10.1159/000026321. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez S, Knopf M A, McGillis J P. J Leukocyte Biol. 2000;67:669–676. doi: 10.1002/jlb.67.5.669. [DOI] [PubMed] [Google Scholar]
- 28.Millet I, Phillips R J, Sherwin R S, Ghosh S, Voll R E, Flavell R A, Vignery A, Rincon M. J Biol Chem. 2000;275:15114–15121. doi: 10.1074/jbc.275.20.15114. [DOI] [PubMed] [Google Scholar]
- 29.Mari B, Guerin S, Far D F, Breitmayer J P, Belhacene N, Peyron J F, Rossi B, Auberger P. FASEB J. 1996;10:309–316. doi: 10.1096/fasebj.10.2.8641564. [DOI] [PubMed] [Google Scholar]
- 30.Rannie G H, Smith M E, Ford W L. Nature (London) 1977;267:321–323. [Google Scholar]
- 31.Damiano B P, Cheung W M, Santulli R J, Fung-Leung W P, Ngo K, Ye R D, Darrow A L, Derian C K, de Garavilla L, Andrade G P. J Pharmacol Exp Ther. 1999;288:671–678. [PubMed] [Google Scholar]
- 32.Kawabata A, Saifeddine M, Al-Ani B, Leblond L, Hollenberg M D. J Pharmacol Exp Ther. 1999;288:358–370. [PubMed] [Google Scholar]
- 33.Andrade-Gordon P, Maryanoff B E, Derian C K, Zhang H C, Addo M F, Darrow A L, Eckardt A J, Hoekstra W J, McComsey D F, Oksenberg D, et al. Proc Natl Acad Sci USA. 1999;96:12257–12262. doi: 10.1073/pnas.96.22.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace J L, MacNaughton W K, Morris G P, Beck P L. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- 35.McCafferty D M, Wallace J L, Sharkey K A. Am J Physiol. 1997;272:G272–G280. doi: 10.1152/ajpgi.1997.272.2.G272. [DOI] [PubMed] [Google Scholar]
- 36.Fiorucci S, Santucci L, Antonelli E, Distrutti E, Del Sero G, Morelli O, Romani L, Federici B, Del Soldato P, Morelli A. Gastroenterology. 2000;118:404–442. doi: 10.1016/s0016-5085(00)70223-x. [DOI] [PubMed] [Google Scholar]
- 37.Fiorucci S, Santucci L, Cirino G, Mencarelli A, Familiari L, Soldato P D, Morelli A. J Immunol. 2000;165:5245–5254. doi: 10.4049/jimmunol.165.9.5245. [DOI] [PubMed] [Google Scholar]
- 38.Van der Heijden P J, Stok W. J Immunol Methods. 1987;103:161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- 39.Galluzzo E, Albi N, Fiorucci S, Merigiola C, Ruggeri L, Tosti A, Grossi C E, Velardi A. Eur J Immunol. 1995;25:2932–2939. doi: 10.1002/eji.1830251033. [DOI] [PubMed] [Google Scholar]
- 40.Wardlaw A C. Practical Statistics for Experimental Biologists. 2nd Ed. New York: Wiley; 2000. [Google Scholar]
- 41.Jacobson N G, Szabo S J, Weber-Nordt R M, Zhong Z, Schreiber R D, Darnell J E, Murphy K M. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thierfelder W E, Van Deursen J M, Yamamoto K, Tripp R A, Sarawar S R, Carson R T, Sangster M Y, Vignali D A, Doherty P C, Grosveld G C, Ihle J N. Nature (London) 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 43.Simpson S J, Shah S, Comiskey M, de Jong Y P, Wang B, Mizoguchi E, Bhan A K, Terhorst C. J Exp Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lesley J, Hyman R, Kincade P. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 45.Huet S, Groux H, Caillou B, Valentin H, Prieur A M, Bernard A. J Immunol. 1989;143:798–801. [PubMed] [Google Scholar]
- 46.Shimizu Y, Van Seventer G, Siraganian R, Wahl L, Shaw S. J Immunol. 1989;143:2457–2463. [PubMed] [Google Scholar]
- 47.DeGrendele H C, Estess P, Picker L J, Siegelman M H. J Exp Med. 1996;183:1119–1127. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikecz K, Brennan F R, Kim J H, Glant T T. Nat Med. 1995;1:558–563. doi: 10.1038/nm0695-558. [DOI] [PubMed] [Google Scholar]
- 49.Wittig B, Seiter S, Schmidt D S, Zuber M, Neurath M, Zoller M. Lab Invest. 1999;79:747–759. [PubMed] [Google Scholar]
- 50.Wittig B M, Johansson B, Zoller M, Schwarzler C, Gunthert U. J Exp Med. 2000;191:2053–2064. doi: 10.1084/jem.191.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wittig B, Schwarzler C, Fohr N, Gunthert U, Zoller M. J Immunol. 1998;161:1069–1073. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure